Abstract
Expression of Regulated endocrine specific protein 18 (Resp18) is localized in numerous tissues and cell types; however, its exact cellular function is unknown. We previously showed that targeted disruption of the Resp18 locus in the Dahl SS (SS) rat (Resp18mutant) results in higher blood pressure (BP), increased renal fibrosis, increased urinary protein excretion, and decreased mean survival time following a chronic (6 weeks) 2% high salt (HS) diet compared with the SS rat. Based on this prominent renal injury phenotype, we hypothesized that targeted disruption of Resp18 in the SS rat promotes an early onset hypertensive-signaling event through altered signatures of the renal transcriptome in response to HS. To test this hypothesis, both SS and Resp18mutant rats were exposed to a 7-day 2% HS diet and BP was recorded by radiotelemetry. After a 7-day exposure to the HS diet, systolic BP was significantly increased in the Resp18mutant rat compared with the SS rat throughout the circadian cycle. Therefore, we sought to investigate the renal transcriptomic response to HS in the Resp18mutant rat. Using RNA sequencing, Resp18mutant rats showed a differential expression of 25 renal genes, including upregulation of Ren. Upregulation of renal Ren and other differentially expressed genes were confirmed via qRT-PCR. Moreover, circulating renin activity was significantly higher in the Resp18mutant rat compared with the WT SS rat after 7 days on HS. Collectively, these observations demonstrate that disruption of the Resp18 gene in the SS rat is associated with an altered renal transcriptomics signature as an early response to salt load.
Keywords: Resp18, Renin, RNA Seq, Dahl SS rat, Blood Pressure
1. Introduction
The interplay among genetic, behavior, and environmental factors plays a vital role in the development and progression of hypertension, also known as high blood pressure (BP). It is well established that upregulation of the renin-angiotensin-aldosterone system (RAAS) pathway increases BP and is a major contributor to water and electrolyte homeostasis [1]. However, advancements in technology provide us with new tools (gene-editing) to uncover additional genes associated with hypertension. The Dahl salt-sensitive (SS) rat is a well-established and clinically relevant experimental model for the study of salt-induced hypertension and renal failure [2]. Quantitative trait locus (QTL) mapping studies conducted in the SS rat have led to the identification of many genetic loci responsible for salt-induced hypertension and renal disease [3]. One such QTL study identified a genomic segment on rat chromosome 9 containing a potential candidate gene for BP and proteinuria, the Regulated Endocrine Specific Protein-18 (Resp18) [4]. Resp18 expression has been identified in numerous tissues and cell types; however, the precise function of this novel endocrine protein is currently unknown [5].
To validate Resp18’s candidacy as causal of hypertension, Resp18mutant rats were generated by targeted disruption of the Resp18 locus in the SS rat [2]. Our previous study showed that the Resp18mutant rat maintained on a high salt (HS) diet (2% NaCl) for 6 weeks exhibited an increase in BP, renal fibrosis, proteinuria and reduced mean survival time [2]. Furthermore, we also observed a significant increase in renal pro-fibrotic gene expression, including Collagen type 1 (Col1a1), Collagen type 3 (Col3a1), and transforming growth factor beta (Tgf-β) in the Resp18mutant rat [2]. From this study, we concluded that Resp18 gene function is vital in BP regulation by modulating proper kidney homeostasis during salt loading.
Based on these studies we concluded that Resp18 gene function is vital in BP regulation by modulating proper kidney homeostasis upon excess salt loading. Thus, the purpose of this study was to test the hypothesis that short-term exposure to a 2% HS diet (7-day) in the Resp18mutant rat, which exhibits a significant increase in BP in response to a chronic salt load, promotes an early onset hypertensive-signaling event through altered signatures of the renal transcriptome in response to HS.
2. Materials and Methods
2.1. Rats
Male Dahl salt-sensitive/Mcw (SS) and Resp18mutant rats were concomitantly bred and raised as separate colonies fed a low salt diet (0.3% NaCl; Harlan Teklad diet 7034) until 6 weeks of age [2]. After 6 weeks of age, the diet was switched to HS (2% NaCl; Harlan Teklad diet 94217) for 7 days. After 7 days of HS, the kidneys were collected and immediately frozen in liquid nitrogen. All rats were kept on a 12:12-h light-dark cycle in a climate-controlled room. Rat chow and water were provided ad libitum. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Toledo, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and followed ARRIVE guidelines.
2.2. Measurement of blood pressure using radiotelemetry
At five weeks of age, SS and Resp18mutant rats were anesthetized using isoflurane and surgically implanted with radiotelemetry transmitters, as previously described [2]. The rats were individually housed after the surgery and allowed to recover for 3 days. Using DSI software and equipment (https://www.datasci.com/), BP data were collected every 5 min, starting a day prior to HS until end of study (after 7 days HS) and analyzed using Dataquest A.R.T 4.2 software.
2.3. RNA Sequencing
2.4. Validation of analysis of RNA- Seq data by qRT-PCR
2.5. Measurement of serum Renin activity
Renin activity was measured in serum obtained from SS and Resp18mutant rats to determine renin activity prior to and after exposure to a 7-day HS diet. Renin activity was measured using a renin assay kit (MAK157-1 KT -Sigma Aldrich, USA), as per manufacturer’s instructions.
