Abstract
Background
Cytomegalovirus (CMV) reactivation is one of the most common infectious complications after allogeneic hematopoietic cell transplant (HCT) and may result in significant morbidity and mortality. Primary prophylaxis with letermovir demonstrated a reduction in clinically significant CMV infections (CS-CMVi) in clinical trials of CMV-seropositive HCT recipients. This study aims at exploring the effect of primary letermovir prophylaxis in this population on the incidence and outcomes of refractory or resistant CMV infections.
Methods
This is a single-center, retrospective cohort study of 537 consecutive CMV-seropositive allogeneic HCT recipients cared for between March 2016 and October 2018. Baseline demographics, HCT characteristics, CMV infections, treatment, and mortality data were collected from the electronic medical record. CMV outcomes were defined according to the recently standardized definitions for clinical trials. Characteristics and outcomes were assessed according to receipt of primary letermovir prophylaxis.
Results
Of 537 patients identified, 123 received letermovir for primary prophylaxis during the first 100 days after HCT; 414 did not. In a multivariate analysis, primary prophylaxis with letermovir was associated with reductions in CS-CMVi (hazard ratio [HR] 0.26; 95% confidence interval [CI], 0.16–0.41), CMV end-organ disease (HR 0.23; 95% CI, 0.10–0.52), refractory or resistant CMV infection (HR 0.15; 95% CI, 0.04–0.52), and nonrelapse mortality at week 48 (HR 0.55; 95% CI, 0.32–0.93). There was neither resistant CMV nor CMV-related mortality in the primary letermovir prophylaxis group.
Conclusions
Primary letermovir prophylaxis effectively prevents refractory or resistant CMV infections and decreases nonrelapse mortality at week 48, as well as CS-CMVi and CMV disease after allogeneic HCT.
Keywords: cytomegalovirus, hematopoietic cell transplant, letermovir, refractory and resistant cytomegalovirus, mortality
Primary letermovir prophylaxis effectively prevents refractory or resistant CMV infections in CMV-seropositive allogeneic HCT recipients and is associated with a lower nonrelapse mortality at 48 weeks after allogeneic HCT.
Human cytomegalovirus (CMV) is a common opportunistic infection in hematopoietic cell transplant (HCT) recipients [1], with clinical manifestations ranging from asymptomatic viremia or DNAemia to end-organ disease such as pneumonitis, retinitis, or colitis [1]. Risk factors for CMV infection and disease after HCT include recipient seropositivity for CMV, umbilical cord blood transplant, conditioning regimens containing antithymocyte globulin, and graft-versus-host disease (GVHD) [2]. Until recently, in the absence of safe chemoprophylactic agents, the main preventive strategy against CMV disease in HCT recipients was preemptive treatment targeting patients with early clinically significant CMV infection (CS-CMVi). Letermovir, a novel antiviral targeting the viral terminase complex, was approved in November 2017 by the US Food and Drug Administration for primary prophylaxis in CMV-seropositive recipients after allogeneic HCT [3], after the phase 2 and 3 clinical trials demonstrated a significant reduction in the incidence of CS-CMVi by week 24 compared with placebo [4, 5]. Because of its efficacy and safety, letermovir has become the standard of care for primary prophylaxis against CMV during the 100 days after transplant; yet, its impact on resistant or refractory CMV infections is not understood.
In 2019, definitions for refractory and resistant CMV infection and disease in transplant recipients for use in clinical trials were published, providing a uniform framework to evaluate response to antiviral therapy [6]. In the past, resistant CMV infections have been reported mostly in solid organ transplant recipients and in patients with acquired immunodeficiency syndrome. In HCT recipients, the reported rates of resistant CMV infections range from 1.7% to 14.5% [7–10]. The most common mutations associated with resistance affect UL97, encoding for protein kinase, and conferring resistance to ganciclovir and valganciclovir, followed by mutation of UL54, encoding for DNA polymerase, and conferring resistance to various DNA polymerase inhibitors, including ganciclovir, valganciclovir, foscarnet, and cidofovir [11]. In comparison, the reported rates of refractory CMV infections in HCT recipients range from 19% to 29% [7, 12, 13]. This wide range of reported rates could be explained by the lack of standardized definitions at the time and by limited access to phenotypic or genotypic testing for resistance to commercially available anti-CMV agents.
To date, no study has evaluated the effect of primary letermovir prophylaxis on the development of refractory or resistant CMV infections in allogeneic HCT recipients, particularly in the context of the new definitions [6]. This study aims to describe the real-life experience with primary letermovir prophylaxis since its systematic implementation at our comprehensive cancer center, as well as its effects on CS-CMVi, CMV end-organ disease, refractory or resistant CMV infections, and mortality in allogeneic HCT recipients.
METHODS
Study Design
We performed a single-center retrospective study of all consecutive CMV-seropositive recipients of allogeneic HCT cared for from March 2016 through October 2018 at The University of Texas MD Anderson Cancer Center. The study was approved by the institutional review board, and a waiver of informed consent was granted.
