Abstract
Alzheimer’s disease (AD) and cerebral small vessel disease (cSVD) are the two main causes of dementia with blood-brain barrier (BBB) breakdown being a common contributor. Recent advances in neuroimaging techniques offer new possibilities to understand how the brain functions in health and disease. This includes methods such as dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) which allows the detection of subtle regional changes in the BBB integrity. The purpose of this work is to provide a review on the recent DCE-MRI findings of subtle BBB leakage focusing on cSVD and AD, including both clinical and pre-clinical studies. Despite being widely used and well-established, we also highlight some of the DCE-MRI challenges and pitfalls faced in the context of dementia inherent to the subtle nature of BBB impairment.
Introduction
Given its unique metabolic needs, the brain is one of the most highly perfused organs in the body with 400- and 0.4-mile-long vascular network in humans and mice, respectively (Begley and Brightman, 2003; Zlokovic, 2008; Montagne et al., 2017). This represents on average one mile of vessels per three grams of human brain tissue and for mice it is even denser, with one mile of vessels per one gram of brain tissue. All brain capillaries are constantly perfused, and it has been estimated that no neuron is more than 10–20 μm away from a capillary (Tsai et al., 2009), which implies that every neuron has its own capillary and thus demonstrates the critical relationship between the vascular and neuronal compartments. Importantly, there is increasing evidence supporting the involvement of brain vascular dysfunction in the early stages of brain disorders such as Alzheimer’s disease (AD) (Montagne et al., 2015, 2017, 2020; van de Haar et al., 2016a, 2016b; Nation et al., 2019; Sweeney et al., 2019a) and cerebral small vessel disease (cSVD) (Zhang et al., 2017b; Wardlaw et al., 2019). Furthermore, it was recently discovered that vascular cells express at least 30 of the top 45 AD risk genes using single-nucleus RNA sequencing on human brain samples (Yang et al., 2021), suggesting that the brain vasculature may play a larger role in the pathogenesis of dementia than was originally thought. Vascular dysfunction can take many forms, involving different cell types comprising the neurovascular unit (NVU), and including disruption of the blood-brain barrier (BBB), which plays a vital role in maintaining brain functions. Many neuroimaging and biofluid biomarker studies, as well as neuropathological studies have revealed the importance of BBB breakdown in the development and progression of common dementias, as extensively reviewed elsewhere (Sweeney et al., 2018a, 2018b, 2019b)
The most advanced method for investigating quantitatively and regionally subtle BBB failure in the living human or rodent brain is dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) using ~1 kDa paramagnetic gadolinium-based contrast agents (GBCAs). DCE-MRI method has become more and more available, and the field has now moved from the easily measured BBB damage in diseases with large permeability leaks (e.g., brain tumors, multiple sclerosis, or strokes), to more subtle disruption in chronic vascular disease and dementia. The slow accumulation of GBCAs from the intravascular into the extracellular extravascular space of the brain can be measured to determine regional BBB permeability, often referred as the blood-to-brain transfer constant, Ktrans. The low permeability is in the range of 10−4 to 10−3 min−1, while permeability in tumors is at least an order of magnitude higher (i.e., 10−2 min−1). There are several mathematical models to compute Ktrans that differ in complexity and assumptions under which they can be applied (Barnes et al., 2016). All the pre- and post-processing steps towards quantifying subtle BBB Ktrans measurements from DCE-MRI datasets have been summarized elsewhere (Montagne et al., 2016; Raja et al., 2018; Thrippleton et al., 2019; Manning et al., 2021).
In this review, we briefly discuss the role of BBB in health and dementia with a focus on cSVD and AD. Next, we examine the recent DCE-MRI studies performed in both humans and animal models relevant to cSVD and AD pathologies. Finally, we comment on the DCE-MRI challenges and pitfalls from image acquisition to data analysis steps in the context of low-permeability applications.
Blood-Brain Barrier in Health and Dementia
The primary role of the healthy BBB is to keep potentially toxic blood-derived components such as cells and pathogens out of the brain (Zlokovic, 2011; Sweeney et al., 2019b). At the same time, the BBB regulates the transport of molecules in-and-out of the brain and thus controls the chemical composition of the neuronal milieu which is essential for proper neuronal function (Zlokovic, 2011; Zhao et al., 2015; Sweeney et al., 2019b). The BBB comprises several components including endothelial cells, pericytes, basement membrane, astrocyte end feet, neuronal projections and surrounding glial cells, which altogether form the NVU (Figure 1). Pericytes, embedded within the basement membrane, wrap around endothelial cells that line the BBB, followed by astrocytic end feet encircling them. The BBB links to neurons in a process called neurovascular coupling where alterations in local blood flow occur in response to changes in neuronal activity (Abbott et al., 2010). Other glial and immune cells such as oligodendrocytes, microglia and perivascular macrophages are also in close and sometimes direct contact with the other NVU components allowing for intimate crosstalk (Abbott et al., 2010; Procter et al., 2021). Brain endothelial cells possess a specialized genetic profile which confer the barriers’ characteristic properties of limiting paracellular permeability (Pfau et al., 2021). Brain endothelial cells have indeed higher expression of junctional proteins such as adherens junctions (e.g., VE-cadherin), tight junctions (e.g., Occludin, Zonula Occludens-1, Claudin-5) and gap junctions, as detailed elsewhere (Stamatovic et al., 2016). Of note, tight junctions are important in limiting movement of small molecules (<0.8 kDa) across the BBB (Nitta et al., 2003).
Figure 1. Blood-brain barrier in health and dementia.
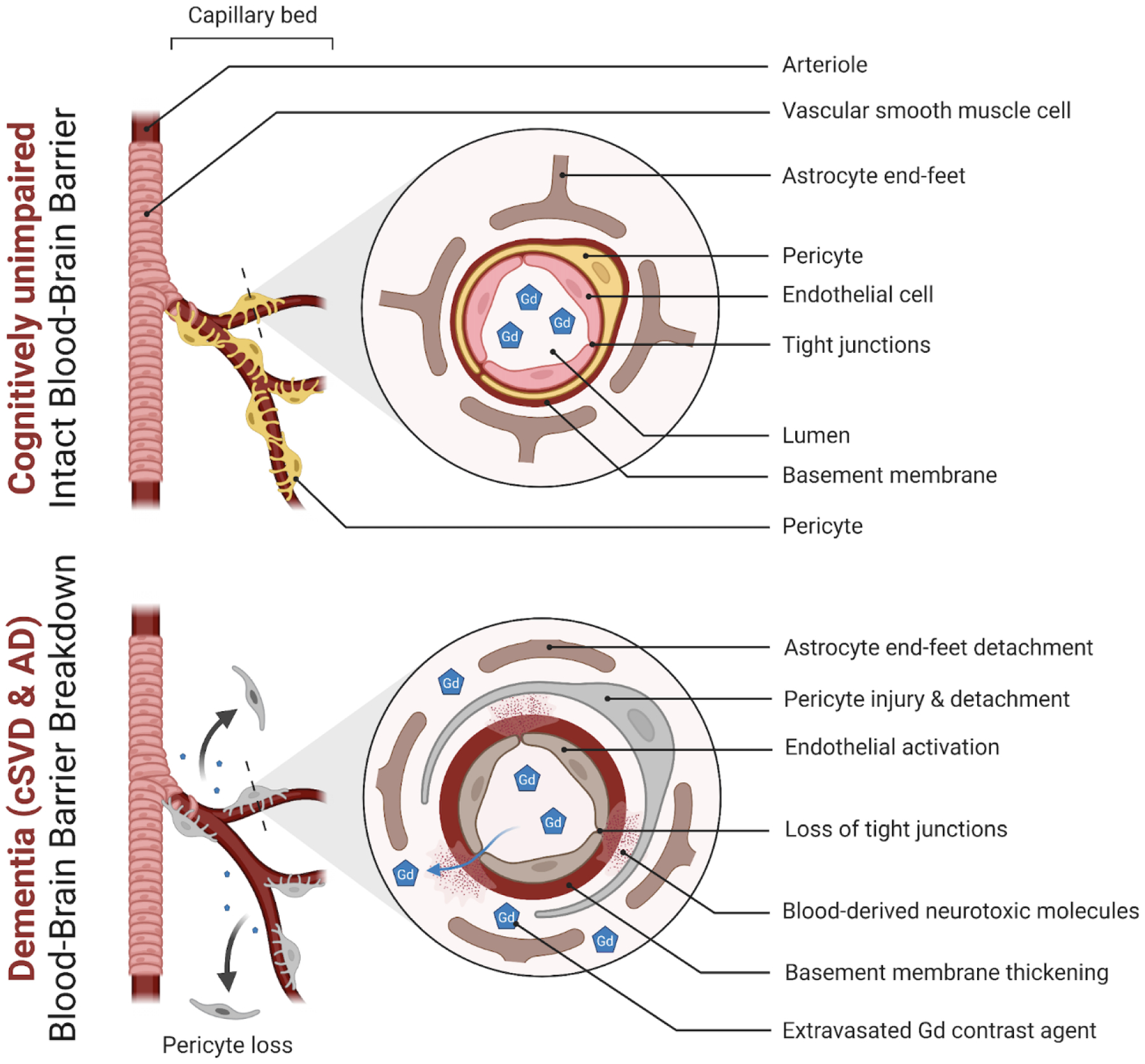
A simplified neurovascular unit (NVU) diagram showing a healthy blood-brain barrier (BBB) with the interactive cellular network at the level of brain capillaries that comprises endothelial cells, pericytes, basal membrane, and astrocyte end-feet (top panel). In dementia, changes to endothelial cells and pericytes lead to loss of function and BBB breakdown with loss of tight junction proteins (bottom panel). Subtle extravasation of Gadolinium (Gd) contrast can be detected using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in both living cerebral small vessel disease (cSVD) and Alzheimer’s disease (AD) participants. Subsequent damage then occurs to the surrounding brain cells such as astrocytes, neurons, and oligodendrocytes contributing to pathology and cognitive decline (Figure created using Biorender.com).
