ABSTRACT
The current COVID-19 pandemic, which continues to spread across the globe, is caused by severe acute respiratory syndrome coronavirus (SARS-Cov-2). Soon after the pandemic emerged in China, it became clear that the receptor-binding domain (RBD) of angiotensin-converting enzyme 2 (ACE2) serves as the primary cell surface receptor for SARS-Cov-2. Subsequent work has shown that diabetes and hyperglycemia are major risk factors for morbidity and mortality in COVID-19 patients. However, data on the pattern of expression of ACE2 on human pancreatic β cells remain contradictory. Additionally, there is no consensus on whether the virus can directly infect and damage pancreatic islets and hence exacerbate diabetes. In this mini-review, we highlight the role of ACE2 receptor and summarize the current state of knowledge regarding its expression/co-localization in human pancreatic endocrine cells. We also discuss recent data on the permissiveness of human pancreatic β cells to SARS-Cov-2 infection.
KEYWORDS: SARS-CoV-2, ACE2, pancreatic β cells, type 2 diabetes, TMPRSS2
1. Introduction
In the early months of 2020, the World Health Organization (WHO) declared COVID-19, which is caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2), a global pandemic.1 SARS-CoV-2 cellular entry is mediated by binding the viral spike S1 protein to the receptor-binding domain (RBD) of the ACE2 on the surface of alveolar epithelial cells.2,3 Additionally, several protease activators, including TMPRSS2 and lysosomal proteases, have been shown to play essential roles in SARS-CoV-2 entry and its subsequent translocation to the cytoplasm of target cells4,5 (Figure 1). SARS-Cov-2 infections are often associated with multiple organ failure, suggesting that the virus targets other cell types besides alveolar epithelial cells.6,7 Hence, researchers are currently investigating additional cell types targeted with SARS-CoV-2. The apparent approach that was followed by many of the recently published studies on this topic was to profile the expression pattern of ACE2 on different tissues, including pancreatic islets.6,7
Figure 1.
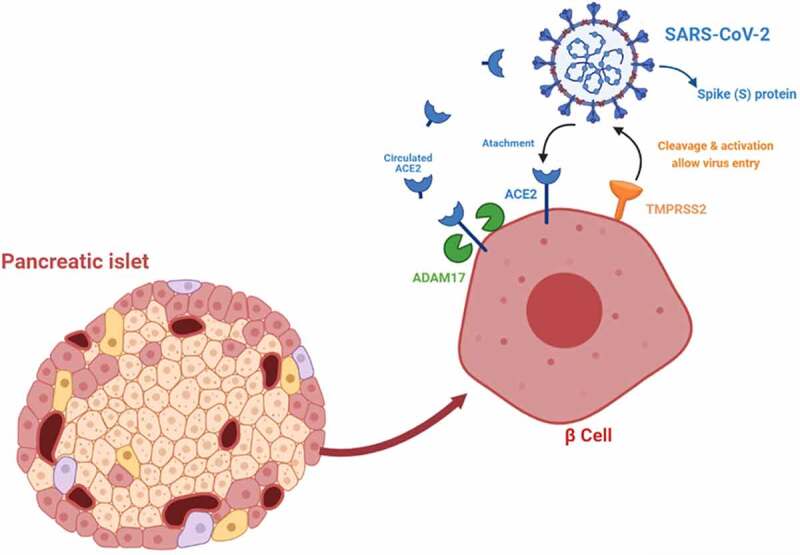
A schematic proposed interaction/uptake of SARS-CoV-2 into pancreatic β-cell. Figure was acquired using biorender.com
Clinical and epidemiological observations have established that people with diabetes infected with COVID-19 are at high risk of developing severe symptoms that may lead to death.8 A substantial percentage of COVID-19 patients suffer from acute hyperglycemia.9 Some of these subjects continued to show uncontrolled glycemia long after glucose-lowering medications were applied. Moreover, increased serum levels of exocrine pancreatic amylases and lipases were reported in severely ill COVID-19 patients.10 Hence, the possibility that this may predispose such patients to excessive inflammation and coagulation system dyshomeostasis, among other adverse consequences.11
The observation that COVID-19-infected diabetics are disproportionately at higher risk of becoming severely ill, along with the observation that non-diabetic COVID-19 patients often develop long-lasting symptoms of aggravated diabetes collectively argue for the possibility that SARS-CoV-2 may target and infect pancreatic islets.10,12–17 However, despite commendable efforts by several research groups across the globe, no conclusive evidence has emerged to rule this possibility in or out. To this end, this mini-review will examine available clinical and experimental observations on the interaction between SARS-CoV-2 and human pancreatic islets and β cells.