Patient Population
We identified all patients who received an allogeneic HCT during the study period. We excluded CMV-seronegative recipients because primary letermovir prophylaxis is approved, and used at our institution, in CMV-seropositive recipients only. Based on our institutional protocol, primary letermovir prophylaxis was implemented starting March 2018 and administered starting day 5 after HCT through day 100 or longer at 480 mg intravenously or orally once daily (240 mg once daily if administered concurrently with cyclosporine), all contingent on insurance authorization. Additionally, haploidentical HCT recipients, donor-mismatched recipients with post-HCT cyclophosphamide, and cord blood HCT recipients received ganciclovir at 5 mg/kg intravenously every 12 hours from admission through day −2, according to institutional protocols. All patients underwent CMV monitoring at least twice weekly by polymerase chain reaction in plasma using the COBAS AmpliPrep/COBAS TaqMan CMV system with a 97 CMV DNA IU/mL limit of detection. Patients with a positive polymerase chain reaction result meeting the institutional threshold for preemptive therapy or with diagnosed end-organ disease were started on appropriate antiviral therapy according to institutional guidelines [2, 14]. Patients were stratified into 2 groups based on whether they received letermovir for primary CMV prophylaxis or not.
Outcomes
The primary outcome of the study was the development of refractory or resistant CMV infection after HCT, as defined by the Resistant Definitions Working Group of the Cytomegalovirus Drug Development Forum, specifically for use in clinical trials in transplant recipients [6]. Briefly, refractory CMV infection is a >1-log10 increase in CMV viremia after at least 2 weeks of appropriate therapy. A probable refractory CMV infection is a persistent or <1-log10 increase in CMV viremia after at least 2 weeks of appropriate therapy. Refractory and probable refractory CMV end-organ disease are defined as worsening in signs and symptoms or lack of improvement, respectively, after 2 weeks of appropriate therapy. Finally, resistant CMV infection requires the detection of a viral genetic mutation known to decrease susceptibility to 1 or more antivirals.
Secondary outcomes included the development of CS-CMVi after HCT, defined as CMV viremia or disease that resulted in initiation of anti-CMV therapy [4]; CMV end-organ disease after HCT; CMV-related mortality; all-cause mortality and nonrelapse mortality at day 100, week 24, and week 48. Additional outcomes included the number of CMV episodes (new CMV viremia or disease after 4 weeks of undetectable viral loads [15]), the administration of anti-CMV agents (ganciclovir, valganciclovir, or foscarnet), and major side effects associated with antiviral therapy, including myelosuppression (50% decrease in absolute neutrophil count or platelet count after initiation of therapy), nephrotoxicity (50% increase in serum creatinine after initiation of therapy), and hepatotoxicity (increase in transaminases to at least 3 times the upper limit of normal after initiation of therapy).
Variables
Demographic and clinical data were obtained from the electronic medical record, including age, sex, race/ethnicity, underlying hematological disease, HCT type and conditioning regimen, donor CMV seropositivity, administration of antithymocyte globulin or post-HCT cyclophosphamide for GVHD prophylaxis, primary graft failure, time from HCT to engraftment, and development of GVHD. For patients who developed CS-CMVi, absolute neutrophil and lymphocyte counts at the onset of CS-CMVi, absolute lymphocyte count at day 40 after HCT, and administration of active GVHD therapy or other immunosuppressants within 30 days before the onset of CS-CMVi were collected.
Statistical Analysis
A χ 2 or Fisher exact test was used to compare categorical variables according to receipt of primary letermovir prophylaxis; a Wilcoxon rank-sum test was used to compare continuous variables between those groups. Similar comparisons were performed in the subset of patients who developed CS-CMVi. A box plot was applied to compare the peak CMV viral load between patients with and without primary letermovir prophylaxis. A competing risk analysis identified independent predictors of the development of CS-CMVi and of refractory or resistant CMV, with death the competing event, estimated and compared the cumulative incidence curves of CS-CMVi and refractory or resistant CMV in the 2 groups. A logistic regression model identified the independent predictors of CMV end-organ disease. A Cox proportional hazards regression model identified the independent predictors of nonrelapse mortality. Because primary graft failure was independently associated with nonrelapse mortality but failed to satisfy the proportional hazards assumption, the Cox regression analysis was stratified into patients with graft failure and patients without graft failure. With the number of patients with graft failure being too small (n = 10) for a valid analysis, we performed a Cox regression analysis on nonrelapse mortality in patients without graft failure only (n = 527, 98% of the study population). Hence, Kaplan-Meier curves for nonrelapse survival were estimated for patients without graft failure and compared between those with and without primary letermovir prophylaxis using a log-rank test. All tests were 2-sided with a significance level of 0.05. The statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
We identified a total of 537 patients cared for between March 2016 and October 2018 who met our inclusion criteria and were included in our analysis. Of the total cohort, 123 patients received primary letermovir prophylaxis and 414 did not. The 2 groups were similar in demographic and transplant characteristics, except for the source of donor stem cells and administration of antithymocyte globulin and post-HCT cyclophosphamide, reflecting a change in practice at our institution over time, with less frequent use of marrow cells and antithymocyte globulin and more frequent use of peripheral blood cells and post-HCT cyclophosphamide. Additionally, the letermovir group had a higher donor CMV seropositivity and a lower rate of skin GVHD (Table 1).
Table 1.
