Abstract
Objective:
Psychostimulants are first line pharmacological treatments for Attention Deficit/Hyperactivity Disorder (ADHD), although symptom reduction varies widely between patients and is poorly understood. We sought to examine whether the resting-state functional connectivity within and between cingulo-opercular, striato-thalamic and default-mode networks was associated with treatment response to psychostimulant medication, and whether this relationship changed with development.
Methods:
Patients (N=110, 196 observations, mean age at first observation = 10.83 years, sd=2.2) and typically developing controls (N=142, 330 observations, mean age at first observation = 10.49 years, sd=2.81) underwent functional neuroimaging on up to five occasions during development (age range 6–17 years). For patients, symptoms were assessed on and off psychostimulant medication (methylphenidate-based treatments: N=132 observations, 67%; amphetamine-based treatments: N=64 observations, 33%) using the Diagnostic Interview for Children and Adolescents for parents. Linear mixed-effects models examined whether resting-state connectivity was associated with treatment response and its interaction with age. Comparisons with typically developing controls were performed to contextualize any significant associations.
Results:
Resting-state connectivity within the cingulo-opercular network was associated with a significant interaction between treatment response and age. Specifically, worse responses to treatment, compared with better responses to treatment and typically developing controls, were associated with an atypical increase in cingulo-opercular connectivity with increasing age from childhood to adolescence.
Conclusion:
This work delineates how resting-state connectivity may be associated over development with response to psychostimulants in ADHD. Functioning and development within the cingulo-opercular network may warrant further investigation as a contributor to differential response to psychostimulants.
Introduction
Psychostimulants, including methylphenidate-based and amphetamine-based agents, are first line pharmacological treatments for attention-deficit/hyperactivity disorder (ADHD) (1–3), one of the most prevalent disorders of childhood (4). While there are important differences in the actions of methylphenidate-based and amphetamine-based medications (5), both classes appear to increase dopamine and norepinephrine action, thus impacting on facets of cognition pertinent to ADHD symptomatology (1, 5). Evidence from randomized double-blind placebo-controlled trials indicates that both subtypes of psychostimulants are efficacious relative to placebo in patients with ADHD (1, 2). Although meta-analytic findings suggests that effect-sizes for treatment response relative to placebo are larger for amphetamine-based (d=−1.02) than methylphenidate-based agents (d=−0.78), the latter are often better tolerated in children and adolescents with the disorder and, of patients who respond well to at least one psychostimulant, the largest portion (48%) respond equally well to both medication types, meaning that methylphenidate-based agents are the most commonly prescribed in this population (1, 2).
Findings from in vivo neuroimaging studies converge on three sets of brain regions that are believed to be particularly relevant to the pathophysiology of ADHD and treatment response to psychostimulants (6–8). Several studies have investigated brain changes in response to acute doses of psychostimulant medication, reporting a normalization of cingulo-opercular and striato-thalamic hypoactivation and default-mode deactivation during task-based fMRI in patients with ADHD (6, 8–13). Abnormalities have also been reported in these networks at rest, although not without inconsistencies in the literature, perhaps in part due to methodological heterogeneity and small sample sizes used in previous studies (14, 15). However, effects of psychostimulants on resting-state connectivity and metabolism within these networks have also been reported (5, 16–20)(see (21) for a critical overview), as have effects on connectivity observed during task-based fMRI (22).
The existing literature has two major limitations. First, symptom reduction in response to psychostimulants varies widely between patients, with an estimated 10–30% not benefiting adequately from treatment, and the scientific literature provides only limited insights on the neural correlates of treatment responsivity (1, 2). Although many neuroimaging studies have examined brain changes when patients are “on” versus “off” psychostimulant medication, little previous work has examined whether individual differences in treatment response are associated with differences in brain functioning, such as when assessed off-medication (8, 12). Since psychostimulants are believed to act via a modulation of functioning within cingulo-opercular, striato-thalamic and default-mode regions, it is plausible that treatment response could be associated with functioning within these regions (8). Second, ADHD is a developmental disorder and the brain continues to develop throughout adolescence (23–25), with cross-sectional comparisons indicating differences in children/adolescents relative to adults with the disorder (26), and longitudinal studies suggesting different neurodevelopmental trajectories in ADHD (27). However, most previous studies used cross-sectional designs and did not examine potential relationships between neurodevelopmental change in brain networks and treatment response to psychostimulants.
