Abstract
DNA molecules containing stretches of contiguous guanine residues can assume a stable configuration in which planar quartets of guanine residues joined by Hoogsteen pairing appear in a stacked array. This conformation, called G4 DNA, has been implicated in several aspects of chromosome behavior including immunoglobulin gene rearrangements, promoter activation, and telomere maintenance. Moreover, the ability of the yeast SEP1 gene product to cleave DNA in a G4-DNA-dependent fashion, as well as that of the SGS1 gene product to unwind G4 DNA, has suggested a crucial role for this structure in meiotic synapsis and recombination. Here, we demonstrate that the HOP1 gene product, which plays a crucial role in the formation of synaptonemal complex in Saccharomyces cerevisiae, binds robustly to G4 DNA. The apparent dissociation constant for interaction with G4 DNA is 2 × 10−10, indicative of binding that is about 1,000-fold stronger than to normal duplex DNA. Oligonucleotides of appropriate sequence bound Hop1 protein maximally if the DNA was first subjected to conditions favoring the formation of G4 DNA. Furthermore, incubation of unfolded oligonucleotides with Hop1 led to their transformation into G4 DNA. Methylation interference experiments confirmed that modifications blocking G4 DNA formation inhibit Hop1 binding. In contrast, neither bacterial RecA proteins that preferentially interact with GT-rich DNA nor histone H1 bound strongly to G4 DNA or induced its formation. These findings implicate specific interactions of Hop1 protein with G4 DNA in the pathway to chromosomal synapsis and recombination in meiosis.
In meiosis, two successive rounds of nuclear division follow a single round of chromosomal DNA replication, reducing the diploid genome to haploidy in preparation for conjugation of the gametes. Faithful recombination between the homologs and their appropriate segregation in the reductional division typically depends on their precise synapsis in prophase of meiosis I. Synapsis typically involves the assembly of the synaptonemal complex (SC), a highly ordered proteinaceous structure consisting of a central region flanked by two lateral elements. Each lateral element serves as the common core for the two sister chromatids derived from each homolog (reviewed in references 18, 32, and 48). An earlier viewpoint held that SC assembly preceded all stages of recombination, juxtaposing the homologs so that crossing over would later occur only between well-aligned homologous sequences. However, temporal and genetic analysis of sporulation in the budding yeast Saccharomyces cerevisiae has revealed that the initial phases of recombination take place much earlier than synapsis and probably contribute to the proper association of the homologs (reviewed in references 18, 19, and 32). Specifically, double-strand breaks (DSBs) are created by the action of the SPO11 gene product in conjunction with the products of several other early meiotic genes well before the assembly of SC (14, 15). Resection of the DSBs exposes a free 3′ single-stranded end that, in association with RecA-like proteins, may participate in a search for the complementary sequence in the homolog (reviewed in reference 19). Concerted strand invasion by both of the free ends created by the DSB leads to the formation of a joint molecule that stably interconnects the homologs (reviewed in reference 18). This configuration appears to persist throughout meiotic prophase and then undergo resolution at the end of the pachytene phase, when SC disassembly occurs and recombinant DNA strands can first be detected (5, 35, 36).
Genetic analysis of meiotic recombination in S. cerevisiae has provided considerable insight into the functional relationships between these processes (reviewed in reference 21). Numerous mutations that abolish DSB formation (such as rad50 and spo11) or later steps of DSB processing (including dmc1 and rad51) generally prevent SC formation (reviewed in references 18 and 32). On the other hand, null mutations in other genes prevent normal synapsis but do not completely abolish recombination. One such gene is ZIP1, which encodes a major structural element of the central core of the SC (43, 44). zip1 mutants are reduced for crossing over about twofold and notably lack chiasma interference, thus implicating the SC in controlling the distribution of crossovers along the chromosomes (42). Other mutations that block synapsis are found in HOP1, RED1, and MEK1, all three of which are required in some manner for the formation and synapsis of the lateral elements (13, 28–30, 40). Hop1 is a DNA-binding protein (17) that appears to act conjointly with Red1 to form a highly condensed core structure that facilitates joining of sister chromatids (40), while Mek1 is a protein kinase controlling the behavior of these other proteins (2, 11, 30). The inability of hop1 or red1 mutations to protect against the meiotic lethality of rad52, which renders DSBs irreparable, indicates that neither HOP1 nor RED1 is essential for DSB formation (12, 24, 26, 29). These findings might be interpreted as evidence that the Hop1-Red1 assemblage acts only in the later stages of recombination, after the DSB has been created. However, the appearance of DSBs is reduced and delayed in hop1 meiosis, suggesting a role for Hop1 in DSB formation (18). These seemingly contradictory findings concerning the role of Hop1 in meiotic synapsis and recombination motivated us to explore the properties of the gene product in vitro.
Biochemical characterization of the HOP1 gene product had previously shown it to be an oligomeric DNA-binding protein with a greater affinity for negatively supercoiled DNA than for nicked circular duplex DNA (17). Potentially, such binding to underwound duplex DNA within the chromosomes could anchor one homolog to the other while the DSB repair pathway acted in parallel to generate the more specific homologous interactions required for recombination. On the other hand, Hop1 has been shown to confer protection against the exonucleolytic degradation of linear duplex DNA that occurs in extracts of meiotic nuclei, suggesting a role for Hop1 in modulating the processing of DSBs (17). In either case, it may benefit our understanding of synapsis to learn whether there is any sequence specificity in the interaction of Hop1 protein with substrate DNA. Although assays for its binding to plasmid DNA and oligonucleotides initially failed to reveal sequence specificity, it was shown that Hop1 binding to double-stranded M13 DNA is competitively inhibited by G-rich oligonucleotides (17). Realization that these G-rich sequences are capable of forming G-quartet structures (G4 DNA) led us to explore the possibility that G4 DNA may be significant to Hop1 binding in vitro and perhaps crucial to its role in meiosis.
The G quartet, the structural unit of G4 DNA, is a nucleic acid motif in which four guanine bases are joined by Hoogsteen pairing in a cyclic planar array. When each base is situated within an uninterrupted track of G residues along its constituent DNA strand, a stack of G quartets can be formed, and this overall assemblage (known as G4 DNA when all four strands are in parallel orientation and as G2′ DNA when two strands are antiparallel) stably joins all four phosphodiester backbones into a unitary structure that strongly resists dissociation (reviewed in reference 49). In this report, henceforth, we will refer to both types as G4 DNA. The ability of natural sequences from immunoglobulin switch regions, gene promoters, and telomeres to form G4 DNA under physiological conditions has attracted considerable attention. Although it has not been proven that G4 DNA exists within the yeast cell, its probable significance to meiosis is evident from the finding that the product of the SEP1/KEM1 gene, which is essential for normal progression through meiotic prophase, displays a G4-DNA-specific nuclease activity (16, 22, 23, 45). In addition, the identification of a resolvase activity in humans (10) and the Sgs1 helicase in S. cerevisiae (41), both of which are able to unwind G4 DNA to single-stranded DNA, further attests to the probable biological significance of G4 DNA in vivo. In the present study, we have assessed the ability of purified Hop1 to interact with G4 DNA and have detected avid binding. We also show here that Hop1 catalyzes the transformation of DNA into this configuration, further implicating G4 DNA in the mechanism of meiotic synapsis and recombination.
MATERIALS AND METHODS
DNA and proteins.
