Abstract
Group B Streptococcus (GBS) is one of the most common organisms causing neonatal sepsis as well as serious infections in adults. Serotyping the organism is important in studying the epidemiology of the disease as well as deciding a course of treatment. There are several methods available for serotyping. Most of them need high-titered sera and are not quantitative. We are reporting a new inhibition enzyme-linked immunosorbent assay (ELISA) for serotyping which is sensitive and specific compared to the conventional methods but does not need high-titered serotype-specific antisera, as the specificity is controlled by the polysaccharide coating on the ELISA plates. The method can also be quantitative, and we have measured polysaccharide elaborated by different serotype V strains. Thus, the inhibition ELISA method will be useful in serotyping for epidemiological studies, assessing virulence, and performing strain selection for vaccine production.
Group B streptococcus (GBS) causes both early- and late-onset infections in neonates with serious sequelae with a case fatality rate of 1.5 per thousand (1a). Epidemiological studies indicate that 4 to 25% of pregnant females are colonized with GBS at delivery and that neonatal sepsis occurs in as many as 2 per 1,000 live births, although the number varies with different populations. GBS can also cause invasive disease in nonpregnant adults as well as an asymptomatic colonization. Projections from surveillance of a multistate population of 10 million during 1990 were used to estimate an incidence of invasive GBS in the United States as 4.0 per 100,000 adults >15 years old (15).
GBS are differentiated from other beta-hemolytic streptococci by Lancefield serological typing. GBS have been classified into nine serotypes on the basis of immunological specificity of cell wall capsular polysaccharides present. Major serotypes include Ia, Ib, II, III, and V. More recently GBS isolates of types IV, VI, VII, and VIII have been identified. These serotypes are further classified based upon the presence of surface proteins (6).
Serotyping is important for our understanding of the epidemiology of GBS disease and in determining the prognosis of the disease in infants. In addition, in order to effectively formulate a multivalent vaccine, we need to understand the serotype distributions prevalent in different parts of the world. Serotyping has been performed by various methods, including immunodiffusion, capillary precipitation (tube and ring), countercurrent immunoelectrophoresis (CIE), slide agglutination, and fluorescence microscopy, which employs GBS type-specific antibodies conjugated to fluorescein isothiocyanate (4, 10). The sensitivities of these methods vary, with CIE being the most sensitive (5, 16). All of these methods require high-titered type-specific antisera, and the typing antigen needs to be prepared from the bacterial cultures by either acid extraction or trypsin treatment. The results are not quantitative. More recently, strain differentiation of isolates of streptococci from bovine mastitis by pulsed-field gel electrophoresis has been published (2). We report here a new inhibition enzyme-linked immunosorbent assay (ELISA) for serotyping the most prevalent GBS serotypes by using a hyperimmune intravenous immune globulin solution (IGIV) containing antibodies to GBS serotypes Ia, Ib, II, and III (7). We have used the same method to serotype type V strains by using an unadsorbed rabbit antiserum to GBS serotype V. This method is simple, sensitive, and quantitative and does not require high-titered antisera.
(This research was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [1].)
MATERIALS AND METHODS
Strains and typing sera.
The GBS strains used in our studies were provided by one of the authors (P.E.), Vincent Fischetti (Rockefeller University, New York, N.Y.), and Rick Schumann (Virion Corporation, Rockville, Md.). The IGIV was obtained by immunizing volunteers with a GBS vaccine containing purified polysaccharides from types Ia, Ib, II, and III and then obtaining plasma during a 2- to 3-month period after immunization. The plasma was pooled into two lots and processed by the Swiss Red Cross into a preparation suitable for intravenous administration (7). This preparation in a lyophilized form was a gift from Gerald Fisher (Uniformed University for Health Sciences, Bethesda, Md.). As the IGIV did not contain antibodies to type V GBS polysaccharide, the inhibition ELISA for serotyping GBS type V strains was performed with rabbit anti-type V serum, which was a gift from Rick Schumann. GBS strains were grown on sheep blood agar plates (Remel, Columbia, Md.) at 37°C overnight, and colonies were transferred to 5 ml of Todd-Hewitt liquid medium (Difco Laboratories, Detroit, Mich.). Frozen cultures were also directly transferred to Todd-Hewitt liquid medium and grown for 20 to 24 h at 37°C. The liquid cultures were then heat killed for 45 min in a 56°C water bath. The cell suspensions were then neutralized by adding 1 M sodium hydroxide, with 10 μl of phenol red used as an indicator. These culture suspensions were used in the inhibition ELISA for serotyping. Immunlon 4 plates (VWR Scientific) were coated with purified GBS polysaccharides of types Ia, Ib, II, III, and V (North AmericanVaccine Inc., Beltsville, Md.). The coating solution consisted of 50 μl of polysaccharide solution (1 mg/ml) and 5 μl of methylated human serum albumin (1 mg/ml) in 10 ml of 1× phosphate-buffered saline made in tissue culture-grade, pyrogen-free sterile water (Bio fluids Inc., Rockville, Md.) containing 0.02% sodium azide (3). A 100-μl aliquot of this solution was added to coat each well of the microtiter plate. Plates were sealed and left overnight at room temperature.
