Abstract
Background
Synaptic injury is a pathological hallmark of neurological impairment in people living with human immunodeficiency virus (HIV, PLWH), a common complication despite viral suppression with antiretroviral therapy (ART). Measurement of synaptic density in living humans may allow better understanding of HIV neuropathogenesis and provide a dynamic biomarker for therapeutic studies. We applied novel synaptic vesical protein 2A (SV2A) positron emission tomographic (PET) imaging to investigate synaptic density in the frontostriatalthalamic region in PLWH and HIV-uninfected participants.
Methods
In this cross-sectional pilot study,13 older male PLWH on ART underwent magnetic resonance imaging (MRI) and PET scanning with the SV2A ligand [11C]UCB-J with partial volume correction and had neurocognitive assessments. SV2A binding potential (BPND) in the frontostriatalthalamic circuit was compared to 13 age-matched HIV-uninfected participants and assessed with respect to neurocognitive performance in PLWH.
Results
PLWH had 14% lower frontostriatalthalamic SV2A synaptic density compared to HIV-uninfected (PLWH: mean [SD], 3.93 [0.80]; HIV-uninfected: 4.59 [0.43]; P = .02, effect size 1.02). Differences were observed in widespread additional regions in exploratory analyses. Higher frontostriatalthalamic SV2A BPND associated with better grooved pegboard performance, a measure of motor coordination, in PLWH (r = 0.61, P = .03).
Conclusions
In a pilot study, SV2A PET imaging reveals reduced synaptic density in older male PLWH on ART compared to HIV-uninfected in the frontostriatalthalamic circuit and other cortical areas. Larger studies controlling for factors in addition to age are needed to determine whether differences are attributable to HIV or comorbidities in PLWH. SV2A imaging is a promising biomarker for studies of neuropathogenesis and therapeutic interventions in HIV.
Keywords: HIV, brain, PET, NeuroHIV, synaptic density
Understanding of the biological substrates of neurological impairment in people with human immunodeficiency virus (HIV) is limited. Here, novel in vivo SV2A positron emission tomographic (PET) imaging demonstrated lower synaptic density in older men with virologically suppressed HIV, potentially revealing a new biomarker of neurologic injury.
(See the Editorial Commentary by Clifford on pages 1412–3.)
Despite the widespread use of antiretroviral therapy (ART), 15% to 50% of the 38 million people living with human immunodeficiency virus (HIV, PLWH) are affected by some form of neurological impairment [1, 2]. Current diagnostic tools, including neuropsychological testing and patient-reported functional status, do not discriminate well between the various etiological bases of neurological impairment in PLWH, including impairments due to aging or comorbid conditions. Despite much research into the underlying neuropathogenesis of HIV and the development of neurological impairments, there are currently no clear biological measures that define etiologic subtypes [3]. This notable lack of biomarkers sensitive to neurocognitive impairment in HIV impedes the ability to effectively understand the causes for and develop treatments for neurocognitive dysfunction in PLWH.
Postmortem studies have revealed synaptodendritic injury as a hallmark neuropathological feature of neurocognitive impairment in PLWH in the ART era, but tools to measure the specific loss of synaptic density have not been widely available [4–6]. Although regional cerebral atrophy, white matter structural abnormalities, and disruptions in functional connectivity are common features of central nervous system (CNS) disease in HIV that suggest a loss of synaptic density, they are highly variable and nonspecific [1, 7–9]. Structural magnetic resonance imaging (MRI) studies in PLWH have found evidence for involvement of many brain regions, most consistently in the frontal cortex, striatum, and thalamus; however, volume in these regions do not clearly correlate with neurological dysfunction in PLWH on ART [10, 11]. Disrupted frontostriatal functional connectivity has also been shown in functional MRI studies [12–14], consistent with early pathological studies demonstrating regional vulnerability to HIV in the dorsal striatum, mid frontal cortex, thalamus, and hippocampus [5, 15–17]. Although these regions have consistently been implicated in studies using different methodologies, measures that are more specific and discriminatory than volumetric analyses are required to understand and intervene with the underlying substrate of neurologic dysfunction in HIV.
