Abstract
Diabetes mellitus is a metabolic disease manifested by hyperglycemia. For patients with type 1 and advanced type 2 diabetes mellitus, insulin therapy is essential. Subcutaneous injection remains the most common administration method. Non-invasive insulin delivery technologies are pursued because of their benefits of decreasing patients’ pain, anxiety, and stress. Transdermal delivery systems have gained extensive attention due to the ease of administration and absence of hepatic first-pass metabolism. Microneedle (MN) technology is one of the most promising tactics, which can effectively deliver insulin through skin stratum corneum in a minimally invasive and painless way. This article will review the research progress of MNs in insulin transdermal delivery, including hollow MNs, dissolving MNs, hydrogel MNs, and glucose-responsive MN patches, in which insulin dosage can be strictly controlled. The clinical studies about insulin delivery with MN devices have also been summarized and grouped based on the study phase. There are still several challenges to achieve successful translation of MNs-based insulin therapy. In this review, we also discussed these challenges including safety, efficacy, patient/prescriber acceptability, manufacturing and scale-up, and regulatory authority acceptability.
Graphical abstract
Keywords: Insulin, Transdermal, Microneedle, Translation, Challenges
Introduction
According to the International Diabetes Federation (IDF) Diabetes Atlas 9th Edition 2019 [1], there are approximately 463 million adults aged 20–79 in the world suffering from diabetes, and this number is predicted to increase to 700 million by 2045. Insulin is essential for patients with type 1 diabetes mellitus (insulin dependence). Patients with advanced type 2 diabetes mellitus (insulin resistance) also need insulin to maintain blood glucose homeostasis [2]. The human insulin molecule (molecular weight 5.8 kD) secreted by pancreatic islet cells is composed of an A chain consisting of 21 amino acids and a B chain consisting of 30 amino acids [2]. At present, subcutaneous (SC) injection through a syringe, insulin pen, and insulin pump is still the most common way in insulin therapy because of the lower cost, more delivery efficiency, and higher bioavailability [3, 4]. Patients still need to inject 2–4 times a day although long-acting insulin (up to 24 h) had been developed. Frequent injection will cause pain and some skin complications in infusion sites, like redness, swelling, infection, and local tissue necrosis, leading to poor patient compliance [5, 6]. In addition, the injection doses are needed to be adjusted in real time to achieve rapid hypoglycemic after a meal and slow but long-term lowering blood glucose before sleep. Sometimes it is difficult to achieve the ideal and precise dose and as a result, hyperglycemia and hypoglycemia often occur. Moreover, as a biological peptide, insulin has poor stability and needs to be kept between 2 and 8 °C during distribution and shelf life storage [7], which increases the difficulties of transportation and storage in some developing countries. Therefore, it has become the focus of insulin drug product development to achieve more stable, convenient, painless, long-acting, and glucose-monitoring insulin preparation.
Scientists are committed to studying non-invasive insulin administration to improve patient compliance, including oral, percutaneous, pulmonary, and nasal [8]. Among them, transdermal delivery system (TDDS) is attractive and offers several benefits: (1) The surface area of skin is almost 1–2 m2, which provides a large surface area for drug administration [9]; (2) compared with painful injections, TDDS is minimally invasive, which facilitates reducing the side effects associated with frequent injection, and consequently increasing patient compliance and improving the life quality of diabetic patients; (3) insulin delivered through the skin can also bypass of hepatic first-pass metabolism and gastrointestinal tract degradation which are major limits of insulin oral administration [10, 11]; (4) TDDS can also continuously release insulin to maintain normal glucose levels for an extended period of time with less glucose fluctuations [10, 12] which significantly reduces the risk of concentration-related side effects.
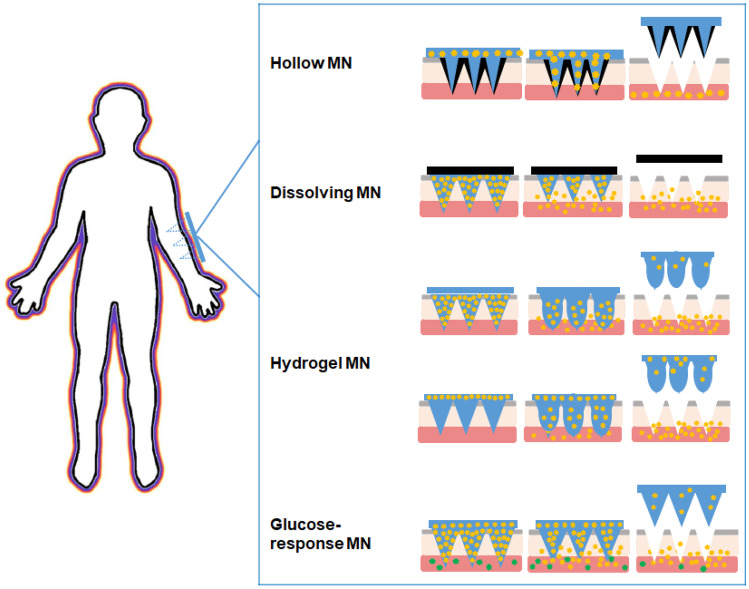
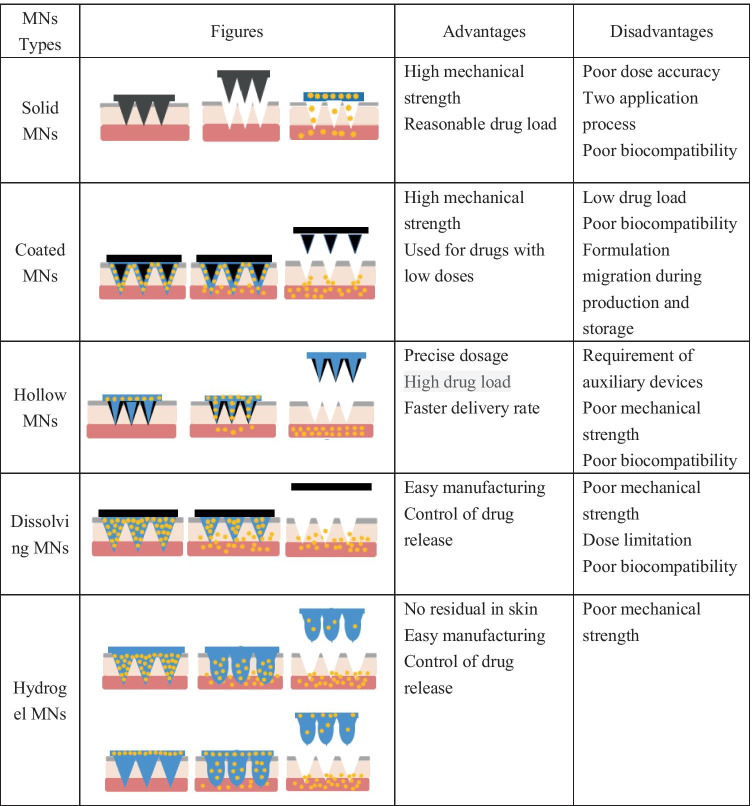
The stratum corneum (SC), located on the outermost layer of the skin, mainly hinders the penetration of macromolecules into the skin. The key of the TDDS is how to make the macromolecules penetrate through SC. Recently, microneedle (MN) technology has provided a new way for transdermal delivery of macromolecular biologics, such as protein, peptide, and gene [13–16]. MN arrays are composed of a plurality of micro-scaled needles with a height of 25 to 2000 μm [11, 17]. MNs can painlessly penetrate SC and access skin epidermis and dermis to release drug. The micro-channels caused by MNs are temporarily presented for drug delivery and can be recovered in a short time after MNs removal, which will prevent long-term skin damage [18]. On the basis of drug delivery mechanism, the MNs are divided into 5 different types, namely hollow MNs, solid MNs, coated MNs, dissolving MNs, and hydrogel MNs [19]. Table 1 outlines the characteristics of different MN types. Among them, solid MNs are drug-free that are designed only to pierce the skin and generate micro-channels. After removing solid MNs, the drug formulation is administrated on the pre-treated skin and the drug substance will enter into the skin through the micro-channels [20]. The actual delivery dosage of solid MNs is hard to control. The resealing time of the micro-channels after solid MNs removed varies with the skin hydration state and skin thickness, which will affect the absorption time of drugs [21]. In addition, the drug applied on the skin surface may be rubbed off by clothing. Coated MNs imply the use of solid MNs array coated with drug solution or dispersion on the needle surface [22]. After application of MNs onto the SC, the formulation which is coated onto the MNs is deposited. The drug can be released in the skin rapidly, while the drug loading is very low [23]. Insulin administration requires a precise dose to improve glycemic control while reducing hypoglycemic events; thus, these two types of MNs may not be suitable for insulin delivery. Hence, the focus of this article will be on hollow MNs, dissolving MNs, and hydrogel MNs and review of their progress in insulin transdermal delivery as well as challenges in clinical translation. Deficiency and over-dosage of insulin both pose a threat to human health. Self-regulating glucose-responsive insulin delivery systems have shown great potential in reducing related risks. The article shall also review the recently developed bio-responsive MNs that can respond to physiological glucose levels and release insulin on demand.
Table 1.
MNs used for insulin transdermal delivery
MNs can improve patient compliance and patients can self-administer. It has great potential as an alternative to traditional insulin therapy. Hollow MNs are indirect auxiliary administration, in which hydrogel and dissolving MN can be directly loaded with insulin for administration, and both can significantly increase the amount of insulin transdermal delivery. Moreover, glucose-responsive MNs can essentially provide precise insulin dosage adapted to the individual’s blood level in real time.
Hollow MNs
Hollow MNs are composed of empty cavity needle (5–70 μm wide) and an external auxiliary device, such as syringe, pump, gas, or electrical assistance, which enable the injection of the liquid formulation into the skin continuously through the needle cavity (usually 10–100 μL/min) [19, 25]. The basic structure of hollow MNs is the same as the conventional subcutaneous injection needle. Compared with other MNs types, the delivery capacity of hollow MNs is higher, since the dose amount and flow rate of hollow MNs can be controlled by external auxiliary device, while the dosage of coated or dissolving MNs is limited to the needle area and needle numbers [26, 27].
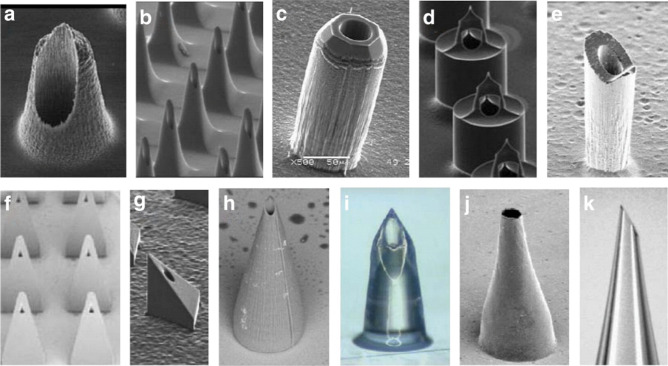
A wide variety of materials can be used to make hollow MNs, including silicon, metal, glass, ceramic, and polymers (Fig. 1) [28, 29]. Polymers with high biocompatibility, such as SU8 polymer [30, 31], clay reinforced polyimide, and metal electroplated polymer, have gained interest in recent years. Hollow MNs can be fabricated by microelectromechanical system (MEMS), such as lithography molding, X-ray photolithography, etching, and laser ablation/cutting [29, 32]. Wang et al. adopted the technique of polymer-based process combined with UV photolithography to prepare a hollow MN array. The whole process was divided into two steps: First, photolithography method was used to prepare polydimethylsiloxane (PDMS) mold, which was with a profile of pyramidal top; second, hollow MNs were fabricated with SU-8 polymer on the built-in PDMS mold [33]. Vinayakumar et al. reported a novel fabrication method of femto second laser micromachining, in which the hollow stainless steel MN array could be fabricated in one step without using masks [34]. By using laser micromachining technology, it is easy to control and manufacture different shapes of MNs in a single exposure device, avoid multilayer masking and patterning, and avoid exposure and chemical etching [1].
Fig. 1.
Hollow MNs fabricated with silicon and polymers. Reprinted with permission from [29].
Copyright 2012, Elsevier
A new type of insulin delivery device composed of a hollow MN array, drug reservoir, and ionic polymer metal composite (IPMC) membrane actuation-based micropump was designed by Richa et al. [35]. This device included two parts. The first part was the reservoir with inlet to the hollow MN array that was fabricated with SU-8 polymer by direct laser writing. The second part was the micropump actuator assembly, which consisted of Nafion membrane sandwiched between two copper electrode rings connected to external electrical supply [35]. The micropump was placed on top of the reservoir. Under electrical actuation, the Nafion membrane would deflect and press liquid in the reservoir and insulin was pumped in from the reservoir and then pushed out through the MNs. The micropump actuation assembly was the core component of this device. In this article, Laser Doppler Vibrometer (LDV) had been used exhaustively to characterize the frequency and voltage response of membrane. In a micropump, the conventional membrane was constrained either circularly or squarely. To increase the deflections, circular Nafion membrane was modified to 8 cuts. Compared with the conventional membrane design, this new MN device achieved a much higher insulin flow rate of 44.8 μL/min. Insulin flow rate could be adjusted in the range of 20–45 μL/min by changing frequency (0.1–0.5 Hz) and voltage (3–6 V), paving the way for painless insulin delivery [35].
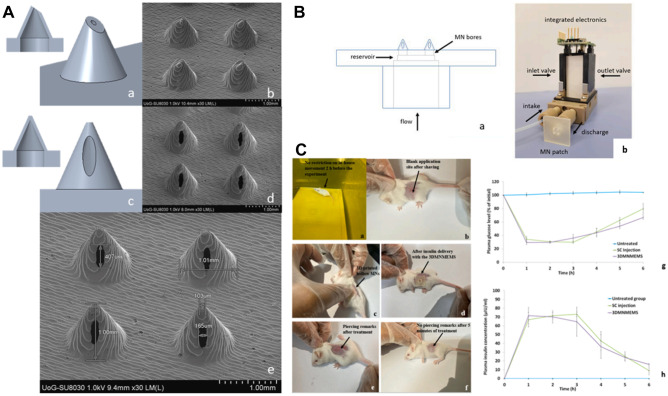
The preparations of hollow MNs need elaborate fabrication process, which are usually complicated, time-consuming, and expensive [36]. In the past 5 years, three-dimensional (3D) printing has attracted more and more attention in MNs manufacturing due to the multiplicity, accuracy, and superior reproducibility in the microscale [37]. A variety of 3D printing technologies are being developed, such as stereolithography (SLA), fused deposition modeling (FDM), liquid crystal display (LCD), selective laser sintering (SLS), and digital light processing (DLP) [38–40]. Economidou et al. adopted the combination of 3D printing technology, MN, and MEMS to design a new device (3DMNMEMS) for controllable insulin delivery (Fig. 2) [41]. They firstly prepared hollow MN patches with laser SLA and photopolymerization-based technology. Then, the hollow MN patch was connected to a diaphragmatic micropump which was acted as a MEMS. The in vitro release test showed that a single dosage of 0.5 IU insulin was fully detected in the receptor in 1 h. The in vivo study on diabetic animal model displayed that 3DMNMEMS delivered insulin demonstrated a similar release profile as the SC injection. Compared with SC injection, the relative pharmacological availability (RPA) of 3DMNMEMS was 105.14%, and the 3DMNMEMS group showed a more moderate decrease (16.1 μIU/mL at 6 h after treatment) of the plasma insulin concentration when compared with SC injection (9 μIU/mL at 6 h after treatment), evincing a more sustained insulin action [41].