2. Role of ACE2 in pancreatic β cell function
ACE is one of the main enzymes that cleave angiotensin I to produce Angiotensin II, the active peptide in the renin-angiotensin system (RAS).18 ACE is a peptidyl dipeptidase that removes two carboxy-terminal amino acids from Ang-I and Ang-1-9.19 ACE2, a homolog of ACE, is a plasma membrane-bound metallocarboxypeptidase that removes a single carboxy-terminal amino acid from oligopeptides like Ang-I and Ang-II to produce Ang-1-9 and Ang-1-7.19 Numerous studies have established that in the RAS system, the protective effects of ACE2 counter the detrimental pressure and tissue remodeling actions of the ACE/angiotensin-II/angiotensin type 1 receptor (Ang-II/AT1R) axis.20 Along these lines, previous work has shown that RAS is heavily involved in regulating insulin secretion, blood flow and cell survival, among other aspects of pancreatic biology.21,22 Pre-treatment of the rat β cell line (INS-1) with Ang (1–7) restored insulin secretion and decreased ROS production following exposure to H2O2.23 Ang (1–7) was also shown to attenuate pancreatic injury through its ability to inhibit apoptosis.24,25 Furthermore, ANG-II (a vasoconstrictor) infusion resulted in hyperglycemia and hyperinsulinemia and decreased insulin secretion in mice.26 In contrast, ANG (1–7) (a vasodilator) was reported to increase pancreatic vascularization and prevent β cell dysfunction.27
Several lines of evidence suggest that ACE2 expression is important for pancreatic cell function, growth and survival, and the maintenance of glucose homeostasis.28 ACE2-deficient mice show impaired glucose tolerance and reduced insulin secretion.26 ACE2 overexpression in INS-1 cells associated with upregulated expression of a considerable number (67) of mitochondrial function-related genes.29 Engagement of ACE2/A1-7/Mas axis in pancreatic β cells was reported to reduce β cell de-differentiation and improve islet microcirculation by suppressing iNOS production/activity.30 The ACE2/A1-7 axis was also reported to inhibit pancreatitis via NO-dependent signals.31
The protective role of ACE2 in β cell growth and function notwithstanding increased morbidity and mortality in COVID-19 patients with diabetes relative to counterparts with no history of diabetes has been amply documented. Loss of ACE2 was reported to promote the deleterious Ang-II/AT1R arm of the RAS system.32 COVID-19 patients tend to exhibit increased levels of serum Ang-II, perhaps indicative of reduced ACE2 cleavage activity.33 In this context, some studies have mused on the possibility that upon entry to the host, the virus binds to the extracellular domain of ACE2 through its spike glycoprotein subunit and reduces its expression.34 Should this be the case, it is possible that reduced ACE2 expression in diabetics could exacerbate the destructive actions of the Ang-II–dependent arm of RAS, leading to pulmonary, bone marrow and gastrointestinal complications that culminate in severe illness and death.32
3. ACE2 expression patterns in human pancreatic islets and β cells
Yang et al., was the first to report ACE2 expression in human pancreas sections with weak expression in exocrine cells, obtained from a brain-dead donor.16 Liu et al. analyzed the expression and distribution of ACE2 in normal pancreas and lung tissues.10 The data revealed that the expression of ACE2 was slightly higher in the pancreas relative to the lungs. Moreover, single-cell RNA-sequencing (scRNA-seq) showed that ACE2 is expressed by both exocrine and pancreatic islets.35 Microarray expression data from human pancreatic islets demonstrated that the expression of ACE2 is lower than ADAM17 or TMPRSS2.15 Fignani et al. tapped into the INNODIA network EUnPOD biobank collection to study the expression of ACE2 in human pancreatic cells and the human insulin-producing cell line, EndoC-βH1.13 Immunohistochemistry staining in paraffin-embedded (FFPE) pancreatic sections showed that ACE2 is clearly expressed in a subset of endothelial cells or pericytes in specific lobules of the exocrine pancreas, some cells of the pancreatic ducts and endocrine pancreatic islets. It is worth noting that pronounced ACE2 expression was detected in a subset of cells within the islet parenchyma in the latter. Subsequent triple immunofluorescence staining showed that ACE2 expression preferentially overlapped with insulin-positive β cells; low/no expression was observed in α cells. Expression of ACE2 receptor at the mRNA level was detected in pancreatic β cells.13 Similar expression profile of ACE2 expression in the human EndoC-βH1 cells to that observed in human pancreatic