Abstract
When 70% of antibiotic users took a 3-strain Lactobacillus probiotic preparation the hospital-wide rate of healthcare-associated Clostridioides difficile infection improved significantly. The incidence of C. difficile infection for those taking the probiotic along with multiple antibiotics or a single high-risk antibiotic was decreased by at least half.
Keywords: Clostridium difficile infection, Primary prevention, Lactobacillus, Probiotic, CL1285
(See the Major Article by Heil et al on pages 1330–7.)
Antibiotics are a mainstay of hospital medicine but each exposure causes changes to the patient’s intestinal microbiota [1]. The use of multiple antibiotics compounds the risk of Clostridioides (Clostridium) difficile infection (CDI) [2], which can manifest as disabling diarrhea and be life threatening [3]. The majority of efforts undertaken by hospitals to avoid CDI focus on 2 broad strategies: minimizing exposure to pathogens (with environmental cleaning and patient isolation) and, minimizing susceptibility to infection (with fewer concurrent antibiotics or high-risk antibiotics) [3, 4]. Probiotics, formulations comprising live microorganisms, offer another complementary approach and aim to improve a patient’s defenses to CDI [5].
Pierre-Le Gardeur Hospital, in a suburb of Montreal, Quebec, Canada, administers a 3-strain Lactobacillus probiotic to all adult inpatients taking antibiotics, as part of their successful strategy to lower the rate of healthcare-associated CDI (HA-CDI) [6, 7]. When this hospital merged administratively with its neighbor, the Centre Hospitalier Régional de Lanaudière, a 335-bed community hospital with endemic CDI, new infection prevention practices were instituted, including the probiotic prophylaxis.
METHODS
Study Setting
Starting in 2016, quinolone antibiotic use was greatly restricted and azithromycin was replaced with doxycycline as the principal treatment for community-acquired pneumonia. One year later, on 16 October 2017, a clinical order set for the 3-strain probiotic preparation was implemented hospital wide, including in the palliative and intensive care units. Adult inpatients prescribed ≥2 days of antibiotics were flagged to receive the probiotic daily within the first 24 hours. Eligible adults took 1 capsule (adults aged ≤49 years) or 2 capsules (adult aged ≥50 years) daily of a probiotic including Lactobacillus acidophilus CL1285, Lacticaseibacillus (Lactobacillus) casei LBC80R, and Lacticaseibacillus (Lactobacillus) rhamnosus CLR2 (Bio-K+ 50 Billion [50 billion colony-forming units per capsule]; Bio-K Plus International) throughout their antibiotic treatment course and for 5 days after treatment ended [6, 8].
Outcome Measures and Analysis
Electronic pharmacy records were collected for all antibiotic or probiotic prescriptions to adult inpatients treated at the hospital from 16 October 2016 through 31 March 2019. Patients with ≥3 loose stools per day and exhibiting signs of possible CDI provided stool samples to the hospital’s microbiology laboratory for C. difficile toxin A and toxin B testing with enzyme immunoassay. HA-CDI cases were reported to the provincial surveillance program if they occurred >48 hours after hospital admission or within 4 weeks after discharge from the hospital [9]. Recurrences occurred when clinical symptoms of CDI emerged within 8 weeks after hospital discharge for a HA-CDI case. The number of cases and patient-days were collected in 4-week periods, including 7 periods for spring through summer (periods 1-7) and 6 for autumn through winter (periods 8-13).
The use of antibiotic classes that historically pose a high risk for CDI was collected; these classes include quinolones (ciprofloxacin, levofloxacin, and moxifloxacin), third-generation cephalosporins (cefixime, cefotaxime, ceftazidime, and ceftriaxone), and carbapenems (ertapenem, imipenem, and meropenem). Statistical comparisons between groups were performed using discrete data in R statistical software (version 3.6.3; epitools, fmsb) though some results were also reported as ratios or percentages. The presentation of results follows the SQUIRE 2.0 publication guidelines [10].
