Abstract
Background
In malaria-endemic areas, pregnant women and especially first-time mothers are more susceptible to Plasmodium falciparum. Malaria diagnosis is often missed during pregnancy, because many women with placental malaria remain asymptomatic or have submicroscopic parasitemia, masking the association between malaria and pregnancy outcomes. Severe maternal anemia and low birthweight deliveries are well-established sequelae, but few studies have confirmed the relationship between malaria infection and severe outcomes like perinatal mortality in high transmission zones.
Methods
Pregnant women of any gestational age enrolled at antenatal clinic into a longitudinal cohort study in Ouelessebougou, Mali, an area of high seasonal malaria transmission. Follow-up visits included scheduled and unscheduled visits throughout pregnancy. Blood smear microscopy and polymerase chain reaction (PCR) analysis were employed to detect both microscopic and submicroscopic infections, respectively. Intermittent preventative treatment in pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) was documented and prompt treatment regardless of symptoms given upon malaria diagnosis.
Results
Of the 1850 women followed through delivery, 72.6% of women received 2 or more IPTp-SP doses, 67.2% of women experienced at least 1 infection between enrollment up to and including delivery. Malaria infection increased the risks of stillbirth (adjusted hazard ratio [aHR] 3.87, 95% confidence interval [CI]: 1.18–12.71) and preterm delivery (aHR 2.41, 95% CI: 1.35–4.29) in primigravidae, and early neonatal death (death within 7 days) in secundigravidae and multigravidae (aHR 6.30, 95% CI: 1.41–28.15).
Conclusions
Malaria treatment after diagnosis, alongside IPTp-SP, is insufficient to prevent malaria-related stillbirth, early neonatal death and preterm delivery (PTD). Although IPTp-SP was beneficial in Mali during the study period, new tools are needed to improve pregnancy outcomes.
Clinical Trials Registration
Keywords: pregnancy malaria, stillbirth, early neonatal death, preterm delivery, intermittent preventative treatment in pregnancy
Pregnant women, particularly primigravidae, are more susceptible to Plasmodium falciparum. We examined associations between malaria infection and pregnancy outcomes. Pregnancy malaria increased the risk of stillbirth and preterm delivery in primigravidae and early neonatal death in later gravidities.
In malaria-endemic areas, pregnant women are more susceptible to malaria infection compared to their nonpregnant counterparts. The unique epidemiology of malaria in pregnancy (PM) is characterized by parity-dependent susceptibility: primigravidae are infected more frequently and with higher placental parasite densities than multigravidae (reviewed in [1]).
During pregnancy, P. falciparum parasites sequester in the placenta by binding to the receptor chondroitin sulfate A (CSA) expressed on the surface of the villous syncytiotrophoblast. With successive pregnancies, women develop specific antibodies that inhibit parasite adhesion to CSA and are associated with reduced prevalence of infection, reduced parasite densities, and improved pregnancy outcomes. The acquisition of specific immunity to placental parasites over successive pregnancy explains the increased susceptibility to malaria infection in primigravidae living in areas of stable malaria transmission [2, 3].
PM is associated with poor outcomes for both the mother and her baby. Severe maternal anemia and low birthweight deliveries (LBW) are well-established sequelae of PM, and both are associated with maternal and infant mortality. The 2019 World Health Organization (WHO) World Malaria Report estimates that 16% of LBW deliveries in sub-Saharan Africa are due to PM [4]. In a multivariate logistic regression model, maternal age and anemia were associated with increased risk of preterm delivery (PTD) in a cross-sectional study of women delivering in Cameroon [5], and in the Gambia, placental malaria was associated with increased odds of PTD [6]. In Malawi, PTD has been associated with malaria infection at first antenatal visit or at delivery, as well as young age, short stature, and maternal anemia [7]. In a large prospective study spanning 3 decades at the border between Thailand and Myanmar, where women were screened by microscopy every 1–2 weeks and treated for infection, P. falciparum and P. vivax infections were associated with PTD and small for gestational age (SGA) deliveries [8]. P. falciparum infection between gestational weeks 24–28 and 28–32 increased the odds of very early PTD (gestational week 28 to <32 weeks), and infections between gestational weeks 28–32 and 32–37 increased the odds of late PTD (between gestational week 32 and <37), regardless of symptoms [8].
