Abstract
We hypothesize that the V̇O2 time constant (τV̇O2) determines exercise tolerance by defining the power output associated with a “critical threshold” of intramuscular metabolite accumulation (e.g. inorganic phosphate), above which muscle fatigue and work inefficiency are apparent. Thereafter, the V̇O2 “slow component”, and its consequences (increased pulmonary, circulatory and neuromuscular demands) determine performance limits.
Keywords: Muscle fatigue, work efficiency, power-duration relationship, mitochondria, inorganic phosphate, oxidative capacity
Graphical Abstract
V̇O2 kinetics determine exercise tolerance by mediating the power output at which muscle metabolite accumulation exceeds a “critical threshold.”
INTRODUCTION
Understanding the physiological determinants of whole-body exercise tolerance remains one of the ultimate targets in exercise physiology. These determinants are particularly pertinent when considered in the context of the continuum of physiological function; underpinning elite sporting performance at one extreme, and predictive of morbidity, mortality and quality of life in chronic disease patients at the other. Identification of parameters of aerobic function, such as the lactate threshold (LT), critical power (CP), and maximum rate of pulmonary oxygen uptake (V̇O2max), have advanced our understanding of the role of O2 transport and utilization in endurance exercise performance (1) and are strong prognostic biomarkers. V̇O2 kinetics, however, have received less attention as an index of exercise tolerance or prognosis. Fast V̇O2 kinetics confers greater ability to meet physical tasks in a physiologic steady-state by reducing the intramuscular metabolism disturbance for any given power output (termed “metabolic stability”; 2, Figure 1), and therefore contributes to fitness, quality of life and survival. V̇O2 kinetics shares mechanisms with LT, CP and V̇O2max, such as mitochondrial volume-density, the capacities for intramuscular oxidative phosphorylation and convective and diffusive O2 delivery (3). However, as V̇O2 kinetics determines the degree of metabolic perturbation (i.e. metabolic stability) during any increase in energy demand, it is more relevant than traditional parameters of aerobic function to the activities of daily living. The purpose of this review is to present contemporary evidence testing the hypothesis that V̇O2 kinetics defines exercise intolerance, by determining the external power available before reaching a “critical threshold” of intramuscular metabolite accumulation, which in turn initiates a “positive feedback loop” of muscle inefficiency and fatigue that presage exercise performance limitations (4–7).
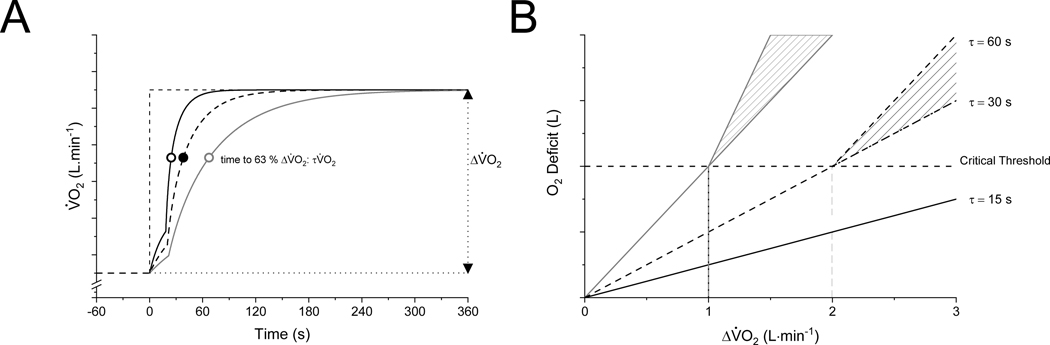
Figure 1.
Schematic representation of: (A) the kinetic V̇O2 response to a moderate-intensity constant-power output test with different values of τV̇O2; and (B) the corresponding interdependent relationship between ΔV̇O2 (AṪP) and τV̇O2 in determining the size of the O2 deficit below (solid line) and above (hatched area) a critical threshold of intramuscular metabolite accumulation. The hatched areas above represent the duration and amplitude dependent changes in ATP demand and V̇O2 for a given constant power exercise. Fast V̇O2 kinetics (τV̇O2 = 15 s) allow high ΔV̇O2 to be achieved in a steady state (below the O2 deficit associated with the critical threshold). Slower V̇O2 kinetics (τV̇O2 = 30 s or 60 s) will result in attaining the O2 deficit associated with the critical threshold at a lower ΔV̇O2 (or power output) (see text for additional details).
V̇O2 KINETICS AND THE O2 DEFICIT
Adenosine triphosphate (ATP) fuels skeletal muscle contraction. However, muscle ATP concentration alone is too low to sustain contractions for more than a few seconds. Therefore, substrate-level and oxidative phosphorylation are recruited at the onset of contractions to supply ATP at rates commensurate with its demand. The relative contribution to the rate of ATP turnover (AṪP) from substrate-level or oxidative phosphorylation is determined by the speed with which V̇O2 responds – the V̇O2 kinetics – which is thus fundamental in determining both the metabolic stress (exercise intensity) and tolerability of any particular power output.
At the onset of constant power exercise, necessitating an instantaneous increase in AṪP, the pulmonary V̇O2 response is finite. Following a short phase I V̇O2 increase, reflecting the limb-lung vascular transit delay, there is a phase II V̇O2 increase that follows an exponential-like profile closely related to the rate of muscle oxygen consumption (8,9), and is typically characterized by a time-constant (τV̇O2; Figure 1A). This finite pulmonary V̇O2 response leaves a substantial deficit of energy provision, which is met by a reduction in oxygen stores (used for oxidative phosphorylation) and substrate-level phosphorylation (phosphocreatine (PCr) breakdown and glycolysis/glycogenolysis forming lactate): a phenomenon termed “the O2 deficit” by Krogh and Lindhard (10).
The magnitude of the O2 deficit at exercise onset is critical since: (i) capacitances of phosphocreatine (PCr), glycolysis/glycogenolysis and O2 stores (dissolved or associated with hemoglobin or myoglobin) are each limited, and small relative to the demands of continuing exercise for more than a few minutes; (ii) it determines the extent of the muscular metabolic perturbation for a given power output e.g. accumulation of hydrogen ions [H+], inorganic phosphate [Pi], adenosine diphosphate [ADP], extracellular [K+], and loss of sarcoplasmic Ca2+ release and sensitivity; (iii) loss of muscle metabolic stability is associated with muscle fatigue and reduced work efficiency. However, it is important to appreciate that the O2 deficit is an important, obligatory component of the normal integrated exercise response, as it prevents rapid, excessive, or even chronic perturbations in multiple systems involved in supporting energy provision. For example, although creatine kinase (CK) inhibition speeds V̇O2 kinetics, ATP homeostasis is compromised and muscle force development is dramatically impaired (11).
At the onset of constant power output exercise, power output determines the AṪP requirement and the steady-state increment in V̇O2 (ΔV̇O2) through ratios mediated by watts/AṪP (W/P) and ATP/molecular oxygen (P/O). For power outputs that reach a steady-state, the O2 deficit can be reasonably estimated by the product of ΔV̇O2 and τV̇O2 (Figure 1, Equation 1), where τV̇O2 is the time constant of the overall V̇O2 response (also termed the mean response time):
| (Equation 1) |
During steady-state cycle ergometry, ΔV̇O2 is related to power by a functional gain of 10 ml·min−1·W−1 (ranging ~9–11 ml·min−1·W−1) (12). This functional gain is similar across sex, age, or state of training. On the other hand, τV̇O2 is affected by state of training, age, or chronic disease, and can vary ~10-fold from the fastest (elite endurance athletes τV̇O2 = 12 s) to the slowest (elderly patients with chronic obstructive pulmonary disease τV̇O2 = 120 s) (3,13). Therefore, the O2 deficit for any given power output is primarily determined by τV̇O2; those with the fastest V̇O2 kinetics will achieve the same power output in a steady-state with a smaller O2 deficit than those with slow V̇O2 kinetics (Figure 1B).
