Abstract
Background
Men who have sex with men (MSM) are at high risk for human papillomavirus (HPV)–related anal cancer. Little is known about the prevalence of low-grade squamous intraepithelial lesions (LSILs) and the anal cancer precursor, high-grade squamous intraepithelial lesions (HSILs), among young MSM with HIV (MSMLWH). HPV vaccination is recommended in this group, but its safety, immunogenicity, and protection against vaccine-type HPV infection and associated LSILs/HSILs have not been studied.
Methods
Two hundred and sixty MSMLWH aged 18–26 years were screened at 17 US sites for a clinical trial of the quadrivalent (HPV6,11,16,18) HPV (qHPV) vaccine. Those without HSILs were vaccinated at 0, 2, and 6 months. Cytology, high-resolution anoscopy with biopsies of lesions, serology, and HPV testing of the mouth/penis/scrotum/anus/perianus were performed at screening/month 0 and months 7, 12, and 24.
Results
Among 260 MSMLWH screened, the most common reason for exclusion was detection of HSILs in 88/260 (34%). 144 MSMLWH were enrolled. 47% of enrollees were previously exposed to HPV16. No incident qHPV type–associated anal LSILs/HSILs were detected among men naive to that type, compared with 11.1, 2.2, 4.5, and 2.8 cases/100 person-years for HPV6,11,16,18–associated LSILs/HSILs, respectively, among those previously exposed to that type. qHPV was immunogenic and safe with no vaccine-associated serious adverse events.
Conclusions
18–26-year-old MSMLWH naive to qHPV vaccine types were protected against incident qHPV type–associated LSILs/HSILs. Given their high prevalence of HSILs, there is an urgent need to vaccinate young MSMLWH before exposure to vaccine HPV types, before initiating sexual activity, and to perform catch-up vaccination.
Keywords: anal human papillomavirus infection, quadrivalent HPV vaccine, anal squamous intraepithelial lesions, men who have sex with men, human immunodeficiency virus
A high proportion of 18-26 year-old men-who-have-sex-with-men-living-with-HIV screened for a quadrivalent HPV (qHPV) vaccine trial had anal low-grade/high-grade squamous intraepithelial lesions (LSIL/HSIL). Vaccinees naive to qHPV types were protected against incident qHPV type-associated-LSIL/HSIL. Vaccination before sexual initiation is critically important.
People living with human immunodeficiency virus (HIV; PLWH) are at elevated risk of anogenital human papillomavirus (HPV) infection and related cancers [1, 2]. Men who have sex with men living with HIV (MSMLWH) are at particularly high risk of anal cancer [3]. We previously reported that more than 95% of 18–26-year-old MSMLWH had anal HPV infection [4]. However, no previous studies have characterized the prevalence of histologic high-grade squamous intraepithelial lesions (hHSILs), the anal cancer precursor, in young MSMLWH.
HPV vaccination is routinely recommended for the prevention of HPV-associated cancers and genital warts in males and females with a target age of 11–12 years and catch-up vaccination to 26 years. Licensed HPV vaccines are effective in preventing HPV infection prior to exposure and vaccination prior to initiating sexual activity optimizes vaccine efficacy [5–9]. Given the risk of anal cancer in MSMLWH, HPV vaccination has been strongly recommended in men up to 26 years of age [10]. While HPV vaccination has been shown to be safe, well tolerated, and immunogenic in PLWH [11–19], some studies have demonstrated lower titers in PLWH compared with similarly aged individuals without HIV [14, 18]. No studies have examined vaccine efficacy as measured by the prevention of incident-persistent HPV infection or incident vaccine type–associated HSILs in PLWH younger than 26 years of age. One study of MSMLWH older than 26 years failed to show efficacy in the prevention of anal HPV infection and incident HSILs [12], likely reflecting a high degree of prior sexual exposure to vaccine HPV types. Given their high risk of anal HPV infection, it is critical to understand the prevalence of anal HSILs and the ability of the vaccine to prevent anal HPV infection and HSILs among young MSMLWH.
METHODS
We performed a phase II, open-label, multicenter trial of the quadrivalent HPV (qHPV; (HPV6,11,16,18) vaccine (Merck and Company, Kenilworth, NJ) in 13- to 26-year-old MSMLWH. For ethical reasons, we did not have a placebo control arm in this study. Our primary objectives were as follows (1) to compare incident HPV 6,11,16,18 infection and HPV 6,11,16,18–associated perianal/anal disease among young MSMLWH with or without exposure to these HPV types prior to vaccination and (2) to compare the data among MSMLWH without evidence of prior HPV exposure to similarly aged HIV-negative men who have sex with men (MSM) in the placebo arm of the Merck V503-020 protocol (NCT00090285) [5] without evidence of prior exposure. The study was conducted by the AIDS Malignancy Consortium (AMC) in collaboration with the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN). Participants were recruited between 2012 and 2015 from 15 US sites. The institutional review board for each site approved the study. Inclusion criteria included male at birth, documented HIV-1 infection, age 13 to 26 years, and at least 1 male sexual partner. We excluded those with cytologic HSIL (cHSIL), atypical squamous cells–cannot rule out HSIL (ASC-H), or histologic HSIL (hHSIL) on screening biopsy. These individuals were referred for treatment and offered the qHPV vaccine.