Fig. 2.
3D printed hollow MN microelectromechanical system (3DMNMEMS). A Hollow MN fabricated by 3D printing. B 3DMNMEMS configuration. C Hypoglycemic effect of 3DMNMEMS in diabetic mouse. Reproduced with permission from [41].
Copyright 2021, Elsevier
Several hollow MN devices are now available in the market [25], and some of them are under clinical evaluation, including MicronJet® (Nanopass Technologies), MicronJet600® (Nanopass Technologies), and Microstructured Transdermal System® (3 M™). Hollow MNs can deliver existing injection formulations, without additional formulation development. Therefore, currently, most of the clinical trials employed the market available hollow MN devices to deliver biological drugs, like insulin and vaccine [25]. Intradermal infusion of insulin with hollow MNs is an effective alternative to traditional insulin injections. It can significantly reduce local irritation and other skin irritation and is resistant to pain. Many literatures and clinical studies have reported that compared with the traditional subcutaneous injection of insulin, intradermal infusion of insulin exhibits better PK/PD characteristics, short time to reach the maximum blood concentration (Cmax), fast onset of action, and high bioavailability [42].
There exit some points for hollow MNs to improve. When the hollow MNs are inserted into the skin, the bore in the needle tip can be easily clogged due to the compactness of the dermal tissue, which will affect the delivery of drugs [43]. The tip design of hollow MNs is very important, since tips with larger openings require a larger insertion force which will cause the fracture of needle, while smaller tips openings are blocked easily [44]. The compressed skin tissue can also give rise to resistance of liquid flow [43, 45]. This can be overcome by the design of side-open hole on the MN tip [46, 47]. Griss et al. firstly designed hollow out-of-wafer-plane silicon MNs which had orifices in the shaft instead of the tip. [48]. This hollow MN array showed low liquid flow resistance, large contact area between fluid and skin tissue, and low risk of clogging. In addition, because of the empty cavity and the brittle materials used, hollow MNs are susceptible to breakage. To achieve safe skin insertion, hollow MNs should be designed with higher breaking force and lower insertion force. Geometry plays an important role on insertion force to avoid needle breakage. Davis et al. firstly experimentally tested and theoretically analyzed the insertion force and breaking force of the insertion of MNs into the skin [49]. They found that the insertion force altered linearly with the tip interface area, while the breaking force improved with the increase of wall thickness, wall angle, and tip radius. The ratio of breaking force to insertion force was considered as the safety margin. The greater the ratio was and the safer MNs were. Davis et al. measured the insertion force of several MNs with different geometries (720 μm height, 30 ~ 80 μm tip radius, and 5 ~ 58 μm wall thickness). The results showed that the insertion force was in the range of 0.1 ~ 3 N [49] and the ratio of breaking force to insertion force increased with increasing wall thickness and decreasing tip radius [49]. These studies provided a theoretical basis for predicting the insertion force and breaking forces, which is helpful for designing reasonable MNs geometrical structure.
Besides, compared with the subcutaneous route, insulin delivery through hollow MNs is less painful, but not painless. Theoretically, MNs should be painless if they are designed to act on the dermis and have no access to blood vessels and nerve fibers [50, 51]. The delivery mechanism of hollow MNs is similar with that of micro-syringes. For hollow MNs, not only the geometry of needles [52, 53] but also the infusion volume and flow rate of liquid formulation can affect the pain of administration [54]. Gupta et al. found that larger pressure applied induced more pain, and MN retraction also increased pain, but lower flow rate and co-administration of hyaluronidase would reduce pain [55].
Dissolving MNs
Recently, dissolving MNs have received more and more attention due to their obvious advantages such as simple preparation, high drug loading, and one-step application and have been extensively used to deliver insulin in literature research. Dissolving MNs are fabricated to incorporate insulin into the water-soluble or bio-degradable polymer matrix and insulin will be released after the dissolving or degradation of the inserted MNs. One of the merits of dissolving MNs is the flexibility of drug loading. Drugs can be loaded into the entire MN array, limited to needle layers, or encapsulated in the needle tip [56]. Moreover, quantitative and controlled drug release can be achieved by adjusting the dissolving profile of the polymer matrix, drug loading, and the distribution of the drug in the needle body [57, 58]. In addition, the solid-state storage of dissolving MNs is also conducive to maintaining the activity of insulin to a certain extent. Insulin encapsulated in MNs fabricated with dextrin can be stored at 40 °C for 1 month with almost no impairment to insulin activity [59], which reduces the demand for harsh cold chain storage during transportation.
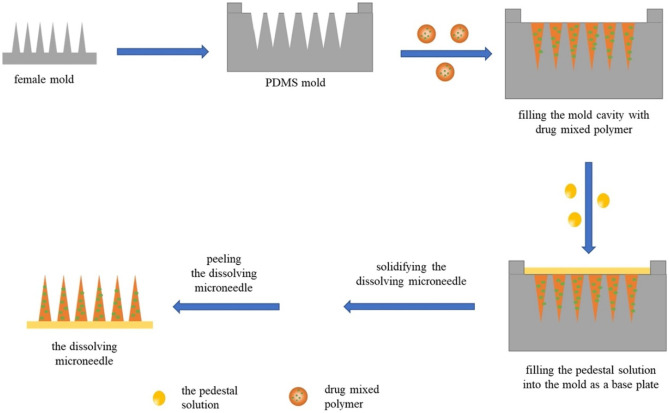
Water-dissolvable polysaccharides, including hydroxypropyl methylcellulose (HPMC), carboxymethyl cellulose (CMC), hydroxypropyl cellulose (HPC), hyaluronic acid (HA), dextran, sodium alginate, amylopectin, and sodium chondroitin sulfate, are mostly used to fabricate dissolving MNs [60–62]. Besides, some polymers with biodegradable properties, like gelatin, poly-γ-glutamic acid (γ-PGA), polyvinyl pyrrolidone (PVP), poly(vinylpyrrolidone-co-methacrylic acid) (PVP-MAA), PVP-cyclodextrin (PVP-CD), polyvinyl pyrrolidone-polyvinyl alcohol (PVP-PVA), are also used to fabricate dissolving MNs [63–65]. Dissolving MNs can be prepared by various micro-molding technologies, such as casting, hot embossing, and injection molding. In micro-molding method, an MN array mold is firstly prepared with tapered MN structure by laser etching, ion etching, or template flipping. And then the polymer solution is filled into the mold. Fill of polymer into mold tip is usually conducted through vacuum or centrifugation. After curing and de-molding, the MN array is obtained (Fig. 3) [66]. This method can expand the scale of production, but it still has some limitations. This fabricating process involves multiple steps, such as master batch preparation, mold manufacturing, and plasticization of thermoplastic polymers, which is time-consuming. Moreover, polymer needs to be plasticized above its glass transition temperature [67], so maybe this method is not suitable for insulin, which is sensitive to heat. To avoid the high temperature for polymer dissolution in micro-molding method, researchers have extensively explored the water-soluble polymers in the fabrication of heat-sensitive drugs loaded dissolving MNs, including HA [68], γ-PGA, mixture of starch and gelatin [69], and mixture of fish gelatin and sucrose [70].
Fig. 3.
Steps of micro-molding method. Reprinted with permission from [66].
Copyright 2021, Elsevier
Some new fabrication methods have been proposed to solve the thermal challenges of melting step in micro-molding methods. N-vinylpyrrolidone, the monomer of PVP, which can be polymerized by UV irradiation at room temperature, has been used to manufacture dissolving MNs. This method can avoid the use of organic solvent. While more than 30 min of UV exposure will be needed to polymerize, which may cause drug degradation [71]. Kathuria et al. used a combination of thermal and photo polymerization to shorten UV exposure time [71]. Hyaluronic acid (HA, 15 kD) was chosen as a model ingredient. N-vinylpyrrolidone solution was pre-polymerized by heating for 2 min at 90 °C and then cooled down to room temperature. Next, HA was added in the N-vinylpyrrolidone solution and the mixture was pipetted onto the PDMS mold, followed by irradiation with UV for 8 min. The pre-polymerization reduced the time of UV exposure and decreased the degradation of HA [71]. This process shortened the preparation time and was beneficial to scale-up production. In micro-molding method, centrifugation or vacuuming is used to fill the polymer solution into needle, while cause poor formation of MNs tip and inability removal of MNs from mold [72]. McGrath et al. used atomized spraying to fill the solution of MNs materials into PDMS mold instead of centrifugation or vacuuming with a two-fluid external mixing nozzle [73]. After spraying, the filled molds could be dried at room temperature. They used this spray method to successfully fabricate dissolving MNs with various sugars, including trehalose, fructose, and raffinose, and polymeric materials, including PVA, PVP, CMC, HPMC, and sodium alginate. This improved micro-molding method allows continuous manufacturing under mild processing conditions and is beneficial for drugs that are sensitive to high temperature or viscosity.
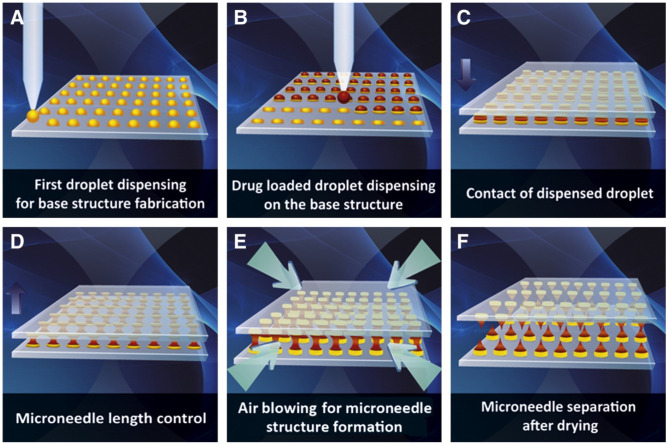
Currently, scientists have explored other mild methods to manufacture dissolving MNs. Kim et al. reported a novel droplet-born air blowing (DAB) method to prepare dissolving MNs [74]. In this method (Fig. 4), base structure of MNs was firstly fabricated by dispensing the drug-free biopolymer droplets (CMC, 90 kDa) to the flat surface, and the droplets containing drugs were dispensed over the base structure. Then, the upper plate was moved downward to contact the drug droplets and thereafter moved upward to elongate the droplets to form the tip of MNs. Air blowing was performed to drive out the water and solidify the MNs. DAB provides a fast and mild MN fabrication method which could be completed within 10 min under a condition of 4 ~ 25 °C. The 6 × 9 MN array containing 0.07 IU insulin was applied to the shaved diabetes mouse. The blood glucose level was dramatically reduced after 60 min and recovered after 120 min. Insulin-loaded MNs showed similar bioavailability (96.6 ± 2.4%) and hypoglycemic profile compared with subcutaneous injection. Shayan et al. also utilized DAB method to fabricate dissolving MNs under room temperature to encapsulate lysozyme which was sensitive to temperature [75]. Trehalose was added as the stabilizer. The results showed that the activity of lysozyme maintained highly up to 99.8% ± 3.8% after MNs fabricated. This indicated that air blow drying had no significant effect on lysozyme activity. And after 12 weeks of storage, the lysozyme activity was maintained at 99.8 ± 3.8% at 4 °C and 96.6 ± 3.0% at 25 °C. These studies display that DAB may be a promising method to encapsulate heat-sensitive biomolecules into MNs.
Fig. 4.
Schematic illustration of droplet-born air blowing (DAB) method. Reprinted with permission from [74].
Copyright 2013, Elsevier
One of the most important parameters of dissolving MNs is mechanical strength, which is essential for successful skin insertion. While dissolving MNs made of water-soluble polymers usually have weaker mechanical strength than insoluble materials such as silicon and metals [76, 77]. MNs’ mechanical strength may be further weakened after drug encapsulation [78, 79]. Park et al. experimentally tested the fracture strength of polymer MNs in the process of skin insertion and found that the fracture strength of MNs increased with the increasing of material elastic modulus and needle base diameter but decreased with the increasing of the MNs length [80]. As for the MNs they studied, when the elastic modulus of the material was less than 1 GPa, the MNs would cause buckling failure before piercing into the skin. These findings provide remarkable information to guide the selection of MNs materials, especially the selection of polymer materials. MNs fabricated with single material typically cannot achieve good hardness and toughness which can be improved by using multiple materials with different physical properties. Yu et al. fabricated insulin dissolving MNs with 3-aminophenylboronic acid–modified alginate and HA to enhance the mechanical strength. The in vitro penetration test showed that encapsulated insulin could be released in the deep skin. In vivo studies showed that the RPA and relative bioavailability (RBA) were both above 90% [81]. CaCO3 encapsulated in polymer matrix had been proved to enhance the mechanical strength before [82]. Liu et al. encapsulated insulin into CaCO3 microparticles and then prepared into dissolving MNs patch with PVP [83]. The MN patch showed excellent mechanical strength and slower insulin release properties due to the pH-sensitive dissolution behavior of CaCO3. The RPA and RBA of insulin were 98.2% and 96.6%, respectively.
Skin elasticity may cause incomplete insertion of the needle body into the skin and resulting in drug waste. In order to solve this issue, Chen et al. designed a MN array containing a supporting structure made from PVA/PVP to improve mechanical strength and overcome the influence from skin deformation during MN insertion process [64]. The needle tip of the MN array was made of insulin-loaded poly-γ-glutamic acid. After inserting the MN array into the skin, both the supporting structure and needle tip could be dissolved in 4 min and then released insulin rapidly. The hypoglycemic effect of the dissolving MN patch on diabetic rats was similar with that of subcutaneous injection, and no significant difference of insulin profile was observed between the first and second administration in diabetic rats, indicating the good reproducibility and accuracy of the MN patch in insulin delivery.
