Abstract
Errors that result from a mismatch between predicted movement outcomes and sensory afference are used to correct ongoing movements through feedback control and to adapt feedforward control of future movements. The cerebellum has been identified as a critical part of the neural circuit underlying implicit adaptation across a wide variety of movements (reaching, gait, eye movements, and speech). The contribution of this structure to feedback control is less well understood. Although it has recently been shown in the speech domain that individuals with cerebellar degeneration produce larger online corrections for sensory perturbations than control participants, similar behavior has not been observed in other motor domains. Currently, comparisons across domains are limited by different population samples and potential ceiling effects in existing tasks. To assess the relationship between changes in feedforward and feedback control associated with cerebellar degeneration across motor domains, we evaluated adaptive (feedforward) and compensatory (feedback) responses to sensory perturbations in reaching and speech production in human participants of both sexes with cerebellar degeneration and neurobiologically healthy controls. As expected, the cerebellar group demonstrated impaired adaptation in both reaching and speech. In contrast, the groups did not differ in their compensatory response in either domain. Moreover, compensatory and adaptive responses in the cerebellar group were not correlated within or across motor domains. These results point to a general impairment in feedforward control with spared feedback control in cerebellar degeneration. However, the magnitude of feedforward impairments and potential changes in feedback-based control manifest in a domain-specific manner across individuals.
SIGNIFICANCE STATEMENT The cerebellum contributes to feedforward updating of movement in response to sensory errors, but its role in feedback control is less understood. Here, we tested individuals with cerebellar degeneration (CD), using sensory perturbations to assess adaptation of feedforward control and feedback gains during reaching and speech production tasks. The results confirmed that CD leads to reduced adaption in both domains. However, feedback gains were unaffected by CD in either domain. Interestingly, measures of feedforward and feedback control were not correlated across individuals within or across motor domains. Together, these results indicate a general impairment in feedforward control with spared feedback control in CD. However, the magnitude of feedforward impairments manifests in a domain-specific manner across individuals.
Keywords: cerebellum, feedback control, feedforward control, limb control, motor control, speech
Introduction
Coordinated movement relies on a combination of feedback control and anticipatory mechanisms. A mismatch between the predicted and actual feedback resulting from a motor command can lead to online corrections as well as adaptation of feedforward control for future movements (Shadmehr and Krakauer, 2008). The cerebellum plays a critical role in this latter process, helping ensure that the predictive system is optimally calibrated. One line of supportive evidence comes from the substantial literature showing markedly impaired performance of individuals with cerebellar degeneration (CD) during sensorimotor adaptation tasks involving upper limb movement (Martin et al., 1996; Smith and Shadmehr, 2005; Tseng et al., 2007; Donchin et al., 2012; Schlerf et al., 2013), gait (Morton and Bastian, 2006), eye movements (Xu-Wilson et al., 2009), and speech (Parrell et al., 2017).
If, and how, the cerebellum contributes to feedback control is less clear. One clue comes from intentional tremor, a prominent feature of CD where low-frequency oscillations occur around the movement end point. Intentional tremor is reduced when movement is produced without visual feedback (Day et al., 1998). Such behavior is broadly consistent with a control system that relies on visual feedback (Beppu et al., 1987) and could occur if the gains on sensory feedback errors are larger in individuals with CD. Larger gains could lead to overcorrections for errors and the need for additional countercorrections. This hypothesis is supported by evidence showing that, relative to controls, individuals with CD produce larger compensatory responses to auditory perturbations of speech (Parrell et al., 2017; Houde et al., 2019; Li et al., 2019).
There is mixed evidence concerning feedback gains in other types of movement. Individuals with CD produce a smaller long-latency muscle response to mechanical perturbations (Kurtzer et al., 2013), consistent with a decreased gain in the response to proprioceptive feedback. However, their feedback-based corrections during split-belt treadmill walking are relatively normal (Morton and Bastian, 2006), suggesting proprioceptive gains are unaffected for at least some tasks. Moreover, individuals with CD have normal feedback gains in a continuous visual tracking task but with a substantial phase lag (Zimmet et al., 2020). Computationally, this is consistent with an increased reliance on (delayed) feedback in the absence of a predictive function for state estimation (Wolpert et al., 1998; Miall et al., 2007).
It is possible that potential increases in feedback gains in nonspeech tasks may be obscured by a ceiling effect. Although the auditory feedback response in speech only partially compensates for the perturbation, the responses to perturbations in tasks such as visually guided movements or walking typically provide nearly complete compensation to the perturbation (Morton and Bastian, 2006; Tseng et al., 2007). As such, reductions in feedback gains could be readily measured, but increases in gain might be hard to detect. Alternatively (or additionally), modification of feedback gains in CD may be task dependent, with variable changes across movement types. This would stand in contrast to feedforward control, where the cerebellum appears to play a similar role in implicit adaptation across domains.
The preceding hypotheses are based on inferences drawn from disparate studies that employ distinct methods and typically focus on a single motor domain. In the present study, we used a 2 × 2 design to evaluate feedback and feedforward control in two motor tasks, one involving visually guided reaching and the other speech production. To avoid potential ceiling effects in the former, we used a task shown previously to induce only partial corrections to the visual perturbation (Körding and Wolpert, 2004). Moreover, by testing the same individuals on all four tasks, we can perform a correlational analysis, focusing on two key questions related to individual differences associated with CD. First, are patterns of impairment similar across the two motor domains and/or forms of control? Second, is the degree of impairment in feedforward control (adaptation) predictive of feedback gains, a signature that would be consistent with the hypothesis that enhanced feedback gains arise as a compensatory mechanism?
Materials and Methods
Participants
Twenty-three individuals with cerebellar degeneration (19 female; age, 37–89 years; mean age 62 years) and 15 age-matched neurobiologically healthy controls (8 female; age, 43–85 years; mean age 61 years) were recruited for the study. One CD participant was excluded because of a potential mild cognitive impairment (see below), leaving 22 individuals in the CD group. The participants had normal or corrected-to-normal vision, and none reported any history of speech or hearing impairments or significant neurologic issues (other than ataxia in the CD group). The participants were naive about the experimental hypotheses, and none had participated in our previous experiment using altered auditory feedback (Parrell et al., 2017). Participants provided informed consent and received financial compensation for their time. The protocol was approved by the institutional review boards at the University of Wisconsin–Madison and the University of California, Berkeley.
The patients were drawn from the Northern California Ataxia Support Group, the Madison Ataxia Support Group, the Kansas City Ataxia Support Group, and individuals who attended the 2018 annual meeting of the National Ataxia Foundation. Recruitment for the latter two groups was conducted in coordination with officials of the associated organizations. In terms of etiology, the CD group was heterogeneous; 11 had a confirmed genetic subtype (3 SCA3, 6 SCA6, 2 SCA8), 1 had a clinically diagnosed genetic subtype (AOA 2), and 11 had been diagnosed as cerebellar ataxia of unknown etiology. Inclusion was limited to individuals who had symptoms of ataxia in combination with genetic confirmation, radiologic findings of cerebellar atrophy, or a medical diagnosis indicating cerebellar atrophy. Although we performed a brief neurologic examination to assess ataxia (see below), the other information was based on the participants' self-reports. We note that individuals who attend support groups are generally very proactive and well informed about their condition. Age-matched control participants were drawn from the greater Berkeley community.
All the participants were administered the Montreal Cognitive Assessment (MoCA) as a gross measure of cognitive impairment. The CD group was additionally administered the Standard Ataxia Rating Scale (SARA) to assess the severity of their ataxia. SARA subscores were calculated for both upper limb control and speech. We did not include a formal assessment of signs of extracerebellar degeneration, although none of the participants, including those with SCA3, exhibited gross symptoms of basal ganglia dysfunction such as rigidity or resting tremor. One individual with CD was excluded because of a MoCA score <18, indicative of moderate cognitive impairment (Table 1).
Table 1.
Characteristics of individuals with cerebellar degeneration
| Diagnosis | Age | MoCA | SARA overall score | SARA upper limb subscore | SARA intention tremor subscore | SARA speech subscore |
|---|---|---|---|---|---|---|
| SCA3 | 61 | 28 | 16 | 5 | 1.5 | 3 |
| SCA3 | 75 | 26 | 16.5 | 5.5 | 1.5 | 0 |
| SCA3 | 65 | 25 | 24 | 7 | 2 | 1 |
| SCA6 | 77 | 22 | 8 | 2 | 1 | 1 |
| SCA6 | 70 | 23 | 7.75 | 3.25 | 0.5 | 0 |
| SCA6 | 60 | 26 | 3 | 0.5 | 0 | 0 |
| SCA6 | 49 | 26 | 1 | 1 | 0 | 0 |
| SCA6 | 65 | 24 | 9 | 3.5 | 0.5 | 0 |
| SCA6 | 57 | 27 | 6 | 3 | 0 | 0 |
| SCA8 | 57 | 27 | 11 | 3.5 | 1 | 0 |
| SCA8 | 53 | 30 | 3.5 | 2 | 0 | 0 |
| AOA2 | 42 | 27 | 22 | 6 | 2.5 | 2 |
| CD, unknown | 57 | 22 | 10 | 4 | 1 | 2 |
| CD, unknown | 65 | 17 | 25 | 6 | 2 | 3 |
| CD, unknown | 89 | 26 | 9.5 | 2 | 0.5 | 4 |
| CD, unknown | 68 | 27 | 23 | 5 | 2 | 1 |
| CD, unknown | 64 | 25 | 8 | 1.5 | 0.5 | 1 |
| CD, unknown | 37 | 27 | 14 | 3 | 1 | 2 |
| CD, unknown | 72 | 27 | 18.5 | 4 | 1.5 | 1 |
| CD, unknown | 64 | 28 | 11 | 3.5 | 0 | 1.5 |
| CD, unknown | 54 | 28 | 8.5 | 4.5 | 0.5 | 3 |
| CD, unknown | 65 | 25 | 9.5 | 2.5 | 1 | 0 |
| CD, unknown | 62 | 30 | 12 | 5.5 | 1 | 1 |
One participant, shown in bold, was excluded because of a MoCA score indicative of moderate cognitive impairment.
