Abstract
Objective(s):
Breast cancer (BC) cells’ ability to metastasize to other tissues increases mortality. The Matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) facilitate cancer cell migration. 5-fluorouracil is a frequently applied chemotherapeutic agent in cancer treatment with destructive side effects on normal tissues. Hence, researchers have focused on finding a way to reduce the dose of chemotherapeutic drugs. Quercetin, a natural polyphenolic compound, has inhibitory effects on proliferation and migration of tumor cells. This study evaluated the effect of the combination of Quercetin and 5-fluorouracil on migration of the MDA-MB-231 breast cancer cell line.
Materials and Methods:
The effect of Quercetin, 5-fluorouracil , and their combination on MDA-MB-231 breast cancer cell proliferation was investigated through MTT assay. Inhibition of tumor cell migration was examined by wound healing assay. Finally, the effect of treatments on gene expression of MMP-2 and MMP-9 was evaluated by quantitative real-time PCR.
Results:
The IC50 values for Quercetin and 5-fluorouracil after 48 hr treatment were 295 μM and 525 μM, respectively. The combination index (CI) for Quercetin and 5-fluorouracil was <1, indicating synergy between them. The combination of Quercetin plus 5-fluorouracil resulted in a significant reduction in migration rate and MMP-2 and MMP-9 gene expressions of MDA-MB-231 cancer cells compared with the individual application of 5-FU.
Conclusion:
Quercetin enhances the suppressory effect of 5-fluorouracil on migration of BC cells. The combination of Quercetin and 5-fluorouracil can be an attractive field for future studies.
Key Words: 5-fluorouracil, Anti-metastatic effect, Breast cancer, Quercetin, Synergism
Introduction
Breast cancer (BC) is the most prevalent cancer among women accounting for over 600000 cancer deaths in 2018 across the globe (1). Fifteen to 20 % of all BC cases are Triple-negative breast cancer (TNBC). Compared with other breast cancer subtypes, TNBC has an earlier relapse after treatment and a greater probability of metastasis (2, 3).
The metastasis of cancer cells all over the body is the main cause of cancer mortality (4-7). This process’s main event is the degradation of the extracellular matrix (ECM) components by proteolytic enzymes such as MMPs(8-12). It has been observed that MMP-2 and MMP-9, also known as gelatinases, have frequently contributed to the metastatic ability of BC cells (11, 13).
Chemotherapy is used as the main treatment for breast cancer (14). However, due to the destructive effects of these drugs on normal cells, the patients have to endure some severe side effects of this treatment (15-18). Therefore, due to their extensive biological activity and low toxicity, natural substances are considered potential alternative treatments for cancers, including BC (19-23). Besides, many studies have focused on combining natural compounds with chemotherapeutic agents to decrease chemotherapy’s adverse effects (20, 21, 24-26).
5-fluorouracil (5-FU) is a uracil nucleotide in which fluorine replaces the hydrogen atom at position 5 and acts as an antimetabolite. 5-FU’s active form can inhibit DNA synthesis and obstruct tumor growth; 5-FU also disrupts some cellular RNAs’ structure and gene expression (27). The inhibitory effect of fluorouracil alone and combined with natural compounds such as resveratrol and EGCG has been shown in various studies on the migration of various cancer cells such as colorectal and gastric cancers (28-32).
Quercetin (Que) is a natural flavonoid that is mainly found in vegetables and fruits such as onions, apples, and broccoli. Que’s therapeutic effect has been shown in multiple diseases, including cardiovascular disease, neurodegenerative disease, and cancer (33-35). It has been widely reported that Que can inhibit migration of various cancer cell lines such as skin, colorectal, and ovarian cancers (36-40). However, the effects of Que’s combination with chemotherapeutic agents such as 5-FU against the migration of highly metastatic BC cells are yet to be clear.
In this study, we evaluated the effects of Que alone and in combination with the widely used chemotherapeutic agent 5-FU on the migration of MDA-MB-231 breast cancer cell line, as a highly invasive BC cell line, and on the expression of MMP-2 and MMP-9 genes in vitro.
Materials and Methods
Cell culture
MDA-MB-231 breast cancer and MRC-5 human normal lung fibroblast cell lines were obtained from the Pasteur Institute (Tehran, Iran). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) with 4.5 g/l glucose, 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin (all reagents obtained from Bio-Idea, Tehran, Iran).
Preparation of treatments
5-FU was purchased from Ebewe Pharma (Unterach, Austria) at 50 mg/ml. To gain the desired concentration, the right amount of drug was added directly to supplement the medium. Que was obtained from Sigma–Aldrich (St. Louis, Missouri, United States). Preparation of Que solutions was performed readily before use at 100 mM through dilution in dimethylsulfoxide (DMSO, Bio-Idea), then the solutions were diluted in the cell culture medium. The concentration of DMSO in the cell culture medium was constantly < 0.1%. Thus it has not affected any cell functions (results not shown).