At screening, participants had a complete medical history and physical examination. We collected separate swabs from the anal canal, perianus, and penis/scrotum for HPV DNA testing. We performed high-resolution anoscopy (HRA) with biopsy of suspected lesions. We enrolled eligible participants within 45 days of screening and administered a questionnaire assessing lifestyle habits. We obtained blood to measure CD4/CD8 levels, HIV viral load, and HPV serology; collected oral Scope (Procter & Gamble) swish/gargle mouthwash specimens for HPV testing and administered the qHPV vaccine at 0, 2, and 6 months. After each injection, participants completed a vaccine report card documenting signs and symptoms and had telephone follow-up to assess for adverse events (AEs). At month 7, we collected blood for CD4/CD8 levels and HIV viral load (VL). At months 7, 12, 18, and 24, we collected blood for HPV serology; anal, perianal, and penile/scrotal swabs; and oral gargles for HPV testing, and performed HRA with biopsy of suspected lesions.
HPV Testing
HPV testing was performed on swabs and rinses with MY09/MY11 L1 consensus primers, as described previously [4]. HPV testing was performed on formalin-fixed paraffin-embedded (FFPE) biopsy samples using sterile technique to avoid cross-contamination, including changing gloves and blades between samples. The first section (4 μm) was set aside and the next 20-μm section was placed into a sterile Eppendorf tube. For DNA preparation we used the Invitrogen by Thermo Fisher Scientific RecoverAll Total Nucleic Acid Isolation Kit (AM1975; Vilnius, Lithuania) following the manufacturer’s instructions. Forty amplification cycles were performed, followed by dot-blotting and probing of amplification mixtures as described previously [4]. Overall, 483 of 690 (70%) anal biopsies collected were retrieved and tested for HPV DNA.
HPV Serology
Sera were tested for antibodies to HPV6,11,16,18 using a competitive Luminex immunoassay [5]. Results were expressed as milliMerck units (mMU)/mL, with positivity cutoffs of 11/8/11/10 mMU/mL, respectively.
Statistical Analysis
We defined being naive to a specified HPV type as seronegative at baseline and DNA-negative at baseline and month 7 at all anatomic locations. We analyzed 2 groups: (1) naive, per-protocol (completed the vaccination series per protocol) and (2) previously exposed (seropositive at baseline or DNA-positive at any anatomic site at either baseline or month 7), per protocol. We compared incident-persistent qHPV type infection and incident qHPV type–associated hLSILs/hHSILs between naive and previously exposed participants. We also compared them with similarly aged naive per-protocol HIV-negative MSM participating in the placebo arm of the Merck V503-020 study (NCT00090285) [5].
Case counting for incident hLSIL/hHSIL analysis occurred at months 12, 18, or 24. Analyses were restricted to those with both a histology and anal biopsy HPV DNA result at a given visit. Cases were considered to be qHPV type–related if HPV6,11,16,18 were detected in the FFPE specimen. Incident-persistent HPV infection was defined as having a positive HPV test at the same anatomic site at 2 consecutive visits after month 7. For this analysis, the previously exposed category only included those who were seropositive and DNA-negative for the specific HPV type. Participants could be in more than 1 HPV type–specific exposure category.
We computed HPV type–specific event rates for incident HPV and lesion endpoints with corresponding 95% confidence intervals (CIs) using Poisson calculations (sample size calculations are found in the Supplementary Material). Given the number of 0 event rates, we compared exposure groups using exact Poisson calculations. P values were 2-sided for comparisons of naive groups with previously exposed groups, whereas comparisons to historical controls were a priori selected to be 1-sided.
RESULTS
Although MSMLWH as young as 13 years old could enroll, all participants were 18–26 years old. We enrolled participants at 17 ATN and AMC study sites. A total of 260 MSMLWH were screened and 111 MSMLWH failed screening, mostly due to ASC-H on cytology, cHSILs, or hHSILs (n = 93). Overall, 34% were excluded for cHSILs or hHSILs at baseline. The cytology and histology results of the screened population are shown in Supplementary Table 1.
