Abstract
Across species, caregivers exert a powerful influence on the neural and behavioral development of offspring. Increasingly, both animal and human research has highlighted specific patterns in caregivers’ behavior that may be especially important early in life, as well as neurobiological mechanisms linking early caregiving experiences with long-term affective behavior. Here we delineate evidence for an early sensitive period during infancy and toddlerhood when caregiver inputs that are predictable and associated with safety may become biologically embedded via influences on corticolimbic circuitry involved in emotion regulation. We propose that these caregiver signals prime corticolimbic circuitry to be receptive to later stage-specific caregiver influences, such as caregivers’ external regulation of children’s emotional reactivity. Following caregiving adversity that disrupts predictability and safety associated with caregivers during this sensitive period, accelerated maturation of corticolimbic circuitry may foreshorten the protracted period of plasticity and caregiver influence that is characteristic of humans. This work has implications for both prevention and intervention efforts for children exposed to early life adversity.
Keywords: caregiving, predictability, safety, corticolimbic circuitry, emotion regulation
Caregivers have a profound impact on children’s neural and behavioral development. Decades of research have shown that stable, nurturing caregiving early in life is essential for children’s healthy socioemotional development, and, conversely, that severe disruption to early caregiving alters long-term development and increases risk for mental health disorders across the lifespan. However, the specific mechanisms by which early caregiving experiences affect long-term neural and behavioral outcomes, and, further, how caregiving in the earliest stages of development influences the potential impact of caregivers on behavioral and neural development at later stages of development, have remained unclear. That is, how does a child’s experience of stable, nurturing caregiving become biologically embedded? How does the nature of caregiving cues that a child receives early in life influence their capacity to optimally benefit from caregiving inputs supporting socioemotional functioning across development?
While it has long been established that the affective quality and content of caregiver signals to developing offspring impact neural development across species (Curley & Champagne, 2016), research has highlighted that specific patterns in caregivers’ behavior—namely, the co-occurrence of predictability and safety—may be particularly important for shaping the development of corticolimbic circuitry involved in emotion regulation and facilitating the caregiver’s ability to serve a regulatory function later in development. Building on extant literature documenting infancy and toddlerhood as a sensitive period for caregiving input more generally, here we review evidence for the hypothesis that a child’s receipt of early caregiving cues that are predictable (i.e., that occur in a way that is expected and reliable) and representative of safety (i.e., that protect a child from danger and are unlikely to cause harm) are essential for receptivity to later stage-specific caregiving influences to promote optimal development. Further, we highlight how these specific caregiving cues may become biologically embedded in the first several years of life and how accelerated maturation of corticolimbic circuitry following adverse caregiving may interfere with opportunities for caregivers’ optimal influence on later stages of development.
Infancy and Toddlerhood as a Sensitive Period for Caregiver Inputs
Via stage-specific inputs, caregivers support children in executing key tasks of typical development from birth through adolescence. Across infancy, infants learn to trust that their primary caregivers’ responses are contingent on their needs and that caregivers have predictable behavior that consistently signals safety. In early infancy, caregivers serve as a critical source of comfort and protection, with a transition to caregivers serving as a source of support for children’s emerging independence, as, increasingly throughout the first year of life, infants begin to explore the world with close caregiver support. During toddlerhood, caregivers continue to establish themselves as predictable sources of comfort and protection as children increasingly negotiate strong, and, at times, competing desires for independence and exploration, and security from close contact with caregivers (Lieberman et al., 2015).
Evidence suggests that caregivers may serve an external regulatory function early in life when corticolimbic circuitry is still developing (Callaghan & Tottenham, 2016a; Gee, 2016; Gee et al., 2014). Corticolimbic circuitry involves the amygdala, which detects emotionally salient stimuli in the environment; the hippocampus, which is involved in learning and memory; and the medial prefrontal cortex, which regulates amygdala reactivity and controls emotion. As this circuitry matures, children’s reliance on caregivers’ provision of external regulation may wane as regulatory abilities become internalized to facilitate independent emotion regulation, and other major attachment figures, such as close peers or romantic partners, take on an increasing role in social buffering (Gee, 2016; Hostinar et al., 2014).