Data from one control participant was excluded from condition 4 because of equipment failure during data collection. The final sample size was based on typical sample sizes in previous studies (Morton and Bastian, 2006; Kurtzer et al., 2013; Parrell et al., 2017; Zimmet et al., 2020). Given the final sample sizes of 22 and 15 for the CD and control groups, respectively, we have a power of 0.75 to detect a large between-group effect (d = 0.8). The larger sample for the CD group has a power of 0.75 to detect a medium effect (d = 0.5) for the within-group correlations. Note that we tested a larger group of individuals in the CD group to have sufficient power to examine predicted correlations within this group of the behavioral measures and clinical symptoms and, more important, a critical prediction concerning the relationship of feedforward and feedback control.
Experimental design
Each participant completed four conditions in a single experimental session that lasted ∼1 h, including breaks of ∼5 min between each condition. Feedforward and feedback control during reaching were assessed in conditions 1 and 2, respectively; similarly, feedforward and feedback control during speech were assessed in conditions 3 and 4, respectively. A schematic of each of the four conditions is shown in Figure 1. The same order of the four conditions was used for each participant. Although this approach introduces order confounds, we opted to keep the order fixed, given that (1) this is preferable for correlational analyses across tasks, as counterbalancing task order introduces additional between-participant variance to these analyses (Goodhew and Edwards, 2019), and (2) the sample size was insufficient to assess effects of task order in a counterbalanced design. Stimulus presentation and data collection was controlled with MATLAB for all conditions.
Figure 1.
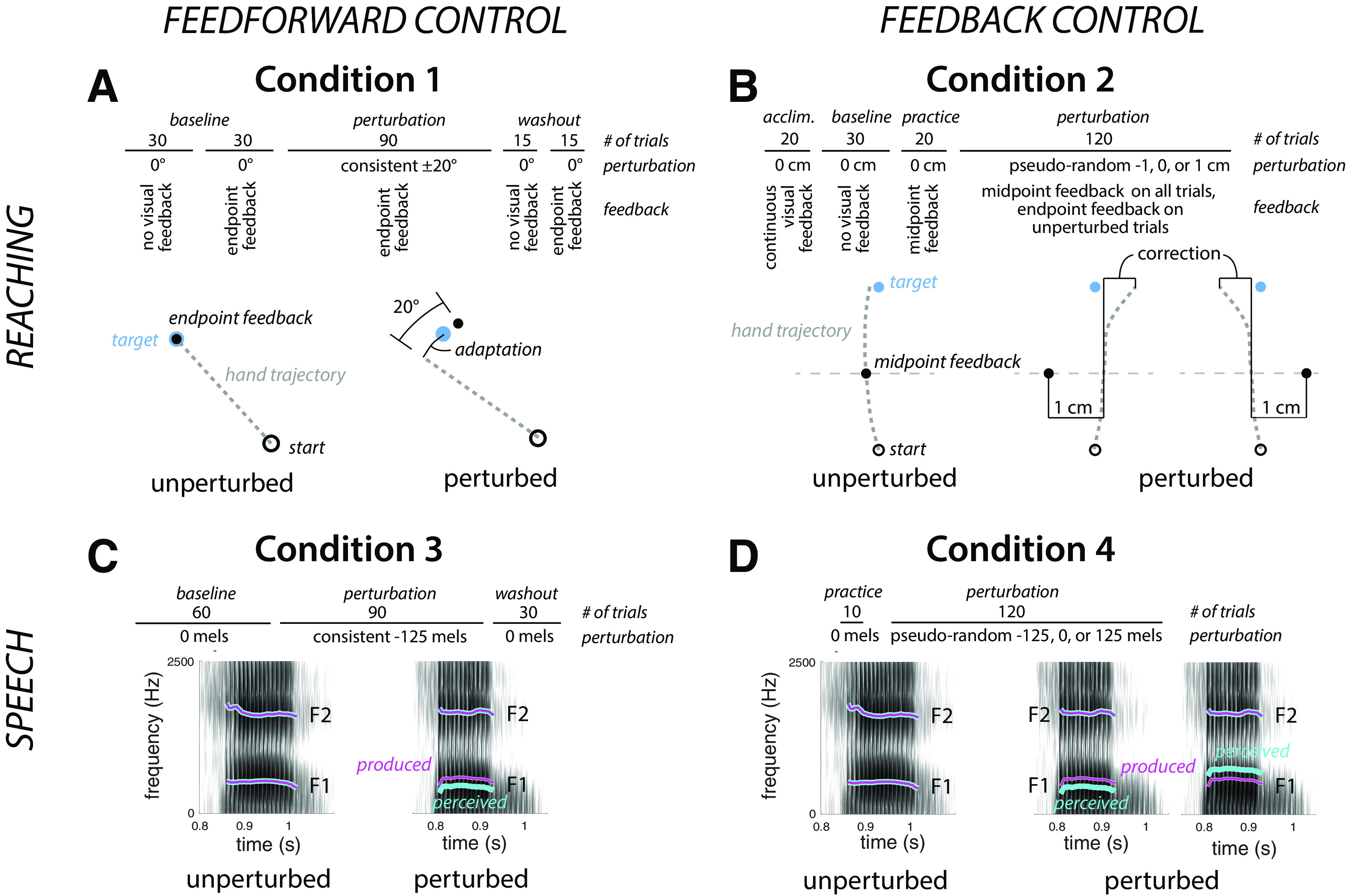
Experimental design. A–D, Top, shows the structure of the experimental session, and bottom, the perturbation. A, Condition 1, feedforward control in reaching, Participants made 8 cm center-out reaches to targets (blue dot) with endpoint feedback. During the perturbation phase, the location of the feedback cursor was rotated by 20° from actual hand position (direction cross-balanced across participants). The dashed line represents the unseen hand movement. B, Condition 2, feedback control in reaching. Participants made 16 cm reaches to a target (blue dot). During the perturbation phase, visual feedback about hand position (black dot) was given at reach midpoint with the feedback shifted by −1, 0, or 1 cm, with the shift on each trial determined in a pseudorandom manner. The thin gray line depicts the location of reach midpoint but was not visible to the participant. C, Condition 3, feedforward control in speech. Participants spoke a single word on each trial, hearing playback of their speech over headphones with minimal delay. During the perturbation phase, feedback of the first formant during the vocalic portion of the utterance was perturbed by imposition of a −125 mel shift (cyan trace superimposed on the spectrogram). D, Condition 4, feedback control in speech. Participants spoke a single word on each trial. During the perturbation phase, auditory perturbations (cyan trace) of −125, 0, or 125 mels were pseudorandomly applied to the first formant during the vocalic portion of the utterance.
Condition 1: Feedforward control in reaching
Participants were seated in front of a 53.2 × 20 cm LCD screen (ASUS) that was horizontally encased in a table frame mounted 27 cm above a 49.3 × 32.7 cm digitizing tablet (Intuos 4XL, Wacom). Participants held a modified air hockey paddle and made reaching movements by sliding the paddle across the table. The position of a stylus embedded in the paddle was recorded by the tablet at 200 Hz. Feedback, when available, was presented in the form of a cursor on the LCD screen. Participants' view of their hand was blocked by the LCD screen. To further limit vision of the upper arm, the experiment was conducted in a darkened room. The experiment was controlled with Psychtoolbox (Brainard, 1997; Pelli, 1997; Kleiner et al., 2007).
Participants made center-out reaches, moving to targets located at a radial distance of 8 cm from the center of the workspace. The start location was marked by a white ring (6 mm diameter). On each trial, the participant moved the digitizing stylus to position the hand within the start location. Visual feedback about the stylus position was provided by a small (3.5 mm diameter) white circle. After the participant had maintained the stylus position within the start location for 500 ms, one of three equally spaced targets (6 mm diameter) appeared (0, 120, or 240°). The participant was instructed to reach, attempting to slice through the target. The instructions emphasized that the movements should be made quickly and, to minimize demands on endpoint (radial) accuracy, should terminate beyond the target location. Reaction time was not emphasized; the movement could be initiated at any time after the presentation of the target.