Cell viability assay (MTT)
The viability of cancerous and normal cells was assessed using the MTT assay. MDA-MB231 and MRC-5 cells were seeded in 96-well plates (4×103 and 104 cells per well, respectively) and incubated at 37 °C in 5% CO2 and kept overnight. Then treatment with various concentrations of Que (25, 50, 200, 350, 500, and 650 μM) and 5-FU (1.5, 6.25, 25, 100, 400, and 1600 μM) was performed and incubated for 24 and 48 hr. The medium containing drugs was then removed, and MTT solution (0.5 mg/ ml) was added to the cells. plates were incubated in the dark for 4 hr. Then, the MTT solution was replaced by 150 μl of DMSO (Merck, Darmstadt, Germany) and left 20 min on the shaker. The optical density (OD) of each well at 570 nm was assessed using a microplate reader (BioTek ELx800 Winooski, Vermont, United States). The cell viability percentage was calculated as the ratio of absorbance of treated and untreated control cells. IC50 was obtained from the proliferation curves produced by Graphpad Prism 8 computer software (La Jolla, CA). The selectivity index (SI) value was calculated for Que and 5-FU using IC50 values obtained from MTT assay results for MDA-MB-231 and MRC5 cell lines using the following formula, SI=IC50 of normal cell line/IC50 of cancer cell line. SI values >1 indicate the drug’s selective effect on cancer cells and lower adverse effects on normal cells.
CI and DRI determination
Combination index (CI) and Dose reduction index (DRI) determination were performed using CompuSyn (Chou and Martin, 2005, Compusyn Inc, USA) to evaluate the synergistic relation between Que and 5-FU. CI=1 represents additive effects, while CI >1 and CI <1 mean antagonism and synergy, respectively. Also, DRI was used to indicate the synergy between drugs and fold change in the reduction of drug dose. DRI >1 is favorable and indicates synergy between drugs.
Migration assay
Cells were grown to form a single layer with about 80% confluency in 6-well plates. Wounds were made in the cultures using the sterile tip of a 200 μl pipette. Cells were then washed using PBS to remove cell debris and non-attached cells. Fresh medium with specific concentrations of Que and 5-FU were added to the cultures. At 0, 24, and 48 hr, wounded areas were photographed in random microscopic zones. The distances of cell migration were obtained through pixel count using the NIH image J software package (National Institutes of Health, Bethesda, USA) through the following formula:
Migration rate = [(T0 – T48)/T0] × 100.
Total RNA extraction and cDNA synthesis
106 cells were used for RNA extraction. Total RNA was isolated using a Hybrid-R RNA isolation kit (GeneAll, Songpa-gu, Seoul, South Korea) in accordance with the manufacturer’s instructions. RNA purity and integrity were confirmed with A260/A280 ratio (~ 1.8-2.0) and agarose gel electrophoresis, respectively. The isolated RNA was eluted in 50 μl DEPC treated water and stored at -70 °C. According to the manufacturer’s instructions, reverse transcription was performed in a 20 μl reaction mixture using the cDNA synthesis kit (Yekta Tajhiz Azuma Tehran, Iran).
Quantitative Real-time PCR
The MMP-2 and -9 gene expression levels were evaluated through Real-time PCR using Syber green kit (Yekta Tajhiz Azuma Tehran, Iran). HPRT was preferred as the internal reference. The sequences of primers are as follow:
MMP-2 F: 5’-CCCAGCCAGAAGCGGAAA-3’ R: 5’- CGAACAGATGCCACAATAAAGC-3’, MMP-9 F: 5’-CCTTTGGACACGCACGAC-3’ R: 5’-CCACCTGGTTCAACTCACTC-3’, HPRT F: 5’ GACCAGTCAACAGGGGACAT 3’ R: 5’ CCTGACCAAGGAAAGCAAAG 3’.
The reaction conditions were as follows: 94 °C for 3 min; 94 °C for 40 sec, 59 °C for 30 sec, 72 °C for 30 sec and 30 cycles; and 72 °C for 5 min.
Statistical analysis
All experiments were performed in three separate tests, and the data were presented as mean±SEM. Statistical analysis was done using IBM SPSS 26 software (Chicago, USA). The results of the experimental groups were compared using one-way ANOVA and LSD post-hoc. A P-value less than 0.05 was considered significant.