2.6. Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Data were analyzed by Student’s t-test or two-way ANOVA (Sidak test), as appropriate, with P value of <0.05 was used as a threshold for statistical significance. Figures were generated with GraphPad Prism software (version 7; GraphPad Software Inc., La Jolla, CA).
3. Results
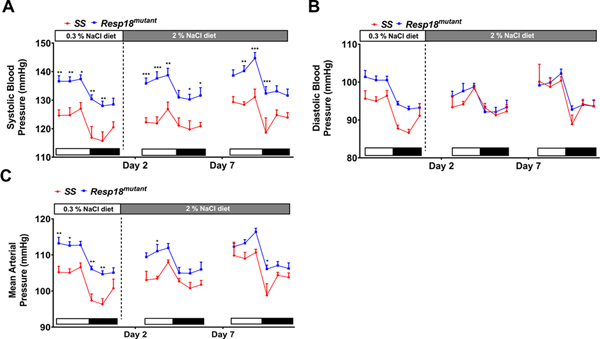
3.1. Resp18mutant rats maintain a significant increase in systolic blood pressure in response to a 7-day HS diet compared with SS counterparts
In the current study, SBP was significantly increased at 7 weeks of age in Resp18mutant rats on a 0.3% salt (LS) diet, and in Resp18mutant rats exposed to 7 days of a HS diet when compared with SS rats (Fig 1A), regardless of light or dark phase of the circadian cycle. DBP was increased in Resp18mutant rats on LS but not in response to the HS diet (Fig. 1B). The mean arterial pressure (MAP) in the Resp18mutant was not increased by HS diet, which was the case in SS rats, but the MAP remained significantly higher in the Resp18mutant than SS rats (Fig 1C).
Figure 1. Resp18mutant rats maintain elevated blood pressure compared with wild-type SS rats after exposure to 2% high salt (HS) diet.
Systolic blood pressure (A), diastolic blood pressure (B), and mean arterial pressure (MAP) (C) were measured using radiotelemetry in SS and Resp18mutant rats (n = 8/group). White bars represent light cycle and black bars represent dark cycle. Data presented are the 4 h average of recordings obtained every 5 min continuously for 24 h. Data represent mean±SEM. Two-way ANOVA with Holm-Šídák was used for comparison. *p<0.05, **p<0.01 ***p<0.001.
3.2. Transcriptome analysis of kidneys from the Resp18mutant rat
As shown on the heat map visualizing results from our RNA-Seq analysis, the renal transcriptome response to a 7-day HS diet was significantly different upon comparison of the Resp18mutant rat to the SS rat (Fig 2A and Supplementary Table 2). Using a volcano plot to infer the overall distribution of differentially expressed renal genes, 25 genes were differentially expressed between the Resp18mutant and SS rats with the threshold set at a p-value <0.05 and with all biological variation removed by DESeq (Fig 2B). Out of the 25 genes, expressions of 12 genes were upregulated and 13 genes were downregulated (Fig 2B). Venn diagram analysis of the RNA-Seq data showed 370 uniquely expressed genes in kidneys from Resp18mutant rat and 294 uniquely expressed genes in kidneys from the SS rat (Fig 2C).