Dissolving MNs can release insulin slowly, providing with a long-term and stable glucose level for diabetics, which is desirable for long-acting insulin. But there are still some issues that need to be considered. The first is the safety concern. In the case of single administration, polymer deposition in the skin may not be a problem. But it is non-negligible for drugs requiring long-term application, such as insulin. Theoretically, repeated application of dissolving MNs may result in polymer deposition and accumulation in skin tissue, which in turn trigger an immune response, and accumulate in the liver or even the whole body [27]. At present, potential effects that result from polymer deposition in the skin are not well studied. Moreover, the current safety evaluation is mainly based on animals and the study period is short. In the study conducted by McCrudden et al., a dissolving MN path with 0.49 cm2 size area was fabricated to deliver ibuprofen sodium [84]. 5 ~ 10 mg of polymer were deposited in rat skin per 1 cm2 MN patch size. When the effective dose in rats was converted to humans, a patch size of 10 cm2 would be needed which meant that 50–100 mg of polymer would deposit in the skin. The author studied the polymers’ biocompatibility in cells and MNs tolerance experiments in rats, and there were no raised concerns. However, these two studies were just performed 24 h in cells, and single administration in rats, the long-term safety was unknown. Vicente-Perez et al. studied the safety of repeat application of dissolving MNs and hydrogel MNs [85]. Dissolving MNs made from methyl-vinyl ether-co-maleic-acid (PMVE/MA, Mw = 1,500,000 Da) and PVP (Mw = 58,000 Da) and hydrogel MNs made from Polyethylene glycol (PEG, Mw = 10,000 Da) were applied once daily for 5 weeks and twice daily for 3 weeks, respectively. In vivo studies in hairless mice showed no observed changes in the appearance and barrier function of skin during the entire study period. Besides, there were also no statistically changed biomarkers related to infection, immunity, and inflammation/irritation (C-reactive protein, immunoglobulin G, interleukin 1-β, and tumor necrosis factor-α) [85]. When reviewing the translation of this kind of MNs into clinic, regulatory authorities may need more information about the amount of polymer remaining in the skin, the rate and pathway of clearance, and the long-term safety.
Hydrogel MNs patch
To solve the problem of polymer deposition in the skin, non-degradable hydrogel MN patch was proposed [86]. Hydrogel MNs are fabricated through the physical or chemical cross-linking of hydrophilic polymers with water swelling properties [82]. When inserted into the skin, needles could rapidly absorb skin interstitial fluid and swell to a hydrogel state, which realizes the slow and continuous release of the preloaded drugs [25, 87]. The transdermal delivery efficiency and drug release behavior could be improved by adjusting the swelling ability of hydrogel polymers through controlling the cross-linking density [88, 89]. The toughness from the cross-linked matrix allowed hydrogel MNs to completely withdrawn from the skin without any deposition, which is expected for the frequent delivery of insulin [87, 90].
Hydrogel MNs are usually prepared by crosslinking of hydrophilic polymers. Natural polymers including agarose, methylcellulose, HA, chitosan, starch, and collagen are commonly used [91, 92]. Synthetic materials, like PVA, hydroxyethyl methacrylate (HEMA), poly(methyl vinyl ether-co-maleic anhydride) (PMVE/MA), and methacrylated hyaluronic acid (MeHA), are also widely used to prepare MNs [93]. The most common technique for hydrogel MNs fabricating is molding [62, 94], which is overall easier and allows for up-scaling production [95]. The polymer solution is poured and filled into a prefabricated mold under heating, pressure, vacuum, or centrifugation, and then the MNs are formed by inducing polymer crosslinking and solidification. The process of polymer crosslinking usually involves chemical reactions, such as esterification [82, 96], and physical induction under high temperature or UV irradiation. Therefore, heat-sensitive drugs, such as insulin, must be prepared in a way that avoids high temperatures. In addition, in the process of insulin encapsulation, chemical crosslinking agents must be carefully selected to avoid the potential interaction between insulin and crosslinking agents, which may affect insulin activity [97].
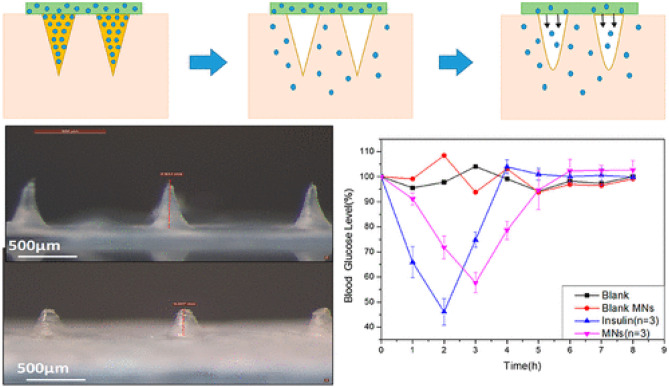
A bullet-shaped MN array containing a swellable tip made from polystyrene-block-poly(acrylic acid) (PS-PAA) and a non-swellable core made from polystyrene (PS) was developed by Seong et al. (Fig. 5) [98]. PS-PAA was also coated on the outside of MNs to form a double-layered structure. Insulin was incorporated into the swellable tips under a mild drop/dry processing based on the reversible swelling/de-swelling properties of MNs. After insertion into the skin, the needles were mechanically interlocked with soft skin tissue through selective distal swelling, which significantly improved the skin adhesion. In vivo pilot study, extended release of insulin could be achieved and consequently leading to a gradual decrease in blood glucose levels [98].
Fig. 5.
Schematic illustration and images of the bullet-shaped double-layered MN patch. The MN array displayed good structural uniformity as marked by the arrow (* to *′). Reproduced with permission from [98].
Copyright 2017, Elsevier
Yang et al. developed a new phase translation MNs patch (PTMs) with PVA as the matrix material and load insulin in the needle body [90]. The PVA solution was subjected to a mild freeze–thaw treatment to form the microcrystalline domains as the cross-linking junctions to obtain the anti-dissolution cross-linking of MNs matrix. Therefore, insulin can be safely loaded in the matrix of the MN tips to ensure sufficient hypoglycemic efficacy [90]. Moreover, dextran and CMC were applied to enrich the diffusion channels to speed up insulin delivery. In vivo study in diabetic pigs showed that PTMs patch carrying 2.0 IU/kg insulin lowered the glucose level for 40% at 2 h point post-administration [90]. The author also showed that the PTMs system can be sterilized by steaming in the final products avoiding the costly aseptic operation in the production process which was a great advancement for scale-up manufacturing [90].
In order to increase the use convenience and patients’ compliance, the size of MNs should not be too large [99]. As a result, the limitation of MNs size and the needle volume hinder to obtain high drug loading. In order to increase the drug loading, some scientists loaded the drug into a separate reservoir other than the needles directly. After being inserted into the skin, the MN needles will rapidly absorb interstitial fluid and swell to hydrogel state with osmotic conduits. Subsequently, it triggers the diffusion of drug molecules from the attached drug-containing reservoir. Owning to the concentration gradient, drug molecules will penetrate through the hydrogel matrix and enter the skin (Fig. 6) [100, 101]. Drug-containing reservoirs are usually designed into polymeric films and lyophilized wafers. This kind of hydrogel MNs has been used to deliver both chemical drugs and bio-macromolecules into the skin, including metformin hydrochloride [102], donepezil hydrochloride [103], insulin [57], ovalbumin [101], and bevacizumab [104].
Fig. 6.
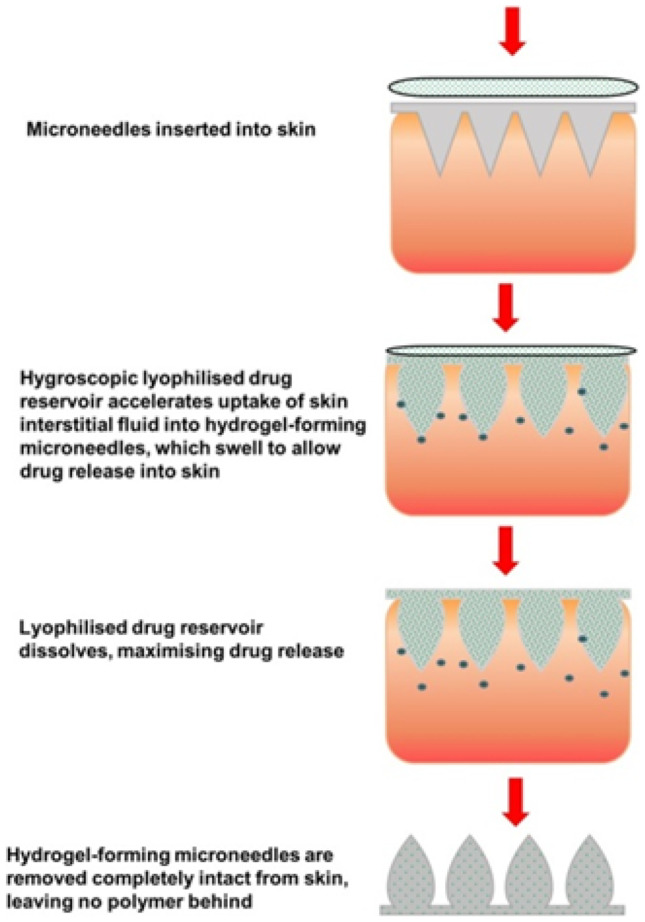
Schematic concept and drug delivery mechanism of hydrogel MNs combined with drug reservoirs [101].
Copyright 2014, PloS one
Donnely et al. reported a novel “integrated MN” consists of a backing layer, a drug-loaded adhesive patch, and a solid crosslinked hydrogel MN array [82]. The MN array was fabricated with PMVE/MA and poly(ethyleneglycol) 10,000 (PEG10000) by using laser-engineered micro-molding method, in which the crosslink temperature was 80 °C. To avoid the high temperature during the manufacturing process of hydrogel MNs, insulin was loaded into an adhesive gel made of PMVE/MA and tripropyleneglycol methyl ether (TPM) and then was cast into adhesive patch under room temperature. The “integrated MN” containing 0.2 IU insulin was formed on the attachment of insulin-loaded adhesive patch to the upper baseplates. After being administrated onto the diabetic rats, the blood glucose level reduced by 10% within 2 h and lowered further to 37% after 12 h [82]. In this insulin-MN system, insulin was released slow, which may be due to the extended diffusion pathway as the insulin reservoir was on the back of MNs arrays, so insulin released may need more time [87].
Recently, silk fibroin has been used to fabricate dissolving MNs due to its better mechanical properties, excellent toughness, and good biocompatibility [105]. Nevertheless, MNs made from unmixed silk fibroin break easily [106] and dissolve quickly [57], which may cause burst release of insulin and transient hypoglycemia. Zhu et al. reported a combined silk fibroin MN patch to solve this problem. This MN patch was fabricated by the two-step micro-molding method under 25 °C. Unmixed silk fibroin was used to make the MN tip and proline-modified fibroin with improved swellability was used to prepare the MN pedestal (Fig. 7) [107]. After the MN patch penetrated the skin, the needle tip could be quickly dissolved by the body fluids and then released insulin. Meanwhile, body fluid contacted the pedestal through the micro-channels formed by the needle tips, encouraging it to swell and subsequently releasing insulin continuously. The results of administration to diabetic rats showed that the blood glucose reached the lowest level in 3 h and returned to the initial level after 6 h. This structure displayed an apparent hypoglycemic effect which could meet the need of rapid hypoglycemic reduction after meal; meanwhile, a sustained release of insulin could be achieved without hypoglycemia risk.
Fig. 7.
Combined silk fibroin MN patch for insulin delivery. Reprinted with permission from [107].
Copyright 2020, American Chemical Society
Compared with dissolving MNs, hydrogel MNs can achieve a more consistent and controlled insulin release profile without polymer deposition in the skin. However, there are still some challenges to be addressed. Due to the incomplete release of loaded insulin from the hydrogel network, the final release amount is difficult to control. Therefore, it is necessary to study the relationship between the release profile and drug loading amount to guide the clinical administration. The insulin utilization ratio is also relatively lower than that of dissolving MNs. Hydrogel MNs made from polymers, like PMVE/MA [82, 102] and MeHA [108], exhibit excellent mechanical strength in the dry state allowing for successful skin penetration. While the negative of these materials is low swelling rate which will cause slow drug release rate. The swelling ability of the polymers is controlled by cross-linking density [109]. Increasing the concentration or cross-linking density can enhance the polymers’ mechanical strength, thus improving the integrity duration of the polymer matrix [110]. Nevertheless, this would determine the polymers’ porosity to be significantly reduced, limiting the modulation of drug release profile [111, 112]. The best application requirement of hydrogel MNs is to achieve a balance between mechanical strength and expansibility by selecting suitable polymer materials and manufacturing process of MNs [113].
Glucose-responsive MNs
The main challenge of insulin therapy is to accurately administer sufficient insulin throughout the day, including meal times and night, to achieve strict blood glucose control while avoiding severe hypoglycemia attacks [114, 115]. In recent years, the closed-loop glucose-responsive MN patch which can detect blood glucose level and adjust the release of insulin in time has become a hotspot in MNs delivery of insulin [116]. Glucose-responsive MNs are effective blood glucose-response systems that link the glucose-sensing and insulin release without the patient’s involvement [117], so as to achieve high efficiency, long-acting, and relatively low dosage. The most important is that glucose-responsive MN can reduce the risk of hypoglycemia [115]. Glucose-responsive MNs are usually composed of glucose-sensing elements, insulin, and cross-linker. Glucose-sensing elements, including glucose oxidase (GOx) [118, 119], glucose-binding protein, and phenylboronic acid (PBA), are widely studied [120].
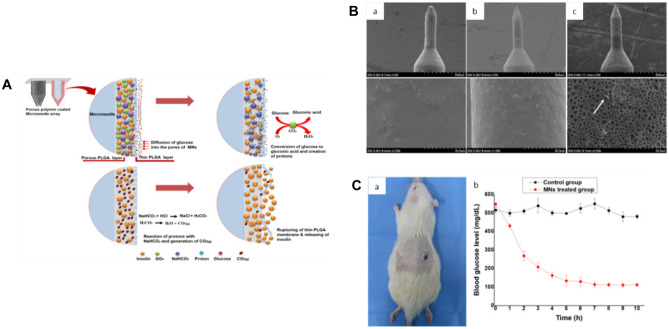
Gox, a flavin-containing glycoprotein with molecular weight of 100–1000 Da, acts as glucose-sensing unit by catalyzing glucose into gluconic acid and hydrogen peroxide (H2O2) through oxidation reaction [116]. This reaction creates local hypoxic, acidity, and high H2O2 environment [115]. These environment conditions can be utilized as biological stimulation to trigger the release of insulin from the MN matrix [87, 115]. Ullah et al. designed a glucose-responsive MN patch using the acidic microenvironment generated by the decomposition of glucose by GOx (Fig. 8) [121]. This MN patch was fabricated with a 7 × 7 stainless steel MN array. A porous polymer layer, which encapsulated glucose-responsive formulation (composed of insulin, GOx, and sodium bicarbonate (NaHCO3)) in the pores, was coated out of the MNs needles by dip-coating method [121]. NaHCO3 was acted as the pH-sensitive element. A PLGA film was coated out of the porous layer to protect the insulin formulation. When inserted into the skin, glucose could diffuse passively through the outer PLGA film and oxidized into gluconic acid by encapsulated GOx, consequently decreasing the local pH. Under the acidic microenvironment, NaHCO3 decomposed into carbon dioxide (CO2) gas [122] which in turn generated pressure inside the pores, causing the PLGA film ruptured and the preloaded insulin released. In vitro release test showed that under hyperglycemic level, almost 53% of the encapsulated insulin was released within the first 1 h [121]. After being applied into diabetic SD rats, the blood glucose level dropped rapidly within 2 h and then decreased slowly to the normal level in the next three hours.
