Abstract
The General Organization of the Veterinary Services in Egypt has adopted a sheeppox vaccination policy to control lumpy skin disease (LSD) in cattle. Over the course of the last two years, recurrent outbreaks were reported, with animals showing severe clinical signs and consequentially higher fatalities than that of cases reported in previous LSD outbreaks. A total of 1050 cattle showing typical clinical signs suggestive of LSD were clinically and pathologically investigated during 2017–2018. Skin nodules were collected and lumpy skin disease virus (LSDV) was screened in collected skin samples using PCR for the RPO-30 gene. Furthermore, the entire P32 protein coding gene was sequenced. Histopathology and immunohistochemistry of the skin nodules were also conducted. The obtained results showed an overall mortality rate of 6.86%. LSDV was confirmed in all the examined nodules as evidenced by immunohistochemistry and positive PCR amplification of the RPO30 gene. Sequencing analysis of the P32 gene revealed a highly conserved nature and genetic stability of the LSDV. The results of the present study show that the current vaccination protocol was not effective for a multitude of reasons. These results also serve as evidence for a strong recommendation of an amendment of homologous vaccine use aside from a complete coverage of cattle populations in order to reduce the incidence of LSD among cattle population in Egypt.
Introduction
LSD is a devastating, high-impact cattle disease. It is characterized by fever and nodules covering all parts of the body, including the mucosal membranes and internal organs, along with generalized lymphadenopathy. It also induces, abortion, damage to hides and a sharp decline in milk production; it also causes sterility in bulls, subsequently leading to drastic economic losses [1].
LSD is caused by a lumpy skin disease virus (LSDV) that is related to the genus Capripoxvirus [2]. Blood-sucking arthropods, mosquitoes and ticks transmit the virus from one animal to another [3].
With its emergence in Egypt in 1988, LSD was seen merely as a typical example of an exotic disease that usually entered the country through importation of live animals. Within a few short years, it transformed into a national crisis with a subsequent establishment of the disease’s enzootic status. The disease then re-emerged in the summer of 1989 [4]. In 2006, LSDV struck most of the governorates in Egypt [5] and it re-appeared again in 2011 and 2014 [6]. According to this study, and in addition to evidence from the above-mentioned reports, it can be seen that the disease continues to spread across the country.
Originally, the disease affected cattle within the continent of Africa. However, since 2012, the disease has spread from these African countries to the Middle East and even some European countries [7]. Outbreaks of LSDV typically occur as epidemics [4]. There are often unexplained gaps of several years between different outbreaks, regardless of humidity, season or abundance of the vectors [8].
LSDV, sheeppox virus (SPPV), and goatpox virus (GTPV) showed genome similarities of at least 96% [9, 10]. For this reason, conventional laboratory tests could not differentiate different capripoxviruses from each other [11]. However, various PCR assays have been recently validated for differentiation between LSDV and SPPV. PRO-30 based PCR provides a simple and quick differentiation between LSDV and SPPV without the requisite of DNA sequencing [12]. The Capripoxvirus P32 gene is a major immunodominant antigen located at the surface of the mature virion [13, 14]. It was identified as the homolog of the vaccinia virus H3L gene. On the other hand, the RPO30 gene is a homologue of the vaccinia virus E4L gene, which encodes DNA-dependent RNA polymerase enzyme [12]. The main objective of this study was to highlight the vaccination failure associated with the spread of the disease in Egypt. Among the objectives of the current study was the nucleotide sequence relatedness screening between the published reference strains and SPPV vaccines and the virus strains detected in this study.
Material and methods
Ethical approval
The animal ethical committee of the Faculty of Veterinary Medicine, Beni-Suef University, Egypt, approved the present study. Clinical samples used in this study were collected after approval of all the owners’ of study animals.
Animals
A total of 1050 of cattle clinically affected with LSD were screened during 2017–2018 from the Beni Suef, Sohag and Aswan Governorates. Diseased animals ranged from the age of 3 months to 5 years and included individually reared cases and cases from dairy farms (Table 1). All affected animals were vaccinated within the national annual vaccination program with the Romanian SPPV vaccine (103 TCID50, Veterinary Serum and Vaccine Research Institute [VSVRI], Egypt). The development of the disease was reported three months after vaccination. Most of the examined animals were reared near different watercourses, ditches and gullies, many of which were found to harbour hard ticks.
Table 1. Number of clinically diseased animals included in the study.
| Animal | Number | Average age |
|---|---|---|
| Pregnant cows | 320 | 3–5 years |
| Lactating non pregnant cows | 570 | 3–5 years |
| Heifers | 82 | 8–13 months |
| Calves | 78 | 3–12 months |
| Total | 1050 |
Samples
Ten skin nodules were aseptically collected from LSDV-infected cattle showing a severe form of the disease. A local subcutaneous infiltration of lidocaine HCl 2% (1ml / 1cm2 of skin) was applied, followed by a small surgical incision of about 0.5 cm x 0.5 cm in diameter which was made to obtain skin samples. Skin nodules were bisected into two equal halves. The first one was suspended in a sterile phosphate buffer saline (pH 7.2) containing gentamicin (50 μg/ml) in the ice box for PCR assays. The other part was placed in a neutral buffered formalin solution of 10% for further histopathological procedures.