Inhibition ELISA.
The steps in the inhibition ELISA procedure are depicted in Fig. 1. Briefly, 160 μl of a broth culture from each isolate (heat killed and neutralized) was dispensed in duplicate into a 96-well Nunc microtiter plate. A 160-μl aliquot of a 1/1,000 dilution of IGIV (lot 006) in serum conjugate buffer (1× phosphate-buffered saline containing 0.5% newborn calf serum, 0.1% Brij 35, 0.05% sodium azide) was added to each sample well and incubated for 40 min at room temperature (3). Immulon 4 plates, previously coated with GBS polysaccharides Ia, Ib, and III, were washed; 100 μl of serum conjugate buffer was dispensed into the first row of each plate (wells A to H) to serve as blanks, and 100 μl of IGIV (1/2,000 dilution) was dispensed into the second row of wells to determine the optical density without inhibitor. One hundred microliters of GBS isolate preincubated with IGIV was added to the polysaccharide-coated plates and incubated for an hour at room temperature. The plates were washed, anti-human immunoglobulin G conjugated to alkaline phosphatase (1/2,000 dilution in the serum conjugate buffer; Sigma) was added, and the plates were incubated for an hour at 37°C. Color was developed with p-nitrophenyl phosphate (1 mg/ml; Sigma) in 1 M Tris, pH 9.8, containing 1 mM MgCl2, and the optical density at 405 nm was measured. An identical procedure was used for GBS type II and type V, except a 1:500 dilution of IGIV was used for serotyping type II strains and a 1/500 dilution of rabbit anti-type V serum was used for serotyping type V strains. The homologous GBS polysaccharide inhibitions with types Ia, Ib, II, and V were 80 to 100%, and the corresponding heterologous inhibitions were 0 to 20%. In the case of type III GBS strains, the homologous inhibition was 60 to 80%. Based on this data, a strain was considered to belong to a serotype when the binding of antibody to the plate coated with the homologous polysaccharide was inhibited by >50%. Strains that did not inhibit antibody binding to GBS polysaccharide types Ia, Ib, II, III, and V were grown on sheep blood agar plates to verify their morphology and were inoculated into liquid Todd-Hewitt medium and grown for 24 h. The inhibition ELISA was repeated with type Ia, Ib, II, III, and V GBS polysaccharides as described above. If there was no inhibition of binding of IGIV with these strains, then they were classified as nontypeable (NT). When cultures showed inhibition of binding to two polysaccharides, the strains were grown on sheep blood agar plates and single colonies were isolated. Five single colonies from each such strain were grown in liquid Todd-Hewitt medium, and the inhibition ELISA was repeated.
FIG. 1.
Serotyping by inhibition ELISA.
RESULTS
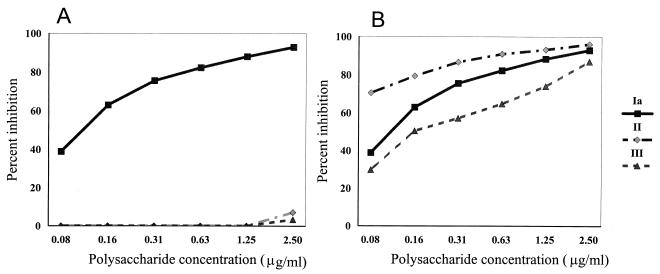
To determine the sensitivity of the inhibition ELISA, serial dilutions of purified polysaccharides of GBS serotypes Ia, Ib, II, and III from 0.08 to 2.50 μg/ml were incubated with IGIV (1/1,000 dilution) for 40 min and then transferred to Immulon 4 plates coated with type Ia, Ib, II, or III GBS polysaccharide. Percent homologous inhibitions with type Ia GBS polysaccharides and heterologous inhibitions with type II and III GBS polysaccharides are shown in Fig. 2A, and percent homologous inhibitions with type Ia, II, and III GBS polysaccharides are shown in Fig. 2B. As can be seen in Fig. 2A, incubation of IGIV with GBS polysaccharide Ia inhibits the binding of IGIV to GBS type Ia polysaccharide-coated plates by >90%, while the heterologous polysaccharide types II and III do not inhibit the binding of IGIV to GBS type Ia polysaccharide-coated plates. Figure 2 depicts the concentration of GBS polysaccharides and percent inhibition of IGIV binding as measured by the inhibition ELISA. Types Ib and V gave results comparable to those shown in Fig. 2B. Taking 50% inhibition as the minimum essential for determining a serotype, the amount of homologous polysaccharide that can be detected at 50% inhibition is about 0.1 μg/ml.