Fig. 8.
Glucose-responsive insulin delivery system using porous-coated MNs. A Schematic illustration; B SEM micrographs of MNs at different magnifications (a before incubation in pH 7.4 PBS at 37 °C; b after incubation in pH 7.4 PBS at 37 °C without glucose; c after incubation in pH 7.4 PBS at 37 °C with 400 mg/dL glucose). C In vivo insulin release in diabetic rats [121].
Copyright 2020, MDPI
GOx-based MN system is acted on enzymatic reaction and exhibits high specificity towards glucose. In addition, the stimulus produced is positively correlated with glucose concentration, which is ideal for detecting glucose concentration accurately [123]. While the inflammation of skin tissues caused by H2O2 is one of the important obstacles in GOx-based glucose-responsive MNs [124]. To eliminate the H2O2 byproduct, Yang et al. designed a mimic multi-enzyme metal–organic framework (MOF)–based stimuli-responsive MNs which could decompose H2O2 while controlling the release of insulin [125]. A mimic multi-enzyme vehicle was constructed by implanting GOx and Co2+ ions into MOFs. GOx in the MOF could catalyze glucose into gluconic acid with the formation of H2O2. The generated gluconic acid lowered the local pH of MOFs, leading to the degradation of MOFs, thereby releasing insulin. At the same time, Co2+ ions in the MOF catalyzed reaction to decompose the byproduct H2O2 [125].
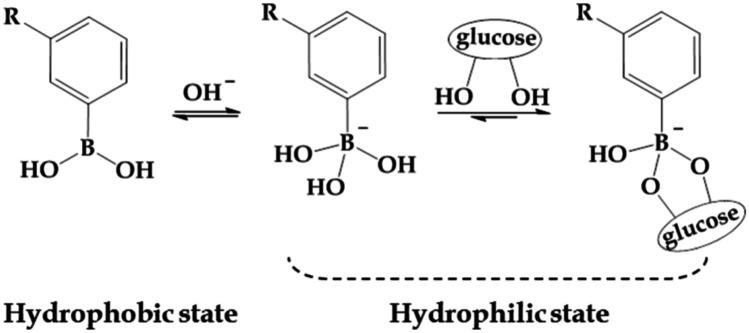
The most used synthetic chemicals in glucose-responsive delivery systems are PBA and its derivatives. PBA is a promising glucose sensor [126]. Compared with GOx and glucose-binding proteins, PBA shows high stability and durability in the physiological environment, making it reliable as glucose sensors in glucose-responsive MNs [115, 127]. The mechanisms of PBA-based glucose-sensing units include reversible conversion with glucose and charge-based swelling. Generally, PBA exists in two forms, charged and uncharged, and those two forms maintain equilibrium. The charged PBA can form a stable structure with glucose via reversible covalent bond (Fig. 9) [127, 128]. At high glucose concentration, the equilibrium will shift to a higher negative charge within the polymeric matrix of MNs. This led to enhanced swelling of MN matrix and triggered the release of preloaded insulin. However, it should be noted that most PBA-based MNs cannot work at physiological pH, because of the higher pKa of PBA (about 8.6) than the physiological pH (7.35–7.45) [129, 130], so high equilibrium constants are difficult to obtain in the body, and the response to glucose tends to be weak. Therefore, in order to lower the pKa and thus increase the response to glucose, the researchers introduced electron-withdrawing groups to benzene ring, including aromatic nucleus [131], sulfonyl group [132], nitryl group [133], halogen atom [134], or chemical groups such as carboxyl group [128] into the polymer [135].
Fig. 9.
Dissociation equilibrium of PBA in aqueous solution [130].
Copyright 2017, MDPI
Yu et al. designed a novel glucose-responsive MN system in which insulin, monomers, and cross-liker were mixed and formed the polymeric matrix by in situ photo-polymerization method [136]. 3-(Acrylamido)PBA (3APBA) was used as the responsive component. This manufacturing process was simple and efficient, and the avoidance of organic solvents and high temperatures was essential for maintaining the biological activity of insulin. In vitro release test showed that insulin released quickly with a glucose concentration of 400 mg/dL, while released relatively slowly at 0 and 100 mg/dL glucose level, reducing the risk of hypoglycemia. This MN patch was applied into STZ-induced diabetic minipigs and the glucose levels could be maintained to a near-normal range for more than 20 h under normal feeding condition [136].
Since the insulin release rate is directly controlled by the insulin concentration and blood glucose levels, it is essential to avoid the possibility of hypoglycemia at the beginning of MNs administration and hyperglycemia at the exhausted phase of loaded insulin. In order to prevent hypoglycemia, GhavamiNejad et al. developed a smart PBA-based glucagon MN patch [137]. In circumstances of hypoglycemia, the transmission of glucagon is enhanced to increase glucose level. When this MN system is given together with a glucose-responsive insulin delivery device, it can ensure safe and accurate blood sugar control. Another promising solution is a dual-drug loading system for insulin and glucagon. Yu et al. reported an insulin-responsive glucagon delivery system on the basis of specific binding between insulin and insulin aptamer [138]. Detailedly, the author firstly synthesized insulin immobilized methacrylated hyaluronic acid (Ins-HA) and insulin aptamer modified glucagon (Apt-Glu). Then, Apt-Glu was connected to Ins-HA via the specific interaction of aptamer and insulin to form an insulin-responsive glucagon conjugated HA (Glu-HA) matrix. With the assist of crosslinker and photoinitiator, MNs were fabricated with Glu-HA matrix by photo-crosslinking method. Under the condition of high insulin concentration, glucagon can be rapidly released from the HA matrix through the competitive binding between free insulin and immobilized insulin on HA [138]. In a chemically induced type 1 diabetic mouse model, the mice were pre-dosed with sufficient insulin to cause profound hypoglycemia. The results showed that mice treated with Glu-HA MNs maintained the glucose level at 80 mg/dL for 2 h, and then slowly returned to hyperglycemia. While the glucose level of mice in the control group continued to drop to a hypoglycemic state (< 70 mg/dL), suggesting that this glucose-responsive insulin delivery MNs can effectively prevent hypoglycemia [138].
Glucose-binding proteins have also been studied as glucose-responsive elements, among which Concanavalin A (Con A) is the most used [139, 140]. Con A is a protein with a molecular weight of 25.5 kDa. Each Con A molecule contains four sugar-binding sites at physiological pH [141] that show strong capability of bonding with α-D-mannopyranose and α-D-glucopyranose by hydroxyl groups [142]. However, due to its toxicity and instability in vivo, the application of Con A is limited [143].
Although these glucose-responsive elements have been widely studied in pre-clinical researches, there are still some problems that need to be considered. GOx can accurately determine or sense glucose concentration; nevertheless, GOx enzyme is very sensitive to environmental factors, such as high temperature, pH, and enzyme action, and exhibits poor stability, so it will affect the sensitivity of glucose sensing and the efficiency of insulin release [144–146]. Although Con A can be easily used to design glucose-responsive MN systems, its inherent limitations of toxicity, poor aqueous solubility and stability, and long response time may limit their usage in clinical translations [147]. PBA is a small molecule that can be easily used to design the different glucose-response systems through various chemicals modifying and incorporating into polymeric matrix to adjust the insulin release on demand [148, 149]. PBA-based glucose-responsive MNs have the advantages of the diversity of matrix composition, the variability of microstructure, and the adjustability of insulin release mechanism [150]. PBA has low specificity to glucose and is susceptible to other polyol molecules in the blood, which will affect the responsive accuracy and response time [135, 150]. Another issue is the biocompatibility. Up to now, there is few data on the safety and toxicity of the PBA-based system [151, 152].
Clinical progress of MNs-based insulin delivery
Some MNs-based insulin delivery systems have been moved into clinic studies, and we analyzed and examined those trials listed on the website ClinicalTrials.gov and classified them by clinical development phase. There are currently only 8 clinical trials related to insulin delivery using MNs. These clinical trials mainly focused on comparing the pharmacokinetics and pharmacodynamics of insulin delivered by MN devices and SC injection (Table 2). Interestingly, despite all the types of MNs discussed before, hollow MNs were the only type of MNs that has moved to clinical stage. Most of them are in Phase 1 and Phase 2 stages. At present, none of the products has reached to commercialization.
Table 2.
Clinical trials and status of insulin delivery using MNs
| NCT No | Condition | MNs type | Phase | Status | Sponsor | Estimate/actual study completion year | Estimate/actual enrollment |
|---|---|---|---|---|---|---|---|
| NCT00553488 | Type 1 Diabetes |
Hollow (BD Research Catheter Set) |
Phase 2 | Completed | Becton, Dickinson and Company | 2008 | 30 |
| NCT00602914 | Type 2 diabetes | Hollow (MicronJet™) | Early Phase 1 | Completed | NanoPass Technologies Ltd | 2009 | 23 |
| NCT01120444 | Type 1 Diabetes |
Hollow (BD Research Catheter Set) |
Phase 1 Phase 2 |
Completed | Becton, Dickinson and Company | 2010 | 20 |
| NCT01061216 | Type 1 Diabetes |
Hollow (BD Research Catheter Set) |
Phase 1 Phase 2 |
Completed | Becton, Dickinson and Company | 2010 | 20 |
| NCT01557907 | Type 1 Diabetes |
Hollow (BD Research Catheter Set) |
Phase 1 Phase 2 |
Completed | Becton, Dickinson and Company | 2012 | 23 |
| NCT00837512 | Type 1 Diabetes | Single, hollow glass MN |
Phase 2 Phase 3 |
Completed | Emory University | 2013 | 16 |
| NCT01684956 | Type 1 Diabetes | Hollow (MicronJet™) | Phase 2 | Unknown | Massachusetts General Hospital | 2017 | 20 |
| NCT02837094 | Type 1 Diabetes | Hollow (Nanopass MNs) | Phase 1 | Active, not recruiting | Cardiff University | 2020 | 8 |
In 2016, Kochba et al. [153] reported an improved insulin pharmacokinetics (PK) profile of patients with type 2 diabetes using Micronjet®, a hollow MN device containing four needles, by comparing with conventional SC injection administration (ClinicalTrials.gov Identifier: NCT01684956). The insulin (Novorapid®) intradermal delivery via Micronjet® showed a shorter Tmax (median 35 vs. 87.5 min (SC) [P < 0.001]), higher early exposure, shorter time to 50% Cmax (median 14.0 min vs. 26.0 min, p = 0.008), slightly higher Cmax (median 80 vs. 55 IU/mL [P = 0.085]), and higher late AUC glucose level which could potentially reduce the risk of postprandial hypoglycaemia.
BD Research Catheter Set was studied in 4 clinical trials. It is a device with 1.5-mm, 34-gauge MN research catheter set. One of the studies was to access the PK and pharmacodynamic postprandial glycemia (PPG) in patients with type 1 diabetes. The patients were given lispro (IL) or regular human insulin (RHI) by MN-based intradermal (ID) injections and SC injections after standard liquid meals (ClinicalTrials.gov Identifier: NCT00553488) [154]. It had been published that administrating insulin ID via hollow MN (BD Research Catheter Set) statistically improves the pharmacodynamic metric related to pharmacodynamic postprandial glycemia (PPG), insulin pharmacokinetics, and pharmacokinetic variability. The 90-min PPG (blood glucose area under the curve for 0–1.5 h) for ID RHI was 14% lower than SC RHI at −17 min (P < 0.0001) and 11% lower than ID RHI at −2 min (P = 0.0006). The Tmax values for IL and RHI were shortened by approximately 50% and 70%, respectively, compared to SC administration. Overall, the PK data of ID IL and ID RHI both displayed faster absorption rate, higher maximum concentration, and shorter systemic circulating duration versus SC administration [154]. The overall tolerability of IL and RHI via ID was good. Another result of the same research team showed that compared with SC injection, the secondary insulin PK endpoint showed faster ID availability in different doses and diets (ΔTmax −16 min, ΔT50 rising −7 min, ΔT50falling −30 min, all p < 0.05) [155] (ClinicalTrials.gov Identifier: NCT01120444). In summary, these findings indicate that, compared with traditional SC injection, MN-based ID insulin administration has a beneficial effect on insulin PK and PPG for both IL and RHI versus SC. With a more consistent pharmacokinetic outcome, the ID administration of insulin promises a better postprandial glucose control [155].
Norman et al. investigated the effectiveness and pain of insulin MNs to 16 children and young adults suffering from Type 1 diabetes by comparing with traditional SC insulin pump catheter [156]. In this phase 2/3 study (ClinicalTrials.gov Identifier: NCT00837512), they utilized a single, hollow glass MN with length less than 1 mm to administrate insulin lispro intradermally. MN produced shorter Tmax with around 32 min and SC delivery used 52 min to achieve Tmax. In addition, insulin onset time and offset time of hollow MN delivery were 22 min faster (p = 0.0004) and 34 min faster (p = 0.017) than SC catheter, respectively. Meanwhile, the injection pain caused by MN was significantly less than SC catheter (ΔVAS = −9.9), but the infusion pain caused by MN was not significantly increased (ΔVAS = 13.2) [156]. Such delivery tool showed superior insulin onset/offset and less injection pain, which could improve the compliance of insulin therapy and speed up the insulin pharmacokinetics to make insulin close-loop more effective.
More recently, a new approach was proposed to treat Type 1 Diabetes by attaching the insulin to gold (Au)-based nanoparticles and administrating intradermally via Nanopass® MNs, through this approach the investigator aims to slow or stop the damage of insulin-producing cells in the pancreas (ClinicalTrials.gov Identifier: NCT02837094). The safety of the treatment with insulin-related peptides linked to gold nanoparticles, including the obvious side effects was investigated in this study. Results had been submitted to ClinicalTrials.gov by the sponsor or investigator but are not yet publicly available.
The MN-based insulin delivery of clinical trials done so far focused on the intradermal administration route, several devices have shown their potential to improve the pharmacokinetics and pharmacodynamics of insulin and meanwhile reduce the risk of needle phobia. However, the participants enrolled in each clinical trial are limited (from 8 to 30 participants). Further study for larger sample size with data of intra-subject reproducibility and long-term safety is required to move this application forward.