The variables that contribute to setting individual V̇O2 kinetics are the subject of comprehensive review elsewhere (3,13) and are not the intended focus of this review. Broadly, the primary site of flux control for muscle oxygen consumption kinetics resides with the rate of ADP feedback to the mitochondrion, the mitochondrial volume-density, the relative activation state of a wide array of enzymes involved in oxidative phosphorylation, and spatial and temporal buffering by PCr (3,13–15). In canine muscle, acute CK inhibition speeds muscle oxygen consumption kinetics (11), suggesting a central role for CK activity in limiting V̇O2 kinetics. More recent evidence indicates that the parallel activation (also termed each-step activation; ESA) of ATP consumption and processes related to mitochondrial and glycolytic enzyme activity, perhaps via Ca2+ accumulation, is likely to exert a major controlling influence on the rate of oxidative phosphorylation induced by [ADP] at the inner-mitochondrial membrane (14,15). Hence, rapid muscle oxygen consumption kinetics require strong parallel activation of ATP-consuming and producing pathways (14,15), high oxidative enzyme activity and sufficient mitochondrial ADP delivery regulated by the CK kinase reaction (11). Pharmacological activation of pyruvate dehydrogenase did not speed muscle oxygen uptake kinetics in most experimental preparations (for discussion see 16), suggesting that reducing equivalent delivery to the electron transport chain is not a limiting factor. There may, however, be a role for mitochondrial enzyme activity to speed V̇O2 kinetics in aged muscles (17). Naturally, O2 is required for oxidative phosphorylation, and therefore mitochondrial O2 delivery has the potential to limit muscle oxygen consumption kinetics. Most evidence suggests that increasing muscle O2 delivery does not speed muscle oxygen consumption or V̇O2 kinetics in young, healthy individuals (13). However, the potential for O2 delivery limitations to V̇O2 kinetics increases in the elderly and chronic disease (3,13). The primacy of any of the factors reviewed in this section for setting the control of V̇O2 kinetics likely also differs with fiber type (13). As such, a relatively greater proportion of type 1 fibers (which possess greater O2 delivery and oxidative capacity, 18) is associated with a lower τV̇O2 and a greater metabolic stability for a given external work rate (19,20).
THE O2 DEFICIT AND EXERCISE INTENSITY
For over 100 years, exercise physiology pioneers such as August Krogh, Archibald Hill, Rodolfo Margaria, David Dill, Bengt Saltin, Brian Whipp and a great many others, focused research efforts on identifying the relationships among the O2 deficit, lactate production and accumulation, and mechanisms of exercise intolerance. It is intuitive that the bioenergetics comprising the O2 deficit play a role in limiting exercise tolerance, but attempts to establish a quantitative link have proven divisive (21,22). Overall, the O2 deficit is unable to explain exercise performance limitations (23). This is in no small part because the O2 deficit is only reliably calculated for power outputs that attain a steady-state or very rapidly achieve V̇O2max (24). However, exercise limitation mainly occurs in non-steady-state physiology. Therefore, attempts to accurately predict exercise tolerance during non-steady-state tasks using the O2 deficit (and by inference V̇O2 kinetics) were destined for failure.
During constant power outputs that result in a metabolic acidosis, W/P decreases (25). This means that there is no single ΔV̇O2 associated with the exercise task, and therefore the assumptions inherent in equation 1 (that V̇O2 reaches a state-state) are no longer valid. In addition, the absence of a V̇O2 steady state necessitates continued ATP provision from O2 deficit-related mechanisms, further driving metabolite accumulation. The delayed increase in V̇O2 that occurs during prolonged heavy and very-heavy intensity exercise (also termed heavy and severe intensity exercise in alternative schemas, 26) is termed the V̇O2 “slow component” (V̇O2SC). This means that for power outputs that exceed some “critical threshold” of O2 deficit accumulation (see below), the O2 deficit is greater than predicted from moderate-intensity exercise, cannot be reliably calculated, and instead ranges between plausible limits, set largely by the lower (~9 ml·min−1·W−1) and upper observed limits for V̇O2 gain (up to ~15 ml·min−1·W−1 in very-heavy intensity exercise) (Figure 1B). Therefore, without a simple means to measure the O2 deficit, it becomes complex to study its effects on exercise tolerance.
THE ‘CRITICAL THRESHOLD AND POSITIVE FEEDBACK’ MODEL THAT LINKS V̇O2 KINETICS TO EXERCISE LIMITATION
Two consequences of exceeding the aforementioned “critical threshold” are peripheral fatigue (27,28) and an increase in AṪP that reduces exercise efficiency (25). These are reflected in the slow components of intramuscular metabolism (progressive rise in [Pi] and progressive fall in [PCr] and pH) and V̇O2 (V̇O2SC) (29–32). The link with muscle fatigue is an essential clue to the development of the hypothesis outlined below. Classical views of the O2 deficit reflect either an exercise limitation caused by “depletion” of stored energy equivalents during exercise (PCr, glycogen, stored O2) or an “accumulation” of one or more metabolic products (e.g. [ADP], [Pi], [H+]) to some level that limits muscle force development and/or velocity and prevents continued exercise. For example, depleting a muscle of PCr or glycogen would cause intolerance due to lack of substrates to support ATP production, or increasing [ADP] or [Pi] to some high level would inhibit the power stroke and/or slow cross-bridge cycling to a degree that prevented contractions from continuing at the force and/or velocity required.
A vital question in the search to understand whether depletion or accumulation mechanisms are better associated with the characteristics of exercise intolerance during large muscle mass exercise, such as cycling, is, how does the muscle “sense” whether it is above or below the “critical threshold” that signals the loss of metabolic stability and precipitates exercise limitation? There is no PCr or glycogen receptor in muscle that can facilitate such signaling. Equally, no mechano- or metabo-receptor exists to sense the power output, AṪP or metabolic rate (muscle oxygen consumption) that, once exceeded, causes a loss of work efficiency. Muscle fatigue (loss of force and/or velocity), on the other hand, can act in a “sensor-like” role by causing an increase in AṪP for a given power output.
There is an association between muscle fatigue and the loss of work efficiency (i.e., decreased W/P causing the V̇O2SC) (33). For instance, the onset of muscle fatigue precedes the emergence of the V̇O2SC and the magnitude of fatigue is correlated with the V̇O2SC amplitude (25). Findings consistent with this effect are also observed during electrically stimulated (34) or voluntary all-out exercise (35). The shared mechanisms between muscle fatigue and inefficiency are not well understood, but may result from the observation that type 1 muscle fibers reach their peak efficiency during very low force/velocity contractions, and at higher force/velocity contractions the efficiency of type 1 fibers decreases below that of type 2 fibers and eventually reaches zero (36). This is presumably because type 1 fibers must be shortened actively by the contractile activity of type 2 fibers (36), i.e., the muscle begins to “work against itself” during fatigue where shortening velocities are reduced. This mechanism may even operate in single fibers that co-express type 1 and type 2 myosin heavy chains. This notion is supported by the observation that V̇O2 remains elevated in fatigued type 1 fibers, whereas V̇O2 declines in parallel with force in fatigued type 2 fibers (37). While additional recruitment of less efficient, more fatigable type 2 fibers may partly explain the association between muscle fatigue and decreased efficiency (38), the onset of muscle fatigue in already-recruited fibers can also signal the onset of increased AṪP demand i.e., fatigue acts like a “sensor” in a feedback loop, triggering the event of increased AṪP demand based on exceeding a critical threshold of metabolite accumulation.
Of the O2-deficit-related metabolites proposed, [Pi] is a prime candidate due to its central role in muscle fatigue (39). Pi inhibits the power stroke of the cross-bridge cycle, i.e. the transition to high-force cross-bridge states. It also decreases myofibrillar Ca2+ sensitivity and can co-precipitate with Ca2+ ions in the sarcoplasmic reticulum (SR), lowering Ca2+ release following excitation (39). Towards the limit of sustained muscle contractions, only a small increase in Pi may be needed to significantly deplete free Ca2+ which interferes with excitation–contraction coupling and thus contributes to limiting muscular work (39).