RESULTS
Patient Characteristics
Electronic pharmacy records were identified for 13 922 adult inpatient visits at which antibiotics were prescribed, representing 4383 unique patients in the 12-month observation period and 6079 patients in the 18-month intervention period. Among those prescribed antibiotics, the proportions of patients aged ≥70 years were consistent during the observation (2142 patients) and intervention (2995 patients) periods (both 49%). There were significant changes to antibiotic prescribing practices. Quinolones were prescribed less frequently during the intervention than during the observation period (odds ratio [OR], 0.59, Table 1). The opposite trend was observed for third-generation cephalosporins (OR, 2.1). The number of antibiotics prescribed per visit was higher during the intervention (1.98 vs. 1.94; P = .009). There was a greater proportion of patients taking an antibiotic that historically poses a high risk for development of CDI during the intervention (OR, 1.2).
Table 1.
Frequency of Exposure to Multiple antibiotics, a High-Risk Antibiotic, and the Probiotic Among Eligible Inpatients During the Observation and Intervention periods and the Incidence of Healthcare-Associated Clostridioides difficile Infection
| Intervention | ||||||
|---|---|---|---|---|---|---|
| Antibiotic Types During Hospitalization | Observation (All) |
Intervention (All) |
OR (95% CI) | No Probiotic | Probiotic | OR (95% CI) |
| All patients using antibiotics, no. | 5666 | 8266 | 2490 | 5776 | ||
| All HA-CDI cases, no. (%) | 84 (1.5) | 73 (0.9) | 0.59 (.43–.81)a | 28 (1.1) | 45 (0.8) | 0.69 (.43– 1.11) |
| 2+ Types | ||||||
| Patients, no. (%) | 3294 (58) | 4965 (60) | 1.08 (1.01–1.16)a | 1100 (44) | 3865 (67) | 2.6 (2.3–2.8)a |
| HA-CDI cases, no. (%) | 73 (2.2) | 61 (1.2) | 0.55 (.39–.77)a | 22 (2.0) | 39 (1.0) | 0.50 (.29–.85)a |
| 3+ Types | ||||||
| Patients, no. (%) | 1362 (24) | 2190 (26) | 1.14 (1.05–1.23)a | 269 (11) | 1921 (26) | 4.1 (3.6–4.7)a |
| HA-CDI cases, no. (%) | 52 (3.8) | 28 (1.3) | 0.33 (.21–.52)a | 8 (3.0) | 20 (1.0) | 0.34 (.15–.79)a |
| 4+ Types | ||||||
| Patients, no. | 419 (7.4) | 673 (8.1) | 1.11 (.98–1.26) | 53 (2.1) | 620 (11) | 5.5 (4.2–7.3)a |
| HA-CDI cases, no. (%) | 32 (7.6) | 11 (1.6) | 0.20 (.10–.40)a | 4 (7.5) | 7 (1.1) | 0.14 (.04–.49)a |
| Any quinolone | ||||||
| Patients, no. (%) | 1334 (24) | 1274 (15) | 0.59 (.54–.64)a | 281 (11) | 993 (17) | 1.6 (1.4–1.9)a |
| HA-CDI cases, no. (%) | 33 (2.5) | 13 (1.0) | 0.41 (.21–.78)a | 5 (1.8) | 8 (0.8) | 0.45 (.15–1.38) |
| Any 3rd-generation cephalosporin | ||||||
| Patients, no. (%) | 849 (15) | 2211 (27) | 2.1 (1.9–2.3)a |
372 (15) |
1839 (32) |
2.7 (2.4–3.0)a |
| HA-CDI cases, no. (%) | 22 (2.6) | 25 (1.1) | 0.43 (.24– 0.77)a |
7 (1.9) | 18 (1.0) | 0.52 (.21–1.24) |
| Any carbapenem | ||||||
| Patients, no. (%) | 336 (6) | 464 (6) | 0.94 (.82–1.09) | 95 (4) | 369 (6) | 1.7 (1.4–2.2)a |
| HA-CDI cases, no. (%) | 17 (5.1) | 8 (1.7) | 0.33 (.14–.77)a | 3 (3.2) | 5 (1.4) | 0.42 (.10–1.79) |
| Any high-risk antibiotic | ||||||
| Patients, no. (%) | 2277 (40) | 3659 (44) | 1.2 (1.1–1.3)a | 720 (29) | 2939 (51) | 3.4 (3.1–3.8)a |
| HA-CDI cases, no. (%) | 57 (2.5) | 39 (1.1) | 0.42 (.28–.63)a | 13 (1.8) | 26 (0.9) | 0.49 (.28–.63)a |
Abbreviations: CI, confidence interval; HA-CDI, healthcare-associated Clostridioides difficile infection; OR, odds ratio.
a Significant at P ≤ .05. Rates and incidences were compared using Fisher exact tests in standard or modified 2-by-2 contingency tables.
