INTRODUCTION:
Serum biomarkers for the diagnosis of minimal hepatic encephalopathy (MHE) in patients with liver cirrhosis would be desirable. In this proof-of-concept study, we investigated the association between MHE and serum levels of neurofilament light chains (sNfL) in patients with liver cirrhosis.
METHODS:
sNfL were studied in patients with liver cirrhosis (with or without MHE) and controls (patients with ischemic stroke, transitory ischemic attack, and healthy individuals). MHE was diagnosed using the Psychometric Hepatic Encephalopathy Score.
RESULTS:
Patients with MHE showed higher sNfL than patients without MHE and controls. In multivariable analyses, higher sNfL were independently associated with the presence of MHE. sNfL had a reliable discriminative power for the detection of MHE with an area under the curve of 0.872.
DISCUSSION:
MHE is associated with higher sNfL.
INTRODUCTION
The diagnosis of minimal hepatic encephalopathy (MHE)—the lowest grade of hepatic encephalopathy (HE)—strictly requires specialized tests, which are time consuming and not widely used. Consequently, serum biomarkers to simplify the diagnosis are an unmet medical need. Most studies have focused on serum ammonia or inflammatory markers because a combination of both is considered the most likely driving force in the development of HE (1,2). However, the diagnostic utility for MHE has not been proven as sufficient in any serum biomarker. Recent preclinical studies have indicated that hyperammonemia and inflammation may cause neuronal cell death and that an episode of overt hepatic encephalopathy (OHE) may result in residual cognitive impairment (3–6). Neurofilament light chains (NfL) are axonal structural proteins that are released as a result of neuroaxonal damage during neurodegeneration and can be detected in serum by high-sensitivity assays (7). Recently, the value of serum NfL (sNfL) as a biomarker has been proven in patients with chronic inflammatory neurological diseases such as multiple sclerosis (8,9). Therefore, in this proof-of-concept study, we investigated the association between MHE and sNfL in patients with liver cirrhosis to potentially identify a future biomarker candidate for MHE.
METHODS
Data of 64 patients with liver cirrhosis recruited during outpatient visits or during elective hepatic venous pressure gradient measurement at the Cirrhosis Center Mainz of the University Medical Center of the Johannes Gutenberg-University were analyzed. In addition, 68 age-matched patients with stroke (n = 29), transitory ischemic attack (n = 29), and healthy individuals (n = 10) recruited at the Department of Neurology served as controls (10). A detailed description of diagnosis of liver cirrhosis and all exclusion criteria can be found in the supplementary methods and elsewhere (11). MHE was diagnosed using the Psychometric Hepatic Encephalopathy Score with established German norms (12).
Blood samples were spun at 2,000 g at room temperature for 10 minutes within 90 minutes after withdrawal at the day of study inclusion and stored in polypropylene tubes at −80 °C until batched sNfL analysis. sNfL were measured by single molecule array HD-1 using the NF-Light Advantage Kit (Quanterix) according to the manufacturer's instructions (9,10).
Quantitative data are expressed as medians with interquartile ranges, and pairwise comparisons for quantitative variables were performed with an unpaired t test or with the Mann-Whitney U test. Categorical variables are given as frequencies and percentages, respectively, and for comparison of 2 or more patient groups, a χ2 test was applied. To identify independent variables associated with MHE, multivariable analyses were conducted using logistic regression models, and only univariable significant variables with biological plausibility (P < 0.05) were included. Multivariable analysis to identify variables associated with higher sNfL levels was conducted using a linear regression model with a stepwise variable selection. The discriminative ability of sNfL for the identification of patients with MHE was analyzed with help of the area under the curve (AUC)-receiver operating characteristics. Correlation analyses were conducted using the Spearman rank correlation or point-biserial correlation. Data were analyzed using IBM SPSS Statistic Version 23.0 (IBM, Armonk, NY) and GraphPad Prism (GraphPad, San Diego, CA).