Challenges
MNs-based insulin delivery systems have been well studied in the past 20 years. However, there are still some limitations remained for clinical translation and further commercialization. Here, we will discuss these translational challenges including safety, efficacy, patient acceptability, MNs manufacturing and scale-up, and regulatory authority acceptability.
Safety
As MNs need to pierce into the skin to work; therefore, it is mandatory that the materials used to fabricate MNs should be inert in nature, non-immunogenic, biocompatible, and stable. Biocompatibility and biosafety evaluation of materials to fabricate MNs are fundamental to translational studies in humans. MNs can be fabricated of metals, silicon, glass, polymers, and other materials. However, some of these materials may cause irritation, toxicity, and other adverse side effects to the skin or human body. Silicon and glass are brittle materials that are easy to brick off during the process of insertion [157] The bio-compatibility of these materials had not been well proven and minimal debris underneath the skin may induce bad inflammation [158]. Metals with strong mechanical strength and hardness, including stainless steel, nickel, and titanium, have also been used in MNs manufacturing [159]. These kinds of materials are not biodegradable and can cause tip waste with biological hazard in the skin [160]. In addition, nickel had been reported to cause allergy and should be prevented in the fabrication of MNs [18]. Polymers usually have high toughness that can insert into the skin with avoidance of brittle fracture [161]. Most polymers are bio-biodegradable which are more advantageous than other materials as they provide the additional benefit of a controllable biodegradation rate. PLA, PGA, and PLGA are commonly used in MNs scaffold applications [162]. However, for the drugs needed to be repeat use frequently, deposition of polymer underneath skin is undesirable. This type of MNs is more suitable for drug delivery that does not require frequent administration, such as vaccines, but it is undesirable for insulin long-term use. Glucose-responsive MNs have been hotspot in insulin therapy, while some safety problems about glucose responsive elements should be considered, as that GOx can cause host immune response [80], Con A shows inherent toxicity limitations, and the safety and toxicity of PBA are also unclear.
Inflammation and irritation are another safety issue of using MNs to deliver insulin. There are some notable reports of granulomas induced by silicon and glass [163, 164]. There are also some studies reported that the dissolving MNs living in the human body may lead to adverse skin effects, such as skin irritation and granulomas [165]. Glucose-responsive MNs with GOx as the reaction element will generate H2O2 which would cause inflammation or even severe tissue damage [124]. Repeated use of MNs on the same site may cause erythema, swelling, and skin irritation, while application at different sites may cause bioavailability variation because of the different thickness [166]. In addition, attention should also be paid to the closure time of pores after MNs removed, as the pores could be a path for pathogenic microbes or toxic substances [167].
Efficacy
The aim of MNs-based insulin drug formulation is to adjust the blood glucose levels to safe ranges without causing adverse reactions such as hypoglycemia. First of all, drug loading is the most important factor affecting the effectiveness of insulin MNs. Solid MNs and coated MNs are limited in insulin delivery due to their inconvenient administration and low drug loading, respectively. For dissolving MNs to be completely inserted into the skin, insulin is only loaded on the needle tip, which causes another problem, that is, low load capacity. The amount of drug loading is limited to the volume of the tip of dissolving MNs. As expected, the cumulative amount of drug administered is proportional to the size area of the MNs. Therefore, when designing a MN array system, it is necessary to consider and optimize the size area, the length, and the shape of the MNs, which can make huge influence on insulin loading on MNs, in order to enhance the insulin loading. For hydrogel MNs, the drug loading may not be a problem, as this kind MNs can be designed with drug reservoir. But because of the diffusion from the reservoir, it may be more suitable for basal blood glucose level maintenance, not the postprandial blood glucose control which needs rapid insulin release. In addition, the hydrogel MNs may exhibit low bioavailability because the insulin in the reservoir may not be fully released.
The stability of insulin in MN system is another problem which can influence the efficacy. Insulin is sensitive to unfavorable environment, like high temperature, extreme pH, trace metal ions, and some buffers, which may occur during MNs production, packaging, and storage, and will induce oxidation of insulin and consequently result in loss of therapeutic effect [168–170]. For instance, studies showed that when insulin conjugated with dextran, it could lose 60–90% native bioactivity [171, 172]. Some studies reported that metal ions could convert the molecular oxygen into more active oxidants, resulting in the oxidative degradation of proteins [173]. The presence of transition metal ions in the auxiliary excipients, as well as the stainless steel equipment used in MNs scale-up production, will both result in significant iron contamination that may lead to the degradation and oxidation of insulin [87]. In addition, some proteins will be oxidized even in an environment with 1% oxygen [174]. Besides, MNs are usually prepared under severe conditions, such as high temperature and ultraviolet radiation, which will also affect the stability of the intentional insulin.
The successful insertion of MNs into the skin without breakage or bending is fundamental for effective insulin delivery, which requires sufficient strength and stiffness of MNs. An optimal MN should be designed with low insertion force, the force required to insert MNs into living the skin and high breaking force, the force needles can withstand before fracturing. In the design of MNs, how to reduce the insertion force is one of the keys for successful MNs design, because the higher insertion force was, the greater force the MNs subjected and the easier to fracture or buckling. Meanwhile, excessive insertion force can also cause pain. Needle geometry, needle height, thickness and tip radius, base diameter, needle density, and MN material are the main factors that influence the mechanical strength of MNs [175]. The mostly used hollow MNs are often broken when inserted into the skin due to insufficient mechanical strength. MNs should not be designed too long or made of relatively weak materials; otherwise, the insertion force may overcome the tension of MNs, and consequently causing the needles to break [176]. Generally, as mentioned above, the hollow MNs with smaller tip radius, acute tip angle, and higher ratio of needle height to the needle bottom width, can be successfully inserted into the skin [177, 178]. Except MNs themselves related factors, skin thickness and elasticity also have effects on the insertion of MNs. Skin is considered as an anisotropic, nonlinear, and viscoelastic composite tissue [179]. Keratin in SC gives the skin stiffness, forming the main barrier of MNs inserting into the skin [180]. Collagen and elastin fibers in the dermis provide the skin strength and flexibility during skin puncture [181]. The insertion of MNs into the skin is influenced by various mechanical forces from the skin, including axial force, transverse force, shear force, friction force, etc. During skin insertion, the tips of the MNs are subjected to axial load. This axial load is basically called the compressive force, and it causes the buckling of MNs which may render MN ineffective. In order to reduce buckling, the tip should be sharp [182]. Some studies showed that the elastic properties of skin could twist around the MNs particularly if the needles are blunt and short, leading to MNs partially or incompletely inserted into SC [183, 184].
The final dose of insulin delivered through MNs is difficult to control, which will affect the hypoglycemic effect of insulin. Some scientific literatures emphasized that dissolving MNs are usually with low mechanical strength, and the dissolving MNs made of a variety of biological materials also have significant differences in skin penetration. The biggest challenge at present is that they cannot be completely inserted into the skin, which leads to drug waste and reduces the efficacy of insulin. To prevent this waste, insulin can be concentrated on the needle tips, but the loading amount will be limited. Chu et al. had also noted that it was difficult to control the insulin encapsulation and delivery of insulin from dissolving MNs, partly due to drug diffusion within the water-soluble MNs matrix during preparation [160]. Therefore, in order to better guide clinical application, the relationship between the final release amount and the load or release time of insulin needs more in-depth research.
For the diabetic patients, long-term insulin SC injection combined with glucose monitoring is necessary. Currently, the development of glucose-responsive MN patches has been extensively studied. These “smart” insulin MN patches have shown excellent drug release performance and therapeutic effects in pre-clinical studies [121, 185]. However, the construction of this system is more complicated, and numerous clinical studies are required to verify whether MNs can work effectively under the physiological environment. Extensive articles reported that MNs-based insulin patches showed significant hypoglycemic effects in animal models. While when the effective dose in animals was converted to humans, the amount of insulin required greatly exceeded the drug-loaded amount of the MNs. The drug loading capacity of MNs should be further improved due to the great difference between the skin structure of the human body and that of animals [116, 150]. Insulin MNs patches that can work successfully in animal models need more related clinical experiments to achieve good hypoglycemic effect in the human body.
Patient acceptability
The ultimate commercial success of a MN-based product depends not only on their ability to operate as designed, but also on their acceptability to patients [186, 187].
The pain generated by drug administration is closely related to patient’s compliance and acceptance [188]. Theoretically, the needle body of the MNs does not reach the deeper dermis and nerve endings, and the puncture pain should be less. Several researchers have studied the pain caused by MNs. In 2001, Kaushik et al. firstly accessed the pain caused by silicon MNs in human subjects [189]. Their results pointed out that the pain generated by MNs was less than that generated by 26-gauge needles [189]. Gill et al. had deeply studied the correlation of pain level and number, length of MN needles in [53]. Their results showed that MNs with length ranging from hundred microns to 1.5 mm produced significantly less pain than hypodermic needles, and the pain score was only 5 ~ 40% of hypodermic needles. The length of the MN needles had the greatest impact on pain, as the length increased 3 times, the pain score increased 7 times. The number of MN needles also affected the pain score. When the number of needles increased 10 times (increase from 5 to 50 needles), the pain increased more than 2 times. While the angle, thickness, and width of the MN tip had no significant effects on pain score [53]. Except application pain, skin reactions induced by MNs had also been studied. Arya et al. studied the effects of applying dissolving MN patches on local skin reactions, reliability of use, and patients’ acceptability [190]. Fifteen human participants were involved in this trial. Results demonstrated that dissolving MN patches showed well tolerance without any pain, erythema, or swelling at the MNs application site and were strongly accepted by human subjects. The study revealed that MN patches were preferred than conventional needle and syringe injection [190].
Another factor that may affect patients’ compliance is the convenience of usage. MN-based insulin delivery system is intended to reduce the incidence of side effects related to frequent injection and improve the quality of life of patients. As a self-applied product, insulin MNs must be easy to use. There were only few hollow MNs devices had been moved into clinical studies, which also requiring external auxiliary device. The administration is more complicated than insulin pens; thus, patients may consider it overly complex. Therefore, more convenient MNs need to be developed for the convenience of patients.
It is also very important for a prescriber to educate the MNs users to administer insulin properly and safely, since the application of MNs will be affected by many anthropogenic factors. Moreover, informative labeling and patient counseling strategies should also be developed [186].
Manufacturing and scale-up
There are now some hollow MN devices on the market and some of them are under clinical evaluation for insulin delivery. For other types of MNs, there may still be some difficulty in scale-up production. Although the manufacture of MNs with simple structures has been simplified and popularized, the manufacture of MNs with multi-component or special structures is still time consuming and technology dependent, requiring delicate operation. In addition, mild fabrication methods are needed to be developed as insulin is a biomolecule. At present, some scientists have optimized traditional preparation methods to produce insulin-loaded MNs under mild conditions, but it is still difficult to scale up. This further limits the translation progress. In order to be able to manufacture MNs on a large scale, further improvements in the existing technologies must ensure (1) a resolution of low-to-mid micron scale (~ 25–100 μm) can be achieved with high accuracy and precision (≤ 5%); (2) the automated process with minimal manual operation; and (3) the flexibility of manufacturing technology to adapt to various materials and fabrication methods [56].
Currently, micro-molding is widely used in MNs fabrication due to the high reproducibility and precision, cost-effectiveness, and the reusability of the molds [56, 87], while several issues need to be solved. In de-molding step, it is easy to break and lead to content variations of drug owning to the brittle character of material, especially in the case of silicon, glass, and ceramics MNs. In order to overcome this problem, “softer” material of mold seems to be a better choice. However, good mold requires well mechanical strength to avoid deformation after repeated use to ensure the accuracy of needle morphology and drug dosage. Shorter service life of “softer” mold means higher cost of manufacture. Besides, when inserted into the skin, the mechanical strength of needle should be strong enough so that it does not break. Several studies evaluated the reliability of MNs based on the ratio between the force at which the MN loses its structural integrity and the insertion force [158]. Moreover, complete cleaning is difficult to achieve in continuous casting process, which will lead to the accumulated residue of drug solution at the surface of mold.
The safety and effectiveness of insulin therapy are highly dose-sensitive and require stricter acceptance criteria for uniformity of content. However, uniformity of content has become one of the biggest barriers for mass manufacture of all kinds of MNs.
Regulatory authority acceptability
Currently, the licensing of MN products is processed on a per-application basis, since there were no production and implementation standards for MNs, which limit the translation and commercialization. Therefore, there is an urgent need to develop universal requirements of MNs based on the classification.
The regulatory requirements determine the acceptance criteria of product quality control and therefore the capital investment of mass manufacture. The likely considerations and potential regulatory requirements for MNs have been discussed [187, 191, 192]. The core question is the classification of MNs, whether MNs will be accepted as a drug delivery system, consumer product, or medical device. Recently, the U.S. Food and Drug Administration (FDA) published the final version of “Regulatory Considerations for Microneedling Products for Aesthetic Use” [193]. In this guidance, besides explicitly or implicitly product declarations or stating, FDA will consider the design and technological characteristics/features of MN product to determine whether MN product meets the requirements of Federal Food, Drug, and Cosmetic Act (FD&C Act) for a device. Specifically, FDA believes that the penetration of the needle through the stratum corneum is the result of the design or technology of a MN product. According to Sect. 201(h) of FD&C Act, it may be “intended to affect the structure or any function of the body”. The critical attributes include needle length, sharpness, and depth of penetration, etc. Although this guidance is classified into cosmetic field, it shows clear indication that the regulatory requirements of MNs as drug delivery system shall fall into the category of device. Meanwhile, FDA describes prefilled drug delivery device in which drug is filled into or otherwise combined with the device and the sole purpose of the device is to deliver drug products containing two or more regulatory products. Prefilled drug syringe, auto-injectors, microneedle “patch,” and MN-based insulin delivery system are examples of this type of product (21 CFR 3.2e) [194]. If MNs are considered to be closer to traditional subcutaneous injections, regulatory agencies may require sterilization of MNs depending on the size of MNs and skin penetration depth [94, 186]. While sterilization procedure, like dry heat, steam sterilization, gamma radiation, or microwave, may affect the stability of insulin. Aseptic manufacturing process can be used, while it is expensive and complicated.
At present, there is no recognized regulatory standard for MNs. For scale-up and commercialization of MNs based products, specific quality standards need to be established within the framework of Good Manufacturing Practices (GMP) [195]. However, there are so far no recognized regulatory acceptance standards of MNs products, and we can only speculate on the possible required Quality Control (QC) tests. Lutton et al. proposed a list of quality specifications applicable to all MNs according to the required specific tests of new drug products and parenteral drug products as required by ICH Harmonised Tripartite Guidelines [186, 192]. The universal specific tests for MNs including dissolution, disintegration, hardness/friability, uniformity of dosage units, water content, microbial limits, sterility, particulate matter, antimicrobial preservative content, extractables, functionality testing of delivery systems, and osmolarity [192]. The MNs’ mechanical characteristics need to be fully studied including axial force, transverse force, and baseplate strength to guarantee the successful insertion and safety for human use. Moreover, for the efficacy, MNs penetration depth in the skin and delivery ability of drugs should also be considered.