In this study, 110 children and adolescents with ADHD underwent assessments of treatment response in tandem with functional neuroimaging scans up to 5 times during development. In addition, 142 typically developing controls underwent longitudinal neuroimaging. An accelerated longitudinal design was used, with the collection of multiple assessments per subject over time, and subjects entering the study at different ages. This design allowed for a time-efficient coverage over the age range of interest, and is well suited for the examination of developmental research questions such as whether treatment effects and their associations with brain functioning change with development (25).
In light of previous work showing changes in functional connectivity with development, as well as pharmaco-fMRI work implicating these networks in the actions of psychostimulants (8, 9), we examined whether resting-state connectivity within and between cingulo-opercular, striato-thalamic and default-mode networks would be associated with treatment response, as well as whether potential relationships changed with development. However, given that this is the first naturalistic study to examine possible associations between resting-state connectivity and long-term treatment response to psychostimulant medication, we did not form specific hypotheses regarding the direction of effects.
Methods
Participants
Patients (N=110, age range 6–17 years old) were selected from an ongoing longitudinal study taking place at the National Institutes of Health, the aim of which is to study the neural and genetic basis of ADHD symptoms using longitudinal data (27, 28). In this analysis, we included data collected from psychostimulant-treated patients with ADHD and typically developing controls between February 2011 and October 2019. Specifically, patients with ADHD had to have good quality neuroimaging data that was collected in tandem with an assessment of symptom response to psychostimulant medication. We defined typically developing controls as those with ≤3 total symptoms and ≤2 symptoms of either hyperactivity/impulsivity or inattention at all timepoints. Principal exclusion criteria included IQ less than 80, neurologic disorders affecting brain structure, current substance dependence, or psychotic disorders.
Study Design
Patients and controls underwent symptom assessments and neuroimaging scans on up to five occasions during childhood and adolescence. A diagnosis of ADHD was established through a clinician led interview with parents (Diagnostic Interview for Children and Adolescents-IV; DICA-IV). This interview determines the number of symptoms of inattention and hyperactivity/impulsivity, with a range from 0 to 9 symptoms in each category (29). Establishing that the child had 6 or more symptoms of hyperactivity/impulsivity and inattention indicated a combined presentation, while ≥6 symptoms of hyperactivity/impulsivity indicated a predominantly hyperactive/impulsive presentation, and ≥6 symptoms of inattention indicated a predominantly inattentive presentation. Changes in symptom profiles with age were ascertained through the same interview at each timepoint. Specifically, parents provided reports on symptoms for the time window shortly after their child had taken psychostimulant medication. Parents also reported symptoms off-medication, reflecting symptoms during days that the child had not taken medication (e.g., at weekends or during school holidays for some children), or in the late afternoon and evening for children who took psychostimulant medication with no breaks. Treatment response was thus defined as percent symptom reduction when rated on- versus off-medication for each patient at each timepoint. Patients did not take psychostimulant medication on the day of scanning. All psychostimulant daily dosages were converted to their oral methylphenidate-equivalent dosage (30, 31), and expressed as methylphenidate-equivalent per kg of body weight per day (daily mg/kg).
Written informed consent was obtained from parents and/or legal guardians, and assent from children, as approved by the institutional review board of the National Human Genome Research Institute. Details regarding excluded data are given in the Supplement.
Preprocessing
fMRI preprocessing was implemented in fMRIPrep and xcpEngine packages (32, 33) (see Supplement). We focused our analyses on three networks (cingulo-opercular, striato-thalamic and default-mode). Full details regarding the rationale for choosing these networks, as well as details on region of interest definitions, are provided in the Supplement. See Supplementary Table 1 and Supplementary Figure 1.
The time courses from all ROIs were correlated with each other to create an ROI-to-ROI connectivity matrix for each subject. Fisher’s r-to-z transformation was then applied to the Pearson correlation coefficients at each cell of the resultant matrices. We examined connectivity averaged within and between a priori defined cingulo-opercular, striato-thalamic and default-mode networks. Within-network connectivity was determined by averaging Fisher-z transformed correlation coefficients for intra-network connections separately for each network, to create three within network connectivity metrics (within cingulo-opercular, within striato-thalamic and within default-mode). Between network connectivity was calculated for each pairwise combination of networks (cingulo-opercular to striato-thalamic, striato-thalamic to default-mode and cingulo-opercular to default-mode) by averaging the Fisher-z transformed correlation coefficients of the relevant inter-network connections.
For completion and to determine which connections were driving network-level findings, exploratory follow-up edgewise analyses were performed at the level of individual ROI-to-ROI connections (see Supplement).
