Abstract
Acute disseminated encephalomyelitis (ADEM) is a demyelinating disease, and there are some data that link this event with various vaccinations. We report a young female admitted to the hospital with headache, fever, back pain, nausea, vomiting, and urinary retention. Two weeks prior, she received the first dose of SARS‐CoV‐2 mRNA vaccine. Brain and spinal cord magnetic resonance imaging (MRI) showed distinctive for ADEM widespread demyelinating lesions. The patient was successfully treated with methylprednisolone.
Acute disseminated encephalomyelitis is a central nervous system demyelinating disease that usually affects the cerebral hemispheres, brainstem, cerebellum, and spinal cord and thus typically presents with multifocal neurologic symptoms. It is widely considered as a monophasic disease with a very rare recurrent variant (multiphasic disseminated encephalomyelitis, MDEM). 1
Viral infections appear to trigger approximately up to three‐quarters of ADEM cases, which manifests in a rapid onset of multifocal neurological deficits. On the other hand, for many years, there have been, and still, there are concerns that vaccinations may cause autoimmune demyelination. Articles published several years ago suggested that about 5% of ADEM events are associated with immunization for varicella, rabies, measles, mumps, rubella, influenza, hepatitis B, Japanese B encephalitis, diphtheria, pertussis, and tetanus. 2 A quite recent report also claims that 61% of ADEM cases developed symptoms 2–31 days after vaccination. 3 However, the results of another decent study in which data almost from 64 million vaccine doses have been explored did not show a significant association between ADEM and prior immunization. 4 The evidence for a causal link between specific acute demyelinating events and any vaccine has been deemed inconclusive by the Institute of Medicine in the United States. 5 ADEM can be classified as an adverse event following immunization (AEFI), defined as any untoward medical occurrence that follows immunization and does not necessarily have a causal relationship with the usage of the vaccine. 6 Vaccinations are crucial for the slowdown of the spread of the COVID‐19 pandemic, so AEFI monitoring and reporting is an essential part of the strategy against SARS‐CoV‐2.
A 19‐year‐old female was admitted to the hospital with complaints of 3‐day severe headache, fever (37.5°C), back and neck pain with nausea and vomiting. She also noticed urinary retention last 2 days. Besides atopic dermatitis and depression, she had no significant medical history, upper respiratory infection, or diarrhea. Neurological examination showed nuchal rigidity, bilateral Babinski signs without other neurological deficit symptoms.
Two weeks prior, she received the first dose of SARS‐CoV‐2 mRNA vaccine (Moderna COVID‐19 Vaccine, ModernaTX, Inc. USA).
Brain magnetic resonance imaging (MRI) showed multiple, poorly demarcated, hyperintense lesions in T2‐weighted and fluid‐attenuated inversion recovery (FLAIR) images located in both brain hemispheres, pons, the medulla oblongata, and cerebellum. Few of them were contrast‐enhanced lesions. Cervical and thoracic MRI revealed a widespread hyperintense area in T2‐weighted and FLAIR images extended from medulla oblongata to Th11 segment with overlapping few contrast‐enhancing lesions (Fig. 1).
Figure 1.
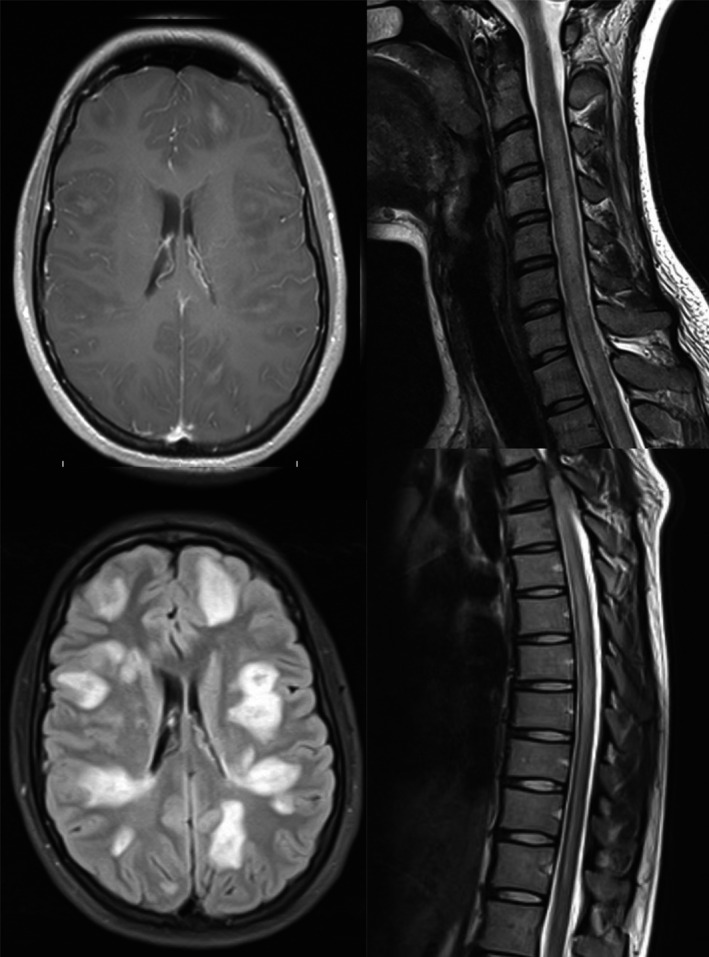
Hyperintense areas shown on MRI brain (FLAIR and post contrast T1‐weighted), cervical and thoracic spine (T2‐weighted) images.