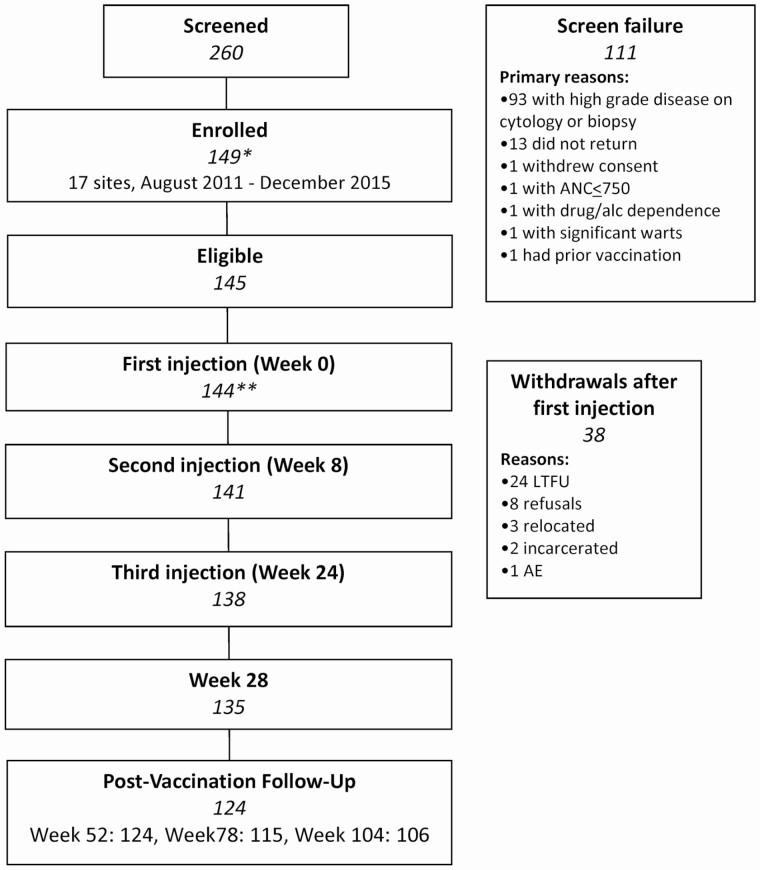
A total of 149 MSMLWH were enrolled (Figure 1): one withdrew prior to treatment, 4 were ineligible, 144 received the first vaccine injection, 141 received the first 2 injections, and 138 received all 3 injections. Adherence to the visit schedule was 85–100%. Of the 38 MSMLWH who received at least 1 injection but did not attend the month 24 visit, 24 were lost to follow-up, 8 withdrew consent, 3 relocated, 2 were incarcerated, and 1 experienced an AE (grade 3 rectal pain) that led to study withdrawal.
Figure 1.
Participant flow in AMC-072. *Four patients were enrolled in error: No treatment per protocol criteria (n = 1), HPV vaccine prior to screening (n = 1), AIN 3 on screening (n = 1), not on HAART for 90 days prior to entry (n = 1). **One patient withdrew/refused prior to beginning protocol therapy. Abbreviations: AE, adverse event; alc, alcohol; AMC, AIDS Malignancy Consortium; AIN, Anal intraepithelial neoplasia; ANC, Absolute neutrophil count; HAART, highly active antiretroviral therapy; HPV, human papillomavirus; LTFU, lost to follow-up.
Most participants were aged 22–26 years (mean, 23 years), 60% were African-American, and 45% were smokers. Eighty-two percent reported receptive anal intercourse within the last 6 months, of whom 46% reported consistent condom use (Table 1). The median nadir CD4 count was 309 cells/mL, median enrollment CD4 level was 594 cells/mL, and 9% had an HIV VL of more than 400 copies/mL.
Table 1.
Demographics of Men Who Have Sex With Men With HIV Enrolled Into the qHPV Vaccine Study
| Variable | Total Na | Values |
|---|---|---|
| Age (years) | 144 | |
| 22–26 | 115 (80) | |
| 18–21 | 29 (20) | |
| Age, median (range), years | 144 | 23 (18–26) |
| Race | 134 | |
| White | 46 (34) | |
| Black | 80 (60) | |
| Other | 8 (6) | |
| Hispanic ethnicity | 144 | 38 (26) |
| Current smoking (some days/every day) | 137 | 62 (45) |
| Last receptive anal sexual intercourseb | 134 | |
| Over 6 months ago | 23 (17) | |
| 1–6 months ago | 36 (27) | |
| Within the past month | 75 (56) | |
| Sexual intercourse with male partner, past 6 months (yes) | 137 | 124 (91) |
| Number of male sexual partners, past 6 months | 137 | |
| 0 | 13 (9) | |
| 1 | 48 (35) | |
| ≥2 | 76 (55) | |
| Number of receptive anal male sexual partners, past 6 months | 137 | |
| 0 | 25 (25) | |
| 1 | 54 (54) | |
| ≥2 | 58 (58) | |
| Frequency of condom use during receptive anal sex with male partner, past 6 months | 137 | |
| No anal sex past 6 months | 25 (18) | |
| Every time | 51 (37) | |
| Sometimes/never | 61 (45) | |
| Number of oral male sexual partners, past 6 months | 136 | |
| 0 | 15 (11) | |
| 1 | 54 (40) | |
| ≥2 | 67 (49) | |
| Frequency of condom use during oral sex, past 6 months | 137 | |
| No oral sex past 6 months | 15 (11) | |
| Sometimes/always | 36 (26) | |
| Never | 86 (63) | |
| Had sexual intercourse with female partner, past 6 months (yes) | 137 | 8 (6) |
| Chlamydia and/or gonorrhea (urethral) infection at baseline (yes) | 141 | 6 (4) |
| Nadir CD4+ count (cells/mm3) | 144 | |
| ≤350 | 83 (59) | |
| >350 | 57 (41) | |
| Nadir CD4+ count, median (range), cells/mm3 | 140 | 309 (10–785) |
| Enrollment CD4+ count (cells/mm3) | 144 | |
| ≤350 | 14 (10) | |
| >350 | 130 (90) | |
| Enrollment CD4+ count, median (range), cells/mm3 | 144 | 594 (237–1520) |
| HIV viral load (copies/mL) | 144 | |
| <400 | 131 (91) | |
| ≥400 | 13 (9) | |
| HIV viral load, median (range), copies/mL | 144 | 0 (0–63000) |
Data are presented as n (%) unless otherwise indicated. Abbreviations: HIV, human immunodeficiency virus; qHPV, quadrivalent human papillomavirus.
aWhen data are missing, the sample size is <144; 7 participants did not complete the behavioral questionnaire.
bThree participants denied ever having receptive anal intercourse and are not included.
