Abstract
Levothyroxine (LT4) is a safe, effective means of hormone replacement therapy for hypothyroidism. Here, we review the pharmaceutical, pathophysiological and behavioural factors influencing the absorption, distribution, metabolism and excretion of LT4. Any factor that alters the state of the epithelium in the stomach or small intestine will reduce and/or slow absorption of LT4; these include ulcerative colitis, coeliac disease, bariatric surgery, Helicobacter pylori infection, food intolerance, gastritis, mineral supplements, dietary fibre, resins, and various drugs. Once in the circulation, LT4 is almost fully bound to plasma proteins. Although free T4 (FT4) and liothyronine concentrations are extensively buffered, it is possible that drug- or disorder-induced changes in plasma proteins levels can modify free hormone levels. The data on the clinical significance of genetic variants in deiodinase genes are contradictory, and wide-scale genotyping of hypothyroid patients is not currently justified. We developed a decision tree for the physician faced with an abnormally high thyroid-stimulating hormone (TSH) level in a patient reporting adequate compliance with the recommended LT4 dose. The physician should review medications, the medical history and the serum FT4 level and check for acute adrenal insufficiency, heterophilic anti-TSH antibodies, antibodies against gastric and intestinal components (gastric parietal cells, endomysium, and tissue transglutaminase 2), and Helicobacter pylori infection. The next step is an LT4 pharmacodynamic absorption test; poor LT4 absorption should prompt a consultation with a gastroenterologist and (depending on the findings) an increase in the LT4 dose level. An in-depth etiological investigation can reveal visceral disorders and, especially, digestive tract disorders.
Keywords: Absorption, Metabolism, Deiodinases, Drugs, LT4 absorption test, Pseudomalabsorption
Introduction
The drug levothyroxine (LT4) constitutes a safe, effective hormone replacement therapy for hypothyroidism caused by autoimmune thyroiditis, partial or total thyroidectomy, radioiodine treatment, or drug side effects [1–5]. LT4 is primarily given per os as a tablet, soft-gel or liquid (drop) formulation, although liquid preparations for intramuscular or intravenous injection are also available. The chronic nature and high prevalence of hypothyroidism (~4% of adults in Western countries), together with the unaltered life expectancy of treated patients, explain why LT4 is one of the world’s most extensively prescribed drugs [6–9]. However, LT4 has a narrow therapeutic window, and around 30% of patients fail to stably achieve the recommended serum level of thyroid-stimulating hormone (TSH; between 0.4 and 4.0 mIU/L, according to the American Thyroid Association guidelines) with a standard, body-weight-based starting dose of LT4 (typically 1.5 to 1.7 µg/kg/day in patients with residual endogenous thyroid function, such as those with auto-immune thyroiditis) [2, 10–12]. The dose of LT4 required by a patient can be predicted from the total body weight, the body mass index (BMI), ideal body weight, and lean body mass, with the latter three parameters providing the most accurate estimates. Several equations have been developed to calculate the dose requirement [13].
Hence, for each individual patient with hypothyroidism, the challenge for the clinician is to find the oral dose of LT4 that relieves the clinical signs and symptoms of hypothyroidism a condition that is typically associated with the restoration of parameters of thyroid hormone (TH) action (primarily the serum TSH level) to within the age-appropriate physiological reference (or target) range.
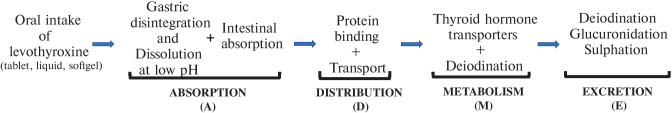
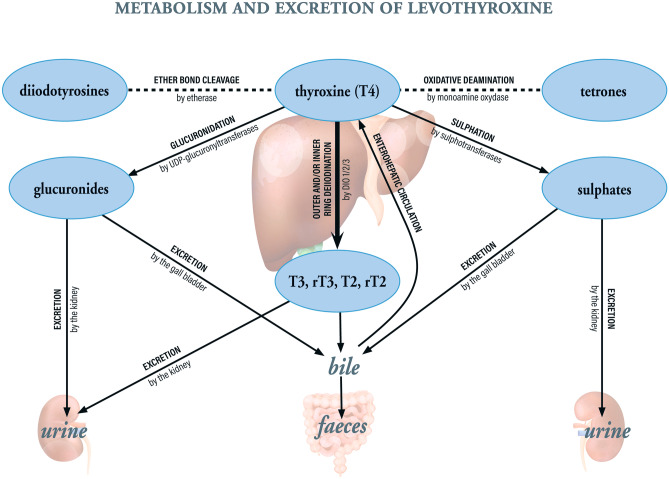
The clinical effectiveness of any drug depends on pharmaceutical, pathophysiological (internal) and behavioural (external) factors. Many of these factors interfere with absorption, distribution, metabolism and excretion (ADME). The overall ADME process for LT4 is summarized in Fig. 1. Oral LT4 (typically formulated as the sodium salt) dissolves in the stomach at low pH but is absorbed mainly in the small intestine (the jejunum and ilium) within three hours of ingestion [14, 15]. The bioavailability of LT4 is 65–80% in fasting euthyroid subjects and hypothyroid patients [16, 17]. Once the LT4 has crossed the intestinal epithelium and reached the circulation, it is almost entirely (~99.9%) bound to plasma proteins (mainly albumin, thyroxine-binding globulin (TBG), transthyretin, and high-density lipoprotein). The mean distribution volume of LT4 is 11–12 L in euthyroid subjects and (due to fluid retention) up to 15 L in hypothyroid patients [18, 19]. T4 is metabolized in several organs, glands, and areas of the brain (mainly the liver but also the thyroid, anterior pituitary, and kidney) and in peripheral tissues (e.g. the muscles) (Fig. 2) [20]. Primarily, deiodination of T4’s outer ring by type 2 deiodinase (DIO2) gives liothyronine (L-tri-iodothyronine or T3, the main biologically active metabolite), and deiodination of the inner ring by type 1 or type 3 deiodinases (DIO1 or DIO3) gives the biologically inactive reverse T3 (rT3) [21]. Around 20% of the ingested dose of LT4 is ultimately eliminated in the stools; this includes T4 and T3 excreted in the bile after glucuronidation or sulphation in the enterohepatic cycle (Fig. 2) [20]. The remaining 80% is excreted in the urine [20]. The half-life of T4 is reportedly 6–7 days in euthyroid subjects and 7–8 days in hypothyroid patients [22]. However, the relationships between levels of TSH, free T4 (FT4) and free T3 (FT3) are not the same in LT4-treated patients as in healthy, euthyroid individuals, and the distribution, metabolism and excretion of exogenous LT4 differ from those of endogenous T4 [23].
Fig. 1.
An overview of ADME processes for orally administered LT4 in the treatment of hypothyroidism
Fig. 2.
Pathways and sites for the metabolism and excretion of LT4
When faced with insufficient initial effectiveness of LT4 or a loss of effectiveness in a previously stable patient, the physician will typically consider increasing the daily dose. However, it is at this moment that the physician should ask him/herself whether LT4’s apparent lack of effectiveness is related to one or more factors that interfere with the drug’s ADME. Of course, one must always seek to distinguish between true malabsorption and pseudomalabsorption; the latter is usually due to poor treatment compliance (i.e. not taking the prescribed LT4). A four- to six-hour LT4 absorption test (i.e. the monitoring of blood T4 levels after the supervised administration of a weight-based dose of LT4) is the mainstay of this distinction [24]. The paracetamol absorption test has also been used as control; given that paracetamol and LT4 differ in their physicochemical properties and absorption mechanisms, malabsorption of both will only be caused by severe dysfunction of the gastrointestinal tract, and so an abnormal LT4 absorption test and a normal paracetamol test is suggestive of poor compliance with LT4 treatment [25].
Hence, the primary objective of the present review was to draw up a comprehensive list of pharmaceutical, pathophysiological (internal) and behavioural (external) factors that influence the absorption, distribution, metabolism and excretion of LT4 and can therefore condition the drug’s effectiveness, safety and required dose. The secondary objective was to provide practical decision support for physicians managing patients with “treatment-refractory” hypothyroidism, unexpectedly poor effectiveness of LT4, or suspected LT4 malabsorption.
Methods
Literature search and analysis
We searched the PubMed database from January 1st, 2000, to April 30th, 2021, with logical combinations of the following terms (in English only): levothyrox*, LT4, L-T4, liothyronin*, T3, tetraiodothyro*, triiodothyro*, absorp*, absorb*, distrib*, transport*, metabol*, eliminat*, excret*, deiodinase*, bioavailab*, titrat*, food*, interacti*, and enterohepat*. For example, a sample search was (levothyrox* OR LT4 OR L-T4 OR liothyronin* OR T3 OR tetraiodothyro* OR triiodothyro*) AND (absorp* OR absorb* OR malabsorb* OR pseudomalabsorb* OR distrib* OR transport* OR metabol* OR eliminat* OR excret* OR bioavailab* OR titrat*) AND hypothyro* NOT rat NOT mouse NOT chicken NOT zebrafish), with the filters Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Comparative Study, Controlled Clinical Trial, Multicenter Study, Observational Study, Pragmatic Clinical Trial, Randomized Controlled Trial, and Humans.
Abstracts were screened for relevance. If an abstract was found to be relevant, the full text article was retrieved and reviewed. We excluded case reports, reviews, and editorials. We arbitrarily classified factors influencing the relationship between the prescribed oral dose of LT4 and the drug’s clinical effectiveness into three broad categories: pharmaceutical factors (the dosing regimen, the time of day taken, the administration route, the pharmaceutical formulation), pathophysiological factors (aetiology, type of hypothyroidism, disease status, concomitant diseases or comorbidities, anthropometric variables, genetic variants, etc.), and behavioural (concomitant drug treatments, foods, compliance, the prescribing physician’s characteristics, etc.) (Table 1). The present study did not involve human subjects and therefore did not require review and approval by an institutional review board.
Table 1.
Classification of factors influencing the effectiveness of LT4 and thus the dose required to achieve the target serum TSH level
| Type of factor | Examples |
|---|---|
| Pharmaceutical | • Pharmaceutical formulation (tablet, gel, liquid, excipients, storage conditions, etc.) |
| • Administration route (oral, intravenous, or intramuscular) | |
| • Dosing regimen (dosing frequency, time of day, before or after a meal, etc.) | |
| • Concomitant administration of other thyroid hormones (e.g. LT4 + LT3 combination therapy) | |
| Pathophysiological (internal) | • Thyroid disorder (type, degree, and progression) and etiology (auto-immune disease, thyroid surgery, radioiodine treatment, etc.) |
| • Comorbidities (type, degree, and progression) | |
| • Age, sex, body mass index, pregnancy, etc | |
| • Genetic variants (in the genes coding for deiodinases, TH transporters or receptors, for example) or possible acquired changes in TH action (a difficult TSH normalization is reported in some patients with congenital hypothyroidism) | |
| • Malabsorption | |
| • Changes in the underlying residual thyroid function | |
| Behavioral (external) | • Concomitant intake of medications, foodstuffs, and food supplements |
| • Poor compliance, pseudomalabsorption, and poor quality of life | |
| • Characteristics of the prescribing physician (medical specialty, country of practice, etc.) |
LT4 levothyroxine, LT3 L-tri-iodothyronine, TH thyroid hormone, TSH thyroid-stimulating hormone
Results
Our search initially identified a total of 741 potentially relevant publications, of which 140 were retrieved and 97 were reviewed. The main reasons for exclusion were animal studies (despite the “Human” and “Clinical Trial” filters in PubMed), case reports, and a lack of data on changes in LT4 ADME.