Primary and Recurrent CDI
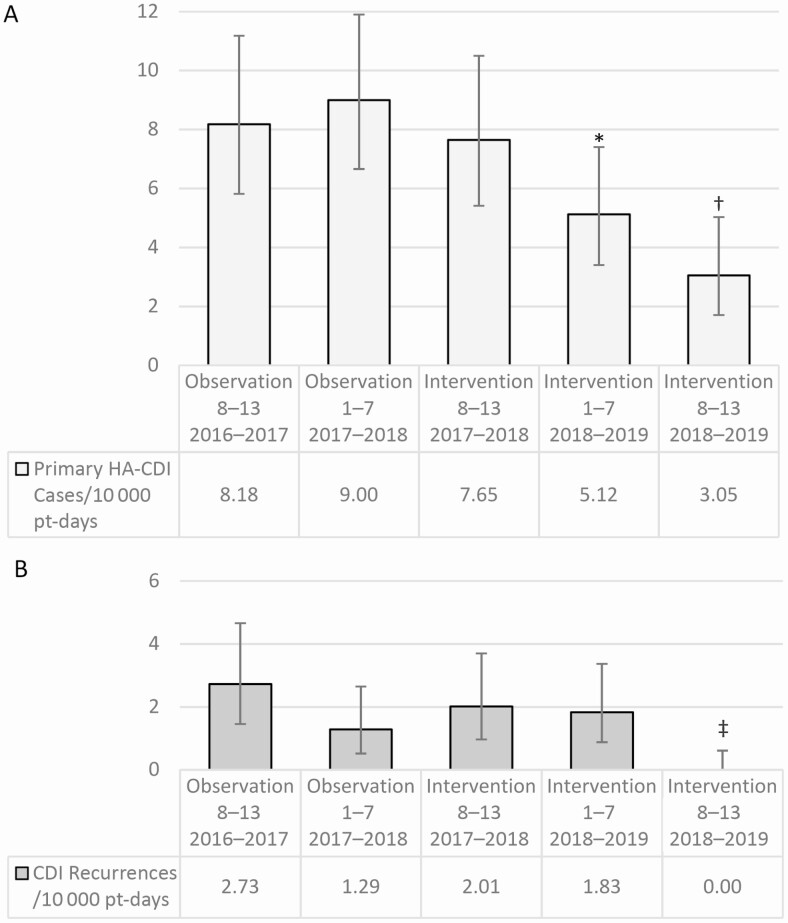
The hospital-wide HA-CDI rate was significantly lower during the intervention period than during the observation period (5.2 vs 8.6 cases per 10 000 patient-days, respectively; P = .002). The rate was significantly lower for the first spring/summer and the second fall/winter of the intervention compared with the preceding seasons (Figure 1). The proportion of HA-CDI cases with recurrences was relatively stable (16 patients [19%] in the observation vs 13 [16%] in the intervention period). A smaller proportion of the probiotic-treated patients had recurrences (6 patients [13%] vs 7 [25%] in the observation period), though this differences was not significant (Supplementary Table 1).
Figure 1.
Hospital-wide rate of primary healthcare-associated Clostridioides difficile (HA-CDI) cases (A) and recurrent CDI episodes (B) per 10 000 patient-days (pt-days) for each semiannual period. The semiannual periods refer to hospital data from the spring through summer [Periods 1-7] or from the autumn through winter [Periods 8-13]. Error bars for simple proportion represent 95% confidence intervals calculated with Fisher exact test. *P < .01 for hospital-wide rate of primary HA-CDI cases versus 1 year earlier; †P < .01 for hospital-wide rate of primary HA-CDI cases versus 1 and 2 years earlier; ‡P < .01 for hospital-wide rate of first recurrent HA-CDI cases versus 1 and 2 years earlier.