Figure 2. Transcriptome response to a 7-day exposure to a 2% high salt (HS) diet is altered in the Resp18mutant rat kidney, relative to wild-type SS rat kidney:
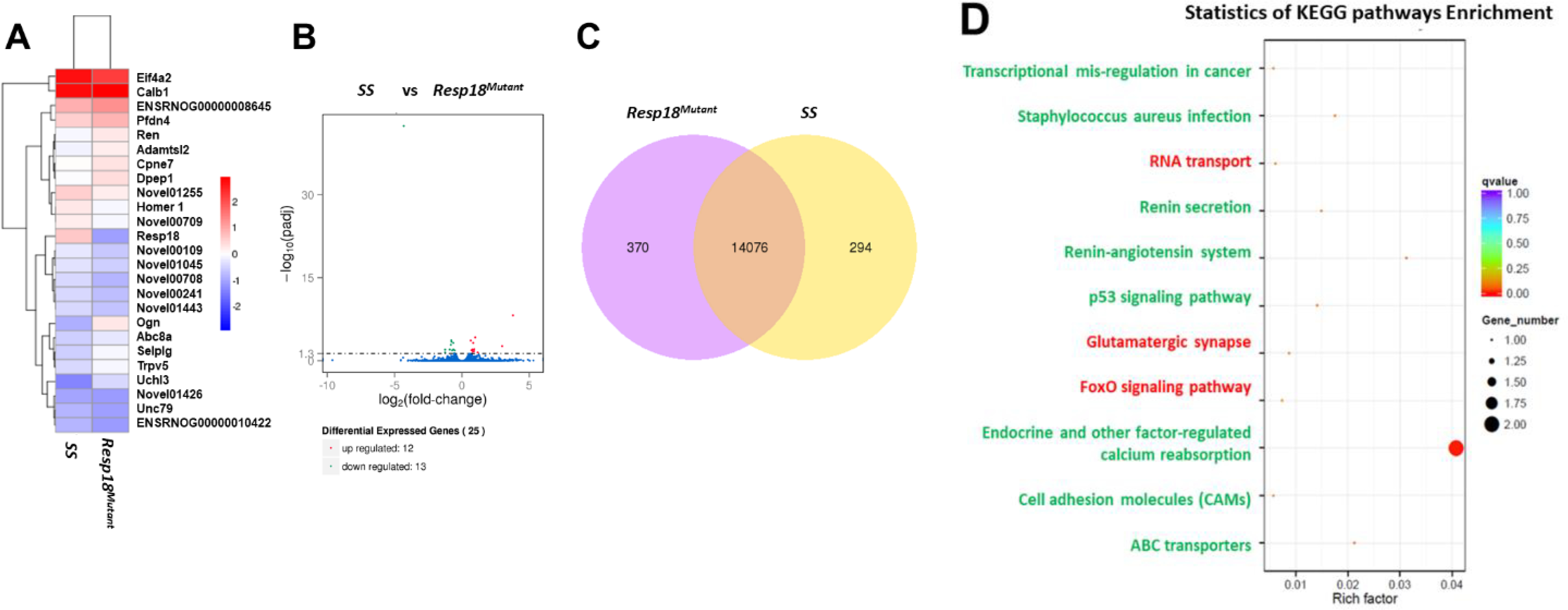
(A) Heat map results of a FPKM cluster analysis, using the log10 (FPKM+1) value. Red denotes renal genes with high expression levels; blue denotes renal genes with low expression levels in the Resp18mutant. (B) Volcano plots x-axis shows the fold-change in gene expression between samples (-log10(padj) and the y-axis shows the statistical significance of the difference (log2 (fold-change). Significant up- and down-regulated genes are highlighted in red and green, respectively. Genes which are not differentially expressed are in blue. (C) Venn diagram analysis with yellow representing uniquely expressed renal genes in the SS rat and purple representing uniquely expressed renal genes in the Resp18mutant rat (D) KEGG analysis of differential gene expression in kidneys. Green text represents pathways that correlate with upregulated genes; red text represents pathways that correlate with downregulated genes. Dot size represents the number of different genes and color represents the q-value.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed to understand the biological relevance of the DEG-enriched pathways. The eight pathways significantly upregulated in the Resp18mutant rat were associated with transcriptional mis-regulation in cancer, Staphylococcus aureus infection, renin secretion, renin-angiotensin system, p53 signaling, endocrine, and other factor-regulated calcium reabsorbing, cell adhesion molecule (CAMs), and ABC transporters pathways (Fig 2D). KEGG pathway analysis also revealed three pathways correlated with significantly downregulated genes that included RNA transport, glutamatergic synapse, and FoxO signaling pathway (Fig 2D).