The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study was approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz. Written informed consent was obtained from all participants.
RESULTS
Baseline characteristics of the patient cohort are displayed in Table 1 and Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A713 (see Supplementary Data file, Supplementary Digital Content 2, http://links.lww.com/CTG/A712). Patients with MHE had highest sNfL as compared to patients with liver cirrhosis but without MHE, patients with ischemic stroke, transitory ischemic attack, or healthy individuals (Figure 1a). Patients with a history of OHE had only numerically higher sNfL as compared to patients with MHE but no history of OHE (P = 0.307) (Figure 1b). sNfL correlated with Psychometric Hepatic Encephalopathy Score (r = −0.575, P < 0.001) and interleukin-6 serum levels (r = 0.544, P < 0.001), while there was a trend for a correlation with ammonia (r = 0.381, P = 0.081) (see Supplementary Figure 1a, b and 2, Supplementary Digital Content 3, http://links.lww.com/CTG/A714). Patients with a combination of higher ammonia and interleukin-6 had significantly higher sNfL than patients without this condition (see Supplementary Figure 3, Supplementary Digital Content 3, http://links.lww.com/CTG/A714). Alcoholic etiology of cirrhosis was not associated with higher sNfL (Figure 1c). In multivariable analyses, sNfL levels were associated with the presence of MHE after adjusting for univariable significant (Table 1) and biologically plausible factors (Table 2). Variables independently associated with higher sNfL were MHE, a higher model for end-stage liver disease and a history of OHE (see Supplementary Table 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A713). In receiver operating characteristic analysis, the discriminative ability of sNfL to identify patients with MHE was high (AUC 0.872 [95% confidence interval 0.773–0.971 P < 0.001] in the total cohort, AUC 0.839 [95% confidence interval 0.711–0.966 P < 0.001] in patients without a history of OHE; Figure 1d,e).
Table 1.
Demographics and clinical characteristics of the patients with liver cirrhosis at study inclusion
| Variable | Total cohort n = 64 | Patients with MHE n = 26 | Patients without MHE n = 38 | P value |
| Age, y (IQR) | 60 (53–65) | 57 (51–66) | 60 (53–62) | 0.795 |
| Male sex, n (%) | 33 (51.6) | 18 (69.2) | 15 (39.5) | 0.025 |
| Etiology | ||||
| Alcohol, n (%) | 36 (56.2) | 20 (76.9) | 16 (42.1) | 0.170 |
| Viral hepatitis, n (%) | 4 (6.3) | 1 (3.8) | 3 (7.9) | |
| NAFLD, n (%) | 9 (14.1) | 1 (3.8) | 8 (21.1) | |
| Cholestatic/autoimmune, n (%) | 3 (4.7) | 0 (0) | 3 (7.9) | |
| Alcohol + other etiology, n (%) | 5 (7.8) | 3 (11.5) | 2 (5.3) | |
| Cryptogenic, n (%) | 7 (10.9) | 1 (3.8) | 6 (15.8) | |
| Median MELD score (IQR) | 13 (9–18) | 15 (11–21) | 12 (8–16) | 0.006 |
| CP A/B/C, n (%) | 34/27/3 (53/42/5) | 7/17/2 (27/65/8) | 27/10/1 (71/26/3) | 0.002 |
| History of ascites, n (%) | 37 (57.8) | 21 (80.7) | 5 (13.2) | 0.002 |
| History of OHE, n (%) | 8 (12.5) | 7 (26.9) | 1 (2.6) | 0.004 |
| MHE, n (%) | 26 (40.6) | 26 (100) | 0 (0) | N/A |
| Thrombocytes, /nL (IQR) | 107 (82–145) | 105 (77–145) | 107 (85–146) | 0.692 |
| Albumin, g/L (IQR) | 31 (23–37) | 26 (21–31) | 33 (27–38) | 0.002 |
| Sodium, mmol/L (IQR) | 138 (135–140) | 137 (132–140) | 138 (137–140) | 0.103 |
| Interleukin-6, pg/mL (IQR)a | 17 (8–31) | 21 (16–82) | 11(5–28) | 0.006 |
| Ammonia, µmol/L (IQR)b | 44 (35–59) | 52 (44–58) | 37 (31–61) | 0.106 |
| sNfL, pg/mL (IQR) | 24.3 (12.1–65.5) | 73.0 (30.4–114.4) | 16 (11–25) | <0.001 |
Data are expressed as medians and interquartile ranges or as frequencies and percentages.