Virus detection
PCR
Extraction of viral DNA was performed on the nodule homogenate using the gSync™ DNA extraction kit, Geneaid (Taiwan) according to the manufacturer’s instructions. PCR runs were performed using PCR master mix (Thermo, Germany) in a total volume of 25μl/reaction. A primer set of the RPO-30 gene that flanks 172bp and 151bp for LSDV and SPPV was used according to Lamien et al. (2011; Table 2). The reaction parameters were denaturation at 94°C for 5 minutes, 35 cycles at 94°C for 45 seconds, 55° C for one minute and 72° C for one minute, with a final extension of 7 minutes. PCR products were visualized with a transilluminator after being electrophoresed in 1.5% agarose gel. Lyophilized sheeppox vaccine virus (Romanian strain) and nuclease-free water were used as positive and negative controls, respectively.
Table 2. Sequence of LSD oligonucleotide primers.
| Primer | Tm | Reference | |
|---|---|---|---|
| RPO30 | F 5’-TCTATGTCTTGATATGTGGTGGTAG-3’ | 55°C | (Lamien et al., 2011) |
| R 5’-AGTGATTAGGTGGTGTATTATTTTCC-3’ | |||
| P32 | F 5’- ATG GCAGAT ATC CCA TTA-3’ | 50°C | (Heine et al., 1999) |
| R 5’ ACTCTCATTGGTGTTCGG-3’ |
Immunohistochemistry
LSD suspected skin samples were subjected to immunohistochemistry staining using Dako Cytomation (Denmark A/S (LSAB+ System-AP)—Edition 06/07—Code K0678). Sections were washed with Tris buffer solution, and the endogenous alkaline phosphatase (AP) was blocked by using 1 mM levamisole. Sections were incubated at room temperature for 30 minutes with normal goat serum to block the non-specific binding immunoglobulins. This was followed by incubation with primary rabbit anti-LSDV polyclonal hyperimmune serum, which was locally prepared and used at a dilution of 1:900 for 1 hour at room temperature. Subsequently, samples were further incubated with biotinylated anti-rabbit monoclonal antibodies and streptavidin AP for 30 minutes each at room temperature. AP substrate was then added for three minutes at room temperature.
Full length sequence of the P32 gene
A PCR for a whole P32 protein coding gene was employed using previously designed primers (Table 2) to confirm the presence of LSDV DNA in all skin samples (n:10) as well as in two archival samples from the 2012 Egypt outbreak; this formed a total of 12 samples [14]. An initial denaturation at 94°C for 5 minutes was followed by 35 cycles at 94°C for 45 seconds, 50° C for one minute and 72° C for one minute, with a final extension of 7 minutes. PCR amplicons of the expected size were excised from the gel and extracted using a Geneaid gel extraction kit (New Taipei City, 22180 Taiwan, China) as according to the manufacturer’s instruction. Purified PCR amplicons were sequenced commercially. Raw sequences were processed using MEGA X [15]. BLAST analysis was initially implemented to establish sequence identity to GenBank accessions. Evolutionary analyses and sequence alignments were conducted using MEGA X software [15]. A tree was generated by the maximum likelihood method with 1,000 bootstrapped data sets. The Kimura 2-parameter was used as a model and the tree was obtained initially by Neighbor-Join and BioNJ algorithms [16]. The maximum composite likelihood (MCL) was estimated as a matrix of pairwise distances.
Histopathological examination
Different tissue specimens were paraffin embedded, fixed in neutral buffered formalin solution 10%; and sectioned at 5–7μm; the specimens were then stained with hematoxylin and eosin according to [17].
Results
Clinical examination
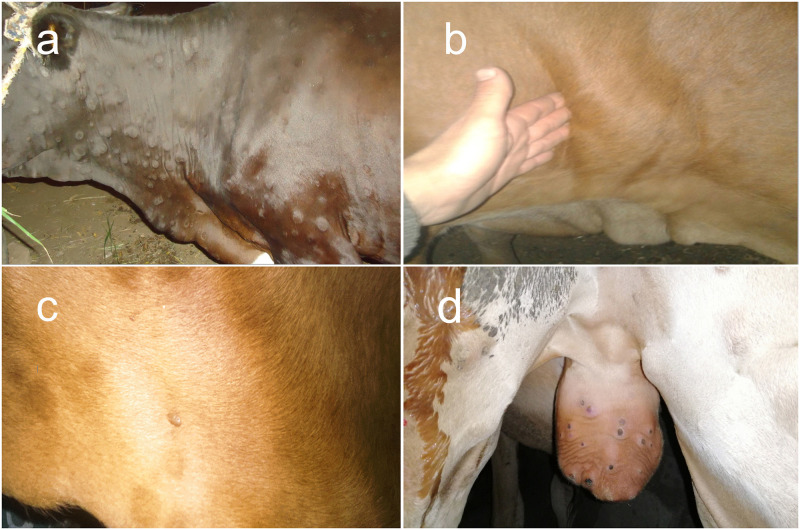
Cattle (n: 1050) from three different governorates (Fig 1) were clinically examined. The animals all showed typical LSD clinical signs, such as nodules on the limbs, head, neck and/or trunk, fever (39.5°C-41.0°C), lymphadenopathy and oedema in the brisket, perineum, genitalia, udder, belly or legs. Typical necrotic lesions with a cone shaped core (sit- fast lesion) were observed (Fig 2). A total of 72 (6.86%) animals succumbed from the acute signs of the disease (Table 3).