Visual feedback about the movement was limited to end point feedback, eliminating the opportunity for visually guided corrections. The feedback cursor disappeared at movement onset and reappeared when the radial distance of the hand movement reached 8 cm. The cursor remained visible for 50 ms, and then was blanked. If participants reached the 8 cm threshold in ≤500 ms, a knocking sound was played, indicating that the movement was fast enough and far enough. If the movement duration was >500 ms, participants heard a recording of the words “too slow.” During the return movement to the start location, visual feedback was withheld until the stylus was within 3 cm of the start position.
Condition 1 consisted of the five phases. (1) A no feedback baseline phase of 30 trials measured movement variability in the absence of visual feedback. (2) A baseline phase of 30 trials had veridical end point feedback of the cursor. (3) A perturbation phase of 90 trials in which the location of the cursor at movement end point was rotated 20° from the true position. The 20° rotation was chosen to limit awareness of the perturbation and minimize the use of explicit reaiming (Bond and Taylor, 2015; Morehead et al., 2015; Werner et al., 2015), and the perturbation was either clockwise or counterclockwise, counterbalanced across participants. (4) An aftereffect phase of 15 trials had was no visual feedback. (5) A washout phase of 15 trials had veridical end point feedback.
The hand angle for each trial was measured as the angle between a line connecting the start location and the hand position at the time of peak radial velocity, relative to the line connecting the start and target locations. To remove intrinsic biases in reaching, the mean hand angle during the baseline phase was subtracted from the heading angle for each trial. We focus on two measures of adaptation, the mean of the last 15 trials of the perturbation phase (asymptote) and the mean of the 15 trials in the aftereffect phase. We additionally measured reaction time (time from target appearance to movement onset) and movement time (time from movement onset to target), as well as the percentage of trials where any part of the cursor representing hand position overlapped with the target (hits).
Condition 2: Feedback control in reaching
The experimental apparatus was identical to that of experiment 1. On each trial, the white ring indicating the start location appeared near the bottom edge of the screen at the horizontal meridian. After this position was maintained for 500 ms, the target appeared at a fixed position, 16 cm in front of the start location. The longer distance was used so that feedback could be presented at the midpoint of the movement, providing sufficient distance (and time) for an online correction.
Condition 2 consisted of five phases. (1) An acclimation phase of 20 trials had continuous veridical feedback. (2) A baseline phase of 30 trials had no visual feedback, either during the movement or at the target distance. (3) A practice phase of 20 trials introduced the method for providing limited visual feedback in which the cursor appeared twice on each trial, first when the radial amplitude of the stylus was 8 cm from the start location (midpoint, duration = 100 ms), and second when the radial amplitude reached 16 cm (end point, duration = 50 ms). Participants were instructed to use what they saw midway through the reach to get as close as possible to the target. (4) A perturbation phase consisted of 120 trials with midpoint feedback. On 50% of the trials, the midpoint feedback was given at the true location of the stylus, and on the remaining 50% of the trials, the midpoint feedback was shifted 1 cm to the left of the stylus position or 1 cm to the right (25% each). Each cluster of four reaches consisted of two unperturbed trials and one trial with the leftward and rightward perturbation in a randomized order. End point feedback was not provided on perturbed trials to minimize learning effects (i.e., anticipatory effects based on prior responses to the perturbation). End point feedback was provided on the unperturbed trials to help participants remain calibrated to the target reaching location. (5) A final phase of 20 trials had no visual feedback. Data from this last phase were not analyzed further.
As in condition 1, participants were instructed to slice through the target. The movement time criterion was increased to 1200 ms. The extra time was used to compensate for the larger amplitude movements and to encourage participants to adopt a movement speed that allowed them time to use the midpoint feedback to make an online correction (if warranted). The knocking sound was played if the target amplitude was reached within 1200 ms, and if the movement duration was between 400 and 1200 ms, the target circle turned green. If the movement was <400 ms, the words “too fast” appeared on the screen. If the movement duration was longer than 1200 ms, the target circle turned red and a recording of the words “too slow” was played.
The focus here was on the online corrections made in response to the horizontally displaced midpoint feedback, that is, the horizontal position of the hand relative to the target at the target distance. However, the raw horizontal displacement would also reflect the horizontal position of the hand at reach midpoint, which may vary between trials. To account for this, we fit the trial-by-trial data of each participant with a linear model that predicted the final horizontal position of the hand on each trial from the horizontal hand position at reach midpoint, the visual perturbation (treated as a categorical variable and coded using separate dummy predictors for each direction), and the interaction between midpoint hand position and the perturbation as follows:
xtarget ∼ β0 + β1xmidpoint + β2perturbation(L) + β3perturbation(R) + β4xmidpoint:perturbation(L) + β5xmidpoint:perturbation(R).
This model allows us to estimate two dependent measures of feedback-based corrections: (1) the magnitude of the correction for the perturbation (direction effect estimates, β2, β3) and (2) corrections for self-produced variability (the midpoint position estimate, β1, and an adjustment in the presence of perturbations, β4, β5). For statistical analysis, the sign of β2 values were flipped so that negative values always reflected a compensatory response to the perturbation, regardless of perturbation direction. Additionally, this method allows us to estimate the effect of the correction for the visual perturbation while accounting for any individual differences in the bias of the overall reach trajectory, an effect captured by the intercept term (β0), which reflects the horizontal displacement relative to the target position for unperturbed reaches. We measured movement time, reaction time, and proportion of trials where the cursor hit the target as for condition 1. Hit proportion was calculated only for unperturbed trials.
Condition 3: Feedforward control in speech
Participants were again seated in front of the horizontally aligned monitor. Participants wore a head-mounted microphone (AKG C520) and close-backed, over-the-ear headphones (Beyerdynamic DT 770). On each trial, participants spoke the word “head” after it appeared on the monitor. The utterance was digitized through a Scarlett 2i2 sound card and processed with Audapter (Cai et al., 2008; Tourville et al., 2013) to synthesize a sound for playback over the headphones. The recording, processing, and playback occurred in near real time (∼20 ms delay). The intensity of the synthesized feedback was set to ∼80 dB based on each participant's comfortable speaking volume, mixed with speech-shaped noise at ∼60 dB to mask air- or bone-conducted direct feedback of the utterance. The actual intensity level of the feedback varied with changes in the intensity of participants' speech.
There were three phases in condition 3. For each phase, the word “head” remained visible for 2.5 s on each trial, and the trials were separated by 1.1–1.3 s (randomly jittered). (1) A baseline phase of 60 trials had speech feedback resynthesized through Audapter with no auditory perturbation applied. (2) A perturbation phase of 100 trials during which a constant shift of −125 mels was applied to the first vowel formant (F1) throughout the utterance, shifting the F1 value of “head” toward that of “hid” (mels are a perceptually calibrated unit of frequency where each step is perceived as equivalently distinct across all frequencies; Stevens et al., 1937). (3) A washout phase of 30 trials had no auditory perturbation.
Vowel onset and offset were labeled for each trial using a semiautomated procedure. First, automatic labels were generated by identifying where the speech amplitude first crossed (onset) or fell below (offset) a participant-specific amplitude value. The automatic labels were then visually inspected and corrected when the waveform and spectrogram indicated that the automatic markings were inappropriate. A semiautomated procedure was used to track the formants during the vocalic phase of the utterance using participant-specific vales for linear predictive coding (LPC) order and pre-emphasis. Formant tracking was performed with Praat (Boersma and Weenink, 2021). Using the Wave Runner software package (Niziolek and Houde, 2015), the tracks were visually inspected, and when they did not align with visible formants on the speech spectrogram, the formant tracking parameters (pre-emphasis, LPC order) were modified.
The primary outcome measure was adaptation of the vocalic portion of the utterances across trials. For each trial, we calculated the average F1 value from 50 to 100 ms after vowel onset. This window avoids the transitional phase of the formant during the word initial consonant (initial 50 ms) as well as any online compensatory response to the perturbation, which typically begins later than 100 ms after vowel onset (Tourville et al., 2008; Cai et al., 2012; Parrell et al., 2017). To facilitate comparisons across participants, the F1 values were normalized with respect to the average F1 value taken over the 50–100 ms window during the second half of the baseline phase (trials 31–60). We chose to use the second half of the baseline phase to allow participants time to acclimate to the processed auditory feedback.
Condition 4: Feedback control in speech
The stimulus set consisted of four words—dead, fed, said, and shed—selected because they share the same vowel /ε/. The condition began with a calibration phase designed to shape the participant's speaking rate so that the produced vowel duration would be between 300 and 500 ms. This criterion was important to ensure there would be sufficient time for feedback-based corrections, shown in previous work to have a latency of ∼150 ms (Tourville et al., 2008; Cai et al., 2012; Parrell et al., 2017). On each trial, one of the four words was displayed. After each utterance, the automated estimate of the vowel duration in milliseconds was displayed on the monitor. This procedure was repeated for 10 trials. If the duration fell outside the 300–500 ms window on >2 of these 10 trials, the procedure was repeated for 10 more trials. When the utterances fell within the criterion window for at least 8 of the 10 trials, the main experiment began.