Characteristics of Patients Who Did or Did Not Receive Letermovir for Primary Prophylaxis
| Primary Letermovir Prophylaxis | ||||
|---|---|---|---|---|
| No | Yes | All Patients | ||
| Characteristic | (n = 414) | (n = 123) | (n = 537) | P Valuea |
| Age, median (range), y | 54 (6–78) | 57 (18–93) | 55 (6–93) | .18 |
| Male, no. (%) | 215 (52) | 64 (52) | 279 (52) | .98 |
| Race/ethnicity, no. (%) | .73 | |||
| White | 272 (66) | 79 (64) | 351 (65) | |
| Black | 29 (7) | 10 (8) | 39 (7) | |
| Hispanic | 68 (16) | 18 (15) | 86 (16) | |
| Asian | 18 (4) | 5 (4) | 23 (4) | |
| Middle Eastern | 22 (5) | 7 (6) | 29 (5) | |
| Other | 5 (1) | 4 (3) | 9 (2) | |
| Underlying disease, no. (%) | .63 | |||
| AML | 187 (45) | 52 (42) | 239 (45) | |
| ALL | 59 (14) | 16 (13) | 75 (14) | |
| MDS | 57 (14) | 14 (11) | 71 (13) | |
| MF | 33 (8) | 10 (8) | 43 (8) | |
| Others | 78 (19) | 31 (25) | 109 (20) | |
| Type of conditioning regimen, no. (%) | .18 | |||
| Myeloablative/reduced-intensity | 401 (97) | 116 (94) | 517 (96) | |
| Nonmyeloablative | 13 (3) | 7 (6) | 20 (4) | |
| Type of transplant, no. (%) | .79 | |||
| MRD | 128 (31) | 37 (30) | 165 (31) | |
| MUD/MMUD | 190 (46) | 58 (47) | 248 (46) | |
| Haploidentical | 74 (18) | 24 (20) | 98 (18) | |
| Cord | 22 (5) | 4 (3) | 26 (5) | |
| Source of stem cells, no. (%) | ||||
| Marrow | 141 (34) | 27 (22) | 168 (31) | .011 |
| Peripheral | 251 (61) | 92 (75) | 343 (64) | .004 |
| Single cord | 1 (0.2) | 0 (0) | 1 (0.2) | >.99 |
| Double cord | 21 (5) | 4 (3) | 25 (5) | .40 |
| Donor CMV seropositivity, no. (%) | 211/407 (52) | 79/122 (65) | 290/529 (55) | .012 |
| N/A or unknown | 7 (2) | 1 (1) | 8 (1) | |
| ATG | 134 (32) | 19 (15) | 153 (28) | <.001 |
| Post-cy | 158 (38) | 78 (63) | 236 (44) | <.0001 |
| Primary graft failure | 9 (2) | 1 (1) | 10 (2) | .47 |
| Time from HCT to engraftment, median (range), d | 15 (7–49) | 15 (7–124) | 15 (7–124) | .34 |
| Any GVHD, no. (%) | 212 (51) | 65 (53) | 277 (52) | .75 |
| Skin | 136/212 (64) | 31/65 (48) | 167/277 (60) | .018 |
| Gastrointestinal | 130/212 (61) | 40/65 (62) | 170/277 (61) | .97 |
| Liver | 9/212 (4) | 7/65 (11) | 16/277 (6) | .066 |
| Ocular | 8/212 (4) | 2/65 (3) | 10/277 (4) | >.99 |
| Acute GVHD, no. (%) | 198 (48) | 60 (49) | 258 (48) | .85 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; CMV, cytomegalovirus; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplant; MDS, myelodysplastic syndrome; MF, myelofibrosis; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; N/A, not available; post-cy, posttransplant cyclophosphamide.
a P values are from the test comparing patients with and without primary letermovir prophylaxis.
Refractory or Resistant CMV
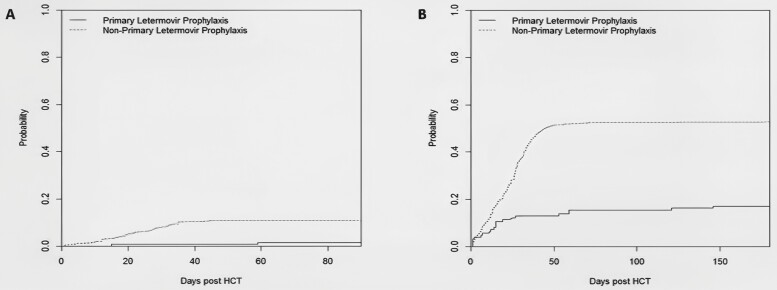
A significantly lower incidence of refractory or resistant CMV infection was observed in patients who received primary letermovir prophylaxis compared with those who did not receive it (2% vs 11%, P = .001, Figure 1A). In the letermovir group, only 2 patients developed probable refractory CMV infection, and none developed refractory or resistant CMV infection (Table 2). One of them underwent genotypic testing for letermovir resistance at UL56, and no mutations were identified. Neither of these patients had CMV end-organ disease. One had gastrointestinal GVHD and died at day 255, whereas the other was still alive at the time of last follow-up.
Figure 1.
Cumulative incidence curves of refractory or resistant CMV (A, P = .001) and clinically significant CMV infection (B, P < .0001) in patients with and without primary letermovir prophylaxis. CMV, cytomegalovirus.
Table 2.