islets. Immunostaining studies in different pancreatic cell lineages including glucagon+ “α” cells, insulin+ “β” cells and somatostatin+ “δ” cells derived from hPSC showed that ACE2 is expressed in α and β cells but not in δ cells.17 scRNA-seq analysis also showed that ACE2 was expressed in acinar cells, ductal cells, β cells and α cells. These findings were further validated by immunohistochemistry in primary human pancreatic islets.17 However, using scRNA-seq from five datasets, including 22 non-diabetic and 8 T2D individuals, Kusmartseva et al. showed that most islet cell subsets express low levels of ACE2. The percentage of ACE2 expressing cells was <2% in endocrine, 4.11% in acinar cells and 5.54% in ductal cells in non-diabetic donors.14
In contrast, using six transcriptional datasets of the human islet, Coate et al. showed no detectable levels of ACE2 in β cells. ACE2 protein was expressed only in the islet and exocrine tissue microvasculature and a subset of pancreatic ducts.12 Lastly, a recent study has demonstrated that both SARS-CoV-2 entry factors (ACE2 and TMPRSS2) are expressed in the pancreatic islets across four different donors.36 Interestingly, co-staining of endocrine cell types for ACE2 revealed that the C-peptide-positive (β cells) has the highest coefficients compared to the α – and δ cells.36 For a summary of these studies see Table 1.
Table 1.
List of the studies investigating the expression of ACE2 in pancreatic islets and β-cells
| Ref | Study design | Method of ACE2 detection | Main findings |
|---|---|---|---|
| Yang et al16 | Pancreatic sections from a 43-year-old male brain-dead organ donor. | Immunohistochemical staining with unspecified ACE2 antibody | Strong staining of ACE2 in pancreatic islets and weak in exocrine tissues. |
| Liu et al | A public database (GTEx) database (https://gtexportal.org) and two ssRNA sequencing data sets of different endocrine cells. | mRNA expression level of ACE2 |
|
| Taneera et al15 | Human pancreatic microarray expression data from 67 donors | mRNA expression in whole islets | ACE2 is expressed at lower levels compared to ADAM17 or TMPRSS2. |
| Fignani et al13 | Fresh, FFPE, or frozen human pancreatic islets from seven brain-dead donors and one T1D donor. Also, the human cell line “EndoC-βH1” was included. | RNA seq, RT-PCR and immunohistochemical staining using anti-ACE2 (cat. MAB933, R&D, USA) |
|
| Yang et al17 | Human Pluripotent Stem-derived endocrine cell types and primary human islets. | Immunohistochemical staining with Anti-ACE2(Abcam, Cat# ab15348 and R & D Systems, Cat# AF933) and ssRNA sequencing |
|
| Kusmartseva et al14 | Public ssRNA-seq from five datasets, including 22 non-diabetic and 8 T2D individuals. Pancreatic tissues from 56 non-diabetic, SARS-CoV-2 negative donors and three patients with fatal COVID-19. |
scRNA-sequencing, fluorescence in situ hybridization, western blotting, and immunolocalization using anti-ACE2 (Abcam Cat# ab108252, Abcam Cat# ab15348;R&D Systems Cat# MAB933 and R&D Systems Cat# AF933) |
|
| Coate et al12 | Tow public bulk RNA-seq dataset from human islets, four ssRNA-seq from human pancreatic and pancreatic tissues normal donors with or without diabetes and COVID-19 decedents after autopsy. | RNA-seq and Immunohistochemical staining using anti-ACE2 (Atlas Antibodies Cat# HPA000288; Abcam Cat# ab15348; R&DCat# AF933 and R&DCat# MAB933). |
|
| Müller et al36 | Pancreatic islets from four different donors and EndoC-βH1 cells. | RT-PCR and immunohistochemistry staining using anti-ACE2 (Abcam Cat # ab15348 and ab92323) |
|
4. The impact of DM on the expression intensity of ACE2 in pancreatic islets
Clinical and experimental evidence supporting the idea that diabetes itself affects ACE2 expression in human pancreatic islets and tissues have been documented. Kusmartseva et al. reported no differences in the expression levels of ACE2 in non-diabetic vs. diabetic donors in any of the islet cell subtypes.14 However, the percentage of ACE2 expressing cells was increased in acinar and ductal cells from 4% in non-diabetic donors to 8% in diabetics.14 Moreover, it has been shown that exposure of EndoC-βH1 cells to a cytokine cocktail (IL-1β, IFNγ, and TNFα) that commonly increased in people with diabetes resulted in an elevation of ACE2 expression levels compared to controls.13 Using microarray expression data, Taneera et al. showed that ACE2 is elevated in diabetic/hyperglycemic (n = 66) islets compared to non-diabetic/normoglycemic (n = 12).15 However, no correlation between ACE2 expression and HbA1c concentration, patient age or body mass index (BMI) was observed.