Statistical analyses
Participant characteristics and treatment response
Analyses were conducted using linear mixed effects models as implemented in the nlme package for R (http://www.r-project.org), while including a random term for each individual that was nested within a random term for each family, thereby accounting for both within-person and within-family dependence (34). This approach was used because our data contains a mixture of multiple observations per subject, measured at different and irregular time periods, as well as single observations per subject. Details on analyses conducted to examine relationships between demographic, clinical and treatment response variables are given in the Supplement.
fMRI analyses
With regards to the brain, in model one we examined whether the development of resting-state connectivity varied as a function of treatment response to psychostimulant medication. We achieved this by regressing each connectivity metric against the interaction between treatment response (% symptom reduction when patients were rated on versus off-medication) and age while controlling for the main effects of age, sex, dosage, off-medication symptom severity, treatment response and motion.
To aid the interpretation of potential associations between the development of resting-state connectivity and treatment response to psychostimulant medication in the context of normative development, we next compared connectivity changes with age between “better” treatment responses (>mean symptom reduction), “worse” treatment responses (<mean symptom reduction) and typically developing controls. In model two we thus regressed connectivity metrics against the interaction between treatment response group (better responses, worse responses, typically developing controls) and age while controlling for the main effects of age, sex, treatment response group and motion. In model three we examined the effects of age on connectivity separately at each level of the group variable. Full model details are given in the Supplement. In addition to examining treatment response by age interactions, we also examined the main effect of treatment response on brain functioning. We corrected for the number of within and between network metrics examined using the Benjamini-Hochberg method (35).
Results
Participant characteristics and treatment response
Demographic details are given in Table 1. There was a higher proportion of females in the typically developing group (61/142,42.96%) compared with the ADHD group (28/110,25.45%) (χ2=8.31,p=0.004), and gender was controlled for in all analyses. Patients were receiving methylphenidate-based medications during 132 observations (67.34%; daily mg/kg mean=0.86, range= 0.14,2.5) and amphetamine-based medications on 64 observations (32.65%; daily mg/kg mean=0.82, range=0.23,2.14). A minority of subjects switched between methylphenidate and amphetamine-based agents during the course of the study (N=7;6.36%), while 69 (62.72%) always received methylphenidate and 34 (30.9%) always received amphetamine-based medications. Details on ADHD presentations are given in the Supplement.
Table 1.
Clinical and demographic characteristics of patients with ADHD (N=110) and typically developing controls (N=142) at each timepoint of the study.
| Characteristic | ADHD (N=110) | TD (N=142) | Test of significance | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Female | 28 | 25.45% | 61 | 42.96 | χ2=8.31, p=0.004 | ||
| N | |||||||
| Time 1 | 110 | 100% | 142 | 100% | - | ||
| Time 2 | 54 | 49.09% | 87 | 61.27% | - | ||
| Time 3 | 19 | 17.27% | 53 | 37.32% | - | ||
| Time 4 | 9 | 8.1% | 33 | 23.24% | - | ||
| Time 5 | 4 | 3.6% | 15 | 10.56% | - | ||
| Mean | SD | Range | Mean | SD | Range | ||
| Age, y | |||||||
| Time 1 | 10.83 | 2.2 | 6–16 | 10.49 | 2.81 | 6–17 | t(250)=1.04, p=0.3 |
| Time 2 | 12.34 | 2.35 | 7–17 | 11.89 | 2.54 | 7–16 | t(139)=1.05, p=0.29 |
| Time 3 | 13.45 | 2.27 | 10–17 | 13.15 | 2.45 | 8–17 | t(70)=0.47, p=0.64 |
| Time 4 | 14.03 | 2.12 | 11–17 | 14.07 | 2.14 | 9–17 | t(40)=0.05, p=0.96 |
| Time 5 | 14.48 | 2.19 | 12–17 | 15.16 | 1.4 | 13–17 | t(19)=0.77, p=0.45 |
| ADHD symptoms | |||||||
| Time 1 | 11.35 | 3.42 | 3–18 | 0.25 | 0.7 | 0–3 | t(250)=37.69, p<0.001 |
| Time 2 | 10.2 | 3.43 | 2–18 | 0.24 | 0.61 | 0–3 | t(139)=26.47, p<0.001 |
| Time 3 | 10.63 | 3.62 | 4–17 | 0.13 | 0.44 | 0–2 | t(70)=20.95, p<0.001 |
| Time 4 | 10.11 | 2.71 | 5–13 | 0.18 | 0.64 | 0–3 | t(40)=19.7, p<0.001 |
| Time 5 | 11.75 | 1.71 | 10–14 | 0.2 | 0.41 | 0–1 | t(19)=25.37, p<0.001 |
| ADHD treatment response (%) | |||||||
| Time 1 | 69.92% | 0.27 | 0–100% | - | - | - | - |
| Time 2 | 67.79% | 0.24 | 11–100% | - | - | - | - |
| Time 3 | 69.02% | 0.24 | 33–100% | - | - | - | - |
| Time 4 | 70.1% | 0.24 | 33–100% | - | - | - | - |
| Time 5 | 61.24% | 0.23 | 35–91% | - | - | - | - |
| Dosage (daily methylphenidate-equivalent mg/kg) | |||||||
| Time 1 | 0.82 | 0.46 | 0.15–2.25 | - | - | - | - |
| Time 2 | 0.86 | 0.43 | 0.14–2.04 | - | - | - | - |
| Time 3 | 0.88 | 0.36 | 0.5–1.86 | - | - | - | - |
| Time 4 | 0.86 | 0.33 | 0.27–1.31 | - | - | - | - |
| Time 5 | 0.88 | 0.2 | 0.66–1.1 | - | - | - | - |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; mg/kg, milligram per kilogram; TD, typically developing; y, years.