Conclusion and perspective
MN technology shows great advantages in pain relief from frequent injections and administration convenience, which can ease diabetic patients from needle phobia under traditional SC insulin injection and therefore improve their life quality.
Many efforts have been made to design and fabricate MN-based insulin delivery systems and numerous great research works have been reported. However, so far, there are still some challenges for clinical application and further commercialization. Firstly, the insulin loading amount needs to be addressed. Considering the bioavailability of existing preclinical insulin MNs and the effective dose in animals, it would require a large dose to be applied to humans, which is currently difficult to achieve. Secondly, the regulations regarding MNs are not fully established, neither the classification of MNs nor the quality standards. Thirdly, there are still challenges for scale-up and manufacturing process for MNs. The production of insulin MNs involves multiple steps, continuous and automatic online control under GMP scale will help to ensure the products consistency, reproducibility, and quality. Some latest technologies will be useful and help overcome those challenges. For example, for the needle shape design and fine-tuning, AI technology could be applied; for the efficiency of the MN manufacturing production, 3D printing technology is promising.
Insulin-controlled administration is a tendency. Numerous researchers are devoted to developing glucose-responsive MNs for insulin delivery. However, there is still a long way to go in clinical translation. It is very important to verify the responsive function in human physiological environment under the premise of ensuring long-term safety.
Although the current MN-based drug delivery system still has some problems to be solved. However, with the continuous development of MNs fabricating technology, the ingenious combination of MNs with other medical devices or intelligent drug delivery systems, and the continuous improvement of the MNs evaluation and regulatory system, MNs will be widely used for the transdermal delivery of insulin and other active molecules. In particular, glucose-responsive MNs have become a research trend that combines novel materials for biosensors or intelligent drug delivery systems.
Author contribution
Jing Zhao and Genying Xu contributed equally to this work. Jing Zhao: Manuscript preparation and writing. Genying Xu: Co-first author, manuscript preparation, and writing. Xin Yao: Manuscript preparation and writing. Huirui Zhou: Manuscript preparation and writing. Boyang Lyu: Manuscript preparation and writing. Shuangshuang Pei: Manuscript preparation and revision. Ping Wen: Manuscript organization, guidance, and revision.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animal experiments.
Consent for publication
All authors agree to the material for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zhao and Genying Xu are first authors.
Contributor Information
Jing Zhao, Email: kay.zhao@prinburybiopharm.com.
Genying Xu, Email: xu.genying@zs-hospital.sh.cn.
Xin Yao, Email: sally.yao@prinburybiopharm.com.
Huirui Zhou, Email: ryan.zhou@prinburybiopharm.com.
Boyang Lyu, Email: boyanglv@prinburybiopharm.com.
Shuangshuang Pei, Email: cathy.pei@prinburybiopharm.com.
Ping Wen, Email: liz.wen@prinburybiopharm.com.
References
- 1.IDF Diabetes Atlas, 9th edn. Brussels, [database on the Internet]. Belgium: International Diabetes Federation. 2019. Accessed: 06 Jun 2021
- 2.Zaykov A, Mayer J, DiMarchi R. Pursuit of a perfect insulin. Nat Rev Drug Discovery. 2016;15(6):425–439. doi: 10.1038/nrd.2015.36. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Wan L, Luo J, Jiang M, Wang K. Advances in subcutaneous delivery systems of biomacromolecular agents for diabetes treatment. Int J Nanomedicine. 2021;16:1261–1280. doi: 10.2147/IJN.S283416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev. 2018;127:119–137. doi: 10.1016/j.addr.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Guo X, Wang W. Challenges and recent advances in the subcutaneous delivery of insulin. Expert Opin Drug Deliv. 2017;14(6):727–734. doi: 10.1080/17425247.2016.1232247. [DOI] [PubMed] [Google Scholar]
- 6.Zhao R, Lu Z, Yang J, Zhang L, Li Y, Zhang X. Drug delivery system in the treatment of diabetes mellitus. Frontiers in bioengineering and biotechnology. 2020;8:880. doi: 10.3389/fbioe.2020.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinemann L, Braune K, Carter A, Zayani A, Krämer LA. Insulin storage: a critical reappraisal. J Diabetes Sci Technol. 2021;15(1):147–159. doi: 10.1177/1932296819900258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen D, Yu H, Wang L, Khan A, Haq F, Chen X, et al. Recent progress in design and preparation of glucose-responsive insulin delivery systems. J Control Release. 2020;321:236–258. doi: 10.1016/j.jconrel.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Long L-y, Zhang J, Yang Z, Guo Y, Hu X, Wang Y. Transdermal delivery of peptide and protein drugs: strategies, advantages and disadvantages. J Drug Delivery Sci Technol. 2020;60:102007. 10.1016/j.jddst.2020.102007.
- 10.Easa N, Alany RG, Carew M, Vangala A. A review of non-invasive insulin delivery systems for diabetes therapy in clinical trials over the past decade. Drug Discovery Today. 2019;24(2):440–451. doi: 10.1016/j.drudis.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. doi: 10.1016/j.addr.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng LC, Gupta M. Transdermal drug delivery systems in diabetes management: a review. Asian J Pharm Sci. 2020;15(1):13–25. doi: 10.1016/j.ajps.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, et al. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Mönkäre J, Pontier M, van Kampen EEM, Du G, Leone M, Romeijn S, et al. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur J Pharm Biopharm. 2018;129:111–121. doi: 10.1016/j.ejpb.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Jeon EY, Lee J, Kim BJ, Joo KI, Kim KH, Lim G, et al. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials. 2019;222:119439. doi: 10.1016/j.biomaterials.2019.119439. [DOI] [PubMed] [Google Scholar]
- 16.Al Sulaiman D, Chang JYH, Bennett NR, Topouzi H, Higgins CA, Irvine DJ, et al. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano. 2019;13(8):9620–9628. doi: 10.1021/acsnano.9b04783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indermun S, Luttge R, Choonara YE, Kumar P, du Toit LC, Modi G, et al. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 18.Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Hatware K, Bhadane P, Sindhikar S, Mishra DK. Recent advances in microneedle composites for biomedical applications: advanced drug delivery technologies. Mater Sci Eng, C. 2019;103:109717. doi: 10.1016/j.msec.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Pradeep Narayanan S, Raghavan S. Solid silicon microneedles for drug delivery applications. The International Journal of Advanced Manufacturing Technology. 2017;93(1):407–422. doi: 10.1007/s00170-016-9698-6. [DOI] [Google Scholar]
- 21.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingrole RSJ, Gill HS. Microneedle coating methods: a review with a perspective. J Pharmacol Exp Ther. 2019;370(3):555–569. doi: 10.1124/jpet.119.258707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 24.Nagarkar R, Singh M, Nguyen HX, Jonnalagadda S. A review of recent advances in microneedle technology for transdermal drug delivery. Journal of Drug Delivery Science and Technology. 2020;59:101923. doi: 10.1016/j.jddst.2020.101923. [DOI] [Google Scholar]
- 25.Tucak A, Sirbubalo M, Hindija L, Rahić O, Hadžiabdić J, Muhamedagić K et al. Microneedles: characteristics, materials, production methods and commercial development. Micromachines. 2020;11(11). 10.3390/mi11110961. [DOI] [PMC free article] [PubMed]
- 26.Roxhed N, Griss P, Stemme G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed Microdevice. 2008;10(2):271–279. doi: 10.1007/s10544-007-9133-8. [DOI] [PubMed] [Google Scholar]
- 27.Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32. doi: 10.1016/j.mser.2016.03.001. [DOI] [Google Scholar]
- 28.Cárcamo-Martínez Á, Mallon B, Domínguez-Robles J, Vora LK, Anjani QK, Donnelly RF. Hollow microneedles: a perspective in biomedical applications. Int J Pharm. 2021;599:120455. doi: 10.1016/j.ijpharm.2021.120455. [DOI] [PubMed] [Google Scholar]
- 29.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P-C, Paik S-J, Chen S, Rajaraman S, Kim S-H, Allen M. Fabrication and characterization of polymer hollow microneedle array using UV lithography into micromolds. J Microelectromech Syst. 2013;22(5):1041–1053. doi: 10.1109/JMEMS.2013.2262587. [DOI] [Google Scholar]
- 31.Vora LK, Courtenay AJ, Tekko IA, Larrañeta E, Donnelly RF. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol. 2020;146:290–298. doi: 10.1016/j.ijbiomac.2019.12.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra R, Bhattacharyya TK. MEMS-based hollow microneedles for transdermal drug delivery. In: Chappel E, editor. Drug Delivery Devices and Therapeutic Systems. Academic Press. 2021;325–44. 10.1016/B978-0-12-819838-4.00007-9.
- 33.Wang PC, Wester BA, Rajaraman S, Paik SJ, Kim SH, Allen MG, Hollow polymer microneedle array fabricated by photolithography process combined with micromolding technique. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Minneapolis, MN, USA: IEEE. 2009;2009:7026–7029. doi: 10.1109/IEMBS.2009.5333317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinayakumar KB, Kulkarni PG, Nayak MM, Dinesh NS, Hegde GM, Ramachandra SG, et al. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J Micromech Microeng. 2016;26(6):065013. doi: 10.1088/0960-1317/26/6/065013. [DOI] [Google Scholar]
- 35.Mishra R, Maiti T, Bhattacharyya T. Feasibility studies on nafion membrane actuated micropump integrated with hollow microneedles for insulin delivery device. J Microelectromech Syst. 2019;28(6):987–996. doi: 10.1109/JMEMS.2019.2939189. [DOI] [Google Scholar]
- 36.Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7(3). 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed]
- 37.Economidou SN, Douroumis D. 3D printing as a transformative tool for microneedle systems: recent advances, manufacturing considerations and market potential. Adv Drug Deliv Rev. 2021;173:60–69. doi: 10.1016/j.addr.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Yadav V, Kumar Sharma P, Suryanarayana Murty U, Mohan NH, Thomas R, Kumar Dwivedy S, et al. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int J Pharm. 2021;605:120815. doi: 10.1016/j.ijpharm.2021.120815. [DOI] [PubMed] [Google Scholar]
- 39.Yeung C, Chen S, King B, Lin H, King K, Akhtar F, et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13(6):064125. doi: 10.1063/1.5127778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xenikakis I, Tsongas K, Tzimtzimis EK, Zacharis CK, Theodoroula N, Kalogianni EP, et al. Fabrication of hollow microneedles using liquid crystal display (LCD) vat polymerization 3D printing technology for transdermal macromolecular delivery. Int J Pharm. 2021;597:120303. doi: 10.1016/j.ijpharm.2021.120303. [DOI] [PubMed] [Google Scholar]
- 41.Economidou S, Uddin M, Marques M, Douroumis D, Sow W, Li H, et al. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalised transdermal drug delivery. Addit Manuf. 2020;38:101815. doi: 10.1016/j.addma.2020.101815. [DOI] [Google Scholar]
- 42.Sušić A, Hrnjica Z, Kajgana I, Mujezinović M, Hasanbegović A, Brčkalo J, et al. editors. Use of hollow microneedle drug delivery systems in treatment of diabetes mellitus. Int Conference on Med Biol Eng Cham: Springer, Cham 2019.