A high proportion of enrollees had LSILs: 35% had cLSILs and 58% had histologic LSILs (hLSILs). Baseline serology and DNA status are shown in Table 2. The proportions who were naive to HPV6,11,16,18 were 22%, 48%, 53%, and 66%, respectively. We examined the impact of testing for HPV DNA at sites other than the anal canal on classification of participants as being naive. HPV6,11,16,18 DNA were detected in the penile or oral sample, but not the anus, in 2 of 132 (1.5%), 1 of 131 (0.8%), 3 of 131 (2.3%), and 1 of 132 participants (0.8%), respectively.
Table 2.
Baseline HPV Serostatus and HPV DNA Status
| HPV6 | HPV11 | HPV16 | HPV18 | HPV 6, 11, 16, or 18 | |
|---|---|---|---|---|---|
| Seropositive/DNA-positive | 51 (35) | 15 (10) | 15 (10) | 4 (3) | 111 (77) |
| Seropositive /DNA-negative | 42 (29) | 49 (34) | 35 (24) | 35 (24) | 0 (0) |
| Seronegative/DNA-positive | 17 (12) | 9 (6) | 16 (11) | 8 (6) | 31 (22) |
| Seronegative/DNA-negative | 32 (22) | 69 (48) | 76 (53) | 95 (66) | 0 (0) |
| Sero-missing/DNA-negative | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 2 (1) |
Data are presented as n (%). “Seropositive” DNA-positive indicates positive for specified HPV type in any of anal, perianal, penile/scrotal, or oral specimens. The denominator for percentages is 144 participants. Abbreviation: HPV, human papillomavirus.
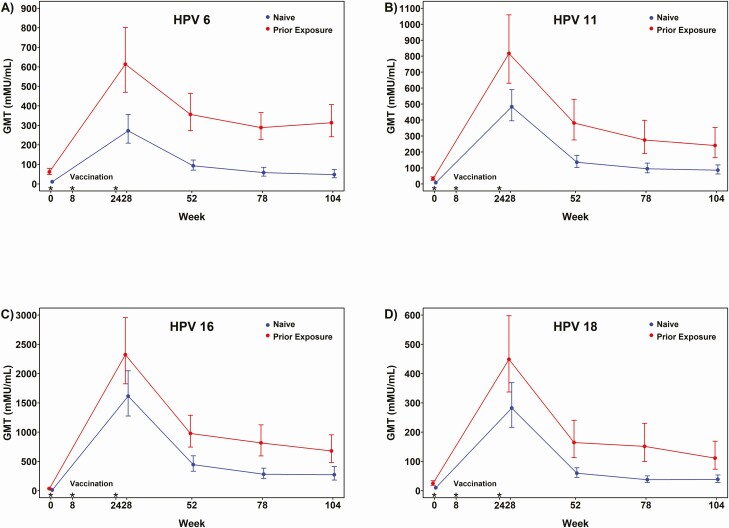
Geometric mean titers generated by qHPV among naive and previously exposed participants at months 7, 12, 18 and 24 are shown in Figure 2. All seroconverted at month 7 to HPV6, 11, and 16, and 99% seroconverted to HPV18. We compared month 7 and month 24 titers with HPV6,11,16,18 by age (<24, ≥24 years), nadir CD4 (≤350, >350 cells/mm3), current CD4 (≤500, >500 cells/mm3), and HIV VL. For baseline seronegative participants, there was a significant difference in titers for HPV18 at month 7 for those with an HIV VL of 75 or less versus more than 75 copies/mL (median, 333 vs 123; P = .011), but there were no other significant differences. Titers were higher for all vaccine types at months 7, 12, 18, and 24 among previously exposed versus naive participants.
Figure 2.
Geometric mean (95% confidence interval) titers to HPV 6 (A), 11 (B), 16 (C), and 18 (D) among naive and previously exposed 18–26-year-old men who have sex with men living with HIV, up to 2 years after initiation of vaccination. Titers in participants naive to a qHPV vaccine HPV type are shown in blue. Titers in participants previously exposed to that HPV type are shown in red. Abbreviations: GMT, geometric mean titer; HIV, human immunodeficiency virus; HPV, human papillomavirus; qHPV, quadrivalent human papillomavirus. Asterisks show when vaccine doses were administered.
Table 3 demonstrates incident hLSILs or hHSILs combined and incident anal/perianal hHSILs in the naive per-protocol and previously exposed per-protocol groups by qHPV vaccine type. The overall incidence of any hHSIL was 29.5 (95% CI: 20.6–42.2) per 100 person-years (PY) regardless of HPV type, and overall incidence of anal/perianal hHSILs/hLSILs was 61.3 (95% CI: 39.1–96.1) per 100 PY. Among the naive per-protocol group, there were no incident cases of qHPV vaccine–related hLSILs/hHSILs up to month 24. Incident lesions associated with each of the 4 vaccine types were detected in the previously exposed per-protocol groups, but the differences between naive per-protocol and previously exposed per-protocol groups were not statistically significant. There was a statistically significant reduction in incident HPV16-associated hHSILs in the naive group compared with the previously exposed group (P = .014).
Table 3.