Fig 1. Areas of investigation and number of animals.
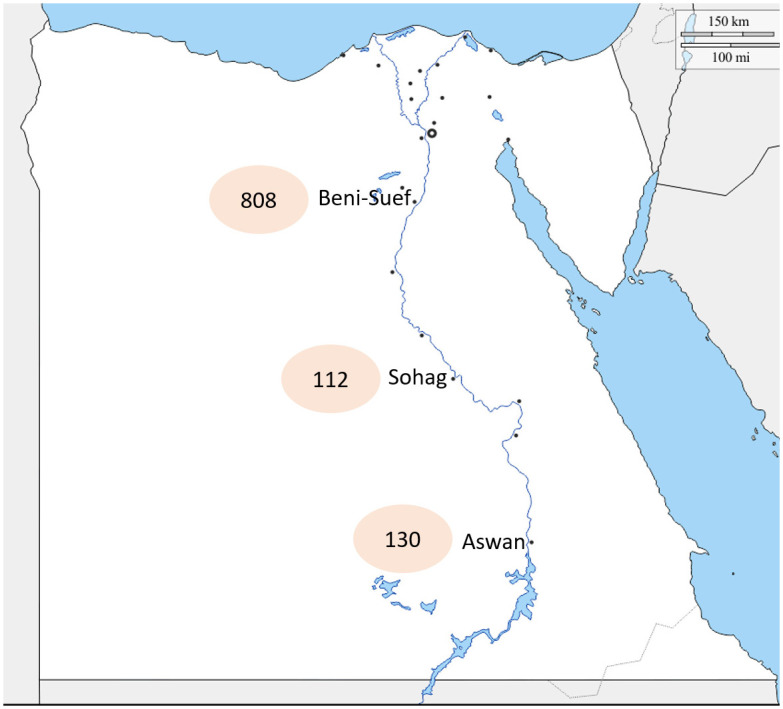
In Beni Suef, 808 animals showed clinical signs of LSD. Twelve calves (3–5 months) and ten pregnant cows were succumbed from infection. In Sohag, 112 animals showed clinical signs of LSD and eighteen cows died. In Aswan, 130 animals showed clinical signs of LSD and thirty-two cows died.
Fig 2. LSDV infected animals.
A) Cutaneous nodules covered the entire body of a LSDV infected cow. B) Enlarged and oedematous pre-scapular lymph node in a LSDV infected cow. C) LSDV infected cow infested with hard ticks. D) A 12-month-old calf with scrotal lesions. Each lesion is surrounded by a zone of necrosis (sitfast).
Table 3. Clinical and epidemiological status of LSDV infected animals.
| Locality | Number of clinical cases | Mortalities (%) | Complications (%) |
|---|---|---|---|
| Beni Suef | 808 | 22 (2.72%) | Pneumonia 44(5.44%) |
| Sohag | 112 | 18 (16.07%) | Enteritis 24(21.43%) |
| Aswan | 130 | 32 (24.62%) | Bloody urine 22(16.92%) |
| Total | 1050 | 72 (6.86%) | Total 90 (8.57%) |
Virus detection: PCR and immunohistochemistry
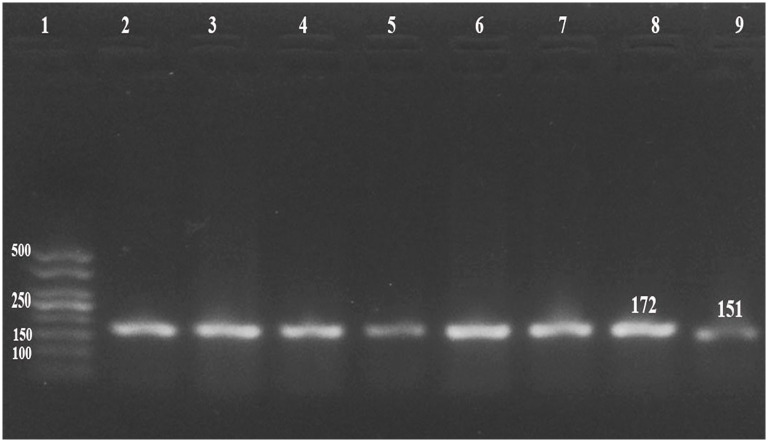
LSDV DNA was successfully amplified by RPO-30 PCR in all of the 10 selected samples as well as for the positive control (Fig 3). Furthermore, the immunohistochemistry confirmed the presence of a viral antigen, as can be seen in the Fig 4g.