The recent development of the [11C]UCB-J radioligand has made noninvasive in vivo estimates of synaptic density possible. [11C]UCB-J binds to the synaptic vesicle glycoprotein 2A (SV2A), which is ubiquitously present in presynaptic nerve terminals throughout the brain [18]. SV2A concentration measured by high-resolution positron emission tomography (PET) imaging effectively reflects the density of nerve terminals, and therefore synaptic density, and has been validated as a measure of synaptic density in prior studies [19]. We capitalized on the availability of this new biomarker to apply the understanding gained by preclinical and autopsy studies in a pilot study investigating synaptic density in living humans with HIV on stable ART. We chose the frontostriatalthalamic circuit, an established functional circuit [20], as our primary region of interest (ROI) based on neuropathological and MRI studies demonstrating regional synaptodendritic injury, brain atrophy, and functional impairment [5, 15–17]. In addition, we hypothesized that loss of synaptic connections in these regions affects neurocognitive function in PLWH despite ART. Therefore, as a secondary analysis, we examined associations between synaptic density in the frontostriatalthalamic circuit and neurocognitive assessments.
METHODS
Participants
Male adults with HIV were recruited from the HIV Reservoirs and Comorbidities study, an established research protocol for neurological studies in PLWH and HIV-uninfected comparison participants at Yale University, between 2019 and 2020. HIV-uninfected participants who had previously completed identical imaging studies in other PET protocols were selected from the Yale PET Center database to achieve adequate sex- and age-matching [21]. HIV participants were required to have documented viral suppression (plasma HIV RNA < 20 copies/mL) on ART for at least 1 year. Exclusion criteria included active substance use disorder and current or past clinically significant neurological illness. Urine toxicology and standardized inventories for alcohol and substance use disorders were used to exclude participants with substance use disorders [22, 23]. Blood and cerebrospinal fluid (CSF) studies were collected as previously described [24]. Interviews were conducted to record ART regimens and historical CD4 nadir. The Yale University Human Investigation Committee and the Radioactive Drug Research Committee approved the study. All participants provided written informed consent before inclusion in the study.
MRI Imaging
All participants underwent structural MRI scanning on a 3-Tesla Siemens Prisma scanner. A 3-dimensional magnetization prepared rapid acquisition gradient echo T1-weighted sequence was used to exclude participants with anatomical abnormalities and for coregistration with PET images to define ROIs. Volumetric segmentation was performed with FreeSurfer 6.0 (Massachusetts General Hospital) image analysis suite [25–27]. FreeSurfer segmented the individual whole head into 109 ROIs. FreeSurfer partially failed on 2 participants due to abnormal intensity in white matter; FreeSurfer failed in the caudate for one of these participants and was redrawn manually. Other mislocated ROIs from those participants were excluded from further analysis.
PET Imaging
Each participant received a [11C]UCB-J PET scan on the High Resolution Research Tomograph (Siemens) for 60 minutes. Before the scan commenced, an arterial line was placed so that the metabolite-corrected arterial input function could be acquired. All PET imaging was performed according to previously described procedures [28].
PET Image Analysis
To control for the contributions of atrophy to the PET outcome measures, Iterative Yang partial volume correction (PVC) was performed on dynamic PET data using FreeSurfer ROIs (seeSupplementary Materials) [29]. Regional volumes were calculated in cortical ROIs delineated by FreeSurfer.
The primary outcome measure was the binding potential (BPND), a measure of specific binding only and the preferred outcome measure in PET studies, calculated for each ROI using the centrum semiovale as the reference region. Arterial data were not available for one HIV participant, so we applied the simplified reference tissue model 2 (SRTM2) to estimate regional BPND values for all participants (seeSupplementary Materials) [30]. For participants with arterial blood sampling, the 1-tissue compartment (1TC) model was applied to compute the volume of distribution (VT) and BPND was derived (BPND = VT ROI / VT reference – 1). The centrum semiovale has been validated as a reference region as it is nearly devoid of SV2A [19, 31].To generate group averaged PET images, the nonlinear registration was used to transform BPND parametric images to automated anatomical labeling template space.
Neuropsychological Assessment
HIV participants completed a 12 domain neuropsychological battery developed to assess cognitive domains affected by HIV, as previously described [24]. Z-scores were determined based on normative values from published studies, or the test developers’ normative data, with participants matched for age and education, and where possible, gender and race.