Treatment response remained stable with age (B=−0.009,t=−1.14,p=0.26,95%CI=−0.03,0.007), and was not associated with psychostimulant dosage (B=0.04,t=0.8,p=0.4,95%CI=−0.05,0.1), sex (B=0.01,t=0.27,p=0.8,95%CI=−0.09,0.1), off-medication symptoms (B=0.0008,t=0.13,p=0.85, 95%CI= −0.01,0.01) or psychostimulant class (methylphenidate or amphetamine-based; B=0.02,t=0.45,p=0.65, 95%CI=−0.07, 0.1). Off-medication symptoms declined with age (B=−0.53,t=−6.3,p<0.001,95%CI=−0.7,−0.3). Psychostimulant methylphenidate equivalent dosage was not associated with age (B=−0.02,t=−1.56,p=0.12,95%CI=−0.04,0.006), psychostimulant class (methylphenidate versus amphetamine; B=0.05,t=0.62,p=0.5,95%CI=−0.1,0.2) or off-medication symptoms (B=0.005,t=0.49,p=0.6,95%CI=−0.02,0.02).
Owing to the accelerated design, some subjects had more scans than others available at the time of analysis (e.g., due to starting the study at an earlier point in time). However, patients with <3 scans did not differ from those with ≥3 scans with regards to treatment response (B=0.02,t= 0.45,p=0.7,95%CI=−0.09,0.1), off-medication symptoms (B=0.02,t=0.28,p=0.79,95%CI=−1.5,1.9), motion (B=−0.05,t=−1.5,p=0.13,95%CI=−0.1,0.02), dosage (B=0.04,t=−0.36,p=0.73,95%CI=−0.2,0.3) or psychostimulant class (methylphenidate or amphetamine; χ2=0.06, p=0.8). The primary finding also remained significant when analyses were restricted to the first two or first three scans (see the online supplement). Thirteen out of 110 patients (11.81%) stopped taking psychostimulant medication permanently while part of the study. Four of these patients stopped treatment and were in remission (≤3 off-medication symptoms); two patients, who were showing “worse” treatment responses (i.e., worse than the mean treatment response), also stopped medication. Finally, seven stopped medication, even though they were showing “better” responses at their last assessment (i.e., better than the mean treatment response).