Multiple studies that examined the relationship between PM and poor birth outcomes failed to find significant associations with stillbirth [9]. However, a meta-analysis based on these studies conducted in areas with varying malaria transmission levels related malaria infection with stillbirth; odds were significantly higher in women with P. falciparum malaria infection at delivery, detected in peripheral or placental blood (OR 1.81 and 1.95, respectively), and in women infected and treated for P. falciparum during pregnancy (OR 1.48) [9].
In malaria-endemic areas, a high proportion of women with PM are asymptomatic, with parasites undetectable by peripheral blood smears. Because diagnosis is based on the presence of parasites in peripheral blood and/or clinical symptoms, women with placental parasitemia often remain undiagnosed. This can mask the association between PM and poor outcomes [10, 11], particularly in cases of miscarriage, which often do not allow placental examination to determine placental infection. In the present study, we assess the relationship between malaria infection detected by blood smear microscopy or polymerase chain reaction (PCR), during pregnancy and at delivery, with pregnancy loss (miscarriage, stillbirth, and early neonatal death), PTD, and SGA. Because primigravidae are infected more frequently and with higher parasite burden than multigravidae, we evaluated the impact of malaria infection on pregnancy outcomes in primigravidae versus secundigravidae and multigravidae.
METHODS
Ethical Approval
A longitudinal cohort study of mother-infant pairs was conducted in Ouélessébougou, Mali, located 80 km south of Bamako, an area of intense seasonal malaria transmission. Study protocol was approved by the institutional review boards at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Ethics Committee of the Faculty of Medicine, Pharmacy, and Dentistry, University of Bamako. Written informed consent was obtained from the study participants or the parents/guardians of pregnant adolescents after receiving a study explanation form and oral explanation from a study clinician in their native language. The protocol is registered at Clinicaltrials.gov under identifier NCT01168271.
Study Population and Clinical Procedures
Pregnant women in Ouélessébougou aged 15–45 years without clinical evidence of chronic or debilitating illness regardless of gestational age were invited to enroll into the study between November 2010 and January 2014. Upon enrollment, women underwent clinical examination including obstetrical examination, a thorough review of medical history including current and previous pregnancies, and assessment of socioeconomic status.
Follow-up included monthly scheduled and unscheduled visits during any time of reported illness. Blood smears and samples for PCR were collected at enrollment, at gestational week 30–32, at delivery and during any illness with symptoms suggesting malaria. Number of visits with and without blood smear are described in Supplementary Table 1. Nearly all malaria infections (98.8%) detected by blood smear microscopy were treated with quinine or with artemether-based therapy according to national guidelines regardless of symptoms (Table 1). Intermittent preventive treatment (IPTp) with sulfadoxine-pyrimethamine (SP) was administered depending on recruitment trimester, which was recorded along with IPTp usage history prior to enrollment (0–3 doses) and any antimalarial drugs to treat intercurrent infections. When antimalarial drugs were given to treat infection, IPTp-SP dosing was delayed until the next scheduled administration. Gestational age was determined by ultrasound examination (Siui CTS-700+), last menstrual period (LMP), and fundal height [12] in 96.8%, 2.8%, and 0.4% of women, respectively. Malaria infection was determined by thick blood smear. Other clinical tests included complete blood count and serologic tests of toxoplasma and syphilis.
Table 1.