Chemicals were of analytical grade, and solutions were prepared using Milli Q pure water. T4 polynucleotide kinase was obtained from New England Biolabs, Beverly, Mass., and biochemicals were from Sigma Chemical Company, St. Louis, Mo. All oligonucleotides used in this study were purchased from Keystone Laboratory Inc., Menlo Park, Calif.; their sequences are listed in Table 1. Oligonucleotides were labeled at their 5′ ends using [γ-32P]ATP and T4 polynucleotide kinase and were isolated by electrophoresis on 8 M urea–8% polyacrylamide gels as described elsewhere (34). G4 DNA was prepared and isolated as described elsewhere (38). Briefly, 32P-labeled substrates were incubated in a buffer containing 20 mM Tris-HCl (pH 8), 120 mM KCl, and 1 mM EDTA at 37°C for 16 h. Samples were electrophoresed at 4°C in 6% nondenaturing polyacrylamide gels at 10 V/cm for 4 h. The band corresponding to G4 DNA was excised, and DNA was eluted by crushing and soaking the gel in TE buffer (10 mM Tris-HCl [pH 7.5], 0.1 mM EDTA) containing 0.3 M NaCl at 40°C for 16 h. The suspension was centrifuged, and G4 DNA in the supernatant was precipitated with ethanol in the presence of 0.3 M ammonium acetate. The pellet was washed with 70% ethanol and resuspended in TE buffer containing 50 mM KCl. G4 DNA was isolated by gel filtration on Sephadex G-50. Aliquots were stored at −20°C in TE buffer containing 50 mM KCl. Circular single-stranded and negatively superhelical DNA were prepared from bacteriophage M13 (20). Linear DNA was prepared by cleaving negatively superhelical DNA with HaeIII as specified by the vendor. The concentration of DNA was estimated at A260 nm and expressed as moles of nucleotide residues. RecA proteins from Escherichia coli and Mycobacterium tuberculosis (20), Hop1 protein from S. cerevisiae (17), and histone H1 (27) were purified, and their concentrations were determined as described previously (17).
TABLE 1.
Oligonucleotides used in this study
| Name | Sequence (5′ to 3′)a | Length (bases) |
|---|---|---|
| 1G4 | CGGTGTGTGGGGATACTCGAGCGGTGTCTGATAGTG | 36 |
| 2G4 | CGGTGTGTGGGGATACTCGAGCGGTGTCTGGGGATG | 36 |
| 4G3 | AATTCTGGGTGTGTGGGTGTGTGGGTGTGTGGGTGTGG | 38 |
| 4G3mut | AATTCTGGGTGTGTGGGAGAGAGGGTGTGTGGGTGTGG | 38 |
| 6G3 | AATTCTGGGTGTGTGGGTGTGTGGGTGTGTGGGTGTGTGGGTGTGTGGGTGTGG | 54 |
| TP | TGGACCAGACCTAGCAGCTATGGGGGAGCTGGGGAAGGTGGGAATGTGA | 49 |
| TPmut | TGGACCAGACCTAGCACTATCTGCAAGTCAAGTTGACTACGTATACATA | 49 |
| OX-1T | ACTGTCGTACTTGATATTTTGGGGTTTTGGGGAATGTGA | 39 |
Tracts of three or four contiguous guanine residues are in boldface.
Electrophoretic mobility shift and competition assays.
The standard buffer (20 μl) for the DNA-binding assay contained 10 to 20 pmol of 32P-labeled single-stranded DNA or G4 DNA in 20 mM Tris-HCl (pH 7.5), 0.1 mM ZnCl2, and Hop1 protein at the indicated concentrations. In experiments involving RecA proteins, reactions were done in a buffer (20 μl) containing 30 mM Tris-HCl (pH 7.5), 1.5 MgCl2, and RecA protein at the indicated concentrations. Unless mentioned, all the reactions were performed at 30°C for 30 min. Samples were loaded onto a 6% polyacrylamide gel and electrophoresed at 4°C in 45 mM Tris-borate buffer (pH 8.3) at 10 V/cm for 4 h. The gel was dried at 60°C on a Whatman 3 mM filter paper, and DNA-protein complexes were visualized by autoradiography.
To assay the effect of competitors, Hop1 protein and serial dilutions of unlabeled competitors were premixed in the standard assay buffer prior to the addition of labeled DNA probe. The reaction mixtures were incubated at 30°C for 30 min. Samples were electrophoresed, and the amount of protein-DNA complexes formed in the absence or presence of competitors was visualized as described above.
Assay for the formation of G4 DNA.
Reactions were carried out in a buffer containing 20 mM Tris-HCl (pH 7.5), 0.1 mM ZnCl2, and 5 pmol of 32P-labeled oligonucleotide in the absence or presence of indicated concentrations of Hop1 protein, or histone H1, for 30 min at 30°C. The reaction was terminated by the addition of proteinase K, KCl, and sodium dodecyl sulfate (SDS) to final concentrations of 0.2 mg/ml, 0.12 M, and 0.2%, respectively. After incubation for 30 min, samples were loaded onto 6% nondenaturing polyacrylamide gel and electrophoresed at 4°C in 45 mM Tris-borate buffer (pH 8.3) at 10 V/cm for 4 h. The formation of G4 DNA was visualized by autoradiography as described above.
DMS interference.
Methylation of single-stranded oligonucleotides was performed by incubating 32P-labeled DNA with 0.05% dimethyl sulfate (DMS) in TE buffer (pH 8) at 24°C for 5 min (partial methylation) or 30 min (full methylation). The reaction was stopped by the addition of 50 μl of solution containing 1.5 M sodium acetate (pH 6), 1 M 2-mercaptoethanol, and 200 μg of yeast tRNA per ml. DNA was precipitated by ethanol and collected by centrifugation at 15,000 × g for 15 min, and the pellet was washed with 70% ethanol. The pellet was dried and resuspended in 20 μl of TE buffer containing 100 mM KCl, and the methylated DNA was used in binding experiments. In the second set of experiments, partially methylated 32P-labeled DNA (5 pmol) was incubated with 100 to 250 nM Hop1 protein at 30°C for 30 min. After addition of proteinase K (0.2 mg/ml), samples were incubated at 30°C for 30 min, loaded onto a 6% nondenaturing polyacrylamide gel, and electrophoresed as described above. The bands corresponding to G4 DNA and its single-stranded form were excised; DNAs were isolated from the crushed gel, precipitated by ethanol, and then subjected to cleavage by incubation with 1 M piperidine at 90°C for 15 min (25). Samples were dried, and the pellets were resuspended in 30 μl of water. This procedure was repeated two more times. The pellets were dissolved in a solution containing 95% deionized formamide, 10 mM EDTA, and 0.05% each bromophenol blue and xylene cyanol. The products were analyzed on 20% polyacrylamide gels in the presence of 7 M urea.
RESULTS
Binding of Hop1 to guanine-rich DNA is sequence specific.