Impact of Letermovir Primary Prophylaxis on Clinical CMV Outcomes
| Primary Letermovir Prophylaxis | ||||
|---|---|---|---|---|
| No | Yes | All Patients | ||
| Outcome | (n = 414) | (n = 123) | (n = 537) | P Valuea |
| CS-CMVi, no. (%) | 221 (53) | 21 (17) | 242 (45) | <.0001 |
| Time from HCT to CS-CMVi, median (range), d | 24 (1–1294) | 15 (1–146) | 23 (1–1294) | .16 |
| CS-CMVi by day 100, no. (%) | 218 (53) | 19 (15) | 237 (44) | <.0001 |
| Late CS-CMVi (beyond day 100), no. (%) | 3 (0.7) | 2 (2) | 5 (0.9) | .32 |
| Peak CMV viral load, median (range), IU/mL | 1485 (136–304 402) | 756 (136–66 398) | 1354 (136–304 402) | .047 |
| Time from first detection of CMV in plasma to initiation of antiviral therapy, mean (range), d | 16 (0–64) | 22 (0–116) | 17 (0–116) | .067 |
| CMV end-organ disease, no. (%) | 83 (20) | 7 (6) | 90 (17) | .0002 |
| Gastrointestinal | 13 (3) | 0 (0) | 13 (2) | .047 |
| Lungs | 51 (12) | 4 (3) | 55 (10) | .004 |
| Retinitis | 2 (0.5) | 0 (0) | 2 (0.4) | >.99 |
| Bone marrow | 25 (6) | 1 (1) | 26 (5) | .018 |
| Otherb | 2 (0.5) | 2 (2) | 4 (1) | .23 |
| R/R CMV, no. (%) | 45 (11) | 2 (2) | 47 (9) | .001 |
| Refractory | 30 (7) | 0 (0) | 30 (6) | .002 |
| Probable refractory | 12 (3) | 2 (2) | 14 (3) | .75 |
| Resistant | 3 (1) | 0 (0) | 3 (1) | >.99 |
| Time from HCT to R/R CMV, median (range), days | 22 (1–44) | 37 (15–59) | 22 (1–59) | .48 |
| All-cause mortality | ||||
| At day 100, no. (%) | 51 (12) | 9 (7) | 60 (11) | .12 |
| At week 24, no. (%) | 81 (20) | 19 (15) | 100 (19) | .30 |
| At week 48, no. (%) | 129 (31) | 35 (28) | 164 (31) | .57 |
| Time to all-cause mortality post-HCT, median (range), d | 183 (1–1279) | 179 (18–726) | 181 (1–1279) | .85 |
| CMV-related mortality, no. (%) | 13 (3) | 0 (0) | 13 (2) | .047 |
| Nonrelapse mortality | ||||
| At day 100, no. (%) | 45 (11) | 8 (7) | 53 (10) | .15 |
| At week 24, no. (%) | 62 (15) | 12 (10) | 74 (14) | .14 |
| At week 48, no. (%) | 88 (21) | 18 (15) | 106 (20) | .11 |
| Time to nonrelapse mortality post-HCT, median (range), days | 174 (1–1279) | 167 (18–565) | 170 (1–1279) | .18 |
Abbreviations: CMV, cytomegalovirus; CS-CMVi, clinically significant cytomegalovirus infection; HCT, hematopoietic cell transplant; R/R, refractory or resistant.
a P values are from the test comparing patients with and without primary letermovir prophylaxis.
bOther sites of CMV end-organ disease include central nervous system and pericardium.
Among the patients who did not receive primary letermovir prophylaxis, 3 developed resistant CMV infections; all had UL54 mutations. An additional 30 patients developed refractory CMV infections, and 12 had probable refractory CMV infections. Of these 45 patients with resistant or refractory infections, 22 had CMV end-organ disease; 30 had GVHD; 20 died by week 48, including 2 from CMV-related causes; and 4 had primary disease relapse. For the entire study cohort, the all-cause mortality rate at week 48 was higher among the patients who developed refractory or resistant CMV (45%) compared with those who did not (29%, P = .02).
A multivariate competing risk analysis (Table 3) identified primary letermovir prophylaxis as an independent protective factor against the development of refractory or resistant CMV infections (adjusted hazard ratio [HR] 0.15; 95% confidence interval [CI], 0.04–0.58; P = .006).
Table 3.
Independent Predictors of Refractory or Resistant CMV by Competing Risk Analysisa
| Predictor | Adjusted HR | 95% CI | P Value |
|---|---|---|---|
| Type of transplant | <.0001 | ||
| MRD | Reference | ||
| MUD/MMUD | 3.87 | 1.15 to 13.03 | |
| Haploidentical | 12.92 | 3.84 to 43.43 | |
| Cord | 4.35 | 0.70 to 27.11 | |
| Donor CMV seropositivity | 0.43 | 0.24 to 0.78 | .005 |
| Letermovir primary prophylaxis | 0.15 | 0.04 to 0.58 | .006 |
Abbreviations: 95% CI, 95% confidence interval; CMV, cytomegalovirus; HR, hazard ratio; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
aDeath is the competing event in the competing risk analysis.
Clinically Significant CMV Infection
Patients who received primary letermovir prophylaxis had a significantly lower incidence of CS-CMVi (17%) compared with those who did not (53%, P < .0001, Figure 1B). There was no significant difference in the timing of CS-CMVi after HCT between the 2 groups (P = .16), and the majority of CS-CMVi occurred before day 100 in both groups (Table 2). Among the patients who received primary letermovir prophylaxis, 74 (60%) received letermovir beyond day 100. A competing risk analysis to identify independent predictors of CS-CMVi (Table 4) revealed a significant protective effect for primary letermovir prophylaxis (adjusted HR 0.26; 95% CI, 0.16–0.41; P < .0001).
Table 4.
Independent Predictors of CS-CMVi by Competing Risk Analysisa
| Predictor | Adjusted HR | 95% CI | P Value |
|---|---|---|---|
| Race/ethnicity | .025 | ||
| White | Reference | ||
| Black | 1.80 | 1.18–2.72 | |
| Hispanic | 1.24 | 0.85–1.81 | |
| Others (including Asian and Middle Eastern) | 1.54 | 1.02–2.33 | |
| Type of transplant | <.0001 | ||
| MRD | Reference | ||
| MUD/MMUD | 2.14 | 1.41–3.25 | |
| Haploidentical | 3.23 | 2.20–4.75 | |
| Cord blood | 1.44 | 0.72–2.90 | |
| ATG | 1.62 | 1.16–2.27 | .005 |
| Letermovir primary prophylaxis | 0.26 | 0.16–0.41 | <.0001 |
Abbreviations: 95% CI, 95% confidence interval; ATG, anti-thymocyte globulin; CS-CMVi, clinically significant cytomegalovirus infection; HR, hazard ratio; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
aDeath is the competing event in the competing risk analysis.