In the context of typical development, the period spanning infancy and toddlerhood may represent a sensitive period during which predictable caregiver inputs associated with safety may be particularly influential in establishing the opportunity for later caregiver modulation of corticolimbic circuitry and emotion (Figure 1). Here we define a sensitive period as a window of heightened neuroplasticity during which specific environmental inputs have an especially strong effect on later functioning (Werker & Hensch, 2015). While growing evidence suggests that normative variation in caregiving experiences tracks with continuous variation in corticolimbic circuitry (e.g., Gee et al., 2014), much of the evidence for an early sensitive period related to caregiving experiences comes from the literature on severe caregiving-related adversity. There is compelling evidence that exposure to caregiving adversity, such as parental deprivation and maltreatment, is more detrimental when it occurs early in life, relative to later periods of development (e.g., Manly et al., 2001). For example, findings from the Bucharest Early Intervention Project (BEIP), a randomized controlled trial of children in institutionalized care that randomly assigned children to be placed in either foster care settings or to remain in institutionalized care settings, has demonstrated that receiving species-expected caregiving input in the first years of life is particularly impactful for both short- and long-term developmental outcomes (Nelson et al., 2007). Children’s experience of parental deprivation—which likely entails exposure to a lack of caregiver-associated predictability and safety—between birth and 24 months, specifically, has been shown to have particularly lasting and severe effects on a broad array of behavioral and neurodevelopmental outcomes (McLaughlin et al., 2015; Cohodes et al., 2020 for a review related to corticolimbic circuitry). Though parental deprivation and maltreatment are multifaceted stressors characterized by both the absence of species-expected inputs and the presence of extreme stress, and which may, themselves, alter the timing of sensitive periods (Gabard-Durnam & McLaughlin, 2020 for a review), these studies present empirical evidence for a potential sensitive period during which a lack of key caregiving inputs, such as predictability and safety, has particularly salient effects on the developing brain.
Figure 1. Delineation of a sensitive period for establishment of caregiver influences on corticolimbic circuitry underlying emotion regulation across development.
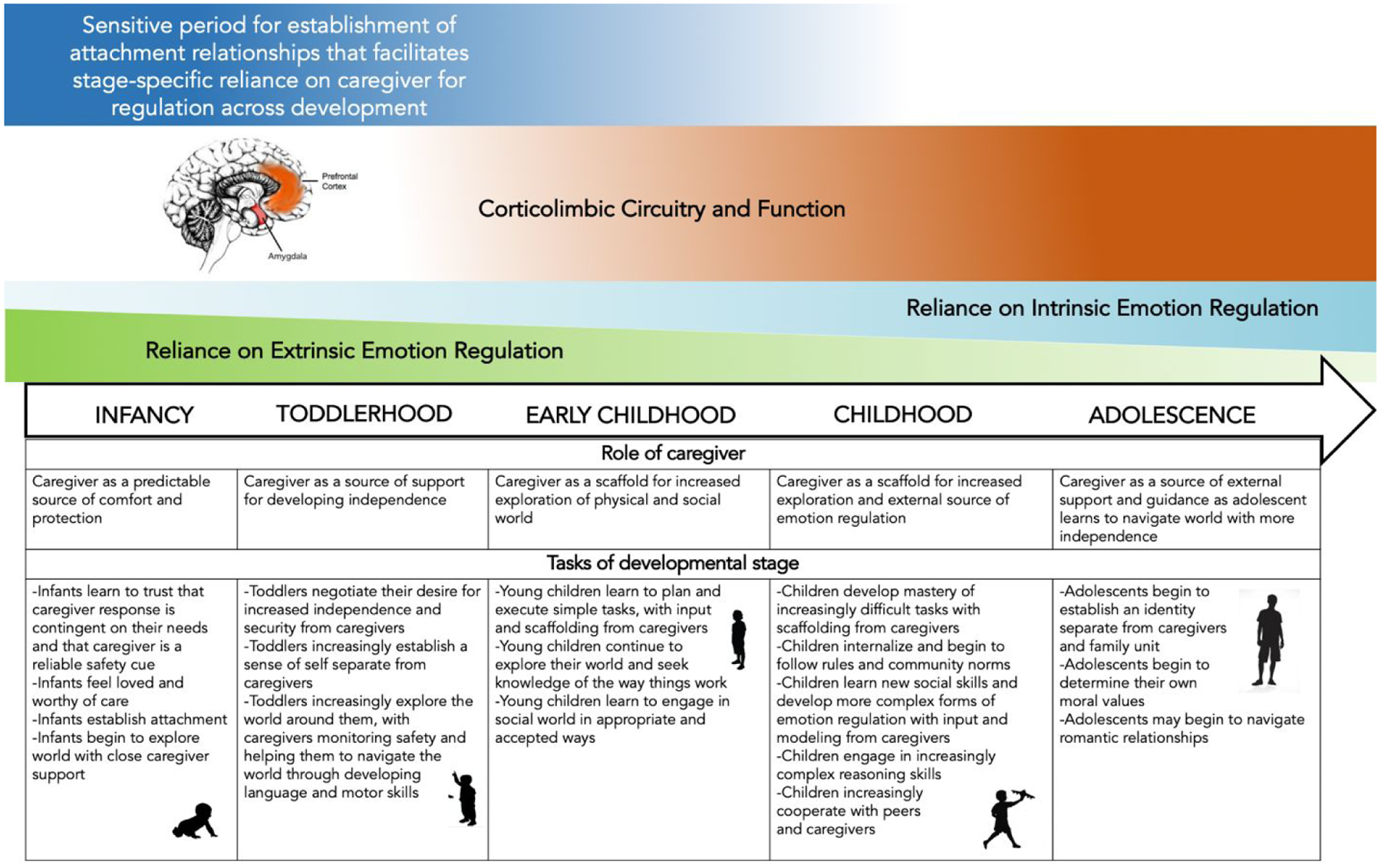