- 43.Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112(3):357–361. doi: 10.1016/j.jconrel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164–182. doi: 10.1016/j.jconrel.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 46.Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Bodhale DW, Nisar A, Afzulpurkar N. Structural and microfluidic analysis of hollow side-open polymeric microneedles for transdermal drug delivery applications. Microfluid Nanofluid. 2010;8(3):373–392. doi: 10.1007/s10404-009-0467-9. [DOI] [Google Scholar]
- 48.Griss P, Stemme G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J Microelectromech Syst. 2003;12(3):296–301. doi: 10.1109/JMEMS.2003.809959. [DOI] [Google Scholar]
- 49.Davis S, Landis B, Adams Z, Allen M, Prausnitz M. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37(8):1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Azmana M, Mahmood S, Hilles AR, Mandal UK, Saeed Al-Japairai KA, Raman S. Transdermal drug delivery system through polymeric microneedle: a recent update. Journal of Drug Delivery Science and Technology. 2020;60:101877. doi: 10.1016/j.jddst.2020.101877. [DOI] [Google Scholar]
- 51.Ita K. Transdermal delivery of drugs with microneedles-potential and challenges. Pharmaceutics. 2015;7(3):90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sezgin B, Ozel B, Bulam H, Guney K, Tuncer S, Cenetoglu S. The effect of microneedle thickness on pain during minimally invasive facial procedures: a clinical study. Aesthetic Surg J. 2014;34(5):757–765. doi: 10.1177/1090820x14532941. [DOI] [PubMed] [Google Scholar]
- 53.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma G, Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Control Release. 2017;251:11–23. doi: 10.1016/j.jconrel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Gupta J, Park SS, Bondy B, Felner EI, Prausnitz MR. Infusion pressure and pain during microneedle injection into skin of human subjects. Biomaterials. 2011;32(28):6823–6831. doi: 10.1016/j.biomaterials.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarbox TN, Watts AB, Cui Z, Williams RO., 3rd An update on coating/manufacturing techniques of microneedles. Drug Deliv Transl Res. 2018;8(6):1828–1843. doi: 10.1007/s13346-017-0466-4. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Zhu M, Zhao L, Kuang D, Kundu SC, Lu S. Insulin-loaded silk fibroin microneedles as sustained release system. ACS Biomater Sci Eng. 2019;5(4):1887–1894. doi: 10.1021/acsbiomaterials.9b00229. [DOI] [PubMed] [Google Scholar]
- 58.Jamaledin R, Makvandi P, Yiu CKY, Agarwal T, Vecchione R, Sun W, et al. Engineered microneedle patches for controlled release of active compounds: recent advances in release profile tuning. Advanced Therapeutics. 2020;3(12):2000171. doi: 10.1002/adtp.202000171. [DOI] [Google Scholar]
- 59.Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29(1):82–88. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Park Y-H, Ha SK, Choi I, Kim KS, Park J, Choi N, et al. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol Bioprocess Eng. 2016;21(1):110–118. doi: 10.1007/s12257-015-0634-7. [DOI] [Google Scholar]
- 61.Saha I, Rai V. Hyaluronic acid based microneedle array: recent applications in drug delivery and cosmetology. Carbohyd Polym. 2021;267:118168. doi: 10.1016/j.carbpol.2021.118168. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed Saeed Al-Japairai K, Mahmood S, Hamed Almurisi S, Reddy Venugopal J, Rebhi Hilles A, Azmana M et al. Current trends in polymer microneedle for transdermal drug delivery. Int J Pharm. 2020;587:119673. 10.1016/j.ijpharm.2020.119673. [DOI] [PMC free article] [PubMed]
- 63.Zhuang J, Rao F, Wu D, Huang Y, Xu H, Gao W, et al. Study on the fabrication and characterization of tip-loaded dissolving microneedles for transdermal drug delivery. Eur J Pharm Biopharm. 2020;157:66–73. doi: 10.1016/j.ejpb.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Chen M-C, Ling M-H, Kusuma SJ. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015;24:106–116. doi: 10.1016/j.actbio.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 65.Lee IC, He JS, Tsai MT, Lin KC. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. Journal of materials chemistry B. 2015;3(2):276–285. doi: 10.1039/c4tb01555j. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Guo R, Wang S, Yang X, Ling G, Zhang P. Fabrication, evaluation and applications of dissolving microneedles. Int J Pharm. 2021;604:120749. doi: 10.1016/j.ijpharm.2021.120749. [DOI] [PubMed] [Google Scholar]
- 67.Eum J, Kim Y, Um DJ, Shin J, Yang H, Jung H. Solvent-free polycaprolactone dissolving microneedles generated via the thermal melting method for the sustained release of capsaicin. Micromachines. 2021;12(2):167. doi: 10.3390/mi12020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu S, Jin MN, Quan YS, Kamiyama F, Katsumi H, Sakane T, et al. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release. 2012;161(3):933–941. doi: 10.1016/j.jconrel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Wu M, Tan D, Liu Q, Xia R, Chen M, et al. A dissolving and glucose-responsive insulin-releasing microneedle patch for type 1 diabetes therapy. Journal of materials chemistry B. 2021;9(3):648–657. doi: 10.1039/d0tb02133d. [DOI] [PubMed] [Google Scholar]
- 70.Vassilieva EV, Kalluri H, McAllister D, Taherbhai MT, Esser ES, Pewin WP, et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv Transl Res. 2015;5(4):360–371. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kathuria H, Kang K, Cai J, Kang L. Rapid microneedle fabrication by heating and photolithography. Int J Pharm. 2020;575:118992. doi: 10.1016/j.ijpharm.2019.118992. [DOI] [PubMed] [Google Scholar]
- 72.Kim M, Park S, Choi S-O. Dual-nozzle spray deposition process for improving the stability of proteins in polymer microneedles. RSC Adv. 2017;7:55350–55359. doi: 10.1039/C7RA10928H. [DOI] [Google Scholar]
- 73.McGrath MG, Vucen S, Vrdoljak A, Kelly A, O’Mahony C, Crean AM, et al. Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration. Eur J Pharm Biopharm. 2014;86(2):200–211. doi: 10.1016/j.ejpb.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 74.Kim JD, Kim M, Yang H, Lee K, Jung H. Droplet-born air blowing: novel dissolving microneedle fabrication. J Control Release. 2013;170(3):430–436. doi: 10.1016/j.jconrel.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 75.Fakhraei Lahiji S, Jang Y, Ma Y, Dangol M, Yang H, Jang M, et al. Effects of dissolving microneedle fabrication parameters on the activity of encapsulated lysozyme. Eur J Pharm Sci. 2018;117:290–296. doi: 10.1016/j.ejps.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Juster H, van der Aar B, de Brouwer H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym Eng Sci. 2019;59(5):877–890. doi: 10.1002/pen.25078. [DOI] [Google Scholar]
- 77.Shercliff HR, Lovatt AM. Selection of manufacturing processes in design and the role of process modelling. Prog Mater Sci. 2001;46(3):429–459. doi: 10.1016/S0079-6425(00)00013-X. [DOI] [Google Scholar]
- 78.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donnelly RF, Morrow DI, Singh TR, Migalska K, McCarron PA, O’Mahony C, et al. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm. 2009;35(10):1242–1254. doi: 10.1080/03639040902882280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park J, Allen M, Prausnitz M. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Yu W, Jiang G, Zhang Y, Liu D, Xu B, Zhou J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater Sci Eng, C Mater Biol Appl. 2017;80:187–196. doi: 10.1016/j.msec.2017.05.143. [DOI] [PubMed] [Google Scholar]
- 82.Donnelly R, Singh T, Garland M, Migalska K, Majithiya R, McCrudden C, et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Func Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu D, Yu B, Jiang G, Yu W, Zhang Y, Xu B. Fabrication of composite microneedles integrated with insulin-loaded CaCO microparticles and PVP for transdermal delivery in diabetic rats. Mater Sci Eng, C Mater Biol Appl. 2018;90:180–188. doi: 10.1016/j.msec.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 84.McCrudden MT, Alkilani AZ, McCrudden CM, McAlister E, McCarthy HO, Woolfson AD, et al. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J Control Release. 2014;180:71–80. doi: 10.1016/j.jconrel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vicente-Perez EM, Larrañeta E, McCrudden MTC, Kissenpfennig A, Hegarty S, McCarthy HO, et al. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur J Pharm Biopharm. 2017;117:400–407. doi: 10.1016/j.ejpb.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Delivery. 2010;17(4):187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X, Wang L, Yu H, Li C, Feng J, Haq F, et al. Preparation, properties and challenges of the microneedles-based insulin delivery system. J Control Release. 2018;288:173–188. doi: 10.1016/j.jconrel.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 88.Donnelly RF, Singh TR, Alkilani AZ, McCrudden MT, O’Neill S, O’Mahony C, et al. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: potential for enhanced patient safety. Int J Pharm. 2013;451(1–2):76–91. doi: 10.1016/j.ijpharm.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiu Y, Qin G, Zhang S, Wu Y, Xu B, Gao Y. Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery. Int J Pharm. 2012;437(1–2):51–56. doi: 10.1016/j.ijpharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 90.Yang S, Wu F, Liu J, Fan G, Welsh W, Zhu H, et al. Phase-transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv Func Mater. 2015;25(29):4633–4641. doi: 10.1002/adfm.201500554. [DOI] [Google Scholar]
- 91.Demir YK, Akan Z, Kerimoglu O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS ONE. 2013;8(10):e77289. doi: 10.1371/journal.pone.0077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong X, Wei L, Wu F, Wu Z, Chen L, Liu Z, et al. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des Dev Ther. 2013;7:945–952. doi: 10.2147/dddt.S44401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Donnelly RF, Majithiya R, Singh TR, Morrow DI, Garland MJ, Demir YK, et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28(1):41–57. doi: 10.1007/s11095-010-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhatnagar S, Gadeela PR, Thathireddy P, Venuganti VVK. Microneedle-based drug delivery: materials of construction. J Chem Sci. 2019;131(9). 10.1007/s12039-019-1666-x.
- 95.Wang QL, Zhu DD, Chen Y, Guo XD. A fabrication method of microneedle molds with controlled microstructures. Mater Sci Eng C Mater Biol Appl. 2016;65:135–142. doi: 10.1016/j.msec.2016.03.097. [DOI] [PubMed] [Google Scholar]
- 96.Demir YK, Metin A, Şatıroğlu B, Solmaz M, Kayser V, Mäder K. Poly (methyl vinyl ether-co-maleic acid) – pectin based hydrogel-forming systems: gel, film, and microneedles. European J Pharm Bio. 2017;117. 10.1016/j.ejpb.2017.04.018. [DOI] [PubMed]
- 97.Demuth PC, Min Y, Huang B, Kramer JA, Miller AD, Dan HB, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013;12(4):367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seong K-Y, Seo M-S, Hwang DY, O’Cearbhaill ED, Sreenan S, Karp JM, et al. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release. 2017;265:48–56. doi: 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 99.Zong Q, Guo R, Dong N, Ling G, Zhang P. Design and development of insulin microneedles for diabetes treatment. Drug Deliv Transl Res. 2021 doi: 10.1007/s13346-021-00981-y. [DOI] [PubMed] [Google Scholar]
- 100.McAlister E, Dutton B, Vora LK, Zhao L, Ripolin A, Zahari DSZBPH, et al. Directly compressed tablets: a novel drug-containing reservoir combined with hydrogel-forming microneedle arrays for transdermal drug delivery. Adv Healthcare Mat. 2021;10(3):2001256. 10.1002/adhm.202001256. [DOI] [PubMed]
- 101.Donnelly RF, McCrudden MTC, Zaid Alkilani A, Larrañeta E, McAlister E, Courtenay AJ, et al. Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery. PLoS ONE. 2014;9(10):e111547. doi: 10.1371/journal.pone.0111547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Migdadi EM, Courtenay AJ, Tekko IA, McCrudden MTC, Kearney MC, McAlister E, et al. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release. 2018;285:142–151. doi: 10.1016/j.jconrel.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kearney MC, Caffarel-Salvador E, Fallows SJ, McCarthy HO, Donnelly RF. Microneedle-mediated delivery of donepezil: potential for improved treatment options in Alzheimer’s disease. Eur J Pharm Biopharm. 2016;103:43–50. doi: 10.1016/j.ejpb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 104.Courtenay AJ, McCrudden MTC, McAvoy KJ, McCarthy HO, Donnelly RF. Microneedle-mediated transdermal delivery of bevacizumab. Mol Pharm. 2018;15(8):3545–3556. doi: 10.1021/acs.molpharmaceut.8b00544. [DOI] [PubMed] [Google Scholar]
- 105.Horan RL, Bramono DS, Stanley JR, Simmons Q, Chen J, Boepple HE et al. Biological and biomechanical assessment of a long-term bioresorbable silk-derived surgical mesh in an abdominal body wall defect model. Hernia: the j hernias and abdominal wall surg. 2009;13(2):189–99. 10.1007/s10029-008-0459-9. [DOI] [PubMed]
- 106.Yin Z, Kuang D, Wang S, Zheng Z, Yadavalli VK, Lu S. Swellable silk fibroin microneedles for transdermal drug delivery. Int J Biol Macromol. 2018;106:48–56. doi: 10.1016/j.ijbiomac.2017.07.178. [DOI] [PubMed] [Google Scholar]
- 107.Zhu M, Liu Y, Jiang F, Cao J, Kundu SC, Lu S. Combined silk fibroin microneedles for insulin delivery. ACS Biomater Sci Eng. 2020;6(6):3422–3429. doi: 10.1021/acsbiomaterials.0c00273. [DOI] [PubMed] [Google Scholar]
- 108.Than A, Liu C, Chang H, Duong PK, Cheung CMG, Xu C, et al. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat Commun. 2018;9(1):4433. doi: 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang M, Hu L, Xu C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip. 2017;17(8):1373–1387. doi: 10.1039/c7lc00016b. [DOI] [PubMed] [Google Scholar]
- 110.Tu Y, Chen N, Li C, Liu H, Zhu R, Chen S, et al. Advances in injectable self-healing biomedical hydrogels. Acta Biomaterialia. 2019;90. 10.1016/j.actbio.2019.03.057. [DOI] [PubMed]
- 111.Kretlow J, Klouda L, Mikos A. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 112.Cirillo G, Curcio M, Nicoletta, Iemma. Injectable hydrogels for cancer therapy over the last decade. Pharmaceutics. 2019;11:486. 10.3390/pharmaceutics11090486. [DOI] [PMC free article] [PubMed]
- 113.Tejashree, Waghule, Gautam, Singhvi, Sunil, Kumar, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed & pharmacotherapy. 2019;109:1249–58. 10.1016/j.biopha.2018.10.078. [DOI] [PubMed]
- 114.Rege NK, Phillips NFB, Weiss MA. Development of glucose-responsive ‘smart’ insulin systems. Curr Opin Endocrinol Diabetes Obes. 2017;24(4):267–278. doi: 10.1097/MED.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Wang Z, Yu J, Kahkoska AR, Buse JB, Gu Z. Glucose-responsive insulin and delivery systems: innovation and translation. Adv Mater. 2020;32(13):e1902004. doi: 10.1002/adma.201902004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen G, Yu J, Gu Z. Glucose-responsive microneedle patches for diabetes treatment. J Diabetes Sci Technol. 2019;13(1):41–48. doi: 10.1177/1932296818778607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bakh NA, Cortinas AB, Weiss MA, Langer RS, Anderson DG, Gu Z, et al. Glucose-responsive insulin by molecular and physical design. Nat Chem. 2017;9(10):937–943. doi: 10.1038/nchem.2857. [DOI] [PubMed] [Google Scholar]
- 118.Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase — an overview. Biotechnol Adv. 2009;27(4):489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112(27):8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsumoto A, Tanaka M, Matsumoto H, Ochi K, Moro-Oka Y, Kuwata H, et al. Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice. Sci Adv. 2017;3(11):eaaq0723. 10.1126/sciadv.aaq0723. [DOI] [PMC free article] [PubMed]
- 121.Ullah A, Choi HJ, Jang M, An S, Kim GM. Smart microneedles with porous polymer layer for glucose-responsive insulin delivery. Pharmaceutics. 2020;12(7):606. doi: 10.3390/pharmaceutics12070606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ke CJ, Lin YJ, Hu YC, Chiang WL, Chen KJ, Yang WC, et al. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials. 2012;33(20):5156–5165. doi: 10.1016/j.biomaterials.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 123.Wang Z, Wang J, Kahkoska AR, Buse JB, Gu Z. Developing insulin delivery devices with glucose responsiveness. Trends Pharmacol Sci. 2021;42(1):31–44. doi: 10.1016/j.tips.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saravanakumar G, Kim J, Kim WJ. Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Advanced Science. 2017;4(1):1600124. doi: 10.1002/advs.201600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang XX, Feng P, Cao J, Liu W, Tang Y. Composition-engineered metal-organic framework-based microneedles for glucose-mediated transdermal insulin delivery. ACS Appl Mater Interfaces. 2020;12(12):13613–13621. doi: 10.1021/acsami.9b20774. [DOI] [PubMed] [Google Scholar]
- 126.Ding Z, Guan Y, Zhang Y, Zhu XX. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter. 2009;5(11):2302. doi: 10.1039/b901910c. [DOI] [Google Scholar]
- 127.Ma Q, Zhao X, Shi A, Wu J. Bioresponsive functional phenylboronic acid-based delivery system as an emerging platform for diabetic therapy. Int J Nanomedicine. 2021;16:297–314. doi: 10.2147/IJN.S284357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsumoto A, Ikeda S, Harada A, Kataoka K. Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions. Biomacromol. 2003;4(5):1410–1416. doi: 10.1021/bm034139o. [DOI] [PubMed] [Google Scholar]
- 129.Ma R, Shi L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: synthesis and applications in drug delivery. Polym Chem. 2014;5. 10.1039/C3PY01202F.