Clinical Outcomes in Patients With CS-CMVi
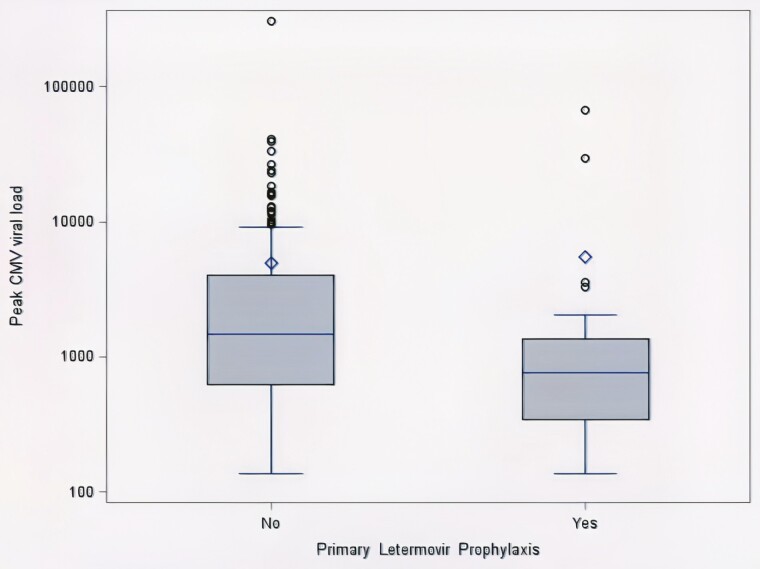
The majority of patients who developed CS-CMVi had 1 episode (92%). None of the patients who received primary letermovir prophylaxis developed a second episode, whereas 9% of those who did not receive letermovir had a second episode (Supplemental Table 1). Foscarnet was administered at a significantly lower rate in the CS-CMVi letermovir group (43% vs 69%, P = .014), likely resulting in the lower incidence of nephrotoxicity during anti-CMV therapy in this group (19% vs 44%, P = .025). None of the patients with CS-CMVi who received letermovir developed hepatotoxicity during anti-CMV therapy (Supplemental Table 1). Among the patients who developed CS-CMVi, those who received primary letermovir prophylaxis had a lower peak CMV viral load (median 756 IU/mL vs 1485 IU/mL, P = .047, Figure 2). The CS-CMVi subset showed no differences according to letermovir prophylaxis with regard to absolute neutrophil or lymphocyte counts at the onset of CMV viremia, active GVHD therapy, or immunosuppressants within 30 days of CS-CMVi.
Figure 2.
Box plot of peak CMV viral load in patients with clinically significant CMV infection with and without primary letermovir prophylaxis (P = .047). The horizontal line in the box interior represents the group median. The diamond represents the group mean. CMV, cytomegalovirus.
CMV End-Organ Disease
Primary letermovir prophylaxis was associated with a lower incidence of CMV end-organ disease (6% vs 20%, P = .0002), with a notable absence of CMV retinitis or gastrointestinal disease in the letermovir group (Table 2). A logistic regression analysis identified primary letermovir prophylaxis as a protective factor (adjusted OR 0.23; 95% CI, 0.10–0.52; P < .001) against CMV disease (Supplemental Table 2).
Mortality
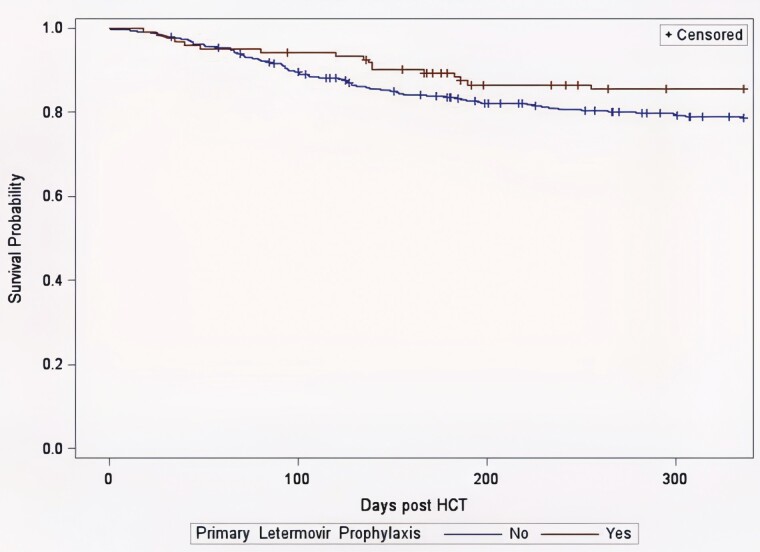
On univariate analysis, there was a trend toward lower all-cause mortality at day 100 in the primary letermovir prophylaxis group (7% vs 12%, P = .12, Table 2), which was less pronounced at weeks 24 and 48. For nonrelapse mortality, there was a survival advantage in the primary letermovir prophylaxis group that persisted from day 100 through weeks 24 and 48 (Table 2), although it was not statistically significant. A Cox regression analysis of the patients without primary graft failure (527 of 537 patients) showed a significantly lower nonrelapse mortality at week 48 in the primary letermovir prophylaxis group (adjusted HR 0.55; 95% CI, 0.32–0.93; P = .025). Risk factors for nonrelapse mortality at week 48 are reported in Tables 5 and 6. A Kaplan-Meier curve for nonrelapse survival of patients without graft failure is shown in Figure 3, with a log-rank P value of .10 for a comparison of the letermovir and nonletermovir groups.