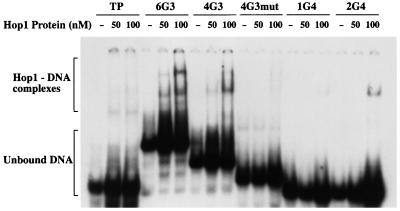
To investigate the molecular function of the HOP1 gene product, we had previously devised methods for abundant overexpression of HOP1 in vegetative cells and for purification of Hop1 protein to homogeneity. We found that purified Hop1 bound efficiently to duplex DNA with little, if any, apparent sequence specificity, but this binding was competitively inhibited by oligonucleotides with G-rich sequences (17). Recognition that the more effective competitor sequences contained multiple stretches of contiguous guanine residues, rendering them capable of assuming the G4 DNA configuration (49), suggested that G quartets may play an important role in the observed DNA binding by Hop1 protein. To explore this, we tested Hop1 binding to a series of oligonucleotides containing one or more tracts of contiguous guanine residues (Table 1). Hop1 protein was incubated with [γ-32P]ATP-labeled oligonucleotides, and the reaction mixtures were separated by nondenaturing gel electrophoresis and analyzed by autoradiography. In a typical experiment (Fig. 1), Hop1 protein displayed a higher affinity for those oligonucleotides with the greater numbers of guanine repeats. A 36-mer bearing a single stretch of four guanine residues (1G4) formed only a barely detectable band of protein-DNA complexes at two Hop1 concentrations tested, whereas a similar 36-mer containing two tracts of guanine residues (2G4) formed a faint but distinct band after incubation with Hop1 at the higher concentration. Other oligonucleotides containing multiple stretches of G residues formed complexes more abundantly. Oligonucleotide 6G3, which contains six segments of guanine repeats, bound Hop1 most efficiently, leading to the formation of two major complexes in addition to several minor species.
FIG. 1.
Hop1 protein binds selectively to oligonucleotides containing contiguous G-rich residues. Reaction mixtures (20 μl) contained 20 mM Tris-HCl (pH 7.5), 0.1 mM ZnCl2, 10 pmol of 32P-labeled oligonucleotide (Table 1), and the specified concentration of Hop1 protein. Samples were incubated at 30°C, separated on a 6% polyacrylamide gel, and visualized by autoradiography.
A simple explanation for these results is that Hop1 protein forms a specific complex with DNA that has undergone G-quartet formation either by zigzag folding within a single oligonucleotide with four or more guanine repeats or by forming a complex of multiple oligonucleotides, each of which may have fewer repeats. To explore complexes of the latter type, we tested oligonucleotide TP, a 49-mer that was used previously to test the G4-DNA-specific nuclease encoded by KEM1/SEP1 (22, 23). Liu et al. (23) had shown that TP forms G4 DNA less efficiently than 2G4. Consistent with this, Hop1 formed complexes with TP less abundantly than with 2G4. No complexes were seen with TPmut (Table 1), which lacks guanine repeats entirely. Together, these experiments suggest that high-affinity binding by Hop1 protein to these oligonucleotides does not depend simply on the presence of G-rich sequences but requires that the DNA acquire the G4 configuration. This possibility was explored further by testing an oligonucleotide (4G3mut) that differs from 4G3 in the sequences intervening between the second and third repeats, as this pattern of residues does not permit G4 DNA formation (49). Consistent with the concept that G4 DNA formation is crucial for stable binding, Hop1 protein clearly bound well to the 38-mer that can form G4 DNA (4G3) but not to the mutant derivative (4G3mut). These findings establish that efficient recognition of G-rich DNA by Hop1 protein requires that the DNA contain a sequence that is capable of forming G4 DNA efficiently.
Hop1 protein displays greater affinity for G4 DNA.
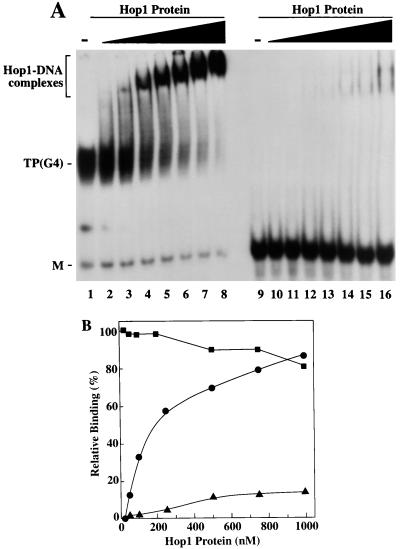
To establish whether Hop1 binding depends on the actual folding of the DNA substrates into the G4 DNA configuration, we tested binding with oligonucleotides that previously had been converted into G4 DNA. This conversion was accomplished by incubating appropriate concentrations of the oligonucleotides at 37°C for 16 h in a buffer containing 120 mM KCl (38). Electrophoresis under nondenaturing conditions and visualization under UV illumination revealed partial conversion of the oligonucleotides into forms with reduced mobility (data not shown), as expected for the behavior of G4 DNA that has formed between pairs of oligonucleotides (49). The band corresponding to G4 DNA was excised from the gel and isolated by electroelution (34). This G4 DNA and the corresponding monomeric form were assayed separately for complex formation with increasing concentrations of Hop1 protein while keeping the DNA concentration constant. Figure 2A shows that the yields of DNA-protein complexes increased with increasing concentrations of Hop1 protein, and there was a concomitant reduction in the amount of free G4 DNA. The autoradiograms were scanned in a laser densitometer for quantification (Fig. 2B). We infer that the high affinity of Hop1 protein for G-rich DNA depends on the ability of that DNA to form G quartets. These findings, as well as the results of similar experiments (data not shown), enable us to quantify the affinity. We estimate that the dissociation constant for binding of Hop1 protein to G4 DNA is on the order of 2 × 10−10 M, indicative that this binding is about 1,000-fold stronger than that shown earlier for its interaction with normal duplex DNA (17).
FIG. 2.
Binding of Hop1 protein to G4 DNA. (A) Electrophoretic mobility shift assays for G4 DNA-Hop1 protein complexes. Ten picomoles of 32P-labeled G4 DNA (left half) or its single-stranded form (49-mer TP DNA; right half) was incubated in the absence or presence of increasing concentrations of Hop1 protein and analyzed by polyacrylamide gel electrophoresis and autoradiography. Lane 1 and 9 represent substrate DNA lacking added Hop1 protein. TP G4 DNA (lanes 2 through 8) or its single-stranded form (lanes 10 through 16) was incubated with 25, 50, 100, 250, 500, 750, and 1,000 nM Hop1 protein, respectively. M, unfolded monomeric form of TP. (B) Quantitation of Hop1 protein binding to G4 DNA and to unfolded precursor TP, as determined by scanning the autoradiograms in panel A with a laser densitometer. ●, TP (G4) DNA-Hop1 protein complexes; ▴, complexes of Hop1 protein with TP without prior folding (right half of panel A); ■, unfolded TP remaining unbound by Hop1 protein after incubation in the presence of G4 DNA (left half of panel A).
Specific requirements for Hop1 binding to G4 DNA.
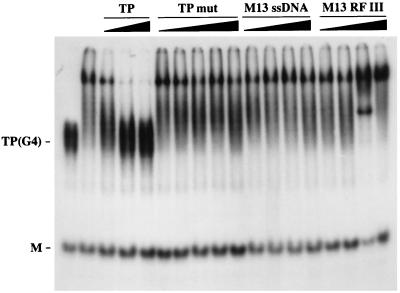
To explore the specificity of Hop1 interaction with G4 DNA, we assayed the binding of 32P-labeled G4 DNA in the presence of unlabeled competitors differing in the ability to form G quartets. As shown in Fig. 3, Hop1 binding was not suppressed by a 10- to 50-fold excess of either of two unlabeled competitors that lack G4 DNA configurations—single-stranded M13 DNA and oligonucleotide TPmut. On the other hand, addition of unlabeled G4 DNA competed effectively, displacing the labeled oligonucleotide from its association with Hop1. Surprisingly, inclusion of duplex M13 DNA in the reaction mixture resulted in increased association of the labeled oligonucleotide with Hop1. The basis for this enhancement is unknown, but it seems plausible that the catalysis of G4 DNA formation by Hop1 protein (described below) is assisted in some manner by undefined sequences within the duplex M13 DNA. Regardless of this apparent enhancement, these experiments clearly demonstrate that G4-DNA-containing oligonucleotides compete for binding to Hop1 protein.