Fig 3. Gel electrophoresis of PCR products.
Gel electrophoresis of PCR products using RPO30 specific primer set using 50bp ladder (with 172 bp expected product for LSDV (Lane 2 to lane 8) and 151 for SPPV (Lane: 9).
Fig 4. Histopathology of skin nodules.
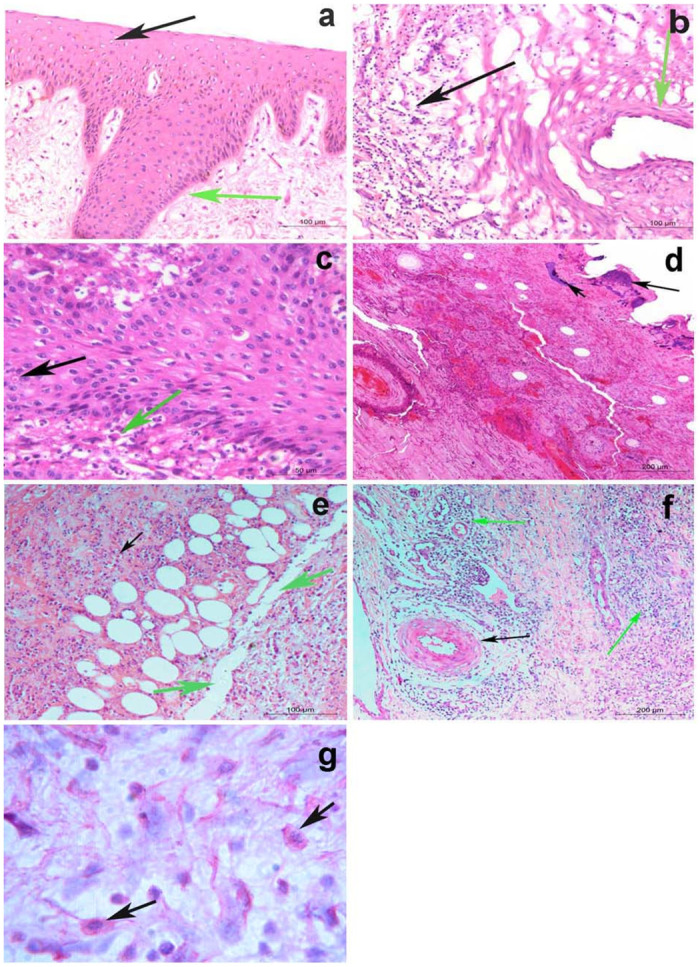
a) Acanthosis (green arrow), associated with hydropic degeneration in keratinocytes of the epidermal layer of the skin (black arrow), while the next dermal layer is normal (H&E; Bar = 100μm). b) Vasculitis (green arrow), associated with massive leucocytic infiltrations in the dermal layer (black arrow) (H&E; Bar = 100μm). c) Intracytoplasmic inclusion body of LSD in the keratinocytes of the epidermal layer (black arrow), while the dermal layer shows massive leucocytic infiltrations mainly by eosinophils (green arrow) (H&E; Bar = 50μm). d) Chromatin condensation and calcium deposits in keratin layer (black arrows), while the next dermal layer shows severe hemorrhages with leucocytic aggregation (H&E; Bar = 200μm). e) Necrosis and dissociation of muscle fibers, fatty infiltrates, and leucocytic infiltration mainly by lymphocytes and few numbers of eosinophils between them (black arrows) (H&E; Bar = 100 μm). f) Dilatation of lymph vessels (LVs.), necrotizing vasculitis (black arrow), and leucocytic infiltration mainly by lymphocytes and few numbers of eosinophils between them (green arrows) (H&E; Bar = 200 μm). g) Red viral particles in macrophage of connective tissue of the dermal layer (black arrows) using alkaline phosphatase immunohistochemistry).