In recent years, there is a growing body of evidence for the contribution of BBB breakdown in the development and progression of common dementias such as AD and cSVD (Figure 1), as comprehensively examined elsewhere (Sweeney et al., 2018b, 2019a; Wardlaw et al., 2019). Just to highlight a few, there is neuropathological evidence of BBB disruption in AD and cSVD brains as indicated by loss of pericyte coverage of the brain capillary wall as well as perivascular accumulation of blood-derived fibrin(ogen) (Halliday et al., 2016; McAleese et al., 2019). Strikingly, a large post-mortem study found that four out of five AD patients had signs of vascular pathology (Toledo et al., 2013). There are also biofluid biomarker studies providing further evidence of BBB damage being an important feature of dementia pathophysiology. For instance, increased levels of the most common biofluid marker of BBB breakdown, albumin quotient (Qalb), were found in AD (as reviewed in (Sweeney et al., 2019b)). Additionally, cerebrospinal fluid (CSF) levels of soluble platelet-derived growth factor receptor-β (sPDGFRβ), a marker of damaged pericytes (Montagne et al., 2015; Sweeney et al., 2020), were found increased with normal aging (Montagne et al., 2015) and markedly accelerated as cognition declined (Montagne et al., 2015, 2020; Nation et al., 2019; Ding et al., 2020). Interestingly, CSF sPDGFRβ is significantly elevated in cognitively unimpaired individuals carrying the E4 variant of apolipoprotein E (APOE) APOE4 (Montagne et al., 2020), the major genetic risk factor for AD, hinting that BBB disruption may be an early marker of cognitive dysfunction and could be considered as a possible driving factor leading to dementia. Besides neuropathological and biofluid biomarkers findings, there are now improved neuroimaging methods such as DCE-MRI which allows for quantifying subtle and local disruption of the BBB in the living human or rodent brain (Raja et al., 2018; Thrippleton et al., 2019). Here, we next summarize the most recent publications using DCE-MRI in the context of cSVD and AD, and applied in both clinical and pre-clinical settings.
DCE-MRI technique
DCE-MRI collects dynamic T1-weighted MRI images that can monitor the change in signal intensity over time. After the collection of some baseline images, a GBCA is injected, this increases the signal intensity proportional to the concentration of the contrast agent. The changes in signal intensity, along with a T1 map (typically measured right before the DCE scan), are used to calculate a quantitative concentration of contrast agent in the blood plasma (Cp) and in the brain tissue, the extracellular extravascular space (Ct). The transport between these two compartments can then be modeled mathematically to solve for various physiological parameters. The most commonly used model for BBB measurements, the Platlak model, has two parameters that are solved for: Ktrans, a transfer constant from the blood plasma to the extravascular extracellular space, and Vp, the volume of blood plasma (Figure 2).
Figure 2. DCE-MRI and BBB assessment.
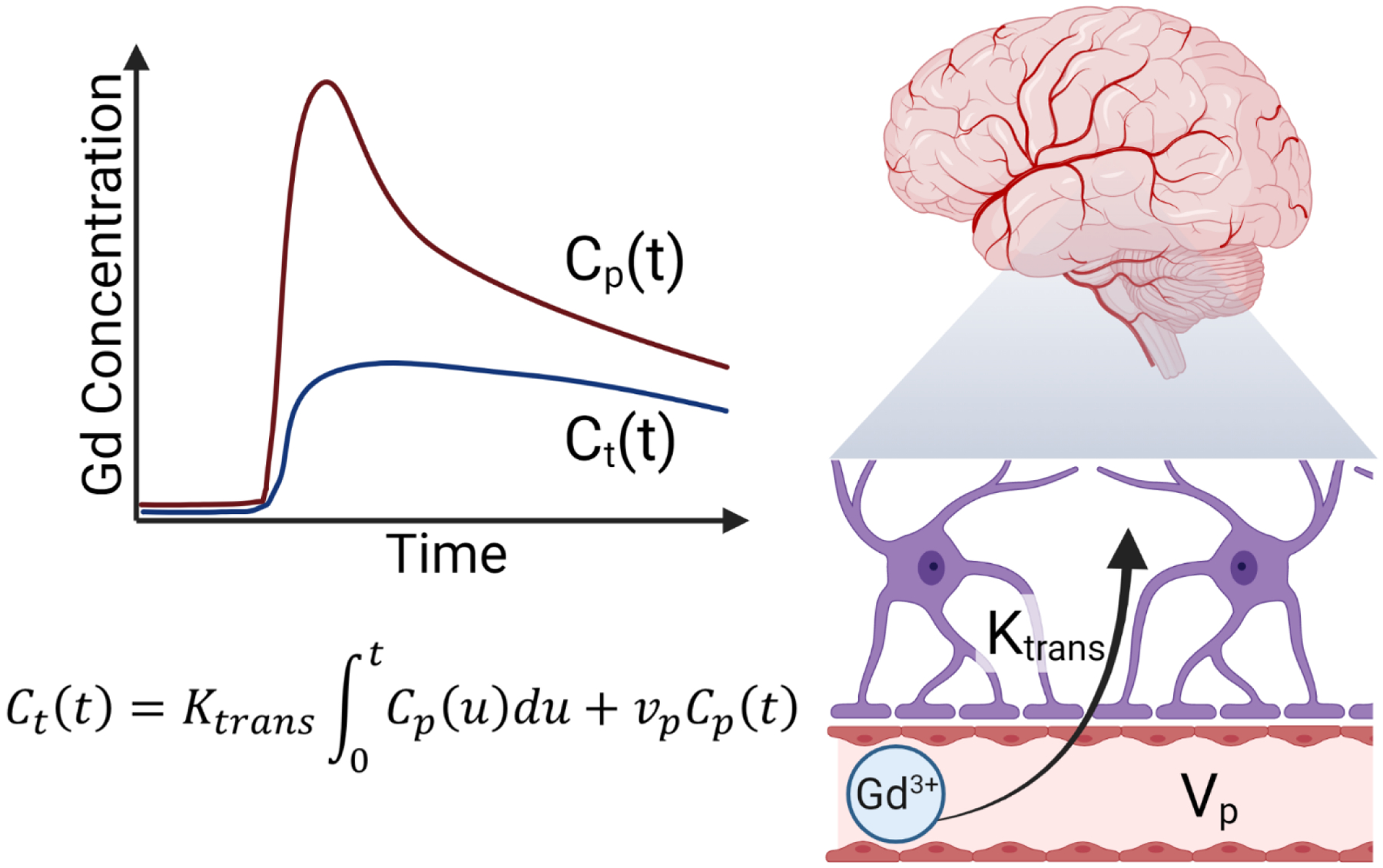
DCE-MRI is used to measure BBB integrity by measuring the concentration of gadolinium contrast agent over time in the blood plasma (Cp) and in the brain tissue (Ct). These measured concentrations are then used to calculate the blood-to-brain transfer coefficient (Ktrans) and the blood volume (vp) using the Patlak mathematical model (Figure created using Biorender.com).
There are many different techniques to acquire T1-maps and T1-weighted MRI images. To our knowledge there has not been a thorough analysis of the tradeoffs or superiority of any given technique. The variety of techniques used is likely a significant source of variability across studies, a rigorous comparison and consolidation to recommended techniques would likely benefit the field. For the dynamic T1-weighted images, spoiled gradient echo (SPGR) is by far the most common sequence used, likely because it is the fastest technique. Fast low angle shot (FLASH), SPGR, fast spoiled gradient echo (FSPGR), and gradient echo (GRE) are generally equivalent. Some papers utilized saturation recovery gradient echo sequences or spin echo sequences which are both somewhat slower but can still give high quality DCE images.
There were three main categories of T1-mapping techniques utilized by studies in this review. Variable flip angle (VFA) typically uses a SPGR sequence with identical parameters to the dynamic T1-weighted acquisition. This is repeated generally three to seven times with different flip angles. This is a very fast technique for T1 mapping, and by utilizing the same sequence type and parameters as the dynamic T1-weighted scan, blood inflow effects and artifacts should be similar between the T1 mapping and dynamic sequence. Variable repetition time (VTR) techniques also use multiple image acquisitions but with different repetition times. These are usually a bit slower than VFA, may have different inflow effects across images, but are less sensitive to B1 inhomogeneity which can cause significant errors in VFA techniques. Finally, inversion recovery techniques such as TAPIR, fast T1 mApping sequence with Partial Inversion Recovery, were utilized in a few studies. These are generally a little slower but give high quality T1 maps.