Low-Grade Squamous Intraepithelial Lesions/High-Grade Squamous Intraepithelial Lesions and High-Grade Squamous Intraepithelial Lesions in the Per-Protocol Population Among those Naive at Baseline Compared With Those Already Exposed
| Per-Protocol: Naive to Specified HPV Typea | Per Protocol: Previously Exposed to Specified HPV Typea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint | No. Included in Analyses | No. of Affected Participants | Person-Years at Risk | Events per 100 Person-Years at Risk | No. Included in Analyses | No. of Affected Participants | Person-Years at Risk | Events per 100 Person-Years at Risk | P b |
| Anal/perianal LSILs/ HSILsc related to | |||||||||
| HPV6 | 15 | 0 | 25.0 | 0.0 | 34 | 6 | 53.9 | 11.1 | .112 |
| HPV11 | 35 | 0 | 55.2 | 0.0 | 28 | 1 | 45.1 | 2.2 | .224 |
| HPV16 | 39 | 0 | 65.3 | 0.0 | 29 | 2 | 44.0 | 4.5 | .079 |
| HPV18 | 47 | 0 | 75.6 | 0.0 | 23 | 1 | 36.3 | 2.8 | .162 |
| Any qHPVd | 9 | 0 | 14.7 | 0.0 | 34 | 7 | 54.4 | 12.9 | .231 |
| Anal/perianal HSILse related to | |||||||||
| HPV6 | 15 | 0 | 25.0 | 0.0 | 56 | 9 | 86.5 | 10.4 | .064 |
| HPV11 | 35 | 0 | 55.2 | 0.0 | 36 | 1 | 57.6 | 1.7 | .745 |
| HPV16 | 39 | 0 | 65.3 | 0.0 | 32 | 4 | 47.4 | 8.4 | .014 |
| HPV18 | 47 | 0 | 75.6 | 0.0 | 24 | 1 | 37.6 | 2.7 | .166 |
| Any qHPVd | 9 | 0 | 13.4 | 0.0 | 63 | 14 | 96.2 | 14.6 | .123 |
Abbreviations: HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; qHPV, quadrivalent human papillomavirus.
aBased on serum at baseline and DNA from baseline and month 7 from any anatomical site. Participants could be in >1 exposure category.
bNaive and previously exposed group comparison of event rates based on exact Poisson calculations (2-sided test).
cAnalyses required having both a histologic result and anal biopsy HPV DNA determination at a given visit. Participants with type-specific anal LSILs at baseline were excluded from this analysis, and HSIL at screening was an exclusion criterion. Case counting occurred after month 7 at months 12, 18, and 24.
dAnalyses required participants to be naive to all 4 types at baseline.
eAnalyses required having both a histologic result and anal biopsy HPV DNA determination at a given visit. Case counting occurred after month 7 at months 12, 18 and 24.
There were no statistically significant differences between the naive per-protocol and previously exposed per-protocol groups (Table 4). There were no incident-persistent infections in the previously exposed per-protocol group. There were 3 cases of incident-persistent HPV16 infection in the naive per-protocol group: 1 had HPV6 at baseline by biopsy and anal swab and HPV16 detected at visits 5 and 6; 1 participant had no qHPV types at baseline but had HPV16 detected on anal swab at visits 4, 5, and 6; and 1 participant had HPV6 at baseline on anal biopsy and HPV16 detected at visits 4, 5, and 6. None had HPV16-associated lesions. The 1 incident HPV18 infection was penile/scrotal.
Table 4.
Incident-Persistent Infection in the Study Population
| Per-Protocol Naive to Specified HPV Typeb | Per-Protocol Previously Exposed to Specified HPV Typeb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint: Persistent Infectiona | No. Included in Analyses | No. of Affected Participants | Person-Years at Risk | Events per 100 Person-Years at Risk | No. Included in Analyses | No. of Affected Participants | Person-Years at Risk | Events per 100 Person-Years at Risk | P c |
| HPV6 | 29 | 1 | 55.5 | 1.8 | 15 | 0 | 29.0 | 0.0 | .671 |
| HPV11 | 55 | 0 | 108.6 | 0.0 | 33 | 0 | 64.9 | 0.0 | >.999 |
| HPV16 | 54 | 3 | 102.9 | 2.9 | 23 | 0 | 46.0 | 0.0 | .386 |
| HPV18 | 74 | 1 | 142.9 | 0.7 | 23 | 0 | 46.1 | 0.0 | .622 |
No. = number of participants with ≥2 follow-up visits. Abbreviation: HPV, human papillomavirus.
aMust have had a positive HPV test at 2 consecutive visits after month 7 (months 12, 18, and 24) at any site (swab DNA anal/perianal, penile/scrotal, oral) and persistent at same site (anal/perianal, penile/scrotal, oral).
bBased on serum at baseline and DNA from baseline and month 7 from any anatomical site. By definition, the previously exposed category included only those who were seropositive, DNA-negative for the specific HPV type. Participants could be in >1 exposure category.
cNaive and previously exposed group comparison of event rates were based on exact Poisson calculations (2-sided test).