Sequence analysis
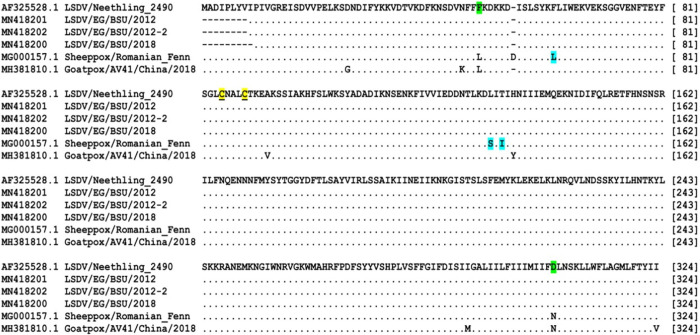
Four out of the ten 2017–2018 samples and two out of the two 2012 samples were positive for P32 gene amplification (1024bp). Out of the twelve samples, only three showed successful readable sequences (one from 2018 and two from 2012). The processed gene sequences were compared with other P32 sequences of capripoxviruses available in the GenBank. Sequence analysis revealed that LSDV obtained in the current study shared 100% identity on both nucleotide and amino acid sequences with LSDV/NI-2490 (AF325528.1) isolated from cattle in 1958. However, nucleotide and amino acid identities were found to be 99% and 98% with the currently used sheeppox vaccinal strain, respectively (Romanian strain) (data not shown). Phylogenetic analysis of capripoxviruses isolated from 1958 to 2018 revealed that members of the genus Capripoxvirus could be classified into three distinct clusters of LSDV, GTPV and SPPV based on their P32 gene nucleotide sequence. LSDV sequences obtained in the present study clustered along with: LSDV/NI-2490/1958 (AF325528.1), LSDV/NW-LW/South Africa/1999 (AF409137.1), LSDV/Kenya/KSGP-0240/1974 (KY702007.1), LSDV/Israel/2012 (KX894508.1), LSDV/Egy/BSU/2012 (MN418201), LSDV/Egy/BSU/2012-2 (MN418202), LSDV/Russia/Dagestan/ 2015 (MH893760.2), LSDV/1015/Egy/2015 (KU298638.1), LSD-Egypt/Ismailia/2016 (KX977487.1) and LSDV/Iran/2016 (KX960780.1) (Fig 5). Deduced amino acid sequences revealed that amino acid residues at position 49(F/L) and 304(D/N) are unique to LSDV while 62(L/F), 132(S/L) and 134(I/T) are unique to SPPV. The most obvious difference between LSDV and SPPV is the presence of an additional aspartic acid at the 55th position of the P32 of the sheeppox virus; the conserved cysteine residues are present at positions 85 and 89 of SPPV protein sequences and at 84 and 88 in LSDV and GTPV protein sequences (Fig 6).
Fig 5. Phylogenetic analysis of P32 gene.
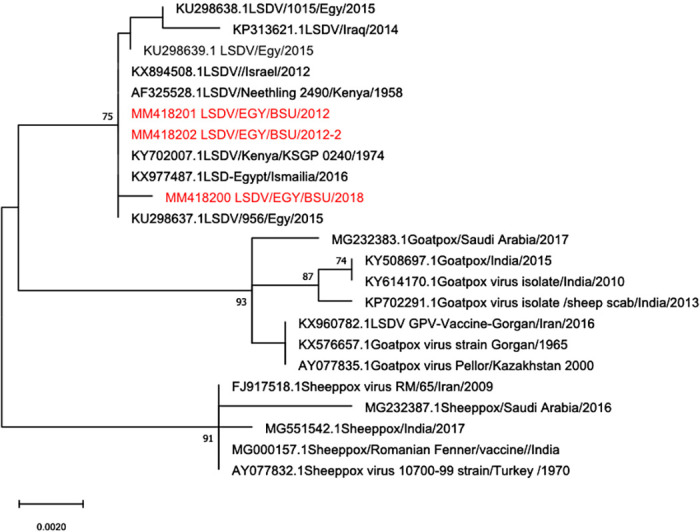
Tree was generated using Mega-X program by the neighbor-joining analysis. Bootstrap confidence values were calculated on 1000 replicates according to the maximum-likelihood approach.
Fig 6. Deduced amino acid sequence of P32 gene.
Dots indicate identical amino acids, green shaded letters denote unique sequences to LSDV, blue shaded letters denote unique sequences to SPPV and yellow underlined letters denote conserved cysteine residues.
Histopathological examination
Biopsy of LSD skin nodules showed acanthosis and vacuolar degeneration in the keratinocytes of the epidermal layer (Fig 4a). Some skin nodules showed dilatation of lymph vessels (LVs), vasculitis and leucocytic infiltration, primarily by lymphocytes (Fig 4b). Intracytoplasmic inclusion bodies were observed in the epidermal layer, while the dermal layer showed massive leucocytic infiltration primarily by eosinophils (Fig 4c). Chromatin condensation and calcium deposits in the keratin layer were observed while the next dermal layer showed severe haemorrhages with leucocytic aggregation (Fig 4d). Necrosis and dissociation of muscle fibers, fatty infiltrates and leucocytic infiltration were observed, primarily by lymphocytes, with few numbers of eosinophils between them. Dilatation of lymph vessels (LVs) and leucocytic infiltration were observed, primarily by lymphocytes, as well as few numbers of eosinophils between them (Fig 4e). Severe necrotizing vasculitis was observed in different areas (Fig 4f). Red viral particles were detected in the macrophage of connective tissue of the dermal layer using immunohistochemistry (Fig 4g).
Discussion
Fever and skin nodules covering the entire skin, lymphadenopathy and oedema were the most significant clinical signs in all suspected cases of LSD during all seasons. This could be explained by the fact that a vector-free season never existed in Egypt, meaning an outbreak could occur all year round and not limited to warm and humid seasons. The existence of different watercourses and water ponds close to cattle populations can also trigger the abundance of the insect vectors, as reported by Tuppurainen (2017). Additionally, the investigated cattle in this study were found to be infested by hard ticks, whose role in LSDV transmission has been previously evaluated [18–21]. A considerable case fatality rate (72/1050, 6.86%) was reported among the examined animals; this rate was higher than that observed in previous Egyptian outbreaks [5, 6, 22]. Epidemiologically, along the two-year (2017–2018) course of this investigation, LSD has been observed only in cattle, which is still considered the main natural host as reported by [23].