Results
Effects of Que and 5-FU on cell proliferation
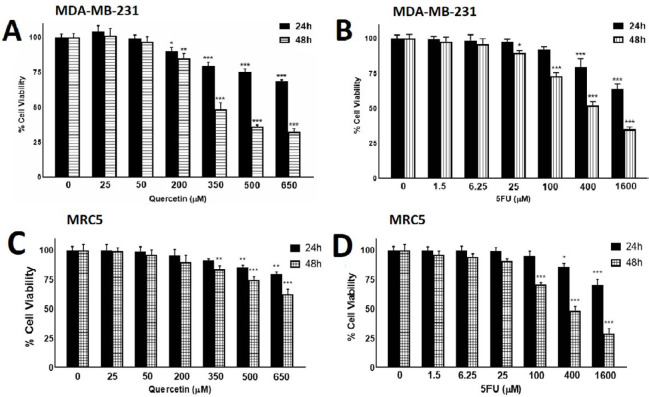
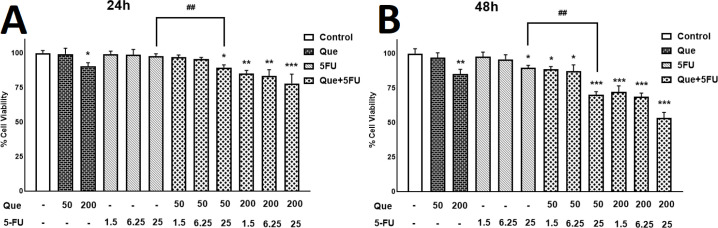
We performed an MTT assay to confirm Que’s ability as an anticancer agent alone and combined with 5-FU. MDA-MB-231 and MRC5 cells were treated with different concentrations of Que and 5-FU for 24 and 48 hr. As revealed in Figures 1A and B, after treatment with Que concentrations above 200 μM and 5-FU concentrations above 25 μM, tumor cells› viability was decreased significantly in a dose-dependent manner. Figures 1C and D represent the effects of Que and 5-FU on proliferation of MRC5 cells for 24 and 48 hr. As shown in Figure 1C, Que concentrations above 200 significantly reduced the viability of normal cells in a dose-dependent manner. As shown in Figure 1D, 5-FU concentrations above 25 μM significantly inhibited the growth of MRC5 cells. IC50 values of Que and 5-FU for MDA-MB-231 and MRC5 cell lines were shown in Table 1. According to these IC50 values, Que has a high selectivity index (SI) (>3.39), and in an opposing manner, 5-FU has a low selectivity index (0.65). In SI analysis, SI values lower than 1 represent more adverse effects on normal cells, and SI values above 2 mean highly selective inhibitory effects on tumor cells compared with normal cells. In the next step, six combination states were produced by three concentrations of 5-FU (1.5, 6.25, and 25 μM), which have no significant effect on MDA-MB-231 growth inhibition and two below IC50 concentration of Que (50 and 200 μM). After treatment with the combination of Que and 5-FU, the viability of cancer cells was reduced significantly compared with treatment with Que or 5-FU alone (Figure 2). To further assess the synergy between Que and 5-FU, the CI value was determined for each combination state. As presented in Table 2, all of the CI values were <1, indicating a synergistic effect between Que and 5-FU. The least CI value (0.33) was for the combination of 50 μM Que and 25 μM 5-FU. Hence, these concentrations were chosen for the rest of the experiments. Also, the DRI value was calculated for the combination of Que and 5-FU to determine the reduction in the applied dose of 5-FU. All DRI values were >1, which is considered desirable (results not shown). To show the effect of the selected combination state on growth inhibition of normal cells, treatment with the combination of 50 μM of Que and 25 μM of 5-FU was performed. As presented in Figure 3, the combination treatment did not significantly increase the cytotoxicity of 5-FU on normal cells compared with 25 μM of 5-FU alone.
Figure 1.
The effects of Quercetin and 5-fluorouracil on growth inhibition of MDA-MB-231and MRC5 cell line
(A) MDA-MB-231 cells were treated with different concentrations of Quercetin for 24 and 48 hr. cell viability was assessed using MTT assay. (B) MDA-MB-231 cells were treated with different concentrations of 5-fluorouracil for 24 and 48 hr. cell viability was assessed using MTT assay. (C) MRC5 human lung fibroblasts as normal cells were treated with different concentrations of Quercetin for 24 and 48 hr. cell viability was assessed using MTT assay. (D) MRC5 cells were treated with different concentrations of 5-fluorouracil for 24 and 48 hr. cell viability was assessed using MTT assay. Results are presented as mean± SEM of at least three independent experiments *P<0.05; **P<0.01; ***P<0.001 significant from control untreated cells
Table 1.
Selectivity index (SI) of Quercetin and 5-fluorouracil for MDA-MB-231 and MRC5 Cell lines
| Cell line | IC50 (µM) | |
|---|---|---|
| 5-FU | Que | |
| MDA-MB-231 MRC5 |
525 348 |
295 >1000 |
| SI | 0.65 | >3.39 |
a Selectivity index
Figure 2.