Evidence from both human and animal studies points to a potential sensitive period spanning infancy and toddlerhood during which caregiver inputs to the developing brain may have a particularly salient impact on the development of corticolimbic circuitry underlying emotion regulation. Specifically, caregiver inputs that are predictable and that are associated with safety may promote typical neurodevelopment such that caregivers are able to support youth emotion regulation via modulation of this circuitry in later developmental stages. Existing evidence from the human literature supports a model in which caregivers regulate human amygdala function during infancy and childhood, but not during adolescence. This shift represents an important transition from reliance on extrinsic to intrinsic emotion regulation as the underlying neural circuitry matures. This transition is also representative of a shift in characteristics of typical caregiver support of child development, as children face novel and compounding developmental challenges at each stage.
Predictability of Caregiving Cues
Cross-species evidence suggests that the predictability of caregiver responsivity to offspring early in life is an important determinant of long-term cognitive and affective outcomes for offspring (Ellis et al., 2009; Glynn & Baram, 2019). Representing one hypothesized pathway by which exposure to predictability of caregiving cues may influence the developing brain, predictable and appropriate caregiver responses to offspring in infancy underpin the development of secure attachment relationships, which, in turn, support social, emotional, and cognitive development (Sroufe, 2005). Indeed, both contingency of caregiver responsivity to infants (Gunnar, 1980) as well as synchrony in infant-caregiver behavior, are key predictors of children’s developmental outcomes (Feldman, 2007).
While the mechanisms supporting the effects of predictable caregiving on neural development have been relatively unexplored in humans, a growing body of evidence in rodents suggests that caregiver predictability may specifically influence the development of corticolimbic circuitry (Glynn & Baram, 2019). Rodent paradigms that manipulate the degree of predictability of maternal care have provided particular insight into the specific neurobiological effects of exposure to unpredictable care in the earliest stages of development. Rodents exposed to unpredictable care exhibit atypical development of the neurobiological systems underpinning emotion-related functioning, including weaker connectivity between the medial prefrontal cortex and the amygdala (e.g., Guadagno et al., 2018). In addition, rodents exposed to unpredictable maternal care show greater amygdala activity, relative to animals raised in typical conditions (Malter Cohen et al., 2013). These findings underscore possible pathways by which caregiving quality—specifically, the degree to which caregiver signals are expected and reliable—may support development of corticolimbic circuitry.