Statistical Analysis
Group differences in SV2A-specific binding (BPND) in the primary brain region (frontostriatalthalamic circuit) were assessed using a 2-tailed, independent-samples t test with P < .05 as a threshold for statistical significance. Multiple regression models were conducted to evaluate the relationship between BPND and HIV status, adjusting for individual potential confounders including age, education, and race, as well as for all three covariates together. In a secondary exploratory analysis, a multivariate analysis of variance was conducted to compare SV2A binding in multiple ROIs between the 2 groups. The relationship between BPND and neurocognitive performance in the HIV group was assessed using Pearson correlations. Statistical analysis and procedures were performed using JMP version 15 (SAS) and SPSS v26 (IBM).
RESULTS
Participants
Thirteen male adults with virologically suppressed HIV completed the study. Comparison imaging data from thirteen male HIV-uninfected participants were selected from the Yale PET Center database based on age and, when possible, race/ethnicity. Demographic and HIV-specific characteristics are shown in Table 1. HIV-uninfected participants included a smaller proportion of non-White participants (77% of the HIV group,31% of the HIV-uninfected group) and were somewhat more highly educated (mean [SD] 13.4 [2.5] years in HIV participants, 15.1 [1.7] years of education in the HIV-uninfected group) but were well matched on age.
Table 1.
Demographics and Clinical Characteristics of Participants
| HIV (n = 13) | HIV-uninfected Participants (n = 13) | P valuea | |
|---|---|---|---|
| Demographic characteristic | |||
| Male sex, no. (%) | 13 (100) | 13 (100) | N/A |
| Age, mean (SD), y | 59.8 (5.1) | 57.3 (6.8) | .29 |
| Non-White race, no. (%) | 10 (77) | 4 (31) | .02 |
| Hispanic, no. (%) | 2 (15) | 0 (0) | .14 |
| BMI, mean (SD), kg/m2 | 28.9 (5.5) | 29.2 (3.1) | .87 |
| Education, mean (SD), yb | 13.4 (2.5) | 15.1 (1.7) | .06 |
| History of SUD, no. (%) | 8 (62) | N/A | N/A |
| History of AUD, no. (%) | 5 (38) | N/A | N/A |
| Smoking | |||
| Current, no. (%) | 5 (38) | N/A | N/A |
| Former, no. (%) | 4 (31) | N/A | N/A |
| HIV-specific characteristic | |||
| CD4+ T cells, median (IQR), cells/μL | 689 (504, 865) | ||
| CD4+/CD8+ ratio, mean (SD) | 1.01 (0.44) | ||
| CD4+ nadir, median (IQR), cells/μLc | 188 (83, 448) | ||
| Plasma HIV RNA < 20 copies/mL, no. (%) | 11 (85) | ||
| CSF HIV RNA < 20 copies/mL, no. (%)d | 9 (75) | ||
| CSF white blood cells, mean (SD), cells/μLd | 3.8 (3.6) | ||
| CSF protein, mean (SD), mg/dLd | 41 (20) | ||
| CSF:blood albumin ratio, mean (SD)d | 5.8 (3.7) | ||
| Duration of HIV infection, mean (SD), y | 23 (16, 28) | ||
| Duration of ART, median (IQR), y | 22 (12, 25) | ||
| Current NRTI, no. (%) | 13 (100) | ||
| Current NNRTI, no. (%) | 4 (31) | ||
| Current integrase inhibitor, no. (%) | 8 (62) | ||
| Current PI, no. (%) | 1 (8) | ||
| Current pharmacokinetic enhancer, no. (%) | 2 (15) |
Abbreviations: ART, antiretroviral therapy; AUD, alcohol use disorder; BMI, body mass index; CSF, cerebrospinal fluid; IQR, interquartile range; HIV, human immunodeficiency virus; IQR, interquartile range; N/A, not applicable; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SUD, substance use disorder.
a P values are Student t test (continuous variables) or χ 2 test (categorical variables).
b n = 11 for the HIV-uninfected participants.
cNadir CD4 count was unavailable for 5 participants.
dOne participant did not have a lumbar puncture.