FIG. 2.
(A) Inhibition of binding of IGIV to GBS polysaccharide type Ia by homologous and heterologous GBS polysaccharides. IGIV (1/1,000 dilution) was incubated with GBS polysaccharide types Ia, II, and III at concentrations of 2.50 to 0.08 μg/ml. For homologous inhibition, 100 μl from the preincubated mixture of type Ia GBS and IGIV was transferred to GBS type Ia polysaccharide-coated Immulon 4 plates. For heterologous inhibition, 100 μl from the preincubated mixture of IGIV with GBS polysaccharide type II or III was transferred to GBS type Ia-coated Immulon 4 plates. (B) Homologous inhibition of binding of IGIV to GBS polysaccharide Ia, II, and III coated plates. IGIV (1/1,000 dilution) was incubated with GBS polysaccharides as described above and then transferred to GBS polysaccharide Ia-, II-, and III-coated plates, respectively. Inhibition ELISA was performed as described in the text.
Validation of the results obtained by the inhibition ELISA method was done by testing 98 isolates (from the collection of P.F.) under code. Eighty-six were invasive isolates, and of these 75 were from a prospective study of early-onset disease (12, 13). Four additional GBS isolates from the Rockefeller University collection were also tested blindly. The isolates were first typed at the University of Minnesota (UM) reference laboratory by immunodiffusion in agarose using Lancefield hot-HCl cell extracts and reference antisera to GBS polysaccharides Ia to VIII (12, 13). These strains were then typed by inhibition ELISA. Table 1 shows the results of typing by the two methods. There was complete agreement between the two methods for the results obtained for serotypes Ia, Ib, II, and III. With regard to type V, there was a discrepancy with two strains. One strain, AP101692, was typed as type V by inhibition ELISA and as type IV with a weak cross-reaction with type V by immunodiffusion. Another strain, MM97002132, was typed as type V by ELISA and as NT by immunodiffusion. Eight other strains were not typeable by ELISA into any of the five types examined. These NT strains were typed as types IV, VI, VII, and VIII by immunodiffusion using sera specific for types not examined by ELISA.
TABLE 1.
Inhibition ELISA and immunodiffusion serotype results
| Typing method | No. of strains determined to belong to GBS serotype:
|
|||||
|---|---|---|---|---|---|---|
| Ia | Ib | II | III | V | NTa | |
| Inhibition ELISA | 32 | 12 | 6 | 19 | 18 | 11 |
| Immunodiffusion | 32 | 12 | 6 | 19 | 16 | 4 |
Nine strains NT by inhibition ELISA were serotyped as IV (n = 5), VI (n = 2), VII (n = 1), and VIII (n = 1) in the UM reference laboratory.
We also received 12 strains with assigned serotypes by capillary precipitation from the Rockefeller University. The results for 10 strains were concordant with those from the Rockefeller Laboratories. The two discordant strains as well as two other concordant strains were retyped blindly at the UM. The results matched those of the inhibition ELISA for all four of the strains. Two strains of GBS type V were provided by Rick Schumann and were typed by the inhibition ELISA. Both strains were correctly identified by inhibition ELISA as type V.
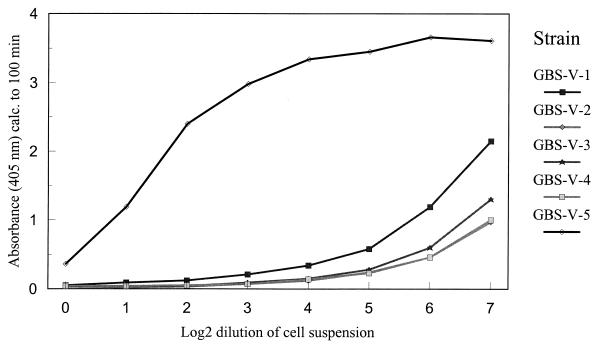
The inhibition ELISA method can also be used to quantitate the amount of polysaccharide present in a culture. This can be very useful in comparing strains from patients and carriers or in selection of strains for vaccine production. Five strains of type V exhibiting different degrees of inhibition by inhibition ELISA serotyping were selected, and 24-h broth cultures were adjusted to the same initial cell density. Inhibition ELISA was performed with serial dilutions of the cultures, and the results are shown in Fig. 3. Three strains have similar inhibition curves, whereas two others showed varying degrees of inhibition, suggesting the production of different amounts of polysaccharide.