Probiotic Exposure and Comparative CDI Incidence
The hospital pharmacy purchased and dispensed 122 000 capsules of the probiotic to 4543 eligible adult antibiotic users for 5766 (70%) of the hospitalizations during the intervention period. The probiotic capsules were generally well tolerated, and no case of Lactobacillus bacteremia after probiotic use was detected.
There were no exclusion criteria for use of the probiotic, but there appears to have been a selection bias toward using the probiotic in older adults and those with an increased antibiotic burden. The majority of patients receiving the probiotic, 2480 (54%), were aged ≥70 years. Patients in this age group were given the probiotic more frequently, (OR, 2.0 [95% confidence interval (CI), 1.8–2.3]). The probiotic was used more frequently among patients taking quinolones, third-generation cephalosporin, or carbapenems (OR, 3.4) (Table 1). Those taking the probiotic received more antibiotics per visit (2.16 on average) than eligible patients who did not receive the probiotic (1.57; P < .001). Most patients receiving multiple antibiotics during the intervention were given a probiotic (OR, 2.6 [95% CI, 2.3–2.8]; P < .001).
The incidence of HA-CDI among antibiotic users decreased significantly during the intervention, compared with the observation period (0.9% vs 1.5%, respectively) (Table 1). Without accounting for the differential risk of eligible patients, the incidence of HA-CDI was similar for those taking the probiotic (OR, 0.69). When riskier courses of antibiotics were prescribed, the probiotic group fared better. Patients exposed to multiple antibiotics per visit had a lower incidence of HA-CDI with the probiotic (OR, 0.50). In particular, patients who received ≥4 types of antibiotic per visit had a remarkably lower incidence of HA-CDI when taking the probiotic (OR, 0.14 [95% CI, .04–.49]) (Supplementary Figure 1). There was a lower incidence of HA-CDI overall during the intervention for patients taking a quinolone (OR, 0.41), a third-generation cephalosporin (OR, 0.43), a carbapenem (OR, 0.33), or any of these high-risk antibiotic classes (OR, 0.42). Patients receiving the probiotic during the intervention with a high-risk antibiotic had a lower incidence of HA-CDI than those who had not taken a probiotic (OR, 0.49) (Table 1).
DISCUSSION
We found that using a pharmacy-driven protocol made it feasible to safely implement this probiotic as an adjunct to antibiotics. The hospital-wide rate of HA-CDI improved by 39% during the intervention relative to the observation period, consistent with findings from other hospitals implementing this probiotic [6, 7, 11].
We attribute the high degree of implementation of the probiotic to the hospital’s streamlining of this process to the hospital pharmacy. Other hospital groups have been challenged in reaching universal adherence to a probiotic guideline [11, 12]; 70% of our eligible patients took the probiotic.
Policy changes to restrict quinolone prescribing started in the observation period and continued through the intervention. It’s unlikely that this shift in prescribing influenced the CDI rates. As the use of quinolones decreased, third-generation cephalosporin use increased. Each of these posed an equal risk of HA-CDI during the observation period (2.5%), yet the incidence of HA-CDI with each of these high-risk antibiotics users was markedly decreased during the intervention (to 1.1%), suggesting an impact from the probiotic intervention. External factors, like changes in community-acquired CDI rates, may have also contributed to this improvement.
The incomplete implementation of the probiotic presented a unique control group to estimate the efficacy of this probiotic during the intervention. However, these comparison groups were not equivalent. There were systematic biases wherein the probiotic was given disproportionately to patients at elevated risk of developing CDI: adults aged ≥70 years (OR, 2.0) and those taking multiple (OR, 2.6) or high-risk (OR, 3.4) antibiotics. The vast majority of patients who received ≥3 antibiotics per visit during the intervention (1921 patients [88%]), were given the probiotic. In light of the disparate use of the probiotic, analyses were made within groups of patients with equivalent types of antibiotics received per visit. The incidence of CDI was cut by at least half when the probiotic was taken with multiple antibiotics. Patients who took ≥1 high-risk antibiotic had a lower incidence of HA-CDI when taking the probiotic during the intervention (0.9%; OR, 0.49).