Significance was set at P < 0.05.
IQR, interquartile range; MELD, model for end-stage liver disease; MHE, minimal hepatic encephalopathy; NAFLD, nonalcoholic fatty liver disease; OHE, overt hepatic encephalopathy; sNfL, serum neurofilament light chains.
Measured in 55 patients.
Measured in 22 patients.
Figure 1.
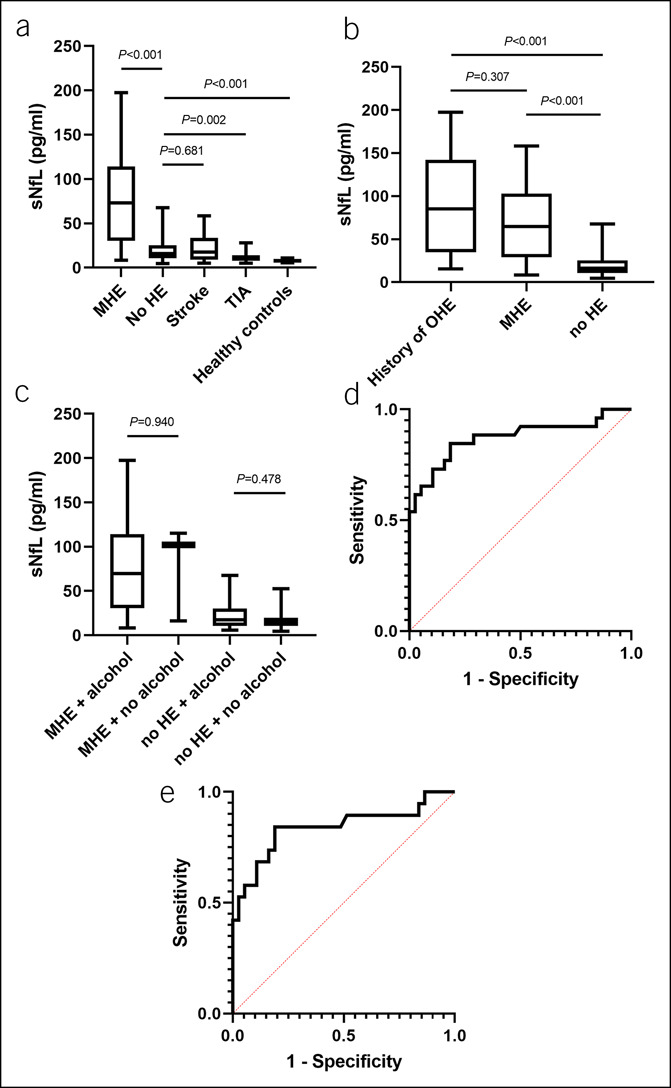
sNfL in different patient cohorts and the discriminative power of sNfL to detect MHE in patients with liver cirrhosis. sNfL in patients with liver cirrhosis with (n = 6) or without MHE (n = 38), in patients with stroke (n = 29), TIA (n = 29), and healthy individuals (n = 10) (a). sNfL in patients with liver cirrhosis and a history of OHE (n = 8), in patients with presence of MHE but no history of OHE (n = 19), and in patients without any history or presence of HE (n = 37) (b). sNfL in patients with or without alcoholic etiology of liver cirrhosis stratified by MHE status (c). Discriminative ability of sNfL to detect MHE in the total cohort of patients with liver cirrhosis (n = 64, AUC = 0.872, 95% CI 0.773–0.971; P < 0.001) (d). Discriminative ability of sNfL to detect MHE in the cohort of patients with liver cirrhosis and no history of OHE (n = 56, AUC 0.839, 95% CI 0.711–0.966; P < 0.001) (e). AUC, area under the curve; HE, hepatic encephalopathy; MHE, minimal hepatic encephalopathy; OHE, overt hepatic encephalopathy; sNfL, serum levels of neurofilament light chains; TIA, transitory ischemic attack.