Age (less than 5 months), pregnancy and blood parasites (Babesia and Theileria species infections) were considered potential risk factors associated with increased fatality. The latter was mostly observed in the Aswan governorate, as it is the most blood parasite enzootic area in Egypt. In addition, some severely affected animals showed severe pneumonia and enteritis. On the other hand, the drastic effect of LSDV may potentially overwhelm the immunity of diseased animals. This supports the presumed immunosuppressive properties of the LSDV, as previously reported [24, 25].
The histopathological findings also revealed serious changes. Haemorrhages and severe destruction of all skin layers in the current study were suggestive of a presumably higher severity of the present 2017–2018 outbreaks. Necrotizing vasculitis of the dermal blood vessels was found to be a major histopathological feature of LSD, as shown in previous studies [26–29].
In this study, 10 (100%) of tested clinical samples were positive for LSDV using PCR (RPO30) and immunohistochemistry. The P32 gene sequences obtained in this study showed no genetic variation from ancestral 1958 LSDV. This finding elucidates the highly conserved nature and the genetic stability of LSDV. Such homology confirmed the highly conserved nature amongst LSDV, SPPV and GTPV [9, 24, 30].
Since 1988, LSD has been persistently reported in Egypt, with severe outbreaks until 2018 despite vaccination campaigns using heterologous vaccine (Romanian sheeppox vaccine). On the basis of current observations and previously reported evidence [31–33], it cannot be ruled out that insufficient and incomplete protection was achieved after heterologous vaccination. On the contrary, other countries successfully experienced vaccination campaign with a homologous LSD vaccine with no further outbreaks were reported [7, 34].
Incomplete vaccination coverage, improper conditions for vaccine storage and transport and the presence of pre-existing immunosuppressive diseases could also be among possible reasons for LSDV outbreaks [34]. There are conflicting results regarding the development of humoral immune response following SPPV vaccine in cattle. No antibody response was detected in cattle following vaccination with SPPV vaccine [35, 36]. However, a partial protection was recorded following challenge with the field strain [35, 36]. In contrast, some studies reported induction of both humoral cell-mediated responses were reported following vaccination of Romanian strain in cattle [37–39]. Humoral antibody response was detected in 2/3 of the vaccinated animals following vaccination with a trivalent vaccine containing SPPV Romania, GTPV and KSGP 0180 [36]. Humoral response was found to be acceptable after using 104.0 TCID50 of RM65 SPP vaccine [40]. Interestingly, it was found that in spite of vaccination, the local immune evasion strategy adopted by capripoxviruses locally alleviates the host’s immune response to allow the virus to replicate at the site of entry [41].
In conclusion, the mass vaccination campaigns in Egypt that were based on the use of a heterologous vaccine (Romanian sheeppox vaccine) provided insufficient levels of protection. This necessitates the use of an alternative strategy in utilizing the homologous LSDV vaccine. A strategic plan is strongly recommended, particularly one that ensures mass coverage of the vaccination among cattle populations all over Egypt.
Supporting information
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting information files. Accession numbers denote nucleotide sequence of LSD P32 gene and all are available in the NCBI database by using the Accession numbers MN418201 MN418202 MN418200.
Funding Statement
The authors acknowledge the support of Taif University Researchers Supporting Project Number (TURSP-2020/11), Taif University, Taif 21944, Saudi Arabia.
References
- 1.Davies FG. Lumpy skin disease of cattle: a growing problem in Africa and the Near East. World Anim Rev. 1991;68(3):37–42. [Google Scholar]
- 2.Buller RM, Arif BM, Black DN, Dumbell KR, Esposito JJ, Lefkowitz EJ, et al. Family Poxviridae. Virus taxonomy: Classification and nomenclature of viruses 8th report ICTV. 2005:117–33.
- 3.Tuppurainen ESM. Epidemiology of lumpy skin disease empres-animal health 360 NO. 47/2017 FAO: FAO, 2017 ISSN 1564-2615.