Group Differences in Synaptic Density in the Frontostriatalthalamic Circuit
HIV participants exhibited significantly lower synaptic density, as defined by [11C]UCB-J SRTM2-derived BPND measurement, in the frontostriatalthalamic circuit, our primary ROI, compared with HIV-uninfected participants. After PVC, frontostriatalthalamic circuit BPND was 14% lower in HIV participants (3.93 [0.80]; 4.59 [0.43]; P = .02) with a large effect size (Cohen’s d = 1.02) (Figure 1A, Supplementary Table 1). This result was largely unchanged when using 1TC-derived BPND or using a weighted average to calculate frontostriatalthalamic BPND (Supplementary Tables 2–4). Frontostriatalthalamic volume was similar in both groups (Supplementary Table 5). A significant group difference remained in the frontostriatalthalamic circuit after the addition of age (P = .03), education (P = .03), or race (P = .03) as a covariate, and effect sizes remained large in magnitude (Cohen’s d range: 0.94–0.99). Group differences did not remain significant when adjusting for age, education, and race together (P = .08).
Figure 1.
Regional mean SV2A binding potential (BPND) after partial volume correction in the (A) frontostriatalthalamic circuit, (B) composite whole gray matter, cortical, and subcortical regions, and (C) all individual secondary regions of interest by group. * P < .05. Error bars represent 95% confidence intervals. Individual data points are shown in panels (A) and (B). C, Exploratory analyses that were not corrected for multiple comparisons. Abbreviation: HIV, human immunodeficiency virus.
Group Differences in Synaptic Density in Secondary HIV-Related Brain Regions and Whole Brain
Exploratory analyses of additional ROIs that have previously been implicated in neurological impairment in PLWH were performed as well. Widespread differences in synaptic density in HIV participants as compared to HIV-uninfected participants were observed, most notably in cortical regions (Figure 1C, Supplementary Table 1), despite similar volumes (Supplementary Table 5). These comparisons were not corrected for multiple comparisons due to the exploratory nature of these analyses in the pilot study. All but one brain region exhibited lower BPND after PVC in HIV participants (percent difference range: 4–22%). We found significantly lower synaptic density in cortical (14.9%), subcortical (11.5%), and whole gray (13.7%) composites (Figure 1B).
Among cortical regions, there was significantly lower synaptic density in HIV participants in the parietal (5.54 [1.49]; 6.77 [0.90]; P = .02), occipital (4.94 [1.16]; 6.04 [0.83]; P = .01), and frontal (5.22 [1.28]; 6.08 [0.67]; P = .045) cortices. We observed significantly lower BPND in the parahippocampal gyrus and temporal pole (both temporal cortex), insula, and posterior cingulate. Subcortical regions with lower BPND in HIV participants included the thalamus, hippocampus, striatum, and caudate. Figure 2 illustrates regional and widespread differences in synaptic density in PLWH compared to HIV-uninfected participants using mean group parametric images of BPND values.
Figure 2.
Whole brain is shown in averaged group SV2A binding potential (BPND) parametric images for HIV-uninfected participants (left) and HIV (middle) participants. Magnetic resonance imaging template (right) for reference. Color scale denotes BPND. Abbreviations: HIV, human immunodeficiency virus; MRI, magnetic resonance imaging.
Associations Between Synaptic Density and Neurocognitive Function in PLWH
Relative to normative population data, PLWH performed below average in all but 2 of the 18 neurocognitive tests (Supplementary Table 6). A global average of z-scores for all tests was calculated for each participant. The mean global average z-score for our sample was 1 standard deviation below the normative average (−1.0 [0.8]), indicating impairment in this group.
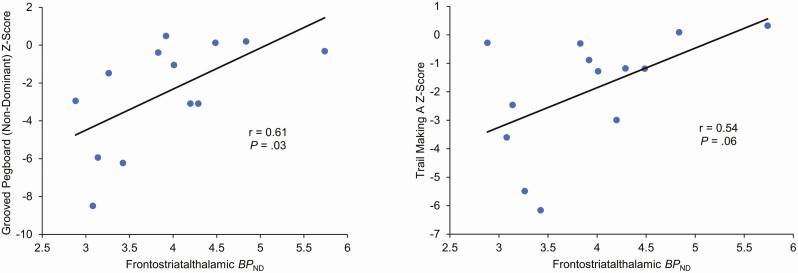
We assessed for correlations between synaptic density in the frontostriatalthalamic circuit and neurocognitive performance in HIV participants (Figure 3). Significant associations were observed between grooved pegboard performance, a measure of motor and executive function, and frontostriatalthalamic BPND (nondominant: r = 0.61, P = .03; dominant: r = 0.54, P = .06). Better performance on the grooved pegboard was also associated with greater hippocampal BPND (nondominant: r = 0.60, P = .03; dominant: r = 0.59, P = .03). There was a trend toward a significant association between Trail Making Test Part A performance, a measure of processing speed, and frontostriatalthalamic (r = 0.54, P = .06) and hippocampal (r = 0.51, P = .08) BPND.