Methods
Search Sources and Selection Criteria
We reviewed the existing literature on DCE-MRI of subtle BBB permeability in aging, cSVD, and AD. The literature available on Pubmed was searched from January 1, 2015 to May 7, 2021. The following combinations of keywords were searched to be in the title, abstract and/or keywords of the article: (Dynamic contrast enhanced MRI) AND (small vessel disease): 30 results; (Dynamic contrast enhanced MRI) AND (Alzheimer’s disease): 29 results; (Dynamic contrast enhanced MRI) AND (aging): 47 results; (Dynamic contrast enhanced MRI) AND (dementia): 32 results; (Dynamic contrast enhanced MRI) AND (cognition): 68 results. In total, 206 references were obtained.
Inclusion and Exclusion Criteria
After exclusion of 71 duplicates, a total of 135 references were obtained from the initial Pubmed search. Out of 135 references, 96 were rejected as they were out of scope. From the 39 remaining publications, 23 were clinical, 3 pre-clinical, 5 methodological recommendations, and 8 review articles. In addition, we included 11 recent DCE-MRI publications that fit the scope of our review but did not appear at the initial search. These include 6 clinical studies, 3 pre-clinical studies, and 2 additional reviews. In total, 50 papers were reviewed in this article. See flow diagram in Figure 3. The main findings, sample characteristics, and protocol used in the clinical studies are detailed in Table 1 (cSVD; 11 publications) and Table 2 (normal aging, mild dementia, and AD; 14 publications), while pre-clinical studies are detailed in Table 3 (6 publications).
Figure 3. Flow Diagram of literature search.
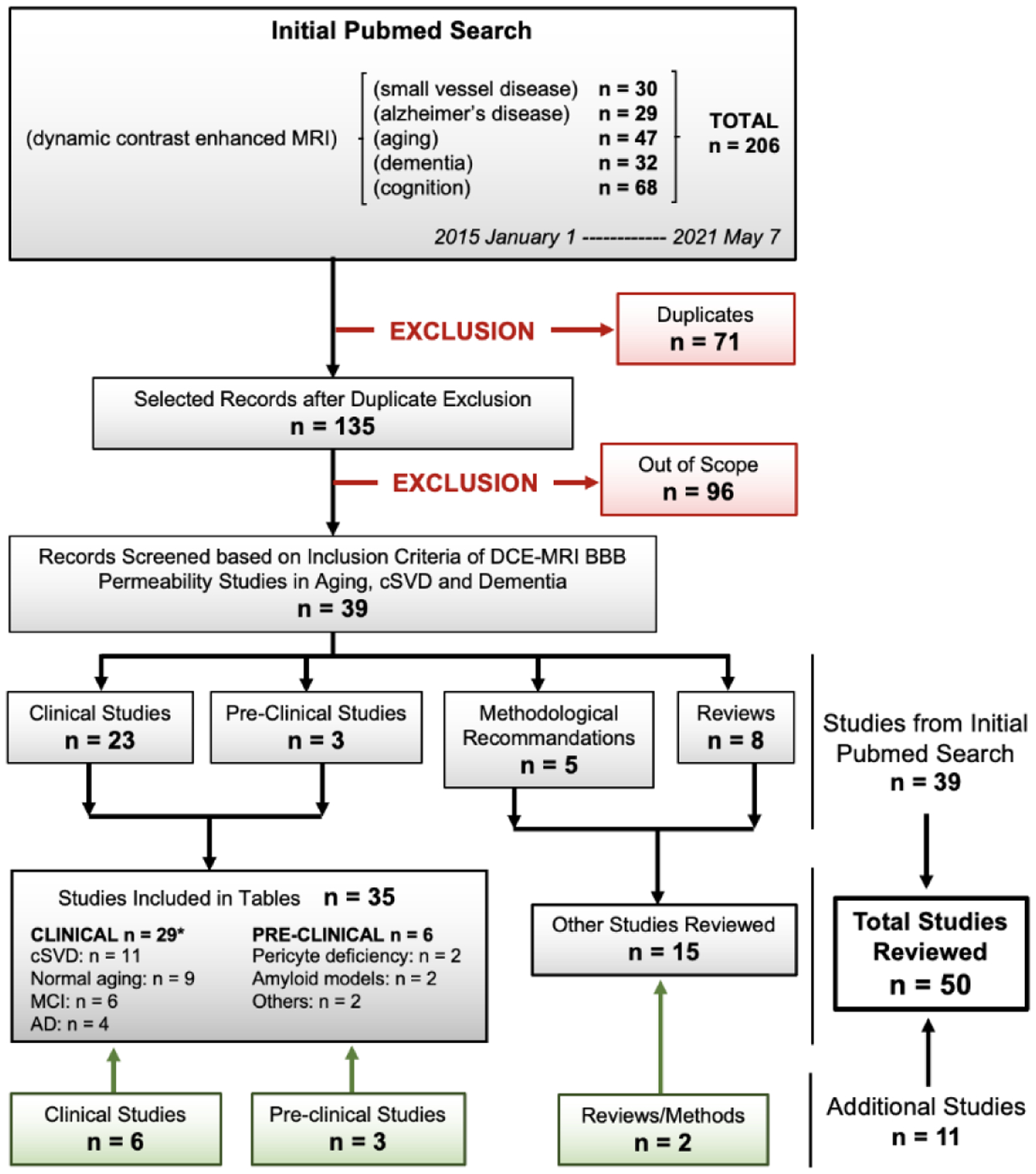
Recent research articles and reviews (January 1, 2015 - May 7, 2021) focusing on blood-brain barrier (BBB) permeability measured by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in normal aging, cerebral small vessel disease (cSVD) or Alzheimer’s disease (AD) were recovered from Pubmed search. In addition to the 39 articles retrieved from the systematic search, 11 additional articles highly relevant to our focus were added (green boxes), leading to a total of 50 reviewed papers. Research articles were categorized into three tables: Tables 1 and 2 summarize DCE-MRI studies performed in cSVD participants (11 publications) and in the AD continuum [including normal aging, mild cognitive impairment (MCI), and AD participants] (14 publications), respectively; Table 3 summarizes pre-clinical DCE-MRI studies in animal models relevant to dementia (6 publications). *Some studies have occurrences for several conditions.
Table 1.
Dynamic contrast-enhanced MRI studies in cerebral small vessel disease.
| Study | Sample Size (Ct/Pt) | Mean Age (Ct/Pt) | DCE Protocol (FS/PS/TA) | CA/Dose (mmol/kg) | VIF | PK Model | Software | Average BBB Ktrans (Ct/Pt; ×10−3 min−1) | Main Findings | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | NAWM | WMH | CGM | |||||||||
| Kerkhofs et al., 2021 | NA/43 | NA/68 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | NK | NK | NA/0.17* | NA/0.18* | NA/0.11* | BBB leakage in the perilesional zone of WMH is related to WM degeneration |
| Shao et al., 2020 | NA/16 | NA/68 | 3T/VFA-SPGR/16min | Dotarem (0.1) | Carotid artery | Patlak | Rocketship | NA/0.76 | NA/0.61 | NK | NA/0.68 | Patterns of BBB permeability for water (Kw) and Gd tracer (Ktrans) are different |
| Uchida et al., 2020 | 21/21 | 46/51 | 3T/VFA-SPGR/5min | Dotarem (0.1) | CCA | Patlak | In-house scripts (MATLAB) | 0.78/0.89 | NK | NK | NK | Iron leakage is associated with increased BBB permeability |
| Wong et al., 2019 | NA/27 | NA/69 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | NK | NK | NA/0.19* | NA/0.20* | NK | Association between BBB impairment, WMH, and hypoperfusion |
| Li et al., 2018 | 31/68 | NA/70 | 3T/VFA-SPGR/NK | Gadolinium (0.1) | SSS | Patlak | nordicICE | NK | 0.01/0.03 | 0.03/0.05 | 0.09/0.14 | BBB leakage is associated with cSVD burden |
| Li et al., 2017 | NA/102 | NA/70 | 3T/VFA-SPGR/NK | Gadolinium (0.1) | SSS | Patlak | nordicICE | NK | 0.01/0.03 | 0.03/0.05 | 0.09/0.14 | BBB leakage is associated with cSVD burden |
| Wardlaw et al., 2017 | NA/201 | NA/67 | 1.5T/VFA-SPGR/24min | Dotarem (0.1) | SSS | Linear mixed model | SAS 9.3 | NK | NK | NK | NK | BBB leakage in NAWM nearing WMH predicts cognitive decline |
| Zhang et al., 2017 | 40/80 | 69/70 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | NK | NK | 0.58*/0.53* | 0.48*/0.47* | 0.82*/0.79* | BBB leakage is associated with cSVD |
| Heye et al., 2016 | NA/201 | NA/66 | 1.5T/VFA-SPGR/24min | Dotarem (0.1) | SSS | Patlak | In-house scripts (MATLAB) | NK | NA/0.30 | NA/0.40 | NK | Patlak model is suited for evaluating subtle BBB leakage, but signal drift is limiting |
| Huisa et al., 2015 | 12/22 | 61/67 | 1.5T-3T/TAPIR/2028min | Magnevist (NK) | SSS | Patlak | In-house scripts (MATLAB) | NK | NK | NK | NK | BBB permeability aeras fluctuates in Binswanger’s disease |
| Rosenberg et al., 2015 | NA/25 | NA/66 | 1.5T/TAPIR/NK | Magnevist (NK) | NK | Patlak | NK | NK | NA/1.87* | NK | NK | BBB permeability is a predictor of Binswanger’s disease |
Abbreviations: BBB: Blood-Brain Barrier; CA: Contrast Agent; CCA: Common Carotid Artery; CGM: Cortical Gray Matter; cSVD: Cerebral Small Vessel Disease; Ct: Controls; DT-SRGRE: Dual-time SRGRE; FS: Field Strength; Gd: Gadolinium; HC: Hippocampus; Ki & Ktrans: Blood-to-Brain Transfer Constant; Kw: Blood-to-Brain Water Exchange Rate; NAWM: Normal Appearing White Matter; NA: Not Applicable; NK: Not Known; PK: Pharmacokinetic; PS: Pulse Sequence; Pt: Patients; SPGR: Spoiled Gradient; SRGRE: Saturation Recovery Gradient Echo; SSS: Superior Sagittal Sinus; TA: Time Acquisition; TAPIR: fast T1 mApping sequence with Partial Inversion Recovery; VFA: Variable Flip Angle; VIF: Vascular Input Function; WMH: White Matter Hyperintensities.