Factors that interfere with the absorption of LT4
Pharmaceutical factors
Several studies have examined the time of day of LT4 intake (independently of food intake). Even though the summaries of product characteristics for LT4 formulations recommend intake on an empty stomach (typically on awakening, and at least 30 min before breakfast), patients are tempted to take their LT4 with (or only shortly before) their breakfast. In this respect, Guglielmi et al. open-label longitudinal study found that taking a liquid LT4 formulation with breakfast (rather than taking LT4 tablets on an empty stomach before breakfast) was associated with greater patient satisfaction, as judged by significant (p < 0.01) improvements in three of the seven subscores in the Underactive Thyroid Treatment Satisfaction Questionnaire [26].
The data on the influence of time of day are inconsistent – at least at first sight. Several studies did not observe an effect. For example, Skelin et al. did not observe significant differences in TSH, FT4 and FT3 levels for three times of day (30 min before breakfast, 60 min before lunch, and at least 120 min after an evening meal) for LT4 tablets [27], and Pirola et al. study of 4 hypothyroid patients treated with liquid LT4 did not find any significant differences in the serum TSH level according to whether the LT4 was taken 30 min before breakfast or at breakfast [28]. Similar findings were reported for serum TSH, FT4 and/or FT3 levels crossover trials [29–31].
In contrast, the results of several crossover studies argued in favour of administration before bedtime, after the evening meal is no longer in the stomach. Srivastava et al. observed significantly lower TSH levels and significantly higher FT3 and FT4 levels than with an equivalent morning dose regimen [32]. Bach-Huynh et al. three-period crossover study of 65 LT4-treated stable hypothyroid patients found that non-fasting LT4 administration was associated with higher and more variable serum TSH concentrations [33]. Lastly, Silva Perez et al. performed a prospective, randomized, open-label, crossover study of LT4 administration on an empty stomach for 90 days vs. LT4 with breakfast for 90 days. The TSH level was higher in the breakfast group than in the fasting group (2.89 vs. 1.9 mIU/L, p = 0.028) [34]. Lastly, Bolk et al. reported that administration of LT4 at bedtime was associated with higher thyroid hormone concentrations and lower TSH concentrations than administration in the morning [35].
Although circadian rhythms may influence LT4 administration [35], we conclude the main variability factor in the time of day is whether or not the stomach contains food or other ingested substances when oral LT4 is administered. Our clinical experience suggests that many patients are unwilling to fast in the morning; hence, LT4 might be more reliably taken at bedtime (i.e. at least 3 to 4 h after the evening meal).
The pharmaceutical formulation of LT4 can interact with factors that modulate the drug’s absorption [36]. In a randomized, single-dose, crossover pharmacokinetic bioequivalence study of 84 healthy subjects, three different orally administered dose-equivalent LT4 formulations (liquid, tablets, and soft-gel capsules) were found to have similar overall exposure rates and area under the concentration–time [37]. The time to peak was shortest (by around 30 min) for the liquid solution – prompting the researchers to suggest that this might reduce drug-LT4 interactions. Other reports from both retrospective and prospective observational studies indicate that orally administered liquid and soft-gel formulations of LT4 are associated with higher serum FT4 levels and lower TSH levels than tablet formulations, even when the overall LT4 dose level is the same – perhaps due to the absence of gastric disintegration [38–44]. Hence, liquid formulations might enable better TSH control and fewer LT4 dose adjustments. A liquid formulation might also reduce the likelihood of drug-drug or drug-supplement interactions. For example, Guglielmi et al. retrospective, real-world analysis of 3965 LT4-treated patients in Italy found that LT4 dose increases before and after a drug-drug indication (mainly due to proton pomp inhibitors and calcium or iron salts) were less likely in individuals taking a liquid LT4 formulation [45]. Furthermore, TSH variability was lower in patients taking the liquid LT4 formulation [45]. However, Benvenga and Di Bari observed abnormally high TSH levels in patients with inappropriate (undiluted) oral administration of liquid LT4—showing that use of a liquid formulation is not a panacea [46].
Lastly, the LT4 dosing frequency has rarely been investigated. In a randomized, crossover study of 14 patients. Bornschein reported that weekly oral LT4 administration (as a possible means of increasing treatment compliance) was associated with transient increases in FT4 levels and (after 6 weeks) a slight decrease in FT3 levels. There were no thyrotoxic symptoms or signs of cardiac problems [47].
In summary, the pharmaceutical formulation of LT4 and the time of day and/or frequency of LT4 administration are easily modifiable parameters that are worth testing when confronted with a potential case of malabsorption.
Pathophysiological factors
It is now clear that a high proportion of LT4-treated patients have a disease or condition (gastroesophageal reflux disease, irritated bowel syndrome, food allergies, lactose intolerance, gastric bypass, H. pylori infection, gastroparesis, coeliac disease, ulcerative colitis, Crohn’s disease, atrophic gastritis, etc.) that can reduce the intestinal absorption of LT4 [24, 48]. To reach an equivalent TSH level, the LT4 dose must be higher if the patient’s intestinal tract is affected by ulcerative colitis [49], coeliac disease [50], lactose intolerance [51], and gastritis (especially Helicobacter pylori–related gastritis and autoimmune gastritis) [43, 52–54], and infections with Giardia lamblia (giardiasis) [55]. In patients with gastritis, a change in LT4 formulation may be beneficial. For example, the administration of a liquid LT4 formulation in patients with hypothyroidism and an ongoing Helicobacter pylori infection was associated with a significantly greater fall in TSH levels, relative to administration of a tablet formulation [43].
Digestive tract surgery in general and bariatric surgery in particular lead to a reduction in BMI, which in turn should prompt a reduction in the LT4 dose requirement. The data are somewhat inconsistent. On one hand, Fierabracci et al. reported a drop in BMI and a significant downward adjustment in the mean total dose of LT4 after surgery [56], and Richou et al. observed a fall in the LT4 dose required to achieve the target TSH level after sleeve gastrectomy [57]. On the other hand, Pedro et al. did not find a difference between restrictive procedures (sleeve gastrectomy or adjustable gastric banding) and malabsorptive procedures (Roux-en-Y gastric bypass) [58], and Rubio et al. did not see a decrease (only a slight delay) in the amount of LT4 absorption after Roux-en-Y gastric bypass surgery [59]. Lastly, Fallahi et al. reported that hypothyroid patients show higher TSH levels after Roux-en-Y gastric bypass or a biliary pancreatic diversion – presumably due to poor LT4 absorption [60].
Again, the “bariatric surgery” factor might interact with the “LT4 formulation” factor: according to Fallahi et al., a switch from a tablet LT4 formulation to a liquid LT4 formulation (at the same dosage) in bariatric surgery patients was associated with a significant fall in the TSH level [60].
Behavioural factors
Here, we consider that behavioural factors included the voluntary ingestion of drugs, foods, and food supplements. As mentioned above, the general presence of food in the stomach (i.e. food intake in the three hours before oral LT4 administration) reduces the LT4 absorption. Furthermore, a high proportion of LT4-treated patients ingest medications, foodstuffs and food supplements that selectively or specifically reduce the intestinal absorption of LT4. These substances include over-the-counter medications (mainly antacids or acid reducers), dietary supplements (primarily calcium and iron salts), foods/beverages high in fibre, iodine or soy, proton pump inhibitors (PPIs), sucralfate, aluminium hydroxide, magnesium hydroxide, calcium salts, ferrous sulphate, raloxifene, cholestyramine, colestipol, lanthanum carbonate, cation exchange resins, and sevelamer [48, 61–63][48], some of which are described in more detail below.
The ingestion of calcium carbonate supplements is associated with lower total T4 (TT4) levels and elevated TSH levels, relative to baseline [65–67]. Indeed, LT4 can bind to calcium carbonate – potentially reducing the bioavailability [66]. After total or completion thyroidectomy for benign disease, the time needed to achieve euthyroid status (usually 3–4 months) was considerably longer and required more dose adjustments in patients taking calcium supplements [64]. Again, liquid formulations of LT4 may be less subject to interference by calcium and iron supplements [39].
Dietary fibre binds LT4 and reduces the drug’s bioavailability in a non-specific manner; in a case series reported by Liel et al. withdrawal of fibre (mainly wholewheat and fibre-enriched bread) was associated with marked reductions in TSH levels and the LT4 dose requirement [68]. However, dietary fibre has many health benefits and should necessarily not be withdrawn; hence, an LT4 dose increase can be considered.
With regard to resins and binders, concomitant administration of LT4 and the non-absorbed, potassium-binding polymer patiromer was found to decrease the area under the curve (AUC) extrapolated to infinity and the peak concentration (Cmax) for T4, according to the results of an open-label crossover study. The interaction was avoided by delaying the patiromer administration by 4 h [69]. Likewise, concomitant intake of the phosphate binder sevelamer hydrochloride significantly decreased the area under the serum TT4 concentration curve [70].
Some antibiotics also interfere with LT4 absorption: in a pharmacokinetic study of healthy volunteers taking a dose of LT4, Goldberg et al. showed that concomitant administration of ciprofloxacin was associated with a significantly lower area under the plasma T4 concentration–time curve [71].
Drugs that modify the stomach’s acidity have also been linked to LT4 malabsorption, although the effect is subject to debate. There is a large body of evidence (from general practice patient databases, electronic medical record reviews, and clinical trials) to indicate that PPIs are prominent interfering substances that raise TSH levels and/or the LT4 dose requirement [63, 72, 73]. In contrast, various clinical studies of one week of treatment with pantoprazole [74], 3 months of treatment with omeprazole [75], and a week’s treatment with esomeprazole [76] did not find significant intergroup differences in the TSH or thyroid hormone levels. We suggest that these apparent disparities in the data might be due to (i) the possible occurrence of interactions with LT4 in a subset of patients only, and/or (ii) a lack of power in some of the randomized studies cited above.
Coffee is a beverage associated with breakfast in many cultures but the strength, amount and composition varies. In Benvenga et al. pharmacokinetic study, taking LT4 with expresso Italian coffee was associated with a lower peak and a lower AUC for serum T4, and failure to achieve target TSH levels. [77]. In contrast, an acute loading test in two patients found that coffee did not modify the pharmacokinetics of LT4 [78]. Lastly, Cappelli et al. reported that a move to taking the LT4 30 min before breakfast for 3 and 6 months did not significantly modify the serum TSH level [79]. Hence, coffee (caffeine) per se does not appear to perturb LT4 uptake.
In Chon et al. pharmacokinetic study of cold or hot milk (another beverage frequently consumed at breakfast), co-administration of LT4 and 2% milk by healthy euthyroid subjects was associated with a significantly lower peak concentration (by around 8%) and a significantly lower area under the curve for TT4 (10%) [80]. However, in a patient population (infants with congenital hypothyroidism), intake of soy formula milk was associated with TSH elevation and a longer time to TSH normalization [81]. Taking LT4 with grapefruit juice was associated with statistically significant decreases (of around 10%) in the peak T4 concentration and the AUC for T4. However, TSH levels did not appear to be affected, and the investigators considered that concomitant grapefruit juice and LT4 intake did not have a clinically relevant effect [82]. It has been suggested that the “grapefruit juice effect” on the absorption of some drugs is due to inhibition of the organic anion transporting polypeptide 1A2 (OATP1A2) transporter in the intestinal mucosa, as has also been suggested for the drug ciprofloxacin [83, 84]. Nevertheless, it should be borne in mind that milk and grapefruit juice will respectively increase and decrease the pH of the stomach, which may introduce a confounding effect.
Although a large number of compounds and pathologies have been suggested as modulators of intestinal absorption of LT4, the biochemical mechanisms remain poorly characterized. In an in vitro study, Zu Schwabedissen et al. sought to determine whether the organic anion transporting polypeptide 2B1 (OATP2B1) was involved in the intestinal absorption of thyroid hormones [85]. The results of competitive counter-flow experiments indicated that T4 and T3 (but not rT3 or T4-gluconuride) were indeed substrates for OATP2B1. The researchers also determined (using PCR gene expression assays and Western blots) that potentially physiological micromolar concentrations of T3 and (to a lesser extent) T4 induced OATP2B1 and DIO1 gene and protein expression in some cell lines. Lastly, Kharrazian et al. found that dozens of raw or cooked food products showed immune cross-reactivity with thyroid hormones and enzymes; however, the putative effect of anti-hormone antibodies on T4 levels remains to be determined [86].