Table 5 shows incident-persistent qHPV vaccine type–associated anal/perianal hLSILs/hHSILs in the naive per-protocol, vaccinated AMC-072 group compared with historical data from naive, per-protocol 18–26-year-old HIV-negative MSM randomized to the V501-020 study placebo arm [5]. Type-associated hLSIL/hHSIL was lower in the AMC-072 group compared with the V501-020 group for all 4 qHPV types. The AMC-072 group had lower incident-persistent HPV infection rates (1.8, 0.0, 2.9, 0.7 per 100 PY for HPV6,11,16,18, respectively) than the V501-020 group (4.5, 1.7, 4.9, 2.7 per 100 PY for HPV6,11,16,18, respectively). There were no statistically significant differences between vaccinated AMC-072 groups and V501-020 groups for incident disease or incident-persistent qHPV type infection associated with each qHPV type, but vaccinated AMC-072 participants had significantly reduced disease in a combined analysis of all 4 qHPV types.
Table 5.
Low-Grade Squamous Intraepithelial Lesions or High-Grade Squamous Intraepithelial Lesions in the Merck 020 Per-Protocol Placebo Group Compared With the AMC-072 Per-Protocol Naive Vaccinated Group
| Per-Protocol Merck 020 Placebo Groupb | Per-Protocol AMC-072 Naive Vaccinated Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint: Anal/ Perianal LSIL/HSILa Related to | No. Included in Analyses | No. of Affected Participants | Person-Years at Risk | Events per 100 Person-Years at Risk | No. Included in Analyses | No. of Affected Participants | Person-Years at Risk | Events per 100 Person-Years at Risk | P c |
| HPV6 | 144 | 10 | 298.5 | 3.4 | 15 | 0 | 25.0 | 0.0 | .447 |
| HPV11 | 144 | 6 | 298.2 | 2.0 | 35 | 0 | 55.2 | 0.0 | .361 |
| HPV16 | 170 | 6 | 341.9 | 1.8 | 39 | 0 | 65.4 | 0.0 | .350 |
| HPV18 | 193 | 4 | 387.4 | 1.0 | 47 | 0 | 75.6 | 0.0 | .490 |
| HPV6, 11, 16, or 18d | 208 | 24 | 411.6 | 5.8 | 58 | 0 | 93.0 | 0.0 | .008 |
Abbreviations: AMC, AIDS Malignancy Consortium; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; qHPV, quadrivalent human papillomavirus.
aAnalyses required having both histology and anal biopsy HPV DNA determination at a given visit. AMC-072 participants with qHPV type–specific AIN1 or perianal condyloma at baseline were removed (HSIL was an exclusion criteria); “baseline” DNA HPV type based on visits 0 and 3. Case counting occurred after month 7 at visits 4, 5, and/or 6.
bThe per-protocol Merck 020 placebo group were HIV-negative men who have sex with men, were naive for a given qHPV type, and did not have LSIL or HSIL at baseline.
cMerck placebo and AMC-072 naive group comparison of event rates based on exact Poisson calculation (1-sided test).
dConsistent with the reported Merck V503-020 combined endpoint, analysis of all participants who were eligible for ≥1 of the individual type-specific analyses. A participant would be counted more than once if multiple lesions of different HPV types developed.
Adverse events were reported by 72% of participants; 92% were grade 1 or 2 and the most common (26%) was injection site reaction (Table 6). Of the 11 serious AEs in 5 participants, none were related to vaccination. The median CD4 level did not decrease and the HIV VL did not increase from baseline to month 7.
Table 6.
Adverse Events in Vaccinated Participants in AMC-072
| Affected Participants, n (%) | |
|---|---|
| Adverse events | 103 (71.5) |
| Affecting >5% | |
| Diarrhea | 8 (5.6) |
| Injection site reaction | 37 (25.7) |
| Infection and infestations—other | 9 (6.3) |
| Neutrophil count decreased | 10 (6.9) |
| Cough | 10 (6.9) |
| Skin and subcutaneous tissue disorders—other | 13 (9.0) |
| Related adverse eventsa | 44 (30.6) |
| Affecting >5%: injection site reaction | 37 (25.7) |
| Any grade 3 adverse eventb | 6 (4.2) |
| Grade 3 injection site reaction | 3 (2.1) |
| Serious adverse events | 5 (3.5) |
| Full listing | |
| Skin infection | 2 (1.4) |
| Gum infection | 1 (0.7) |
| Vomiting | 1 (0.7) |
| Nausea | 1 (0.7) |
| Homicidal ideation | 1 (0.7) |
| Suicidal ideation | 1 (0.7) |
| Suicide attempt | 1 (0.7) |
| Potentially related serious adverse events | 0 (0) |
Abbreviation: AMC, AIDS Malignancy Consortium.
aRelated adverse events were those that were deemed definitely, probably, or possibly related to vaccine.
bGrade 3 was the highest severity for any treatment-related adverse event.
DISCUSSION
Consistent with our previous report showing high rates of anal HPV infection in MSMLWH [4], here we report that more than 34% of this population had anal HSILs at screening for this qHPV vaccination study. Further, among those without anal HSILs who were enrolled in the study, 74% had LSILs. The significance of having an HSIL at a young age is unknown because the incidence of anal cancer is low in those younger than 26 years and prospective studies of anal HSILs in this group have not been performed. Studies are needed to inform guidelines to recommend screening, such as the Anal Cancer/HSIL Outcomes Research (ANCHOR) Study, which is designed to determine if screening for and treating anal HSILs is effective in reducing the incidence of anal cancer. Similar to other, older populations [20], anal cytology in young MSMLWH had limited sensitivity for the detection of HSILs (Supplementary Table 1), unless the threshold for screening included Atypical squamous cells of undetermined significance (ASC-US), and anal cytology underestimated the level of disease detected.