Study Population Characteristics
| Enrollment Trimester (%) | Gestational Age at Delivery (weeks)b Mean (range) | At least 2 Doses IPTp-SP n (%) | ITN % | Malaria Treatmentc Mean (range) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | PM statusa | n (%) | Gestational Age at Enrollment (weeks) Mean (range) | 1st | 2nd | 3rd | ||||
| Primigravid | PM− | 116 | 20.6 (7.1–36.0) | 20.7 | 57.8 | 21.5 | 38.9 (32.0–41.6) | 83 (72.2) | 50.0 | |
| PM+ | 318 (73.3%) | 20.8 (5.1–36.1) | 13.5 | 72.0 | 14.5 | 38.7 (29.6–42.0) | 214 (67.1) | 39.6 | 1.3 (0–5) | |
| Secundigravid | PM− | 118 | 20.5 (5.1–36.0) | 21.2 | 59.3 | 19.5 | 39.0 (31.6–42.3) | 91 (77.7) | 53.4 | |
| PM+ | 241 (67.1%) | 20.6 (6.3–36.0) | 12.9 | 72.2 | 14.9 | 39.1 (32.1–42.7) | 175 (72.3) | 52.3 | 1.2 (0–4) | |
| Multigravid | PM− | 373 | 20.6 (5.0–36.0) | 16.6 | 66.8 | 16.6 | 39.6 (32.4–43.0) | 279 (75.0) | 52.5 | |
| PM+ | 684 (64.7%) | 20.5 (5.6–39.0) | 15.8 | 69.2 | 15.1 | 39.5 (30.3–42.7) | 498 (72.7) | 55.7 | 1.1 (0–3) | |
Abbreviations: IPTp-SP, intermittent preventative treatment in pregnancy with sulfadoxine-pyrimethamine; ITN, insecticide treated net; PM, malaria in pregnancy.
a PM−: no infections; PM+ at least 1 infection detected by blood smear microscopy or polymerase chain reaction (PCR).
b Gestational age at delivery includes viable singleton births.
cMean and range of treatment rounds with antimalarial drugs.
Clinical Definitions
PM was defined as the presence of any parasite detected by a peripheral or placental blood smear or infection detected by nested PCR. Symptomatic malaria was defined as fever (temperature >37.5°C) in the presence of any parasitemia on blood smear. Miscarriage was defined as pregnancy ending before gestational week 28, stillbirth as a delivery of a nonviable baby at a gestational age of ≥28 weeks, and early neonatal death as death occurring in the first week of life. Preterm delivery was defined as birth prior to gestational age of 37 weeks. Small for gestational age (SGA) was defined according to INTERGROWTH-21 standards [13].
Laboratory Methods
Blood Smear
Blood films were stained with 10% Giemsa solution and examined by microscopy. A blood smear was deemed negative if no parasite was detected after examination of at least 100 high power fields in the thick smear.
DNA Extraction and Polymerase Chain Reaction
DNA was extracted from frozen blood sample with QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer’s instructions. PCR to detect Plasmodium parasites was performed as previously described targeting small subunit ribosomal RNA (ssrRNA) [14]. PCR was performed on frozen blood samples with negative blood smears collected at fixed time points: enrollment, gestational week 30–32, delivery, and 17% of blood smear positive samples.
Statistical Analyses
Data were collected on standardized forms, then manually entered and verified using DataFax (version 4.2, Clinical DataFax Systems, Inc., Hamilton, Ontario, Canada). The observational data were formatted into an analytical data set in a way to reduce bias. The formatting involved making cohort risk-sets in the following manner. First, the time scale was made into calendar time, where time = 1 is the first day of the study. Second, cohort risk-sets were made for each miscarriage, stillbirth, early neonatal death, and preterm delivery event. Populating each cohort risk-set starts with the individual who experienced the event. The calendar time of the event was identified, and then a 14-day window (±7 days around the event calendar day) was applied around the calendar time of the delivery date. Anyone with a visit recorded within that 14-day window was then included in the cohort risk-set. Each risk-set was given a unique event-number. In this fashion, we obtained temporally focused infection information. Once this is assimilated, further formatting is undertaken to reflect the competing risks feature of this dataset. We stacked all 385 risk-sets (Supplementary Table 2) into one analytic data set and then fitted a Lunn & McNeil Method B model [15]. The model was adjusted for factor associated with malaria infection: insecticide treated net (ITN) usage, IPTp administered within 3 weeks of the visit. Statistical significance was defined as P < .05.