Figure 3.
Association between BPND in the frontostriatalthalamic circuit and neuropsychological test scores. Correlations were computed using Pearson’s r. Abbreviation: BP, binding potential.
Associations Between Synaptic Density and Demographic and Clinical Measures in PLWH
There was no association between age and frontostriatalthalamic BPND among HIV participants. No other associations were observed in a correlation analysis of the relationship between frontostriatalthalamic synaptic density and other demographic and clinical measures reported in Table 1, including duration of HIV infection and CD4 nadir.
Discussion
In this pilot study, we evaluated synaptic density in PLWH using in vivo SV2A PET imaging. Until now, assessment of synaptic density in HIV has been limited to investigations using postmortem brain tissue. To our knowledge, this is the first study to provide in vivo evidence of significantly lower synaptic density in PLWH in the frontostriatalthalamic circuit, an area consistently implicated in structural and functional MRI studies of neurocognitive dysfunction in PLWH. Exploratory analyses, uncorrected for multiple comparisons, hint at more widespread loss of synaptic density in PLWH. Furthermore, in this small study, lower frontostriatalthalamic BPND was associated with poorer performance on a standardized measure of motor and executive function. This study provides compelling initial evidence that SV2A PET imaging as a research modality can measure in vivo differences in synaptic density in PLWH. Findings of this study provide additional evidence that the frontostriatalthalamic circuit may be implicated in the neuropathology of HIV.
Although SV2A PET imaging is a recent development, it has already been used to study synaptic density in Alzheimer’s disease [32], depression [33], post-traumatic stress disorder (PTSD) [33], Parkinson’s disease (PD) [34], schizophrenia [35], and epilepsy [36]. Among other notable findings, one study found large reductions in synaptic density in the seizure onset zone (SOZ) relative to the contralateral side, providing a biomarker for accurately identifying the SOZ in the presurgical evaluation of these patients. In PD, lower synaptic density was observed at earlier stages of PD than previously known. Collectively, these studies have led to novel insights in our understanding of the pathogenesis of these disorders and have already demonstrated utility as biomarkers for treatment monitoring and disease progression. Notable associations with cognitive function testing have been identified in these studies as well. In the current study, we applied this tool to identify synaptic density as a potential biomarker associated with neurocognitive impairment in PLWH.
The etiology of CNS dysfunction in people with well-treated HIV is likely multifactorial [37]. Contributing factors for CNS dysfunction in PLWH include legacy effects from CNS damage during initial HIV infection [38], ongoing neuroinflammation and immune activation [39], ART toxicities [40], HIV escape and persistence [41], lifestyle factors, noninfectious comorbidities [42], accelerated aging [43], and mental health disorders. A challenge to better understanding neurocognitive impairment in HIV is separating out key confounders (ie, mood disorders, polypharmacy, substance use) that are common in PLWH [44] from disease-related factors that contribute to brain injury [45]. This has been particularly difficult due to the absence of a sensitive measure of brain pathology.
Synaptodendritic injury, a known feature of neurocognitive dysfunction in PLWH, is associated with severity of cognitive injury, with some studies indicating this correlation is stronger than other classic pathologic markers of HIV encephalitis, such as microglial nodules and multinucleated giant cells [46, 47]. There are several proposed mechanisms of synaptic degeneration in HIV, including an inflammatory milieu generated by proliferating immune cells, toxic impact of viral proteins, viral replication, and exposure to methamphetamines and opiates [46, 48–50]. Understanding of synaptodendritic injury and other pathological changes in the brain during HIV infection has been limited by lack of appropriate research modalities.