As mentioned in the section on the time of day of LT4 administration, taking LT4 on an empty stomach rules out a large number of variability factors and is advisable in cases of suspected malabsorption. Factors that reportedly reduce or slow the absorption of LT4 are listed in Table 2 and summarized in Fig. 3.
Table 2.
Factors that modulate the absorption of LT4. (↓ = decrease, ↑ = increase, ↔ = no change)
| Factor | Category | Subcategory | Effect | Suggested clinical strategy | References |
|---|---|---|---|---|---|
| Ulcerative colitis | Pathophysiological | pathological | ↑ in the LT4 dose required to achieve the target TSH level | Screen for ulcerative colitis | [49] |
| Coeliac disease | Pathophysiological | pathological | ↑ in the LT4 dose required to achieve the target TSH level | Screen for coeliac disease | [50] |
| Bariatric surgery | Pathophysiological | pathological | ↓ in BMI, ↓ in the LT4 dose but ↓ in LT4 absorption, ↑ in the time to serum T4 peak, ↑ in mean steady-state TSH levels | Monitor TSH and thyroid hormone levels periodically during weight loss and adjust as required | [56–60] |
| Autoimmune gastritis | Pathophysiological | pathological | ↑ in the LT4 dose required to achieve the target TSH level | Screen for serum anti-parietal cell antibodies | [53] |
| Helicobacter pylori infection | Pathophysiological | pathological | ↑ in the LT4 dose required to achieve the target TSH level | Screen for a Helicobacter pylori infection and eradicate if present. Consider a switch to a liquid LT4 | [43, 52, 73] |
| Proton pump inhibitors (e.g. omeprazole, lansoprazole, esomeprazole, and pantoprazole) | Behavioral | medications | Effect subject to debate: ↑ in TSH levels reported in studies of omeprazole and lansoprazole but no significant effect or no consensus for esomeprazole and pantoprazole | Check for PPI treatment, check the TSH level, and increase the dose level of LT4 if TSH is elevated | [72–76] |
| Tyrosine kinase inhibitors (e.g. imatinib and sorafenib) | Behavioral | medications |
↑ in TSH levels with imatinib ↓ in serum FT4 and FT3 with sorafenib, after adjustment for LT4 dose and bodyweight. ↓ in T3/T4 and T3/rT3, possibly due to an increase in DIO3 activity |
Check for the use of tyrosine kinase inhibitors; if so, increase the LT4 dose level | [113, 115] |
| Alendronate | Behavioral | medications | ↔ no significant effect or no consensus | An effervescent formulation of alendronate supplement is unlikely to interfere with concomitant LT4 treatment | [133] |
| Patiromer (potassium-binding resin) | Behavioral | medications | ↓ in LT4 absorption | Delay patiromer administration by several hours in patients taking LT4 | [69] |
| Ciprofloxacin (antibiotic) | Behavioral | medications | ↓ in plasma T4 | Check for concomitant use of ciprofloxacin; if so, increase the LT4 dose level | [71] |
| Rifampin (antibiotic) | Behavioral | medications | ↑ in plasma T4 | Check for concomitant use of rifampin; if so, decrease the LT4 dose level | [71, 117] |
| Simvastatin (statin) | Behavioral | medications | ↔ no significant effect or no consensus | No action required because an interaction is unlikely | [134] |
| Colesevelam HCl (bile acid sequestrant) | Behavioral | medications | ↓ in serum T4 | Check for the use of the bile acid sequestrant colesevelam; if so, increase the LT4 dose level | [123] |
| Lanthanum carbonate (phosphate binder) | Behavioral | medications | ↓ in serum T4 | Check for the use of phosphate binders; if so, increase the LT4 dose level | [123] |
| Sevelamer hydrochloride (phosphate binder) | Behavioral | medications | ↓ in serum T4 | Check for the use of phosphate binders; if so, increase the LT4 dose level | [70] |
| Fluoxetine and sertraline (selective serotonin reuptake inhibitors) | Behavioral | medications | ↓ in serum T3 and T4 | Check for the use of the SSRIs (small effect); if so, increase the LT4 dose level | [129] |
| Famotidine (H2 antihistamine, antacid) | Behavioral | medications | ↔ no significant effect or no consensus | No action required because an interaction is unlikely | [76] |
| Oral gonadotropin (infertility treatment) | Behavioral | medications | ↔ no significant effect or no consensus | No action required because an interaction is unlikely | [139] |
| Calcium supplements | Behavioral | dietary supplements | ↑ in TSH levels, ↓ in LT4 absorption | Calcium carbonate supplements should be taken 6 to 8 h after LT4. Also consider a switch to a liquid LT4 formulation in patients taking calcium carbonate supplements | [64–67] |
| Iron supplements | Behavioral | dietary supplements | ↑ in TSH levels, ↑ in LT4 dose adjustments required to achieve the target TSH level | Screen for the use of iron or mineral calcium supplements | [39] |
| Food ingestion (breakfast) | Behavioral | foodstuffs | ↔ no significant negative effect or no consensus for breakfast in general but some components of the meal (listed in the lines below, e.g. cow’s milk) have effects | Check that LT4 is taken at the same time of day, regardless of whether this is before or during breakfast. Check TSH levels more frequently in patients taking LT4 with food | [27–31] |
| Grapefruit juice | Behavioral | foodstuffs | ↓ in T4 peak and AUC. ↔ for TSH levels | Monitor TSH levels | [82] |
| Coffee | Behavioral | foodstuffs | ↔ no significant effect or no consensus | Consider a switch to a liquid formulation if the patient persists in drinking coffee at the same time as the LT4 dose | [77–79] |
| Cow’s milk | Behavioral | foodstuffs | ↓ in LT4 absorption | Check that the patient is avoiding cow’s milk and similar substances at the time of the LT4 dose | [80] |
| Soy milk (formula) | Behavioral | foodstuffs | ↑ in TSH and the time to TSH normalization | Check that the patient is avoiding milk and similar substances at the time of the LT4 dose | [81] |
| Dietary fibre (wholewheat and fiber-enriched bread) | Behavioral | foodstuffs | ↑ in TSH levels and the LT4 dose requirement | Consider withdrawing dietary fiber | [68] |
| Curcumin extract | Behavioral | foodstuffs | ↔ no significant effect or no consensus | A curcumin supplement is unlikely to interfere with LT4 treatment | [132] |
| Vitamin C | Behavioral | foodstuffs | ↓ in TSH levels and ↑ in FT4 and TT3 | Consider supplementation with vitamin C in patients with malabsorption | [141, 142] |
LT4 levothyroxine, TSH thyroid-stimulating hormone, BMI body mass index, T4 thyroxine, FT3 free liothyronine, FT4 free thyroxine, rT3 reverse liothyronine, DIO3 type 3 deiodinase, AUC area under the curve
Fig. 3.
A summary of anatomic and functional changes that can reduce or slow the absorption of LT4 in the gastrointestinal tract
Factors that interfere with the distribution of T4
Pharmaceutical factors
We did not find any evidence to suggest that once T4 is absorbed and crosses the epithelium, its distribution is significantly modulated by LT4-related pharmaceutical variables (formulation, dosing frequency, time of day, etc.).
Pathophysiological factors
It is well known that the mean distribution volume of LT4 is greater in hypothyroid patients (up to 15 L) than in euthyroid subjects (typically 11–12 L) – possibly as a result of an increase in the volume of intracellular water [18, 19].
As mentioned above, Zu Schwabedissen et al. in vitro cell-based study showed that T4 and T3 were substrates of the drug transporter OATP2B1. Although the researchers focused on uptake across the intestinal epithelium (i.e. absorption), OATP2B1 is also expressed in other tissues – notably the liver. Hence, variations in OATP2B1 gene transcription and translation might alter the pharmacodynamics of thyroid hormones more broadly – including their tissue distribution [85].
Behavioural factors
A number of drugs modify the amount of T4 bound to plasma proteins. Firstly, therapeutic levels of phenytoin, carbamazepine, salsalate, salicylate, furosemide, fenclofenac, ethacrynic acid, and heparin induce the displacement of T4 from the binding proteins in vitro [87, 88]. However, give the large amounts of plasma proteins in the circulation, Burch considered that the in vivo effects of this displacement would be negligible [88].
In postmenopausal women taking LT4, the initiation of oestrogen therapy was associated with increases in serum TBG, TT4 and TSH, and a decrease in the serum FT4 concentration [89]. The researchers suggested that an oestrogen-induced increase in serum TBG might transiently decrease the FT4 concentration and thus reduce the entry of thyroid hormones into cells. In contrast, Shifren et al. reported that transdermal oestrogen therapy (as opposed to oral administration) exerts minimal effects on the binding proteins and then on TT4 and FT4 [90].
At this point, it makes sense to mention the effect of pregnancy on LT4, which is also mainly mediated by oestrogen. The sharp rise in oestrogen levels during the first weeks of pregnancy leads to an elevation of TBG levels and thus contributes to a greater requirement for LT4 [91].
A number of other medications and drugs of abuse reportedly increase TBG levels: selective oestrogen receptor modulators, tamoxifen, heroin, methadone, 5-fluorouracil, and mitotane [88]. Hence, patients taking these drugs (especially mitotane) may require an LT4 dose increase. Conversely, exposure to glucocorticoids, niacin, and androgens leads to a fall in TBG levels and thus a decrease in the LT4 requirement [88, 92].
Factors that reportedly modulate the distribution of LT4 are listed in Table 3.
Table 3.
Factors that modulate the distribution, metabolism, and excretion of LT4 (↓ = decrease, ↑ = increase)
| Distribution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Category | Subcategory | Observations/putative mechanism | Suggested action | References | |||||
| Postmenopausal treatment with estrogen | Behavioral | medications | ↑ in serum TT4, TBG and TSH. ↓ in serum FT4, possibly due to an increase in serum TBG levels | Increase the LT4 dose if estrogen therapy is initiated | [89] | |||||
| Activity of the drug transporter OATP2B1 | Pathophysiological | physiological | ↑ in OATP2B1 gene expression levels in an in vitro (cell culture) study upon exposure to thyroid hormones | Systematic OATP2B1 genotyping is not recommended. Treat the patient on the basis of other data | [85] | |||||
| Metabolism | ||||||||||
| Factor | Category | Subcategory | Observations/putative mechanism | Suggested action | References | |||||
| Selenium deficiency | Pathophysiological | pathological | ↓ in FT3, ↑ in glutathione peroxidase, which competes with deiodinases for selenium | Screen for oxidative stress | [109] | |||||
| Oxidative stress | Pathophysiological | pathological | ↓ in serum malondialdehyde levels upon treatment with LT4 | Screen for oxidative stress | [111] | |||||
| Pregnancy | Pathophysiological | physiological | ↑ in FT4 during pregnancy in women with hypothyroidism. The main determinants of FT4 were weight and age | Adjust LT4 dose for bodyweight changes during pregnancy | [138] | |||||
| Excretion | ||||||||||
| Factor | Category | Subcategory | Observations/putative mechanism | Suggested action | References | |||||
| Nephrotic syndrome | Pathophysiological | pathological | ↑ in proteinuria and the renal excretion of T4 bound to transport proteins | Screen for nephrotic syndrome | [118, 119] | |||||
| Bile acid sequestrants (cholestyramine, colestipol and colesevelam) | Behavioral | medications | ↑ enterohepatic recycling of T4 | Screen for the use of bile acid sequestrants | [120, 121] | |||||
| Colesevelam | Behavioral | medications | Formation of a colesevelam-T4 complex in the stomach, with ↑ excretion in the feces | Screen for the use of colesevelam | [122] | |||||
| Phenobarbital, phenytoin, carbamazepine, and rifampin | Behavioral | medications | ↑ in the levels of the enzyme responsible for the glucuronidation of LT4 | Screen for the use of phenobarbital, phenytoin, carbamazepine, and rifampin | [116, 117] | |||||
TT4 total thyroxine, TBG thyroxine-binding globulin, TSH thyroid-stimulating hormone, FT4 free thyroxine, LT4 levothyroxine, OATP2B1 organic anion transporting polypeptide 2B1, FT3 free liothyronine, FT4 free thyroxine, T4 thyroxine
Factors that interfere with the metabolism of LT4 and T4
Pharmaceutical factors
We did not find any evidence to suggest that the metabolism of T4 is significantly modulated by LT4-related pharmaceutical variables (formulation, dosing frequency, time of day, etc.).