Condition 4 had two phases: (1) a practice phase of 10 trials with veridical auditory feedback and (2) a perturbation phase of 120 trials. During the perturbation phase, participants heard veridical feedback on 50% of trials, a +125 mel shift of F1 (moving the vowel toward that in “had”) on 25% of trials, and a −125 mel shift of F1 (moving the vowel toward that in “hid”) on the remaining 25% of trials. Each group of four trials consisted of two unperturbed trials and one trial each of positive and negative F1 perturbations, randomly ordered. Stimuli were presented in a random order (selection with replacement), with the constraint that the total number of each stimulus word was as equal as possible across the experimental condition.
Vowels were tracked as in experiment 3. The primary outcome measure was the online correction to the perturbation, calculated following standard approaches (Cai et al., 2012; Niziolek and Guenther, 2013; Parrell et al., 2017; Daliri et al., 2020). First, an average baseline F1 trajectory was calculated for each stimulus word from the unperturbed productions of that word. Second, the F1 trajectory from each perturbed trial was normalized by subtracting the appropriate word-specific average baseline F1 trajectory, giving a normalized F1 response for each perturbed trial. Trajectories from trials with upward and downward F1 perturbations were separately averaged to generate an average F1 response in each direction. To generate a composite feedback response, the sign of the average response to the upward perturbation was flipped so that positive values always reflected a compensatory response to the perturbation, regardless of perturbation direction. Note that because of the normalization process (and lack of an explicit target), this differs slightly from the approach in experiment 2, where midpoint variability had to be accounted for. To quantify the magnitude of the compensatory response, the mean value of each average F1 response trajectory between 200 and 300 ms after vowel onset was calculated. This window begins well after the expected 150 ms latency of the compensatory response (Tourville et al., 2008; Cai et al., 2012; Parrell et al., 2017).
Statistical analysis
Statistical analysis for all conditions was conducted in R (R Core Team, 2020) using mixed ANOVAs or Welch's two-sample t tests. When necessary, post hoc tests were conducted with Tukey's HSD correction. Where appropriate, one-sample t tests with Holm–Bonferroni corrections were used to determine whether the responses for each group differed significantly from zero. Means are reported with SDs. Effect sizes are given as Hedges' g or partial η2.
For conditions 1 and 3, a mixed ANOVA was used that included group (CD vs control) and phase (end of perturbation and aftereffects), as well as the interaction between the two factors. Similarly, for condition 4, the mixed ANOVA included group (CD vs control) and perturbation direction (up and down), as well as the interaction between the two.
For condition 2, separate ANOVAs were used to evaluate differences between the CD and control group in (1) the magnitude of the correction in response to the perturbation and (2) the magnitude of correction in response to self-produced variability. These were conducted as second-level analyses on the β coefficients estimated in the first-level analyses that were conducted within each individual (see above). The dependent variable for the perturbation correction model was the direction effect coefficient, estimated separately for each perturbation direction (β2, β3). In addition to the perturbation direction factor (left vs right), this model included a group factor as well as the interaction between perturbation direction and group. For the variability correction, the dependent variable was the midpoint coefficient representing the magnitude of correction for variability at reach midpoint in unperturbed (β1), left (β1+β4), and right (β1+β5) conditions. In addition to the main variable of interest, group, this model also included perturbation condition (no perturbation, left, right). Welch's t tests were used to compare the CD and control groups on the reaction time, movement time, and hit percentage in unperturbed trials.
To assess the relationship between feedback and feedforward control, we conducted a set of planned correlational analyses using data from pairs of conditions. One set of correlations compared the measures of feedback and feedforward control within each motor domain (i.e., one correlation between the two reaching tasks and a second between the two speech tasks). We also performed a second set of correlations of similar forms of control across the motor domains (i.e., one correlation of the feedforward measures from the reaching and speech tasks and a second correlation of the feedback measures from the reaching and speech tasks). Because the primary aim of these analyses is to investigate the possibility that changes in motor behavior because of cerebellar degeneration are correlated across sensory domains and control systems, we limited these correlational analyses to the data from the CD group. We also examined the extent to which our experimental measures of feedforward and feedback control in the CD group were related to ataxia symptom severity, as measured by SARA speech and upper limb subscores.
Results
Condition 1: Feedforward control in reaching
In the baseline phase with visual feedback, the CD group was substantially more variable than controls (SD of 6.2 ± 2.0° vs 4.0 ± 0.8°, t(31.6) = 4.6 p < 0.0001, g = −0.89) and less accurate (percentage of targets hit, 67 ± 25% vs 87 ± 15%, t(35.8) = −3.0, p = 0.005, g = 1.28). The CD group also produced slower movements than controls (347 ± 148 ms vs 264 ± 61 ms, t(31.6) = 2.4, p = 0.02, g = 0.67) and had longer reaction times (544 ± 116 ms vs 442 ± 58 ms, t(34.0) = 3.6, p = 0.001, g = 1.02).
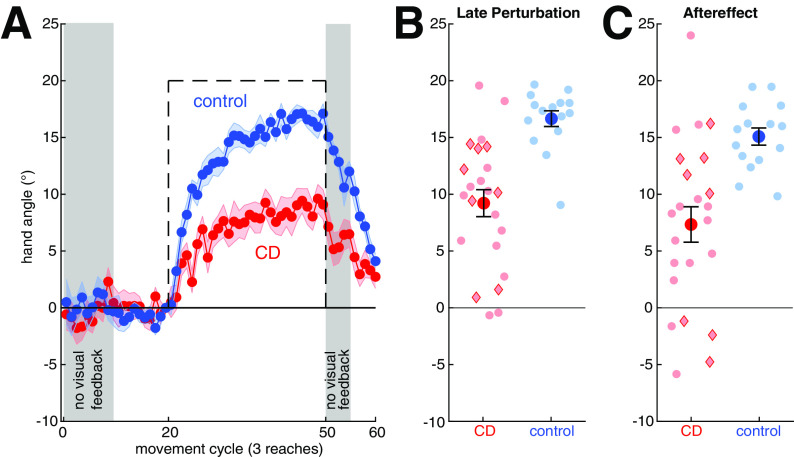
Our principle outcome measure, adaptation, was operationalized as the change in heading angle following the introduction of a 20° rotation of the feedback cursor. As can be seen in Figure 2, the perturbation induced a gradual change in the heading angle, with the functions appearing to reach or approach asymptote by the end of the perturbation trials. Adaptation in the control participants compensated for ∼83% of the perturbation; the comparable figure for the CD participants was only 45%. A decline in adaptation is visible throughout the aftereffect phase in which there was no visual feedback and the following washout phase in which veridical feedback was reintroduced. In Figure 2 and subsequent figures, red diamonds are used to indicate individuals with SCA6 and SCA8, two genetic subtypes in which the degeneration is largely confined to the cerebellum proper; red circles indicate CD individuals with other diagnoses. As can be seen, the relatively pure cerebellar participants fall within a distribution similar to that of the other CD participants.
Figure 2.
In the visuomotor rotation task, the cerebellar degeneration (CD) group reached a lower level of adaptation and exhibited a smaller aftereffect relative to the control group. A, Hand angle over the course of the experimental condition. Means and SDs are shown for control participants (blue) and individuals with cerebellar degeneration (red). Visual feedback was withheld in the shaded phases. The perturbation is shown with a dashed black line. B, C, Individual data (semitransparent dots) and group means ± SE for the CD and control participants during the last 15 reaches of the perturbation phase (B) and 15-trial aftereffect phase in which the visual feedback was withheld (C). Red diamonds indicate individuals with SCA6 and SCA8; red circles indicate the other CD participants.
Although we did not conduct any postsession interviews, we assume that adaptation was implicit with negligible contributions from strategic aiming (e.g., in the opposite direction of the perturbation). Not only does the size of the perturbation fall within a range in which learning is thought to be driven largely by implicit adaptation (Bond and Taylor, 2015; Morehead et al., 2015; Werner et al., 2015) but there is only a slight decrease in hand angle at the start of the aftereffect block. Moreover, we did not observe a sustained increase in reaction times following the introduction of the perturbation, a cardinal signature of aiming (McDougle and Taylor, 2019), in our data (comparing first 10 trials of the perturbation to the baseline, p > 0.94 for both groups).