Denote Ki -> Ktrans conversion using Ki = Ktrans/[1-hematocrit] with hematocrit = 0.45.
Table 2.
Dynamic contrast-enhanced MRI studies in Alzheimer’s disease-continuum.
| Study | Sample Size (Ct/Pt) | Mean Age (Ct/Pt) | DCE Protocol (FS/PS/TA) | CA/Dose (mmol/kg) | VIF | PK Model | Software | Average BBB Ktrans (Ct/Pt; ×10−3 min−1) | Main Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | NAWM | WMH | CGM | ||||||||||
| Normal aging | Ha et al., 2021 | 34/NA | 65/NA | 3T/VFA-SPGR/10min | Gadovist (0.1) | SSS | Patlak | nordicICE | 0.56/NA | 0.33/NA | NK | NK | Regional BBB Ktrans differences exist in cognitively normal elderly adults |
| Moon et al., 2021 | 75/NA | 67/NA | 3T/GRE/10mi n | Gadovist (1.0) | SSS | Patlak | nordicICE | NK | NK | NK | NK | Females are protected from early BBB breakdown in cortex | |
| Montagne et al., 2020 | 206#/NA | 69/NA | 3T/VFA-SPGR/16min | Dotarem (0.05) | ICA | Patlak | Rocketship | 1.38/NA | 1.90/NA | NK | 1.43/NA | Increased BBB permeability in the MTL of cognitively unimpaired APOE4 carriers | |
| Verheggen et al., 2020a | 57/NA | 66/NA | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | NK | 0.61E-3*/NA | NK | 0.50E-3*/NA | Increased BBB permeability with aging | |
| Verheggen et al., 2020b | 57/NA | 66/NA | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | 0.94E-3*/NA | 0.61E-3*/NA | NK | 0.50E-3*/NA | BBB leakage is associated with memory decline in normal aging | |
| Li et al., 2019 | 109/NA | 70/NA | 3T/VFA-SPGR/NK | Gadolinium (0.1) | SSS | Patlak | nordicICE | NK | 0.03/NA | NK | NK | Increased BBB permeability correlates with BGEPVS | |
| Montagne et al., 2019 | 46/NA | 69/NA | 3T/VFA-SPGR/16min | Multihance (0.05) / Dotarem (0.05) | CCA | Patlak | Rocketship | 1.12/NA | NK | NK | NK | Increased hippocampal BBB permeability with normal aging | |
| Montagne et al., 2015 | 24/NA | 63/NA | 3T/VFA-SPGR/16min | Multihance (0.05) | CCA | Patlak | Rocketship | 1.18/NA | 2.06/NA | NK | 1.26/NA | BBB breakdown starts in HC | |
| MCI | Li et al., 2021 | 21/26 | 67/71 | 3T/VFA-SPGR/NK | Gadolinium (0.1) | SSS | Patlak | nordicICE | NK | 0.02/0.03 | 0.03/0.06 | 0.09/0.16 | Increased BBB permeability in WMH is linked to CI |
| Freeze et al.,2020 | 32/34 | 72/69 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | 0.40E-3*/0.16E-3* | 0.67E-3*/1.03E-3* | 0.69E-3*/0.72E-3* | 0.54E-3*/0.47E-3* | Increased BBB permeability in WMH is linked to CI | |
| Montagne et al., 2020 | 206/39 | 69/72 | 3T/VFA-3D-VIBE/16min | Dotarem (0.05) | CCA | Patlak | Rocketship | 1.38/1.69 | 1.90/2.13 | NK | 1.43/1.42 | Accelerated BBB breakdown in the MTL of APOE4 carriers | |
| Montagne et al., 2019 | 40/12 | 74/75 | 3T/VFA-SPGR/16min | Multihance (0.05) / Dotarem (0.05) | CCA | Patlak | Rocketship | 1.36/1.70 | NK | NK | NK | Increased hippocampal BBB permeability in MCI | |
| Nation et al., 2019 | 42/20 | 74/73 | 3T/VFA-SPGR/16min | Multihance (0.05) / Dotarem (0.05) | CCA | Patlak | Rocketship | 1.38/1.67 | 2.23/2.39 | NK | 1.32/1.35 | Increased hippocampal BBB permeability in MCI independent of amyloid and tau pathology | |
| Montagne et al., 2015 | 18/21 | 73/72 | 3T/VFA-SPGR/16min | Multihance (0.05) | CCA | Patlak | Rocketship | 1.28/1.64 | 2.19/2.30 | NK | 1.29/1.38 | BBB breakdown starts in HC and may contribute to CI | |
| AD | Freeze et al., 2020 | 32/14 | 72/72 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | 0.40E-3*/0.25E-3* | 0.67E-3*/0.44E-3* | 0.69E-3*/0.56E-3* | 0.54E-3*/0.41E-3* | Increased BBB permeability is related to cSVD severity in AD patients |
| Van de Haar et al., 2017 | 17/7 | 76/74 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | NK | 0.04*/0.04*‡ | NK | 4.00E-3*/0.057*‡ | Global BBB leakage in patients with early AD that is associated with cognitive decline | |
| Van de Haar et al., 2016a | 17/7 | 76/74 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | NK | 0.04/0.04‡ | 0.03/0.06‡ | 4.00E-3/0.05‡ | Brain-wide increased BBB permeability in AD patients is associated with CI | |
| Van de Haar et al., 2016b | 16/7 | 76/75 | 3T/DT-SRGRE/25min | Gadovist (0.1) | SSS | Patlak | In-house scripts (MATLAB) | NK | NK | NK | 0.18/0.27‡ | AD patients show increased BBB permeability in cortex | |
Abbreviations: AD: Alzheimer’s Disease; APOE4: Apolipoprotein E4; BG-EPVS: Enlarged Perivascular Spaces in the Basal Ganglia; BBB: Blood-Brain Barrier; CA: Contrast Agent; CCA: Common Carotid Artery; CDR: Clinical Dementia Rating; CGM: Cortical Gray Matter; CI: Cognitive Impairment; cSVD: Cerebral Small Vessel Disease; Ct: Controls; DT-SRGRE: Dual-time SRGRE; FS: Field Strength; FSPGR: Fast SPGR; GRE: Gradient Echo; HC: Hippocampus; ICA: Internal Carotid Artery; Ki & Ktrans: Blood-to-Brain Transfer Constant; MCI: Mild Cognitive Impairment; MTL: Median Temporal Lobe; NAWM: Normal Appearing White Matter; NA: Not Applicable; NK: Not Known; PK: Pharmacokinetic; PS: Pulse Sequence; Pt: patients; sPDGFRβ: Soluble Platelet-Derived Growth Factor Receptor β; SPGR: Spoiled Gradient; SRGRE: Saturation Recovery Gradient Echo; SSS: Superior Sagittal Sinus; TA: Time Acquisition; VFA: Variable Flip Angle; VIBE: Volumetric Interpolated Breath-Hold; VIF: Vascular Input Function; WMH: White Matter Hyperintensities.
Denote Ki -> Ktrans conversion using Ki = Ktrans/[1-hematocrit] with hematocrit = 0.45;
Denotes all cognitively normal (CDR=0) participants;
MCI and AD groups combined.
Table 3.