Association between Caregiver Presence and Safety
Caregivers’ inputs to offspring are multifaceted; predictability of cues in the first several years of life is necessary but not solely sufficient for priming neural circuitry for caregivers to play an optimal role across development. Caregiving cues must also be associated with safety. Early in life, interactions with caregivers provide opportunities to learn about the degree to which a caregiver’s presence is associated with the attenuation of fear (Moriceau & Sullivan, 2006). Over the course of repeated shared experiences between children and caregivers beginning immediately following birth and extending across postnatal development, caregivers’ consistent buffering of offspring fear (e.g., via physical presence and related attenuation of physiological reactivity; Callaghan & Tottenham, 2016a) reinforces the association between caregiver presence and safety. Caregivers’ successful attenuation of offspring fear, in turn, enhances the efficacy of the caregiver as a buffer and further instantiates caregivers as safety signals. It is important to note that a caregiver signaling that they are safe is distinct from a caregiver protecting a child from all possible dangers or preventing a child from seeking opportunities for exploration. Overprotective behaviors may signal to the child that the world is a dangerous place and interfere with the child’s normative development of independent regulation of anxiety. Here we focus on the normative development of the association between caregiver presence and safety.
Cross-species evidence provides insight into the specific neurobiological mechanisms by which caregivers buffer offspring fear and stress reactivity. In humans, caregiver presence suppresses cortisol reactivity (Hostinar et al., 2014) and phasically strengthens connections between the medial prefrontal cortex and the amygdala to dampen amygdala reactivity (Gee et al., 2014) during childhood. These findings are consistent with evidence that caregiver presence suppresses corticosterone and amygdala activity in developing rodents (Moriceau & Sullivan, 2006). Perhaps due in part to the potency of caregiver buffering, offspring approach stimuli associated with their caregiver, even when those stimuli are inherently aversive (Moriceau & Sullivan, 2006; Tottenham et al., 2019). Facilitation of approach behavior via caregiver-related cues may further promote early attachment and ensure that offspring remain close to their caregivers.
By establishing that their presence is associated with the attenuation of fear during infancy, caregivers lay the groundwork for later caregiver modulation of corticolimbic circuitry and emotion regulation in a stage-specific manner across development. Offspring rely on caregivers to play a more active role in facilitating emotion regulation and, relatedly, buffering amygdala reactivity during childhood, whereas caregivers shift to take on a supporting role as emotion regulation becomes more internalized during adolescence (Gee, 2016; Gee et al., 2014). Studies documenting the long-term effects of disrupted caregiving offer compelling evidence for the hypothesis that establishing an association between caregiver presence and safety facilitates optimal caregiver modulation of corticolimbic circuitry later in development. Even though attachment relationships can be established in the context of threatening cues (e.g., Perry & Sullivan, 2014), caregiving adversity during infancy interferes with caregiver buffering. Specifically, pups exposed to maltreatment by their caregivers (e.g., rough handling of pups, stepping on pups) do not show expected suppression of fear-related behavior in the presence of their caregiver during infancy, and, further, exhibit weakened caregiver buffering during the adolescent period, relative to their non-maltreatment-exposed counterparts (Opendak et al., 2019; Robinson-Drummer et al., 2019). Similarly, among non-human primates, infant maltreatment is associated with less effective maternal buffering of cortisol reactivity (Sanchez et al., 2015). Focusing on human development, on average, children exposed to caregiver deprivation early in life do not exhibit reduced amygdala reactivity in the presence of their adoptive caregivers (Callaghan et al., 2019).
Despite the narrative proffered by this pattern of findings—namely that failure to form associations between caregivers and safety-related cues early in life is associated with diminished influence of caregiver presence in later stages of development, emerging evidence points to malleability in the impact of early disruption to caregiving relationships. Of note, Callaghan and colleagues (2019) found that, while the majority of children with caregiving-related adversity did not exhibit attenuation of amygdala reactivity in the presence of their caregivers, 40% of children who had experienced early caregiving adversity did, in fact, exhibit this age-expected modulation. Individual differences emerged, with greater security in the caregiver-child relationship associated with greater caregiver-related attenuation of amygdala reactivity (Callaghan et al., 2019). These findings suggest that, while the absence of caregiving cues that are reliably associated with safety early in life appears to disrupt offspring receptivity to later caregiver buffering, there is also the potential for later plasticity and reshaping. Despite “missing” the opportunity for exposure to safety-related caregiving cues in the first several years of life, the observation of buffering among children exposed to early caregiver adversity suggests that these children may have learned to associate their adoptive caregivers with safety during a later phase of development. Indeed, research utilizing rodent models of augmented caregiving suggests that exposure to subsequent optimal care is associated with neurodevelopment that supports adaptive responses to stress (e.g., Singh-Taylor et al., 2018). Together, these studies raise the possibility that, while specific patterns of caregiver inputs in the earliest stages of life may be crucial for priming neural circuitry to be more receptive to later caregiver modulation, high quality care following attachment disruption may foster plasticity in the capacity for buffering.