Table 2.
Logistic regression analyses of predictors for the presence of minimal hepatic encephalopathy (MHE) in patients with liver cirrhosis
| Model 1a | P value | Model 2b | P value | |
| OR (95% CI) | OR (95% CI) | |||
| sNfL | 1.077 (1.027–1.130) | 0.002 | 1.071 (1.022–1.123) | 0.004 |
| MELD score | 0.863 (0.692–1.077) | 0.193 | 0.875 (0.706–1.084) | 0.223 |
| Albumin serum levels | 0.924 (0.819–1.042) | 0.196 | 0.920 (0.818–1.036) | 0.169 |
| History of OHE | 5.235 (0.341–80.284) | 0.235 |
CI, confidence interval; MELD, model for end-stage liver disease; OHE, overt hepatic encephalopathy; OR, odds ratio; sNfL, serum neurofilament light chains.
Model 1 included sNfL, MELD score, albumin serum levels, and history of OHE.
In model 2, patients with a history of OHE were excluded, and the model included the variables: sNfL, MELD score, and albumin serum levels.
DISCUSSION
In this proof-of-concept study, we demonstrate that sNfL are independently associated with MHE in patients with liver cirrhosis. Our data may therefore provide indirect evidence of a potential slight neuroaxonal damage in these patients.
Although it was the prevailing opinion for a long time that neuroaxonal cell death is minimal or even nonexistent in patients with HE and that HE is completely reversible, this was challenged in a clinical study by Bajaj et al., demonstrating that episodes of OHE are associated with persistent and even cumulative deficits in working memory, response inhibition, as well as learning (3). Moreover, recent preclinical studies have indicated that hyperammonemia may cause neurite degeneration and cell death of cultured neurons (4,13). In addition, a study simulating the clinical course of episodic HE in rats has demonstrated the existence of neuronal death in the cerebellum in immunohistochemical analysis (5). Other data have indicated that peripheral inflammation leading to neuroinflammation results in a loss of cerebellar neurons and may occur at early stages of liver diseases and worsens in patients with cirrhosis and HE (6). Our current findings are in line with these studies hereby demonstrating potential neuroaxonal damage in patients with MHE as reflected by higher sNfL.
It has to be acknowledged that the current study is limited by its cross-sectional design, and therefore, causality of higher sNfL and presence of MHE cannot be proven. In addition, we did not screen our patients according to a prespecified protocol for other triggers of elevated sNfL, such as cerebral microangiopathy or polyneuropathy. Moreover, our study is limited by lack of longitudinal data, and therefore, we are unable to investigate fluctuations in sNfL or effects of treatment (e.g., lactulose) and liver function. Last, our cohort mainly consists of patients with an alcoholic etiology of their liver cirrhosis, which may be a potential confounder. The effect of a history of alcohol abuse on sNfL has to be investigated in future studies in more detail.
In conclusion, our study provides indirect evidence that neuroaxonal damage may be present in patients with liver cirrhosis and MHE, and in turn, sNfL may be a potential future biomarker for MHE.
CONFLICTS OF INTEREST
Guarantor of the article: Christian Labenz, MD.