In this study, we found a large magnitude reduction in synaptic density in PLWH after correction for cerebral atrophy despite long-term ART compared with age-matched HIV-uninfected participants. Lower synaptic density in the frontostriatalthalamic pathway is consistent with findings of atrophy in structural MRI studies of PLWH [10, 11] as well as functional MRI studies demonstrating compromised connectivity in this circuit and associations with cognitive impairment [12–14]. This was detected despite the relatively small sample and withstood correction for age and brain atrophy, indicating the potential for SV2A PET imaging as a useful marker of synaptic density in PLWH. Furthermore, even in this small pilot study a significant association was observed between synaptic density in the frontostriatalthalamic circuit and performance on the grooved pegboard test, a measure of motor integration and executive function [51]. Frontostriatal circuits, along with their connections to the thalamus, are known to mediate motor and oculomotor function [52], both of which are important in the grooved pegboard test. In HIV, there is evidence that basal ganglia gray matter (ie, striatum) atrophy is associated with motor dysfunction [53]. There is further evidence of the association between abnormal frontostriatal connectivity and motor and executive dysfunction from studies in PD and schizophrenia [54–56].
Results of this initial study highlight the need for future investigations. Our ongoing longitudinal SV2A study will further elucidate interactions between synaptic loss due to aging with loss due to HIV, including a potential accelerated loss of synaptic density in adults aging with HIV [57]. Furthermore, combining longitudinal SV2A imaging of synaptic density with translocator protein PET imaging of microglia and cerebrospinal fluid measures of immune activation will determine the extent to which neuroinflammation impacts synaptic density and neurocognitive function. SV2A PET imaging may also be useful in elucidating the etiology of CNS injury and cognitive impairment in other acute and chronic viral infections that impact the brain.
Limitations
This pilot study was designed to detect overall group differences between PLWH and age- and sex-matched historical controls; larger studies with prospectively enrolled controls matched for additional clinical and demographic factors are needed to determine the contribution of HIV disease versus comorbidities in PLWH to our findings. There was a significant difference in the racial composition of each group; however, we have no plausible biological reason to expect a difference in synaptic density due to race alone, and the group difference remained significant when race was used as a covariate. Further, frontostriatalthalamic BPND was not associated with race in our cohort, and there is no published evidence that synaptic density in HIV is associated with race, nor have there been studies showing a relationship between synaptic density and race more generally. Drug abuse is known to cause synaptodendritic changes, and although there were no associations between synaptic density and history of substance or alcohol abuse among HIV participants, we were not able to account for these variables in our comparative analyses as this data was not available from the HIV-uninfected group.
Similarly, studies with larger samples are needed to further investigate associations between synaptic density and neurocognitive performance, as well as associations with other HIV-associated lab and clinical parameters, such as CD4 nadir and duration of HIV infection, which were not observed in this small study. Finally, our sample was older and entirely male, limiting generalizability. Although a homogeneous cohort is an advantage for this analysis given the small sample size, future studies should focus on recruiting more diverse samples, including women and younger individuals [21].
CONCLUSION
We used novel PET imaging of SV2A to assess synaptic density in a cohort of older male, virologically suppressed HIV participants and demographically similar HIV-uninfected participants, demonstrating lower synaptic density in the frontostriatalthalamic circuit, among other brain regions, in the HIV group. The detection of synaptodendritic injury independent of brain atrophy in PLWH with viral suppression, in association with reduced neurocognitive function, suggests a potentially reversible neuropathology in some PLWH. These pilot observations raise questions regarding the etiology of the differences observed between the 2 groups, which need to be determined in larger studies examining mechanisms underlying synaptic density in diverse populations of PLWH. This method provides promise as a valuable in vivo molecular biomarker of CNS dysfunction in HIV, which may affect up to half of the 38 million PLWH worldwide, as well as an outcome measure for trials of disease-modifying therapies in this population.
Supplementary Material
Notes
Acknowledgments. The authors thank the research volunteers for their participation and dedication to the study, as well as the staff of the Yale PET Center and Yale MRI Center for their invaluable assistance. J. J. W. acknowledges financial support from Yale University for the G.D. Hsiung, PhD Student Research Fellowship and the Infectious Diseases Society of America (IDSA)/HIV Medicine Association (HIVMA) for the GERM Medical Student award.