- 4.Davies FG. Lumpy skin disease, an African capripox virus disease of cattle. Br Vet J. 1991;147(6):489–503. doi: 10.1016/0007-1935(91)90019-J [DOI] [PubMed] [Google Scholar]
- 5.Awadin W, Hussein H, Elseady Y, Babiuk S, Furuoka H. Detection of lumpy skin disease virus antigen and genomic DNA in formalin-fixed paraffin-embedded tissues from an Egyptian outbreak in 2006. Transboundary and emerging diseases. 2011;58(5):451–7. Epub 2011/06/28. doi: 10.1111/j.1865-1682.2011.01238.x . [DOI] [PubMed] [Google Scholar]
- 6.Amin A, El-Nahas E, El-Mashed A. Pathological and virological studies on an outbreak of lumpy skin disease among cattle in Kalubia Governorate-Egypt. J Adv Vet Res. 2015;5(4):165–75. [Google Scholar]
- 7.European-Food-Safety-Authority, Calistri P, DeClercq K, Gubbins S, Klement E, Stegeman A, et al. Lumpy skin disease: III. Data collection and analysis. EFSA Journal. 2019;17(3):e05638. doi: 10.2903/j.efsa.2019.5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, et al. Capripoxvirus diseases: current status and opportunities for control. Transboundary and emerging diseases. 2017;64(3):729–45. doi: 10.1111/tbed.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tulman ER, Afonso CL, Lu Z, Zsak L, Kutish GF, Rock DL. Genome of lumpy skin disease virus. J Virol. 2001;75(15):7122–30. doi: 10.1128/JVI.75.15.7122-7130.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tulman ER, Afonso CL, Lu Z, Zsak L, Sur JH, Sandybaev NT, et al. The genomes of sheeppox and goatpox viruses. J Virol. 2002;76(12):6054–61. Epub 2002/05/22. doi: 10.1128/jvi.76.12.6054-6061.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitching RP. Passive protection of sheep against capripoxvirus. Res Vet Sci. 1986;41(2):247–50. Epub 1986/09/01. . [PubMed] [Google Scholar]
- 12.Lamien CE, Le Goff C, Silber R, Wallace DB, Gulyaz V, Tuppurainen E, et al. Use of the Capripoxvirus homologue of Vaccinia virus 30 kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate Goat poxvirus from Sheep poxvirus. Vet Microbiol. 2011;149(1–2):30–9. Epub 2010/12/01. doi: 10.1016/j.vetmic.2010.09.038 . [DOI] [PubMed] [Google Scholar]
- 13.Zinoviev VV, Tchikaev NA, Chertov O, Malygin EG. Identification of the gene encoding vaccinia virus immunodominant protein p35. Gene. 1994;147(2):209–14. Epub 1994/09/30. doi: 10.1016/0378-1119(94)90067-1 . [DOI] [PubMed] [Google Scholar]
- 14.Heine HG, Stevens MP, Foord AJ, Boyle DB. A capripoxvirus detection PCR and antibody ELISA based on the major antigen P32, the homolog of the vaccinia virus H3L gene. Journal of immunological methods. 1999;227(1–2):187–96. Epub 1999/09/15. doi: 10.1016/s0022-1759(99)00072-1 . [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular biology and evolution. 2018;35(6):1547–9. Epub 2018/05/04. doi: 10.1093/molbev/msy096 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16(2):111–20. doi: 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- 17.Bancroft JD, Gamble M. Theory and practice of histological techniques: Elsevier Health Sci; 2008. [Google Scholar]
- 18.Lubinga JC, Tuppurainen ESM, Coetzer JAW, Stoltsz WH, Venter EH. Transovarial passage and transmission of LSDV by Amblyomma hebraeum, Rhipicephalus appendiculatus and Rhipicephalus decoloratus. Experimental and applied acarology. 2014;62(1):67–75. doi: 10.1007/s10493-013-9722-6 [DOI] [PubMed] [Google Scholar]
- 19.Lubinga JC, Tuppurainen ESM, Mahlare R, Coetzer JAW, Stoltsz WH, Venter EH. Evidence of Transstadial and Mechanical Transmission of Lumpy Skin Disease Virus by A mblyomma hebraeum Ticks. Transboundary and emerging diseases. 2015;62(2):174–82. doi: 10.1111/tbed.12102 [DOI] [PubMed] [Google Scholar]
- 20.Rouby SR, Hussein KH, Aboelhadid SM, EL-Sherif AM. Role of rhipicephalus annulatus tick in transmission of lumpy skin disease virus in naturally infected cattle in Egypt. Advanced Animal Veterinary Science. 2017;5(4):185–91. [Google Scholar]
- 21.Sprygin A, Pestova Y, Wallace DB, Tuppurainen E, Kononov AV. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019;269:197637. Epub 2019/06/04. doi: 10.1016/j.virusres.2019.05.015 . [DOI] [PubMed] [Google Scholar]
- 22.House JA, Wilson TM, El Nakashly S, Karim IA, Ismail I, El Danaf N, et al. The isolation of lumpy skin disease virus and bovine herpesvirus-4 from cattle in Egypt. J Vet Diagn Invest. 1990;2(2):111–5. Epub 1990/04/01. doi: 10.1177/104063879000200205 . [DOI] [PubMed] [Google Scholar]