The potential role of [Pi] in mediating the “critical threshold” was recently provided by studies in silico using a validated model of myocellular bioenergetics (6,40). By defining a “critical” (i.e. threshold) and a “peak” (i.e. limiting) [Pi], Korzeniewski & Rossiter (6) demonstrated that exceeding a critical [Pi] during an exercise transition can signal an increase in the requirements for AṪP, i.e., reducing W/P. This additional AṪP results in a self-propagating positive feedback loop where additional AṪP turnover results in increased [Pi], which causes fatigue and additional AṪP turnover, and so on. During exercise that causes [Pi] to accumulate to slightly above critical [Pi] this positive feedback loop may stabilize (analogous to the heavy intensity domain), but at higher work rates the feedback loop continues to drive [Pi] to greater values until a peak [Pi] is reached (analogous to the very-heavy intensity domain) and exercise intolerance ensues (40,41). These features are consistent with experimental findings of heavy and very-heavy intensity exercise and are emergent properties of the system driven by a single “critical threshold” of metabolite accumulation. Within the model, heavy-intensity exercise represents the portion of the aerobic range where [Pi] increases above the critical [Pi] value during the rest-to-work transition, generating fatigue and additional AṪP, but the magnitude of the additional AṪP is not sufficient to provide an inexorable increase in [Pi] and AṪP with time, thereby allowing metabolic stability. Whether muscle metabolism can stabilize or not once critical [Pi] has been exceeded is thus dependent on the difference between the instantaneous [Pi] and critical [Pi], as this drives the increase in AṪP with time.
Simulations using a “critical [Pi]” to trigger a reduction in work efficiency revealed an inherent hyperbolic relationship between ATP usage activity (AUT; related to external power output) and the tolerable duration of exercise (Figure 2). This hyperbolic behavior is consistent with experimental data across a wide array of exercise modes or species (reviewed by 42), with an asymptote termed critical power (CP), reflecting the highest power output at which a metabolic stability can be maintained, and a curvature constant (W’), reflecting a finite volume of work that can be performed above CP. Additional simulations to limit or enhance myocellular O2 availability resulted in the changes in V̇O2 kinetics, CP and exercise tolerance that were anticipated based on human experimental data (6). Within the model, critical [Pi] is set at 18 mM and peak [Pi] at 25 mM, as these reflect the experimentally observed values at the onset of the metabolite/V̇O2 slow components and at task failure, respectively (6,40). Although these values are likely to vary between different muscle fiber types, exercise modes, and individuals, it is important to point out that varying the values of critical [Pi] from ~16–20 mM and peak [Pi] from ~22.5–27.5 mM resulted in similarly close conformation to experimentally observed power-duration data (40,41).
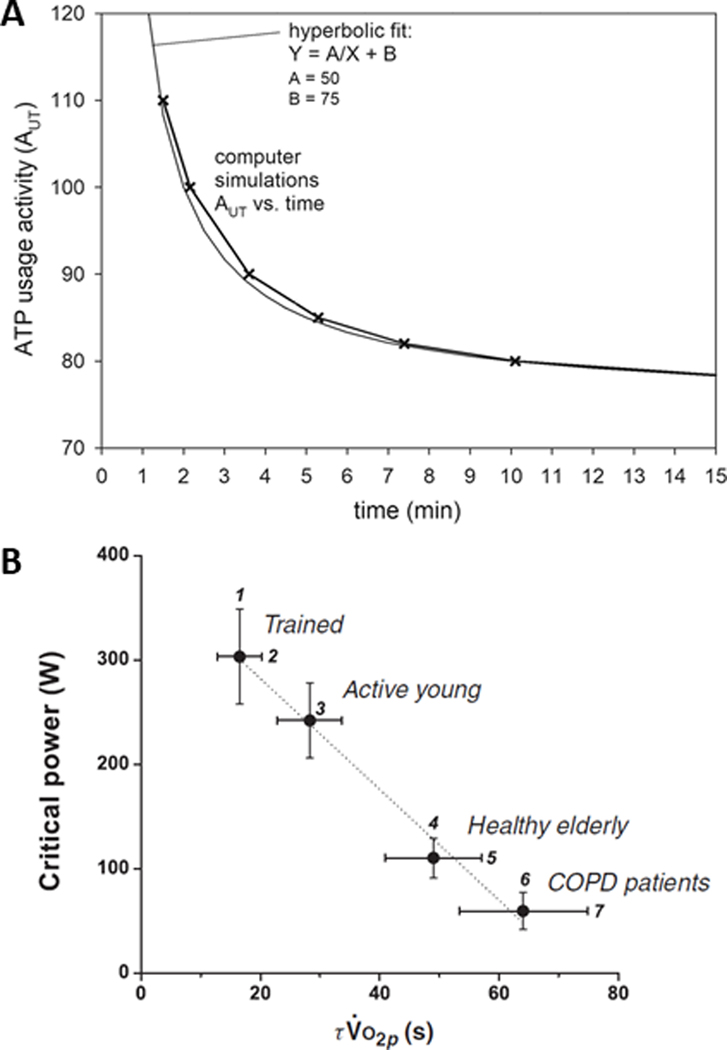
Figure 2.
A: Simulated relationship between ATP usage activity (AUT) (herein termed AṪP) and the tolerable duration of exercise. One AUT unit corresponds roughly to 3 watts of power output. A hyperbolic fit of the simulated AUT–duration relationship is also shown. The asymptote of this hyperbola is (Parameter B = 75; the critical ATP usage activity) corresponds approximately to critical power (CP) of 222 W of total mechanical power or 210 W of external power. The curvature constant (Parameter A = 50) corresponds to the total work available above CP before intolerance, i.e. W’. Reproduced with permission from (6). B: Critical power (CP) as a function of the fundamental phase pulmonary oxygen uptake kinetics time constant (τV̇O2p) during cycle ergometry across populations differing in aerobic function. The figure is derived from 35 reports published between 1982 and 2008. Reproduced with permission from (3).
These data provide theoretical support for the “critical threshold and positive feedback loop” hypothesis, placing [Pi] as the central mediator, responsible for both the initiation of the positive feedback loop and AUT/CP threshold that ultimately results in termination of exercise once [Pi] reaches some predetermined peak [Pi] beyond which the task is limited. The concept of a critical [Pi], proposed by Korzeniewski & Rossiter (6), thus provides a plausible candidate mechanism linking the traditional “accumulation” hypothesis of the O2 deficit to our current understanding of the power-duration relationship and exercise performance.
As the authors point out, the notion that a single variable i.e., [Pi] or other mechanisms based on actions of a single metabolite (43) is responsible for the complex integrative physiology of exercise limitation is clearly an oversimplification. Rather, these simulations demonstrate that for a single metabolite, with known fatigue-inducing ability, exceeding a “critical threshold” can set in motion a wide array of muscle and systemic bioenergetic behaviors that are consistent with directly measured experimental observations; specifically the shape of the power-duration relationship and the observed V̇O2 kinetics (6). Hence, in this context, [Pi] represents a surrogate marker of wide-ranging metabolic processes involved in muscle fatigue, that is likely dependent on more than one variable.
This model of exercise intolerance results in (at least) two inferences. Firstly, work inefficiency is initiated when a degree of muscle metabolic perturbation (i.e. critical [Pi]) is exceeded. Since the V̇O2 kinetics determine the degree of muscle metabolic perturbation incurred during the transition from rest-to-exercise, it then follows that V̇O2 kinetics determine the AUT at which the critical threshold is exceeded, and hence are a central mediator of the properties of the bioenergetic system including the size of the heavy-intensity domain and the characteristics of the power-duration curve. Specifically, faster V̇O2 kinetics at exercise onset (i.e. lower τV̇O2) reduces the O2 deficit, and therefore the rate of metabolite accumulation for a given power output, such that a greater power output would be achieved before the critical threshold is exceeded, compared with when V̇O2 kinetics is slower. The result is that τV̇O2 is a primary mediator of CP through its relationship with the O2 deficit (5), the rate and magnitude of metabolite accumulation, and the power output that causes the attainment of the critical threshold for [Pi] that, in turn, causes fatigue and the onset of inefficiency (6,41).