To validate our RNA-Seq results, qRT-PCR was used to analyze a subset of genes that demonstrated a significant differential expression. Through qRT-PCR analysis, we validated the differential expressions of numerous up-regulated and down-regulated genes in the HS-exposed Resp18mutant rat (Fig 3A&B). We also found eight novel transcripts that were differentially expressed between Resp18mutant and SS rats.
Figure 3. qRT-PCR validation of RNA seq:
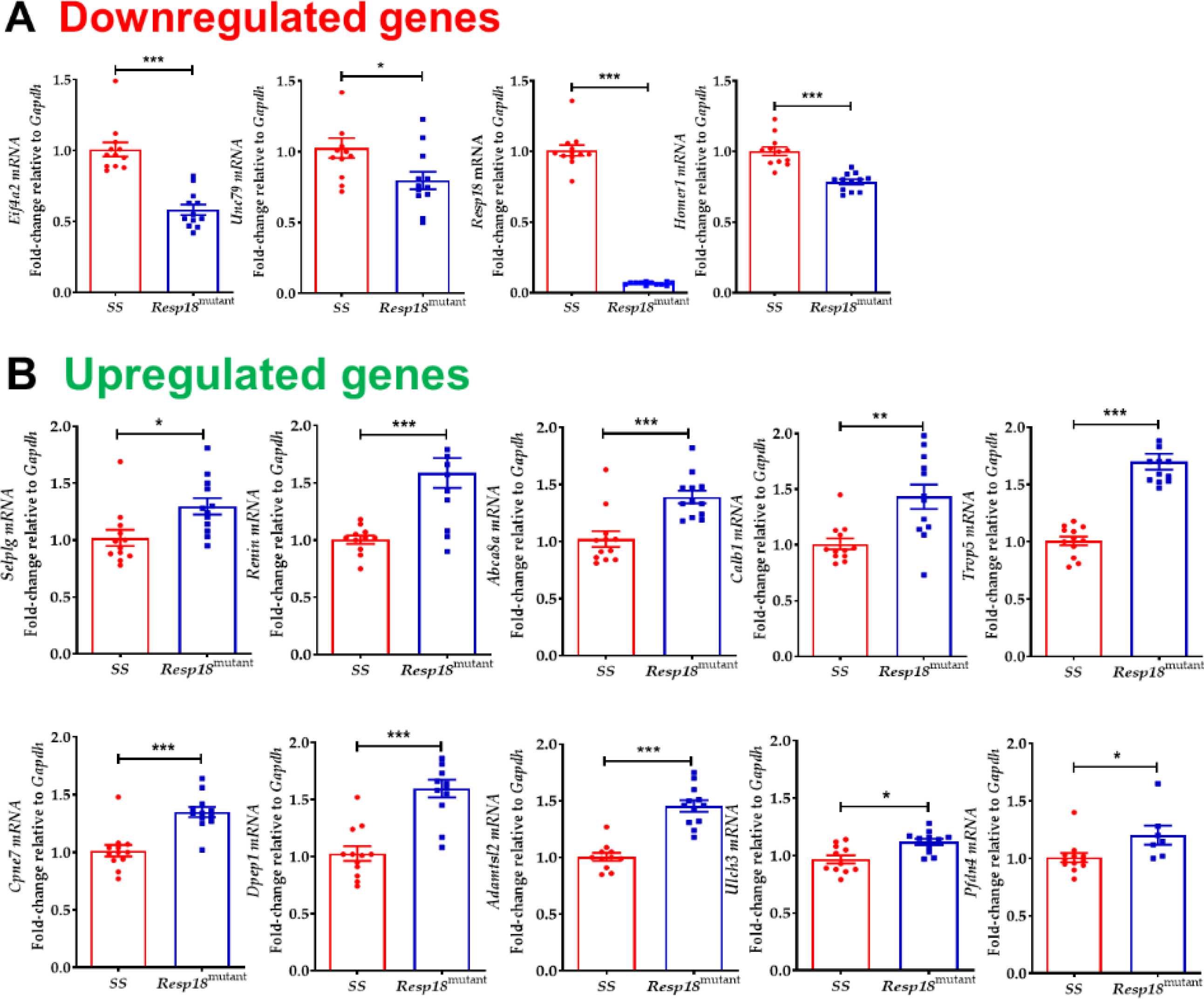
(A) downregulated or (B) upregulated in Resp18mutant rats compared with the SS rats (n=4/grp in duplicates). Data represent mean±SEM. Statistical significance was calculated by unpaired two-tailed t-test *p<0.05, **p<0.01 ***p<0.001.
3.3. Circulating renin activity is increased in the Resp18mutant rat in response to 7-day salt load
The renin-angiotensin-aldosterone system (RAAS) plays a pivotal role in regulating water and electrolyte balance and BP [6]. RNA-Seq analysis with validation by qRT-PCR demonstrated that renal renin gene expression was significantly upregulated in the Resp18mutant rat compared with the SS rat. Circulating renin activity before HS diet was not different between Resp18mutant and SS rat (Fig 4A). However, after 7days on HS, circulating renin activity was significantly increased in Resp18mutant compared with SS rat (Fig 4B).
Figure 4: Renin levels in the Resp18mutant rat after a 7-day exposure to a 2% high salt (HS) diet:
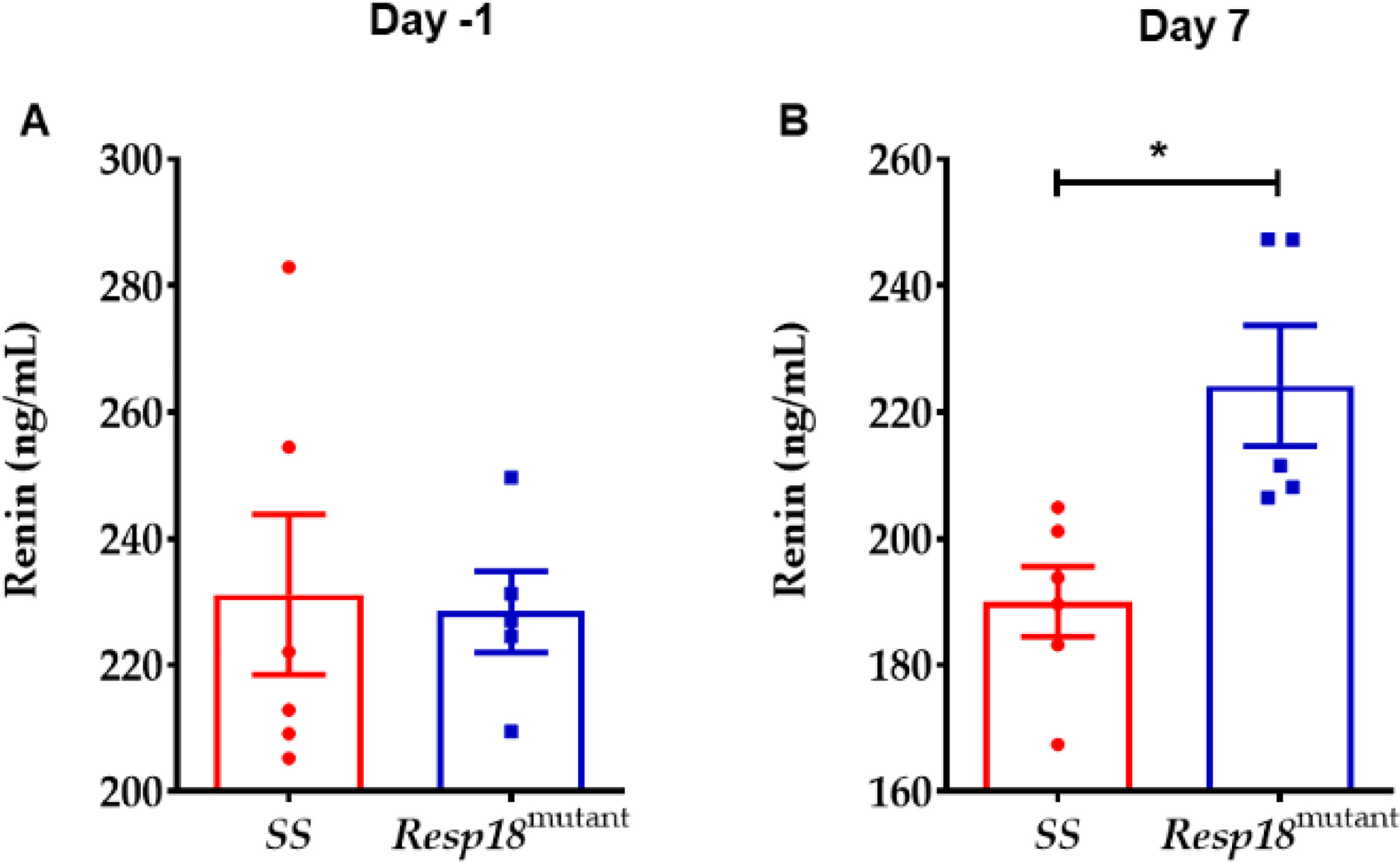
(A) Serum samples collected from Resp18mutant and SS rats prior to exposure to the HS (n=5–6/grp) and after 7 days on HS (n=5–6/grp). Data represent mean±SEM. Statistical significance was calculated unpaired two-tailed t-test **p<0.05.
4. Discussion
Based on our previous findings, which suggest that the Resp18 gene plays an essential role in decelerating the progression of salt-induced hypertension and renal injury, [2] this study tested the hypothesis that 7 days of HS starting at 6 weeks of age promotes early transcriptomic changes in the kidney of the Resp18mutant rat. RNA-Seq revealed 25 renal DEG’s between Resp18mutant and SS rats. In total, 13 genes were downregulated, and 12 genes were upregulated and were confirmed through qRT-PCR analysis. Among these DEGs, six are closely associated with BP and included Homer1 [7], Selplg [8], Abca8a [9], Dpep1 [10], Calb1 [11], and Ren [12]. Yet, for some, the relevance for altered expression confirmed by qRT-PCR was not immediately clear.