fMRI Results
Network results
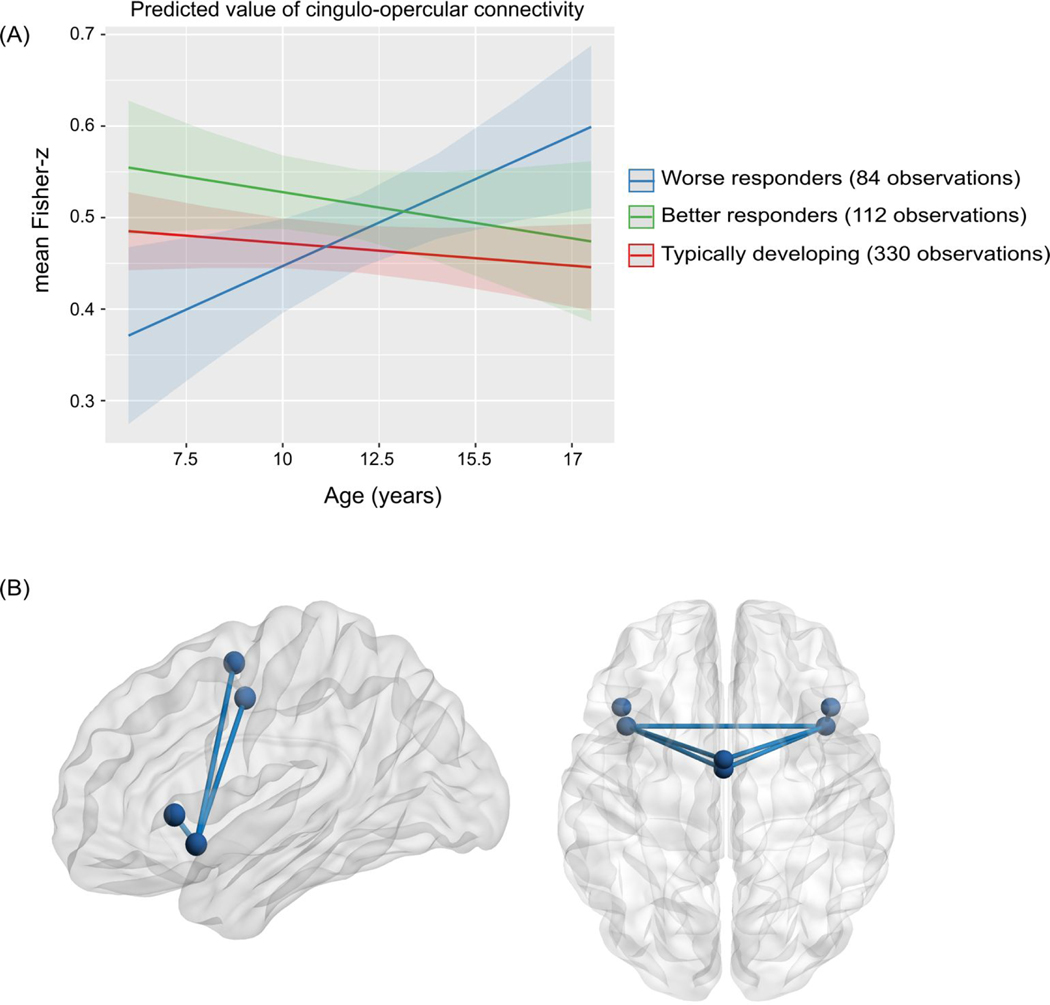
Model one showed that resting-state connectivity within the cingulo-opercular network was explained by a significant interaction between age and treatment response (B=−0.07,t=−3.4,adjusted p=0.006,95%CI=−0.1,−0.03). No other within or between network metrics were significant even at an uncorrected threshold (p>0.1).
In model two, a significant interaction between group and age on cingulo-opercular connectivity was found (F(2,266)=4.86,p=0.008). Specifically, age-related changes in connectivity associated with worse psychostimulant responses (i.e., a treatment response less than the mean; 84 observations from N=63, 25.4% female, mean age=12.25 years) differed significantly from changes associated with better psychostimulant responses (i.e., a treatment response greater than the mean; 112 observations from N=76, 27.63% female, mean age=11.33 years; B=−0.03,t=−2.79,p=0.006,95%CI=−0.04,−0.008) and the typically developing controls (B=0.02,t=2.93,p=0.004,95%CI=0.007,0.04). In contrast, a better response to psychostimulant treatment was associated with age-related stability in cingulo-opercular connectivity that was similar to connectivity observed in the typically developing group (B=−0.003,t=−0.51,p=0.6,95%CI=−0.02,0.01).
Model three showed that worse responses to psychostimulant medication were associated with increased connectivity with increasing age (B=0.02,t=2.6,p=0.02,95%CI=0.004,0.04). Connectivity associated with better responses did not change with age (B=−0.007,t=−1.06,p=0.3,95%CI=−0.02,0.007), nor did it for the typically developing controls (B=−0.005,t=−1.49,p=0.14,95%CI=−0.01,0.001).
There were no significant main effects of treatment response or treatment response by age interactions for any other network metrics (all p>0.1). See Figure 1. Sensitivity analyses and robustness checks, including those relating to motion, number of scans available, psychostimulant subtypes, symptom sub-scales and comorbid disorders are provided in the Supplement. See Supplementary Table 2.
Figure 1. Shows the significant interaction between age and treatment response on cingulo-opercular connectivity.
(A). The graph shows the predicted estimates derived from the linear mixed-effects model for the effect of the interaction of age and treatment response group (worse responders, better responders and typically developing controls) on cingulo-opercular connectivity (F(2, 266)= 4.86, p=0.008). The y-axis represents the predicted mean of the Fisher-z transformed correlation coefficients for the cingulo-opercular network based on model estimates, and separate lines indicate group. Error-bars represent 95% confidence intervals. (B). Shows sagittal (left) and axial (right) views of the cingulo-opercular network. Spheres represent nodes (regions of interest) for each of the cingulo-opercular regions. Lines represent cingulo-opercular edges (connections between nodes) that were significant at a nominal p<0.05 uncorrected threshold in the follow-up edgewise analysis.