Dynamic contrast-enhanced MRI studies in animal models relevant to dementia.
| Study | Model (species) | Sample Size (Ct/Tg) | Age (months) | DCE Protocol (FS/PS/TA) | CA/Dose (mmol/kg) | VIF | PK Model | Software | Average BBB Ktmns (Ct/Tg; ×10−3 min−1) | Main Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | CTX | THAL | CC | |||||||||||
| Pericyte deficiency | Nikolakop oulou et al., 2019 | Pericyte-Cre-DTR (mouse) | 5/6 | 2–3 | 7T/VFA-FLASH/15min | Magnevist (0.5) | CCA | Patlak | Rocketship | 0.59#/1.13# | 0.68#/1.23# | NK | NK | Increased BBB permeability after pericyte ablation |
| Montagne et al., 2018 | PdgfrbF7/F7 (mouse) | 5–7/5–7 | 1–1.5, 3–4, 9–12 | 7T/VTR-FLASH/35min | Magnevist (0.5) | CCA | Patlak | Rocketship | NK | NK | NK | 0.60/0.86, 0.58/1.05, 0.70/1.22 | Increased BBB permeability in pericyte-depleted WM regions | |
| Amyloid models | Dickie et al., 2021 | TgF344-AD (rat) | 16/13, 5/7, 7/8 | 13, 18, 21 | 7T/VFA-SPGR/10min | Dotarem (0.5) | SSS | Patlak | R | NK | NK | NK | NK | AD genotype did not lead to Ktrans changes, but higher water permeability (Kw) was observed |
| Montagne et al., 2021 | 5xFAD; TR-APOE3/4 (mouse) | 12-14 FAD−/13-16 FAD+ | 18–24 | 7T/VFA-FLASH/25min | Gd-DTPA (BioPAL) (0.5) | CCA | Patlak | Rocketship | 0.68/0.98$ | 0.69/0.91$ | NK | NK | Increased BBB permeability in APOE4 AD mice, independent of amyloid pathology | |
| Wang et al., 2018 | SHR (rat) | 5/5 | 17 | 7T/Spin Echo/35min | Magnevist (0.2) | NK | Extended Tofts | DCE@urLAB | 8.40/31.60 | 5.20/30.20 | NK | NK | Increased BBB permeability and Aβ accumulation in HC and CTX of SHR rats | |
| Xu et al., 2017 | Diabetes (rhesus monkey) | 6/5 | 13 years | 3T/SRGRE/10 min | MultiHance (0.1) | SSS | Extended Tofts | DCE@urLAB | 3.32/7.91 | 3.27/8.11 | 3.30/7.47 | NK | Increased BBB permeability in diabetic monkeys | |
Abbreviations: Aβ: Amyloid-beta; AD: Alzheimer’s Disease; APOE4: Apolipoprotein E4; BBB: Blood-Brain Barrier; CA: Contrast Agent; CCA: Common Carotid Artery; cSVD: Cerebral Small Vessel Disease; Ct: Controls; CTX: Cortex; DTR: Diphtheria Toxin Receptor; DT-SRGRE: Dual-time SRGRE; FAD: Familial Alzheimer’s Disease; FLASH: Fast Low Angle Shot; FS: Field Strength; HC: Hippocampus; Ki & Ktrans: Blood-to-Brain Transfer Constant; Kw: Blood-to-Brain Water Exchange Rate; NK: Not Known; Pdgfrb: Platelet-Derived Growth Factor Receptor b; PK: Pharmacokinetic; PS: Pulse Sequence; SHR: Spontaneously Hypertensive Rat; SPGR: Spoiled Gradient; SRGRE: Saturation Recovery Gradient Echo; SSS: Superior Sagittal Sinus; TA: Time Acquisition; Tg: Transgenic Animals; TR: Targeted Replacement; THAL: Thalamus; VFA: Variable Flip Angle; VIF: Vascular Input Function; VTR: Variable Time Repetition.
BBB Ktrans values from 15 days post Diphtheria Toxin treatment;
BBB Ktrans values from TR-APOE4;5xFAD (Tg) vs TR-APOE3;5xFAD (Ct) mice.
DCE-MRI in cSVD
Cerebral SVD covers a wide array of pathologies involving the dysfunction of the small vessels of the brain. Clinical manifestations include stroke, cognitive impairment, or gait disturbance. Many studies reported BBB impairment in cSVD patients, particularly in the white matter (WM) (Thrippleton et al., 2019). A growing number of neuroimaging studies have described subtle BBB breakdown in the living cSVD brain using DCE-MRI technique, as summarized in Table 1 and further discussed below.
White matter hyperintensities (WMH) are a common finding in the elderly population and a key feature of cSVD. While their pathogenesis remains yet unclear, BBB leakage is the most accepted hypothesis. In their 2017 article, Li et al. assessed the BBB permeability in a sample of 102 patients with low, medium, or high cSVD burden and found that global BBB permeability was associated with higher WMH burden (Li et al., 2017). The same year, Wardlaw’s group also found a relationship between BBB permeability and WMH burden in a cohort of 201 cSVD patients (Wardlaw et al., 2017). Notably, they highlighted the fact that the healthy WM tissue surrounding WMH presented increased BBB permeability, suggesting that BBB disruption could precede further extensions of the WMH lesions. This result was recently confirmed by Kerkhofs et al. who demonstrated that BBB leakage nearing WMH is related to changes in WM diffusivity, an MRI-based diffusion marker of tissue degeneration possibly caused by local BBB damage (Kerkhofs et al., 2021). Furthermore, Wong et al. observed a negative correlation between cerebral blood flow (CBF) and BBB leakage in the tissue surrounding WMH in 27 cSVD patients (Wong et al., 2019).
DCE-based differences in BBB leakage due to normal aging and cSVD may be very subtle as illustrated by the work of Zhang et al. who did not observe a significant difference in BBB leakage rate (Zhang et al., 2017a). However, leakage extent was found to be higher in cSVD patients. Also, Binswanger’s disease (BD) belongs to the cSVD spectrum and is characterized by an extensive involvement of WM and impairment of executive functions. Differentiating BD from other cSVD-related conditions is often difficult; hence, Rosenberg et al. aimed to define more specific biomarkers. Among these, WM BBB permeability was found to be increased in BD patients compared to a combined group of patients having multiple infarcts, mixed AD/vascular cognitive impairment (vCI), or leukoaraiosis (Huisa et al., 2015; Rosenberg et al., 2015).
A known consequence of BBB disruption is the increasing occurrence of microbleeds (Wardlaw et al., 2019). Uchida et al. successfully correlated iron accumulation detected by quantitative susceptibility mapping MRI to local BBB leakage in patients with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL), emphasizing how alternate imaging techniques could support DCE-MRI findings (Uchida et al., 2020). Another alternate method would be to measure vessel density, diameter, and size from the R2 and R2* relaxation rates upon GBCA injection (Choi et al., 2020)
In a cohort of older subjects at risk for cSVD, Shao et al. compared DCE-MRI to an arterial spin labeling (ASL)-based technique allowing measurement of water permeability across the BBB (Shao et al., 2020). Interestingly, they found only few correlations between Ktrans and kw, suggesting that the mechanisms regulating the permeability of water or contrast agents across the BBB are likely to be different.
DCE-MRI in the AD-continuum
The vascular contribution to AD pathophysiology is increasingly recognized. As such, BBB breakdown is considered an important player in the development and progression of the most common cause of dementia (Sweeney et al., 2018a, 2018b, 2019b). Thus, it is not surprising to see a growing number of DCE-MRI studies performed in the normal aging to AD spectrum, as reviewed in Table 2 and examined hereafter.
Normal Aging
In 2015, Montagne et al. first showed an age-dependent BBB leakage starting in the hippocampus in a cohort of 24 healthy individuals ranging from 23 to 91 years of age (Montagne et al., 2015), which was further confirmed in a larger cohort of 46 cognitively unimpaired participants (Montagne et al., 2019). Another group also observed that global BBB impairment was correlated with age (Verheggen et al., 2020a) and cognitive decline (Verheggen et al., 2020b) in a sample of 57 healthy elderly individuals. Interestingly, Moon et al. investigated gender-related differences in BBB permeability in a sample of 75 elderly patients (51 females). They showed that while women are better protected than men with regards to age-related BBB disruption thanks to the protective effect of estrogens, they are also more sensitive to late BBB disruption in the occipital cortex, where the estrogen receptor is more expressed (Moon et al., 2021). Another recent DCE study spotted subtle differences between brain regions in a cohort of 35 cognitively normal elderly participants, with the lowest and highest Ktrans values in the frontal and occipital WM, respectively(Ha et al., 2021). Of note, no differences between left and right hemispheres were detected. Finally, enlarged perivascular spaces (EPVS) are a common finding in normal aging, and highly increased EPVS load is a feature of both cSVD and AD. In a sample of 109 middle-aged to elderly participants, Li et al. found that BBB leakage and EPVS burden were associated in the basal ganglia (Li et al., 2019).
Mild Dementia
Although regional BBB leakage seems to occur during normal aging, Montagne et al. also reported that hippocampal BBB disruption worsened as participants’ cognition declined (Montagne et al., 2015, 2019). A following study from the same group observed a local increase of BBB permeability in the medial temporal lobe (MTL) (including the hippocampi and parahippocampal gyri) of 42 patients with mild cognitive impairment (MCI) compared to 20 age-matched cognitively unimpaired controls (Nation et al., 2019). Interestingly, the authors demonstrated that MTL vascular leakage is an independent, early biomarker of cognitive impairment unrelated to amyloid-β (Aβ) and tau pathology, as the positivity for the respective CSF biomarkers Aβ42 and phosphorylated tau (pTau) did not affect the results.
Recently, the same authors questioned the relation between APOE4 genotype and BBB permeability in a cohort of 245 elderly MCI and cognitively unimpaired participants (Montagne et al., 2020). Increased BBB permeability in the MTL was confirmed in MCI and further accelerated in APOE4 carriers. Interestingly, cognitively unimpaired individuals carrying APOE4 had substantially higher MTL BBB Ktrans values compared non-carriers (Montagne et al., 2020), supporting the involvement of BBB dysfunction early in the course of AD.