Homer is upregulated in coronary artery disease and plays a role in the regulation of G protein-coupled receptors [13]. Yet, intra-renal Homer1 gene expression was downregulated in the Resp18mutant rat after a 7-day exposure to HS. Selectin P Ligand (Selplg) gene expression was upregulated in the kidney of the Resp18mutant rat, in response to HS. Decreased Selplg binding capacity, can lead to fewer leukocyte—endothelium and leukocyte—platelet complexes and potentially reduce the risk of stroke [8]. Single nucleotide polymorphisms (SNPs) within the ABCC8 gene, a member of the superfamily of ATP-binding cassette (ABC) transporters, were found to be associated with pulmonary arterial hypertension [14]. Dpep1, another differentially expressed gene encodes for a membrane-bound enzyme in the kidney, which is involved in the hydrolysis of dipeptides, such as glutathione and other similar proteins [15]. Furthermore, Dpep1 regulates leukotriene activity by catalyzing the conversion of leukotriene D4 to leukotriene E4 [16]. Leukotrienes are pro-inflammatory mediators and are also potential contributors to hypertension [17]. Calb1 plays a role in Ca2+ transport and anti-calcification process. In the kidney, Calb1 regulates renal tubular Ca2+ reabsorption [18], along with other partners such as Trpv5, Trpv6, Pmca1b calcium pump, and Ncx1 exchanger [19]. Interestingly, in our studies we found that Trpv5 was upregulated in Resp18mutant rat kidneys. A SNP identified in the TRPV5 gene is closely associated with stone multiplicity in calcium nephrolithiasis patients [20]. Another differentially expressed gene, such as Adamts like 2, a secreted glycoprotein binds to the cell surface and extracellular matrix, also interacts with latent transforming growth factor beta binding protein 1 [21]. Furthermore, through substitution mapping studies, genetic loci, including genes such as Pfdn4 [22], Cpne7 [23], Eif4a2 [24], and Unc79 [25], are significantly associated with BP, stress-related neuroendocrine reactivity, and urinary albumin excretion quantitative trait locus.