Pathophysiological factors
Given the deiodinases’ key roles in the metabolism and action of thyroid hormones, the genetic basis for potential differences in activity has been subject to much scrutiny – albeit with conflicting results, as described below.
With regard to DIO1, Panicker et al. suggested that the DIO1 rs2235544 C allele was correlated with elevated FT3 and low FT4 levels in both LT4-treated patients and in the general population [93]. In contrast, significant relationships between the DIO1 genotypes on one hand and thyroid hormone parameters (LT4 dose, FT3, FT4, TSH, etc.) were not observed by Santoro et al. [94] and Arici et al. [95].
For DIO2, Appelhof et al. studied 41 LT4-treated patients with primary autoimmune hypothyroidism before and during a randomized clinical trial of LT4 monotherapy vs. L-tri-iodothyronine (LT3) + LT4 combination therapy [96]. The researchers reasoned that well-being, neurocognitive functioning, and preference might be related to local T3 levels in the brain, which are regulated by DIO2 activity. However, the patient’s status for two DIO2 variants (DIO2-ORFa-Gly3Asp and DIO2-Thr92Ala) was not associated with thyroid hormone levels or the stated preference for combination therapy. Likewise, Santoro et al. did not find a significant relationship between DIO2 genotypes and the patients’ T4 dose level [94]. Indeed, the absence of an association between the genotype and thyroid hormone level was confirmed in many other studies [93, 97–99]. In contrast, Castagna et al. reported that heterozygosity and homozygosity for the DIO2 Thr92Ala polymorphism were significantly associated with low FT3 levels—thus suggesting a rationale for LT4 + LT3 combination therapy [100]. This was confirmed by Cantara et al. [101]. Associations between the genotype and thyroid hormone variables were also reported by Torlontano et al. (for the DIO2 rs225014 Ala/Ala variant) [102], Arici et al. (for the rs225014 TT and rs225015 GG genotypes) [95] and Carlé et al. (for the rs225014 polymorphism in DIO2 and the rs17606253 polymorphism in MCT10/SLC16A10) [103].
Indirect effects on DIOs have also been suggested. In a study of 40 adult patients with hypothyroidism due to Hashimoto disease, Papanas et al. found that the serum interleukin (IL)-6 level was significantly and positively correlated with the LT4 dose, the LT4 dose per kg body weight, and the serum tumour necrosis factor alpha level but was significantly and negatively correlated with the serum TT3 level and the serum T3/T4 ratio [104]—suggesting that IL-6 inhibited the deiodination of T3 and rT3.
With regard to other enzymes and transporters, Santoro et al. investigated UGT1A1 and UGT1A3 genotypes in a group of LT4-treated patients having undergone total thyroidectomy and radioiodine therapy [94]. The T4 dose was found to be associated with the UGT1A haplotype; this was attributed to low UGT1A1 expression and T4 glucuronidation in carriers of the UGT1A1 rs8175347 allele. In a regression model, however, the UGT1A haplotype accounted for only 2% of the total variability in T4 dose level and was not considered to be clinically relevant. With regard to the monocarboxylate transporter 10 (MCT10, an amino acid transporter), Cantara et al. reported that patients homozygous or heterozygous for the rs17606253 polymorphism in SLC16A10 had significantly lower FT3 levels than other individuals [101]. Roef et al. study in Belgium found that the rs5937843 (G/T) polymorphism in the SLC16A2 gene coding for the monocarboxylate transporter 8 (MCT8, which transports T3 and T4 specifically) was inversely associated with FT4 levels in men but not in women. Similarly, the rs6647476 (T/C) polymorphism in MCT8 was inversely associated with FT3 levels but again only in men [105]. It is noteworthy that inactivating mutations in SLC16A2 produce a severe X-linked syndrome (Allan-Herndon-Dudley syndrome) with a lack of TH in target brain regions and excess TH in the peripheral tissues [106].
Cardiovascular disease and heart failure are associated with elevated levels of oxidative stress and the selenoprotein glutathione peroxidase, which in turn have been linked to selenium (Se) deficiency, competition for Se, and low deiodinase activity [107, 108]. Fraczek-Jucha et al. reported that serum Se and FT3 levels were not correlated even when the prevalence of Se deficiency was high (74.6%) [109]. Yang et al. single-centre study of a cohort of ambulatory patients in the US found that the dose of LT4 did not differ significantly when comparing patients with heart failure vs. those without [110]. Lastly, Chakrabarti et al. found that serum malondialdehyde levels (a marker of oxidative stress) fell during treatment with LT4 [111]. Se supplementation did not have a significant additional effect.
Behavioural factors
The metabolism of T4 can be significantly modulated by behavioural factors, such as medication use. Firstly, the deiodinases’ action might also be affected by various drugs or other compounds: Olker et al. in vitro screening study of recombinant DIOs found that 411 (22.5%) of the 1851 compounds from the ToxCast Phase 2 and e1k libraries inhibited DIO1, DIO2 and/or DIO3 by more than 20%, and that 228 (12.5%) inhibited a DIO by at least 50% [112]. The most potent inhibitors had IC50 values in the micromolar range, and 81 chemicals were specific inhibitors (i.e. inhibition in only one of the three deiodinases).
With regard to a putative effect of tyrosine kinase inhibitors (TKIs) on DIO3, Abdulrahman et al. found that 26 weeks of sorafenib treatment in 21 LT4-treated patients with progressive non-medullary thyroid carcinoma was associate with falls in serum FT4 and FT3 levels and the T3/T4 and T3/rT3 ratios [113]. The researchers suggested that these changes were due to a sorafenib-induced increase in DIO3 activity. An increase in DIO3 activity can also be observed in consumptive hypothyroidism syndrome (associated with tumours such as hepatic hemangioendotheliomas, gliomas, neuroblastomas, colon carcinomas, gastrointestinal stromal tumours, and fibrous tumours), gestational hypothyroidism and treatments with oestrogens. More generally, thyroid dysfunction is a well-known adverse effect of TKIs in euthyroid individuals – although not necessarily by modifying the metabolism of LT4 –and so may increase the requirement for LT4 in individuals with hypothyroidism [114, 115].
Treatments with phenobarbital, phenytoin, carbamazepine, and rifampin induce an increase in the levels of the enzyme responsible for the glucuronidation of LT4 [116, 117]. In Goldberg et al. pharmacokinetic study, for example, the concomitant administration of rifampin and LT4 was associated with a significantly higher area under the plasma T4 concentration–time curve [71].
The factors that reportedly modulate the metabolism of LT4 are summarized in Table 3.
Factors that interfere with the excretion of LT4 and T4
Pharmaceutical factors
We did not find any reports on the specific modulation of T4 excretion by LT4-related pharmaceutical variables (formulation, dosing frequency, time of day, etc.).
Pathophysiological factors
With regard to the specific modulation of T4 excretion by pathophysiological variables, the occurrence of nephrotic syndrome with marked proteinuria may increase the renal excretion of T4 bound to transport proteins like TBG. Hence, the LT4 requirement would increase in this context [118, 119].
Behavioural factors
The bile acid sequestrants cholestyramine, colestipol and colesevelam decrease the T4 concentration – probably via the augmented enterohepatic recycling of T4 [120, 121], although cholestyramine additionally forms a complex with T4 in the stomach and thus increases the amount of T4 excreted in the feces [122]. The serum TT4 concentration curve is decreased by around 95% and 40% by the administration of the bile acid sequestrant colesevelam and lanthanum, respectively, relative to LT4 taken alone [123]. As mentioned above, treatments with phenobarbital, phenytoin, carbamazepine, and rifampin induce an increase in the levels of the enzyme responsible for the glucuronidation of LT4, and thus will modify (albeit indirectly) the latter’s excretion [116, 117].
Factors that reportedly modulate the excretion of LT4 are summarized in Table 3.
Lastly, factors that reportedly modulate the effectiveness of LT4 but whose mechanisms are unknown are listed in Table 4 [124].
Table 4.
Other factors that modulate the effectiveness of LT4 (mechanisms unknown). (↓ = decrease, ↑ = increase)
| Factor | Category | Subcategory | Observations/putative mechanism | Suggested action | References |
|---|---|---|---|---|---|
| Female sex | Pathophysiological | physiological | ↑ Changes in estrogen levels during events such as pregnancy and menopause might decrease the FT4 concentration and thus reduce the entry of thyroid hormones into cells | Monitor the LT4 maintenance dose in female patients as a function of life events | [89–91] |
| Kidney transplantation | Pathophysiological | pathological | Kidney transplantation was associated with a marked fall in the required dose of LT4. Patients who subsequently return to dialysis required an LT4 dose increase | Decrease the LT4 dose after kidney transplantation | [124] |
| Food antigens | Pathophysiological | foodstuffs | ↑ in immune cross-reactivity between thyroid hormones/enzymes and raw or cooked food products, as a possible trigger for thyroid disorders | Consider repeating the thyroid hormone assays with kits/techniques | [86] |
| Zinc | Pathophysiological | foodstuffs | ↑ in serum FT3 after supplementation with Zn (alone or combined with Se) | Consider zinc supplementation in overweight or obese patients with hypothyroidism | [135] |
FT3 free liothyronine, FT4 free thyroxine, LT4 levothyroxine, T4 thyroxine
Factors that do not appear to worsen the ADME parameters of LT4
Many drugs and foods appear to have no effect on LT4 or an effect that is subject to debate. In a study of eight obese, diabetic postmenopausal women with primary hypothyroidism, Isidro et al. found that after 3 months of treatment with metformin, the mean ± SD TSH level (1.18 ± 0.36 µIU/ml) was significantly lower than at baseline (3.11 ± 0.50 µIU/ml). The mean FT4 level was slightly but not significantly higher [125]. Metformin treatment has a TSH-lowering effect in patients with LT4-treated or untreated polycystic ovary syndrome or in LT4-treated patients with diabetes mellitus [126, 127]. The TSH-lowering effect in untreated patients (i.e. those not taking LT4) means that the metformin is unlikely to act by modifying LT4 absorption. Animal studies have also shown that the low TSH levels are not due to a metformin-induced increase in LT4 absorption [128]. Hence, metformin’s effect (or lack of effect) on TSH and FT4 levels requires further investigation.
De Carvalho et al. performed a prospective, controlled study of fluoxetine or sertraline initiation in 28 patients with major depression and hypothyroidism and 29 patients with major depression and normal thyroid function. Patients on fluoxetine experienced a significant fall in the TT3 level after 15 and 30 days of treatment and the TT4 levels after 15, 30 and 90 days, although the values remained in the euthyroid range. Sertraline had similar but weaker effects [129]. Other substrates that do not appear to influence the rate and extent of LT4 absorption and its effects include soy isoflavones [130], the antacid famotidine, the lipid-lowering compound ezetimibe [76], the anticoagulant warfarin [131], a curcumin supplement for osteoarthritis [132], the osteoporosis drug alendronate [133], and simvastatin [134]. Interestingly, supplementation with Zn (alone or combined with Se) for 12 weeks was associated with a significant increase in serum FT3 levels but had no significant effects on TT3, FT4, TT4 or TSH levels [135]. Although Zn is known to interact with thyroid hormone systems in a complex manner, it is not clear whether Zn supplementation influences deiodinase levels or activity [136].