Upon hospitalization, the cerebrospinal fluid (CSF) white blood cell (WBC) count was 294 × 106/L (reference range, 0–5 × 106/L): 91% lymphocytes (reference range, 40%–80%), 8% monocytes (reference range 15%–45%), 1% neutrophils (reference range 0%–6%); protein levels were 648 mg/L (reference range, 200–400 mg/L) and red blood cell (RBC) count was 77/µL. CSF was negative for antibodies to major pathogens and cultures of bacteria and fungi; genome sequencing also revealed no pathogens (Neisseria meningitidis, Streptococcus pneumoniae, group B streptococcus, Haemophilus influenzae, Listeria monocytogenes, HSV1, HSV2, VZV, CMV, EBV, HHV6). The reverse transcription real‐time PCR (RT‐PCR) and the antigen test were used to detect active SARS‐CoV‐2 infections.
Oligoclonal bands in blood and CSF were negative, and blood antibodies, including anti‐aquaporin‐4 and anti‐myelin oligodendrocyte glycoprotein, were also negative. Empirical therapy with ceftriaxone and acyclovir was started. After establishing a diagnosis of ADEM, patient was treated with methylprednisolone (MPS). The therapeutic plasma exchange (TPE) was also initiated, but the procedure has been stopped because of allergic reactions. The clinical status improved after MPS. Control lumbar puncture was done 12 days after the first one; CSF WBC count was 61 × 106/L and protein levels were 338 mg/L. She was discharged from the hospital without any symptoms except a mild headache. On follow‐up after 40 days, she complained of only mild headache.
We are in a worldwide SARS‐CoV‐2 pandemic, COVID‐19 infection could be complicated by many neurological symptoms and disorders: Guillain‐Barre syndrome, encephalopathy, cerebrovascular diseases, meningitis, neuralgia, ataxia, or epileptic crisis. 7 , 8 In the literature, there are also reports of patients with ADEM associated with confirmed COVID‐19 disease. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
Our patient manifested a typical radiological pattern for ADEM with extensive, diffuse demyelinating lesions in the brain and along all cervical and thoracic spinal cord. The CSF examination results were specific for ADEM with pleocytosis and the absence of oligoclonal bands. However, clinical urinary retention without lower limbs motor or sensory deficits is quite rare.
Panicker reported a bigger group of 61 patients with ADEM, where lower urinary tract dysfunction was found in 20 (33%) of them (16 patients had urinary retention), but mostly in patients with lower paraparesis. 18
In the literature, we found only two cases of patients with ADEM following SARS‐CoV‐2 vaccination; two women both received inactivated SARS‐CoV‐2 vaccine of Sinovac (Vero Cells, Beijing Institute of Biological Products Co., Ltd., Beijing, China). 19 , 20
The first woman revealed symptoms 2 weeks after vaccination; she presented somnolence and memory decline and improved after steroids and iv immunoglobulins therapy. The second one was admitted to the hospital after the first tonic‐clonic seizure one month after vaccination, she had typical scattered, demyelinating lesions in the brain, but because of lack of encephalopathy, it was called ADEM‐like presentation.
The reported cases do not impair the importance of COVID‐19 vaccinations with global SARS‐CoV‐2 pandemic, where the risk of neurologic complications, hospitalization, and even death of infected patients is still prevailing. But the clinicians should be aware of the potential implications of this rare neurological condition.