Statistically, we first confirmed that the sign-dependent changes in heading angle were significantly different from zero, the signature of adaptation. When measured during the final trials of the perturbation block, the heading angle values were different from zero for both the control (16.6 ± 2.7°, t(14) = 24.1, p < 0.0001, g = 5.90) and CD groups (9.2 ± 5.7°, t(22) = 7.8, p < 0.0001, g = 1.56). The adapted response persisted into the aftereffect block (control: 15.1 ± 2.9°, t(14) = 20.0, p < 0.0001, g = 4.87; CD: 7.3 ± 7.5°, t(22) = 4.7, p < 0.0001, g = 0.95), although the value was smaller than observed at the end of the perturbation block for both groups (F(1,36) = 7.4, p = 0.01, η2 = 0.17). Across both the perturbation and aftereffect phases, adaptation was significantly greater in the control group (F(1,36) = 20.1, p < 0.0001, η2 = 0.36) and the Group × Phase interaction was not significant (F(1,36) = 0.05, p = 0.82, η2 = 0.001). These results are in accord with previous results (Krakauer et al., 2019) in showing that adaptation of feedforward control for reaching is impaired in individuals with CD.
Condition 2: Feedback control during reaching
As in condition 1, there were some kinematic differences between the two groups. The most salient group difference was in movement accuracy: control participants hit the target more often than the CD participants on the unperturbed trials (control: 85 ± 10%, CD: 70 ± 24%, t(31.9) = 2.7, p = 0.01, g = 0.75). There were small differences between the groups in reach curvature, with controls showing a slight leftward shift from midpoint to target on unperturbed trials and the CD group showing a slight rightward shift on these trials (control: −0.17 ± 0.193 cm, CD: 0.13 ± 0.52 cm, t(30.2) = 2.5, p = 0.02, g = 0.69, Fig. 3E). Reaction times were also slower in the CD group (control: 452 ± 79 ms, CD: 525 ± 138 ms, t(35.5) = 2.08, p = 0.045, g = 0.61), whereas movement time was similar between groups (control: 717 ± 114 ms, CD: 722 ± 114 ms, t(30.1) = 0.14, p = 0.89, g = 0.05).
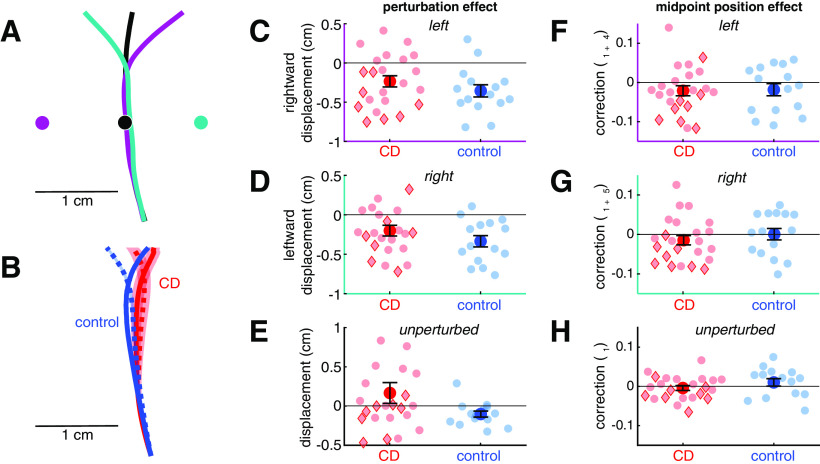
Figure 3.
In the reaching feedback control task, both the cerebellar degeneration (CD) and control groups showed a robust on-line correction to perturbed visual feedback presented at the midpoint of the reach, with no group differences. A, Example data from one control participant showing average reach trajectories for unperturbed reaches (black), reaches with a 1 cm rightward visual perturbation (teal), and reaches with a 1 cm leftward visual perturbation (purple). Dots in corresponding colors show the location of the visual feedback. The corrective response only partially corrects for the perturbation. B, Reach trajectories for the CD group (red) and control group (blue) aligned to the same starting point, showing mean responses to leftward perturbations (solid lines) and rightward perturbations (dashed lines). Shaded areas represent SE across participants. C, D, Compensatory response to the feedback perturbation. E, Estimate of the change in horizontal hand position from reach midpoint on unperturbed trials. F–H, Estimates of the effect of true hand position at reach midpoint on the compensatory response for leftward (F), rightward (G), and unperturbed (H) trials. Across all plots, compensatory responses have negative values. Semitransparent dots represent individuals. Red diamonds indicate individuals with SCA6 and SCA8; red circles indicate the other CD participants.
Our principle dependent measure, online corrective responses, was operationalized as a lateral shift in the trajectory in response to perturbed feedback that was presented at the midpoint of the movement, with the direction of the perturbation randomized across trials. As can be seen in the data from a representative control participant (Fig. 3A), the hand trajectory deviated in the opposite direction of the perturbation, a signature of a feedback-based response. The lateral shift accounted for ∼33% of the perturbation magnitude in the control group and 21% in the ataxic group (Fig. 3C,D). These corrective feedback responses were significantly different from zero in both groups in response to the leftward perturbation (CD: −0.21 ± 0.34 cm, t(22) = 3.3, p = 0.003, g = 0.67; control: −0.35 ± 0.30 cm, t(14) = 4.6, p = 0.0005, g = 1.12) and rightward perturbation (CD: −0.20 ± 0.32 cm, t(22) = 2.9, p = 0.007 g = 0.60; control: −0.34 ± 0.28 cm, t(14) = 4.7, p = 0.0003, g = 1.15). Although the magnitude of the feedback-based response was smaller for the CD group, this difference was not significant (F(1,36) = 2.8, p = 0.1, η2 = 0.07). There was no difference between the responses to the two perturbation directions (F(1,36) = 0.16, p = 0.69, η2 = 0.004) and the Group × Direction interaction was not significant (F(1,36) = 0.009, p = 0.93, η2 = 0.0002). These results indicate that feedback-based corrective responses to visual perturbations are not enhanced in individuals with CD; indeed, as a group the trend was for attenuated feedback responses.
In a second measure of feedback control, we looked at adjustments in the reach trajectory in response to motor variability (estimated as the change in horizontal position from reach midpoint to endpoint unrelated to the visual perturbation; model parameters β1, β1+β4, and β1+β5). Although both groups showed a slight shift in trajectory in the compensatory direction in response to the position of the cursor at reach midpoint (Fig. 3F–H), this shift was not significantly different from zero after correction for multiple comparisons (CD: −0.010 ± 0.035, p = 0.05, g = −0.30; control: −0.009 ± 0.042, p = 0.24, g = −0.21). There was no difference between the two groups (F(1,36) = 0.01, p = 0.91, η2 = 0.0004), nor any interaction between group and direction (F(2,72) = 2.4, p = 0.10, η2 = 0.06). However, there was a significant effect of perturbation direction (F(2,72) = 7.3, p = 0.001, η2 = 0.17) such that the shift in trajectory was greater in the presence of leftward perturbations (−0.021 ± 0.0332) compared with trials with no perturbation (0.001 ± 0.038; p = 0.008, g = 0.62). There was a similar trend in trials with rightward perturbations (−0.010 ± 0.036), but this did not reach significance (p = 0.15, g = 0.33). In sum, there is little evidence that either group corrected for self-produced variability at reach midpoint.
Condition 3: Feedforward control in speech
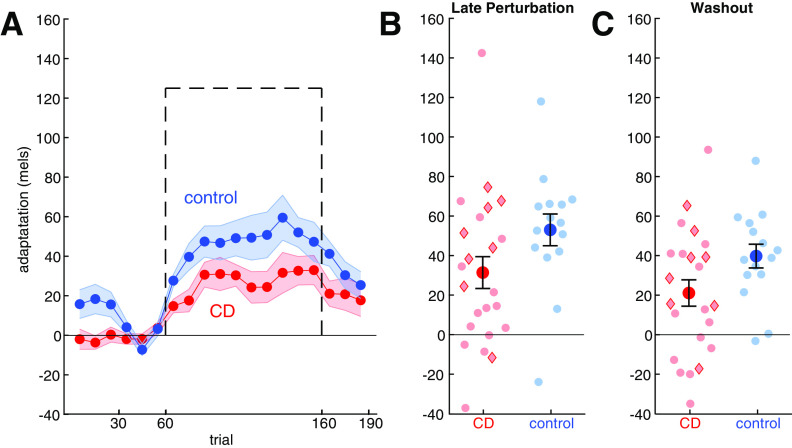
Adaptation was operationalized as the change in F1 in response to a −125 mel F1 shift in the feedback heard by participants, introduced via the real-time resynthesis of their utterances. As can be seen in Figure 4A, this auditory perturbation caused a gradual change in F1 that opposed the perturbation. At asymptote, the adaptive response had reached ∼40% of the perturbation in the control group and 25% in the CD group. The lower degree of compensation relative to reaching is consistent with previous studies (Houde and Jordan, 1998; Purcell and Munhall, 2006; Parrell et al., 2017). Note that unlike condition 1, postperturbation trials (washout phase) always included veridical feedback; we did not include a no-feedback phase as masking auditory feedback with noise leads to substantial changes in speech (Lombard, 1911; Summers et al., 1988).
Figure 4.
In the speech adaptation task, the cerebellar degeneration (CD) group adapted less than controls. A, Change in first formant over the course of the experimental condition. Means and SEs are shown for control (blue) and CD participants (red). For each participant, the change was calculated relative to the mean F1 value during the second half of the baseline phase, with positive values corresponding to changes in the opposite direction of perturbation. The perturbation is shown with a dashed black line (sign flipped). B, C, Group means ± SE (solid dots) and individual data points (semitransparent dots) of adaptive response during the last 10 trials of the perturbation phase and first 10 trials of the washout phase. Red diamonds indicate individuals with SCA6 and SCA8; red circles indicate the other CD participants.