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers R01MH125396, R21MH118023, and R21MH118109 to S. S., grant number R01NS094253 to R. E. C., grant number K23MH118999 to S. F. F.). This publication was also supported by Clinical and Translational Science Award (CTSA) grant number UL1TR001863 from the National Center for Advancing Translational Science (NCATS), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Potential conflicts of interest. S. S. receives grant funding from the NIH/ National Institute of Mental Health (NIMH) and directs a clinical trial within the AIDS Clinical Trials Group network that receives study medications donated by ViiV Healthcare, Inc. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. CHARTER Study 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 2011; 17:176–83. [DOI] [PubMed] [Google Scholar]
- 3. Chan P, Hellmuth J, Spudich S, Valcour V. Cognitive impairment and persistent CNS injury in treated HIV. Curr HIV/AIDS Rep 2016; 13:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiley CA, Masliah E, Morey M, et al. Neocortical damage during HIV infection. Ann Neurol 1991; 29:651–7. [DOI] [PubMed] [Google Scholar]
- 5. Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol 1997; 42:963–72. [DOI] [PubMed] [Google Scholar]
- 6. Everall IP, Heaton RK, Marcotte TD, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol 1999; 9:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gongvatana A, Schweinsburg BC, Taylor MJ, et al. ; Charter Group . White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 2009; 15:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker JT, Sanders J, Madsen SK, et al. ; Multicenter AIDS Cohort Study . Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011; 5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaganti JR, Heinecke A, Gates TM, Moffat KJ, Brew BJ. Functional connectivity in virally suppressed patients with HIV-associated neurocognitive disorder: a resting-state analysis. AJNR Am J Neuroradiol 2017; 38:1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Israel SM, Hassanzadeh-Behbahani S, Turkeltaub PE, Moore DJ, Ellis RJ, Jiang X. Different roles of frontal versus striatal atrophy in HIV-associated neurocognitive disorders. Hum Brain Mapp 2019; 40:3010–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kallianpur KJ, Jahanshad N, Sailasuta N, et al. ; SEARCH010/RV254 Study Group . Regional brain volumetric changes despite 2 years of treatment initiated during acute HIV infection. AIDS 2020; 34:415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ipser JC, Brown GG, Bischoff-Grethe A, et al. ; Translational Methamphetamine AIDS Research Center (TMARC) Group . HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J Int Neuropsychol Soc 2015; 21:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 2008; 188:337–47. [DOI] [PubMed] [Google Scholar]
- 14. Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS 2014; 28:803–11. [DOI] [PubMed] [Google Scholar]
- 15. Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The relationship of quantitative brain magnetic resonance imaging measures to neuropathologic indexes of human immunodeficiency virus infection. Arch Neurol 1994; 51:1129–35. [DOI] [PubMed] [Google Scholar]
- 16. Meeker RB, Thiede BA, Hall C, English R, Tompkins M. Cortical cell loss in asymptomatic cats experimentally infected with feline immunodeficiency virus. AIDS Res Hum Retroviruses 1997; 13:1131–40. [DOI] [PubMed] [Google Scholar]
- 17. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007; 8:33–44. [DOI] [PubMed] [Google Scholar]
- 18. Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 1994; 14:5223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finnema SJ, Nabulsi NB, Eid T, et al. Imaging synaptic density in the living human brain. Sci Transl Med 2016; 8:348ra96. [DOI] [PubMed] [Google Scholar]
- 20. Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 2002; 53:647–54. [DOI] [PubMed] [Google Scholar]
- 21. Carson R, Naganawa M, Matuskey D, et al. Age and sex effects on synaptic density in healthy humans as assessed with SV2A PET. J Nucl Med 2018; 59:541-. [Google Scholar]
- 22. Group WAW. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002; 97:1183–94. [DOI] [PubMed] [Google Scholar]
- 23. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 24. Farhadian SF, Mistry H, Kirchwey T, et al. Markers of CNS injury in adults living with HIV with CSF HIV not detected vs detected <20 Copies/mL. Open Forum Infect Dis 2019; 6:ofz528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9:179–94. [DOI] [PubMed] [Google Scholar]
- 26. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000; 97:11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006; 32:180–94. [DOI] [PubMed] [Google Scholar]