- 130.Zhao L, Huang Q, Liu Y, Wang Q, Wang L, Xiao S, et al. Boronic acid as glucose-sensitive agent regulates drug delivery for diabetes treatment. Materials (Basel) 2017;10(2):170. doi: 10.3390/ma10020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Springsteen G, Wang B. A detailed examination of boronic acid–diol complexation. Tetrahedron. 2002;58(26):5291–5300. doi: 10.1016/S0040-4020(02)00489-1. [DOI] [Google Scholar]
- 132.Li X, Pennington J, Stobaugh JF, Schöneich C. Synthesis of sulfonamide- and sulfonyl-phenylboronic acid-modified silica phases for boronate affinity chromatography at physiological pH. Anal Biochem. 2008;372(2):227–236. doi: 10.1016/j.ab.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan J, Springsteen G, Deeter S, Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears. Tetrahedron. 2004;60(49):11205–11209. doi: 10.1016/j.tet.2004.08.051. [DOI] [Google Scholar]
- 134.Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem Int Ed. 2012;51:2124–2128. doi: 10.1002/anie.201108617. [DOI] [PubMed] [Google Scholar]
- 135.Huang Q, Wang L, Yu H, Ur-Rahman K. Advances in phenylboronic acid-based closed-loop smart drug delivery system for diabetic therapy. J Control Release. 2019;305:50–64. doi: 10.1016/j.jconrel.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 136.Yu J, Wang J, Zhang Y, Chen G, Mao W, Ye Y, et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nature biomedical engineering. 2020;4(5):499–506. doi: 10.1038/s41551-019-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.GhavamiNejad A, Li J, Lu B, Zhou L, Lam L, Giacca A, et al. Glucose-responsive composite microneedle patch for hypoglycemia-triggered delivery of native glucagon. Adv Mater. 2019;31(30):e1901051. doi: 10.1002/adma.201901051. [DOI] [PubMed] [Google Scholar]
- 138.Yu J, Zhang Y, Sun W, Kahkoska A, Wang J, Buse J, et al. Insulin-responsive glucagon delivery for prevention of hypoglycemia. Small. 2017;13(19):1603028. doi: 10.1002/smll.201603028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brownlee M, Cerami A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science (New York, NY) 1979;206(4423):1190–1191. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- 140.Brownlee M, Cerami A. Glycosylated insulin complexed to Concanavalin A. Biochemical basis for a closed-loop insulin delivery system. Diabetes. 1983;32(6):499–504. 10.2337/diab.32.6.499. [DOI] [PubMed]
- 141.Edelman GM, Cunningham BA, Reeke GN, Becker JW, Wang W. The covalent and three-dimensional structure of Concanavalin A. Proc Natl Acad Sci USA. 1972;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sappia LD, Piccinini E, Marmisollé W, Santilli N, Maza E, Moya S et al. Integration of biorecognition elements on PEDOT platforms through supramolecular interactions. Adv Mater Interfaces. 2017:1700502. 10.1002/admi.201700502.
- 143.Ibrahim SRM, Sirwi A, Eid BG, Mohamed SGA, Mohamed GA. Summary of natural products ameliorate Concanavalin A-induced liver injury: structures, sources, pharmacological effects, and mechanisms of action. Plants (Basel, Switzerland). 2021;10(2). 10.3390/plants10020228. [DOI] [PMC free article] [PubMed]
- 144.Harris JM, Reyes C, Lopez GP. Common causes of glucose oxidase instability in in vivo biosensing: a brief review. J Diabetes Sci Technol. 2013;7(4):1030–1038. doi: 10.1177/193229681300700428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu Q, Wang L, Yu H, Wang J, Chen Z. Organization of glucose-responsive systems and their properties. Chem Rev. 2011;111(12):7855–7875. doi: 10.1021/cr200027j. [DOI] [PubMed] [Google Scholar]
- 146.Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010;10(5):4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim JJ, Park K. Glucose-binding property of pegylated Concanavalin a. Pharm Res. 2001;18(6):794–799. doi: 10.1023/A:1011084312134. [DOI] [PubMed] [Google Scholar]
- 148.Wu W, Mitra N, Yan EC, Zhou S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano. 2010;4(8):4831–4839. doi: 10.1021/nn1008319. [DOI] [PubMed] [Google Scholar]
- 149.Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem Int Ed Engl. 2012;51(9):2124–2128. doi: 10.1002/anie.201106252. [DOI] [PubMed] [Google Scholar]
- 150.Yang J, Cao Z. Glucose-responsive insulin release: analysis of mechanisms, formulations, and evaluation criteria. J Control Release. 2017;263:231–239. doi: 10.1016/j.jconrel.2017.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2012;64(3):49–60. doi: 10.1016/S0169-409X(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 152.Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem Int Ed. 2012;51(9):2124–2128. doi: 10.1002/anie.201106252. [DOI] [PubMed] [Google Scholar]
- 153.Kochba E, Levin Y, Raz I, Cahn A. Improved insulin pharmacokinetics using a novel microneedle device for intradermal delivery in patients with type 2 diabetes. Diabetes Technol Ther. 2016;18(9):525–531. doi: 10.1089/dia.2016.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pettis RJ, Hirsch L, Kapitza C, Nosek L, Hövelmann U, Kurth HJ, et al. Microneedle-based intradermal versus subcutaneous administration of regular human insulin or insulin lispro: pharmacokinetics and postprandial glycemic excursions in patients with type 1 diabetes. Diabetes Technol Ther. 2011;13(4):443–450. doi: 10.1089/dia.2010.0183. [DOI] [PubMed] [Google Scholar]
- 155.McVey E, Hirsch L, Sutter DE, Kapitza C, Dellweg S, Clair J, et al. Pharmacokinetics and postprandial glycemic excursions following insulin lispro delivered by intradermal microneedle or subcutaneous infusion. J Diabetes Sci Technol. 2012;6(4):743–754. doi: 10.1177/193229681200600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Norman JJ, Brown MR, Raviele NA, Prausnitz MR, Felner EI. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2013;14(6):459–465. doi: 10.1111/pedi.12031. [DOI] [PubMed] [Google Scholar]
- 157.He X, Sun J, Zhuang J, Xu H, Liu Y, Wu D. Microneedle system for transdermal drug and vaccine delivery: devices, safety, and prospects. Dose-Response. 2019;17(4):1559325819878585. doi: 10.1177/1559325819878585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O’Mahony C. Structural characterization and in-vivo reliability evaluation of silicon microneedles. Biomed Microdevice. 2014;16(3):333–343. doi: 10.1007/s10544-014-9836-6. [DOI] [PubMed] [Google Scholar]
- 159.Ali R, Mehta P, Arshad MS, Kucuk I, Chang MW, Ahmad Z. Transdermal microneedles—a materials perspective. AAPS PharmSciTech. 2019;21(1):12. doi: 10.1208/s12249-019-1560-3. [DOI] [PubMed] [Google Scholar]
- 160.Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010;99(10):4228–4238. doi: 10.1002/jps.22140. [DOI] [PubMed] [Google Scholar]
- 161.Lee JW, Han MR, Park JH. Polymer microneedles for transdermal drug delivery. J Drug Target. 2013;21(3):211–223. doi: 10.3109/1061186x.2012.741136. [DOI] [PubMed] [Google Scholar]
- 162.Sabir MI, Xu X, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44(21):5713–5724. doi: 10.1007/s10853-009-3770-7. [DOI] [Google Scholar]
- 163.Kubo K, Tsukasa N, Uehara M, Izumi Y, Ogino M, Kitano M, et al. Calcium and silicon from bioactive glass concerned with formation of nodules in periodontal-ligament fibroblasts in vitro. J Oral Rehabil. 1997;24(1):70–75. doi: 10.1046/j.1365-2842.1997.00462.x. [DOI] [PubMed] [Google Scholar]
- 164.Eskeland G, Langmark F, Husby G. Silicon granuloma of the skin and subcutaneous tissues. The American Journal of Surgery. 1966;112(1):119–123. doi: 10.1016/S0002-9610(66)91294-3. [DOI] [PubMed] [Google Scholar]
- 165.Jeong HR, Lee HS, Choi IJ, Park JH. Considerations in the use of microneedles: pain, convenience, anxiety and safety. J Drug Target. 2017;25(1):29–40. doi: 10.1080/1061186x.2016.1200589. [DOI] [PubMed] [Google Scholar]
- 166.Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59(11):1152–1161. doi: 10.1016/j.addr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 167.Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29. doi: 10.1111/j.2042-7158.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 168.Broom WA, Coulthard CE, et al. Some pharmacological and chemotherapeutic properties of notatin. Br J Pharmacol Chemother. 1946;1(4):225–233. doi: 10.1111/j.1476-5381.1946.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 169.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2(4):289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 170.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Baudys M, Letourneur D, Liu F, Mix D, Jozefonvicz J, Kim SW. Extending insulin action in vivo by conjugation to carboxymethyl dextran. Bioconjug Chem. 1998;9(2):176–183. doi: 10.1021/bc970180a. [DOI] [PubMed] [Google Scholar]
- 172.Sakamoto Y, Akanuma Y, Kosaka K, Jeanrenaud B. Comparative effects of native insulin and insulin-dextran complexes on the metabolism of adipose tissue. Biochem Biophys Acta. 1977;498(1):102–113. doi: 10.1016/0304-4165(77)90091-5. [DOI] [PubMed] [Google Scholar]
- 173.Li S, Patapoff TW, Overcashier D, Hsu C, Nguyen TH, Borchardt RT. Effects of reducing sugars on the chemical stability of human relaxin in the lyophilized state. J Pharm Sci. 1996;85(8):873–877. doi: 10.1021/js950456s. [DOI] [PubMed] [Google Scholar]
- 174.Benson HAE, McIldowie M, Prow T. Magnetophoresis: skin penetration enhancement by a magnetic field. In: Dragicevic N, Maibach HI, editors. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. vol Chapter 12: Springer-Verlag Berlin Heidelberg. 2017;195–206. 10.1007/978-3-662-53273-7_12.
- 175.Davis SP, Prausnitz MR, Allen MG, editors. Fabrication and characterization of laser micromachined hollow microneedles. TRANSDUCERS '03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems. Digest of Technical Papers (Cat. No.03TH8664); 2003 8–12 June 2003.
- 176.Al-Qallaf B, Das DB. Optimization of square microneedle arrays for increasing drug permeability in skin. Chem Eng Sci. 2008;63(9):2523–2535. doi: 10.1016/j.ces.2008.02.007. [DOI] [Google Scholar]
- 177.Coulman SA, Birchall JC, Alex A, Pearton M, Hofer B, O’Mahony C, et al. In vivo, in situ imaging of microneedle insertion into the skin of human volunteers using optical coherence tomography. Pharm Res. 2011;28(1):66–81. doi: 10.1007/s11095-010-0167-x. [DOI] [PubMed] [Google Scholar]
- 178.Kalluri H, Choi S-O, Guo XD, Lee JW, Norman J, Prausnitz MR. Evaluation of microneedles in human subjects. In: Dragicevic N, I. Maibach H, editors. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. Berlin, Heidelberg: Springer Berlin Heidelberg. 2017;325–40. 10.1007/978-3-662-53273-7_20.
- 179.Schriefl AJ, Zeindlinger G, Pierce DM, Regitnig P, Holzapfel GA. Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface. 2012;9(71):1275–1286. doi: 10.1098/rsif.2011.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Benítez JM, Montáns FJ. The mechanical behavior of skin: structures and models for the finite element analysis. Comput Struct. 2017;190:75–107. doi: 10.1016/j.compstruc.2017.05.003. [DOI] [Google Scholar]
- 181.Makvandi P, Kirkby M, Hutton ARJ, Shabani M, Yiu CKY, Baghbantaraghdari Z, et al. Engineering microneedle patches for improved penetration: analysis, skin models and factors affecting needle insertion. Nano-Micro Letters. 2021;13(1):93. doi: 10.1007/s40820-021-00611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sawon MA, Samad MF. Design and optimization of a microneedle with skin insertion analysis for transdermal drug delivery applications. Journal of Drug Delivery Science and Technology. 2021;63:102477. doi: 10.1016/j.jddst.2021.102477. [DOI] [Google Scholar]
- 183.Verbaan FJ, Bal SM, van den Berg DJ, Dijksman JA, van Hecke M, Verpoorten H, et al. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J Control Release. 2008;128(1):80–88. doi: 10.1016/j.jconrel.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 184.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wu M, Zhang Y, Huang H, Li J, Liu H, Guo Z, et al. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater Sci Eng, C. 2020;117:111299. doi: 10.1016/j.msec.2020.111299. [DOI] [PubMed] [Google Scholar]
- 186.Kirkby M, Hutton A, Donnelly R. Microneedle mediated transdermal delivery of protein, peptide and antibody based therapeutics: current status and future considerations. Pharm Res. 2020;37(6):117. doi: 10.1007/s11095-020-02844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Donnelly R. Clinical translation and industrial development of microneedle-based products. In: Ryan F. Donnelly TRRS, Eneko Larrañeta, and Maelíosa T.C. McCrudden, editor. Microneedles for Drug and Vaccine Delivery and Patient Monitoring, First Edition.: John Wiley & Sons Ltd. 2018;307–22. 10.1002/9781119305101.ch11.
- 188.Guillot AJ, Cordeiro AS, Donnelly RF, Montesinos MC, Garrigues TM, Melero A. Microneedle-based delivery: an overview of current applications and trends. Pharmaceutics. 2020;12(6). 10.3390/pharmaceutics12060569. [DOI] [PMC free article] [PubMed]
- 189.Kaushik S, Hord A, Denson D, McAllister D, Smitra S, Allen M, et al. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92:502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 190.Arya J, Henry S, Kalluri H, McAllister D, Pewin W, Prausnitz M. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128. 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed]
- 191.Nguyen T, Oh Y, Kim Y, Shin Y, Baek S, Park J. Progress in microneedle array patch (MAP) for vaccine delivery. Hum Vaccin Immunother. 2021;17:316–327. doi: 10.1080/21645515.2020.1767997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lutton RE, Moore J, Larrañeta E, Ligett S, Woolfson AD, Donnelly RF. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv Transl Res. 2015;5(4):313–331. doi: 10.1007/s13346-015-0237-z. [DOI] [PubMed] [Google Scholar]
- 193.Administration USDoHaHSFaD, Health CfDaR. Regulatory considerations for microneedling products. The U.S Food and drug administration. 2020.
- 194.Administration UFaD. Combination products therapeutic and diagnostic products that combine drugs, devices, and/or biological products. 2018.
- 195.Richter-Johnson J, Kumar P, Choonara Y, du Toit L, Pillay V. Therapeutic applications and pharmacoeconomics of microneedle technology. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):359–369. doi: 10.1080/14737167.2018.1485100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.