Support for this notion was recently provided by the theoretical study of Korzeniewski & Rossiter (41), which simulated system metabolic responses in “trained” and “untrained” muscle. Specifically, at an ATP usage activity of 65 (which roughly corresponds to 195 W), in untrained muscle V̇O2 kinetics was slower (τV̇O2 = 29.2 s), leading to a slow component in [Pi] and attainment of peak [Pi] (25 mM) initiating task failure in approximately ~8 minutes. In trained muscle at the same ATP usage activity, V̇O2 kinetics was faster (τV̇O2 = 22.8 s), no [Pi] slow component was observed and Pi stabilized below the critical [Pi] value of 18 mM (end-exercise [Pi] = 16.7 mM), and hence, the system displayed moderate-intensity behavior (36). It is worth noting here that this hypothesis means that CP does not represent a unique power output, nor does it represent a unique metabolic rate or V̇O2 (c.f. 44), but rather a critical level of muscle metabolite accumulation that is associated with fatigue induction and work inefficiency. In turn, this triggers a positive feedback loop that determines exercise tolerance via its subsequent relationships with peak [Pi] and AUT. This is consistent with findings that CP delineates power outputs that result in progressive muscle fatigue from those that do not (27,28).
The second inference from this is hypothesis is that when the power output is high enough for the loss of metabolic stability to be progressive, the tolerable duration of exercise above CP is a function of the rate with which [Pi] attains peak [Pi]. An emergent property of this system, regardless of the rate of [Pi] accumulation, appears to be a fixed volume of work (i.e., W’) underlying the attainment of peak [Pi]. This may in part be a function of the fact that increases in [Pi] have a diminishing return on propagation of inefficiency as exercise progresses, in much the same way that increases in [Pi] in isolated muscle preparations has a diminishing effect on force (45). In other words, W/P decreases more for a given increase in [Pi] just above the critical threshold, compared with when [Pi] approaches peak. It should also be noted that should the proximal cause of exercise limitation be located somewhere other than the active muscle (e.g. limitation due to dyspnea or pain), then the peak [Pi] (or peak combination of accumulating metabolites) will be a consequence rather than a cause of exercise termination.
Murgatroyd et al. (5) characterized relationships between τV̇O2 and CP by normalizing exercise intensity across individuals such that the tolerable duration of exercise was uniform (6 minutes). They demonstrated a strong, inverse correlation between τV̇O2 and CP (R2 = 0.90) (and also a strong, positive association between the V̇O2SC and W’; R2 = 0.76; see below), wholly consistent with the predictions of the critical threshold and positive feedback model of exercise intolerance. However, until recently, a quantitative mechanism to explain this link was lacking.
EVIDENCE FOR THE CRITICAL THRESHOLD: τV̇O2 AND CRITICAL POWER
A wealth of cross-sectional evidence exists to support an association between τV̇O2 and CP. Cross-sectional comparisons in endurance athletes, the elderly, and in patients with chronic diseases that affect the O2 transport and utilization pathways indicate that where τV̇O2 is small (i.e. fast V̇O2 kinetics), CP is correspondingly large, and where τV̇O2 is large (i.e. slow V̇O2 kinetics), CP is correspondingly small (Figure 2B) (3). Indeed, when this analysis is performed across populations, the relationship is strong, inverse, and appears linear (Figure 2B) (3). Furthermore, V̇O2 kinetics is faster in type 1 compared to type 2 fibers (46), consistent with the positive relationship between percentage type 1 fiber composition and CP (32). Moreover, interventional studies also support a causative link: endurance training both reduces τV̇O2 (47) and increases CP (48), whereas in hypoxia τV̇O2 is increased (49) and CP is decreased (50), in agreement with in silico predictions (6). Increasing pedal cadence (thereby increasing type 2 muscle fiber activation) increases τV̇O2 (51) and reduces CP (44).
Thus, there is strong rationale and a large body of cross-sectional evidence in humans consistent with the hypothesis of a mechanistic link between τV̇O2 and CP. This proposal has been further validated by studies with targeted experimental interventions to acutely speed or slow τV̇O2 within an individual and observe the effect on CP. Specifically, when τV̇O2 was increased, CP was concomitantly decreased (43,52), and when τV̇O2 was reduced, CP was concomitantly increased (53–55). The changes observed in CP following each intervention occurred both with (53–55) and without (43,52) concomitant changes in muscle O2 delivery, suggesting that τV̇O2 exerts a determining effect on CP that is independent of the already-established effect of muscle O2 delivery (31). Moreover, a priming-exercise-induced speeding of V̇O2 kinetics also increased CP in a group of patients with type 1 diabetes (55). Hence, there appears to be a consistency in the τV̇O2-CP relationship across both distinct human populations differing in aerobic function (i.e. trained and untrained healthy individuals, healthy elderly, type 1 diabetes and COPD, Figure 2B) (3,55), as well as conditions of altered muscle O2 availability (43,52–54,56). Together these data support that the τV̇O2-CP relationship is ubiquitous across both health and disease, and CP is mediated by τV̇O2 through its relationship with a critical threshold of O2-deficit-related intramuscular metabolite accumulation.
Stronger evidence for a determining effect of τV̇O2 on CP, however, would arguably come from demonstrating a relationship between changes in τV̇O2 (ΔτV̇O2) and CP (ΔCP) following an intervention. Evidence for this was provided by studies that determined τV̇O2 and CP during supine exercise in normoxic and hyperoxic conditions, and also used repeated bouts of moderate intensity exercise in both conditions to allow precise characterization of the relationship between ΔτV̇O2 and ΔCP (53). Consistent with previous data, the lower τV̇O2 in hyperoxia occurred concomitantly with an increase in CP; despite this, ΔCP and ΔτV̇O2 were not linearly related (r = −0.45). However, it was notable that in this (53) and previous studies utilizing the supine position (52,54) the linear relationship between τV̇O2 and CP was absent in normoxic conditions, unlike the linear relationship seen with exercise in the upright position (52–54). Whilst such findings may question the role of τV̇O2 in determining CP, it is likely that supine exercise introduces a kinetic dissociation between muscle oxygen consumption and pulmonary V̇O2 kinetics, due to the lower baseline perfusion and slower O2 delivery kinetics during exercise in this position (3). Such a dissociation would serve to obscure the relationship between ΔCP and ΔτV̇O2. With respect to this latter point, in hyperoxia, the relationship between τV̇O2 and CP in the supine position was restored (r = −0.89) (53).
In a further study, hyperoxia increased indices of muscle O2 availability assessed via NIRS (i.e., [oxyhaemoglobin + oxymyoglobin]) and increased CP during upright cycle exercise, despite τV̇O2 being unchanged between normoxia and hyperoxia (56). This finding suggests that microvascular O2 availability, in addition to τV̇O2, is an independent determinant of CP. This finding is consistent with the predictions of the critical threshold and positive feedback model, when considering that the degradation of [PCr] and accumulation of [ADP] and [Pi] during exercise are inversely related to FiO2 (57). These effects may have been particularly pertinent in type 2 muscle, which operates at lower microvascular (58) and interstitial O2 pressures (18) at rest and during contractions when compared to type 1 muscle. Changes thereof afforded by hyperoxia would act to increase intramyocyte O2 pressures, thereby promoting metabolic stability and reducing Δ[Pi] for a given increment in metabolic rate (57).