5. Permissiveness of pancreatic β cells to SARS-Cov-2 infection
Although most studies that addressed pancreatic β cell susceptibility to SARS CoV-2 have argued in the affirmative.13,15–17,21 other studies have argued in the negative.12,14 Numerous studies have established that viral infections are risk factors in Type 1 diabetes.37 For example, viruses like Coxsackievirus B, rotavirus, and cytomegalovirus were previously shown to infect and destroy pancreatic β cells.38–40 Immunohistochemistry and in situ hybridization studies have previously reported the presence of SARS-CoV in pancreatic tissues of patients who died due to SARS infections.41 Therefore, it is within the possibility for SARS-CoV-2 to gain entry to pancreatic β cells through ACE2 to cause significant damage and impair insulin secretion. Yan et al. have already demonstrated that human pancreatic islets are permissive to pseudo-entry and infection by SARS-CoV-2.17 The presence of SARS-CoV-2 spike protein in β and α cells was further confirmed by immunohistochemical staining. A very recent study showed ex vivo infection of isolated human pancreatic islets (n = 4) to SARS-CoV-2 led to a detectable expression of viral spike and nucleocapsid proteins at day 3. Although some C-pep/chromogranin A cells exhibited double positivity with the viral proteins, most SARS-CoV-2-infected cells appeared to lack pancreatic hormone expression.36 Moreover, postmortem examination in COVID-19 patients documented the presence of SARS-CoV-2 nucleocapsid protein in pancreatic exocrine cells and β cell in four subjects, further suggesting that human pancreatic tissues are possible targets of SARS-CoV-2 infection.
It is worth noting that ADAM17 and TMPRSS2 were found to play an essential role in the permissiveness of pancreatic β cells to SARS-Cov-242 (Figure 1). The transmembrane serine protease 2 TMPRSS2 was reported to prime spike protein and ACE2 cytoplasmic tail cleavage; a crucial step that enhances viral uptake by target cells.43 Mechanisms that have been proposed to explain how TMPRSS2 promotes viral entry include the cleavage of SARS-S and the subsequent activation of S protein for membrane fusion and/or the cleavage of ACE2 as means of promoting viral uptake through the cathepsin L-dependent pathway.43 The main function of ADAM17 is to cleave and release the Ectodomain of ACE2 in the circulation; a process that culminates with ACE2 shedding.4,5,44 Several lines of evidence suggest that ADAM17 activity contributes to SARS-CoV-2 infection and COVID-19 comorbidities such as elevated plasma levels of ACE2 in old men as well as patients with chronic pulmonary inflammation, renal disorders, or diabetes mellitus.45 In this context, it is important to emphasize that increased activity of ADAM17 has been correlated with hyperglycemia, which increases insulin resistance.46 Moreover, diabetic patients with ADAM17 upregulation exhibited increased insulin receptor resistance.44 Taken together, these observation suggest that the interaction between ACE2, ADAM17, and TMPRSS2 may represent a sidestep that determines the course of COVID-19 disease. More work is still needed to better configure the ACE2/ADAM17/TMPRSS2 axis especially as it relates to severe COVID-19 disease.