FIG. 3.
Relative quantitation of polysaccharides elaborated by five different strains of type V GBS. The strains were grown in 5 ml of Todd-Hewitt liquid medium for 24 h, heat inactivated, and neutralized as described in Materials and Methods. One hundred microliters of IGIV (1:1,000 dilution) was incubated with serial dilutions of suspensions from different strains and 100 μl was transferred to GBS type V polysaccharide-coated plates for ELISA.
DISCUSSION
Serotyping methods that have been used in the last 30 years for typing GBS include immunodiffusion, capillary tube precipitation, and CIE (4, 10, 14). Pulsed-field gel electrophoresis is a new addition and was found to be a reliable method for typing the most common streptococci which cause bovine mastitis, but it requires specialized equipment (2). The relative sensitivities of capillary precipitation (tube and ring), slide agglutination, immunodiffusion, and fluorescent antibody techniques are similar, as are the specificities of all these methods. They all require high-titered type-specific antisera which are not commercially available. CIE is more sensitive and less time-consuming than these other serotyping methods. CIE has an additional advantage of using the patient specimen directly, without requiring overnight growth of cultures or an extraction procedure (10, 14). CIE detected 14 μg of antigen per ml when a commercial antiserum from Difco was used and 0.7 μg of GBS type III polysaccharide per ml when a high-titered burro antiserum to type III GBS polysaccharide (serum not commercially available) was used (14). None of these methods are more than semiquantitative.
ELISA methods have been used for the measurement of innumerable antigens and antibodies (11). We report here an inhibition ELISA for serotyping GBS strains. Initially, high-titered type-specific antibodies were not available in the laboratory, but a GBS hyperimmune IGIV was available. However, it is important to note that the inhibition ELISA can also be performed with rabbit antisera raised against individual serotypes, as we have shown with rabbit antisera to type V.
Purified type-specific GBS polysaccharides and not whole bacteria are used for coating the plates which determine the specificity of the assay and not the type of antisera that is used. In the inhibition ELISA using purified GBS polysaccharides from different serotypes, the amount of homologous polysaccharide detected at 50% inhibition was 0.1 μg/ml, which could be quantitated with inhibition curves. With regards to specificity, the inhibition ELISA is as specific as the conventional methods. Comparison of serotyping of samples that were earlier analyzed by immunodiffusion and tube precipitation methods revealed that the results obtained by the inhibition ELISA agreed with the analysis of the five serotypes. In the case of two strains whose inhibition ELISA results were type V and whose immunodiffusion results showed one strain to be nontypeable and the other to be type IV with a slight cross-reaction with type V, it was possibly an issue of sensitivity of the methods, as inhibition ELISA may be more sensitive. For the other strain, one cannot exclude the possibility that it is truly type IV with a cross-reaction to type V antiserum.
Forty samples in duplicate can be assayed at one time in a microtiter plate. The process can also be automated for washing plates when a large number of plates are being handled at one time. We have serotyped over 1,600 strains of GBS by this method. This procedure is suitable for large-scale serotyping either with IGIV or with antiserum that has been raised to different serotypes of GBS.
An interesting outcome of this procedure is that the amount of polysaccharide made by a given strain can be quantitated by the inhibition ELISA by using dilution curves with pure polysaccharides as references, similar to the method reported by Holm and Hakansson (11). As shown in Fig. 2, different type V strains cause inhibition to different extents, depending on the amount of capsular polysaccharide present. This can be useful for studying strains isolated from patients with invasive disease and healthy carriers to see if there are inherent differences among them. It is well known that capsular polysaccharide plays a role in virulence although it is not the only factor. Hakansson et al. (9) isolated high- and low-density subpopulations of GBS and observed that the low-density variants had enhanced virulence. Among the strains that have been serotyped by inhibition ELISA, there are strains with varying inhibitions among all the serotypes that have been tested, and further studies are planned to pursue these differences.
ACKNOWLEDGMENTS
We thank Gerald Fisher for hyperimmune globulin, Vincent Fischetti for GBS strains, and Richard Schumann for type V GBS strains and antisera. We also thank Michael Schmitt and Bruce Mead from the Center for Biologics Evaluation and Research (CBER) for critical review of the manuscript.