FIG. 3.
Competitive inhibition of Hop1 protein binding to G4 DNA. Samples contained 10 pmol of 32P-labeled TP (as G4 DNA), 0.5 μM Hop1 protein, and the indicated concentrations of unlabeled competitor DNA substrates. The relative mass of unlabeled TP DNA was 10, 50, or 100 pmol; that of TPmut DNA or M13 DNA in single-stranded or linear duplex form was 50, 100, 250, or 500 pmol. Reaction mixtures were incubated and analyzed as described for Fig. 1. TP(G4) denotes G4 DNA prepared from 49-mer TP DNA, M is its constituent monomer, and TPmut is the mutant analogue. M13 RF III is linear duplex DNA generated by cleaving form I M13 DNA with HaeIII, and M13 ssDNA is the positive strand isolated from virions.
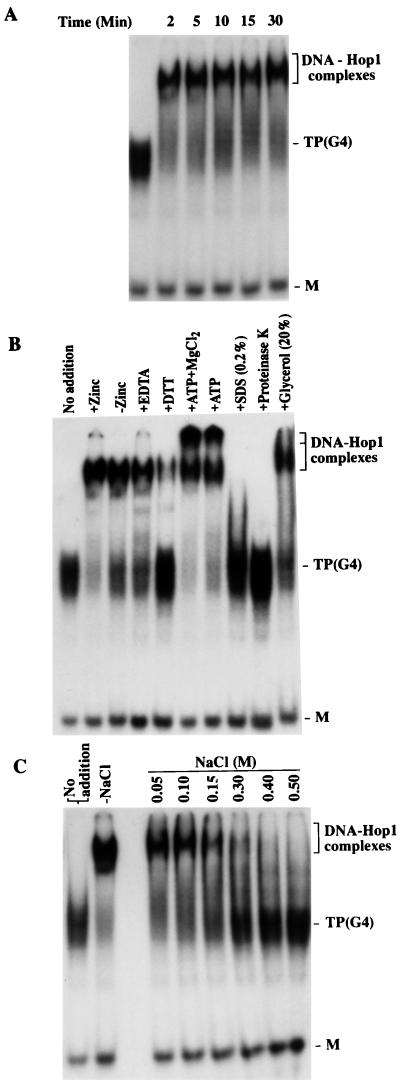
Other parameters controlling Hop1 binding were explored by varying the reaction conditions. Figure 4A shows that binding had already occurred strongly within the briefest incubation period tested (2 min) and did not change appreciably with longer incubations. We also explored the effect of added zinc ion because (i) the Hop1 sequence contains an apparent zinc ion-binding motif (12), (ii) zinc is detectable in the purified protein, and (iii) addition of zinc ion was already shown to affect DNA binding (17). Figure 4B shows that added Zn2+ led to a moderate increase in complex formation, while EDTA addition decreased the yield. It can also be seen here that complexes were absent if the reaction mixture was treated with proteinase K or 0.2% SDS, consistent with the need for Hop1 protein to be present and in native conformation. Addition of dithiothreitol decreased binding, but it is unknown whether this reflects an effect on zinc ion chelation caused by modifying the cysteine residues. Addition of ATP caused a decreased mobility of the complex, especially at higher Mg2+ levels, but much of the label failed to migrate beyond the gel pocket, suggesting the formation of an insoluble aggregate of unknown nature. Finally, although addition of NaCl below 150 mM had no effect, binding decreased at higher concentrations and was abolished at 0.5 M (Fig. 4C).
FIG. 4.
Characterization of binding of Hop1 protein to G4 DNA. Reactions were carried out in a standard assay buffer (20 μl) containing 10 pmol of TP G4 DNA and 0.5 μM Hop1 protein, plus the indicated additional treatments, as described in Materials and Methods. (A) Kinetics of Hop1 protein binding to G4 DNA with no added constituents. (B) Complex formation with G4 DNA in the absence or presence of zinc (0.1 mM), EDTA (5 mM), dithiothreitol (DTT; 10 mM), ATP (5 mM), MgCl2 (10 mM), proteinase K (0.2 mg/ml), or SDS or glycerol at the indicated concentrations. (C) Effect of NaCl added at the indicated concentrations. TP(G4) and M denote G4 DNA prepared from TP oligonucleotide and its unfolded constituent monomer, respectively.
RecA proteins and histone H1 do not display stable G4 DNA binding.
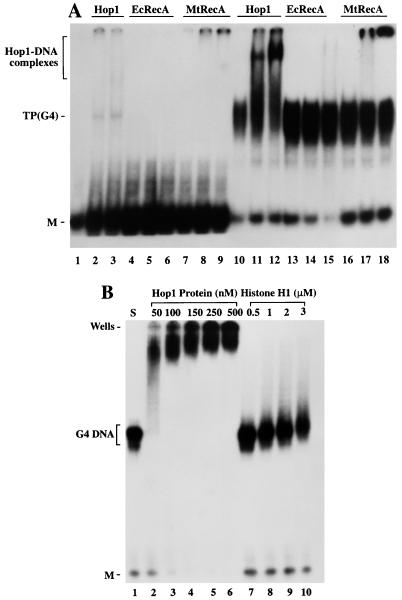
It had previously been shown that certain strand exchange proteins, including not only the RecA proteins of bacteria but also a yeast homolog (Rad51), display significantly higher affinity for single-stranded DNA substrates with higher GT contents (46, 47). In light of the present evidence that Hop1 displays a preferential affinity for G4 DNA, we wished to determine whether these strand exchange proteins might interact with their DNA substrates in a similar manner. As a test of this possibility, the same oligonucleotides that had been shown to bind Hop1 were also incubated with certain RecA proteins. We tested not only RecA of E. coli but also that of M. tuberculosis, which has a genome that is especially rich in GC content (1) and might therefore be expected to favor this mode of interaction. However, no bands indicative of RecA-G4 DNA complexes could be detected by gel electrophoresis for the E. coli protein (Fig. 5A). In the presence of the M. tuberculosis RecA protein, no bands were seen within the gel, although a small proportion of the label remained in the gel pocket, where it presumably was bound to insoluble material. Aside from this ambiguous result, we conclude that conditions suitable for demonstration of Hop1-G4 DNA binding fail to provide any convincing evidence for a similar mode of binding by RecA proteins.
FIG. 5.