We first confirmed that both groups adapted during the perturbation phase (CD: 31 ± 39 mels, t(1,22) = 3.9, p = 0.0007, g = 0.79; control: 53 ± 31 mels, t(1,14) = 6.6, p < 0.0001, g = 1.61; Fig. 4B) and maintained their adapted state during the initial part of the washout phase (CD: 21 ± 32 mels, t(1,22) = 3.1, p = 0.005, g = 0.64; control: 40 ± 23 mels, t(1,14) = 6.6, p < 0.0001, g = 1.62; Fig. 4C). The CD group exhibited less adaptation than the control participants (F(1,36) = 4.7, p = 0.036, η2 = 0.12), and this difference was similar in both phases (Group × Phase interaction: F(1,36) = 0.06, p = 0.80, η2 = 0.002). In sum, we find that sensorimotor adaptation in speech is impaired in individuals with CD, similar to the impairment observed for reach adaptation in condition 1 and consistent with previous findings (Parrell et al., 2017).
Condition 4: Feedback control in speech
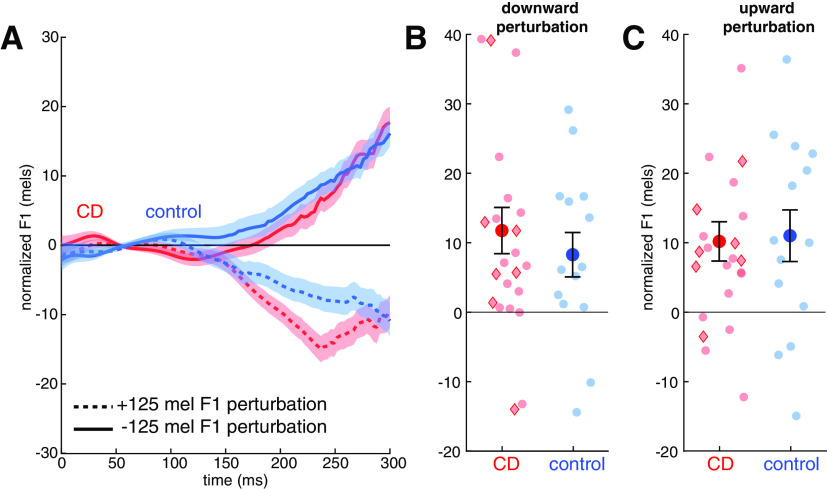
Online corrective responses for speech were operationalized as the change in F1 during the time window from 200 to 300 ms after vowel onset in response to an upward or downward perturbation of F1, randomized across trials. The data are plotted relative to F1 values measured on unperturbed trials. As can be seen in Figure 5A, the nonpredictable auditory perturbations resulted in compensatory responses that opposed the perturbation in both groups. The magnitude of the corrective response (7.6% in controls, 9.2% in the ataxic group) was similar to that typically observed in response to auditory perturbations of speech and much lower than observed for the unpredictable perturbations during reaching used in condition 2.
Figure 5.
In the speech feedback control task, both the cerebellar degeneration (CD) group and controls corrected for formant perturbations in both directions, with no difference between the two groups. A, Normalized formant trajectories in response to a downward F1 perturbation (solid lines) or upward perturbation (dotted lines) in the control (blue) and CD (red) groups. Shaded area indicates SE. Data are normalized to the formant trajectories produced in trials with no perturbation. B, C, Magnitude of F1 values in trials with a downward (B) or upward (C) perturbation, averaged over the time window spanning from 200 to 300 ms following onset of vocalic portion of the utterances. Compensatory responses are expressed as positive values in both cases. Group means ± SE are shown as solid dots; Semitransparent dots represent individuals. Red diamonds indicate individuals with SCA6 and SCA8; red circles indicate the other CD participants.
The change on perturbed trials was significantly larger than zero in response to upward and downward perturbations in the CD group (up: 10.2 ± 13.6 mels, t(1,22) = 3.6, p = 0.002, g = 0.73; down: 11.8 ± 16.0 mels, t(1,22) = 3.5, p = 0.002, g = 0.71) and control group (up: 11.0 ± 14.4 mels, t(1,13) = 2.9, p = 0.02, g = 0.72; down: 8.2 ± 12.4 mels, t(1,13) = 2.9, p = 0.02, g = 0.72; Fig. 5B,C). Although the mean values were larger in the CD group, and individuals in this group showed the largest compensatory response, the difference between the two groups was not significant (F(1,35) = 0.35, p = 0.56, η2 = 0.009). There were no differences between the responses to the two perturbation directions (F(1,35) = 0.1, p = 0.74, η2 = 0.003) and the Group × Direction interaction was not significant (F(1,35) = 0.07, p = 0.80, η2 = 0.002). These results suggest feedback gains for auditory perturbations in speech are similar in individuals with CD and healthy controls, consistent with the reaching results in experiment 2. Of note, the null effects here are inconsistent with the results from previous studies involving speech articulation (Parrell et al., 2017) and vocal pitch production (Houde et al., 2019; Li et al., 2019) in which individuals with CD were found to show an enhanced feedback response.
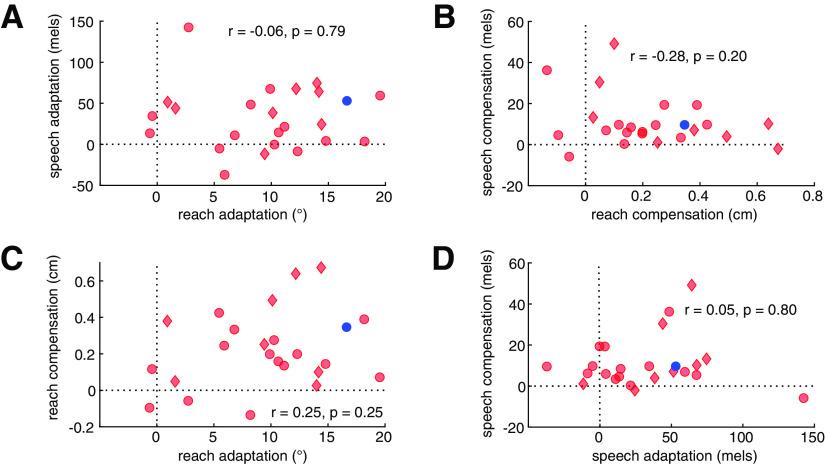
Feedforward and feedback control within and across motor domains
By testing each participant in all four conditions, we can compare the measures of feedback and feedforward control within and across task domains. Because our focus in this analysis is how deficits in these domains may be correlated in individuals with cerebellar degeneration, we limit this analysis to the 22 individuals with CD.
The between-domain comparisons assess the similarity of impairment (or lack thereof) between reaching and speech. Although the ataxic group adapted less than the controls at the group level, there was no significant correlation within the ataxic group between the magnitude of adaptation between speech and reaching (Fig. 6A). Similarly, the magnitude of feedback-based compensation (Fig. 6B) was unrelated across motor domains.
Figure 6.
Correlational analyses of feedforward adaptation and feedback-based compensation in individuals with cerebellar degeneration (CD). A–D, Within the CD group, there were no correlations in behavioral measures obtained across (A, B) or within (C, D) motor domain. Plots show individual CD participants as red symbols (red diamonds, SCA6 and SCA8; red circles, other CD participants) and the mean of the control participants as a blue dot. To simplify interpretation, the sign of compensation in reaching has been flipped so that larger positive values reflect greater compensation, as for all other measures. A, Adaptation in reaching and speech. B, Compensation in reaching and speech. C, Adaptation and compensation in reaching. D, Adaptation and compensation in speech.
The within-domain correlations provide a test of the hypothesis that feedback gains may increase in response to impaired feedforward control. In this case, we would expect a negative correlation between these measures so that individuals who are more impaired in feedforward control should be more likely to exhibit a greater reliance on feedback control. This relationship might hold even if there is no overall increase in compensatory responses when comparing the CD and control groups. Contrary to this prediction, the relationship between feedforward adaptation and feedback-based compensation was not significant in either reaching (Fig. 6C) or speech (Fig. 6D).
We additionally tested whether any of the measures of feedforward and feedback control correlated with ataxia severity as assessed with the SARA. Neither a summary measure of overall upper limb ataxia nor intentional tremor severity correlated with reach adaptation or compensation (all p > 0.2). Similarly, overall speech impairment was not correlated with speech compensation (r = −0.24, p = 0.26). We did observe a positive correlation between the SARA measure of speech impairment and speech adaptation, with lower levels of adaptation to the auditory perturbation associated with greater speech impairment, although this was not significant after correction for multiple comparisons (r = 0.45, p = 0.03). Participant age was not correlated with either SARA scores in the CD group or the behavioral measures in either the CD group or controls (all p > 0.31).