Women with hypothyroidism are generally advised to increase the dose of LT4 by 30 to 50% (depending on the residual endogenous thyroid function) because of weight gain, a larger T4 pool (an elevated TBG level), transfer to the foetus, and increased clearance of T4 (due to placental DIO3 activity) [137]. Haddow et al. found that FT4 levels were higher in pregnant women treated for hypothyroidism than in pregnant euthyroid women with equivalent TSH levels, as is also the case when comparing non-pregnant women treated for hypothyroidism and non-pregnant euthyroid women with equivalent TSH levels; hence, pregnancy per se did not change the high FT4 levels associated with LT4 treatment [138]. Davis et al. compared TSH levels in 5 hypothyroid women who conceived naturally and 4 who conceived after gonadotropin stimulation or with oral medications for ovulation induction. The post-conception TSH level and LT4 doses did not differ significantly when comparing the two group. The researchers concluded that fertility treatment did not require additional LT4, relative to that recommended for natural pregnancy [137, 139]. Indeed, in real life, it is necessary to monitor thyroid function (TSH) in LT4-treated women participating in an assisted reproductive technology program because the associated, transient, high oestrogen level might increase the LT4 requirement [140].
Some substances may even improve LT4 absorption. For example, Antunez and Licht found that after 2 months of co-administration of LT4 tablets with 1 g of vitamin C, the patients’ TSH levels had fallen by an average of around 70% for the same LT4 dose [141]. The effect of vitamin C was confirmed by Jubiz and Ramirez in a longitudinal study of 31 patients [142]. The researchers suggested that vitamin C might increase the solubility of LT4 in the stomach [142]. Thus, vitamin C is the only nutrient positively associated with LT4 absorption and can thus be proposed as a co-adjuvant in patients with gastrointestinal pathologies and LT4 malabsorption.
Discussion
Our review of the literature enabled us to draw up an extensive list of pharmaceutical, pathophysiological and behavioural factors that influence the absorption, distribution, metabolism and excretion of LT4. The great majority of these factors affected the absorption or (to a lesser extent) the metabolism (Fig. 4). When the physician is faced with a case of an abnormally high TSH level in a patient reportedly taking the recommended, weight-adjusted dose of LT4 (greater than 2 µg/kg/day or high dose to obtain and maintain a normal TSH concentration), we recommend considering the aspects summarized in Fig. 4.
Fig. 4.
A decision tree for patients with an abnormally high TSH level but who are reportedly taking the recommended, weight-adjusted dose of LT4
The first consideration is to assay the serum FT4 level. An elevated serum FT4 level may indicate the recent resumption (in the previous week) of LT4 treatment or an LT4 dose increase or the T4 administration 60–90 min before the blood withdrawal; normally, the physician will be aware of these events and can review the patient’s TSH and FT4 levels 4 to 6 weeks later. Thyroid hormone resistance syndromes are rare but can be considered once the patient’s endocrine profile is well documented; to gain a fuller clinical picture and rule out hereditary factors, it may be worth measuring TSH and FT4 levels in the patient’s family members [143]. Autonomous TSH secretion by a TSH-secreting pituitary adenoma can also be considered [144]. Lastly, patients with congenital hypothyroidism (due to GLIS3 mutations, for example) can acquire resistance to the action of thyroid hormones [145]. A normal serum FT4 level should prompt the physician to check for acute adrenal insufficiency (especially in patients treated for chronic and primary adrenal disease) or the presence of heterophilic anti-TSH antibodies. Lastly, a low serum FT4 level may be related to a dosing error, an LT4 storage problem, LT4 malabsorption, or pseudo-malabsorption (i.e. poor compliance with LT4 treatment).
For patients with a normal or low serum FT4 level and an elevated TSH level, the next step is a detailed interview in which the patient is questioned about his/her personal and family medical histories (notably any previous surgical operations involving the digestive tract), pregnancy, and medication habits. In particular the patients should be asked to describe how and when he/she takes the LT4 (the time of day, before/during/after meals, with a drink other than water, etc.) and any other source of variability. A medication review (i.e. the systematic appraisal of all aspects of a patient’s medication management, with a view to optimization and better outcomes) may be of value – especially in older adults who might not recall the full list of their medications in an interview [146]. A possible decrease in residual thyroid function (in patients with autoimmune thyroiditis) should be considered.
If the patient interview has not revealed an obvious factor for a normal or low LT4 concentration, several laboratory tests can be prescribed. Those will include a screen for antibodies against gastric components (e.g. circulating IgGs against parietal cells [54] and intestinal antibodies against endomysium or tissue transglutaminase 2 as specific markers of coeliac disease [147]) and for Helicobacter pylori infection. An abnormally high level of rT3 (an analyte not assayed on a routine bases) suggests that conversion of T4 to rT3 by DIO3 is favored over the production of T3 in the context of consumptive hypothyroidism [148]. The feces can be screened for steatorrhoea (rare in hypothyroid patients) as a sign of small intestine disease and possible malabsorption. Urine dipsticks can be used to screen for a nephrotic syndrome with proteinuria.
If the clinical problem has not been revealed by the patient interview and/or laboratory assays, the next step is performance of a pharmacodynamic LT4 absorption test (LAT). Although many different protocols have been used, a LAT typically involves the oral administration (under medical supervision) of LT4 with water in the morning after fasting for at least 8 h. Venous blood samples are then taken 1, 2, 3, 4, 5 and 6 h after the LT4 and are assayed for T4. However, the lack of standardization means that LAT parameters vary from one centre to another: the acute oral LT4 dose (a fixed dose of 1000 µg vs. the patient’s normal daily dose), the measurement of TT4 or FT4, the equation used to calculate absorption, the threshold for a normal result (typically 60% or 65% of the administered dose at the 3 h time point), and the list of contraindications (mainly ischemic heart disease) [24, 149–152].
A positive LT4 absorption test result (i.e. normal absorption) is suggestive of the patient-related problems mentioned above: a dosing error, a storage error, and/or poor compliance. However, there are several other possible explanations for an normal LT4 absorption test result: (i) an increase in the digestive excretion of T4 via stimulation of the enterohepatic cycle (e.g. by cholestyramine), (ii) an increase in DIO3 activity, (iv) the effects of medications (such as phenytoin, phenobarbital, rifampin, and nicardipine), or (v) possible consequences of endocrine disruptors (bisphenol A, and per/polyfluoroalkyl compounds) [153].
In fact, the endocrine disruptors bisphenol A (2,2-bis(4-hydroxyphenyl)propane) and other bisphenols (such as bisphenol B) are structurally similar to T4 and T3 and now known to have several effects on the thyroid hormone system [154, 155]. In addition to antagonizing nuclear thyroid receptors and thus interfering with hormone-stimulated transcriptional activity, bisphenols also appear to directly influence gene expression in the thyroid and pituitary gland [156]. Furthermore, da Silva et al. reported that bisphenol A inhibits the activities of DIO1 and DIO2 in vitro and (when administered orally to adult male Wistar rats) is associated with significantly lower hepatic DIO1 activity (but not brown adipose tissue DIO2 activity) [157]. Serum T4 levels were abnormally high in rats treated with bisphenol A, while T3 remained unchanged [157]. Although hydroxylated and halogenated bisphenols bind competitively to thyroid hormone transport proteins like TBG and transthyretin [158], the low binding constants (relative to T3 and T4) and low bisphenol concentrations in vivo might not have a major influence on transport in LT4-treated patients [159].
An abnormally slow or low increase in T4 levels in the absorption test should prompt a consultation with a gastroenterologist, who will typically screen for H. pylori-related gastritis (using the carbon-13 urea breath test), autoimmune gastritis (a test for anti-parietal cell antibodies, plus a gastroscopic assessment of the atrophy of gastric mucosa), coeliac disease, lactose intolerance (using the hydrogen breath test), parasitic disease (giardiasis), and steatorrhoea (which increases the fecal loss of LT4).
If true LT4 malabsorption has been ruled out, the physician can consider a diagnosis of pseudo-malabsorption. Rather than poor LT4 storage or a poor dosing technique, the primary cause for pseudo-malabsorption is likely to be poor compliance for psychological reasons. Indeed, this poor compliance might not be readily acknowledged by the patient. Lips et al. have recommended a subtle, non-confrontational approach to solving the problem of pseudo-malabsorption [160]. The physician should explain the treatment of hypothyroidism, emphasize the benefits of good compliance and, conversely, highlight the harmful consequences of chronic hypothyroidism and therefore of poor compliance. If this subtle approach does not resolve the problem, other possible approaches include changes in the pharmaceutical form for oral administration, weekly oral ingestion of LT4 under the supervision of medical staff, or exceptionally (and preferably at an expert centre) the parenteral infusion of LT4.
Our literature search did not identify any publications on interactions between bacterial or viral infections and the effectiveness of LT4. The initial data collected during the pandemic of coronavirus 2019 disease suggest that treated hypothyroidism is neither a risk factor nor a severity factor for infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [161]. Nevertheless, it has been suggested that thyroid status should be reassessed in euthyroid pregnant women infected by SARS-CoV-2 in their first trimester of pregnancy, notably if they are at high risk of thyroid dysfunction or have a history of thyroid autoimmune disease [162, 163].
The present study had a number of limitations. Firstly, only the PubMed database was searched. Secondly, only publications in English were screened.
Perspectives for further research include the broader application of in silico simulations of oral drug absorption and the effect of drug interactions [164]. There is also scope for better evaluating the risk of malabsorption in newly treated patients, using rapid screening questionnaires. For example, Bellastella et al. recently developed and tested a seven-question “Evaluation of Malabsorption in Patients with Hypothyroidism” (EMPATHY) questionnaire [165]. The questions covered gastritis, gastroesophageal reflux disease, H. pylori infection, bowel disease, food allergies, dietary habits linked to a high soy intake, alcohol intolerance or addiction, nickel allergy, and intolerance of gluten, histamine, lactose, citric acid or corn starch. Use of the EMPATHY questionnaire was associated with a lower requirement for subsequent dose adjustments.
Acknowledgements
Editorial support was provided by David Fraser (Biotech Communication SARL, Ploudalmézeau, France) and funded by Merck Serono SAS (Lyon, France), an affiliate of Merck KGaA (Darmstadt, Germany).
Abbreviations
- ADME
Absorption, distribution, metabolism and excretion
- AUC
Area under the curve
- BMI
Body mass index
- EMPATHY
Evaluation of malabsorption in patients with hypothyroidism
- DIO1
Type 1 deiodinase
- DIO2
Type 2 deiodinase
- DIO3
Type 3 deiodinase
- FT3
Free T3
- FT4
Free T4
- LT4
Levothyroxine
- LAT
LT4 absorption test
- T3
L-tri-iodothyronine
- MCT
Monocarboxylate transporter
- OATP1A2
Organic anion transporting polypeptide 1A2
- OATP2B1
Organic anion transporting polypeptide 2B1
- Cmax
Peak concentration
- PPI
Proton pump inhibitor
- rT3
Reverse T3
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SNP
Single nucleotide polymorphism
- SD
Standard deviation
- TH
Thyroid hormone
- TKI
Tyrosine kinase inhibitor
- TSH
Thyroid-stimulating hormone
- TBG
Thyroxine-binding globulin
- TT3
Total T3
- TT4
Total T4
Author contribution
All authors wrote the manuscript; P.C. designed the research; all authors performed the research; all authors analyzed the data.
Funding
The present research was funded by an unrestricted educational grant from Merck Serono SAS (Lyon, France), an affiliate of Merck KGaA (Darmstadt, Germany).
Data availability
The datasets (search query and list of publications) are presented in the main manuscript.