Edgewise results
No findings survived correction for multiple comparisons at the level of individual ROI-to-ROI connections. Connections that were significant at an uncorrected p<0.05 threshold are presented in Supplementary Table 3, and, in line with the network level findings, include multiple connections between regions of the cingulo-opercular network.
Discussion
In this work, we aimed to examine whether treatment response to psychostimulant medication was associated with resting-state connectivity within a set of cingulo-opercular, striato-thalamic and default-mode networks implicated in pathophysiological models of ADHD and in the therapeutic actions of psychostimulants, as well as whether the associations changed with development. We found that psychostimulant response showed overall stability with age. At a neural level, we found that cingulo-opercular functioning was associated with worse treatment responses in a way that changed with development.
In this work we assessed symptoms on and off-medication at each timepoint meaning that we could separate symptom change due to psychostimulants from age-related symptom reduction. We found that response to psychostimulants remained stable with age in our observational study. This finding is in line with a recent double-blind randomized placebo-controlled treatment discontinuation trial, in which methylphenidate discontinuation in children and adolescents who had undergone treatment for at least two years was associated with a worsening of ADHD symptoms, as compared with treatment continuation (36). Findings are also in line with pharmacoepidemiological work showing long-term associations between psychostimulants and decreases in injuries, accidents and possibly substance abuse (see (37) for a critical review of this literature). Therefore, while causal inferences cannot be made given our observational design, the present findings are consistent with prior reports of stability in the efficacy of psychostimulant treatment with increasing age in children and adolescents with ADHD.
With regards to the brain, we found an association between psychostimulant treatment response and functional connectivity within the cingulo-opercular network. Better treatment responses were associated with a stable level of connectivity between nodes of this network during childhood and adolescence, whereas worse responses were associated with increased connectivity with increasing age. Moreover, typically developing children and adolescents showed age-related stability in cingulo-opercular connectivity, which tracked closely with the trajectory associated with better responses to psychostimulants. These findings are interesting in light of the literature reporting a normative segregation of networks during adolescence involving increasing within-network and decreasing between-network connectivity with age (23). Since network segregation has been proposed to be adaptive and associated with improvements in cognitive functioning with age, the present findings of increasing within-network cingulo-opercular connectivity only in the worse treatment responders might be deemed surprising (23). However, findings from longitudinal research on the development of within cingulo-opercular network connectivity have been mixed and the fact that cingulo-opercular connectivity, at least as defined here, did not increase with age in the typically developing controls indicates that this increase associated with worse responses is atypical (38, 39). While the exact nature of the relationship between functional connectivity, cognitive and behavioral development is unknown, it is generally accepted that complex cognition and behaviors depend upon a carefully balanced interplay between multiple interconnected networks (23, 24). Consequently, the functioning of a given network is proposed to be highly dependent upon the inputs and constraints provided by patterns of connectivity within and between other closely interdependent networks (23). Therefore, connectivity patterns associated with worse treatment responses might be interpreted as developmentally atypical and potentially out of line with ongoing structural, functional and neurochemical development in other brain regions and networks, as well as with ongoing age-related changes in behavioral maturation and environmental demands (24).
That associations with treatment response were observed within the cingulo-opercular network is interesting in light of randomized placebo-controlled fMRI studies of acute methylphenidate effects in medication-naïve ADHD boys showing a normalization of functioning in this network (10, 11, 13, 22, 40), as well as improvements in task performance (11, 13, 40), when under methylphenidate relative to placebo. Normalizing activation within the cingulo-opercular network therefore appears to be an important candidate mechanism of the acute effects of psychostimulant medication (6, 8, 11). Few studies have examined whether the pattern or degree of acute neural changes associated with taking psychostimulants depend upon neural functioning as assessed off-medication (12). However, based on the present findings, the hypothesis emerges that psychostimulants may be better able to normalize recruitment within cingulo-opercular regions during cognitive demands when resting-state connectivity is already more in line with typical development. Alternatively, patients with already typical functioning in a network that undergoes modulation by psychostimulants may experience “better than typical” connectivity when medicated, which might allow them to compensate for their ADHD symptoms. No associations with treatment response were observed involving connectivity of the striato-thalamic and default-mode networks, despite previous work showing changes in these networks in response to acute doses of psychostimulants (9, 10, 12, 13). However, in this study subjects were scanned during the temporary cessation of psychostimulant medication, and it is likely that treatment response also depends upon changes in neural functioning that are induced by psychostimulant medication (12).