The observations highlighted in this section are also consistent with the previously reported positive association between CP and percentage type 1 muscle fiber composition (32): type 1 fibers possess faster V̇O2 kinetics when compared to type 2 fibers (46), which dictates improved muscle metabolic stability during exercise (59). Hence, individuals possessing a relatively greater proportion of type 1 muscle fibers possess faster V̇O2 kinetics (19), and hence would experience a lower metabolic strain on each individual fiber when exercising at a given external power output, thereby explaining the positive association between CP and type 1 muscle fiber composition (32). Taken together, across a series of studies (3,5,43,52–56) there is strong evidence that τV̇O2 is an independent mediator of CP.
EVIDENCE FOR THE POSITIVE FEEDBACK LOOP: V̇O2SC AND W’
The proposed model of exercise intolerance predicts that during work rates where fatigue-related metabolite accumulation cannot be stabilized, the tolerable duration of exercise is a function of W’ ‘utilization’. This acts through the rate of “accumulation” of intramuscular metabolites to achieve peak, or limiting, values. Therefore, the rate at which work efficiency is lost (reflected in the speed/magnitude of the V̇O2SC) and limiting conditions are attained e.g., reflected in V̇O2max, would determine W’. Thus, evidence for a relationship between V̇O2SC and W’, would further support this concept.
A large body of empirical evidence demonstrating a relationship between the amplitude of the V̇O2SC and W’ exists. For example, the positive relationship noted between the amplitude of the V̇O2SC and W’ for constant work rate exercise (5) is also present during all-out exercise (32). Endurance training reduces both the amplitude of the V̇O2SC at a fixed submaximal work rate (47,60), and also decreases W’ (48,61). Glycogen depletion reduces W’ (62) and also reduces the amplitude of the V̇O2SC at a fixed submaximal work rate (63). Also, prior exhaustive severe-intensity exercise reduces both the V̇O2SC and W’ during subsequent exercise following a brief (i.e. 2 min) recovery period, without affecting either τV̇O2 or CP (64). However, when the performance of prior exercise is carefully selected to increase subsequent exercise performance, W’ is increased in concert with a reduced V̇O2SC (65). Rather than argue against a mechanistic relationship between the V̇O2SC and W’, such observations instead highlight that quantification of the amplitude of the V̇O2SC per se, especially when it is incompletely evolved, is a simplistic metric that does not fully capture its role in reflecting the loss of muscle efficiency and thus W’. Interventions that alter the magnitude of W’ therefore also impact the amplitude and/or rate of progression of the V̇O2SC, thereby influencing exercise tolerance.
On the other hand, Goulding et al. showed that initiating exercise from an elevated baseline work rate in the supine position (i.e. work-to-work exercise) reduced the amplitude of the V̇O2SC but increased W’ (52). However, in these conditions, CP was reduced without a concomitant reduction in V̇O2max. Hence, there would have been a greater potential to increase V̇O2 above the V̇O2 associated with CP, thus increasing W’. However, the effects of this intervention include a slower τV̇O2, an increased “gain” of the fundamental phase of V̇O2, and a delayed emergence of the V̇O2SC, which together complicate quantification of the V̇O2SC amplitude. Hyperoxia reduces the rate of development of the V̇O2 (56) and intramuscular (31) slow components, but also decreases W’. In this latter scenario, both CP and the fundamental V̇O2 amplitude are raised in hyperoxia (31,56), which would reduce the potential for the V̇O2SC to develop. The extant literature is therefore largely consistent with the notion that the V̇O2SC and W’ are mechanistically related.
INTEGRATION: DIFFERENT EXERCISE MODALITIES
At the core of the proposed model of exercise tolerance is the notion that CP is an emergent property of a system that occurs once a critical threshold of muscle metabolite accumulation and fatigue induction is exceeded. If so, the critical metabolite accumulation threshold would remain constant even when the relationship between power output and metabolic rate was dissociated. This notion is supported by studies that alter both pedal cadence during cycle exercise and the work:recovery ratios during intermittent exercise. Barker et al. (44) demonstrated that CP was lower at 100 rpm when compared to 60 rpm, however, the V̇O2 measured at each pedal-rate specific CP did not differ between the two pedal rates. Thus, the critical threshold of muscle metabolite accumulation required for sustained propagation of AṪP, within an individual, can be achieved at different external power output and pedal rate combinations.
Intermittent exercise, wherein periods of supra-CP work are interspersed with periods of recovery, dissociates the power output from the systemic (V̇O2, [L-]) and intramuscular ([PCr], [Pi], and pH) (66) responses. Hence, exercise tolerance at a given very-heavy or severe intensity power output is greater using intermittent compared to continuous work and is associated with a reduced intramuscular metabolic strain (66). Specifically, shortening the work:recovery durations at a high power output (corresponding to 110% V̇O2max) increased exercise tolerance from ~4 minutes to being reached in a steady-state (the experiment was stopped after ~30 minutes) (66). Indeed, with very short work:recovery durations, the peak of the V̇O2 fluctuation can be constrained below the LT and as such, the bioenergetic responses reflect those expected of moderate intensity exercise (66) (Figure 4). Thus, it is proposed that the time course of the V̇O2 response at exercise onset and the associated rate of metabolite accumulation determines exercise tolerance, even during intermittent exercise. During intermittent exercise, despite the high AṪP, the V̇O2 kinetics in combination with a limited work bout duration can constrain the associated metabolite accumulation below the critical threshold, or slow the propagation of the positive feedback loop, thereby increasing exercise tolerance. Hence, it is not a fait accompli at exercise onset that intolerance will eventually ensue. Rather, it is the temporal responses of V̇O2 (which in turn mediate the intramuscular bioenergetic responses) that determine whether or not intolerance occurs, depending on whether or not exercise is continued for long enough to allow the [Pi] (or some combination of muscle metabolites) to exceed critical limits.
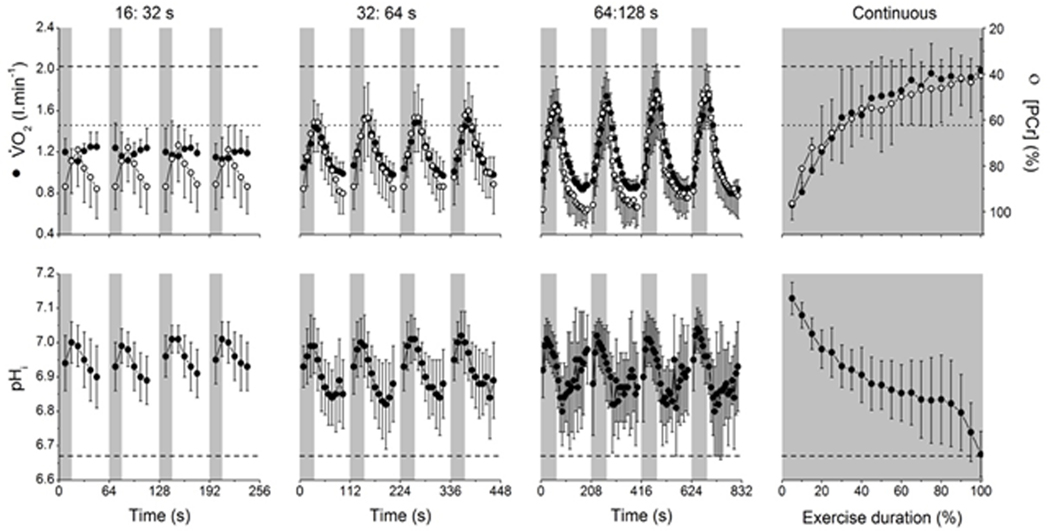
Figure 4.
V̇O2 (filled circles, top row), PCr (clear circles, top row) and intramuscular pH (pHi; bottom row) responses to work:recovery durations of 16:32 s (first column), 32:64 s (second column), 64:128 s (third column) or continuous exercise (fourth column) at an external work rate corresponding to 110% peak incremental test power. Lactate threshold (LT) from the ramp incremental test is shown by the dotted line (top row), as is V̇O2 max (top row, dashed line) and pHi at task failure from the continuous exercise protocol (bottom row, dashed line). Note that during the 16:32 s protocol, V̇O2 never exceeds the LT and the fluctuations in pHi and PCr are small, i.e. consistent with moderate intensity exercise. The peak V̇O2 amplitude exceeds the LT in the 32:64 and 64:128 s intermittent protocols and during continuous exercise, and this is accompanied by a metabolic acidosis (decline in pHi), consistent with a greater metabolic strain in these protocols. Reproduced with permission from (66).