RESULTS
Study Population
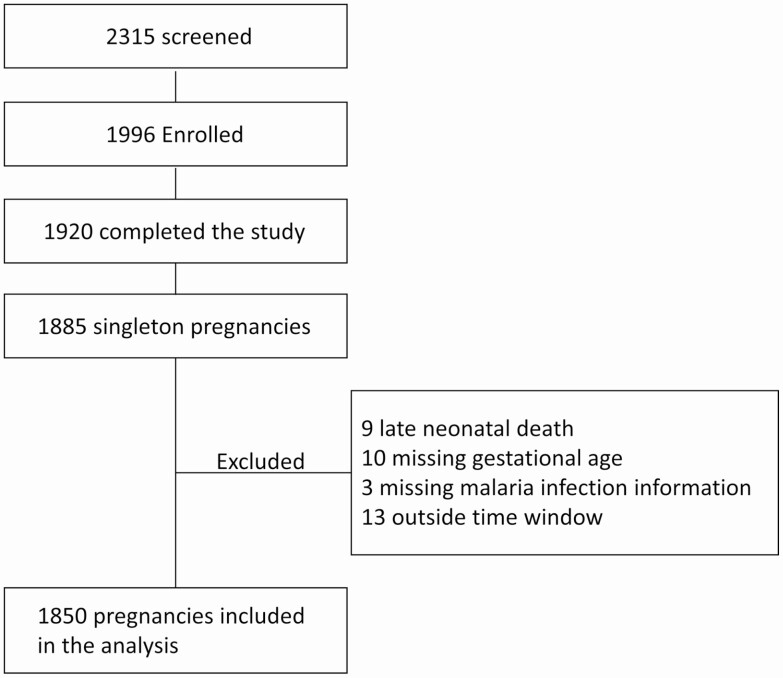
In total, 1996 pregnant women were enrolled into a longitudinal study of mothers and their newborn children in Ouélessébougou, Mali. Of the 1920 women who completed the study, 1885 had singleton pregnancy; 1850 were included in the analysis (Figure 1). Most women were recruited during the second trimester of pregnancy (Table 1). Although percentage of women enrolled in first trimester was higher among uninfected women, mean and median gestational age at enrollment was similar between infected and uninfected women.
Figure 1.
Flow diagram of study participants included in the analysis
Most women experienced at least 1 malaria infection detected by blood smear or PCR analysis of peripheral blood collected during pregnancy and placental blood at delivery (Table 1). Overall, 32.9%, 41.6%, and 25.6% of women had 0, 1, and ≥2 infections, respectively, during antenatal or delivery assessments. At enrollment, 53.8% were BS−/PCR−, 27.7% BS+ and 17.6% BS−/PCR+ (Supplementary Table 3).
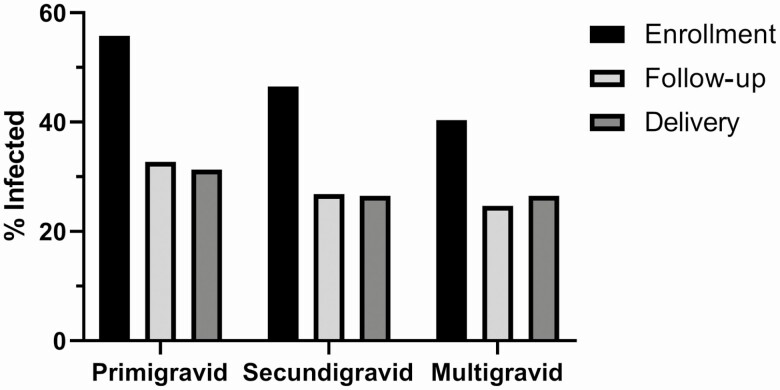
The highest proportion of infections were identified at enrollment in all gravidities, and the proportions were significantly higher at enrollment for primigravidae (P < .0001) secundigravidae (P < .0001), and multigravidae (P < .0001) (Figure 2). Similar reduction in infection rate during follow-up visits or at delivery was observed in women that received or did not receive antimalarial treatment at enrollment, possibly reflecting the follow-up care in the study (Supplementary Figure 1).
Figure 2.
Percentage of malaria-infected women at enrollment, at follow-up visits and at delivery. After enrollment, women were followed up to and including delivery. Infection was determined by blood smear microscopy or PCR in peripheral blood and placental blood at delivery. Numbers that follow in parentheses indicate number of women with infection information at enrollment, at follow-up visits and at delivery (1850, 1612, 1846). Abbreviation: PCR, polymerase chain reaction.