6. Impact of ARBs/ACE inhibitors or anti-diabetic drugs on ACE2 expression in β-cells
Two of the most widely used drugs in diabetes and heart failure are angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARBs) are known to increase the Angiotensin II level and can indirectly activate the ACE2.47 Consequently, during COVID-19 pandemic, there was a raised concern regarding patients receiving these medications ACEI or ARBs, which elevate ACE2 expression and could be more susceptible to infection with SARS-COV-2. Although there are no reports on the impact of ARBs/ACE inhibitors on ACE2 expression in pancreatic β-cells, ARBs/ACE inhibitors showed no effect on ACE2 expression in the human heart.48 It was also reported that no relation between ACE inhibitors or ARBs and the susceptibility to COVID-19 in humans.49 Interestingly, patients on ARBs/ACE inhibitors have been shown to have a better prognosis and experience less inflammation during COVID-19 infection, which supports the use of ARBs/ACE inhibitors compared to other antihypertensive drugs in treating COVID-19 patients.50,51
Additionally, there is a lack of studies on the impact of or anti-diabetic drugs such as insulin, metformin, sulfonylureas and dapagliflozin on ACE2 expression in human pancreatic islets. The anti-diabetic drugs, SGLT2 and DPP-4 inhibitors, showed no increased risk of COVID-19 infection, suggesting that they are safe for type 2 diabetic subjects during the COVID-19 pandemic.52 At the moment, there is no solid evidence on a particular drug that could attenuate or exacerbate the outcomes of COVID-19 disease.
7. Technical issues of concern
Contradictory data on the expression of ACE2 in human pancreatic β cells could be attributed to several variables, including the quality of islets (cause of death, enzymatic preparation, culture conditions, tissues processing, etc.) and the sensitivity of antibodies used as per the respective study. The localization of ACE2 in endocrine cells was observed using two out of three different antibodies tested in one study.13 Some commercial antibodies failed to detect the short-ACE2 isoform in human β cells.13,14 It is also worth noting that scRNA-seq sequencing data often fails to detect the full range of genes that are usually detected and counted using bulk cell-based RNA sequencing approaches.53 Several studies that relied on scRNA-seq missed multiple key genes involved in pancreatic β cell function, including SLC30A8 and TCF7L2.54,55 Hence, further refinement of these techniques is required to generate reproducible data, be it pancreatic islet preparation and processing, antibody sensitivity, or scRNA-seq data robustness.
8. Concluding remarks
Experimental data accumulated over the last decade suggest that ACE2 plays an essential role in pancreatic β cell function and survival. The majority of studies that examined ACE2 expression patterns in pancreatic islets indicate that it is expressed at low levels. Although several lines of evidence suggest that pancreatic β cells could be susceptible to infection by SARS-CoV-2, data on this interaction between pancreatic β cells and SARS-Cov-2 remain inconclusive. Additionally, the mechanism(s) underlying the hyperglycemia-related symptoms manifest in non-diabetic COVID-19 patients required further clarification. Hence, to understand the pathogenesis of SARS‐CoV‐2, more studies/samples from COVID‐19 patients after autopsy or ultrasound-guided tissue biopsies are warranted to validate the presence of the SARS‐CoV‐2 and co-localization of ACE2 in pancreatic β-cells. Notably, such effort should be made in a large number of collected pancreata with/without diabetes from different geographic/ethnic populations. Great cautions must be considered when pancreatic tissue is collected from cadaver donors to avoid autolysis and thus influence the expression of SARS‐CoV‐2 target receptors. Also, different ACE2 antibodies are required for a better expression profile of ACE2 expression in pancreatic islets. Finally, more studies are needed to investigate the effect of anti-diabetic drugs on the expression of ACE2 in human pancreatic islets.
Acknowledgments
This study is supported by research grants 2001090176 and CoV19-0305/MH University of Sharjah. The authors thank Sham Abdrabh for helping with the figure.
Funding Statement
This work was supported by the University of Sharjah [2001090176]; University of Sharjah [CoV19-0305/MH].