INTEGRATION: WHOLE-BODY MECHANISMS
The computations by Korzeniewski & Rossiter (6,41) represent cellular bioenergetics that averages multiple cells with a range of different properties into a single, chimeric, cell model (6,40,41). Recent studies have argued that CP reflects a “boundary” layer i.e. a range of power outputs, rather than sharp threshold between heavy and very heavy intensities (Pethick et al;(67)). It is possible to conceive of sharp “threshold” behaviors when the bioenergetics of a single muscle fiber are considered. However, because there is a continuum of fiber biogenetic properties involved in whole body exercise, it is difficult to justify sharp threshold behavior when thousands of muscle fibers are combined, even without considering the effects of motor unit recruitment patterns (that is inevitably involved). Nevertheless, the observed bioenergetics behaviors in silico agree well with experimental data from complex in vivo systems with heterogeneous bioenergetic responses among fibers and muscles. Therefore, although a simplification, the lumped bioenergetics responses of the computations from Korzeniewski & Rossiter (6,40,41) result in bioenergetic system properties that cohere well to experimental findings of more complex systems that contain a range of fiber types, recruitment profiles and blood flow distribution patterns (6,40,41,67).
Extrapolating these muscle cellular bioenergetic properties to the integrative physiologic responses of an exercising human is, naturally, fraught with complexity. One key consideration is the role of muscle neural afferents in limiting whole body exercise. Group III/IV mechano- and metaboreceptors are involved in sensing peripheral fatigue processes (such as those represented in computation by the critical threshold), and respond by constraining motoneuronal output and thus, place limits on neuromuscular fatigue development (68). Inhibition of group III/IV afferents using intrathecal fentanyl increases the “peak” [Pi] intramuscular metabolite perturbation that is tolerable over the course of a given exercise bout (68). The alteration in the proposed mechanism that would reflect the action group III/IV afferent inhibition would be to increase “peak” [Pi], i.e. increasing the amount of work that may be performed above CP, i.e. W’. This is consistent with experimental observations (68). Hence, group III/IV afferents play a central role in limiting W’, especially during large muscle mass exercise. However, this relationship may be modulated by a reduction in work efficiency with group III/IV afferent inhibition (68–70), a finding consistent with the proposed link between muscle fatigue and decreased efficiency. Further research is therefore necessary to uncover precisely how these mechanisms, along with input from other systems (e.g., the ventilatory system), integrate at the level of the whole organism to determine the point of exercise intolerance.
CONCLUSIONS
Here we review the experimental evidence to support the concept that a critical threshold and positive feedback loop model of muscle metabolite accumulation determines supra-CP exercise tolerance by constraining it to the limits defined by the power-duration curve. We show that the classical view that the O2 deficit is associated with accumulation of fatigue-related metabolites, can qualitatively and quantitatively explain the shape of the power-duration relationship and therefore that V̇O2 kinetics is a central player in mediating exercise tolerance: fast V̇O2 kinetics allow improved metabolic stability, lesser metabolite accumulation and greater CP. There is a wide array of correlative evidence supporting a strong relationship between τV̇O2 and CP. Moreover, recent acute interventional studies also support that a change in τV̇O2 results in an appropriate change in CP. Exceeding CP is associated with the progressive loss of metabolic stability, muscle fatigue and reduction in work efficiency. Muscle fatigue, determined by the magnitude of muscle metabolite accumulation, is proposed to act in the way that a sensor might act in a feedback loop, by triggering reductions in work efficiency and shaping whether a particular power output is below or above CP. Exceeding this critical threshold initiates a positive feedback loop that may stabilize (heavy intensity) or propagate with diminishing return (very heavy intensity) the demand for ATP synthesis and therefore the rate or amplitude of the V̇O2SC. Interventions that modify either the rate or amplitude of the V̇O2SC are shown to also alter the capacity for supra-CP exercise (W’). In silico simulation studies of the human bioenergetic system offer a mechanism underpinning the proposed model through [Pi] accumulation (or some combination of fatigue-inducing metabolites). These simulations suggest that exceeding critical [Pi] causes fatigue and lowers work efficiency from which emerge the parameters of the power-duration relationship. Future experiments are required to test this hypothesis in vivo.
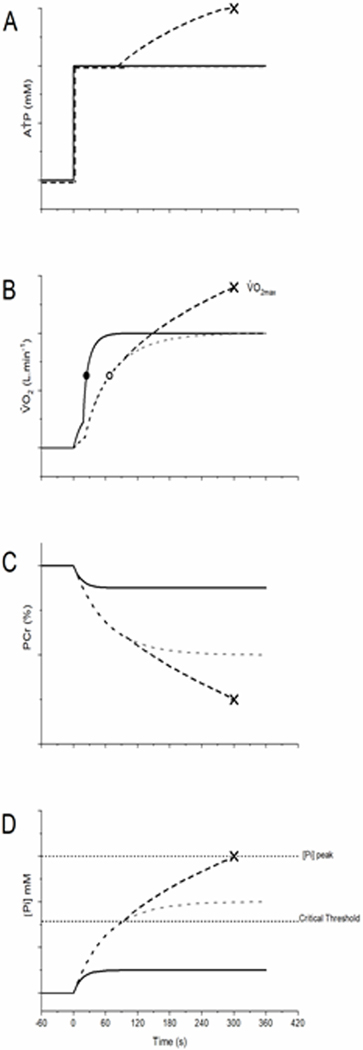
Figure 3.
A schematic of the AṪP (A), V̇O2 (B), PCr (C) and [Pi] responses for participants with fast (τV̇O2 = 15 s; solid line) and slow V̇O2 kinetics (τV̇O2 = 60 s; dashed line). For a given AṪP, a steady-state is achieved rapidly in all variables with a small τV̇O2. The greater rate of O2 deficit accumulation associated with a large τV̇O2 leads to increased PCr depletion and Pi accumulation, to the extent that Pi accumulation (and other muscle metabolites, not shown) exceeds a critical threshold. Exceeding the critical threshold induces fatigue, which generates inefficiency and increases AṪP for a given power output. This initiates a positive feedback loop where increased AṪP, causes increased metabolite accumulation, which causes increased fatigue, which causes further increased AṪP, and so on until peak limits are reached (indicated by X). Stimulation of oxidative phosphorylation by metabolite accumulation above the critical threshold results in an increase in the O2 cost of the exercise, development of the V̇O2SC, and eventual attainment of V̇O2max.
KEY POINTS.
Understanding the physiological determinants of exercise tolerance remains an important goal for exercise physiologists; however, these determinants remain unclear
Here, we present contemporary quantitative evidence that exercise intolerance is mediated via a “critical threshold” and “positive feedback” mechanism, which links V̇O2 kinetics to the ability to sustain exercise
Specifically, the fundamental phase of V̇O2 kinetics determines the power output at which O2-deficit-related metabolite accumulation (e.g. [inorganic phosphate]) exceeds critical limits
Exceeding this “critical threshold” causes muscle fatigue and initiation of a positive feedback loop - a self-driving, reciprocal mechanism of diminishing return - which causes the loss of work efficiency, increased ATP demand, further fatigue, and so on
Once the critical threshold is exceeded, tolerable duration is a function of the propagation rate of this positive feedback loop, with intolerance occurring once peak pulmonary, circulatory or neuromuscular limits, or associated symptoms, are reached
Acknowledgments:
RPG was supported by an International Research Fellowship, Japan Society for Promotion of Sciences, Tokyo, Japan and a European Foundation for the Study of Diabetes (EFSD) Boehringer Ingelheim European Research Programme grant. HBR is supported by grants from NIH (R01HL151452, P50HD098593, R01DK122767, P2CHD086851) and the Tobacco Related Disease Research Program (T31IP1666). He reports consulting fees from Omniox Inc., and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech and Regeneron.