Accelerated Development Following Disruption to Predictable and Safe Caregiving Cues
Acceleration of corticolimbic circuit development is a mechanism by which exposure to caregiving adversity—characterized by a lack of predictability and safety—during an early sensitive period may undermine offspring responsivity to caregiver buffering at later stages of development. Disruption to reliable and safe caregiving cues early in life is associated with accelerated maturation of the hippocampus and amygdala in both rodents (Bath et al., 2016; Manzano Nieves et al., 2020) and humans (Gee et al., 2013). One possibility is that unpredictable care triggers precocious activation of the stress response system, which could subsequently lead to accelerated maturation of corticolimbic circuitry (Callaghan & Tottenham, 2016b; Gee et al., 2013). Though much remains unknown about the function of acceleration, earlier maturation appears to represent an ontogenetic adaptation in the context of a harsh and unpredictable caregiving environment (Callaghan & Tottenham, 2016b; Ellis et al., 2009; Gee et al., 2013). However, despite evidence for some initial advantage (e.g., Gee et al., 2013), accelerated corticolimbic development may have long-term consequences for brain development and mental health. A protracted period of plasticity and caregiver influence in human development confers various advantages, and in the affective domain, may provide opportunities for learning safety signals that serve a later anxiolytic function (Yang et al., 2012). Foreshortening this period of immature corticolimbic function and plasticity may ultimately limit subsequent influences of caregiver inputs to corticolimbic circuitry and reduce opportunities for learning and adaptation later in development. Finally, representing a potential mechanism by which exposure to caregiving adversity may confer risk for the development of mental health disorders across the lifespan, behavioral adaptations that proved to be effective in the context of harsh caregiving conditions may undermine future adaptive coping in response to the novel challenges of each new developmental stage (Gee, 2016).
Future Directions
Here we have highlighted cross-species evidence for a potential early sensitive period for caregiver inputs to the developing brain related to predictability and safety of caregiving cues. Specifically, during infancy and toddlerhood, typical caregiving may facilitate an opportunity for optimal caregiver modulation of corticolimbic circuitry across subsequent stages of development, which is hypothesized to promote children’s development of an increasingly intrinsic capacity for emotion regulation. As cross-species research continues to target increased understanding of the ways in which specific features of caregiving “get under the skin,” several important questions remain.
First, though human research has established associations between exposure to predictable caregiving and generalized developmental outcomes (e.g., working memory or cognitive control), future research is needed to test the specific hypothesis that exposure to predictable caregiving in the first several years of life is associated with both stronger caregiver modulation of corticolimbic circuitry later in development and with a more protracted period of modulation by caregiver presence. Second, though research has begun to delineate the effects of early caregiving adversity on caregiver modulation of amygdala reactivity across development, studies to date have not examined how specific aspects of caregiving adversity (e.g., unpredictability and/or the extent to which caregivers exhibit behaviors associated with safety versus threat), experienced in the earliest stage of human development, confer risk for diminished caregiver modulation of this circuitry later in life (Cohodes et al., 2020). Moreover, additional research is necessary to specifically test the neural mechanisms by which these particular features—such as predictable signals or learned safety—become encoded (Meyer et al., 2019) and influence the processing of later caregiving cues (Opendak et al., 2020). Third, future studies that shed light on the specific mechanisms by which interventions can facilitate recovery of the establishment of caregiver capacities for later circuit modulation—despite missed opportunities for key inputs during the early sensitive period—will inform interventions for children exposed to early adversity.