Declarations
Conflicts of interest
Philippe Caron has received consulting fees, honoraria for lectures and/or research funding from Merck Serono SAS and Laboratoires Genevrier; Solange Grunenwald has received consulting fees, honoraria for lectures and/or research funding from Merck Serono SAS, ESAI France, and Ipsen France; Luca Persani has received consulting fees, honoraria for lectures and/or research funding from Merck Serono and Sandoz Italy; Françoise Borson-Chazot has received consulting fees, honoraria for lectures and/or research funding from Merck Serono SAS and ESAI France; Remy Leroy has received consulting fees, honoraria for lectures and/or research funding from Merck Serono SAS; Leonidas Duntas has received consulting fees, honoraria for lectures and/or research funding from Merck Serono and Berlin-Chemie.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hennessey JV. The emergence of levothyroxine as a treatment for hypothyroidism. Endocrine. 2017;55(1):6–18. doi: 10.1007/s12020-016-1199-8. [DOI] [PubMed] [Google Scholar]
- 2.Jonklaas J, et al. Guidelines for the treatment of hypothyroidism: Prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateo RCI, Hennessey JV. Thyroxine and treatment of hypothyroidism: Seven decades of experience. Endocrine. 2019;66(1):10–17. doi: 10.1007/s12020-019-02006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biondi B, Cooper DS. Thyroid hormone therapy for hypothyroidism. Endocrine. 2019;66(1):18–26. doi: 10.1007/s12020-019-02023-7. [DOI] [PubMed] [Google Scholar]
- 5.Villar HC, et al. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev. 2007;3:CD003419. 10.1002/14651858.CD003419.pub2. [DOI] [PMC free article] [PubMed]
- 6.Tichy EM, et al. National trends in prescription drug expenditures and projections for 2020. Am J Health Syst Pharm. 2020;77(15):1213–1230. doi: 10.1093/ajhp/zxaa116. [DOI] [PubMed] [Google Scholar]
- 7.Garmendia Madariaga A, et al. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J Clin Endocrinol Metab. 2014;99(3):923–931. doi: 10.1210/jc.2013-2409. [DOI] [PubMed] [Google Scholar]
- 8.Aoki Y, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002) Thyroid. 2007;17(12):1211–1223. doi: 10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- 9.Hollowell JG, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 10.Okosieme OE, et al. Adequacy of thyroid hormone replacement in a general population. QJM. 2011;104(5):395–401. doi: 10.1093/qjmed/hcq222. [DOI] [PubMed] [Google Scholar]
- 11.Zaborek NA, et al. The optimal dosing scheme for levothyroxine after thyroidectomy: A comprehensive comparison and evaluation. Surgery. 2019;165(1):92–98. doi: 10.1016/j.surg.2018.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virili C, et al. Gastrointestinal malabsorption of thyroxine. Endocr Rev. 2019;40(1):118–136. doi: 10.1210/er.2018-00168. [DOI] [PubMed] [Google Scholar]
- 13.Duntas LH, Jonklaas J. Levothyroxine dose adjustment to optimise therapy throughout a patient’s lifetime. Adv Ther. 2019;36(Suppl 2):30–46. doi: 10.1007/s12325-019-01078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays MT. Thyroid hormone and the gut. Endocr Res. 1988;14(2–3):203–224. doi: 10.3109/07435808809032986. [DOI] [PubMed] [Google Scholar]
- 15.Hays MT. Localization of human thyroxine absorption. Thyroid. 1991;1(3):241–248. doi: 10.1089/thy.1991.1.241. [DOI] [PubMed] [Google Scholar]
- 16.Hasselstrom K, et al. The bioavailability of thyroxine and 3,5,3'-triiodothyronine in normal subjects and in hyper- and hypothyroid patients. Acta Endocrinol (Copenh) 1985;110(4):483–486. doi: 10.1530/acta.0.1100483. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism. 1977;26(1):1–8. doi: 10.1016/0026-0495(77)90121-4. [DOI] [PubMed] [Google Scholar]
- 18.Parving HH, et al. Mechanisms of edema formation in myxedema–increased protein extravasation and relatively slow lymphatic drainage. N Engl J Med. 1979;301(9):460–465. doi: 10.1056/NEJM197908303010902. [DOI] [PubMed] [Google Scholar]
- 19.Wiig H, Reed RK, Tenstad O. Interstitial fluid pressure, composition of interstitium, and interstitial exclusion of albumin in hypothyroid rats. Am J Physiol Heart Circ Physiol. 2000;278(5):H1627–H1639. doi: 10.1152/ajpheart.2000.278.5.H1627. [DOI] [PubMed] [Google Scholar]
- 20.Peeters RP, Visser TJ. Metabolism of thyroid hormone. In: Feingold KR, et al. editors. Endotext. South Dartmouth (MA); 2000.
- 21.Bianco AC, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 22.Colucci P, et al. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur Endocrinol. 2013;9(1):40–7. 10.17925/EE.2013.09.01.40. [DOI] [PMC free article] [PubMed]
- 23.Hoermann R, et al. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf) 2014;81(6):907–915. doi: 10.1111/cen.12527. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales KM, et al. The levothyroxine absorption test: a four-year experience (2015–2018) at The Mayo Clinic. Thyroid. 2019;29(12):1734–1742. doi: 10.1089/thy.2019.0256. [DOI] [PubMed] [Google Scholar]
- 25.Thynne TR, Doogue MP. A dose of paracetamol for the levothyroxine absorption test. Clin Endocrinol (Oxf) 2013;78(6):968–969. doi: 10.1111/cen.12211. [DOI] [PubMed] [Google Scholar]
- 26.Guglielmi R, et al. Shift from levothyroxine tablets to liquid formulation at breakfast improves quality of life of hypothyroid patients. Endocr Metab Immune Disord Drug Targets. 2018;18(3):235–240. doi: 10.2174/1871530318666180125155348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelin M, et al. Effect of timing of levothyroxine administration on the treatment of hypothyroidism: A three-period crossover randomized study. Endocrine. 2018;62(2):432–439. doi: 10.1007/s12020-018-1686-1. [DOI] [PubMed] [Google Scholar]
- 28.Pirola I, et al. TSH evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30 min before breakfast. J Endocrinol Invest. 2018;41(11):1301–1306. doi: 10.1007/s40618-018-0867-3. [DOI] [PubMed] [Google Scholar]
- 29.Marina M, et al. Circulating concentrations of free thyroxine after an oral intake of liquid LT4 taken either during fasting conditions or at breakfast. Acta Biomed. 2016;87(3):247–252. [PMC free article] [PubMed] [Google Scholar]
- 30.Morelli S, et al. Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine. 2016;52(3):571–578. doi: 10.1007/s12020-015-0788-2. [DOI] [PubMed] [Google Scholar]
- 31.Cappelli C, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: Results of the TICO study. Thyroid. 2016;26(2):197–202. doi: 10.1089/thy.2015.0422. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava S, et al. A crossover study evaluating effect of timing of levothyroxine on thyroid hormone status in patients of hypothyroidism. J Assoc Physicians India. 2018;66(9):37–40. [PubMed] [Google Scholar]
- 33.Bach-Huynh TG, et al. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94(10):3905–3912. doi: 10.1210/jc.2009-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez CL, et al. Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid. 2013;23(7):779–784. doi: 10.1089/thy.2012.0435. [DOI] [PubMed] [Google Scholar]
- 35.Bolk N, et al. Effects of evening vs morning levothyroxine intake: a randomized double-blind crossover trial. Arch Intern Med. 2010;170(22):1996–2003. doi: 10.1001/archinternmed.2010.436. [DOI] [PubMed] [Google Scholar]
- 36.Fallahi P, et al. Advancements in the treatment of hypothyroidism with L-T4 liquid formulation or soft gel capsule: An update. Expert Opin Drug Deliv. 2017;14(5):647–655. doi: 10.1080/17425247.2016.1227782. [DOI] [PubMed] [Google Scholar]
- 37.Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung. 2012;62(12):631–6. 10.1055/s-0032-1329951. [DOI] [PubMed]
- 38.Benvenga S, Capodicasa G, Perelli S. l-Thyroxine in an oral liquid or softgel formulation ensures more normal serum levels of free T4 in patients with central hypothyroidism. Front Endocrinol (Lausanne) 2017;8:321. doi: 10.3389/fendo.2017.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benvenga S, Di Bari F, Vita R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine. 2017;56(1):138–145. doi: 10.1007/s12020-017-1244-2. [DOI] [PubMed] [Google Scholar]
- 40.Fallahi P, Ferrari SM, Antonelli A. Oral L-thyroxine liquid versus tablet in patients with hypothyroidism without malabsorption: A prospective study. Endocrine. 2016;52(3):597–601. doi: 10.1007/s12020-015-0836-y. [DOI] [PubMed] [Google Scholar]
- 41.Brancato D, et al. Comparison of TSH levels with liquid formulation versus tablet formulations of levothyroxine in the treatment of adult hypothyroidism. Endocr Pract. 2014;20(7):657–662. doi: 10.4158/EP13418.OR. [DOI] [PubMed] [Google Scholar]
- 42.Trimboli P, et al. Thyroxine treatment with softgel capsule formulation: Usefulness in hypothyroid patients without malabsorption. Front Endocrinol (Lausanne) 2018;9:118. doi: 10.3389/fendo.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribichini D, et al. Tablet and oral liquid L-thyroxine formulation in the treatment of naive hypothyroid patients with Helicobacter pylori infection. Endocrine. 2017;57(3):394–401. doi: 10.1007/s12020-016-1167-3. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara R, et al. Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: A population-based study during the years 2009–2015. Endocrine. 2017;58(1):143–152. doi: 10.1007/s12020-017-1242-4. [DOI] [PubMed] [Google Scholar]
- 45.Guglielmi V, et al. Drug interactions in users of tablet vs. oral liquid levothyroxine formulations: A real-world evidence study in primary care. Endocrine. 2018;59(3): 585–92. 10.1007/s12020-017-1412-4. [DOI] [PubMed]
- 46.Benvenga S, Di Bari F. Intestinal absorption and buccal absorption of liquid levothyroxine. Endocrine. 2017;58(3):591–594. doi: 10.1007/s12020-017-1250-4. [DOI] [PubMed] [Google Scholar]
- 47.Bornschein A, et al. Treating primary hypothyroidism with weekly doses of levothyroxine: A randomized, single-blind, crossover study. Arq Bras Endocrinol Metabol. 2012;56(4):250–258. doi: 10.1590/s0004-27302012000400006. [DOI] [PubMed] [Google Scholar]
- 48.McMillan M, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: Results of the CONTROL surveillance project. Drugs R D. 2016;16(1):53–68. doi: 10.1007/s40268-015-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virili C, et al. Ulcerative colitis as a novel cause of increased need for levothyroxine. Front Endocrinol (Lausanne) 2019;10:233. doi: 10.3389/fendo.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins D, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125(3):278–282. doi: 10.1016/j.amjmed.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Munoz-Torres M, Varsavsky M, Alonso G. Lactose intolerance revealed by severe resistance to treatment with levothyroxine. Thyroid. 2006;16(11):1171–1173. doi: 10.1089/thy.2006.16.1171. [DOI] [PubMed] [Google Scholar]
- 52.Bugdaci MS, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16(2):124–130. doi: 10.1111/j.1523-5378.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 53.Checchi S, et al. L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab. 2008;93(2):465–469. doi: 10.1210/jc.2007-1544. [DOI] [PubMed] [Google Scholar]
- 54.Lenti MV, et al. Autoimmune gastritis. Nat Rev Dis Primers. 2020;6(1):56. doi: 10.1038/s41572-020-0187-8. [DOI] [PubMed] [Google Scholar]
- 55.Castellana M, et al. Prevalence of gastrointestinal disorders having an impact on tablet levothyroxine absorption: Should this formulation still be considered as the first-line therapy? Endocrine. 2020;67(2):281–290. doi: 10.1007/s12020-019-02185-4. [DOI] [PubMed] [Google Scholar]