More than 70% of women received at least 2 doses of IPTp. The percentage of infected primigravidae that received at least 2 doses of IPTp was slightly lower due to other anti-malarial treatments given for active infections (Table 1). Overall, 54.6% primigravidae, 47.9% secundigravidae, and 44.2% multigravidae received at least 1 round of antimalarial drug treatment. The percentage of women with severe anemia (hemoglobin <7) at enrollment was low (1.2%), and anemia was not included as a covariate in the analysis relating malaria infection with pregnancy outcomes.
Among primigravid women, 3.5%, 3.2%, 2.8%, 11.5%, and 14.2% of pregnancies resulted in miscarriage, stillbirth, early neonatal death, PTD, and SGA, respectively. The respective figures among multigravid women were 2.6%, 1.9%, 0.8%, 3.0%, and 8.8%, and among secundigravid women, 2.2%, 1.4%, 0.8%, 6.7%, and 8.5% (Table 2).
Table 2.
Pregnancy Outcomes Stratified by Maternal Gravidity
| Group | Outcome | N (%) | Infected at Enrollment, FU, Deliveryc by BSd (n) | Infected at Enrollment, FU, Deliveryd by PCRe (n) | Infected Enrollment, FU, Delivery by BS or PCR (n) |
|---|---|---|---|---|---|
| Primigravid | Miscarriage a | 15 (3.5) | 4, 1, 2 | 3, 1, 3 | 7, 2, 5 |
| Stillbirtha | 14 (3.2) | 3, 4, 4 | 2, 0, 3 | 5, 4, 7 | |
| Early neonatal deatha | 12 (2.8) | 7, 2, 1 | 2, 1, 1 | 9, 3, 2 | |
| Preterm deliveryb | 45 (11.5) | 19, 9, 18 | 6, 1, 2 | 25, 10, 20 | |
| Small for gestational ageb | 56 (14.2) | 26, 21, 10 | 11, 6, 13 | 37, 27, 23 | |
| Secundigravid | Miscarriagea | 8 (2.2) | 1, 2, 3 | 2, 0, 0 | 3, 2, 3 |
| Stillbirtha | 5 (1.4) | 1, 1, 0 | 0, 0, 0 | 1, 1, 0 | |
| Early neonatal deatha | 3 (0.8) | 0, 0, 2 | 1, 0, 0 | 1, 0, 2 | |
| Preterm deliveryb | 23 (6.7) | 12, 8, 5 | 3, 3, 1 | 15, 11, 6 | |
| Small for gestational ageb | 29 (8.5) | 13, 7, 8 | 4, 3, 5 | 17, 10, 13 | |
| Multigravid | Miscarriagea | 28 (2.6) | 5, 0, 1 | 3, 0, 4 | 8, 0, 5 |
| Stillbirtha | 20 (1.9) | 7, 1, 1 | 1, 1, 3 | 8, 2, 4 | |
| Early neonatal deatha | 8 (0.8) | 1, 3, 1 | 2, 0, 2 | 3, 3, 3 | |
| Preterm deliveryb | 30 (3.0) | 4, 3, 3 | 9, 0, 3 | 13, 3, 6 | |
| Small for gestational ageb | 89 (8.8) | 13, 16, 15 | 27, 3, 3 | 40, 19, 18 |
Abbreviations: BS, blood smear; FU, follow-up; PCR, polymerase chain reaction.
a Percent of all pregnancies.
bPercent of viable deliveries.
cBased on peripheral and placental blood results.
dBlood smear microscopy.
ePositive by PCR of samples with a negative blood smear microscopy.
Malaria Infection Predicts Poor Pregnancy Outcomes
A competing risk model was fitted to examine whether P. falciparum infection during pregnancy predicts miscarriage, stillbirth, early neonatal death, PTD, and SGA. The model was adjusted for ITN usage, chemoprophylaxis (IPTp-SP). Hemoglobin type (AC and AS compared to AA) did not modify hazard ratios and was not included in the final model. To relate gravidity-specific effects, models were stratified by gravidity. In the model adjusted for gravidity, there were no differences between secundigravidae and multigravidae; therefore, the analysis is presented for primigravidae and for secundigravidae/multigravidae (Table 3).
Table 3.