The effect of combinations of Quercetin and 5-fluorouracil on growth inhibition of MDA-MB-231
(A) Viability of MDA-MB-231 cells after treatment with Quercetin (50 and 200μM) combined with 5-fluorouracil (1.5, 6.25, and 25μM) for 24 hr was measured using MTT assay. (B) Viability of MDA-MB-231 cells after treatment with Quercetin (50 or 200μM) combined with 5-fluorouracil (1.5, 6.25, or 25μM) for 48 hr was measured using MTT assay. Results are presented as mean± SEM of at least three independent experiments *P<0.05; **P<0.01; ***P<0.001 significant from control untreated cells, and ##P<0.01 significant from 5-FU-alone treated cells
Table 2.
CI b and DRI c values determined for various combinations of Quercetin and 5-fluorouracil. CI >1 Antagonism; CI=1 Additive; CI <1 Synergistic effect between drugs
| 5-FU (µM) | Quer (µM) | CI |
|---|---|---|
| 1.5 | 50 | 0.45 |
| 6.25 | 50 | 0.53 |
| 25 | 50 | 0.33 |
| 1.5 | 200 | 0.74 |
| 6.25 | 200 | 0.7 |
| 25 | 200 | 0.47 |
b Combination index; c Dose-reduction index
Figure 3.
Effects of Quercetin and 5-fluorouracil combination on the proliferation of MRC5 cell line
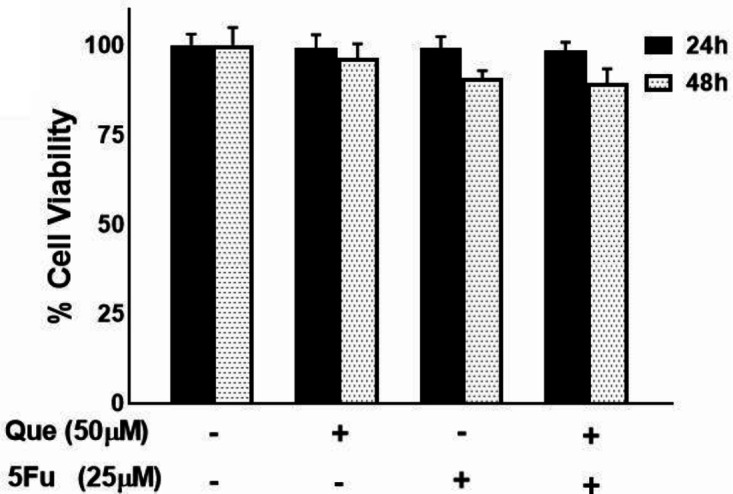
MRC5 cells were treated with the combination of selected concentrations of Quercetin (50μM) and 5-fluorouracil (25 μM). Viability was determined by MTT assay. Results are presented as mean±SEM of at least three independent experiments
Effects of Que and 5-FU on the migration of breast cancer cells
To evaluate the migration rate of tumor cells treated with Que and 5-FU, a wound-healing assay was performed. MDA-MB-231 cells were treated with Que and 5-FU alone and combined for 24 and 48 hr. As shown in Figure 4, Que inhibited cancer cells’ migration, leaving the wound closure of 71.9% and 92.5% for 24 and 48 hr, respectively. So did 5-FU with the wound closure of 57.6% and 71.2% for 24 and 48 hr, respectively. Also, the combination of Que and 5-FU resulted in further inhibition of tumor cell migration, reducing the wound closure to 29.3% and 36.1% for 24 and 48 hr, respectively. These results show that Que co-delivered with 5-FU could enhance the chemotherapeutic agent’s effects on the inhibition of MDA-MB-231 cell migration.
Figure 4.