A study by Li et al. showed that BBB leakage in WMH was significantly higher in vascular MCI individuals, an uncommon condition where the sole cause for cognitive impairment is vascular pathology, when compared to age- and sex-matched healthy controls (Li et al., 2021). Additionally, Freeze et al. investigated the link between BBB leakage, WMH, and processing speed in a cohort of 80 elderly participants, including 34 MCI as well as 14 AD patients (Freeze et al., 2020). The authors found an association between increased BBB permeability in WMH and cognitive impairment suggesting that local BBB breakdown may trigger WM lesions which could then explain the reduction in information processing speed (Freeze et al., 2020).
Alzheimer’s Disease
In their AD cohort, Freeze et al. found that BBB disruption throughout the whole brain was associated with cSVD severity, independently of cognitive status (Freeze et al., 2020). The group of Backes recently studied BBB dysfunction in AD using DCE-MRI method. For instance, they confirmed the relationship between BBB disruption and early AD in a cohort of 33 elderly, whom 9 presented MCI and 7 AD diagnosis. Indeed, AD patients showed increased BBB leakage in the whole brain, which worsened with cognitive impairment (van de Haar et al., 2016a). In a follow-up study, the same authors also observed a general decrease in CBF in the gray matter of early AD patients, which correlated with increased BBB leakage in a cohort of 14 MCI/AD and 16 age-matched controls (van de Haar et al., 2016b, 2017).
DCE-MRI in pre-clinical models relevant to dementia
As MRI is becoming more accessible, DCE method is also increasingly applied in a variety of rodent models that are relevant to dementia (see Table 3).
Pericyte Deficiency
Pericytes are a major component of the BBB, especially at the capillary level where the smooth muscle layers are lacking (Procter et al., 2021). Pericytes wrap around endothelial cells that line the BBB, and their dysfunction is thought to play a critical role in aggravating dementia (Sweeney et al., 2019b; Uemura et al., 2020). Analyses of post-mortem brains and CSF samples provided substantial evidence supporting early pericyte loss in AD (Baloyannis and Baloyannis, 2012; Sagare et al., 2013; Sengillo et al., 2013; Miners et al., 2018), mild dementia (Montagne et al., 2015, 2020; Nation et al., 2019), stroke (Yemisci et al., 2009; Hall et al., 2014), and cSVD (Ghosh et al., 2015; Montagne et al., 2018). Using DCE-MRI in a chronic mouse model of pericyte deficiency, Montagne et al. reported a global and age-dependent increase in BBB permeability with the highest Ktrans values found in WM structures (Montagne et al., 2018). Also, they observed CBF reduction and EPVS in the WM of young pericyte-deficient mice, which are common findings in the aged brain (Montagne et al., 2018). In another model of acute pericyte ablation, the same group reported a substantial circulatory failure with acute BBB disruption and reduced CBF in the cortex and hippocampus, followed by rapid neuronal loss (Nikolakopoulou et al., 2019). These pre-clinical results emphasize the role of pericytes in BBB homeostasis and promote the use of DCE-MRI to detect subtle BBB changes in the living rodent brains.
Amyloid Models
Although several studies found increased BBB permeability to gadolinium contrast agent in rodent models of AD (Montagne et al., 2017), Dickie et al. failed to observe BBB Ktrans changes in old TgF344-AD rats compared to wildtype controls but did find an increase in water permeability (Dickie et al., 2021). It suggests that old TgF344-AD rats do not develop BBB breakdown with loss of tight junction proteins as one would assume, but rather show an increase in water exchange rate that is facilitated by dedicated water channel, predominantly aquaporin-4 (AQP4) in astrocytes and aquaporin-1 (AQP1) in endothelial and mural cells. Of note, it is important to clarify that Kw and Ktrans are measuring two very different processes, one being the water exchange rate across the BBB and the other one the BBB permeability to gadolinium contrast (Shao et al., 2020).
As shown in humans carrying APOE4 (Montagne et al., 2020), the same group found that, compared to APOE3, APOE4 accelerates BBB breakdown, loss of CBF, neuronal loss, and behavioral deficits independently of Aβ using aged APOE knock-in mice crossed with 5xFAD mice (Montagne et al., 2021). Such results support that BBB disruption may occur earlier than anticipated in AD and be an early contributor to the pathogenesis of the disease, thus emphasizing the need for appropriate methods to detect subtle BBB leakage in humans.
Vascular Risk Factors
Several conditions related to lifestyle are associated with BBB disruption. Wang et al. found a sharp increase of BBB Ktrans measured in the cortex and hippocampus of aged spontaneously hypertensive rats compared with controls (Wang et al., 2018). Similarly, diabetes increased BBB leakage in the cortex, hippocampus, and thalamus of aged rhesus monkeys (Xu et al., 2017). While BBB disruption occurring due to normal aging cannot be prevented, moderating the lifestyle factors known to affect vessel wall integrity could delay the occurrence of clinical consequences.
DCE-MRI Challenges and Pitfalls in Dementia
The small amounts of leakage that occur in cSVD and AD bring the inherent difficulty of detecting and quantifying very small signal changes in DCE-MRI. This is a significant technical challenge, but steady progress is being made in identifying and mitigating the various challenges. Here we highlight some of the principal challenges that can prevent accurate data. For a more thorough review of the technical challenges, recommended acquisition, and processing steps, see the recent review by (Thrippleton et al., 2019).
Motion
Patient motion is a significant concern over the course of a 10–20-minute DCE acquisition. Even small changes in head position can cause changes in tissue type in a given voxel, particularly at parenchyma-CSF boundaries. These changes show up as gradual increases or decreases in signal intensity. This will bias measured Ktrans values higher or lower, even causing Ktrans to have negative values. Figure 4 shows an example of a measured percent signal change over 12 minutes DCE acquisition without any contrast injection, so the expected signal change is zero. Images are shown with and without motion correction co-registration algorithms, and show signal changes up to 25% near CSF boundaries. These changes occur even though there is minimal motion (<0.5 mm of displacement in any direction) but are almost entirely corrected with co-registration. Motion correction with co-registration is recommended for all DCE studies (Thrippleton et al., 2019), and more recent publications have demonstrated errors in fit parameters of >200% in some voxels including many negative Ktrans values from even low motion that are almost entirely corrected with co-registration (Bernal et al., 2021). We have found co-registration is more effective when a brain mask is applied first, as scalp motion can be independent of brain motion and DCE measurements in the scalp are usually of little interest.
Figure 4. Dynamic contrast-enhanced magnetic resonance imaging and motion correction.
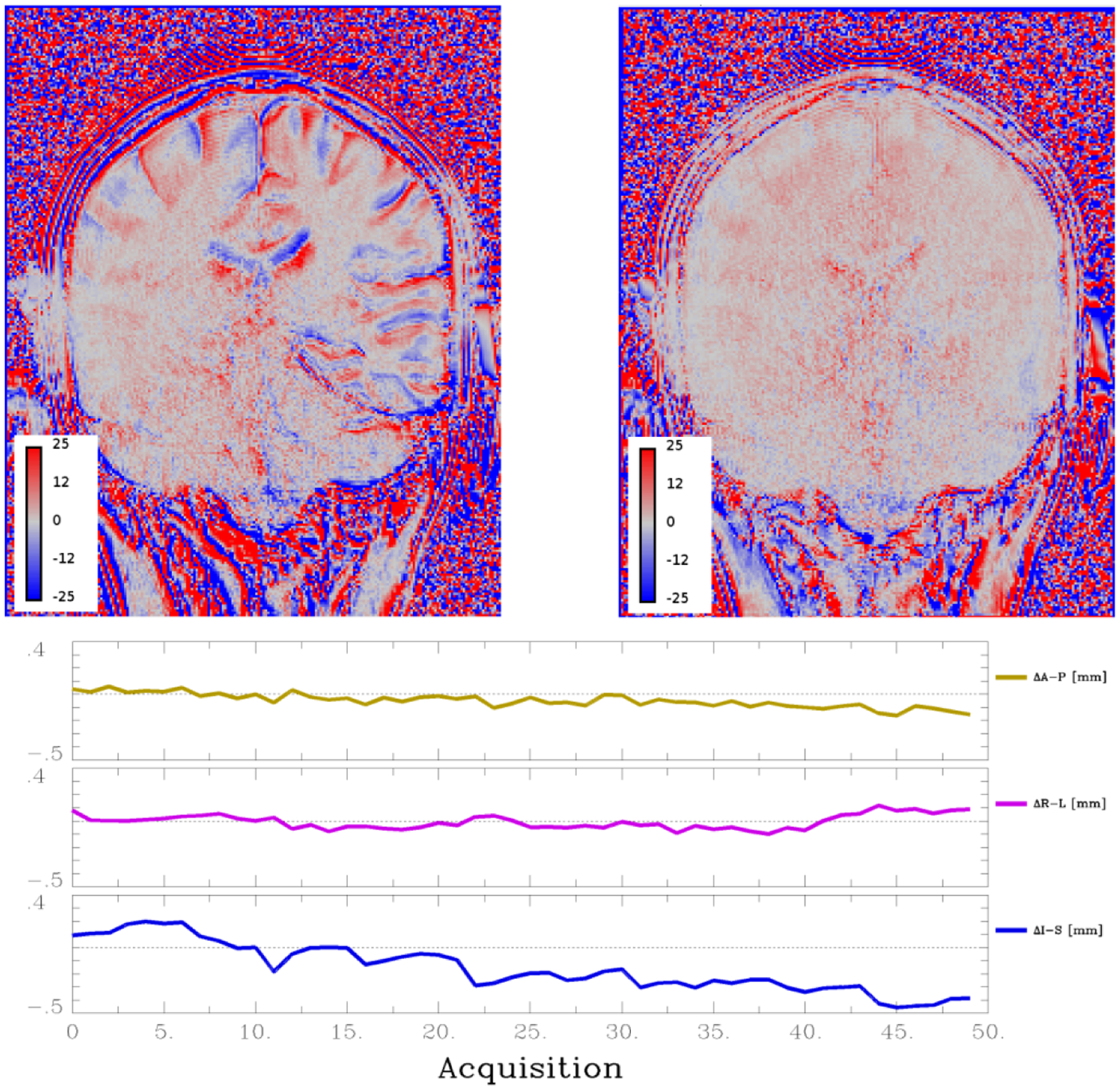
Dynamic contrast-enhanced (DCE) protocol acquisition without contract injection shows percent signal change calculated from a linear fit over time (top images). Left image is the ‘without motion correction’ and shows signal changes up to 25%, particularly at cerebrospinal fluid (CSF)-tissue interface, due to small motions of subjects’ head. Right image is the same dataset after motion correction, which shows almost all signal change is eliminated. Bottom graphs show calculated brain motion relative to the first image along the three principal axis (A-P, anterior-posterior; R-L, right-left; I-S, inferior-superior). Even <0.5 mm of motion can induce significant signal changes (Images courtesy of SRB).