Specific author contributions: Performed research: C.L., M.N., P.K., S.E., L.K., J.M.S., M.-A.W., and F.L. Contributed to acquisition of data: C.L., M.N., P.K., S.E., and F.L. Designed the experiments and analyzed the data: C.L., P.K., S.E., M.-A.W., and F.L. Contributed reagents/materials/analysis tools: C.L., P.R.G., S.B., and F.L. Wrote the article: C.L., S.E., M.-A.W., and F.L. Statistical analysis: C.L. All authors approved the final version of the manuscript and the authorship list.
Financial support: None to report.
Potential competing interests: None to report.
Supplementary Material
ACKNOWLEDGMENTS
This study contains parts of the medical thesis of Paula Kämper. This work was not supported by any grant or funding source. C.L. is supported by the Clinical Research Fellowship Program by the Mainz Research School of Translational Biomedicine.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A713, http://links.lww.com/CTG/A712, and http://links.lww.com/CTG/A714.
Marcus-Alexander Wörns and Felix Lüssi are senior coauthors.
Contributor Information
Michael Nagel, Email: michael.nagel@unimedizin-mainz.de.
Paula Kämper, Email: paula.kaemper@gmx.de.
Sinah Engel, Email: sinah.engel@unimedizin-mainz.de.
Stefan Bittner, Email: stefan.bittner@unimedizin-mainz.de.
Leonard Kaps, Email: leonard.kaps@unimedizin-mainz.de.
Peter R. Galle, Email: peter.galle@unimedizin-mainz.de.
Jörn M. Schattenberg, Email: joern.schattenberg@unimedizin-mainz.de.
Marcus-Alexander Wörns, Email: marcus-alexander.woerns@klinikumdo.de.
Felix Lüssi, Email: luessi@uni-mainz.de.
REFERENCES
- 1.Montagnese S, Biancardi A, Schiff S, et al. Different biochemical correlates for different neuropsychiatric abnormalities in patients with cirrhosis. Hepatology 2011;53:558–66. [DOI] [PubMed] [Google Scholar]
- 2.Tapper EB. Predicting overt hepatic encephalopathy for the population with cirrhosis. Hepatology 2019:70(1):403–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj JS, Schubert CM, Heuman DM, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010;138:2332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klejman A, Wegrzynowicz M, Szatmari EM, et al. Mechanisms of ammonia-induced cell death in rat cortical neurons: Roles of NMDA receptors and glutathione. Neurochem Int 2005;47:51–7. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lezana T, Oria M, Romero-Gimenez J, et al. Cerebellar neurodegeneration in a new rat model of episodic hepatic encephalopathy. J Cereb Blood Flow Metab 2017;37:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balzano T, Forteza J, Molina P, et al. The cerebellum of patients with steatohepatitis shows lymphocyte infiltration, microglial activation and loss of purkinje and granular neurons. Sci Rep 2018;8:3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–89. [DOI] [PubMed] [Google Scholar]
- 8.Engel S, Steffen F, Uphaus T, et al. Association of intrathecal pleocytosis and IgG synthesis with axonal damage in early MS. Neurol Neuroimmunol Neuroinflamm 2020;7(3):e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel S, Friedrich M, Muthuraman M, et al. Intrathecal B-cell accumulation and axonal damage distinguish MRI-based benign from aggressive onset in MS. Neurol Neuroimmunol Neuroinflamm 2019;6(5):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uphaus T, Bittner S, Groschel S, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke 2019;50:3077–84. [DOI] [PubMed] [Google Scholar]
- 11.Labenz C, Toenges G, Huber Y, et al. Development and validation of a prognostic score to predict covert hepatic encephalopathy in patients with cirrhosis. Am J Gastroenterol 2019;114:764–70. [DOI] [PubMed] [Google Scholar]
- 12.Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001;34:768–73. [DOI] [PubMed] [Google Scholar]
- 13.Cai Z, Zhu X, Zhang G, et al. Ammonia induces calpain-dependent cleavage of CRMP-2 during neurite degeneration in primary cultured neurons. Aging (Albany NY) 2019;11:4354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