We found that, among participants without anal HSILs detected at screening, a substantial proportion had already been exposed to qHPV vaccine types, particularly HPV6 (78%) and 16 (47%). However, despite the high rate of prior exposure to vaccine HPV types, more than half of young MSMLWH were DNA- and seronegative for HPV16, the type predominantly associated with anal cancer, and therefore could be protected if vaccinated.
Little is known about the performance of the qHPV vaccine in young MSMLWH. Similar to data reported in older women living with HIV [16], our data demonstrate that there were no cases of incident qHPV vaccine type–associated hLSILs or hHSILs among naive vaccinated individuals during the 2-year duration of the study. There was also a statistically significant reduction in HPV16-associated hHSILs among individuals naive to HPV16 compared with previously exposed individuals, a clinically important finding given the dominant role of HPV16 in the pathogenesis of anal cancer. The significance of the 3 cases of incident-persistent HPV16 infection is not clear at this time. Further follow-up would have been needed to determine if this became associated with an incident lesion.
Most of the comparisons between naive and previously exposed populations were not statistically significant. Our ability to demonstrate vaccine efficacy was limited by the small sample size of analyzable participants for each qHPV vaccine type. Factors limiting the size of our analyzable study population included a lower-than-expected number of HPV-naive individuals by type, since the sample size projected 100 naive individuals per type, and a lower-than-expected rate of events among those who had been exposed to each HPV type. Some participants were excluded from infection and disease outcome analyses since those with the prevalent endpoint were removed for the incidence calculation and we did not have access to all biopsies for HPV DNA determination.
We also compared our data in the naive vaccinated AMC-072 group with a historical control consisting of the naive placebo group of the V503-020 study. The AMC-072 participants were MSMLWH while the V503-020 participants were HIV-negative MSM. Compared with the vaccinated AMC-072 group, at baseline the V501-020 placebo group was younger, had fewer sexual partners, and had no evidence of any anogenital lesions. The groups also differed by race, with a higher proportion of AMC-072 participants being African-American. Despite being a lower-risk population than our AMC-072 participants, the V501-020 placebo group had a higher incidence of hHSILs associated with all 4 qHPV types than the vaccinated AMC-072 group. These differences were not statistically significant for individual subtypes, primarily due to the size of the study subpopulations, but were significant in a combined analysis of all 4 qHPV types. The level of the titers generated by vaccination among MSMLWH in AMC-072 and the HIV-negative MSM in Merck 020 were similar, and about half of those observed in similarly aged heterosexual men in Merck 020 [5].
The qHPV vaccine was safe and well tolerated by young MSMLWH, and the titers generated suggest that protection should be as long-lasting as in other vaccinated cohorts. There were no differences in titer based on current/nadir CD4 level or HIV VL, other than lower titers to HPV18 among those with a higher HIV VL at month 7. Previously exposed MSMLWH also showed sustained higher titers than naive MSMLWH, consistent with the immunologic memory demonstrated in other populations, including older MSMLWH [21].
Population-based studies among MSM in the United States demonstrate low rates of vaccine initiation, ranging from 13% to 37.6% [22, 23]. It has been estimated that vaccinating 70% of boys will protect 91% of sexual partnerships in MSM [23]. Our findings of no qHPV type–associated LSILs/HSILs among naive vaccinated individuals, that more than half of young MSMLWH were naive to HPV16 at baseline, and the high proportion with HPV infection and disease in the absence of vaccination support recent guidelines for HPV vaccination [10]. These guidelines encourage vaccine initiation at 9–12 years. Catch-up HPV vaccination for all persons through age 26 years, including MSM, is also critical but our data highlight the importance of vaccinating prior to onset of sexual activity whenever possible.
There are several strengths of this study. This was a prospective, phase II multisite study with 2 years of follow-up with rigorous clinical endpoints. The HPV endpoints used to define the naive population and incident-persistent HPV infection included assessment of HPV infection at multiple anatomic locations. The study was performed by clinicians highly experienced in performing HRA and HRA-guided biopsy. There were also several limitations. In addition to sample size limitations, for ethical reasons we were not able to have a placebo control. Our study excluded those with HSILs at screening, and our results likely overestimate the value of vaccinating the entire population of 18–26-year-old MSMLWH. Our results may therefore not be generalizable to all young MSMLWH. We followed participants for only 24 months and were not able to retrieve all biopsies to perform HPV testing, reducing our analyzable dataset.
In summary, young MSMLWH have a very high risk of HPV-related HSILs and a large proportion have been previously exposed to qHPV types. Our data show that qHPV vaccination is safe, well tolerated, and immunogenic in this population, and prevents incident HPV infection and hHSILs associated with vaccine HPV types to which the individual has not yet been exposed. It is expected that the nonavalent HPV vaccine currently in use should perform similarly to the qHPV vaccine in preventing HPV16 and 18, and to protect against the additional 5 HPV types. Our data confirm the need to continue efforts to vaccinate all boys and young men as early as possible prior to sexual exposure to HPV, and to target young MSMLWH for catch-up vaccination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all of the study participants, staff members of the study sites, and Emmes Corporation for their invaluable and enthusiastic support of this study.