wound healing assay of MDA-MB-231 breast cancer cell line treated with 5-fluorouracil and Quercetin
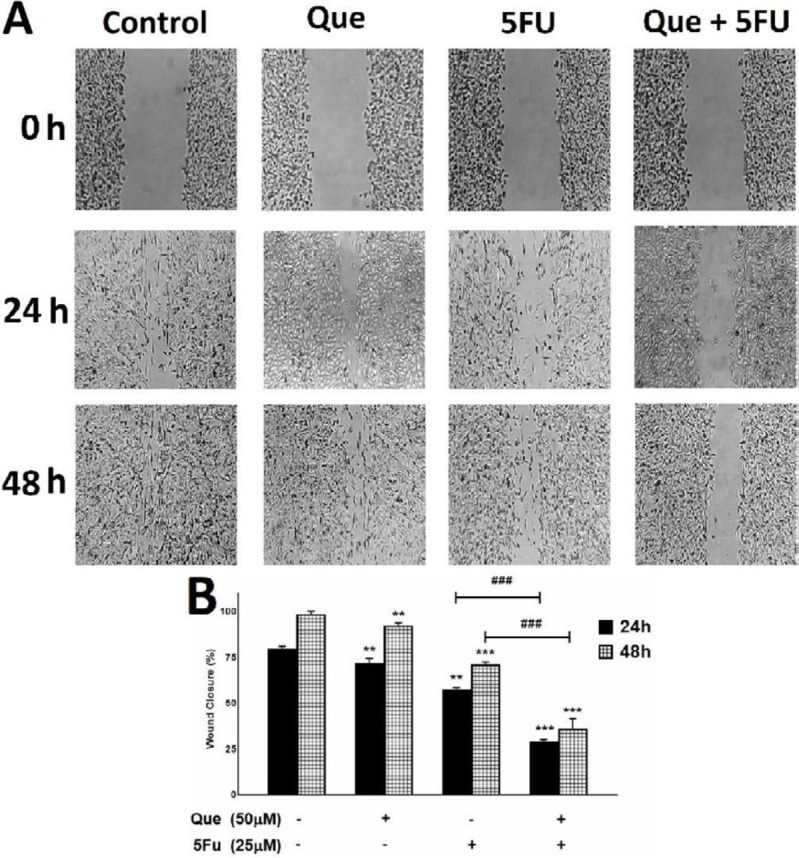
Quercetin and 5-fluorouracil inhibited migration of tumor cells alone and in combination. (A) image of MDA-MB-231 cells migration following treatment with Quercetin (50 μM), 5-fluorouracil (25 μM), and their combination for 48h. (B) quantitative analysis of the anti-migrative effect of Quercetin (50 μM), 5-fluorouracil (25 μM), and their combination for 48 hr. Results are presented as mean± SEM of at least three independent experiments **P<0.01; *** P<0.001 significant from control untreated cells ; and ### P<0.001 significant from 5-FU-alone treated cells
Effects of Que and 5-FU on the expression of MMP-2 and MMP-9 genes
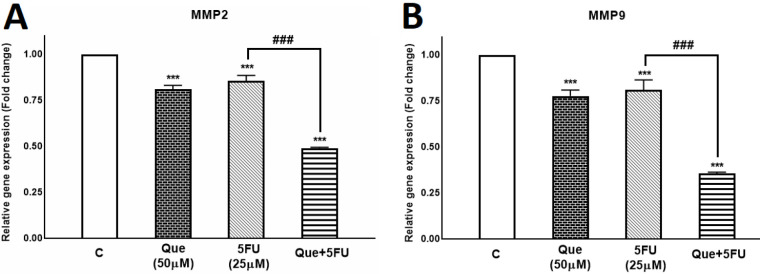
MMP-2 and MMP-9 have key roles in breast cancer cells’ metastasis; therefore, the effects of Que and 5-FU on MMP-2/-9 gene expression were assessed alone and in combination. As shown in Figure 5, 5-FU reduced the expression of MMP-2/-9 genes to 0.85 and 0.8 fold, respectively. Also, Que decreased expression of MMP-2 and MMP-9 genes to 0.8 and 0.77 fold, respectively. However, the combination of Que and 5-FU reduced the expression of the MMP-2 gene to 0.48 fold and the expression of the MMP-9 gene to 0.35 fold. According to these results, Que and 5-FU alone reduced the expression of MMP-2 /-9 genes significantly, and the combination of Que and 5-FU reduced the expression of these genes more significantly compared with each drug alone.
Figure 5.
Quercetin enhanced the effect of 5-fluorouracil on the Matrix metalloproteinase-2 and Matrix metalloproteinase-9 gene expression in the MDA-MB-231 breast cancer cell line
Quercetin and 5-fluorouracil decreased gene expression of Matrix metalloproteinase-2 and Matrix metalloproteinase-9 alone and in combination. (A) Expression of Matrix metalloproteinase-2 gene was evaluated in MDA-MB-231 untreated control, treated with Quercetin(50 μM), 5-fluorouracil (25 μM), and the combination of Quercetin plus 5-fluorouracil using quantitative Real-time PCR. (B) Expression of Matrix metalloproteinase-9 gene was evaluated in MDA-MB-231 untreated control, treated with Quercetin(50 μM), 5-fluorouracil (25 μM), and the combination of Quercetin plus 5-fluorouracil using quantitative Real-time PCR. . Results are presented as mean± SEM of at least three independent experiments ***P<0.001 significant from control untreated cells and ### P<0.001 significant from 5-FU-alone treated cells
Discussion
BC is one of the deadliest cancers worldwide (41). Cancer cell metastasis is a major factor in increasing cancer mortality (5). Hence, one of the major challenges is finding ways to suppress this capacity in cancer cells. MMP-2 and MMP-9 enzymes are two major factors in the occurrence of cell migration by facilitating their exit from the tissue through ECM degradation. Increased expression of these enzymes in various cancer cells has been frequently shown and resulted in increased invasion properties (42). 5-FU is a frequently used chemotherapeutic agent. In its initial use, 5-FU effectively inhibits the proliferation of cancer cells. However, long-term application of 5-FU causes resistance in cancer cells and reduces the inhibitory effect of this drug along with destructive effects on normal tissues (15, 43). Therefore, finding a way to decrease the dose of chemotherapeutic agents while conserving their therapeutic effects has become a hot topic of research. Que has been shown to inhibit viability and reduce migration in different cancer cell lines, such as colorectal and HeLa cells. Studies have also shown that Que can increase the sensitivity of various cancer cell lines such as colorectal, esophageal, and Hela cells to 5-FU (44-47). In this research, we studied the effects of a combination of 5-FU and Que on the inhibition of growth and migration of the MDA-MB-231 breast cancer cell line. Our results indicate a synergistic effect between Que and 5-FU in inhibiting breast cancer cells’ proliferation and migration.