In our study, we chose to focus on the role of renin, well known for its role in BP regulation and renal function in the SS rat, a low renin model of hypertension. KEGG pathway analysis revealed upregulation of the intra-renal Ren gene expression in the Resp18mutant rat versus the SS rat on HS diet. Exposure to a HS diet suppresses serum renin activity and circulating angiotensinogen in the SS rat, suggesting that the SS rat is a model of low renin hypertension [26]. Yet, Kobori et al., reported that intrarenal angiotensinogen is increased in the SS rat on HS, indicative of tissue-specific inappropriate activation of the intrarenal RAAS [26]. In our study, circulating renin activity prior to HS in the Resp18mutant rat was comparable to the SS rat. However, following a 7-day exposure to HS diet circulating renin activity was significantly elevated in the Resp18mutant rat, an increase that also correlated with a significant increase in SBP and intra-renal Ren gene expression compared with the SS rat. Whether intra-renal Ren gene expression was elevated prior to HS is not yet known. An increase in Ren gene expression and circulating levels of renin are known to promote hypertension and other cardiovascular and renal complications [27]. Thus, the factors that contribute to increased BP in the Resp18mutant rat on LS remain unknown although up-regulation of intra-renal Ren is not yet ruled out, as a contributory factor. Collectively, these data suggest a differential response of intra-renal Ren gene expression in the Resp18mutant rat compared with the SS rat on HS diet. Whether upregulation of other components of the classical intrarenal RAAS in the Resp18mutant rat correlates with increased circulating and intra-renal renin is not yet known. However, these findings suggest that exposure to a HS diet promotes an early transcriptomic response that favors the rise in BP and altered gene expression of known factors, such as renin and unknown players (eight novel transcripts). The effect of systemic activation of the RAAS on secondary immune activation and inflammation in the Resp18mutant rat is also unclear and remains a focus of ongoing studies.
Little is known regarding the cellular function of Resp18; however, using a bioinformatics approach, it was discovered that RESP18 shares sequence homology with the luminal region of IA-2, a DCV (dense core vesicle) transmembrane protein involved in insulin secretion [28]. Furthermore, RESP18 expression is found in the lumen of DCVs [29], collectively suggesting that Resp18 may play a role in hormone or peptide secretion. Based on findings from our study, Resp18 may play a role in the control of renin secretion and the regulation of BP in response to a HS diet in the Resp18mutant rat. To date, there is only one FDA-approved direct renin inhibitor (aliskiren) on the market for the treatment of hypertension [30]. Additional studies will be required to validate the relationship between Resp18 and renin synthesis and secretion, and activation of the RAAS and its downstream components in the onset and progression of increased BP and hypertension.
The SS rat is a well-established experimental model utilized for study of salt-sensitive hypertension. Historically considered a model of low renin, the SS rat is implicated to mimic the etiology of hypertension in individuals non-responsive to ACE inhibition, most likely due to suppression of the RAAS [26]. In our study, circulating renin activity was elevated in response to HS in the Resp18mutant rat, compared with the SS rat, suggesting that mutation of Resp18 alters the ability of salt to suppress the systemic RAAS. Whether intra-renal Ren gene expression is elevated in the Resp18mutant rat compared with SS rat, prior to HS is not yet known. Yet, the sustained increase in BP regardless of salt intake implicates a critical role for Resp18 in the long-term control of BP. Moreover, our findings suggest that increased BP in the Resp18mutant rat that persists in response to salt load may involve “global” in addition to tissue-specific actions of the RAAS. Thus, insight using this novel model of cardiorenal risk may lead to the identification of yet unexplored therapeutics for the treatment of high BP that occurs even in the absence of salt load.
In summary, targeted disruption of the Resp18 locus in SS rats leads to a hypertensive phenotype even after short-term exposure (7-day) to a HS diet. RNA-Seq analysis, confirmed by qRT-PCR, demonstrated up-regulation of renal Ren gene expression. However, unlike the Dahl SS rat, our study showed a significant increase in circulating renin activity in the Resp18mutant rat. Resp18 shares sequence homology with IA-2 a, DCV involved in the secretion of hormones and neurotransmitters. Based on our current study, we speculate that Resp18 gene plays a pivotal role in systemic and intra-renal renin synthesis and/or secretion, and thus, regulation of BP upon exposure to a HS diet. One limitation of our study involved the study of male but not female Resp18mutant rats. It is well established that sex alters the phenotypic response to HS in the SS rat. Whether sex alters BP in response to LS or HS in the female Resp18mutant rat is not yet known. Future studies will determine the phenotypic response in the female Resp18mutant. Moreover, aging may be another variable that also influences the progression and severity of cardiorenal risk that occurs even under conditions of reduced salt intake.
Supplementary Material
Highlights.
The study highlights the physiological role of Resp18, a novel player in salt-induced hypertension.
The study emphasizes the early transcriptomic signature of genes involved in HS-induced hypertension in Resp18 mutant rat kidneys.
This study also shows that targeted disruption of the Resp18 locus in Dahl SS rat (Resp18mutant) up-regulates RAAS.
Our study highlights the use of this novel model cardiorenal risk may lead to the identification of yet unexplored therapeutic targets for the treatment of high BP that occurs even in the absence of salt load.
5. Acknowledgments
This work was supported by an American Heart Association Scientist Development Grant (16SDG27700030), US National Institutes of Health (5 R01 DK119652), and the startup funds from the University of Toledo College of Medicine and Life Sciences.