Competing Risk Analysis on the Effect of Malaria Infection on Pregnancy Outcome
| Group | Risk Factor | Outcome | Crude HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Primigravid | Infection | Miscarriage | .75 (.28–1.95) | .5 | .75 (.27–2.06) | .6 |
| Stillbirth | 3.80 (1.06–13.67) | .04 | 3.87 (1.18–12.71) | .03 | ||
| Early neonatal death | .75 (.17–3.28) | .7 | .69 (.16–2.97) | .6 | ||
| PTD | 2.35 (1.34–4.11) | .003 | 2.41 (1.35–4.29) | .003 | ||
| SGA | 1.53 (.83–2.81) | .2 | 1.50 (.83–2.72) | .2 | ||
| ITN use | 1.18 (.84–1.67) | .3 | ||||
| IPTp-SP | .27 (.15–.50) | <.0001 | ||||
| Secundigravid and multigravid | Infection | Miscarriage | .81 (.38–1.73) | .6 | .78 (.36–1.67) | .5 |
| Stillbirth | .71 .23–2.14) | .5 | .68 (.22–2.08) | .5 | ||
| Early neonatal death | 5.86 (1.38–24.85) | .02 | 6.30 (1.41–28.15) | .02 | ||
| PTD | 1.70 (.88–3.30) | .1 | 1.72 (.86–3.41) | .1 | ||
| SGA | 1.38 (.89–2.14) | .2 | 1.38 (.89–2.15) | .2 | ||
| ITN use | .87 (.65–1.17) | .4 | ||||
| IPTp-SP | .12 (.06–.22) | <.0001 |
Lunn & McNeil competing risks model was used to assess the effect of infection on pregnancy outcomes. Adjusted HR: model was adjusted for IPTp and ITN usage. Abbreviations: CI, confidence interval; GA, gestational age; HR, hazard ratio; IPTp-SP, intermittent preventative treatment in pregnancy with sulfadoxine-pyrimethamine; ITN, insecticide-treated net; PTD, preterm delivery; SGA, small for gestational age.
Malaria infection predicted increased risk of stillbirth (adjusted hazard ratio [aHR] 3.87, P = .03) and PTD (aHR 2.41, P = .003) in primigravidae (Table 3). Among secundigravidae and multigravidae, PM increased the risk of early neonatal death (aHR 6.30, P = .02). Infection increased the risk of SGA in both primigravidae and other gravidae, but these relationships were not statistically significant. IPTp-SP reduced the risk of poor outcomes by 73% in primigravidae and 88.3% in other gravidae during the 3 weeks after administration; 17% of patent infections were symptomatic. Symptomatic infection as a covariate in the model did not modify the HR of adverse pregnancy outcomes and was not included in the final model. Other factors (eg, advanced human immunodeficiency virus [HIV] disease and syphilis) were ruled out as contributors to fetal loss, because no women were infected with either pathogen. Hypertensive disorders in pregnancy (gestational hypertension, preeclampsia, and eclampsia) that increase the risk of poor pregnancy outcomes were uncommon (22 women) and were not included in the model.
DISCUSSION
PM is associated with poor outcomes for both mothers and their children. According to the WHO 2019 World Malaria Report, prevalence is currently highest in West and Central Africa (~35% of infected pregnant women) [4]. In this observational study of pregnant women and their offspring, the highest rate of infection was observed at enrollment, and 67.2% of women experienced at least 1 infection during the study. We evaluated the impact of PM on miscarriage, stillbirth, early neonatal death, PTD, and SGA. In competing risks analysis adjusted for factors that may influence pregnancy outcomes, such as malaria prevention (eg, IPTp-SP and ITN), malaria infection predicted increased risk of stillbirth and PTD among primigravidae and of early neonatal death among secundigravidae and multigravidae. Notably, the increased risk of stillbirth, PTD and early neonatal death was observed in a longitudinal cohort of women who received antimalarial drugs to treat both asymptomatic and symptomatic infections, in addition to chemoprophylaxis (IPTp-SP) delivered through routine antenatal care.