To investigate Que and 5-FU’s synergy in inhibiting cancer cell growth, we performed a cell proliferation assay. Our results showed that both Que and 5-FU could individually reduce the proliferation of tumor cells. However, the cytotoxic effect of the combination of Que and 5-FU on BC cells is significantly more than that of individual drugs. In other words, Que enhances the inhibitory effect of 5-FU on the proliferation of BC cells. CI and DRI values were calculated to further examine the synergy between Que and 5-FU. According to our results, CI values for all combinations between Que and 5-FU were <1, which further confirms Que and 5-FU’s synergistic effect. The lowest CI value (0.33) was related to 50 μM of Que and 25 μM of 5-FU, indicating the highest synergy level at this combination between Que and 5-FU. To confirm the non-toxic effect of this combination, we examined the effects of the mentioned combination on the growth of MRC5 human normal lung fibroblast cells, and results showed that this combination did not significantly reduce normal cell viability compared with each drug alone. The DRI value indicates the reduction in drug dose in the combined state compared with individual drugs’ implication while maintaining the inhibitory effect. Therefore, DRI values >1 reflect the synergy between drugs that results in a reduced dose of the chemotherapeutic agent. According to our results, the DRI values for 5-FU in combination with Que were >1. Therefore, the dose of 5-FU in combination with Que is reduced while maintaining its inhibitory effect on the proliferation of cancer cells. These results are in line with previous works reporting the synergistic effects between 5-FU and other polyphenolic compounds such as resveratrol, EGCG, and Que in colorectal, OSCC, and esophageal cancers (30, 44, 48).
We performed a wound healing assay to evaluate the effects of Que, 5-FU, and their combination on BC cells’ migration. Our results indicated that the rate of BC cell migration is significantly reduced after treatment with Que and 5-FU by 6% and 27%, respectively. However, migration of tumor cells treated with the combination of Que and 5-FU showed a significant reduction (62%) compared with Que and 5-FU alone, which is consistent with the results of our proliferation assay. Decreased tumor cell migration by 5-FU may be due to its effect on the structure and gene expression of cellular RNAs involved in tumor cell migration. Also, a significant reduction in tumor cell migration after treatment with the combination of Que and 5-FU can be due to Que’s ability to increase the sensitivity of tumor cells to 5-FU, which leads to a more significant inhibitory effect of 5-FU on their migration. These results are in line with the previous works on the inhibitory effects of 5-FU in combination with other polyphenol compounds such as resveratrol, kaempferol, and EGCG on colorectal and OSCC cancer cells migration (30, 48, 49).
Increased expression of gelatinase enzymes in metastatic tumor cells has been shown in various studies (50-53). Therefore, we assessed the effects of Que, 5-FU, and their combination on the expression of MMP-2 and MMP-9 genes. Our results showed that expression of MMP-9 and MMP-2 genes treated with Que and 5-FU decreased significantly. However, these genes’ expression showed a significant decrease after treatment with Que and 5-FU’s combination compared with the treatment with Que and 5-FU individually. These results are in agreement with the results of the migration assay. The results obtained in this experiment are consistent with the results of previous studies regarding the effect of the combination of 5-FU and natural compounds on the expression of mmp2 and mmp9 genes in tumor cells (54-58). Our results indicate that 5-FU, Que, and their combination can affect tumor cells’ migration at the gene expression level. However, Que and 5-FU’s combination has a more significant effect on the gene expression of gelatinase enzymes than Que and 5-FU alone, which suggests the synergistic effect between them.
Conclusion
Our results showed that not only Que can reduce the growth and migration of BC cells, it can also synergize with 5-FU as a chemosensitizing agent that results in the reduction of 5-FU dose and also further reduces the proliferation and migration capacity of BC cells. These findings introduce Que as a new therapeutic agent in the treatment of breast cancer. However, further in vitro and in vivo studies are needed to clarify the continuation of the route.
Acknowledgment
The research was financially supported by the Cellular and Molecular Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The results described in this paper were part of Mohammadreza Roshanazadeh thesis (grant no. CMRC - 9811).