RecA proteins and histone H1 fail to form G4 DNA complexes that are stable to electrophoresis. (A) Assays with Hop1 protein in comparison with the RecA proteins. Reactions were performed in assay buffer (20 μl) containing 20 mM Tris-HCl (pH 7.5), 10 pmol of 32P-labeled TP oligonucleotide (lanes 1 to 9) or its G-quartet structure, TP G4 DNA (lanes 10 to 18), plus the proteins indicated above each lane. Lanes 1 and 10 are controls lacking any added protein. As a positive control, Hop1 protein was substituted at a concentration of 50 nM (lanes 2 and 11) or 100 nM (lanes 3 and 12) in the presence of 0.1 mM ZnCl2. Identical G4 DNA samples were incubated with RecA protein from E. coli (EcRecA) at a concentration of 100 nM (lanes 4 and 13), 250 nM (lanes 5 and 14), or 500 nM (lanes 6 and 15) and with that from M. tuberculosis (mtRecA) at 100 nM (lanes 7 and 16), 250 nM (lanes 8 and 17), or 500 nM (lanes 9 and 18) in the presence of 1.5 mM ATP and 2 mM MgCl2. (B) Histone H1 fails to bind G4 DNA under these conditions. Reactions were carried out with TP G4 DNA with Hop1 or histone H1 at concentrations indicated above each lane. Samples were separated on a 6% polyacrylamide gel and visualized by autoradiography as described in Materials and Methods. M, unfolded TP monomer.
Additional support for specificity in the binding of G4 DNA by Hop1 protein is evident from comparisons with binding assays for the positively charged chromosomal protein, histone H1, which has previously been shown to enhance G4 DNA formation. In this experiment (Fig. 5B), a fixed concentration of G4 DNA was incubated with increasing concentrations of either Hop1 or histone H1. Reaction mixtures were analyzed by performing nondenaturing gel electrophoresis and determining the mobilities of protein-DNA complexes by autoradiography. Figure 5B shows that low concentrations of Hop1 protein produced shifted complexes of distinct mobilities. In contrast, incubation of G4 DNA with increasing amounts of histone H1, even at 60-fold-higher concentration, did not alter the mobilities of either G4-DNA or its unfolded constituent oligonucleotide.
Hop1 protein promotes the formation of G4 DNA.
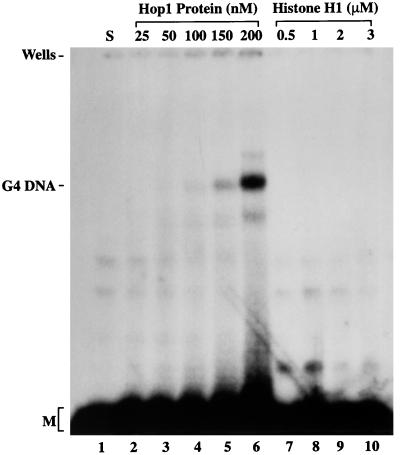
Evidence described above (Fig. 2A) had shown that Hop1 protein has a much higher affinity for G4 DNA than for oligonucleotide that had not previously been converted into the folded configuration. This might be interpreted as indicating that Hop1 binds stably only to G4 DNA and that the small amount of DNA-protein complexes seen for the unfolded control represented binding to a subfraction of the oligonucleotide that had formed G4 DNA spontaneously before incubation with Hop1. On the other hand, perhaps weak Hop1-DNA interactions that are unstable to electrophoresis might gradually have induced the formation of G4 DNA, which thereby gained the potential for stable Hop1 binding. To explore the latter possibility, we incubated oligonucleotide TP with increasing concentrations of Hop1 protein and then treated the reaction mixtures with proteinase K to remove the protein, so that the underlying DNA configuration could be analyzed separately from binding. Nondenaturing gel electrophoresis (Fig. 6) showed that Hop1 protein did indeed promote the formation of stable G4 DNA in a manner similar to that previously shown for S. cerevisiae Rap1 (9) and for the β subunit of Oxytricha telomere-binding protein (7).
FIG. 6.
Hop1 protein promotes the formation of stable G4 DNA. Reactions were performed in a standard assay buffer containing 20 mM Tris-HCl (pH 7.5), 0.1 mM ZnCl2, and 5 pmol of 32P-labeled TP 49-mer in the absence (lane 1) and presence of indicated concentrations of Hop1 protein or histone H1 at 30°C. After incubation for 30 min, proteinase K was added to a final concentration of 0.2 mg/ml and incubation was continued for an additional 30 min. Samples were analyzed on a 6% polyacrylamide gel and visualized by autoradiography. G4 DNA indicates the position of the folded form, M is that of its unfolded constituent monomer, and S denotes substrate DNA.
Previous studies indicated that polycations and highly charged basic proteins such as histone H1 promote stable association of Oxytricha telomeric DNA into dimers and tetramers involving G quartets (7). However, it has been noted that G4 DNA formation that is mediated by basic proteins occurs only at high protein concentrations and requires prolonged periods of incubation (90 to 180 min). To determine whether histone H1 has the capacity to promote the formation of G4 DNA under conditions used in this study, TP oligonucleotide was incubated with increasing concentrations of histone H1. Histone H1 failed to promote the formation of G4 DNA even at 60-fold-higher concentration than Hop1 protein (Fig. 6). Thus, folding of oligonucleotide TP into G4 DNA conformation appears not to be an inherent feature of histone H1 or strand exchange proteins.
An experiment reported above (Fig. 5) indicates not only that the RecA proteins fail to bind G4 DNA stably in the manner shown for Hop1 protein but also that they are unable to induce G4 DNA formation. Because no stable DNA-protein complexes were formed with the RecA proteins (lanes 4 to 9), any free G4 DNA that might have been induced should have been evident on the gel without our having to subject these samples to proteolysis. There being no band at the position of G4 DNA in these lanes, we conclude that the RecA proteins and histone H1 do not catalyze G4 DNA formation under these conditions.
Methylation interference.
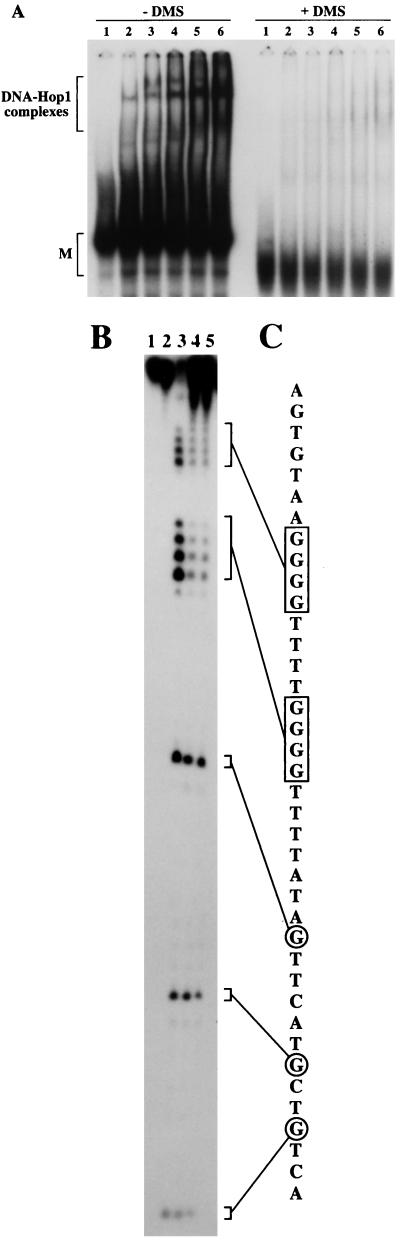
It has previously been shown that folded G-quartet structures involve Hoogsteen base pairing between guanine residues (37). To identify those guanine residues that are important for the binding of Hop1 and for the formation of G4 DNA by Hop1 protein, methylation interference assays were carried out using DMS as the methyl donor. Unmethylated or methylated 32P-labeled OX-1T 39-mer was incubated with increasing concentrations of Hop1 protein. Reaction mixtures were analyzed by nondenaturing gel electrophoresis, and the protein DNA complexes were visualized by autoradiography. The results in Fig. 7A demonstrate that methylation of guanine residues led to a considerable decrease in Hop1 binding to DNA. To ascertain that loss of binding did not result from random modification of the substrate and to identify the residues involved in G-quartet formation, we carried out methylation interference assays with partially methylated DNA. Oligonucleotide OX-1T that had been treated with DMS for 5 min was incubated with 100 or 250 nM Hop1 protein. DNAs were deproteinized by incubation with proteinase K, analyzed by nondenaturing gel electrophoresis, and visualized by autoradiography. The bands corresponding either to G4 DNA or to the fraction that had remained in single-stranded form were excised from the gel, and DNAs were isolated as described in Materials and Methods. DNAs were treated with piperidine, and the cleavage pattern was analyzed on a 20% polyacrylamide gel in the presence of 7 M urea. Figure 7B shows that guanine residues were uniformly cleaved in the single-stranded DNA. By contrast, two tracts of guanine residues were less efficiently cleaved in the sample corresponding to G4 DNA. The intensities of bands corresponding to guanine residues in the 5′ end that are not involved in the formation of G4 DNA serve as internal controls. A similar pattern of protection of guanine residues was observed in methylation protection assays (data not shown). These findings ascertain that the DNA species capable of binding Hop1 protein with high affinity must contain arrays of unmethylated deoxyguanine residues, as is the case for G4 DNA.