In high endemic areas, women may be infected with parasites sequestering in the placenta without detectable parasites on their peripheral blood smear. Most patent infections were afebrile in this cohort, as we previously reported for a pregnancy registry in this area [16]. The strengths of this study include the longitudinal follow-up of women including routine, unscheduled, and delivery visits that captured malaria infection episodes, the use of both blood smear microscopy and PCR to identify infection, and competing risks analyses of poor outcomes stratified by gravidity. These features enabled us to estimate the impact of malaria infection at any time during pregnancy on birth outcomes.
In nonendemic countries, the miscarriage rate is high during the first weeks of gestation and substantially reduced by the end of the first trimester [17]; most women in our study area attend their first antenatal clinic visit after first trimester. Factors that increased miscarriage risk in this cohort include gravidity (primigravidae and multigravidae with >4 pregnancies) [18] and maternal age (<20 and >35 years old) [18, 19]. In a study conducted on the Thai-Myanmar border (a low malaria transmission area), first-trimester infections with P. falciparum or P. vivax increased the odds of miscarriage 2.7 (95% CI: 2.1–3.4) and 3.1 (95% CI: 2.4–3.9), respectively [20]. It is unknown whether malaria infection during the first trimester has a similar impact on miscarriage in high transmission areas (such as in sub-Saharan Africa). In the present study, the majority of women enrolled during their second trimester and the delayed antenatal clinic presentation may have limited our ability to find a significant association between early malaria infection and miscarriage.
Multiple factors contribute to stillbirth and neonatal death, like hypertensive disorders in pregnancy, maternal body-mass-index, and systemic infections [21]. Globally, the estimated population attributable fraction of malaria is 8.2%, and in sub-Saharan Africa, the estimate is 19.7% [22]. A previous study reported a significantly increased risk of stillbirth in P. falciparum-infected pregnant women living in a low transmission area at the Thai-Myanmar border [23]. Furthermore, P. falciparum infection, especially in the third trimester, significantly increased antepartum stillbirth but was not associated with intrapartum stillbirth [23]. In the current study, information to classify stillbirth as antepartum or intrapartum was not collected; therefore, all stillbirth cases were included. Previous studies associated PM with reduced fetal growth; in a low malaria transmission zone, each infection increased the odd of SGA by 1.13 [21]. Here malaria infection increased the risk of SGA in all gravidity groups, but the relationships were not significant. IPTp-SP within 3 weeks of a visit significantly reduced the risk of poor pregnancy outcomes, supporting monthly IPTp in areas where SP is still efficacious.
ITN usage had no effect on pregnancy outcomes in our analyses. Roughly 50% of women in our cohort reported ITN usage, and this practice had recently increased in pregnant women [16]. Because chemoprevention with SP is not recommended in first trimester, women should be encouraged to use good quality ITN starting early in pregnancy to delay time to first infection [24].
In primigravid women who are not immune to placental parasites, parasite sequestration in the placenta is associated with inflammatory cell infiltrates and increased pro-inflammatory cytokines such as tumor necrosis factor (TNF) and interferon (IFN), and chemokines such as CXCL9 and CXCL10 [25]. In a previous analysis of inflammatory responses to malaria infection in a subset of this population, we reported that high peripheral blood CXCL9 levels during pregnancy predicted increased risk of pregnancy loss and PTD. At delivery, high interleukin (IL)-1β levels were associated with pregnancy loss and PTD, and high IL-10 levels with increased pregnancy loss [25]. Although women in our study were treated for malaria regardless of symptoms and most received at least 2 doses of IPTp-SP, these results suggest that treatment of microscopic infections alongside IPTp-SP is insufficient to prevent inflammatory immune responses prior to diagnosis and treatment, resulting in stillbirth and PTD. This is the first report that in secundigravidae and multigravidae, PM was associated with early neonatal death; the mechanisms associated with this outcome remain to be studied.
In summary, malaria infection in primigravid women significantly increased the risks of stillbirth and PTD in a region of Mali with intense seasonal transmission of malaria. In secundigravid and multigravid women, malaria infection increased the risk of early neonatal death. These data were collected during an observational longitudinal study in which women were actively and passively screened for parasitemia, treated with antimalarial drugs for any infection detected by microscopy, and the majority received at least 2 doses of IPTp-SP. This highlights the need for additional measures to protect pregnant women, such as a PM vaccine that can confers protection throughout pregnancy, to reduce the heavy disease burden associated with PM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Percentage of malaria-infected women at follow up visits and at delivery stratified by treatment type at enrollment. A. no treatment; B. IPTp-SP: C. Artemether-based (Art) treatment or Artemether-based and IPTp-SP (Art + SP); D. Quinine (Q) or Quinine and IPTp-SP (Q + SP).