Antibiotics are the critical modifiable risk factor for HA-CDI, but there are other important risk factors not considered in these analyses, for example, the use of proton pump inhibitors, antihistamines, and antacids [3]. Prescription records also could not provide patient-level data on comorbid illness or frailty, which are relevant to CDI risk. Future studies could use a broader electronic database to confirm these findings.
In conclusion, the experience in our this confirms the effectiveness of this probiotic preparation for the primary prevention of HA-CDI, as described in multiple controlled, clinical studies. The incidence of HA-CDI was lower by at least half for patients who took the 3-strain Lactobacillus probiotic with multiple-antibiotic regimens or regimens that included high-risk antibiotics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. P. J. M. and N. S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: P. J. M. Acquisition of data: P. J. M. Analysis or interpretation of data: P. J. M. and N. S. Drafting of manuscript: P. J. M., N. S., J. C. S., and E. J. C. G. Critical revision of the manuscript for important intellectual content: P. J. M., N. S., J. C. S., and E. J. C. G. Statistical analysis: N. S.
Potential conflicts of interest. N. S. is a full-time employee of Bio-K Plus International; he had no role in the design and conduct of the study, collection and management of the data, approval of the manuscript, or decision to submit the manuscript for publication. P. J. M., J. C. S., and E. J. C. G. are scientific advisory board members of Bio-K Plus International. J. C. S. has received speaker bureau honoraria from Bio-K Plus International, unrelated to the conduct of this study. None of the authors own a financial interest in Bio-K Plus International.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota—a systematic review. J Infect 2019; 79:471-89. [DOI] [PubMed] [Google Scholar]
- 2. Tartof SY, Rieg GK, Wei R, Tseng HF, Jacobsen SJ, Yu KC. A comprehensive assessment across the healthcare continuum: risk of hospital-associated Clostridium difficile infection due to outpatient and inpatient antibiotic exposure. Infect Control Hosp Epidemiol 2015; 36:1409-16. [DOI] [PubMed] [Google Scholar]
- 3. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987-94. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein EJ, Johnson S, Maziade PJ, et al. Pathway to prevention of nosocomial Clostridium difficile infection. Clin Infect Dis 2015; 60(suppl 2):S148-58. [DOI] [PubMed] [Google Scholar]
- 5. Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One 2018; 13:e0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis 2015; 60 (suppl 2):S144-7. [DOI] [PubMed] [Google Scholar]
- 7. McFarland LV, Ship N, Auclair J, Millette M. Primary prevention of Clostridium difficile infections with a specific probiotic combining Lactobacillus acidophilus, L. casei, and L. rhamnosus strains: assessing the evidence. J Hosp Infect 2018; 99:443-52. [DOI] [PubMed] [Google Scholar]
- 8. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010; 105:1636-41. [DOI] [PubMed] [Google Scholar]
- 9. Institut National de Santé Publique du Québec (INSPQ). Comité de Surveillance Provinciale des Infections Nosocomiales (SPIN). Diarrhées associées au Clostridium difficile: résultats de surveillance 2018-2019.https://www.inspq.qc.ca/infections-nosocomiales/spin/dacd/surveillance-2018-2019. Accessed 1 April 2021.
- 10. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016; 25:986-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trick WE, Sokalski SJ, Johnson S, et al. Effectiveness of probiotic for primary prevention of Clostridium difficile infection: a single-center before-and-after quality improvement intervention at a tertiary-care medical center. Infect Control Hosp Epidemiol 2018; 39:765-70. [DOI] [PubMed] [Google Scholar]
- 12. Wombwell E, Patterson ME, Bransteitter B, Gillen LR. The effect of Saccharomyces boulardii primary prevention on risk of hospital onset Clostridioides difficile infection in hospitalized patients administered antibiotics frequently associated with Clostridioides difficile infection. Clin Infect Dis 2020;ciaa808. doi: 10.1093/cid/ciaa808c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.