- 56.Fierabracci P, et al. Weight loss and variation of levothyroxine requirements in hypothyroid obese patients after bariatric surgery. Thyroid. 2016;26(4):499–503. doi: 10.1089/thy.2015.0473. [DOI] [PubMed] [Google Scholar]
- 57.Richou M, et al. Levothyroxine dose adjustment in hypothyroid patients following gastric sleeve surgery. Ann Endocrinol (Paris) 2020;81(5):500–506. doi: 10.1016/j.ando.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Pedro J, et al. The effect of the bariatric surgery type on the levothyroxine dose of morbidly obese hypothyroid patients. Obes Surg. 2018;28(11):3538–3543. doi: 10.1007/s11695-018-3388-4. [DOI] [PubMed] [Google Scholar]
- 59.Rubio IG, et al. Levothyroxine absorption in morbidly obese patients before and after Roux-En-Y gastric bypass (RYGB) surgery. Obes Surg. 2012;22(2):253–258. doi: 10.1007/s11695-011-0452-8. [DOI] [PubMed] [Google Scholar]
- 60.Fallahi P, et al. TSH normalization in bariatric surgery patients after the switch from L-Thyroxine in tablet to an oral liquid formulation. Obes Surg. 2017;27(1):78–82. doi: 10.1007/s11695-016-2247-4. [DOI] [PubMed] [Google Scholar]
- 61.Benvenga S, et al. A minimum of two years of undertreated primary hypothyroidism, as a result of drug-induced malabsorption of l-thyroxine, may have metabolic and cardiovascular consequences. J Clin Transl Endocrinol. 2019;16:100189. doi: 10.1016/j.jcte.2019.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abu Hashim H, Foda O, Ghayaty E. Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: A meta-analysis of randomized trials. Eur J Obstet Gynecol Reprod Biol. 2017;219:45–52. 10.1016/j.ejogrb.2017.10.003. [DOI] [PubMed]
- 63.Trifiro G, et al. Drug interactions with levothyroxine therapy in patients with hypothyroidism: Observational study in general practice. Clin Drug Investig. 2015;35(3):187–195. doi: 10.1007/s40261-015-0271-0. [DOI] [PubMed] [Google Scholar]
- 64.Atruktsang TS, et al. Identifying predictors of prolonged levothyroxine dose adjustment after thyroidectomy. J Surg Res. 2019;242:166–171. doi: 10.1016/j.jss.2019.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morini E, et al. In thyroxine-replaced hypothyroid postmenopausal women under simultaneous calcium supplementation, switch to oral liquid or softgel capsule L-thyroxine ensures lower serum TSH levels and favorable effects on blood pressure, total cholesterolemia and glycemia. Endocrine. 2019;65(3):569–579. doi: 10.1007/s12020-019-01908-x. [DOI] [PubMed] [Google Scholar]
- 66.Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283(21):2822–2825. doi: 10.1001/jama.283.21.2822. [DOI] [PubMed] [Google Scholar]
- 67.Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21(5):483–486. doi: 10.1089/thy.2010.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liel Y, Harman-Boehm I, Shany S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab. 1996;81(2):857–859. doi: 10.1210/jcem.81.2.8636317. [DOI] [PubMed] [Google Scholar]
- 69.Lesko LJ, et al. Evaluation of the potential for drug interactions with patiromer in healthy volunteers. J Cardiovasc Pharmacol Ther. 2017;22(5):434–446. doi: 10.1177/1074248417691135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17(8):763–765. doi: 10.1089/thy.2007.0060. [DOI] [PubMed] [Google Scholar]
- 71.Goldberg AS, et al. Ciprofloxacin and rifampin have opposite effects on levothyroxine absorption. Thyroid. 2013;23(11):1374–1378. doi: 10.1089/thy.2013.0014. [DOI] [PubMed] [Google Scholar]
- 72.Sachmechi I, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13(4):345–349. doi: 10.4158/EP.13.4.345. [DOI] [PubMed] [Google Scholar]
- 73.Centanni M, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354(17):1787–1795. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- 74.Dietrich JW, et al. Absorption kinetics of levothyroxine is not altered by proton-pump inhibitor therapy. Horm Metab Res. 2006;38(1):57–59. doi: 10.1055/s-2006-924980. [DOI] [PubMed] [Google Scholar]
- 75.Abi-Abib RC, Vaisman M. Is it necessary to increase the dose of levothyroxine in patients with hypothyroidism who use omeprazole? Arq Bras Endocrinol Metabol. 2014;58(7):731–6. 10.1590/0004-2730000002997. [DOI] [PubMed]
- 76.Ananthakrishnan S, et al. The effect of famotidine, esomeprazole, and ezetimibe on levothyroxine absorption. Thyroid. 2008;18(5):493–498. doi: 10.1089/thy.2007.0381. [DOI] [PubMed] [Google Scholar]
- 77.Benvenga S, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18(3):293–301. doi: 10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- 78.Vita R, et al. A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine. 2013;43(1):154–160. doi: 10.1007/s12020-012-9772-2. [DOI] [PubMed] [Google Scholar]
- 79.Cappelli C, et al. Oral liquid levothyroxine treatment at breakfast: A mistake? Eur J Endocrinol. 2014;170(1):95–99. doi: 10.1530/EJE-13-0693. [DOI] [PubMed] [Google Scholar]
- 80.Chon DA, et al. Concurrent milk ingestion decreases absorption of levothyroxine. Thyroid. 2018;28(4):454–457. doi: 10.1089/thy.2017.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conrad SC, Chiu H, Silverman BL. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child. 2004;89(1):37–40. doi: 10.1136/adc.2002.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lilja JJ, Laitinen K, Neuvonen PJ. Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005;60(3):337–341. doi: 10.1111/j.1365-2125.2005.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arakawa H, et al. Active intestinal absorption of fluoroquinolone antibacterial agent ciprofloxacin by organic anion transporting polypeptide, Oatp1a5. Biopharm Drug Dispos. 2012;33(6):332–341. doi: 10.1002/bdd.1809. [DOI] [PubMed] [Google Scholar]
- 84.Glaeser H, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 85.Zu Schwabedissen HEM, et al. Thyroid hormones are transport substrates and transcriptional regulators of organic anion transporting polypeptide 2B1. Mol Pharmacol. 2018;94(1):700–12. 10.1124/mol.117.111161. [DOI] [PubMed]
- 86.Kharrazian D, Herbert M, Vojdani A. Immunological reactivity using monoclonal and polyclonal antibodies of autoimmune thyroid target sites with dietary proteins. J Thyroid Res. 2017;2017:4354723. doi: 10.1155/2017/4354723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Surks MI, DeFesi CR. Normal serum free thyroid hormone concentrations in patients treated with phenytoin or carbamazepine. A paradox resolved JAMA. 1996;275(19):1495–1498. doi: 10.1001/jama.1996.03530430039036. [DOI] [PubMed] [Google Scholar]
- 88.Burch HB. Drug effects on the thyroid. N Engl J Med. 2019;381(8):749–761. doi: 10.1056/NEJMra1901214. [DOI] [PubMed] [Google Scholar]
- 89.Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344(23):1743–1749. doi: 10.1056/NEJM200106073442302. [DOI] [PubMed] [Google Scholar]
- 90.Shifren JL, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14(6):985–994. doi: 10.1097/gme.0b013e31803867a. [DOI] [PubMed] [Google Scholar]
- 91.Alexander EK, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351(3):241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 92.Arafah BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann Intern Med. 1994;121(4):247–251. doi: 10.7326/0003-4819-121-4-199408150-00002. [DOI] [PubMed] [Google Scholar]
- 93.Panicker V, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94(5):1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 94.Santoro AB, et al. Effect of UGT1A1, UGT1A3, DIO1 and DIO2 polymorphisms on L-thyroxine doses required for TSH suppression in patients with differentiated thyroid cancer. Br J Clin Pharmacol. 2014;78(5):1067–1075. doi: 10.1111/bcp.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arici M, et al. Association between genetic polymorphism and levothyroxine bioavailability in hypothyroid patients. Endocr J. 2018;65(3):317–323. doi: 10.1507/endocrj.EJ17-0162. [DOI] [PubMed] [Google Scholar]
- 96.Appelhof BC, et al. Polymorphisms in type 2 deiodinase are not associated with well-being, neurocognitive functioning, and preference for combined thyroxine/3,5,3'-triiodothyronine therapy. J Clin Endocrinol Metab. 2005;90(11):6296–6299. doi: 10.1210/jc.2005-0451. [DOI] [PubMed] [Google Scholar]
- 97.Al-azzam SI, et al. The role of type II deiodinase polymorphisms in clinical management of hypothyroid patients treated with levothyroxine. Exp Clin Endocrinol Diabetes. 2013;121(5):300–305. doi: 10.1055/s-0032-1331695. [DOI] [PubMed] [Google Scholar]
- 98.Heemstra KA, et al. Thr92Ala polymorphism in the type 2 deiodinase is not associated with T4 dose in athyroid patients or patients with Hashimoto thyroiditis. Clin Endocrinol (Oxf) 2009;71(2):279–283. doi: 10.1111/j.1365-2265.2008.03474.x. [DOI] [PubMed] [Google Scholar]
- 99.Wouters HJ, et al. No effect of the Thr92Ala polymorphism of Deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017;27(2):147–155. doi: 10.1089/thy.2016.0199. [DOI] [PubMed] [Google Scholar]
- 100.Castagna MG, et al. DIO2 Thr92Ala reduces Deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017;102(5):1623–1630. doi: 10.1210/jc.2016-2587. [DOI] [PubMed] [Google Scholar]
- 101.Cantara S, et al. Variants in MCT10 protein do not affect FT3 levels in athyreotic patients. Endocrine. 2019;66(3):551–556. doi: 10.1007/s12020-019-02001-z. [DOI] [PubMed] [Google Scholar]
- 102.Torlontano M, et al. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J Clin Endocrinol Metab. 2008;93(3):910–913. doi: 10.1210/jc.2007-1067. [DOI] [PubMed] [Google Scholar]
- 103.Carlé A, et al. Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment - data using a blind, randomized, clinical study. Eur Thyroid J. 2017;6(3):143–151. doi: 10.1159/000469709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papanas N, et al. Thyroxine replacement dose in patients with Hashimoto disease: A potential role for interleukin-6. Cytokine. 2006;35(3–4):166–170. doi: 10.1016/j.cyto.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 105.Roef GL, et al. Associations between single nucleotide polymorphisms in thyroid hormone transporter genes (MCT8, MCT10 and OATP1C1) and circulating thyroid hormones. Clin Chim Acta. 2013;425:227–232. doi: 10.1016/j.cca.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 106.Sarret C, Petit O, Tonduti D. Allan-Herndon-Dudley syndrome. In: Adam MP, et al. editors. GeneReviews (R).Seattle (WA); 1993. [PubMed]
- 107.de Lorgeril M, Salen P. Selenium and antioxidant defenses as major mediators in the development of chronic heart failure. Heart Fail Rev. 2006;11(1):13–17. doi: 10.1007/s10741-006-9188-2. [DOI] [PubMed] [Google Scholar]
- 108.Dubois-Deruy E, et al. Oxidative stress in cardiovascular diseases. Antioxidants (Basel). 2020;9(9). 10.3390/antiox9090864. [DOI] [PMC free article] [PubMed]
- 109.Fraczek-Jucha M, et al. Low triiodothyronine syndrome and serum selenium status in the course of acute myocardial infarction. Pol Merkur Lekarski. 2019;47(278):45–51. [PubMed] [Google Scholar]
- 110.Yang T, et al. Comparison of levothyroxine dosing in patients with and without heart failure. Endocr Res. 2020;45(1):50–57. doi: 10.1080/07435800.2019.1645165. [DOI] [PubMed] [Google Scholar]