The current study informs the understanding of the neural correlates of response to psychostimulants, in a naturalistic setting. The findings suggest that characterization of developing functional connectivity may help us to understand differences in psychostimulant responsivity. However, the study does not inform clinical decisions about the choice of psychostimulants, nor does it provide predictors of treatment response over time. Clinical translation of the findings depends not only on independent replication, but also the ability to characterize developing functional connectivity within a much shorter, clinically useful time frame than is used in the current study. Furthermore, the costs of fMRI data need to be weighed against any gain in predictive power, particularly as psychostimulants act quickly, and adverse effects usually cease when the medication is stopped (1, 3). Finally, given the observational design, we cannot make causal statements about the direction of the relationship between treatment response and developing functional connectivity. Therefore, we argue that rather than setting the stage for use of fMRI in clinical decision-making in the near future, the contribution of the present study is to further our understanding of potential biological processes associated with differences in treatment response to psychostimulant medication, which has been limited.
Further limitations of the present work include the fact that data were collected as part of a naturalistic observational study, and therefore the sample was heterogenous with regards to prescribed psychostimulant medications, duration of treatment and other clinical and demographic variables. Relatedly, we included patients on methylphenidate and amphetamine-based agents which have partly distinct mechanisms of action (5). However, we found that the relationship between cingulo-opercular connectivity, age and treatment response did not change significantly according to psychostimulant class (see Supplement). An additional limitation is that, since patients were receiving long term psychostimulant treatment, this may imply that they had been receiving at least some benefit from the drug. Therefore, findings may not generalize to very poor treatment responders who are more likely to cease taking the medication (2). In addition, ratings of ADHD symptoms were based on interviews with parents; therefore, they might have been biased by expectation effects of parents, who were not blind to treatment. Moreover, we did not have objective data regarding treatment adherence and relied on parental report. Finally, as this analysis used data collected as part of a natural history longitudinal cohort study, sample size was not determined according to a formal a priori power analysis. However, observed power estimates indicated that the study had adequate power to detect medium to large effect sizes for the interaction of interest in the present study, in line with the detected effects (see Supplement).
In summary, we provide the first longitudinal investigation of the relationship between brain connectivity and treatment response to psychostimulants in youth with ADHD. We report that worse responses to psychostimulant treatment may be tied to developmentally atypical functioning within a cingulo-opercular network that has been implicated in both ADHD and psychostimulant action. The findings therefore speak to a potential factor contributing to differences in response to psychostimulant medication.
Supplementary Material
Acknowledgments:
The study was funded by the Intramural Programs of the National Institute of Mental Health and National Human Genome Research Institute (Z01- HG200378).
Footnotes
Disclosures: The authors report no financial relationships with commercial interests.
References
- 1.Cortese S: Pharmacologic Treatment of Attention Deficit–Hyperactivity Disorder. N Engl J Med 2020; 383:1050–1056 [DOI] [PubMed] [Google Scholar]
- 2.Cortese S, Adamo N, Del Giovane C, et al. : Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018; 5:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuddas A, Banaschewski T, Coghill D, et al. : ADHD treatment. Oxf Textb Atten Deficit Hyperact Disord 2018; 379 [Google Scholar]
- 4.Polanczyk GV, Willcutt EG, Salum GA, et al. : ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 2014; 43:434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faraone SV: The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev 2018; 87:255–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubia K: Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci 2018; 12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukito S, Norman L, Carlisi C, et al. : Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med 2020; 50:894–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubia K, Alegria AA, Cubillo AI, et al. : Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry 2014; 76:616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liddle EB, Hollis C, Batty MJ, et al. : Task-related default mode network modulation and inhibitory control in ADHD: Effects of motivation and methylphenidate. J Child Psychol Psychiatry 2011; 52:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubillo A, Smith AB, Barrett N, et al. : Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb Cortex 2014; 24:174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalczyk OS, Cubillo AI, Smith A, et al. : Methylphenidate and atomoxetine normalise fronto-parietal underactivation during sustained attention in ADHD adolescents. Eur Neuropsychopharmacol 2019; 29:1102–1116 [DOI] [PubMed] [Google Scholar]