Disclaimer. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck and Co. J. M. P. reports receipt of research grant support from Merck and Co. in his conflict-of-interest disclosure.
Financial support. This work was supported by the AIDS Malignany Consortium (AMC) of the National Cancer Institute (grant number UM1CA121974) and the Adolescent Trials Network for HIV/AIDS Interventions (ATN) of the National Institute of Child Health and Human Development (grant numbers U01 HD 040533 and U01 HD 040474). The quadrivalent HPV vaccine was provided and HPV geometric mean titers were measured through the Investigator-Initiated Studies Program of Merck & Co, Inc.
Potential conflicts of interest. S. E. G. reports speaker fees and grants from Merck. S. E. G. also reports grants from Inovio, Medtronic Inc, and Antiva, and consultant fees from THD America, outside the submitted work. T. M. D. reports advisory board fees from BD and Roche and consultant fees from TheVax and Boston Scientific, outside the submitted work. E. Y. C. reports Gilead Science and Merck Sharp & Dohme and advisory board fees, outside the submitted work. J. M. P. is a scientific advisory board member for Merck and Co and Inovio and reports travel support from Merck and Co, personal fees and grants from Vir Biotech, and stock options from Virion Therapeutics, outside the submitted work. E. A. S. reports a lecture honorarium from Physicians Research Network; an honorarium and reimbursement for travel (2019) from the British Association for Sexual Health and HIV; conference fees (2018, 2019) from EUROGIN; and an honorarium (2018) and conference fees (2019) from ASCCP, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Colón-López V, Shiels MS, Machin M, et al. . Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018; 36:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahale P, Engels EA, Coghill AE, Kahn AR, Shiels MS. Cancer risk in older persons living with human immunodeficiency virus infection in the United States. Clin Infect Dis 2018; 67:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverberg MJ, Lau B, Justice AC, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012; 54:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn JA, Belzer M, Chi X, et al. ; AIDS Malignancy Consortium and Adolescent Medicine Trials Network for HIV/AIDS Interventions . Pre-vaccination prevalence of anogenital and oral human papillomavirus in young HIV-infected men who have sex with men. Papillomavirus Res 2019; 7:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palefsky JM, Giuliano AR, Goldstone S, et al. . HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–85. [DOI] [PubMed] [Google Scholar]
- 6. Giuliano AR, Palefsky JM, Goldstone S, et al. . Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joura EA, Leodolter S, Hernandez-Avila M, et al. . Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 2007; 369:1693–702. [DOI] [PubMed] [Google Scholar]
- 8. Harper DM, Franco EL, Wheeler CM, et al. ; HPV Vaccine Study Group . Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–55. [DOI] [PubMed] [Google Scholar]
- 9. Olsson SE, Restrepo JA, Reina JC, et al. . Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res 2020; 10:100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2019; 68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilkin T, Lee JY, Lensing SY, et al. . Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis 2010; 202:1246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkin TJ, Chen H, Cespedes MS, et al. . A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS Clinical Trials Group Protocol A5298. Clin Infect Dis 2018; 67:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brophy J, Bitnun A, Alimenti A, et al. ; HPV in HIV Study Group . Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in girls living with HIV. Pediatr Infect Dis J 2018; 37:595–7. [DOI] [PubMed] [Google Scholar]
- 14. Levin MJ, Moscicki AB, Song LY, et al. ; IMPAACT P1047 Protocol Team . Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr 2010; 55:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levin MJ, Huang S, Moscicki AB, et al. ; IMPAACT P1085 Protocol Team . Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: effect of a fourth dose of vaccine. Vaccine 2017; 35:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McClymont E, Lee M, Raboud J, et al. ; CTN 236 HPV in HIV Study Team . The efficacy of the quadrivalent human papillomavirus vaccine in girls and women living with human immunodeficiency virus. Clin Infect Dis 2019; 68:788–94. [DOI] [PubMed] [Google Scholar]
- 17. Denny L, Hendricks B, Gordon C, et al. . Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine 2013; 31:5745–53. [DOI] [PubMed] [Google Scholar]
- 18. Kojic EM, Kang M, Cespedes MS, et al. . Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014; 59:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cespedes MS, Kang M, Kojic EM, et al. . Anogenital human papillomavirus virus DNA and sustained response to the quadrivalent HPV vaccine in women living with HIV-1. Papillomavirus Res 2018; 6:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dias Goncalves Lima F, Viset JD, Leeflang MMG, Limpens J, Prins JM, de Vries HJC. The accuracy of anal swab-based tests to detect high-grade anal intraepithelial neoplasia in HIV-infected patients: a systematic review and meta-analysis. Open Forum Infect Dis 2019; 6:ofz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellsworth GB, Lensing SY, Ogilvie CB, et al. . A delayed dose of quadrivalent human papillomavirus vaccine demonstrates immune memory in HIV-1-infected men. Papillomavirus Res 2018; 6:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reiter PL, McRee AL, Katz ML, Paskett ED. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health 2015; 105:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fairley CK, Zou H, Zhang L, Chow EPF. Human papillomavirus vaccination in men who have sex with men—what will be required by 2020 for the same dramatic changes seen in heterosexuals. Sex Health 2017; 14:123–5. [DOI] [PubMed] [Google Scholar]
- 24. Loretan C, Chamberlain AT, Sanchez T, Zlotorzynska M, Jones J. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014–2017. Sex Transm Dis 2019; 46:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.