Table 5.
Patient Characteristics According to Nonrelapse Mortality Status at Week 48
| Nonrelapse Mortality at Week 48 | ||||
|---|---|---|---|---|
| No | Yes | All Patients | ||
| Characteristic | (n = 431) | (n = 106) | (n = 537) | P Valuea |
| Age, median (range), y | 54 (6–93) | 57 (21–78) | 55 (6–93) | .035 |
| Male, no. (%) | 234 (54) | 45 (42) | 279 (52) | .029 |
| Race/ethnicity, no. (%) | .617 | |||
| White | 274 (63) | 77 (72) | 351 (66) | |
| Black | 33 (8) | 6 (6) | 39 (7) | |
| Hispanic | 71 (16) | 15 (14) | 86 (16) | |
| Asian | 20 (5) | 3 (3) | 23 (4) | |
| Middle Eastern | 25 (6) | 4 (4) | 29 (5) | |
| Other | 8 (2) | 1 (1) | 9 (2) | |
| Underlying disease, no. (%) | .779 | |||
| AML | 194 (45) | 45 (43) | 239 (45) | |
| ALL | 61 (14) | 14 (13) | 75 (14) | |
| MDS | 53 (12) | 18 (17) | 71 (13) | |
| MF | 34 (8) | 9 (8) | 43 (8) | |
| Others | 89 (21) | 20 (19) | 109 (20) | |
| Type of conditioning regimen, no. (%) | .091 | |||
| Myeloablative/reduced-intensity | 412 (96) | 105 (99) | 517 (96) | |
| Nonmyeloablative | 19 (4) | 1 (1) | 20 (4) | |
| Type of transplant, no. (%) | <.001 | |||
| MRD | 149 (35) | 16 (15) | 165 (31) | |
| MUD/MMUD | 193 (45) | 55 (52) | 248 (46) | |
| Haploidentical | 66 (15) | 32 (30) | 98 (18) | |
| Cord | 23 (5) | 3 (3) | 26 (5) | |
| Source of stem cells, no. (%) | ||||
| Marrow | 124 (29) | 43 (40) | 167 (31) | .019 |
| Peripheral | 284 (66) | 60 (57) | 344 (64) | .040 |
| Single cord | 1 (0) | 0 (0) | 1 (0) | .620 |
| Double cord | 22 (5) | 3 (3) | 25 (5) | .319 |
| Donor CMV seropositivity, no. (%) | 236 (56) | 54 (51) | 290 (55) | .370 |
| ATG, no. (%) | 114 (26) | 39 (37) | 153 (28) | .035 |
| Post-cy, no. (%) | 188 (44) | 48 (45) | 236 (44) | .757 |
| Primary letermovir prophylaxis, no. (%) | 105 (24) | 18 (17) | 123 (23) | .105 |
| CS-CMVi, no. (%) | 178 (41) | 64 (60) | 242 (45) | <.001 |
| CMV end-organ disease, no. (%) | 54 (12) | 36 (34) | 90 (17) | <.001 |
| R/R CMV, no. (%) | 30 (7) | 17 (16) | 47 (9) | .003 |
| Primary graft failure, no. (%) | 5 (1) | 5 (5) | 10 (2) | .015 |
| Any GVHD, no. (%) | 219 (51) | 58 (55) | 277 (52) | .471 |
| Acute GVHD, no. (%) | 202 (47) | 56 (53) | 258 (48) | .271 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; CS-CMVi, clinically significant cytomegalovirus infection; GVHD, graft-versus-host disease; MDS, myelodysplastic syndrome; MF, myelofibrosis; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; N/A, not available; post-cy, posttransplant cyclophosphamide; R/R, refractory or resistant.
a P value is from the test comparing patients who survived at week 48 versus patients with nonrelapse mortality at week 48.
Table 6.
Independent Predictors of Nonrelapse Mortality Within 48 Weeks by Cox Regression Analysis
| Predictors | Adjusted HR | 95% CI | P Value |
|---|---|---|---|
| Age (every 1-y increase) | 1.02 | 1.001–1.03 | .033 |
| Sex | .024 | ||
| Male | Reference | ||
| Female | 1.58 | 1.06–2.35 | |
| Type of transplant | <.0001 | ||
| MRD | Reference | ||
| MUD/MMUD | 2.42 | 1.38–4.24 | |
| Haploidentical | 4.27 | 2.30–7.93 | |
| Cord | 1.70 | 0.48–5.99 | |
| Letermovir primary prophylaxis | 0.55 | 0.32–0.93 | .025 |
This analysis was performed for the patients without graft failure (n = 527). Data analysis was not performed for the patients with graft failure owing to the small number of patients in that group (n = 10).
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio. MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Figure 3.
Kaplan-Meier curves of nonrelapse survival of patients with and without primary letermovir prophylaxis. The Kaplan-Meier nonrelapse survival analysis was performed in the patients without graft failure (n = 527). The P value was .10 from a log-rank test comparing the patients with and without primary letermovir prophylaxis. However, after adjusting for the potential confounders, a multivariate Cox regression analysis showed a significant difference in nonrelapse survival between these 2 groups of patients (P = .025).