- 111.Chakrabarti SK, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20(5):674–678. doi: 10.4103/2230-8210.190555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olker JH, et al. Screening the ToxCast phase 1, phase 2, and e1k chemical libraries for inhibitors of Iodothyronine deiodinases. Toxicol Sci. 2019;168(2):430–442. doi: 10.1093/toxsci/kfy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abdulrahman RM, et al. Sorafenib-induced hypothyroidism is associated with increased type 3 deiodination. J Clin Endocrinol Metab. 2010;95(8):3758–3762. doi: 10.1210/jc.2009-2507. [DOI] [PubMed] [Google Scholar]
- 114.Fallahi P, et al. Thyroid dysfunctions induced by tyrosine kinase inhibitors. Expert Opin Drug Saf. 2014;13(6):723–733. doi: 10.1517/14740338.2014.913021. [DOI] [PubMed] [Google Scholar]
- 115.de Groot JW, et al. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther. 2005;78(4):433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 116.Curran PG, DeGroot LJ. The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocr Rev. 1991;12(2):135–150. doi: 10.1210/edrv-12-2-135. [DOI] [PubMed] [Google Scholar]
- 117.Kim HI, et al. Effect of rifampin on thyroid function test in patients on levothyroxine medication. PLoS One. 2017;12(1):e0169775. doi: 10.1371/journal.pone.0169775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Benvenga S, et al. Do not forget nephrotic syndrome as a cause of increased requirement of levothyroxine replacement therapy. Eur Thyroid J. 2015;4(2):138–142. doi: 10.1159/000381310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwong N, et al. Severity of proteinuria is directly associated with risk of hypothyroidism in adults. J Clin Endocrinol Metab. 2021;106(2):e757–e762. doi: 10.1210/clinem/dgaa872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shakir KM, et al. The use of bile acid sequestrants to lower serum thyroid hormones in iatrogenic hyperthyroidism. Ann Intern Med. 1993;118(2):112–113. doi: 10.7326/0003-4819-118-2-199301150-00006. [DOI] [PubMed] [Google Scholar]
- 121.Solomon BL, Wartofsky L, Burman KD. Adjunctive cholestyramine therapy for thyrotoxicosis. Clin Endocrinol (Oxf) 1993;38(1):39–43. doi: 10.1111/j.1365-2265.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 122.Northcutt RC, et al. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208(10):1857–1861. doi: 10.1001/jama.1969.03160100047012. [DOI] [PubMed] [Google Scholar]
- 123.Weitzman SP, Ginsburg KC, Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19(1):77–79. doi: 10.1089/thy.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas MC, Mathew TH, Russ GR. Changes in thyroxine requirements in patients with hypothyroidism undergoing renal transplantation. Am J Kidney Dis. 2002;39(2):354–357. doi: 10.1053/ajkd.2002.30556. [DOI] [PubMed] [Google Scholar]
- 125.Isidro ML, et al. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine. 2007;32(1):79–82. doi: 10.1007/s12020-007-9012-3. [DOI] [PubMed] [Google Scholar]
- 126.Cappelli C, et al. Thyreotropin levels in diabetic patients on metformin treatment. Eur J Endocrinol. 2012;167(2):261–265. doi: 10.1530/EJE-12-0225. [DOI] [PubMed] [Google Scholar]
- 127.Rotondi M, et al. Thyroidal effect of metformin treatment in patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2011;75(3):378–381. doi: 10.1111/j.1365-2265.2011.04042.x. [DOI] [PubMed] [Google Scholar]
- 128.Hu X, et al. Metformin affects thyroid function in male rats. Oncotarget. 2017;8(64):107589–95. 10.18632/oncotarget.22536. [DOI] [PMC free article] [PubMed]
- 129.de Carvalho GA, et al. Effects of selective serotonin reuptake inhibitors on thyroid function in depressed patients with primary hypothyroidism or normal thyroid function. Thyroid. 2009;19(7):691–697. doi: 10.1089/thy.2008.0261. [DOI] [PubMed] [Google Scholar]
- 130.Persiani S, et al. Evaluation of levothyroxine bioavailability after oral administration of a fixed combination of soy isoflavones in post-menopausal female volunteers. Drug Res (Stuttg) 2016;66(3):136–140. doi: 10.1055/s-0035-1555784. [DOI] [PubMed] [Google Scholar]
- 131.Wood MD, et al. An evaluation of the potential drug interaction between warfarin and levothyroxine. J Thromb Haemost. 2014;12(8):1313–1319. doi: 10.1111/jth.12626. [DOI] [PubMed] [Google Scholar]
- 132.Hu S, et al. Interaction study between antiplatelet agents, anticoagulants, thyroid replacement therapy and a bioavailable formulation of curcumin (Meriva(R)). Eur Rev Med Pharmacol Sci. 2018;22(15):5042–46. 10.26355/eurrev_201808_15647. [DOI] [PubMed]
- 133.Bone HG, et al. Pharmacokinetics of coadministration of levothyroxine sodium and alendronate sodium new effervescent formulation. Osteoporos Int. 2017;28(5):1745–1752. doi: 10.1007/s00198-017-3941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abbasinazari M, Nakhjavani M, Gogani S. The effects of simvastatin on the serum concentrations of thyroid stimulating hormone and free thyroxine in hypothyroid patients treated with levothyroxine. Iran J Med Sci. 2011;36(2):80–83. [PMC free article] [PubMed] [Google Scholar]
- 135.Mahmoodianfard S, et al. Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: A randomized double-blind controlled trial. J Am Coll Nutr. 2015;34(5):391–399. doi: 10.1080/07315724.2014.926161. [DOI] [PubMed] [Google Scholar]
- 136.Severo JS, et al. The role of zinc in thyroid hormones metabolism. Int J Vitam Nutr Res. 2019;89(1–2):80–88. doi: 10.1024/0300-9831/a000262. [DOI] [PubMed] [Google Scholar]
- 137.Li SW, Chan SY. Management of overt hypothyroidism during pregnancy. Best Pract Res Clin Endocrinol Metab. 2020;34(4):101439. doi: 10.1016/j.beem.2020.101439. [DOI] [PubMed] [Google Scholar]
- 138.Haddow JE, et al. An inverse relationship between weight and free thyroxine during early gestation among women treated for hypothyroidism. Thyroid. 2015;25(8):949–953. doi: 10.1089/thy.2015.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davis LB, Lathi RB, Dahan MH. The effect of infertility medication on thyroid function in hypothyroid women who conceive. Thyroid. 2007;17(8):773–777. doi: 10.1089/thy.2007.0065. [DOI] [PubMed] [Google Scholar]
- 140.Poppe K, et al. 2021 European thyroid association guideline on thyroid disorders prior to and during assisted reproduction. Eur Thyroid J. 2021;9(6):281–295. doi: 10.1159/000512790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Antúnez PB, Licht SD. Vitamin C improves the apparent absorption of levothyroxine in a subset of patients receiving this hormone for primary hypothyroidism. Rev Argent Endocrinol Metab. 2011;48.
- 142.Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab. 2014;99(6):E1031–E1034. doi: 10.1210/jc.2013-4360. [DOI] [PubMed] [Google Scholar]
- 143.Persani L, Campi I. Syndromes of resistance to thyroid hormone action. Exp Suppl. 2019;111:55–84. doi: 10.1007/978-3-030-25905-1_5. [DOI] [PubMed] [Google Scholar]
- 144.Campi I, et al. The differential diagnosis of discrepant thyroid function tests: Insistent pitfalls and updated flow-chart based on a long-standing experience. Front Endocrinol (Lausanne) 2020;11:432. doi: 10.3389/fendo.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jetten AM. GLIS1-3 transcription factors: critical roles in the regulation of multiple physiological processes and diseases. Cell Mol Life Sci. 2018;75(19):3473–3494. doi: 10.1007/s00018-018-2841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Medicines optimisation: The safe and effective use of medicines to enable the best possible outcomes. Accessed at 2015. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26180890. [PubMed]
- 147.Maglio M, Troncone R. Intestinal anti-tissue Transglutaminase2 autoantibodies: pathogenic and clinical implications for Celiac disease. Front Nutr. 2020;7:73. doi: 10.3389/fnut.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.LoPresti JS, et al. Alterations in 3,3'5'-triiodothyronine metabolism in response to propylthiouracil, dexamethasone, and thyroxine administration in man. J Clin Invest. 1989;84(5):1650–1656. doi: 10.1172/JCI114343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewandowski KC, et al. Adequate timing and constant supervision are the keys for successful implementation of levothyroxine or levothyroxine/paracetamol absorption test. Thyroid Res. 2020;13:5. doi: 10.1186/s13044-020-00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun GE, et al. The clinical utility of free thyroxine in oral levothyroxine absorption testing. Endocr Pract. 2014;20(9):925–929. doi: 10.4158/EP13487.OR. [DOI] [PubMed] [Google Scholar]
- 151.Ghosh S, et al. Levothyroxine absorption test to differentiate pseudomalabsorption from true malabsorption. Eur Thyroid J. 2020;9(1):19–24. doi: 10.1159/000504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simsir IY, Soyaltin UE, Ozgen AG. Levothyroxine absorption test results in patients with TSH elevation resistant to treatment. Endocrine. 2019;64(1):118–21. 10.1007/s12020-019-01889-x. [DOI] [PubMed]
- 153.Bhattacharya S, et al. Anticancer drug-induced thyroid dysfunction. Eur Endocrinol. 2020;16(1):32–9. 10.17925/EE.2020.16.1.32. [DOI] [PMC free article] [PubMed]
- 154.Gorini F, et al. Bisphenols as environmental triggers of thyroid dysfunction: Clues and evidence. Int J Environ Res Public Health. 2020;17(8). 10.3390/ijerph17082654. [DOI] [PMC free article] [PubMed]
- 155.Milczarek-Banach J, et al. Exposure to Bisphenol A analogs and the thyroid function and volume in women of reproductive age-cross-sectional study. Front Endocrinol (Lausanne) 2020;11:587252. doi: 10.3389/fendo.2020.587252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gentilcore D, et al. Bisphenol A interferes with thyroid specific gene expression. Toxicology. 2013;304:21–31. doi: 10.1016/j.tox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 157.da Silva MM, et al. Inhibition of Type 1 Iodothyronine Deiodinase by Bisphenol A. Horm Metab Res. 2019;51(10):671–677. doi: 10.1055/a-0919-3879. [DOI] [PubMed] [Google Scholar]
- 158.Kudo Y, et al. In vitro and in vivo analysis of the thyroid system-disrupting activities of brominated phenolic and phenol compounds in Xenopus laevis. Toxicol Sci. 2006;92(1):87–95. doi: 10.1093/toxsci/kfj204. [DOI] [PubMed] [Google Scholar]
- 159.Cao J, et al. In vitro fluorescence displacement investigation of thyroxine transport disruption by bisphenol A. J Environ Sci (China) 2011;23(2):315–321. doi: 10.1016/s1001-0742(10)60408-1. [DOI] [PubMed] [Google Scholar]
- 160.Lips DJ, et al. Diagnosis and treatment of levothyroxine pseudomalabsorption. Neth J Med. 2004;62(4):114–118. [PubMed] [Google Scholar]
- 161.Brix TH, et al. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol. 2021;9(4):197–199. doi: 10.1016/S2213-8587(21)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sarahian N, Naz MSG, Ramezani TF. Following SARS-CoV-2 in the first trimester of pregnancy, what should we do in the 2nd, 3rd trimesters, and postpartum in terms of thyroid assessment? Endocrine. 2021. 10.1007/s12020-021-02678-1. [DOI] [PMC free article] [PubMed]
- 163.van Gerwen M, et al. Outcomes of patients with hypothyroidism and COVID-19: A retrospective cohort study. Front Endocrinol (Lausanne) 2020;11:565. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kocic I, et al. A case study on the in silico absorption simulations of levothyroxine sodium immediate-release tablets. Biopharm Drug Dispos. 2012;33(3):146–159. doi: 10.1002/bdd.1780. [DOI] [PubMed] [Google Scholar]
- 165.Bellastella G, et al. EMPATHY: A new tool for identifying the most suitable thyroxine formulation in hypothyroid patients. Thyroid. 2019;29(7):928–933. doi: 10.1089/thy.2018.0493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets (search query and list of publications) are presented in the main manuscript.