Statement of Ethics
No human or animal experiments were performed in this research.
Authors’ Contributions
Title of manuscript: Quercetin synergistically potentiates the anti-metastatic effect of 5- Fluorouracil on MDA-MB-231 breast cancer cell line
Author list: Mojtaba Rashidi, Hossein Babaahmadi Rezaei, Mohammadreza Roshanazadeh
Study conception or design: M R and MR R; Data analyzing and draft manuscript preparation: H BR and MR R; Critical revision of the paper: H BR; Supervision of the research:M R; Final approval of the version to be published: M R, H BR, and MR R.
Conflicts of Interest
We have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control. 2010;17:173–176. doi: 10.1177/107327481001700305. [DOI] [PubMed] [Google Scholar]
- 3.Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8:957. doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Lord SJ, Kiely BE, Pearson S-A, Daniels B, O’Connell DL, Beith J, et al. Metastatic breast cancer incidence, site and survival in Australia, 2001–2016: a population-based health record linkage study protocol. BMJ Open. 2019;9:e026414. doi: 10.1136/bmjopen-2018-026414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad A, Hart I. Mechanisms of metastasis. Crit Rev Oncol Hematol. 1997;26:163–173. doi: 10.1016/s1040-8428(97)10002-6. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly SK, Cabrera R, Mao SP, Christin JR, Wu B, Guo W, et al. Rac3 regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J Cell Biol. 2017;216:4331–4349. doi: 10.1083/jcb.201704048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 12.Köhrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer. 2009;9:1–20. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eiseler T, Döppler H, Yan IK, Goodison S, Storz P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009;11:1–12. doi: 10.1186/bcr2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shewach DS, Kuchta RD. Introduction to cancer chemotherapeutics. Chem Rev. 2009;109:2859–2861. doi: 10.1021/cr900208x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altun I, Sonkaya A. The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran J Public Health. 2018;47:1218–1219. [PMC free article] [PubMed] [Google Scholar]
- 16.Love RR, Leventhal H, Easterling DV, Nerenz DR. Side effects and emotional distress during cancer chemotherapy. Cancer. 1989;63:604–612. doi: 10.1002/1097-0142(19890201)63:3<604::aid-cncr2820630334>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Monsuez J-J, Charniot J-C, Vignat N, Artigou J-Y. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010;144:3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Munro AJ, Guanjian L, Liu GJ. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst Rev . 2005:1–24. doi: 10.1002/14651858.CD004540.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boik J. Natural Compounds in Cancer Therapy: Oregon Medical Press Princeton, MN ; 2001. [Google Scholar]
- 20.Lin S-R, Fu Y-S, Tsai M-J, Cheng H, Weng C-F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int J Mol Sci. 2017;18:1412. doi: 10.3390/ijms18071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin SR, Chang CH, Hsu CF, Tsai MJ, Cheng H, Leong MK, et al. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br J Pharmacol. 2020;177:1409–1423. doi: 10.1111/bph.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 23.von Schwarzenberg K, Vollmar AM. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013;332:295–303. doi: 10.1016/j.canlet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Amin AR, Wang D, Zhang H, Peng S, Shin HJC, Brandes JC, et al. Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: potential role of p53. J Biol Chem. 2010;285:34557–34565. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rejhová A, Opattová A, Čumová A, Slíva D, Vodička P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem. 2018;144:582–594. doi: 10.1016/j.ejmech.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 28.Latif YA, El-Bana M, Hussein J, El-Khayat Z, Farrag AR. Effects of resveratrol in combination with 5-fluorouracil on N-methylnitrosourea-induced colon cancer in rats. Comp Clin Path. 2019;28:1351–1362. [Google Scholar]
- 29.Lee SH, Koo BS, Park SY, Kim YM. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol Med Rep. 2015;12:2777–2783. doi: 10.3892/mmr.2015.3675. [DOI] [PubMed] [Google Scholar]
- 30.López EP-F, García FG, Jornet PL. Combination of 5-Florouracil and polyphenol EGCG exerts suppressive effects on oral cancer cells exposed to radiation. Arch Oral Biol. 2019;101:8–12. doi: 10.1016/j.archoralbio.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:1–14. doi: 10.1155/2014/424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang H, Zeng L, Wang J, Zhang X, Ruan Q, Wang J, et al. Reversal of 5-fluorouracil resistance by EGCG is mediate by inactivation of TFAP2A/VEGF signaling pathway and down-regulation of MDR-1 and P-gp expression in gastric cancer. Oncotarget. 2017;8:82842. doi: 10.18632/oncotarget.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ (1-42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson AJ, Symons JD, Jalili T. Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv Nutr. 2012;3:39–46. doi: 10.3945/an.111.001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angst E, Park JL, Moro A, Lu Q-Y, Lu X, Li G, et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas. 2013;42:223. doi: 10.1097/MPA.0b013e318264ccae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dihal AA, de Boer VC, van der Woude H, Tilburgs C, Bruijntjes JP, Alink GM, et al. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J Nutr. 2006;136:2862–2867. doi: 10.1093/jn/136.11.2862. [DOI] [PubMed] [Google Scholar]