DISCUSSION
This study describes the largest single-center cohort of allogeneic HCT recipients receiving primary letermovir prophylaxis published to date. We report a significant decrease in the incidence of refractory or resistant CMV infections in patients receiving primary letermovir prophylaxis, a finding that has not been described yet. We also found significant decreases in CS-CMVi, CMV end-organ disease, peak CMV viral load, use of foscarnet, subsequent nephrotoxicity, and nonrelapse mortality at week 48 in patients receiving primary letermovir prophylaxis.
Refractory and resistant CMV infections carry significant morbidity and mortality in HCT recipients, with poor clinical outcomes despite aggressive therapy that can often be toxic [16]. In previous studies, the incidence of refractory CMV infections ranged between 19% and 29% [7, 12, 13], and resistant CMV infections ranged from 1.7% to 14.5% [7–10]. In our study, the group that did not receive letermovir prophylaxis had a 6% rate of refractory, 3% probable refractory, and 1% resistant CMV infections. These differences from the previously reported rates could in large part be explained by the adoption of standardized definitions [6] after the prior studies were conducted, as well as differences in the HCT population, type of HCT, time to immune reconstitution, development of GVHD, and prophylactic and therapeutic approaches to GVHD, all of which are established risk factors for CMV resistance [17]. Notably, all 3 cases of resistant CMV in our cohort, none of whom had received primary letermovir prophylaxis, had UL54 mutations, conferring resistance to foscarnet, consistent with our institutional practice of favoring foscarnet over ganciclovir, particularly in the immediate posttransplant period, owing to its lower risk of myelosuppression. This is in contrast with the literature reporting UL97 mutations more commonly than UL54 mutations [7, 11, 18, 19], which probably reflects different practices across various institutions.
Our study showed a decrease in the incidence of CS-CMVi with primary letermovir prophylaxis, from 53% to 17%, consistent with the results of the phase 3 clinical trial of primary letermovir prophylaxis (42% to 18%) [4] and additional retrospective studies (22%–69% to 0%–22%) [20–25]. The majority of the CS-CMVi occurred before day 100 in our cohort, in line with previous studies [26, 27]. Remarkably, the increase in CS-CMVi noted in the phase 3 clinical trial after letermovir discontinuation at day 100 [4] was not noted in our study, probably because 60% of our patients on primary letermovir prophylaxis received letermovir beyond day 100. Additionally, we demonstrated a significant decrease in CMV end-organ disease associated with primary letermovir prophylaxis (decreasing from 20% to 6%), which has been shown in 1 retrospective study of high-risk patients (no CMV disease in the primary letermovir prophylaxis group compared with 5% in the control group) [24]. Last, we describe a significant decrease in the peak CMV viral load in association with primary letermovir prophylaxis, a finding noted in 2 other retrospective studies [23, 24].
Analyzing the association between primary letermovir prophylaxis and mortality, our study showed numerically lower all-cause mortality and nonrelapse mortality through week 48. The association of letermovir with lower nonrelapse mortality at week 48 achieved statistical significance on Cox regression analysis. The relationship between CMV reactivation and mortality after HCT, independently of preemptive therapy, is well described [26, 28]. Our results are consistent with the phase 3 clinical trial of letermovir prophylaxis, demonstrating a significantly lower all-cause mortality at week 24 in the letermovir group (from 16% to 10%, P = .03) [4]. This was reproduced in a mortality analysis of the same trial focusing on the patients with undetectable CMV viral load at randomization, in which all-cause mortality was significantly lower in the letermovir arm at week 24; the study was not powered enough to detect a difference at week 48 [29]. One retrospective study evaluated nonrelapse mortality at day 200, with no significant difference between patients who received letermovir for prophylaxis and those who did not [23]. The lower nonrelapse mortality in the primary letermovir prophylaxis group in our study could be explained by the prevention of CS-CMVi and the need for preemptive therapy and its associated toxicities, the lower incidence of refractory or resistant CMV infections, and the lower CMV viral load in the letermovir group, similar to the mortality analysis of the letermovir phase 3 trial [28, 29].
Our study has multiple strengths. It is the largest real-life cohort of allogeneic HCT recipients who received primary letermovir prophylaxis published to date using standardized definitions for CMV outcomes, particularly CMV disease [15] and refractory or resistant CMV infections [6]. Furthermore, our cohort included all CMV-seropositive allogeneic HCT recipients, whereas most retrospective studies focused on high-risk HCT populations receiving letermovir. Nevertheless, our study has some limitations, owing to its retrospective nature and single-center design. CMV resistance testing was limited, particularly in the nonletermovir group. Finally, there was some heterogeneity in both groups of our cohort related to the GVHD prophylactic regimens and the types of HCT performed owing to changes in clinical practice over time.
In conclusion, our cohort study showed that primary letermovir prophylaxis in allogeneic HCT recipients effectively prevents refractory or resistant CMV infections and decreases nonrelapse mortality at week 48. Our study also confirms the findings of prior studies with significant reductions in CS-CMVi, CMV disease, and peak CMV viral loads.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions: R. F. C. designed the project. J. S., F. K., T. L. S., V. H., F. F., and S. A. collected the data. J. S. and Y. J. analyzed the data. R. C., E. S., K. R., and E. A. H. interpreted the data. J. S., F. K., Y. J., and R. F. C. wrote the manuscript. All the authors revised and approved the final version of the manuscript.
Acknowledgments. The manuscript was edited by Sarah Bronson, ELS, of the Research Medical Library at The University of Texas MD Anderson Cancer Center.