Signal Drift
Small amounts of signal intensity drift over time can be a significant source of error as they can be comparable to the amount of signal intensity change from the contrast agent (Barnes et al., 2016). A frequent cause of signal drift is temperature changes of the MRI hardware and electronics. In animal acquisitions this can be caused by thermal support systems for the animal (particularly blowing warm air into the bore), and care should be taken to achieve a steady temperature and not make adjustments for the entirety of the acquisition. In humans, newer wide bore systems (70 cm bore diameter) are more susceptible to heat transfer from the gradients, and gradient heavy sequences (such as diffusion tensor imaging) can potentially cause heating and signal drift after they are run (as the gradients cool). Signal drift is very system-dependent and therefore it is recommended to acquire some datasets without contrast injection to evaluate any signal drift on the scanner that will be used. If signal drift is a problem on a particular system, it can be monitored and corrected to some extent with constant signal intensity phantom added to the field of view (Barnes et al., 2016; Thrippleton et al., 2019). This is usually a water tube dopped with a small amount of gadolinium contrast agent. Care should be taken to make sure the phantom is large enough that the signal can be reliably measured, so small movements are not mistaken for signal drift.
Vascular Input Function
The measurement of the vascular input function is a critical step that can have a large effect on the final Ktrans values. It can be challenging to make accurate measurements for a variety of reasons including: motion artifacts of the flowing blood, small size of vessels causing partial volume errors, inflow of fresh blood that has seen few radiofrequency (RF) pulses and hasn’t reached steady state, and insufficient temporal resolution to accurately measure the first pass of the contrast bolus. While each of these can be a source of considerable error, most can be minimized by simple acquisition choices. A coronal acquisition, measuring in the superior sagittal sinus (SSS), and a slow contrast agent injection (greater than the temporal resolution) generally gives good results. The coronal acquisition exposes the blood to many RF pulses ensuring it will reach steady state, as the 2D excitation slab will extend through the neck, and possibly to the heart depending on the angle of the slab (Thrippleton et al., 2019). Measuring in the SSS minimizes the flow speed and associated artifacts compared to arterial measurements. The blood in the SSS has also been exposed to more RF pulses traveling through the brain, so is more likely to achieve steady state. The SSS is also relatively large and stationary so will minimize partial volume errors. The venous measurements have been shown to be very similar to arterial measurements and are largely just shifted by 5–7 seconds (Sourbron et al., 2009; Foottit et al., 2010). For acquisitions with temporal resolution greater than 7 seconds there will be little measurable difference. Finally, injecting the contrast agent over a longer duration will minimize errors in measuring the first pass of a bolus injection (Manning et al., 2021).
Conclusion
DCE-MRI underlined the occurrence of local, subtle, and progressive BBB leakage in aging. One of the plausible causal links to cognitive impairment and dementia is through the passage of blood-derived neurotoxic compounds from the blood to brain tissues, causing progressive damage in strategic regions such as the WM and medial temporal lobe. The integrity of the BBB depends on the cohesion of all its components, especially at the capillary level which represents the largest surface for blood contact. Pericytes are found on the whole vascular tree and are key actors in the regulation of vasomotricity and BBB permeability. APOE4 genotype leads to faster BBB disruption, which could prevent waste removal and trigger amyloid-related pathologies such as AD. Inter-regional differences, as well as gender-related differences in the development of BBB disruption may lead to a more precise interpretation of the experimental results. Pre-clinical transgenic models, as well as clinical cohorts, have helped to connect vascular aging to cognitive impairment and led to important insights on the physiology and disease processes. However, detecting earlier stages of BBB breakdown is challenging and may require additional technical innovations in DCE-MRI acquisition and processing techniques. Alternate methods evaluating the water permeability of the BBB does not necessarily correlate with DCE-MRI, thus emphasizing the complexity of the exchanges occurring across the BBB and the need for additional studies to understand these mechanisms.
Highlights.
BBB breakdown is a major driving force in dementia pathology.
DCE-MRI is the most advanced method to investigate BBB leakage in the living human and rodent brain.
Many different DCE-MRI techniques and imaging processing methods exist.
Detecting subtle BBB breakdown using DCE-MRI can be challenging.
Acknowledgements
Audrey Chagnot is supported by grants from the Ministère de l’Enseignement Supérieur et de la Recherche and INSERM (French National Institute for Health and Medical Research) (HCERES U1237-2017/2022) and by the special grant from the Agence Nationale de la Recherche (ANR), MRGly (ANR-17-CE37-0010). Samuel R Barnes is supported by a GRASP award from Loma Linda University and National Institute of Aging 2R44AG059478-03. Axel Montagne is supported by UK Dementia Research Institute which receives its funding from DRI Ltd, funded by the Medical Research Council, Alzheimer’s Society and Alzheimer Research UK. We also thank Michael G Harrington for careful reading of the manuscript. Figures 1 and 2 were created using BioRender.com (Toronto, Canada).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Baloyannis IS (2012) The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci 322:117–121. [DOI] [PubMed] [Google Scholar]
- Barnes SR, Ng TSC, Montagne A, Law M, Zlokovic BV, Jacobs RE (2016) Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med 75:1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood-brain barrier. Prog Drug Res Fortschritte Arzneimittelforschung Progres Rech Pharm 61:39–78. [DOI] [PubMed] [Google Scholar]
- Bernal J, Valdés-Hernández M d. C, Escudero J, Heye AK, Sakka E, Armitage PA, Makin S, Touyz RM, Wardlaw JM, Thrippleton MJ (2021) A four-dimensional computational model of dynamic contrast-enhanced magnetic resonance imaging measurement of subtle blood-brain barrier leakage. NeuroImage 230:117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-I, Ryu C-W, Kim S, Rhee HY, Jahng G-H (2020) Changes in Microvascular Morphology in Subcortical Vascular Dementia: A Study of Vessel Size Magnetic Resonance Imaging. Front Neurol 11:545450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie BR, Boutin H, Parker GJM, Parkes LM (2021) Alzheimer’s disease pathology is associated with earlier alterations to blood-brain barrier water permeability compared with healthy ageing in TgF344-AD rats. NMR Biomed:e4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Liu X, Li X, Wen M, Li Y, Han Y, Huang L, Liu M, Zeng H (2020) Hypercapnia exacerbates the disruption of the blood-brain barrier by inducing interleukin-1β overproduction in the blood of hypoxemic adult rats. Int J Mol Med 46:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foottit C, Cron GO, Hogan MJ, Nguyen TB, Cameron I (2010) Determination of the venous output function from MR signal phase: feasibility for quantitative DCE-MRI in human brain. Magn Reson Med 63:772–781. [DOI] [PubMed] [Google Scholar]
- Freeze WM, Jacobs HIL, de Jong JJ, Verheggen ICM, Gronenschild EHBM, Palm WM, Hoff EI, Wardlaw JM, Jansen JFA, Verhey FR, Backes WH (2020) White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging 85:113–122. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N (2015) Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 78:887–900. [DOI] [PubMed] [Google Scholar]
- Ha IH, Lim C, Kim Y, Moon Y, Han SH, Moon WJ (2021) Regional Differences in Blood-Brain Barrier Permeability in Cognitively Normal Elderly Subjects: A Dynamic Contrast-Enhanced MRI-Based Study. Korean J Radiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV (2016) Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 36:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heye AK, Thrippleton MJ, Armitage PA, Valdés Hernández MDC, Makin SD, Glatz A, Sakka E, Wardlaw JM (2016) Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage 125:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA (2015) Long-Term Blood-Brain Barrier Permeability Changes in Binswanger Disease. Stroke 46:2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhofs D, Wong SM, Zhang E, Staals J, Jansen JFA, van Oostenbrugge RJ, Backes WH (2021) Baseline Blood-Brain Barrier Leakage and Longitudinal Microstructural Tissue Damage in the Periphery of White Matter Hyperintensities. Neurology 96:e2192–e2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li Y, Zuo L, Hu W, Jiang T (2021) Increase of blood-brain barrier leakage is related to cognitive decline in vascular mild cognitive impairment. BMC Neurol 21:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li M, Yang L, Qin W, Yang S, Yuan J, Jiang T, Hu W (2019) The relationship between blood-brain barrier permeability and enlarged perivascular spaces: a cross-sectional study. Clin Interv Aging 14:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li M, Zuo L, Shi Q, Qin W, Yang L, Jiang T, Hu W (2018) Compromised Blood-Brain Barrier Integrity Is Associated With Total Magnetic Resonance Imaging Burden of Cerebral Small Vessel Disease. Front Neurol 9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li M, Zhang X, Shi Q, Yang S, Fan H, Qin W, Yang L, Yuan J, Jiang T, Hu W (2017) Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol 264:1474–1481. [DOI] [PubMed] [Google Scholar]