Discussion
We tested a group of CD participants across a series of tasks to evaluate the impact of cerebellar degeneration on feedforward and feedback control in two motor domains, reaching and speech. Individuals with cerebellar degeneration showed a marked impairment in feedforward control relative to controls, manifested as reduced adaptation in response to a sensory perturbation that remained constant from trial to trial. Feedback control, measured as the on-line response to a variable perturbation, was intact in both task domains.
Multimodal impairment in feedforward control in individuals with cerebellar degeneration
Our principle positive result, that individuals with cerebellar degeneration are impaired in adapting their motor behavior in the presence of sensory prediction errors, is consistent with prior neuropsychological studies involving upper limb control and speech. These results are consistent with the hypothesis that the cerebellum provides a domain-general mechanism for generating sensory predictions and using error information to keep this predictive system well calibrated. We assume this process operates in an automatic and implicit manner. Adaptation in speech is highly likely to be an implicit process (Munhall et al., 2009; Kim et al., 2020; Lametti et al., 2020) as are adaptive responses in reaching in response to perturbations similar to those used in the current study (Bond and Taylor, 2015; Morehead et al., 2015; Werner et al., 2015).
Although adaptation was impaired in both reaching and speech at the group level, we did not observe correlations between the two motor domains. It is possible that the null results in the correlational analyses reflect statistical limitations with our design. Given that we only tested each condition a single time, we do not have estimates of the reliability of our estimates of adaptation and compensation in each domain, and the upper bound for a correlation analysis is set by the reliability of each measure. There is also concern that we lack sufficient power given our sample size of 22 individuals with CD, although Bayes factor values (0.46–0.88) indicate that we have some evidence against the predicted correlations. However, previous work has identified a similar dissociation in individuals with CD between adaptation to dynamic (force field) and kinematic (visuomotor rotation) perturbations during reaching in both behavior and cerebellar localization (Rabe et al., 2009; Donchin et al., 2012). Our results provide further evidence that dissociable regions of the cerebellum are involved in different forms of adaptation. Given existing evidence of speech localization in the cerebellum (Urban et al., 2003; Ackermann, 2008; Peeva et al., 2010; Mariën et al., 2014), we would anticipate that deficits in speech adaptation would be associated with intermediate portions of lobule VI bilaterally, relative to more lateralized regions of lobule V/VI that have been associated with deficits in limb adaptation (Rabe et al., 2009; Donchin et al., 2012).
Future work examining patterns of cerebellar damage in patients will be needed to directly test this hypothesis. A detailed analysis of structural MRIs would also provide the means to assess the contribution of extracerebellar structures to feedforward and feedback control. Given our heterogeneous sample, we expect that the pathology in at least some of the participants with CD extended to extracerebellar structures. Although this qualifies our assumption that the observed deficits are associated with cerebellar dysfunction, we did observe that individuals with genetic variants in which the pathology is relatively restricted to the cerebellum (SCA6 and SCA8) performed qualitatively similarly to the rest of the sample. As such, we think it reasonable to attribute the impairments in feedforward control to the cerebellar pathology.
Intact but not enhanced feedback control in individuals with cerebellar degeneration
Feedback-based corrections for errors were similar in magnitude between individuals with cerebellar degeneration and controls for both speech and reaching. Although this result agrees with estimates of feedback gains in a continuous visuomotor tracking task (Zimmet et al., 2020), the absence of a difference between the CD and control groups on the speech task fails to replicate our previous finding showing an enhanced feedback response (Parrell et al., 2017). Our previous results had led to the hypothesis that the enhanced feedback response reflected a compensatory mechanism to help offset the disruptive effects of impairment in feedforward control. The failure to observe enhanced feedback in the current study in both domains argues against this compensatory hypothesis. The absence of a correlation between the feedforward and feedback measures within both motor domains also argues against a compensatory hypothesis.
There are a few issues to consider in terms of the discrepancy between our results and previous studies as well as the interpretation of the null results regarding the compensatory hypothesis. First, we may have failed to find any changes in feedback control in individuals with CD simply because of sampling issues. Our sample of this population (n = 22) is consistent with, or larger than, many previous studies (Morton and Bastian, 2006; Parrell et al., 2017; Houde et al., 2019; Li et al., 2019; Zimmet et al., 2020), and we were adequately powered to detect relatively large between-group effects (0.75 power to detect d of 0.8). However, the effect size of the expected increase in feedback gains in certain domains, as in our previous work on oral articulation, may be relatively small. Although we did find a numerically larger feedback response for speech in the CD group compared with controls, this difference was smaller than in our previous work (Parrell et al., 2017). Thus, we may simply have been underpowered to detect changes associated with CD.
Second, it may be that increased feedback gains are a secondary, and somewhat sporadic, effect of cerebellar degeneration. The consistent and striking deficit in feedforward control points to inaccurate or attenuated predictive signals. In this case, some individuals may learn to rely more on sensory feedback to help with movement accuracy as a compensatory mechanism for impairments in feedforward control. It may be that our sample did not include enough participants with altered feedback gains to show an effect at the group level. If this is the case, we might still expect to find some evidence that individuals with larger feedback gains have greater impairments in feedforward control, even if there were no group differences. However, we found no evidence of this negative correlation, making this account less likely.
A final potential explanation is that increases in feedback gains are domain specific, whereas impairments in feedforward control are more general, at least at the group level. The strongest example of higher feedback gains in individuals with CD comes from work on pitch control, where the on-line response to a pitch perturbation is roughly twice as large in a CD group compared with a control group (Houde et al., 2019; Li et al., 2019). This increase may result from an inherent reliance on auditory feedback for pitch control, compared with higher reliance on feedforward control for speech and reaching, as evidenced by the fact that pitch control rapidly degenerates after postlingual hearing loss, whereas oral articulation is better maintained (Cowie and Douglas-Cowie, 1983; Lane and Webster, 1991). Thus, cerebellar degeneration may cause increased feedback gains only in those motor domains that are already primarily reliant on sensory-feedback-based control.
These results raise a broader question regarding the role of the cerebellum in feedback control. Traditionally, the cerebellum has been thought to be involved in both feedforward and feedback aspects of motor control (Herrick, 1924; Guenther, 2016). However, our results suggest limited changes in feedback control in individuals with CD. It is possible that feedback control and feedforward control are localized in different cerebellar subregions (Evarts and Thach, 1969; Allen and Tsukahara, 1974) and that cerebellar degeneration in our sample was localized to areas critical for feedforward control. Alternatively, it may be that the cerebellum plays a central role in feedforward control but a more modulatory role in feedback control. Specifically, the cerebellum may be needed to generate appropriate corrective commands in a dynamic context, when the state of the moving body must be estimated; in contrast, it may not be essential under static conditions when the state can be more directly inferred from sensory feedback (Wolpert et al., 1998; Miall et al., 2007). Consistent with this view, individuals with CD produce appropriately scaled changes in precision grip force to discretely presented changes in load but not to continuously varying, but unpredictable, load changes (Nowak et al., 2004; Brandauer et al., 2010). Future work could test these possibilities using MRI imaging coupled with more complex reaching and speech tasks.
Conclusions
Adaptation of feedforward control based on sensory errors is impaired in individuals with cerebellar degeneration in both reach and speech. Contrary to our initial hypothesis and data from vocal pitch control, we found no evidence for increased feedback gains in either domain. However, these results, together with those from a recent study of upper extremity control (Zimmet et al., 2020), motivate further investigation into how feedback gains may be differentially affected by the specific demands of different motor tasks, as well as to determine the variability in feedback control associated with cerebellar dysfunction.
Footnotes
This work was supported by the National Institues of Health Grant R01 DC017091 to B.P. and R.I., Grant R35 NS116883 to R.I., Grant K12 HD055931 to H.E.K, and Core Center Grant U54 HD090256 to the Waisman Center.
The authors declare no competing financial interests.