- 28. Finnema SJ, Nabulsi NB, Mercier J, et al. Kinetic evaluation and test-retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab 2018; 38:2041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erlandsson K, Buvat I, Pretorius PH, Thomas BA, Hutton BF. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys Med Biol 2012; 57:R119–59. [DOI] [PubMed] [Google Scholar]
- 30. Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 2002; 22:1440–52. [DOI] [PubMed] [Google Scholar]
- 31. Rossano S, Toyonaga T, Finnema SJ, et al. Assessment of a white matter reference region for (11)C-UCB-J PET quantification. J Cereb Blood Flow Metab 2020; 40:1890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mecca AP, Chen M-K, O’Dell RS, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimer’s Dement 2020; 16:974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holmes SE, Scheinost D, Finnema SJ, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun 2019; 10:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matuskey D, Tinaz S, Wilcox KC, et al. Synaptic changes in parkinson disease assessed with in vivo imaging. Ann Neurol 2020; 87:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Onwordi EC, Halff EF, Whitehurst T, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun 2020; 11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finnema SJ, Toyonaga T, Detyniecki K, et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: a [(11) C]UCB-J positron emission tomography study. Epilepsia 2020; 61:2183–93. [DOI] [PubMed] [Google Scholar]
- 37. Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep 2015; 12:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winston A, Spudich S. Cognitive disorders in people living with HIV. Lancet HIV 2020; 7:e504–13. [DOI] [PubMed] [Google Scholar]
- 39. Rubin LH, Sacktor N, Creighton J, et al. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 2018; 32:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 2011; 76:1403–9. [DOI] [PubMed] [Google Scholar]
- 41. Spudich S, Robertson KR, Bosch RJ, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest 2019; 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schouten J, Wit FW, Stolte IG, et al. ; AGEhIV Cohort Study Group . Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 43. Goodkin K, Miller EN, Cox C, et al. ; Multicenter AIDS Cohort Study . Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV 2017; 4:e411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castilho JL, Rebeiro PF, Shepherd BE, et al. Mood disorders and increased risk of noncommunicable disease in adults with HIV. J Acquir Immune Defic Syndr 2020; 83:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guaraldi G, Malagoli A, Calcagno A, et al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr 2018; 18:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bryant AK, Ellis RJ, Umlauf A, et al. Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS 2015; 29:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moore DJ, Masliah E, Rippeth JD, et al. ; HNRC Group . Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 2006; 20:879–87. [DOI] [PubMed] [Google Scholar]
- 48. Bandaru VV, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol 2011; 6:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boska MD, Dash PK, Knibbe J, et al. Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice. Mol Neurodegener 2014; 9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bryant AK, Moore DJ, Burdo TH, et al. Plasma soluble CD163 is associated with postmortem brain pathology in human immunodeficiency virus infection. AIDS 2017; 31:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tolle KA, Rahman-Filipiak AM, Hale AC, Kitchen Andren KA, Spencer RJ. Grooved pegboard test as a measure of executive functioning. Appl Neuropsychol Adult 2020; 27:414–20. [DOI] [PubMed] [Google Scholar]
- 52. Morris LS, Kundu P, Dowell N, et al. Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex 2016; 74:118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Küper M, Rabe K, Esser S, et al. Structural gray and white matter changes in patients with HIV. J Neurol 2011; 258:1066–75. [DOI] [PubMed] [Google Scholar]
- 54. Bohnen NI, Kuwabara H, Constantine GM, Mathis CA, Moore RY. Grooved pegboard test as a biomarker of nigrostriatal denervation in Parkinson’s disease. Neurosci Lett 2007; 424:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Viher PV, Docx L, Van Hecke W, et al. Aberrant fronto-striatal connectivity and fine motor function in schizophrenia. Psychiatry Res Neuroimaging 2019; 288:44–50. [DOI] [PubMed] [Google Scholar]
- 56. Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist 2004; 10:525–37. [DOI] [PubMed] [Google Scholar]
- 57. Cole JH, Caan MWA, Underwood J, et al. ; Comorbidity in Relations to AIDS (COBRA) Collaboration . No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) Project. Clin Infect Dis 2018; 66:1899–909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.