Author Contributions
J.T., W.A., and M.H. conceived and writing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G.. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020;81(5):537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu, N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty. 2020;9(1):1–7. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuschieri S, Grech S. COVID-19 and diabetes: The why, the what and the how. J Diabetes Complications. 2020;34(9):107637. doi: 10.1016/j.jdiacomp.2020.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clinical Gastroenterology and Hepatology. 2020;18(9):2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liao Y-H, Zheng J-Q, Zheng C-M, Lu K-C, Chao Y-C. Novel molecular evidence related to COVID-19 in patients with diabetes mellitus. Journal of Clinical Medicine. 2020;9(12):3962. doi: 10.3390/jcm9123962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020;32(6):1028–1040.e4. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 2020;11:876. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusmartseva I, Wu W, Syed F, Van Der Heide V, Jorgensen M, Josep, P, Tang X., Candelario-Jalil E, Yang C, Nick H. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32(6):1041–1051.e6. doi: 10.1016/j.cmet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taneera J, El-Huneidi W, Hamad M, Mohammed AK, Elaraby E, Hachim MY. Expression profile of SARS-CoV-2 host receptors in human pancreatic islets revealed upregulation of ACE2 in diabetic donors. Biology. 2020;9(8):215. doi: 10.3390/biology9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J-K, Lin -S-S, Ji X-J, Guo L-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L., Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X., Tang X, Zhu J, Zhao Z, Jaffré, F. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(125–36):e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302(2):193–202. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batlle D, Jose Soler M, Ye M. ACE2 and diabetes: ACE of ACEs? Diabetes. 2010;59(12):2994–2996. doi: 10.2337/db10-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young MJ, Clyne CD, Chapman KE. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. Journal of Endocrinology. 2020;247(2):R45–R62. doi: 10.1530/JOE-20-0260. [DOI] [PubMed] [Google Scholar]
- 21.Brar GS, Barrow BM, Watson M, Griesbach R, Choung, E, Welch, A, Ruzsicska, B, Raleigh, DP, Zraika, S. Neprilysin is required for angiotensin-(1–7)’s ability to enhance insulin secretion via its proteolytic activity to generate angiotensin-(1–2). Diabetes. 2017;66(8):2201–2212. doi: 10.2337/db16-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chodavarapu H, Chhabra KH, Xia H, Shenoy V, Yue X, Lazartigues E. High-fat diet-induced glucose dysregulation is independent of changes in islet ACE2 in mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2016;311(6):R1223–R33. doi: 10.1152/ajpregu.00362.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Liu C, Wang L, Cao X, Wang YY, Yang JK. Antioxidant effect of angiotensin (1‑7) in the protection of pancreatic β cell function. Mol Med Rep. 2016;14(3):1963–1969. doi: 10.3892/mmr.2016.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I–Converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59(10):2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui L, Liu R, Li C, Yu X, Liu X, Hou F, Chi C, Yin C, Wang C. Angiotensin(17) attenuates caerulein induced pancreatic acinar cell apoptosis. Mol Med Rep. 2017;16(3):3455–3460. doi: 10.3892/mmr.2017.6982. [DOI] [PubMed] [Google Scholar]
- 26.Shoemaker R, Yiannikouris F, Thatcher S, Cassis L. ACE2 deficiency reduces beta-cell mass and impairs beta-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab. 2015;309(7):E621–31. doi: 10.1152/ajpendo.00054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ihoriya C, Satoh M, Kuwabara A, Sasaki T, Kashihara N. Angiotensin II regulates islet microcirculation and insulin secretion in mice. Microcirculation. 2014;21(2):112–123. doi: 10.1111/micc.12094. [DOI] [PubMed] [Google Scholar]
- 28.Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine. 2008;34(1–3):56–61. doi: 10.1007/s12020-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 29.Shi T-T, Yang F-Y, Liu C, Cao X, Lu J, Zhang X-L, Yuan M-X, Chen C, Yang, J-K. Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic β-cells. Biochem Biophys Res Commun. 2018;495(1):860–866. doi: 10.1016/j.bbrc.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 30.Xuan X, Gao F, Ma X, Huang C, Wang Y, Deng H, Wang S, Li W, Yuan, L. Activation of ACE2/angiotensin (1-7) attenuates pancreatic beta cell dedifferentiation in a high-fat-diet mouse model. Metabolism. 2018;81:83–96. doi: 10.1016/j.metabol.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Liu R, Qi H, Wang Y, Cui L, Wen Y, Li H, Yin C. The ACE2-angiotensin-(1-7)-Mas axis protects against pancreatic cell damage in cell culture. Pancreas. 