REFERENCES
- 1.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008. January 1;586(Pt 1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grassi B, Porcelli S, Salvadego D, Zoladz JA. Slow VO₂ kinetics during moderate-intensity exercise as markers of lower metabolic stability and lower exercise tolerance. Eur J Appl Physiol. 2011. March;111(3):345–55. [DOI] [PubMed] [Google Scholar]
- 3.Rossiter HB. Exercise: Kinetic Considerations for Gas Exchange. In: Comprehensive Physiology [Internet]. John Wiley & Sons, Inc.; 2010. Available from: http://onlinelibrary.wiley.com/doi/10.1002/cphy.c090010/abstract [DOI] [PubMed] [Google Scholar]
- 4.Murgatroyd SR, Wylde LA. The power–duration relationship of high-intensity exercise: from mathematical parameters to physiological mechanisms. J Physiol. 2011. May 15;589(Pt 10):2443–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murgatroyd SR, Ferguson C, Ward SA, Whipp BJ, Rossiter HB. Pulmonary O2 uptake kinetics as a determinant of high-intensity exercise tolerance in humans. Journal of Applied Physiology. 2011. June 1;110(6):1598–606. [DOI] [PubMed] [Google Scholar]
- 6.Korzeniewski B, Rossiter HB. Exceeding a “critical” muscle Pi: implications for [Formula: see text] and metabolite slow components, muscle fatigue and the power-duration relationship. Eur J Appl Physiol. 2020. May 20; [DOI] [PubMed] [Google Scholar]
- 7.Korzeniewski B, Zoladz JA. Possible factors determining the non-linearity in the VO2-power output relationship in humans: theoretical studies. Jpn J Physiol. 2003. August;53(4):271–80. [DOI] [PubMed] [Google Scholar]
- 8.Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996. March;80(3):988–98. [DOI] [PubMed] [Google Scholar]
- 9.Benson AP, Grassi B, Rossiter HB. A validated model of oxygen uptake and circulatory dynamic interactions at exercise onset in humans. Journal of Applied Physiology. 2013. September 1;115(5):743–55. [DOI] [PubMed] [Google Scholar]
- 10.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913. October 17;47(1–2):112–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassi B, Rossiter HB, Hogan MC, Howlett RA, Harris JE, Goodwin ML, et al. Faster O₂ uptake kinetics in canine skeletal muscle in situ after acute creatine kinase inhibition. J Physiol (Lond). 2011. January 1;589(Pt 1):221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkerson DP, Koppo K, Barstow TJ, Jones AM. Effect of work rate on the functional “gain” of Phase II pulmonary O2 uptake response to exercise. Respir Physiol Neurobiol. 2004. September 15;142(2–3):211–23. [DOI] [PubMed] [Google Scholar]
- 13.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012. April;2(2):933–96. [DOI] [PubMed] [Google Scholar]
- 14.Korzeniewski B, Rossiter HB. Each-step activation of oxidative phosphorylation is necessary to explain muscle metabolic kinetic responses to exercise and recovery in humans. J Physiol. 2015. December 15;593(24):5255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of Calcium on the Oxidative Phosphorylation Cascade in Skeletal Muscle Mitochondria. Biochemistry. 2013. April 23;52(16):2793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marwood S, Constantin-Teodosiu D, Casey E, Whyte M, Boobis L, Bowtell J. No acetyl group deficit is evident at the onset of exercise at 90% of maximal oxygen uptake in humans. J Sports Sci. 2010;28(3):267–79. [DOI] [PubMed] [Google Scholar]
- 17.Gurd BJ, Peters SJ, Heigenhauser GJF, LeBlanc PJ, Doherty TJ, Paterson DH, et al. Prior heavy exercise elevates pyruvate dehydrogenase activity and muscle oxygenation and speeds O2 uptake kinetics during moderate exercise in older adults. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009. September 1;297(3):R877–84. [DOI] [PubMed] [Google Scholar]
- 18.Colburn TD, Hirai DM, Craig JC, Ferguson SK, Weber RE, Schulze KM, et al. Transcapillary PO2 gradients in contracting muscles across the fibre type and oxidative continuum. The Journal of Physiology. 2020;598(15):3187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pringle JSM, Doust JH, Carter H, Tolfrey K, Campbell IT, Sakkas GK, et al. Oxygen uptake kinetics during moderate, heavy and severe intensity “submaximal” exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol. 2003. May;89(3–4):289–300. [DOI] [PubMed] [Google Scholar]
- 20.Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996. October;81(4):1642–50. [DOI] [PubMed] [Google Scholar]
- 21.Medbø JI, Tabata I. Anaerobic energy release in working muscle during 30 s to 3 min of exhausting bicycling. J Appl Physiol. 1993. October;75(4):1654–60. [DOI] [PubMed] [Google Scholar]
- 22.Bangsbo J. Oxygen deficit: a measure of the anaerobic energy production during intense exercise? Can J Appl Physiol. 1996. October;21(5):350–63; discussion 364–369. [DOI] [PubMed] [Google Scholar]
- 23.Billat VL, Hamard L, Koralsztein JP. The influence of exercise duration at VO2 max on the off-transient pulmonary oxygen uptake phase during high intensity running activity. Arch Physiol Biochem. 2002. December;110(5):383–92. [DOI] [PubMed] [Google Scholar]
- 24.Ozyener F, Rossiter HB, Ward SA, Whipp BJ. Negative accumulated oxygen deficit during heavy and very heavy intensity cycle ergometry in humans. Eur J Appl Physiol. 2003. September;90(1–2):185–90. [DOI] [PubMed] [Google Scholar]
- 25.Cannon DT, White AC, Andriano MF, Kolkhorst FW, Rossiter HB. Skeletal muscle fatigue precedes the slow component of oxygen uptake kinetics during exercise in humans. J Physiol (Lond). 2011. February 1;589(Pt 3):727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill DW, Poole DC, Smith JC. The relationship between power and the time to achieve.VO(2max). Med Sci Sports Exerc. 2002. April;34(4):709–14. [DOI] [PubMed] [Google Scholar]
- 27.Keir DA, Copithorne DB, Hodgson MD, Pogliaghi S, Rice CL, Kowalchuk JM. The slow component of pulmonary O2 uptake accompanies peripheral muscle fatigue during high-intensity exercise. Journal of Applied Physiology. 2016. June 23;121(2):493–502. [DOI] [PubMed] [Google Scholar]
- 28.Burnley M. Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J Appl Physiol. 2009. March;106(3):975–83. [DOI] [PubMed] [Google Scholar]
- 29.Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol. 2008. February;294(2):R585–593. [DOI] [PubMed] [Google Scholar]
- 30.Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988. September;31(9):1265–79. [DOI] [PubMed] [Google Scholar]
- 31.Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol. 2010. April;95(4):528–40. [DOI] [PubMed] [Google Scholar]
- 32.Vanhatalo A, Black MI, DiMenna FJ, Blackwell JR, Schmidt JF, Thompson C, et al. The mechanistic bases of the power-time relationship: muscle metabolic responses and relationships to muscle fibre type. J Physiol (Lond). 2016. August 1;594(15):4407–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassi B, Rossiter HB, Zoladz JA. Skeletal muscle fatigue and decreased efficiency: two sides of the same coin? Exerc Sport Sci Rev. 2015. April;43(2):75–83. [DOI] [PubMed] [Google Scholar]
- 34.Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of VO2 kinetics. J Appl Physiol. 2008. August;105(2):575–80. [DOI] [PubMed] [Google Scholar]
- 35.Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Regul Integr Comp Physiol. 2011. March;300(3):R700–707. [DOI] [PubMed] [Google Scholar]