Given the impact of caregiving adversity in the first years of life, policy- and public health-related efforts should focus on implementing structural changes that prevent ruptures to young children’s attachment relationships, which inherently compromise their sense of safety and predictability. From an intervention perspective, the extant literature suggests that young children who have experienced caregiving adversity during infancy and toddlerhood may benefit from dyadic (child-caregiver) interventions focused on providing children with opportunities to play and talk about traumatic exposures that may have compromised their sense of safety and predictability within their primary attachment relationships, and, reciprocally, providing caregivers with opportunities to reaffirm their association with safety and predictability, and to scaffold young children’s emerging understanding of caregivers’ capacity for repair (Lieberman et al., 2015). We also highlight the need to continue developing evidence-based interventions that consider both the developmental needs of children in infancy and toddlerhood (e.g., treatments that rely on play, in addition to verbal communication) and the potential inherent in this developmental period for forming new associations between caregivers and signaling of safety/predictability due to enhanced neural plasticity and young children’s reliance on caregiver support for nearly all aspects of functioning. Further delineating mechanisms by which enriched caregiving environments may allow children to re-establish predictability and safety with caregivers following adversity will also inform the design of additional targeted interventions for children exposed to early caregiving adversity.
Conclusions
A wealth of cross-species evidence has demonstrated that early caregiving experiences can shape brain and behavioral development across the life course. While caregiving influences are particularly salient early in life, caregivers play a central role in tasks of typical development throughout childhood and adolescence. Interactions between caregivers and children during infancy and toddlerhood form a foundation that allows the caregiver to effectively take on stage-specific support roles across development. A rapidly evolving literature highlights predictability and safety as two key aspects of caregiver behaviors early in life that are essential for healthy development and that facilitate age-appropriate caregiver inputs to development. Specifically, encoding of predictable, safe caregiving cues during an early sensitive period may shape corticolimbic development and support caregivers’ role in guiding emotional learning and regulation later in development. Future research will be essential to translate emerging work on the neurobiological pathways by which predictability and safety of caregiving cues become embedded early in life from animal models to human development, with implications for informing prevention and intervention efforts for children exposed to caregiving adversity early in life.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Director’s Early Independence Award (DP5OD021370), Brain & Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression; NARSAD) Young Investigator Award, Jacobs Foundation Early Career Research Fellowship, and the Society for Clinical Child and Adolescent Psychology (Division 53 of the American Psychological Association) Richard “Dick” Abidin Early Career Award and Grant to D.G.G.; and National Science Foundation Graduate Research Fellowship Program Award (NSF DGE1752134), The Society for Clinical Child and Adolescent Psychology (Division 53 of the American Psychological Association) Donald Routh Dissertation Grant, the American Psychological Foundation Elizabeth Munsterberg Koppitz Child Psychology Graduate Fellowship, a Dissertation Funding Award from the Society for Research in Child Development, and a Dissertation Research Award from the American Psychological Association to E.M.C.
References
- Bath KG, Manzano-Nieves G, & Goodwill H (2016). Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and Behavior, 82, 64–71. 10.1016/j.yhbeh.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B, Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, & Tottenham N (2019). Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: An examination across 3-years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 0(0). 10.1016/j.bpsc.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; An empirical investigation of caregiver buffering of offspring amygdala reactivity following early caregiving adversity.
- Callaghan BL, & Tottenham N (2016a). The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology, 41(1), 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016b). The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. 10.1016/j.cobeha.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohodes EM, Kitt ER, Baskin-Sommers A, & Gee DG (2020). Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Developmental Psychobiology. [DOI] [PubMed] [Google Scholar]; A review of key dimensions of early adversity (including predictability and caregiver involvement) impacting the development of corticolimbic circuitry.
- Curley JP, & Champagne FA (2016). Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Frontiers in Neuroendocrinology, 40, 52–66. 10.1016/j.yfrne.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009). Fundamental dimensions of environmental risk. Human Nature, 20(2), 204–268. [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48(3–4), 329–354. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L, & McLaughlin KA (2020). Sensitive periods in human development: Charting a course for the future. Current Opinion in Behavioral Sciences, 36, 120–128. 10.1016/j.cobeha.2020.09.003 [DOI] [Google Scholar]
- Gee DG (2016). Sensitive periods of emotion regulation: Influences of parental care on frontoamygdala circuitry and plasticity: Sensitive periods of emotion regulation. New Directions for Child and Adolescent Development, 2016(153), 87–110. 10.1002/cad.20166 [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, & Tottenham N (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, & Tottenham N (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25(11), 2067–2078. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]; An empirical demonstration of a neurobiological mechanism by which caregivers regulate offspring anxiety in childhood.