This work was supported by the Division of Epidemiology, Statistics, and Preventive Research at the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md., and by the Postgraduate Research Participation Program at CBER administered by the Oak Ridge Institute for Science and Education, Oak Ridge, Tenn.
REFERENCES
- 1.Arakere G, Frasch C E. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. A new method for serotyping of group B Streptococcus, abstr. D-88; p. 154. [Google Scholar]
- 1a.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 3rd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 742–811. [Google Scholar]
- 2.Baseggio N, Mansell P D, Browning J W, Browning G F. Strain differentiation of isolates of streptococci from bovine mastitis by pulsed-field gel electrophoresis. Mol Cell Probes. 1997;11:349–354. doi: 10.1006/mcpr.1997.0126. [DOI] [PubMed] [Google Scholar]
- 3.Bhushan R, Anthony B F, Frasch C E. Estimation of group B streptococcus type III polysaccharide-specific antibody concentrations in human sera is antigen dependent. Infect Immun. 1998;66:5848–5853. doi: 10.1128/iai.66.12.5848-5853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cropp C B, Zimmerman R A, Jelinkova J, Auernheimer A H, Bolin R A, Wyrick B C. Serotyping of group B streptococci by slide agglutination, fluorescence microscopy, and microimmunodiffusion. J Lab Clin Med. 1974;84:594–603. [PubMed] [Google Scholar]
- 5.Dajani A S. Rapid identification of beta hemolytic streptococci by counterimmuno-electrophoresis. J Immunol. 1973;110:1702–1705. [PubMed] [Google Scholar]
- 6.Ferrieri P, Flores A E. Surface protein expression in group B streptococcal invasive isolates. In: Horaud T, Bouvet A, Leclercq R, de Montclos H, Sicard M, editors. Streptococci and the host, proceedings of the XIIIth Lancefield International Symposium on Streptococci and Streptococcal Diseases. New York, N.Y: Plenum Press; 1997. pp. 635–637. [Google Scholar]
- 7.Fisher G W, Hemming V G, Gloser H P, Bachmayer H, Von Pilar C E, Wilson S R, Baron P A. Polyvalent group B streptococcal immune globulin for intravenous administration: overview. Rev Infect Dis. 1990;12(Suppl. 4):S483–S491. doi: 10.1093/clinids/12.supplement_4.s483. [DOI] [PubMed] [Google Scholar]
- 8.Flores A E, Ferrieri P. Molecular diversity among the trypsin resistant surface proteins of group B streptococci. Zbl Bakteriol. 1996;285:44–51. doi: 10.1016/s0934-8840(96)80021-1. [DOI] [PubMed] [Google Scholar]
- 9.Hakansson S, Bergholm A, Holm S E, Wagner B, Wagner M. Properties of high and low density subpopulations of group B streptococci: enhanced virulence of the low density variant. Microb Pathog. 1988;5:345–355. doi: 10.1016/0882-4010(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 10.Hill H R, Riter M E, Menge S K, Johnson D R, Matsen J M. Rapid identification of group B streptococci by counterimmunoelectrophoresis. J Clin Microbiol. 1975;1:188–191. doi: 10.1128/jcm.1.2.188-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm S E, Hakansson S. A simple and sensitive enzyme immunoassay for determination of soluble type-specific polysaccharide from group B streptococci. J Immunol Methods. 1988;106:89–94. doi: 10.1016/0022-1759(88)90275-x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson D R, Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984;19:506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin F-Y C, Clemens J D, Azimi P H, Regan J A, Weisman L E, Philips III J B, Rhoads G G, Clark P, Brenner R A, Ferrieri P. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J Infect Dis. 1998;177:790–792. doi: 10.1086/517810. [DOI] [PubMed] [Google Scholar]
- 14.Quentin R, Huet H, Wang F-S, Geslin P, Goudeau A, Selander R K. Characterization of Streptococcus agalactiae strains by multilocus enzyme genotype and serotype: identification of multiple virulent clone families that cause invasive neonatal disease. J Clin Microbiol. 1995;33:2576–2581. doi: 10.1128/jcm.33.10.2576-2581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuchat A, Wenger J D. Epidemiology of group B streptococcal disease. Epidemiol Rev. 1994;16:374–402. doi: 10.1093/oxfordjournals.epirev.a036159. [DOI] [PubMed] [Google Scholar]
- 16.Siegel J D, McCracken G H., Jr Detection of group B streptococcal antigens in body fluids of neonates. J Pediatr. 1978;93:491–492. doi: 10.1016/s0022-3476(78)81174-3. [DOI] [PubMed] [Google Scholar]