Conflict of Interest
All authors declare that there is no conflict of interest.
Funding Information
No funding information provided.
References
- 1. Nishiyama M, Nagase H, Tomioka K, et al. Clinical time course of pediatric acute disseminated encephalomyelitis. Brain Dev. 2019;41:531‐537. doi: 10.1016/j.braindev.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 2. Bennetto L, Scolding N. Inflammatory/post‐infectious encephalomyelitis. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 1):i22‐i28. doi: 10.1136/jnnp.2003.034256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pellegrino P, Carnovale C, Perrone V, et al. Acute disseminated encephalomyelitis onset: evaluation based on vaccine adverse events reporting systems. PLoS One. 2013;8:e77766. doi: 10.1371/journal.pone.0077766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baxter R, Lewis E, Goddard K, et al. Acute demyelinating events following vaccines: a case‐centered analysis. Clin Infect Dis. 2016;63:1456‐1462. doi: 10.1093/cid/ciw607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stratton K, Ford A, Rusch E, Clayton EW, eds. Institute of Medicine Adverse Effects of Vaccines: Evidence and Causality. National Academies Press; 2012. [PubMed] [Google Scholar]
- 6. Global manual on surveillance of adverse events following immunization. Accessed 31 May 2021. https://www.who.int/vaccine_safety/publications/Global_Manual_on_Surveillance_of_AEFI.pdf?ua=1
- 7. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7:875‐882. doi: 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Correia AO, Feitosa PWG, Moreira JLDS, Nogueira SÁR, Fonseca RB, Nobre MEP. Neurological manifestations of COVID‐19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res. 2020;37:27‐32. doi: 10.1016/j.npbr.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Assunção FB, Fragoso DC, Donoso Scoppetta T, Martins Maia AC. COVID‐19‐associated acute disseminated encephalomyelitis‐like disease. Am J Neuroradiol. 2020;40:E21‐E23. doi: 10.3174/ajnr.A6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID‐19‐associated acute disseminated encephalomyelitis (ADEM). J Neurol. 2020;267:2799‐2802. doi: 10.1007/s00415-020-09951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdi S, Ghorbani A, Fatehi F. The association of SARS‐CoV‐2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416:117001. doi: 10.1016/j.jns.2020.117001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahmirzaei S, Naser MA. Association of COVID‐19 and acute disseminated encephalomyelitis (ADEM) in the absence of pulmonary involvement. Autoimmun Rev. 2021;20:102753. doi: 10.1016/j.autrev.2021.102753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID‐19. BMJ Case Rep. 2020;13:e239597. doi: 10.1136/bcr-2020-239597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green C, Morrison H, Smith P, et al. Teaching neuroimages: COVID‐19‐associated acute disseminated encephalomyelitis with corpus callosal hemorrhage. Neurology. 2021;96:e307‐e308. doi: 10.1212/WNL.0000000000011001 [DOI] [PubMed] [Google Scholar]
- 15. Varadan B, Shankar A, Rajakumar A, et al. Acute hemorrhagic leukoencephalitis in a COVID‐19 patient‐a case report with literature review. Neuroradiology. 2021;63:653‐661. doi: 10.1007/s00234-021-02667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopes CCB, Brucki SMD, Passos Neto CEB, et al. Acute Disseminated Encephalomyelitis in COVID‐19: presentation of two cases and review of the literature. Arq Neuropsiquiatr. 2020;78:805‐810. doi: 10.1590/0004-282x20200186 [DOI] [PubMed] [Google Scholar]
- 17. McCuddy M, Kelkar P, Zhao Y, Wicklund D. Acute demyelinating encephalomyelitis (ADEM) in COVID‐19 infection: a case series. Neurol India. 2020;68:1192‐1195. [DOI] [PubMed] [Google Scholar]
- 18. Panicker JN, Nagaraja D, Kovoor J, Nair KPS, Subbakrishna DK. Lower urinary tract dysfunction in acute disseminated encephalomyelitis. Mult Scler. 2009;15:1118‐1122. doi: 10.1177/1352458509106614 [DOI] [PubMed] [Google Scholar]
- 19. Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021;1:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ozgen Kenangil G, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis‐like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089‐1091. doi: 10.1007/s13760-021-01699-x [DOI] [PMC free article] [PubMed] [Google Scholar]