- Glynn LM, & Baram TZ (2019). The influence of unpredictable, fragmented parental signals on the developing brain. Frontiers in Neuroendocrinology, 53, 100736. 10.1016/j.yfrne.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; An overview of cross-species examinations of the effects of unpredictable care on offspring neurodevelopment and behavior.
- Guadagno A, Kang MS, Devenyi GA, Mathieu AP, Rosa-Neto P, Chakravarty M, & Walker C-D (2018). Reduced resting-state functional connectivity of the basolateral amygdala to the medial prefrontal cortex in preweaning rats exposed to chronic early-life stress. Brain Structure and Function, 223(8), 3711–3729. [DOI] [PubMed] [Google Scholar]
- Gunnar MR (1980). Contingent stimulation: A review of its role in early development. In Coping and health (pp. 101–119). Springer. [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin, 140(1), 256–282. 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AF, Ghosh Ippen C, & Van Horn P (2015). Don’t hit my mommy: A manual for Child-Parent Psychotherapy with young children exposed to violence and other trauma. Washington, DC: Zero to Three. [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, & Casey BJ (2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences, 110(45), 18274–18278. 10.1073/pnas.1310163110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, & Cicchetti D (2001). Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology, 13(4), 759–782. [PubMed] [Google Scholar]
- Manzano Nieves G, Bravo M, Baskoylu S, & Bath KG (2020). Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. ELife, 9, e55263. 10.7554/eLife.55263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, & Nelson CA (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences, 112(18), 5637–5642. 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, & Gee DG (2019). Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, & Sullivan RM (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience, 9(8), 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, & Guthrie D (2007). Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science, 318(5858), 1937–1940. 10.1126/science.1143921 [DOI] [PubMed] [Google Scholar]
- Opendak M, Robinson-Drummer P, Blomkvist A, Zanca RM, Wood K, Jacobs L, Chan S, Tan S, Woo J, Venkataraman G, Kirschner E, Lundström JN, Wilson DA, Serrano PA, & Sullivan RM (2019). Neurobiology of maternal regulation of infant fear: The role of mesolimbic dopamine and its disruption by maltreatment. Neuropsychopharmacology, 1. 10.1038/s41386-019-0340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; An empirical examination of caregiver buffering of offspring fear following caregiving adversity in rodents.
- Opendak M, Theisen E, Blomkvist A, Hollis K, Lind T, Sarro E, Lundström JN, Tottenham N, Dozier M, Wilson DA, & Sullivan RM (2020). Adverse caregiving in infancy blunts neural processing of the mother. Nature Communications, 11(1), 1119. 10.1038/s41467-020-14801-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R, & Sullivan RM (2014). Neurobiology of attachment to an abusive caregiver: Short-term benefits and long-term costs. Developmental Psychobiology, 56(8), 1626–1634. 10.1002/dev.21219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Opendak M, Blomkvist A, Chan S, Tan S, Delmer C, Wood K, Sloan A, Jacobs L, Fine E, Chopra D, Sandler C, Kamenetzky G, & Sullivan RM (2019). Infant Trauma Alters Social Buffering of Threat Learning: Emerging Role of Prefrontal Cortex in Preadolescence. Frontiers in Behavioral Neuroscience, 13. 10.3389/fnbeh.2019.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, & Howell BR (2015). Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Social Neuroscience, 10(5), 512–526. 10.1080/17470919.2015.1087426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton JL, Noam Y, Simeone K, Cope J, Chen Y, Mortazavi A, & Baram TZ (2018). NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Molecular Psychiatry, 23(3), 648–657. 10.1038/mp.2016.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA (2005). Attachment and development: A prospective, longitudinal study from birth to adulthood. Attachment & Human Development, 7(4), 349–367. 10.1080/14616730500365928 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Flannery J, Caldera C, & Sullivan RM (2019). Parental presence switches avoidance to attraction learning in children. Nature Human Behaviour, 3(10), 1070–1077. 10.1038/s41562-019-0656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, & Hensch TK (2015). Critical periods in speech perception: New directions. Annual Review of Psychology, 66, 173–196. [DOI] [PubMed] [Google Scholar]
- Yang E-J, Lin EW, & Hensch TK (2012). Critical period for acoustic preference in mice. Proceedings of the National Academy of Sciences of the United States of America, 109 Suppl 2, 17213–17220. 10.1073/pnas.1200705109 [DOI] [PMC free article] [PubMed] [Google Scholar]