Footnotes
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- [1].Lifton RP, Gharavi AG, Geller DS, Molecular mechanisms of human hypertension, Cell 104 (2001) 545–556. 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- [2].Kumarasamy S, Waghulde H, Cheng X, Haller ST, Mell B, Abhijith B, Ashraf UM, Atari E, Joe B, Targeted disruption of regulated endocrine-specific protein ( Resp18) in Dahl SS/Mcw rats aggravates salt-induced hypertension and renal injury, Physiol Genomics 50 (2018) 369–375. 10.1152/physiolgenomics.00008.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Joe B, Shapiro JI, Molecular mechanisms of experimental salt-sensitive hypertension, J Am Heart Assoc 1 (2012) e002121. 10.1161/JAHA.112.002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garrett MR, Meng H, Rapp JP, Joe B, Locating a blood pressure quantitative trait locus within 117 kb on the rat genome: substitution mapping and renal expression analysis, Hypertension 45 (2005) 451–459. 10.1161/01.HYP.0000154678.64340.7f. [DOI] [PubMed] [Google Scholar]
- [5].Atari E, Perry MC, Jose PA, Kumarasamy S, Regulated Endocrine-Specific Protein-18, an Emerging Endocrine Protein in Physiology: A Literature Review, Endocrinology 160 (2019) 2093–2100. 10.1210/en.2019-00397. [DOI] [PubMed] [Google Scholar]
- [6].Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM, Classical Renin-Angiotensin system in kidney physiology, Compr Physiol 4 (2014) 1201–1228. 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fulop G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z, Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment, Geroscience 39 (2017) 385–406. 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Volcik KA, Catellier D, Folsom AR, Matijevic N, Wasserman B, Boerwinkle E, SELP and SELPLG genetic variation is associated with cell surface measures of SELP and SELPLG: the Atherosclerosis Risk in Communities Carotid MRI Study, Clin Chem 55 (2009) 1076–1082. 10.1373/clinchem.2008.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chauvet C, Menard A, Xiao C, Aguila B, Blain M, Roy J, Deng AY, Novel genes as primary triggers for polygenic hypertension, J Hypertens 30 (2012) 81–86. 10.1097/HJH.0b013e32834dddb1. [DOI] [PubMed] [Google Scholar]
- [10].Takeuchi F, Akiyama M, Matoba N, Katsuya T, Nakatochi M, Tabara Y, Narita A, Saw WY, Moon S, Spracklen CN, Chai JF, Kim YJ, Zhang L, Wang C, Li H, Li H, Wu JY, Dorajoo R, Nierenberg JL, Wang YX, He J, Bennett DA, Takahashi A, Momozawa Y, Hirata M, Matsuda K, Rakugi H, Nakashima E, Isono M, Shirota M, Hozawa A, Ichihara S, Matsubara T, Yamamoto K, Kohara K, Igase M, Han S, Gordon-Larsen P, Huang W, Lee NR, Adair LS, Hwang MY, Lee J, Chee ML, Sabanayagam C, Zhao W, Liu J, Reilly DF, Sun L, Huo S, Edwards TL, Long J, Chang LC, Chen CH, Yuan JM, Koh WP, Friedlander Y, Kelly TN, Bin Wei W, Xu L, Cai H, Xiang YB, Lin K, Clarke R, Walters RG, Millwood IY, Li L, Chambers JC, Kooner JS, Elliott P, van der Harst P, C. International Genomics of Blood Pressure, Chen Z, Sasaki M, Shu XO, Jonas JB, He J, Heng CK, Chen YT, Zheng W, Lin X, Teo YY, Tai ES, Cheng CY, Wong TY, Sim X, Mohlke KL, Yamamoto M, Kim BJ, Miki T, Nabika T, Yokota M, Kamatani Y, Kubo M, Kato N, Interethnic analyses of blood pressure loci in populations of East Asian and European descent, Nat Commun 9 (2018) 5052. 10.1038/s41467-018-07345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dunbar DR, Khaled H, Evans LC, Al-Dujaili EA, Mullins LJ, Mullins JJ, Kenyon CJ, Bailey MA, Transcriptional and physiological responses to chronic ACTH treatment by the mouse kidney, Physiol Genomics 40 (2010) 158–166. 10.1152/physiolgenomics.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Culver S, Li C, Siragy HM, Intrarenal Angiotensin-Converting Enzyme: the Old and the New, Curr Hypertens Rep 19 (2017) 80. 10.1007/s11906-017-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jing X, Chen SS, Jing W, Tan Q, Yu MX, Tu JC, Diagnostic potential of differentially expressed Homer1, IL-1beta, and TNF-alpha in coronary artery disease, Int J Mol Sci 16 (2014) 535–546. 10.3390/ijms16010535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bohnen MS, Ma L, Zhu N, Qi H, McClenaghan C, Gonzaga-Jauregui C, Dewey FE, Overton JD, Reid JG, Shuldiner AR, Baras A, Sampson KJ, Bleda M, Hadinnapola C, Haimel M, Bogaard HJ, Church C, Coghlan G, Corris PA, Eyries M, Gibbs JSR, Girerd B, Houweling AC, Humbert M, Guignabert C, Kiely DG, Lawrie A, MacKenzie Ross RV, Martin JM, Montani D, Peacock AJ, Pepke-Zaba J, Soubrier F, Suntharalingam J, Toshner M, Treacy CM, Trembath RC, Vonk Noordegraaf A, Wharton J, Wilkins MR, Wort SJ, Yates K, Graf S, Morrell NW, Krishnan U, Rosenzweig EB, Shen Y, Nichols CG, Kass RS, Chung WK, Loss-of-Function ABCC8 Mutations in Pulmonary Arterial Hypertension, Circ Genom Precis Med 11 (2018) e002087. 