- 36.Barclay CJ. Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J Physiol. 1996. December 15;497(Pt 3):781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hepple RT, Howlett RA, Kindig CA, Stary CM, Hogan MC. The O2 cost of the tension-time integral in isolated single myocytes during fatigue. Am J Physiol Regul Integr Comp Physiol. 2010. April;298(4):R983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krustrup P, Söderlund K, Mohr M, González-Alonso J, Bangsbo J. Recruitment of fibre types and quadriceps muscle portions during repeated, intense knee-extensor exercise in humans. Pflugers Arch. 2004. October;449(1):56–65. [DOI] [PubMed] [Google Scholar]
- 39.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008. January;88(1):287–332. [DOI] [PubMed] [Google Scholar]
- 40.Korzeniewski B. Pi-induced muscle fatigue leads to near-hyperbolic power-duration dependence. Eur J Appl Physiol. 2019. October;119(10):2201–13. [DOI] [PubMed] [Google Scholar]
- 41.Korzeniewski B, Rossiter HB. Factors determining training-induced changes in V̇O2max, critical power and V̇O2 on-kinetics in skeletal muscle. Journal of Applied Physiology [Internet]. 2020. November 19 [cited 2020 Nov 25]; Available from: https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00745.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC. Critical power: implications for determination of V˙O2max and exercise tolerance. Med Sci Sports Exerc. 2010. October;42(10):1876–90. [DOI] [PubMed] [Google Scholar]
- 43.Goulding RP, Roche DM, Marwood S. Elevated baseline work rate slows pulmonary oxygen uptake kinetics and decreases critical power during upright cycle exercise. Physiol Rep [Internet]. 2018. July 23 [cited 2018 Aug 6];6(14). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6056736/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker T, Poole DC, Noble ML, Barstow TJ. Human critical power–oxygen uptake relationship at different pedalling frequencies. Experimental Physiology. 2006. May 1;91(3):621–32. [DOI] [PubMed] [Google Scholar]
- 45.Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985. November;48(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. The Journal of General Physiology. 1982. January 1;79(1):147–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demarle AP, Slawinski JJ, Laffite LP, Bocquet VG, Koralsztein JP, Billat VL. Decrease of O2 deficit is a potential factor in increased time to exhaustion after specific endurance training. Journal of Applied Physiology. 2001. March 1;90(3):947–53. [DOI] [PubMed] [Google Scholar]
- 48.Gaesser GA, Wilson LA. Effects of continuous and interval training on the parameters of the power-endurance time relationship for high-intensity exercise. Int J Sports Med. 1988. December;9(6):417–21. [DOI] [PubMed] [Google Scholar]
- 49.Hughson RL, Kowalchuk JM. Kinetics of oxygen uptake for submaximal exercise in hyperoxia, normoxia, and hypoxia. Can J Appl Physiol. 1995. June;20(2):198–210. [DOI] [PubMed] [Google Scholar]
- 50.Simpson LP, Jones AM, Skiba PF, Vanhatalo A, Wilkerson D. Influence of hypoxia on the power-duration relationship during high-intensity exercise. Int J Sports Med. 2015. February;36(2):113–9. [DOI] [PubMed] [Google Scholar]
- 51.Pringle JSM, Doust JH, Carter H, Tolfrey K, Jones AM. Effect of pedal rate on primary and slow-component oxygen uptake responses during heavy-cycle exercise. J Appl Physiol. 2003. April;94(4):1501–7. [DOI] [PubMed] [Google Scholar]
- 52.Goulding RP, Roche DM, Marwood S. “Work-to-Work” exercise slows pulmonary oxygen uptake kinetics, decreases critical power, and increases W’ during supine cycling. Physiol Rep. 2018. November;6(21):e13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goulding RP, Roche DM, Marwood S. Hyperoxia speeds pulmonary oxygen uptake kinetics and increases critical power during supine cycling. Exp Physiol. 2019. May 4; [DOI] [PubMed] [Google Scholar]
- 54.Goulding RP, Roche DM, Marwood S. Prior exercise speeds pulmonary oxygen uptake kinetics and increases critical power during supine but not upright cycling. Exp Physiol. 2017. September 1;102(9):1158–76. [DOI] [PubMed] [Google Scholar]
- 55.Goulding RP, Roche DM, Scott SN, Koga S, Weston PJ, Marwood S. Limitations to exercise tolerance in type 1 diabetes: the role of pulmonary oxygen uptake kinetics and priming exercise. Journal of Applied Physiology [Internet]. 2020. March 26 [cited 2020 Mar 28]; Available from: https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00892.2019 [DOI] [PubMed] [Google Scholar]
- 56.Goulding RP, Roche DM, Marwood S. Effect of Hyperoxia on Critical Power and V[Combining Dot Above]O2 Kinetics during Upright Cycling. Med Sci Sports Exerc. 2019. December 5; [DOI] [PubMed] [Google Scholar]
- 57.Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999. April;86(4):1367–73. [DOI] [PubMed] [Google Scholar]
- 58.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol (Lond). 2003. June 1;549(Pt 2):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Söderlund K, Hultman E. ATP and phosphocreatine changes in single human muscle fibers after intense electrical stimulation. Am J Physiol. 1991. December;261(6 Pt 1):E737–741. [DOI] [PubMed] [Google Scholar]
- 60.Cleuziou C, Perrey S, Lecoq AM, Candau R, Courteix D, Obert P. Oxygen uptake kinetics during moderate and heavy intensity exercise in humans: the influence of hypoxia and training status. Int J Sports Med. 2005. June;26(5):356–62. [DOI] [PubMed] [Google Scholar]
- 61.Jenkins DG, Quigley BM. Endurance training enhances critical power. Med Sci Sports Exerc. 1992. November;24(11):1283–9. [PubMed] [Google Scholar]
- 62.Miura A, Sato H, Sato H, Whipp BJ, Fukuba Y. The effect of glycogen depletion on the curvature constant parameter of the power-duration curve for cycle ergometry. Ergonomics. 2000. January;43(1):133–41. [DOI] [PubMed] [Google Scholar]
- 63.Carter H, Pringle JSM, Boobis L, Jones AM, Doust JH. Muscle glycogen depletion alters oxygen uptake kinetics during heavy exercise. Med Sci Sports Exerc. 2004. June;36(6):965–72. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson C, Whipp BJ, Cathcart AJ, Rossiter HB, Turner AP, Ward SA. Effects of prior very-heavy intensity exercise on indices of aerobic function and high-intensity exercise tolerance. Journal of Applied Physiology. 2007. September 1;103(3):812–22. [DOI] [PubMed] [Google Scholar]
- 65.Burnley M, Davison G, Baker JR. Effects of priming exercise on VO2 kinetics and the power-duration relationship. Med Sci Sports Exerc. 2011. November;43(11):2171–9. [DOI] [PubMed] [Google Scholar]
- 66.Davies MJ, Benson AP, Cannon DT, Marwood S, Kemp GJ, Rossiter HB, et al. Dissociating external power from intramuscular exercise intensity during intermittent bilateral knee-extension in humans. J Physiol (Lond). 2017. 01;595(21):6673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pethick J, Winter SL, Burnley M. Physiological Evidence that the Critical Torque Is a Phase Transition Not a Threshold. Med Sci Sports Exerc. 2020. May 4; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broxterman RM, Hureau TJ, Layec G, Morgan DE, Bledsoe AD, Jessop JE, et al. Influence of group III/IV muscle afferents on small muscle mass exercise performance: a bioenergetics perspective. The Journal of Physiology. 2018;596(12):2301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broxterman RM, Layec G, Hureau TJ, Amann M, Richardson RS. Skeletal muscle bioenergetics during all-out exercise: mechanistic insight into the oxygen uptake slow component and neuromuscular fatigue. J Appl Physiol. 2017. May 1;122(5):1208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broxterman RM, Layec G, Hureau TJ, Morgan DE, Bledsoe AD, Jessop JE, et al. Bioenergetics and ATP Synthesis during Exercise: Role of Group III/IV Muscle Afferents. Med Sci Sports Exerc. 2017. December;49(12):2404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]