- 12.Peterson BS, Potenza MN, Wang Z, et al. : An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry 2009; 166:1286–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubia K, Halari R, Mohammad A-M, et al. : Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry 2011; 70:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutcubasi B, Metin B, Kurban MK, et al. : Resting-state network dysconnectivity in ADHD: A system-neuroscience-based meta-analysis. World J Biol Psychiatry 2020; 1–74 [DOI] [PubMed] [Google Scholar]
- 15.Cortese S, Aoki YY, Itahashi T, et al. : Systematic Review and Meta-analysis: Resting State Functional Magnetic Resonance Imaging Studies of Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry 2020; [DOI] [PubMed] [Google Scholar]
- 16.Yoo JH, Kim D, Choi J, et al. : Treatment effect of methylphenidate on intrinsic functional brain network in medication-naïve ADHD children: A multivariate analysis. Brain Imaging Behav 2018; 12:518–531 [DOI] [PubMed] [Google Scholar]
- 17.Silk TJ, Malpas C, Vance A, et al. : The effect of single-dose methylphenidate on resting-state network functional connectivity in ADHD. Brain Imaging Behav 2017; 11:1422–1431 [DOI] [PubMed] [Google Scholar]
- 18.An L, Cao X-H, Cao Q-J, et al. : Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology 2013; 38:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battel L, Kieling RR, Kieling C, et al. : Intrinsic brain connectivity following long-term treatment with methylphenidate in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2016; 26:555–561 [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Kelly C, Castellanos FX, et al. : Neural correlates of symptom improvement following stimulant treatment in adults with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2016; 26:527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira-Sanchez V, Franco AR, Vieira D, et al. : Systematic Review: Medication Effects on Brain Intrinsic Functional Connectivity in Patients With Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry 2020; [DOI] [PubMed] [Google Scholar]
- 22.Rubia K, Halari R, Cubillo A, et al. : Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009; 57:640–652 [DOI] [PubMed] [Google Scholar]
- 23.Bassett DS, Xia CH, Satterthwaite TD: Understanding the emergence of neuropsychiatric disorders with network neuroscience. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:742–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mareschal D, Johnson M, Sirois S, et al. : Neuroconstructivism: How the brain constructs cognition. Oxford University Press, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Shaw P, Gilliam M, Liverpool M, et al. : Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry 2011; 168:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoogman M, Muetzel R, Guimaraes JP, et al. : Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry 2019; 176:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudre G, Sharp W, Kundzicz P, et al. : Predicting the course of ADHD symptoms through the integration of childhood genomic, neural, and cognitive features. Mol Psychiatry 2020; 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudre G, Frederick J, Sharp W, et al. : Mapping associations between polygenic risks for childhood neuropsychiatric disorders, symptoms of attention deficit hyperactivity disorder, cognition, and the brain. Mol Psychiatry 2019; 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reich W: Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000; 39:59–66 [DOI] [PubMed] [Google Scholar]
- 30.Swenson M: Stimulant Equivalency Table [Internet] 2019; Available from: https://www.uacap.org/uploads/3/2/5/0/3250432/stimulant_equivalency.pdf [Google Scholar]
- 31.Kevin M. Nasky: Stimulant Dose Conversion Calculator Beta [Internet]. 2019. Available from: https://psychopharmacopeia.com/stimulant_conversion.php [Google Scholar]
- 32.Ciric R, Rosen AF, Erus G, et al. : Mitigating head motion artifact in functional connectivity MRI. Nat Protoc 2018; 13:2801–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteban O, Markiewicz CJ, Blair RW, et al. : fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 2019; 16:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro J, Bates D, DebRoy S, et al. : nlme: Linear and Nonlinear Mixed Effects Models [Internet]. 2020. Available from: https://CRAN.R-project.org/package=nlme [Google Scholar]
- 35.Benjamini Y, Hochberg Y: Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995; 57:289–300 [Google Scholar]
- 36.Matthijssen A-FM, Dietrich A, Bierens M, et al. : Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry 2019; 176:754–762 [DOI] [PubMed] [Google Scholar]
- 37.Chang Z, Ghirardi L, Quinn PD, et al. : Risks and Benefits of Attention-Deficit/Hyperactivity Disorder Medication on Behavioral and Neuropsychiatric Outcomes: A Qualitative Review of Pharmacoepidemiology Studies Using Linked Prescription Databases. Biol Psychiatry 2019; 86:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sylvester CM, Whalen DJ, Belden AC, et al. : Shyness and Trajectories of Functional Network Connectivity Over Early Adolescence. Child Dev 2018; 89:734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teeuw J, Brouwer RM, Guimarães JP, et al. : Genetic and environmental influences on functional connectivity within and between canonical cortical resting-state networks throughout adolescent development in boys and girls. NeuroImage 2019; 202:116073 [DOI] [PubMed] [Google Scholar]
- 40.Rubia K, Halari R, Christakou A, et al. : Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc B Biol Sci 2009; 364:1919–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.