- 38.Maciejczyk A, Surowiak P. Quercetin inhibits proliferation and increases sensitivity of ovarian cancer cells to cisplatin and paclitaxel. Ginekol Pol. 2013;84:590–595. doi: 10.17772/gp/1609. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta A, Ghosh S, Das S. Modulation of DMBA induced genotoxicity in bone marrow by quercetin during skin carcinogenesis. J Exp Clin Cancer Res: CR. 2001;20:131–134. [PubMed] [Google Scholar]
- 40.Senthilkumar K, Arunkumar R, Elumalai P, Sharmila G, Gunadharini DN, Banudevi S, et al. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3) Cell Biochem Funct. 2011;29:87–95. doi: 10.1002/cbf.1725. [DOI] [PubMed] [Google Scholar]
- 41.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 42.Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68:3853–3868. doi: 10.1007/s00018-011-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuczynski EA, Sargent DJ, Grothey A, Kerbel RS. Drug rechallenge and treatment beyond progression-implications for drug resistance. Nat Rev Clin Oncol. 2013;10:571. doi: 10.1038/nrclinonc.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuang-Xin L, Wen-Yu W, Yao C, Xiao-Yan L, Yun Z. Quercetin enhances the effects of 5-fluorouracil-mediated growth inhibition and apoptosis of esophageal cancer cells by inhibiting NF-κB. Oncol Lett. 2012;4:775–778. doi: 10.3892/ol.2012.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.M Elsadek BE, Abdel Aziz MA, M El-Deek SE, M Mahdy MM, Hussein MR. Combination therapy with quercetin and 5-Fluorouracil ameliorates 1, 2-Dimethylhydrazine induced carcinogenesis in the colon of wistar rats. Bulletin of Egyptian Soc Physiol Sci. 2017;37:227–244. [Google Scholar]
- 46.Sundaram MK, Raina R, Afroze N, Dhupkar A, Kaur NP, Arte A, et al. Combinational use of phytochemicals and chemotherapeutic drugs enhance their therapeutic potential on human cervical cancer cells. Int J Cancer Manag. 2019;12:e91783. [Google Scholar]
- 47.Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. 2011;68:1449–1457. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 48.Chung SS, Dutta P, Austin D, Wang P, Awad A, Vadgama JV. Combination of resveratrol and 5-flurouracil enhanced anti-telomerase activity and apoptosis by inhibiting STAT3 and Akt signaling pathways in human colorectal cancer cells. Oncotarget. 2018;9:32943. doi: 10.18632/oncotarget.25993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riahi-Chebbi I, Souid S, Othman H, Haoues M, Karoui H, Morel A, et al. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci Rep. 2019;9:1–20. doi: 10.1038/s41598-018-36808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta (BBA)-Reviews on Cancer. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Merdad A, Karim S, Schulten H-J, Dallol A, Buhmeida A, Al-Thubaity F, et al. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014;34:1355–1366. [PubMed] [Google Scholar]
- 52.El-Badrawy MK, Yousef AM, Shaalan D, Elsamanoudy AZ. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J Bronchology Interv Pulmonol. 2014;21:327–334. doi: 10.1097/LBR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 53.Hung W-C, Tseng W-L, Shiea J, Chang H-C. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer lett. 2010;288:156–161. doi: 10.1016/j.canlet.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 54.Huang L, Cai YJ, Lung I, Leung BC, Burd A. A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. J Plast Reconstr Aesthet Surg. 2013;66:e251–e259. doi: 10.1016/j.bjps.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Ma L, Xu Y, Liu Y, Li W, Cai J, et al. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020;11:1–13. doi: 10.1038/s41419-020-2675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu Z, Ma K, Dong B, Zhao C, Che C, Dong C, et al. The synergistic antitumor effect of Huaier combined with 5-Florouracil in human cholangiocarcinoma cells. BMC Complement Altern Med. 2019;19:1–12. doi: 10.1186/s12906-019-2614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Chen H, Wang M, Song X, Ding F, Zhu J, et al. Effects of glabridin combined with 5-fluorouracil on the proliferation and apoptosis of gastric cancer cells. Oncol Lett. 2018;15:7037–7045. doi: 10.3892/ol.2018.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afrin S, Giampieri F, Forbes-Hernández TY, Gasparrini M, Amici A, Cianciosi D, et al. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic Biol Med. 2018;126:41–54. doi: 10.1016/j.freeradbiomed.2018.07.014. [DOI] [PubMed] [Google Scholar]