Notes
Acknowledgments. We thank the women in Ouélessébougou, Mali, for participation in the study. J. Patrick Gorres assisted in editing this article, Rathy Mohan managed the clinical database, and staff at the community health center supported follow-up of study participants.
Financial support. This work was funded by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 2. Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature 1998; 395:851–2. [DOI] [PubMed] [Google Scholar]
- 3. Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun 2003; 71:6620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. World malaria report. Available at: https://www.who.int/publications-detail/world-malaria-report-2019. Accessed December 2020.
- 5. Tako EA, Zhou A, Lohoue J, Leke R, Taylor DW, Leke RF. Risk factors for placental malaria and its effect on pregnancy outcome in Yaounde, Cameroon. Am J Trop Med Hyg 2005; 72:236–42. [PubMed] [Google Scholar]
- 6. Okoko BJ, Ota MO, Yamuah LK, et al. Influence of placental malaria infection on foetal outcome in the Gambia: twenty years after Ian Mcgregor. J Health Popul Nutr 2002; 20:4–11. [PubMed] [Google Scholar]
- 7. Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiol Infect 2006; 134:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore KA, Simpson JA, Wiladphaingern J, et al. Influence of the number and timing of malaria episodes during pregnancy on prematurity and small-for-gestational-age in an area of low transmission. BMC Med 2017; 15:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health 2017; 5:e1101–12. [DOI] [PubMed] [Google Scholar]
- 10. Fried M, Muehlenbachs A, Duffy PE. Diagnosing malaria in pregnancy: an update. Expert Rev Anti Infect Ther 2012; 10:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bardají A, Sigauque B, Bruni L, et al. Clinical malaria in African pregnant women. Malar J 2008; 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. White LJ, Lee SJ, Stepniewska K, et al. Estimation of gestational age from fundal height: a solution for resource-poor settings. J R Soc Interface 2012; 9:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384:857–68. [DOI] [PubMed] [Google Scholar]
- 14. Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 1993; 58:283–92. [DOI] [PubMed] [Google Scholar]
- 15. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995; 51:524–32. [PubMed] [Google Scholar]
- 16. Andemel N, Gaoussou S, Barry A, et al. Adverse pregnancy outcomes among women presenting at antenatal clinics in Ouélessébougou, Mali. Reprod Health 2020; 17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol 2012; 94:417–23. [DOI] [PubMed] [Google Scholar]
- 18. Dellicour S, Aol G, Ouma P, et al. Weekly miscarriage rates in a community-based prospective cohort study in rural western Kenya. BMJ Open 2016; 6:e011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magnus MC, Karlstad Ø, Parr CL, et al. Maternal history of miscarriages and measures of fertility in relation to childhood asthma. Thorax 2019; 74:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGready R, Lee SJ, Wiladphaingern J, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis 2012; 12:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito M, Briand V, Min AM, McGready R. Deleterious effects of malaria in pregnancy on the developing fetus: a review on prevention and treatment with antimalarial drugs. Lancet Child Adolesc Health 2020; 4:761–74. [DOI] [PubMed] [Google Scholar]
- 22. Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016; 387:587–603. [DOI] [PubMed] [Google Scholar]
- 23. Moore KA, Fowkes FJI, Wiladphaingern J, et al. Mediation of the effect of malaria in pregnancy on stillbirth and neonatal death in an area of low transmission: observational data analysis. BMC Med 2017; 15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hounkonnou C, Djènontin A, Egbinola S, et al. Impact of the use and efficacy of long lasting insecticidal net on malaria infection during the first trimester of pregnancy: a pre-conceptional cohort study in southern Benin. BMC Public Health 2018; 18:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fried M, Kurtis JD, Swihart B, et al. Systemic inflammatory response to malaria during pregnancy is associated with pregnancy loss and preterm delivery. Clin Infect Dis 2017; 65:1729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.