2015;44(2):266–272. doi: 10.1097/MPA.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 32.Obukhov AG, Stevens BR, Prasad R, Calzi SL, Boulton ME, Raizada MK., Oudit GY, Grant M.B. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020;69(9):1875–1886. doi: 10.2337/dbi20-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z. Li J, Li J, Feng, C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhry, F, Lavandero, S, Xie, X., Sabharwal, B, Zheng, Y-Y, Correa, A, Narula, J, Levy, P. Manipulation of ACE2 expression in COVID-19. Open Heart. 2020;7(2):e001424. doi: 10.1136/openhrt-2020-001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest. 2013;123(3):1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozz, CP, Read, C. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nature Metabolism. 2021;1–17. [DOI] [PubMed] [Google Scholar]
- 37.VVehik K, Lynch KF, Wong MC, Tian X, Ross MC, Gibbs RA, Ajami NJ, Petrosino JF, Rewers M, Toppari, J. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med. 2019;25(8):1865–1872. doi: 10.1038/s41591-019-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anagandula M., Richardson S.J., Oberste M.S., Sioofy‐Khojine AB, Hyöty H, Morgan, NG, Korsgren, O, Frisk, G. Infection of human islets of Langerhans with two strains of Coxsackie B virus serotype 1: assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. J Med Virol. 2014;86(8):1402–1411. doi: 10.1002/jmv.23835. [DOI] [PubMed] [Google Scholar]
- 39.Honeyman MC, Coulson BS, Stone NL. Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman, PG, Harrison LC. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49(8):1319–1324. doi: 10.2337/diabetes.49.8.1319. [DOI] [PubMed] [Google Scholar]
- 40.Forrest J, Menser M, Burgess J. High frequency of diabetes mellitus in young adults with congenital rubella. The Lancet. 1971;298(7720):332–334. doi: 10.1016/S0140-6736(71)90057-2. [DOI] [PubMed] [Google Scholar]
- 41.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zipeto D, da Fonseca Palmeira J, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salem ES, Grobe N, Elased KM. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. American Journal of Physiology-Renal Physiology. 2014;306(6):F629–F39. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, Yamamoto, N, Sasazuki T, Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proceedings of the National Academy of Sciences. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Federici M, Hribal M., Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V. Timp3 deficiency in insulin receptor–haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-α. J Clin Invest. 2005;115(12):3494–3505. doi: 10.1172/JCI26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.South AM, Brady TM, Flynn JT. ACE2 (angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and Ang II (Angiotensin II) receptor blocker use during the pandemic: the pediatric perspective. Hypertension. 2020;76(1):16–22. doi: 10.1161/HYPERTENSIONAHA.120.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman-Edelstein M, Guetta T, Barnea A, Waldman M, Ben-Dor N, Barak Y, Kornowski R, Arad M, Hochhauser E, Aravot, D. Expression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the renin angiotensin system. Cardiovasc Diabetol. 2021;20(1):1–14. doi: 10.1186/s12933-021-01275-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. New England Journal of Medicine. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, Cai J, Yang R, Han J, Huang Y. Effects of angiotensin II receptor blockers and ACE (angiotensin-converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: a single-center retrospective study. Hypertension. 2020;76(1):51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 51.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang,Z, Li. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerging Microbes & Infections. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sainsbury C, Wang J, Gokhale K, Acosta‐Mena, D, Dhalla S, Byne N, Chandan JS, Anand A, Cooper, J Okoth K. Sodium-glucose co-transporter-2 inhibitors and susceptibility to COVID-19: a population-based retrospective cohort study. Diabetes Obes Metab. 2021;23(1):263–269. doi: 10.1111/dom.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasad RB, Groop L. Single-Cell Sequencing of Human Pancreatic Islets—New Kids on the Block. Cell Metab. 2016;24(4):523–524. doi: 10.1016/j.cmet.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Segerstolpe Å, Palasantza A, Eliasson P, Andersson E-M, Andréasson A-C, Sun, Picelli S, Sabirsh A, Clausen M, Bjursell MK. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Li C, Gromada J. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 2016;24(4):608–615. doi: 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]