- 23.Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, et al. Capripoxvirus diseases: current status and opportunities for control. Transboundary and emerging diseases. 2017;64(3):729–45. doi: 10.1111/tbed.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kara PD, Afonso CL, Wallace DB, Kutish GF, Abolnik C, Lu Z, et al. Comparative sequence analysis of the South African vaccine strain and two virulent field isolates of lumpy skin disease virus. Arch Virol. 2003;148(7):1335–56. doi: 10.1007/s00705-003-0102-0 [DOI] [PubMed] [Google Scholar]
- 25.Hunter P, Wallace D. Lumpy skin disease in southern Africa: a review of the disease and aspects of control. J S Afr Vet Assoc. 2001;72(2):68–71. doi: 10.4102/jsava.v72i2.619 [DOI] [PubMed] [Google Scholar]
- 26.Tuppurainen ESM, Venter EH, Coetzer JAW. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort J Vet Res. 2005;72(2):153–64. doi: 10.4102/ojvr.v72i2.213 [DOI] [PubMed] [Google Scholar]
- 27.Sanz-Bernardo B, Haga IR, Wijesiriwardana N, Hawes PC, Simpson J, Morrison LR, et al. Lumpy skin disease is characterized by severe multifocal dermatitis with necrotizing fibrinoid vasculitis following experimental infection. Vet Pathol. 2020;57(3):388–96. doi: 10.1177/0300985820913268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prozesky L, Barnard BJ. A study of the pathology of lumpy skin disease in cattle. Onderstepoort J Vet Res. 1982;49(3):167–75. Epub 1982/09/01. . [PubMed] [Google Scholar]
- 29.Tageldin MH, Wallace DB, Gerdes GH, Putterill JF, Greyling RR, Phosiwa MN, et al. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Trop Anim Health Prod. 2014;46(1):241–6. doi: 10.1007/s11250-013-0483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mafirakureva P, Saidi B, Mbanga J. Incidence and molecular characterisation of lumpy skin disease virus in Zimbabwe using the P32 gene. Trop Anim Health Prod. 2017;49(1):47–54. Epub 2016/09/28. doi: 10.1007/s11250-016-1156-9 . [DOI] [PubMed] [Google Scholar]
- 31.Stram Y, Kuznetzova L, Friedgut O, Gelman B, Yadin H, Rubinstein-Guini M. The use of lumpy skin disease virus genome termini for detection and phylogenetic analysis. J Virol Methods. 2008;151(2):225–9. doi: 10.1016/j.jviromet.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 32.Tuppurainen ESM, Oura C. Lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transboundary and emerging diseases. 2012;59(1):40–8. doi: 10.1111/j.1865-1682.2011.01242.x [DOI] [PubMed] [Google Scholar]
- 33.Gelaye E, Belay A, Ayelet G, Jenberie S, Yami M, Loitsch A, et al. Capripox disease in Ethiopia: Genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antiviral Res. 2015;119:28–35. Epub 2015/04/25. doi: 10.1016/j.antiviral.2015.04.008 . [DOI] [PubMed] [Google Scholar]
- 34.Molini U, Aikukutu G, Khaiseb S, Haindongo NN, Lilungwe AC, Cattoli G, et al. Molecular characterization of lumpy skin disease virus in Namibia, 2017. Arch Virol. 2018;163(9):2525–9. Epub 2018/06/06. doi: 10.1007/s00705-018-3891-x APPROVAL: This article does not contain animal studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamdi J, Bamouh Z, Jazouli M, Boumart Z, Tadlaoui KO, Fihri OF, et al. Experimental evaluation of the cross-protection between Sheeppox and bovine Lumpy skin vaccines. Scientific reports. 2020;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kafafy M, El Soally S, Aboul-Soud E, Zaghloul M, Mikhael C. Preparation of trivalent vaccine against lumpy skin disease using different capripox viral strain. Veterinary Medical Journal (Giza). 2018;64(1):23–38. [Google Scholar]
- 37.Varshovi HR, Norian R, Azadmehr A, Afzal Ahangaran N. Immune response characteristics of Capri pox virus vaccines following emergency vaccination of cattle against lumpy skin disease virus. Iranian Journal of Veterinary Science and Technology. 2017;9(2):33–40. [Google Scholar]
- 38.Mikhael C, Ibrahim M, Saad M. Efficacy of Alternative Vaccination with Attenuated Sheep Pox and Inactivated Lumpy Skin Disease Vaccines against Lumpy Skin Disease. Suez Canal Veterinary Medical Journal SCVMJ. 2016;21(2):125–42. [Google Scholar]
- 39.Norian R, Afzal Ahangaran N, Azadmehr A. Evaluation of humoral and cell-mediated immunity of two capripoxvirus vaccine strains against lumpy skin disease virus. مجله ویروس شناسی ایران. 2016;10(4):1–11. [Google Scholar]
- 40.Abutarbush SM, Tuppurainen ES. Serological and clinical evaluation of the Yugoslavian RM 65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transboundary and emerging diseases. 2018;65(6):1657–63. doi: 10.1111/tbed.12923 [DOI] [PubMed] [Google Scholar]
- 41.Abu-El-Saad AA, Abdel-Moneim AS. Modulation of macrophage functions by sheeppox virus provides clues to understand interaction of the virus with host immune system. Virology journal. 2005;2:22. Epub 2005/03/24. doi: 10.1186/1743-422X-2-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]