FIG. 7.
Methylation of the N7 group of guanine modifies binding of Hop1 protein to DNA. (A) Mobility shift assay of Hop1 protein binding to DMS-modified and unmodified DNA. Reactions were performed in a standard assay buffer containing 20 mM Tris-HCl (pH 7.5), 0.1 mM ZnCl2, and 10 pmol of 32P-labeled OX-1T oligonucleotide [either unmodified (−DMS) or modified (+DMS)] in the absence (lane 1) or presence of Hop1 protein at a concentration of 25 (lane 2), 50 (lane 3), 100 (lane 4), 250 (lane 5), or 500 nM (lane 6), respectively. Samples were incubated and analyzed as described in the legend to Fig. 1. M denotes the position of unfolded monomer. (B) Methylation interference footprinting identifies the involvement of contiguous dG residues in the formation of G quartets. Five picomoles of partially methylated and radiolabeled OX-1T oligonucleotide was incubated with 100 nM Hop1 protein at 30°C for 30 min and then deproteinized by incubation with proteinase K (0.2 mg/ml) for 30 min at 30°C. G4 DNA and single-stranded DNA were then separated by polyacrylamide gel electrophoresis as described in the legend to Fig. 6 and isolated from the gel. Aliquots of these isolates were treated with piperidine, subjected to the Maxam-Gilbert chemical sequencing reaction, and analyzed on a polyacrylamide gel in the presence of 7 M urea as described in Materials and Methods. Single-stranded DNA (lane 3) and G4 DNA (lane 4) isolates display differential intensities of G-specific cleavage in the poly(dG) segments. Control aliquots of the single-stranded DNA (lane 1) and G4 DNA (lane 2) samples were not treated with piperidine. The aliquot in lane 5 was treated identically to that in lane 4 except the G4 DNA was formed by incubation with 250 nM Hop1 protein. (C) Summary of methylation interference and cleavage pattern. The guanine residues involved in G-G Hoogsteen base pairing are boxed, while those residues that are not involved are circled.
DISCUSSION
Hop1 protein is known from genetic analysis to play a crucial role in meiotic synapsis (11, 17). In this study, we have explored the possibility that the interaction of Hop1 with chromosomal DNA involves its specific affinity for G4 DNA. We have found not only that oligonucleotides capable of forming G quartets are most effectively bound by Hop1 protein but also that preformation of G4 DNA strongly enhances binding. These findings suggest that the crucial function of Hop1 in yeast meiosis involves its interaction with G4 DNA.
Previous studies have suggested a role for G4 DNA in meiotic synapsis. Sen and Gilbert (37) demonstrated that four identical molecules containing stretches of guanine residues could be stably joined in parallel by the formation of G quartets between them. On this basis, they made the intriguing suggestion that meiotic synapsis might entail the joining of the chromatids to one other in this manner. Although their model focused on the possibility that G4 DNA would join all four chromatids, the ability of oligonucleotides containing two or more guanine repeats to form G4 DNA by dimerization (such as several of the oligonucleotides used in this work) raises the possibility that pairs of duplexes with neighboring repeats would be joined to each other. Later studies have revealed that the product of the KEM1/SEP1 gene acts as a DNase with specificity toward G4 DNA, thus suggesting that Kem1/Sep1 serves to process G4 DNA formed earlier in meiotic prophase (22). Detailed phenotypic analysis has revealed a pachytene-phase arrest in kem1/sep1 mutants, indicating that hydrolysis of G4 DNA may be required for further progression through meiosis (45). Furthermore, there was a striking incidence of nonhomologous synapsis in these mutants, perhaps indicative of a requirement for Kem1/Sep1-mediated DNA hydrolysis in the desynapsis of mispaired chromatids. Together, these findings favor the possibility that G4 DNA plays a crucial role in meiotic synapsis and recombination.
How might the Hop1 protein and G4 DNA act together to promote synapsis? A full understanding will require direct analysis of G4 DNA in meiotic cells, but certain possible roles can be inferred from the mutant phenotypes and cytological analysis. The HOP1, RED1, and MEK1 genes define a single epistasis group, as multiple mutants for these genes generally reduce recombination to about the same extent as individual mutants (32). These findings suggest that the products of all three genes collaborate in executing a common function, and phenotypic analysis indicates that this function is important for proper synapsis and crossing over between homologs. Immunochemical staining reveals that Hop1 and Red1 colocalize discontinuously along the chromosomes early in synapsis, with the Red1 staining pattern progressively coalescing into a more nearly continuous array along each bivalent as Hop1 is progressively lost (40). The specificity of genetic interactions between HOP1 and RED1 suggests a direct interaction between the proteins (8, 13), as confirmed by two-hybrid experiments (13). Since Hop1 is a DNA-binding protein with specificity for G4 DNA sequences, we feel it likely that Hop1 binds directly to the chromatids via this interaction (perhaps also playing a role in the formation of G4 DNA) and later dissociates from the DNA in a manner that leaves Red1 interactions intact.
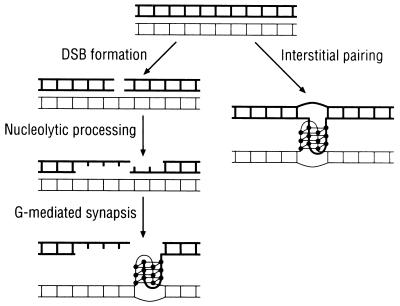
There appear to be at least two classes of DNA transactions that might well depend on G4 DNA and involvement of Hop1 protein (Fig. 8): (i) broad-scale interactions along the length of intact duplexes and (ii) more specific functions involved in the formation or processing of DSBs. With regard to the former, it has been argued convincingly (18, 32) that homologous chromatids interact rather nonspecifically along their length prior to the establishment of the sequence-specific interactions involved in gene conversion and crossing over. Since DNA normally is negatively supercoiled in vivo (50) and Hop1 preferentially binds DNA of this type (17), we suggest that Hop1 interacts with underwound duplex DNA in G-rich regions and mediates the folding of appropriate segments into G quartets, some of which may interconnect homologous duplexes. Alternatively, the duplex DNA may transiently become single-stranded during a genome-wide search for homologous sequences, thereby contributing to the formation of G4 DNA. Consistent with this notion, there is evidence for the unwinding of heterologous and homologous duplex DNA during search for homology by E. coli RecA protein (33). A prevalence of such interactions might preferentially align homologous segments due to the identity in their patterns of G-rich sequences. Alternatively, homologous alignment of this sort might serve to maintain juxtaposition of sister chromatids, thereby providing a structural basis for distinguishing the sister duplex from either homolog as a precondition for the preferential exclusion of sister chromatid crossovers (18, 32).