References
- Ackermann H (2008) Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci 31:265–272. 10.1016/j.tins.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Allen GI, Tsukahara N (1974) Cerebrocerebellar communication systems. Physiol Rev 54:957–1006. 10.1152/physrev.1974.54.4.957 [DOI] [PubMed] [Google Scholar]
- Beppu H, Nagaoka M, Tanaka R (1987) Analysis of cerebellar motor disorders by visually- guided elbow tracking movement 2. Contribution of the visual cues on slow ramp pursuit. Brain 110:1–18. 10.1093/brain/110.1.1 [DOI] [PubMed] [Google Scholar]
- Bond KM, Taylor JA (2015) Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J Neurophysiol 113:3836–3849. 10.1152/jn.00009.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, Weenink D (2021) Praat: doing phonetics by computer [Computer program]. Version 6.0.46. [Google Scholar]
- Brainard DH (1997) The psychophysics toolbox. Spat Vis 10:433–436. [PubMed] [Google Scholar]
- Brandauer B, Timmann D, Häusler A, Hermsdörfer J (2010) Influences of load characteristics on impaired control of grip forces in patients with cerebellar damage. J Neurophysiol 103:698–708. 10.1152/jn.00337.2009 [DOI] [PubMed] [Google Scholar]
- Cai S, Boucek M, Ghosh S, Guenther FH, Perkell J (2008) A system for online dynamic perturbation of formant trajectories and results from perturbations of the mandarin triphthong/iau/. Paper presented at the Eighth International Seminar on Speech Production, Strasbourg, France, December. [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Tiede MK, Guenther FH, Perkell JS (2012) Weak responses to auditory feedback perturbation during articulation in persons who stutter: evidence for abnormal auditory-motor transformation. PLoS One 7:e41830. 10.1371/journal.pone.0041830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie RID, Douglas-Cowie E (1983) Speech production in profound postlingual deafness. In: Hearing science and hearing disorders (Lutman ME, ed), pp 183–230. London: Academic. [Google Scholar]
- Daliri A, Chao S-C, Fitzgerald LC (2020) Compensatory responses to formant perturbations proportionally decrease as perturbations increase. J Speech Lang Hear Res 63:3392–3407. 10.1044/2020_JSLHR-19-00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Harding AE, Marsden CD (1998) Influence of vision on upper limb reaching movements in patients with cerebellar ataxia. Brain 121:357–372. 10.1093/brain/121.2.357 [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D (2012) Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol 107:134–147. 10.1152/jn.00007.2011 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Thach WT (1969) Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol 31:451–498. 10.1146/annurev.ph.31.030169.002315 [DOI] [PubMed] [Google Scholar]
- Goodhew SC, Edwards M (2019) Translating experimental paradigms into individual-differences research: contributions, challenges, and practical recommendations. Conscious Cogn 69:14–25. 10.1016/j.concog.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Guenther FH (2016) Neural control of speech. Cambridge, MA: MIT. [Google Scholar]
- Herrick CJ (1924) Origin and evolution of the cerebellum. Arch NeurPsych 11:621–652. 10.1001/archneurpsyc.1924.02190360009001 [DOI] [Google Scholar]
- Houde JF, Jordan IM (1998) Sensorimotor adaptation in speech production. Science 279:1213–1216. 10.1126/science.279.5354.1213 [DOI] [PubMed] [Google Scholar]
- Houde JF, Gill JS, Agnew Z, Kothare H, Hickok G, Parrell B, Ivry RB, Nagarajan SS (2019) Abnormally increased vocal responses to pitch feedback perturbations in patients with cerebellar degeneration. J Acoust Soc Am 145:EL372. 10.1121/1.5100910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Wang H, Max L (2020) It's about time: Minimizing hardware and software latencies in speech research with real-time auditory feedback. J Speech Lang Hear Res 63:2522–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D (2007) “What's new in Psychtoolbox-3?” Perception 36 ECVP Abstract Supplement. [Google Scholar]
- Körding KP, Wolpert DM (2004) Bayesian integration in sensorimotor learning. Nature 427:244–247. 10.1038/nature02169 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Hadjiosif AM, Xu J, Wong AL, Haith AM (2019) Motor learning. Compr Physiol 9:613–663. 10.1002/cphy.c170043 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Trautman P, Rasquinha RJ, Bhanpuri NH, Scott SH, Bastian AJ (2013) Cerebellar damage diminishes long-latency responses to multijoint perturbations. J Neurophysiol 109:2228–2241. 10.1152/jn.00145.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametti DR, Quek MYM, Prescott CB, Brittain J-S, Watkins KE (2020) The perils of learning to move while speaking: one-sided interference between speech and visuomotor adaptation. Psychon Bull Rev 27:544–552. 10.3758/s13423-020-01725-8 [DOI] [PubMed] [Google Scholar]
- Lane H, Webster JW (1991) Speech deterioration in postlingually deafened adults. J Acoust Soc Am 89:859–866. 10.1121/1.1894647 [DOI] [PubMed] [Google Scholar]
- Li W, Zhuang J, Guo Z, Jones JA, Xu Z, Liu H (2019) Cerebellar contribution to auditory feedback control of speech production: evidence from patients with spinocerebellar ataxia. Hum Brain Mapp 40:4748–4758. 10.1002/hbm.24734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard E (1911) Le signe de l'élévation de la voix. Annale des maladies de l'oreille et du larynx 37:101–109. [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Küper M, Leggio M, Marvel C, Molinari M, Murdoch BE, Nicolson RI, Schmahmann JD, Stoodley CJ, Thürling M, Timmann D, Wouters E, et al. (2014) Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum 13:386–410. 10.1007/s12311-013-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT (1996) Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119 (Pt 4):1183–1198. 10.1093/brain/119.4.1183 [DOI] [PubMed] [Google Scholar]
- McDougle SD, Taylor JA (2019) Dissociable cognitive strategies for sensorimotor learning. Nat Commun 10:40. 10.1038/s41467-018-07941-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Christensen LOD, Cain O, Stanley J (2007) Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5:e316. 10.1371/journal.pbio.0050316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Qasim SE, Crossley MJ, Ivry R (2015) Savings upon re-aiming in visuomotor adaptation. J Neurosci 35:14386–14396. 10.1523/JNEUROSCI.1046-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ (2006) Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26:9107–9116. 10.1523/JNEUROSCI.2622-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhall GK, MacDonald EN, Byrne SK, Johnsrude I (2009) Talkers alter vowel production in response to real-time formant perturbation even when instructed not to compensate. J Acoust Soc Am 125:384–390. 10.1121/1.3035829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niziolek C, Houde J (2015) Wave_viewer: First release (v1.0). Zenodo. doi: 10.5281/zenodo.13839. [DOI] [Google Scholar]
- Niziolek CA, Guenther FH (2013) Vowel category boundaries enhance cortical and behavioral responses to speech feedback alterations. J Neurosci 33:12090–12098. 10.1523/JNEUROSCI.1008-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Hermsdörfer J, Rost K, Timmann D, Topka H (2004) Predictive and reactive finger force control during catching in cerebellar degeneration. Cerebellum 3:227–235. 10.1080/14734220410019057 [DOI] [PubMed] [Google Scholar]
- Parrell B, Agnew Z, Nagarajan S, Houde JF, Ivry RB (2017) Impaired feedforward control and enhanced feedback control of speech in patients with cerebellar degeneration. J Neurosci 37:9249–9258. 10.1523/JNEUROSCI.3363-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto-Castanon A, Anton J-LL, Nazarian B, Alario F-XX (2010) Distinct representations of phonemes, syllables, and supra-syllabic sequences in the speech production network. Neuroimage 50:626–638. 10.1016/j.neuroimage.2009.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG (1997) The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10:437–442. [PubMed] [Google Scholar]
- Purcell DW, Munhall KG (2006) Adaptive control of vowel formant frequency: evidence from real-time formant manipulation. J Acoust Soc Am 120:966–977. 10.1121/1.2217714 [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O (2009) Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101:1961–1971. 10.1152/jn.91069.2008 [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB (2013) Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol 109:1164–1173. 10.1152/jn.00654.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW (2008) A computational neuroanatomy for motor control. Exp Brain Res 185:359–381. 10.1007/s00221-008-1280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R (2005) Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93:2809–2821. 10.1152/jn.00943.2004 [DOI] [PubMed] [Google Scholar]
- Stevens SS, Volkmann J, Newman EB (1937) A scale for the measurement of the psychological magnitude pitch. The Journal of the Acoustical Society of America 8:185–190. [Google Scholar]
- Summers WV, Pisoni DB, Bernacki RH, Pedlow RI, Stokes MA (1988) Effects of noise on speech production: acoustic and perceptual analyses. J Acoust Soc Am 84:917–928. 10.1121/1.396660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH (2008) Neural mechanisms underlying auditory feedback control of speech. Neuroimage 39:1429–1443. 10.1016/j.neuroimage.2007.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, Cai S, Guenther F (2013) Exploring auditory-motor interactions in normal and disordered speech. Proc Mtgs Acoust 19:060180. 10.1121/1.4800684 [DOI] [Google Scholar]
- Tseng Y-WW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ (2007) Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98:54–62. 10.1152/jn.00266.2007 [DOI] [PubMed] [Google Scholar]
- Urban PP, Marx J, Hunsche S, Gawehn J, Vucurevic G, Wicht S, Massinger C, Stoeter P, Hopf HC (2003) Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch Neurol 60:965–972. 10.1001/archneur.60.7.965 [DOI] [PubMed] [Google Scholar]
- Werner S, Aken BC, van Hulst T, Frens MA, Geest JN, van der Strüder HK, Donchin O (2015) Awareness of sensorimotor adaptation to visual rotations of different size. PLoS ONE 10:e0123321. 10.1371/journal.pone.0123321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cogn Sci 2:338–347. 10.1016/s1364-6613(98)01221-2 [DOI] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R (2009) Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29:12930–12939. 10.1523/JNEUROSCI.3115-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet AM, Cao D, Bastian AJ, Cowan NJ (2020) Cerebellar patients have intact feedback control that can be leveraged to improve reaching. Elife 9:e53246. 10.7554/eLife.53246 [DOI] [PMC free article] [PubMed] [Google Scholar]