Financial support. This work was partly supported by the National Cancer Institute at the National Institutes of Health (grant number P30CA016672).
Potential conflicts of interest. K. R. reports licensing deals with Takeda and Affimed; she serves on the scientific advisory board of GemoAb and the data and safety monitoring board of Kiadis. R. F. C. reports research grants paid to his institution from Merck, Chimerix, Shire/Takeda, Novartis, Oxford Immunotec, Ansun Biopharma, Pulmotec, AiCuris, Xenex, Karius, Genentech, Janssen, and Viracor; he serves as a consultant for Merck, Chimerix, Clinigen, Genentech, Pulmotec, Ansun Biopharma, Janssen, Shire/Takeda, Partner Therapeutics, and Oxford Immunotec. E. A. H. reports a research grant paid to her institution from Merck. All other authors declare no competing financial interests. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Parody R, Martino R, Rovira M, et al. ; Infectious/Non-infectious Complications Subcommittee of the Grupo Español de Trasplante Hematopoyético (GETH) . Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 2006; 12:734–48. [DOI] [PubMed] [Google Scholar]
- 2. Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett 2014; 342:1–8. [DOI] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration. Approval Package for Letermovir. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209939Orig1s000,209940Orig1s000Approv.pdf. Accessed 27 October 2020.
- 4. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377:2433–44. [DOI] [PubMed] [Google Scholar]
- 5. Chemaly RF, Ullmann AJ, Stoelben S, et al. ; AIC246 Study Team . Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 2014; 370:1781–9. [DOI] [PubMed] [Google Scholar]
- 6. Chemaly RF, Chou S, Einsele H, et al. ; Resistant Definitions Working Group of the Cytomegalovirus Drug Development Forum . Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis 2019; 68:1420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 2001; 97:867–74. [DOI] [PubMed] [Google Scholar]
- 8. Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209:557–61. [DOI] [PubMed] [Google Scholar]
- 9. Hantz S, Garnier-Geoffroy F, Mazeron MC, et al. ; French CMV Resistance Survey Study Group . Drug-resistant cytomegalovirus in transplant recipients: a French cohort study. J Antimicrob Chemother 2010; 65:2628–40. [DOI] [PubMed] [Google Scholar]
- 10. Allice T, Busca A, Locatelli F, Falda M, Pittaluga F, Ghisetti V. Valganciclovir as pre-emptive therapy for cytomegalovirus infection post-allogenic stem cell transplantation: implications for the emergence of drug-resistant cytomegalovirus. J Antimicrob Chemother 2009; 63:600–8. [DOI] [PubMed] [Google Scholar]
- 11. Hakki M, Chou S. The biology of cytomegalovirus drug resistance. Curr Opin Infect Dis 2011; 24:605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almyroudis NG, Jakubowski A, Jaffe D, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2007; 9:286–94. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura R, Battiwalla M, Solomon S, et al. Persisting posttransplantation cytomegalovirus antigenemia correlates with poor lymphocyte proliferation to cytomegalovirus antigen and predicts for increased late relapse and treatment failure. Biol Blood Marrow Transplant 2004; 10:49–57. [DOI] [PubMed] [Google Scholar]
- 14. Tomblyn M, Chiller T, Einsele H, et al. ; Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention . Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum . Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 16. Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 2016; 100:e74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016; 128:2624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khawaja F, Batista MV, El Haddad L, Chemaly RF. Resistant or refractory cytomegalovirus infections after hematopoietic cell transplantation: diagnosis and management. Curr Opin Infect Dis 2019; 32:565–74. [DOI] [PubMed] [Google Scholar]
- 19. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant 2008; 22:162–70. [DOI] [PubMed] [Google Scholar]
- 20. Lin A, Flynn J, DeRespiris L, et al. Letermovir for prevention of cytomegalovirus reactivation in haploidentical and mismatched adult donor allogeneic hematopoietic cell transplantation with post-transplant cyclophosphamide for graft-versus-host disease prophylaxis. Transplant Cell Ther 2021; 27:85.e1–85.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Studer U, Khanna N, Leuzinger K, et al. Incidence of CMV replication and the role of letermovir primary/secondary prophylaxis in the early phase after allogeneic hematopoietic stem cell transplantation–a single centre study. Anticancer Res 2020; 40:5909–17. [DOI] [PubMed] [Google Scholar]
- 22. Sharma P, Gakhar N, MacDonald J, et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transplant 2020; 55:780–6. [DOI] [PubMed] [Google Scholar]
- 23. Anderson A, Raja M, Vazquez N, Morris M, Komanduri K, Camargo J. Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transplant 2020; 34:e13866. [DOI] [PubMed] [Google Scholar]
- 24. Johnsrud JJ, Nguyen IT, Domingo W, Narasimhan B, Efron B, Brown JW. Letermovir prophylaxis decreases burden of cytomegalovirus (CMV) in patients at high risk for CMV disease following hematopoietic cell transplant. Biol Blood Marrow Transplant 2020; 26:1963–70. [DOI] [PubMed] [Google Scholar]
- 25. Lin A, Maloy M, Su Y, et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: real-world experience. Transpl Infect Dis 2019; 21:e13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chemaly RF, El Haddad L, Winston DJ, et al. Cytomegalovirus (CMV) cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: the REACT Study. Clin Infect Dis 2020; 71:2365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Haddad L, Ariza-Heredia E, Shah DP, et al. The ability of a cytomegalovirus ELISPOT assay to predict outcome of low-level CMV reactivation in hematopoietic cell transplant recipients. J Infect Dis 2019; 219:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis 2020; 70:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.