FIG. 8.
Hypothetical mechanism of formation and/or stabilization of G4 DNA by Hop1 protein. In the model, bold and light DNA molecules represent homologs and closed circles denote guanine residues. Once a DSB is produced at a hot spot by Spo11 endonuclease, the free ends are resected by exonuclease and/or helicase activities to generate a 3′ overhang. Subsequently, Hop1 protein, by binding to the guanine repeats in the 3′ overhang and to the G-rich strand in the partially unwound homologous duplex DNA, promotes the formation of intermolecular G4 DNA. Alternatively (right side), interstitial interactions between chromatids could be mediated by the formation of intermolecular G-G pairing, regardless of whether the flanking nucleotide sequences are homologous. We suggest that such interactions might either join homologs (as diagrammed here) to facilitate interhomolog recombination or join sister chromatids in a manner that delimits sister chromatid exchange.
A second set of Hop1 interactions with G4 DNA might serve more specifically in DSB repair and recombination. It has been reported that DSBs are reduced in number and delayed in appearance in hop1 meiosis (18). This might mean that Hop1-dependent interhomolog interactions of the type discussed above serve to promote DSB formation, especially in G-rich regions that define the G isochores where DSBs are prevalent (3, 14, 34). On the other hand, it is difficult to exclude the possibility that DSBs actually were being formed at the usual rate but were short lived in the absence of Hop1 function, as might have been the case if the stability of unresected DSBs in rad50S meiosis depends on HOP1. A failure to modulate resection appropriately may lead to extensive exonucleolytic attack on both 5′ and 3′ ends, producing a greatly expanded gap that might then be repaired from the sister chromatid. This could hinder interhomolog recombination and leave no genetic signal other than the possible excision of duplications (18). Circumstantial evidence favoring a role for Hop1 in DSB processing is seen in the aforementioned finding (17) that exonucleolytic attack on linearized duplex DNA in nuclear extracts is blocked by addition of purified Hop1. The preferential binding of Hop1 to duplex DNA relative to single-stranded DNA (17) might permit its rapid assembly on the duplex directly adjacent to the single-stranded tail being generated by resection, possibly by forming G quartets and/or associating with them in a manner that would inhibit further resection. Accordingly, a possible explanation for the prevalence of G isochores in the vicinity of recombinational hotspots (3) is that the flanking DNA must contain sufficient guanine repeats to aid in a resection-limiting action of this sort. Additionally, G4 DNA formed within the single-stranded tail and bound by Hop1 might play a direct role in searching for the homolog and promoting strand invasion. Regarding the free ends of chromosomal DNA molecules, it should also be noted that telomeres, which are especially rich in sequences capable of G4 DNA formation, probably play an important role in synapsis (31), providing yet another potentially important substrate for interaction with Hop1 protein.
Recent studies have shown that ATP-dependent strand exchange proteins, such as the prototypic RecA protein and its homolog Rad51, interact preferentially with GT-rich DNA (46, 47), and we found in this study that this affinity is independent of G4-DNA. In contrast, the binding of G-rich DNA by Hop1 protein appears to reflect the ability of the DNA to adopt the G4 DNA conformation by a mechanism that does not require ATP. Despite these distinctive modes of interaction with chromosomal DNA during meiosis, both RecA-like proteins and Hop1 perform their complementary functions in meiotic recombination within G-rich DNA. It therefore seems possible that an individual recombination event entails the successive interactions of the same guanine residues with each class of protein. The probable significance of G4 DNA to recombination is also underscored by the demonstration that LR1, a B-cell-specific DNA-binding factor, interacts specifically with sequences capable of forming G4 DNA in the region undergoing Ig switch recombination (6). In the future, a more detailed analysis of how Hop1 protein interacts with chromosomal DNA in vivo may help to elucidate the role of G4 DNA in meiotic synapsis and recombination.
ACKNOWLEDGMENTS
We thank Mary Kironmai, Moreshwar Vaze, and R. Ajay Kumar for generous gifts of purified Hop1 and RecA proteins used in the initial stages of this study.
This research was supported by a fellowship from the American Cancer Society, Yamagiwa-Yoshida fellowship (administered by UICC, Geneva, Switzerland), a grant from the Department of Science and Technology, New Delhi, to K.M., and an NIH grant (GM-18541) to B.B.
REFERENCES
- 1.Anderson S G E, Sharp P M. Codon usage in the Mycobacterium tuberculosis complex. Microbiology. 1996;142:915–925. doi: 10.1099/00221287-142-4-915. [DOI] [PubMed] [Google Scholar]
- 2.Bailis J M, Roeder G S. Synaptonemal complex morphogenesis and sister-chromatid cohesion require Mek1-dependent phosphorylation of a meiotic chromosomal protein. Genes Dev. 1998;12:3551–3563. doi: 10.1101/gad.12.22.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudat F, Nicolas A. Clustering of meiotic double-strand breaks on yeast chromosome III. Proc Natl Acad Sci USA. 1997;94:5213–5218. doi: 10.1073/pnas.94.10.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergerat A, de Massey B, Gadelle D, Varoutas P-C, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 5.Collins I, Newlon C S. Meiosis-specific formation of joint DNA molecules containing sequences from homologous chromosomes. Cell. 1994;76:65–75. doi: 10.1016/0092-8674(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey A, Sun H, Hanakahi L A, Maizels N. G4-DNA binding by LR1 and its subunits, nucleolin and hnRNP D: a role for G-G pairing in immunoglobulin switch recombination. J Biol Chem. 1999;274:1066–1071. doi: 10.1074/jbc.274.2.1066. [DOI] [PubMed] [Google Scholar]
- 7.Fang G, Cech T R. The β subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell. 1993;74:875–885. doi: 10.1016/0092-8674(93)90467-5. [DOI] [PubMed] [Google Scholar]
- 8.Friedman D B, Hollingsworth N M, Byers B. Insertional mutations in the yeast HOP1 gene: evidence for multimeric assembly in meiosis. Genetics. 1994;136:449–464. doi: 10.1093/genetics/136.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giraldo R, Rhodes D. The yeast telomere-binding protein RAP1 binds to and promotes the formation of DNA quadruplexes in telomeric DNA. EMBO J. 1994;13:2411–2420. doi: 10.1002/j.1460-2075.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington C, Lan Y, Akman S A. The identification and characterization of a G4-DNA resolvase activity. J Biol Chem. 1997;272:24631–24636. doi: 10.1074/jbc.272.39.24631. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth N M, Byers B. HOP1: a yeast meiotic pairing gene. Genetics. 1989;121:445–462. doi: 10.1093/genetics/121.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingsworth N M, Goetsch L, Byers B. The HOP1 gene encodes a meiosis-specific component of synaptonemal complex. Cell. 1990;61:73–84. doi: 10.1016/0092-8674(90)90216-2. [DOI] [PubMed] [Google Scholar]