10.1161/CIRCGEN.118.002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nafar M, Kalantari S, Samavat S, Rezaei-Tavirani M, Rutishuser D, Zubarev RA, The novel diagnostic biomarkers for focal segmental glomerulosclerosis, Int J Nephrol 2014 (2014) 574261. 10.1155/2014/574261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yamaya M, Sekizawa K, Terajima M, Okinaga S, Ohrui T, Sasaki H, Dipeptidase inhibitor and epithelial removal potentiate leukotriene D4-induced human tracheal smooth muscle contraction, Respir Physiol 111 (1998) 101–109. 10.1016/s0034-5687(97)00099-6. [DOI] [PubMed] [Google Scholar]
- [17].Samuelsson B, Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation, Science 220 (1983) 568–575. 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- [18].Sooy K, Kohut J, Christakos S, The role of calbindin and 1,25dihydroxyvitamin D3 in the kidney, Curr Opin Nephrol Hypertens 9 (2000) 341–347. 10.1097/00041552-200007000-00004. [DOI] [PubMed] [Google Scholar]
- [19].Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ, Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport, EMBO J 25 (2006) 2978–2988. 10.1038/sj.emboj.7601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khaleel A, Wu MS, Wong HS, Hsu YW, Chou YH, Chen HY, A Single Nucleotide Polymorphism (rs4236480) in TRPV5 Calcium Channel Gene Is Associated with Stone Multiplicity in Calcium Nephrolithiasis Patients, Mediators Inflamm 2015 (2015) 375427. 10.1155/2015/375427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D, Ge G, Greenspan DS, Bonnet D, Le Merrer M, Munnich A, Apte SS, Cormier-Daire V, ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation, Nat Genet 40 (2008) 1119–1123. 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duong C, Charron S, Xiao C, Hamet P, Menard A, Roy J, Deng AY, Distinct quantitative trait loci for kidney, cardiac, and aortic mass dissociated from and associated with blood pressure in Dahl congenic rats, Mamm Genome 17 (2006) 1147–1161. 10.1007/s00335-006-0086-7. [DOI] [PubMed] [Google Scholar]
- [23].Llamas B, Contesse V, Guyonnet-Duperat V, Vaudry H, Mormede P, Moisan MP, QTL mapping for traits associated with stress neuroendocrine reactivity in rats, Mamm Genome 16 (2005) 505–515. 10.1007/s00335-005-0022-2. [DOI] [PubMed] [Google Scholar]
- [24].Garrett MR, Joe B, Yerga-Woolwine S, Genetic linkage of urinary albumin excretion in Dahl salt-sensitive rats: influence of dietary salt and confirmation using congenic strains, Physiol Genomics 25 (2006) 39–49. 10.1152/physiolgenomics.00150.2005. [DOI] [PubMed] [Google Scholar]
- [25].Schulz A, Schlesener M, Weiss J, Hansch J, Wendt N, Kossmehl P, Grimm D, Vetter R, Kreutz R, Protective effect of female gender on the development of albuminuria in a polygenetic rat model is enhanced further by replacement of a major autosomal QTL, Clin Sci (Lond) 114 (2008) 305–311. 10.1042/CS20070300. [DOI] [PubMed] [Google Scholar]
- [26].Kobori H, Nishiyama A, Abe Y, Navar LG, Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet, Hypertension 41 (2003) 592–597. 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Munoz-Durango N, Fuentes CA, Castillo AE, Gonzalez-Gomez LM, Vecchiola A, Fardella CE, Kalergis AM, Role of the Renin-Angiotensin-Aldosterone System beyond Blood Pressure Regulation: Molecular and Cellular Mechanisms Involved in End-Organ Damage during Arterial Hypertension, Int J Mol Sci 17 (2016). 10.3390/ijms17070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sosa L, Torkko JM, Primo ME, Llovera RE, Toledo PL, Rios AS, Flecha FL, Trabucchi A, Valdez SN, Poskus E, Solimena M, Ermacora MR, Biochemical, biophysical, and functional properties of ICA512/IA-2 RESP18 homology domain, Biochim Biophys Acta 1864 (2016) 511–522. 10.1016/j.bbapap.2016.01.013. [DOI] [PubMed] [Google Scholar]
- [29].Zhang G, Hirai H, Cai T, Miura J, Yu P, Huang H, Schiller MR, Swaim WD, Leapman RD, Notkins AL, RESP18, a homolog of the luminal domain IA-2, is found in dense core vesicles in pancreatic islet cells and is induced by high glucose, J Endocrinol 195 (2007) 313–321. 10.1677/JOE-07-0252. [DOI] [PubMed] [Google Scholar]
- [30].Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP, Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients, Circulation 111 (2005) 1012–1018. 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.