- Manning C, Stringer M, Dickie B, Clancy U, Valdés Hernandez MC, Wiseman SJ, Garcia DJ, Sakka E, Backes WH, Ingrisch M, Chappell F, Doubal F, Buckley C, Parkes LM, Parker GJM, Marshall I, Wardlaw JM, Thrippleton MJ (2021) Sources of systematic error in DCE-MRI estimation of low-level blood-brain barrier leakage. Magn Reson Med. [DOI] [PubMed] [Google Scholar]
- McAleese KE, Graham S, Dey M, Walker L, Erskine D, Johnson M, Johnston E, Thomas AJ, McKeith IG, DeCarli C, Attems J (2019) Extravascular fibrinogen in the white matter of Alzheimer’s disease and normal aged brains: implications for fibrinogen as a biomarker for Alzheimer’s disease. Brain Pathol Zurich Switz 29:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JS, Schulz I, Love S (2018) Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 38:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A et al. (2018) Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 24:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Montagne A et al. (2020) APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Huuskonen MT, Rajagopal G, Sweeney MD, Nation DA, Sepehrband F, D’Orazio LM, Harrington MG, Chui HC, Law M, Toga AW, Zlokovic BV (2019) Undetectable gadolinium brain retention in individuals with an age-dependent blood-brain barrier breakdown in the hippocampus and mild cognitive impairment. Alzheimers Dement J Alzheimers Assoc 15:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV (2016) Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol (Berl) 131:687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Nikolakopoulou AM, Huuskonen MT, Sagare AP, Lawson EJ, Lazic D, Rege SV, Grond A, Zuniga E, Barnes SR, Prince J, Sagare M, Hsu CJ, LaDu MJ, Jacobs RE, Zlokovic BV (2021) APOE4 accelerates advanced stage vascular and neurodegenrative disorder in old Alzheimer’s mice via cyclophilin A independently of amyloid-β. Nat Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med 214:3151–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y, Lim C, Kim Y, Moon W-J (2021) Sex-Related Differences in Regional Blood-Brain Barrier Integrity in Non-Demented Elderly Subjects. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, Huuskonen MT, Sagare AP, Lazic D, Sweeney MD, Kong P, Wang M, Owens NC, Lawson EJ, Xie X, Zhao Z, Zlokovic BV (2019) Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 22:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau SJ, Langen UH, Fisher TM, Prakash I, Nagpurwala F, Lozoya RA, Lee W-CA, Wu Z, Gu C (2021) Vascular and perivascular cell profiling reveals the molecular and cellular bases of blood-brain barrier heterogeneity. bioRxiv:2021.04.26.441465. [Google Scholar]
- Procter T, Williams A, Montagne A (2021) Interplay between brain pericytes and endothelial cells in dementia. Am J Pathol. [DOI] [PubMed] [Google Scholar]
- Raja R, Rosenberg GA, Caprihan A (2018) MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies. Neuropharmacology 134:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Prestopnik J, Adair JC, Huisa BN, Knoefel J, Caprihan A, Gasparovic C, Thompson J, Erhardt EB, Schrader R (2015) Validation of biomarkers in subcortical ischaemic vascular disease of the Binswanger type: approach to targeted treatment trials. J Neurol Neurosurg Psychiatry 86:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV (2013) Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4:2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol Zurich Switz 23:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Jann K, Ma SJ, Yan L, Montagne A, Ringman JM, Zlokovic BV, Wang DJJ (2020) Comparison Between Blood-Brain Barrier Water Exchange Rate and Permeability to Gadolinium-Based Contrast Agent in an Elderly Cohort. Front Neurosci 14:571480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourbron S, Heilmann M, Biffar A, Walczak C, Vautier J, Volk A, Peller M (2009) Bolus-tracking MRI with a simultaneous T1- and T2*-measurement. Magn Reson Med 62:672–681. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV (2016) Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4:e1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD et al. (2019a) Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 15:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV (2018a) The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21:1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Pachicano M, Harrington MG, Joe E, Chui HC, Schneider LS, Montagne A, Ringman JM, Fagan AM, Morris JC, Pa J, Nation DA, Toga AW, Zlokovic BV (2020) A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid. Alzheimers Dement J Alzheimers Assoc 16:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV (2018b) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019b) Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev 99:21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrippleton MJ, Backes WH, Sourbron S, Ingrisch M, van Osch MJP, Dichgans M, Fazekas F, Ropele S, Frayne R, van Oostenbrugge RJ, Smith EE, Wardlaw JM (2019) Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimers Dement J Alzheimers Assoc 15:840–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ (2013) Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain J Neurol 136:2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D (2009) Correlations of Neuronal and Microvascular Densities in Murine Cortex Revealed by Direct Counting and Colocalization of Nuclei and Vessels. J Neurosci 29:14553–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Kan H, Sakurai K, Arai N, Inui S, Kobayashi S, Kato D, Ueki Y, Matsukawa N (2020) Iron leakage owing to blood-brain barrier disruption in small vessel disease CADASIL. Neurology 95:e1188–e1198. [DOI] [PubMed] [Google Scholar]
- Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ (2020) Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front Aging Neurosci 12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Haar HJ, Burgmans S, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Hofman PAM, Verhey FRJ, Backes WH (2016a) Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 281:527–535. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Jansen JFA, Jeukens CRLPN, Burgmans S, van Buchem MA, Muller M, Hofman PAM, Verhey FRJ, van Osch MJP, Backes WH (2017) Subtle blood-brain barrier leakage rate and spatial extent: Considerations for dynamic contrast-enhanced MRI. Med Phys 44:4112–4125. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Wong SM, Hofman PAM, Burgmans S, Verhey FRJ, Backes WH (2016b) Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging 45:190–196. [DOI] [PubMed] [Google Scholar]
- Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild EHBM, Palm WM, Postma AA, Jansen JFA, Verhey FRJ, Backes WH (2020a) Increase in blood-brain barrier leakage in healthy, older adults. GeroScience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen ICM, de Jong JJA, van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, Backes WH (2020b) Imaging the role of blood-brain barrier disruption in normal cognitive ageing. GeroScience 42:1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang R, Tao C, Xu Z, Chen W, Wang C, Song L, Zheng J, Gao F (2018) Blood-Brain Barrier Disruption and Perivascular Beta-Amyloid Accumulation in the Brain of Aged Rats with Spontaneous Hypertension: Evaluation with Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Korean J Radiol 19:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, Muñoz-Maniega S, Sakka E, Shuler K, Dennis MS, Thrippleton MJ (2017) Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement 13:634–643. [Google Scholar]
- Wardlaw JM, Smith C, Dichgans M (2019) Small vessel disease: mechanisms and clinical implications. Lancet Neurol 18:684–696. [DOI] [PubMed] [Google Scholar]
- Wong SM, Jansen JFA, Zhang CE, Hoff EI, Staals J, van Oostenbrugge RJ, Backes WH (2019) Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 92:e1669–e1677. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zeng W, Sun J, Chen W, Zhang R, Yang Z, Yao Z, Wang L, Song L, Chen Y, Zhang Y, Wang C, Gong L, Wu B, Wang T, Zheng J, Gao F (2017) The quantification of blood-brain barrier disruption using dynamic contrast-enhanced magnetic resonance imaging in aging rhesus monkeys with spontaneous type 2 diabetes mellitus. NeuroImage 158:480–487. [DOI] [PubMed] [Google Scholar]
- Yang AC et al. (2021) A human brain vascular atlas reveals diverse cell mediators of Alzheimer’s disease risk. bioRxiv:2021.04.26.441262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T (2009) Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 15:1031–1037. [DOI] [PubMed] [Google Scholar]
- Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JFA, Jeukens CRLPN, Hofman PAM, van Oostenbrugge RJ, Backes WH (2017a) Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology 88:426–432. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D (2017b) Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163:1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]