- 13.Hollingsworth N M, Ponte L. Genetic interactions between HOP1, RED1, and MEK1 suggest that MEK1 regulates assembly of axial element components during meiosis in the yeast Saccharomyces cerevisiae. Genetics. 1997;147:33–42. doi: 10.1093/genetics/147.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacq C, Alt-Morbe J, Andre B, Arnold W, Bahr A, Bellesta J P G, Bargues M, et al. The nucleotide sequence of Saccharomyces cerevisiae chromosome IV. Nature. 1997;387:75–78. [PubMed] [Google Scholar]
- 15.Keeney S, Giroux C N, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Ljungdahl P O, Fink G R. kem1 mutations affect nuclear fusion in Saccharomyces cerevisiae. Genetics. 1990;126:799–812. doi: 10.1093/genetics/126.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kironmai K M, Muniyappa K, Friedman D B, Hollingsworth N M, Byers B. DNA-binding activities of Hop1 protein: a synaptonemal complex component from Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:1424–1435. doi: 10.1128/mcb.18.3.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleckner N. Meiosis: How could it work? Proc Natl Acad Sci USA. 1996;93:8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleckner N, Weiner B M. Potential advantages of unstable interactions for pairing of chromosomes in meiotic, somatic, and premeiotic cells. Cold Spring Harbor Symp Quant Biol. 1993;58:553–565. doi: 10.1101/sqb.1993.058.01.062. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R A, Vaze M B, Chandra N R, Vijayan M, Muniyappa K. Functional characterization of the precursor and spliced forms of RecA protein of Mycobacterium tuberculosis. Biochemistry. 1996;35:1793–1802. doi: 10.1021/bi9517751. [DOI] [PubMed] [Google Scholar]
- 21.Kupiec M, Byers B, Esposito R E, Mitchell A P. Meiosis and sporulation in S. cerevisiae. In: Pringle J R, Broach J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. Cell cycle and cell biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 889–1036. [Google Scholar]
- 22.Liu Z, Gilbert W. The yeast KEM1 gene encodes a nuclease specific for G4 tetraplex DNA: Implication of in vivo functions for this novel DNA structure. Cell. 1994;77:1083–1092. doi: 10.1016/0092-8674(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Frantz J D, Gilbert W, Tye B-K. Identification and characterization of a nuclease activity specific for G4 tetra-stranded DNA. Proc Natl Acad Sci USA. 1993;90:3157–3161. doi: 10.1073/pnas.90.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao-Draayer Y, Galbraith A M, Pittman D L, Cool M, Malone R E. Analysis of meiotic recombination pathways in the yeast Saccharomyces cerevisiae. Genetics. 1996;144:71–86. doi: 10.1093/genetics/144.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 26.Nag D K, Scherthan, Rockmill B, Bhargava J, Roeder G S. Heteroduplex DNA formation and homolog pairing in yeast meiotic mutants. Genetics. 1995;141:75–86. doi: 10.1093/genetics/141.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramdas J, Mythili E, Muniyappa K. Nucleosomes on linear duplex DNA allow homologous pairing but prevent strand exchange promoted by RecA protein. Proc Natl Acad Sci USA. 1991;88:1344–1348. doi: 10.1073/pnas.88.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockmill B, Roeder G S. RED1: a yeast gene required for the segregation of chromosomes during the reductional division of meiosis. Proc Natl Acad Sci USA. 1988;85:6057–6061. doi: 10.1073/pnas.85.16.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockmill B, Roeder G S. Meiosis in asynaptic yeast. Genetics. 1990;126:563–574. doi: 10.1093/genetics/126.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockmill B, Roeder G S. A meiosis-specific protein kinase homolog required for chromosome synapsis and recombination. Genes Dev. 1991;5:2392–2404. doi: 10.1101/gad.5.12b.2392. [DOI] [PubMed] [Google Scholar]
- 31.Rockmill B, Roeder G S. Telomere-mediated chromosome pairing during meiosis in budding yeast. Genes Dev. 1998;12:2574–2586. doi: 10.1101/gad.12.16.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roeder G S. Meiotic chromosomes: it takes two to tango. Genes Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- 33.Rould E, Muniyappa K, Raddiing C M. Unwinding of heterologous DNA by RecA protein during the search for homologous sequences. J Mol Biol. 1992;226:127–139. doi: 10.1016/0022-2836(92)90129-8. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schwacha A, Kleckner N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994;76:51–63. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 36.Schwacha A, Kleckner N. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1135. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 37.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334:364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 38.Sen D, Gilbert W. Guanine quartet structures. Methods Enzymol. 1992;211:191–199. doi: 10.1016/0076-6879(92)11012-8. [DOI] [PubMed] [Google Scholar]
- 39.Sharp P M, Lloyd A T. Regional base composition variation along yeast chromosome III: evolution of chromosome primary structure. Nucleic Acids Res. 1993;21:179–183. doi: 10.1093/nar/21.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith A V, Roeder G S. The yeast Red1 protein localizes to the cores of meiotic chromosomes. J Cell Biol. 1997;136:957–967. doi: 10.1083/jcb.136.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun H, Bennet R J, Maizels N. The Saccharomyces cerevisiae Sgs1 helicase efficiently unwinds G-G paired DNAs. Nucleic Acids Res. 1999;27:1978–1984. doi: 10.1093/nar/27.9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sym M, Roeder G S. Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell. 1994;79:283–292. doi: 10.1016/0092-8674(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 43.Sym M, Roeder G S. Zip1-induced changes in synaptonemal complex structure and polycomplex assembly. J Cell Biol. 1995;128:455–466. doi: 10.1083/jcb.128.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sym M, Engebrecht J, Roeder G S. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993;72:365–378. doi: 10.1016/0092-8674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- 45.Tishkoff D X, Rockmill B, Roeder G S, Kolodner R D. The sep1 mutant of Saccharomyces cerevisiae arrests in pachytene and is deficient in meiotic recombination. Genetics. 1995;139:495–509. doi: 10.1093/genetics/139.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tracy R B, Kowalczykowski S C. In vitro selection of preferred DNA pairing sequences by the Escherichia coli RecA protein. Genes Dev. 1996;10:1890–1903. doi: 10.1101/gad.10.15.1890. [DOI] [PubMed] [Google Scholar]
- 47.Tracy R B, Baumohl J K, Kowalczykowski S C. The preference for GT-rich DNA by the yeast Rad51 protein defines a set of universal pairing sequences. Genes Dev. 1997;11:3423–3431. doi: 10.1101/gad.11.24.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Wettstein D, Rasmussen S W, Holm P B. The synaptonemal complex in genetic segregation. Annu Rev Genet. 1984;18:331–413. doi: 10.1146/annurev.ge.18.120184.001555. [DOI] [PubMed] [Google Scholar]
- 49.Williamson J R. G-quartet structures in telomeric DNA. Annu Rev Biophys Biomol Struct. 1994;23:703–730. doi: 10.1146/annurev.bb.23.060194.003415. [DOI] [PubMed] [Google Scholar]
- 50.Yanagida M, Sternglanz R. Genetics of DNA topoisomerases. In: Cozzarelli N R, Wang J C, editors. DNA topology and its biological implications. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 299–320. [Google Scholar]