Introduction
The cannabinoid type 1 receptor (CB1R) inverse agonist, rimonabant, had several beneficial effects in individuals with metabolic disorder, but exacerbated anxiety and depressive symptoms in susceptible individuals and was removed from clinical use [1]. Since the psychiatric adverse effects of rimonabant are likely mediated centrally, the peripherally-restricted CB1R neutral antagonist, AM6545, was developed with the hopes of avoiding psychiatric side effects. In rodents, AM6545 shares the beneficial cardiometabolic properties of rimonabant, but lacks effects in CNS-mediated assays for anxiety, food palatability, cannabinoid tetrad, or withdrawal [2–8].
Considerable evidence has accumulated demonstrating that endogenous activation of the CB1R in several brain regions dampens stress-induced hypothalamic–pituitary–adrenal (HPA) axis activation [9]. In particular, rimonabant has been shown to increase basal and stress-induced HPA axis activation [10] and inhibit recovery of the axis to baseline following stress [11]. A peripherally restricted drug, such as AM6545, should not accumulate in higher brain regions and, therefore, should not share the effects of rimonabant to potentiate HPA axis activity unless there is another site of action. The purpose of these studies was to determine whether AM6545 affects stress-induced HPA axis activation and to explore the role of the CB1R in the effect.
Materials and Methods
Male and female, adult (>8weeks) ICR mice were used in these studies. Wild-type mice were purchased from Harlan Laboratories (Madison, WI); wild-type and CB1R null ICR mice were bred in house, as previously described [12] from founders obtained from Roche Bioscience (Palo Alto, CA, USA). Genotypes were determined using polymerase chain reaction to amplify DNA isolated from ear clips as previously described [13].
All mice were given ad libitum access to standard mouse chow and water. Animals were housed on a 12:12-hour light:dark cycle with lights on at 0600 hours in American Association for Accreditation of Laboratory Animal Care (AAALAC) approved facilities. Experiments were performed between 0600 and 1200. All experiments were carried out in accordance with the NIH Guide for the Use and Care of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
All drugs were administered via intraperitoneal (i.p.) injection in a volume of 0.1ml per 25g body weight 30min before the initiation of restraint. Mice were injected with vehicle, rimonabant (5 and 10mg/kg) or AM6545 (5 and 10mg/kg) administered in a 1:1:8 dimethyl sulfoxide:emulphor:saline or 1:1:18 ethanol:emulphor:saline vehicle.
Mice were stressed by restraint using 3M Durapore tape to anchor the proximal tail to a bench top in a lighted room for 30min. After restraint, the mice were returned to their home cages for 20 or 30min depending on the experiment. In some studies, mice were sacrificed by swift cervical dislocation followed by decapitation for the collection of trunk blood into EDTA coated tubes. In other studies, tail blood samples were taken as described previously [11]. Blood samples were centrifuged at 10,000xg for 30seconds and plasma or serum was removed and frozen at −20°C until assayed. Corticosterone and ACTH concentrations were determined by commercial radioimmunoassays (MP Biomedicals, Solon, OH). Compared samples were measured in the same assays. The intra-assay coefficients of variation (provided by the manufacturer) for corticosterone and ACTH are less than 10% and 15%, respectively.
Statistical analyses were performed using GraphPad Prism v6/v8 (San Diego, CA, USA). One- or two-way ANOVA was used followed by post hoc Tukey’s or Dunnett’s t-test as appropriate. Data are presented as the mean ± SEM. Comparisons in which p values were less than 0.05 were considered statistically significant.
Results and Discussion
In agreement with previous results [9–11], treatment of mice with rimonabant prior to restraint resulted in a significant increase in serum corticosterone compared to vehicle treated males (Figure 1a) and females (Figure 1b). The same doses of AM6545 also significantly potentiated restraint-induced increases in corticosterone.
Fig. 1.
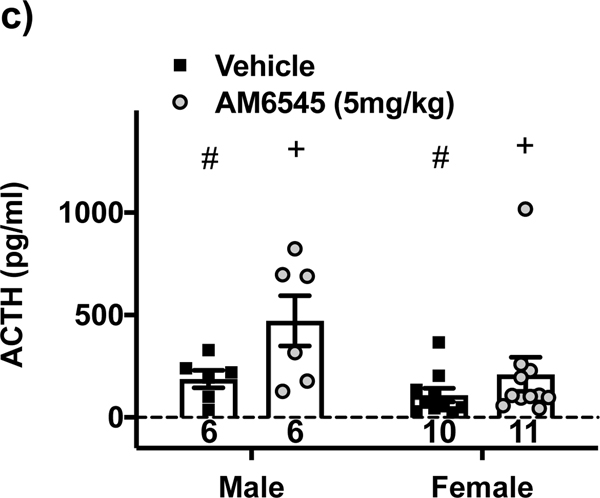
AM6545 potentiates HPA axis activity similar to rimonabant, except there is a non-CB1R component. Male and female ICR mice were injected i.p. with rimonabant (5 or 10 mg/kg), AM6545 (5 or 10 mg/kg) or vehicle 1:1:8 dimethyl sulfoxide:emulphor:saline at the doses indicated 30 min prior to a 30 min restraint. Mice were sacrificed 20 min after restraint ended; trunk or tail blood was collected, and corticosterone or ACTH concentrations were determined using RIA. There are significant differences in corticosterone between a) male groups (F4,64 = 11.9; p<0.0001) and b) female groups (F4,64 = 21; p<0.0001). c) ACTH concentrations were significantly different for main effects of drug treatment (F1,29 = 6.0; p<0.05) and sex (F1,29 = 4.7; p<0.05), however, in the female group one mouse treated with AM6545 had an ACTH value of approximately 1000 pg/ml and so was evaluated as an outlier. According to Grubb’s test the value has a probability of 0.01 – 0.05 of being a statistical outlier (Z = 2.95 and 3.28 with an α = 0.05 and 0.01, respectively). If that data point were removed, ACTH concentrations were significantly different for main effects of drug treatment (F1,28 = 7.5; p<0.05); sex (F1,28 = 14.2; p<0.001); with an interaction (F1,28 = 5.6; p<0.05) that post-test comparisons found vehicle was different than AM6545 for male (p<0.05), but not female mice (p>0.05). d) Restraint-induced corticosterone concentrations in male and female CB1R null mice demonstrated a significant main effect of AM6545 (F1,26 = 8.6; p<0.01) and sex (F1,26 = 25.7; p<0.0001). e) However, blood collected 30 min after recovery when rimonabant or 1:1:18 ethanol:emulphor:saline vehicle was injected 30 min prior to the onset of a 30 min restraint stress in 6–9 month old CB1R null mice demonstrated no significant effect of rimonabant (F1,21 = 0.1; p>0.05) but a significant effect of sex (F1,21 = 50.9; p<0.0001). Bars with vertical lines represent mean data with S.E.M. Significant differences are denoted by **p<0.01 or ***p<0.001 for drug different from vehicle treated group, +p<0.05 or ++p<0.01 for main effects of drug treatment, #p<0.05 or ###p<0.001 for main effects of sex. Sample sizes are given in each bar.
In light of the restriction of AM6545 to the periphery [2], one explanation of these data is that AM6545 acts to increase corticosterone release via a direct effect on the adrenal cortex. CB1R mRNA is present in rodent [14] and human [15] adrenals and CB1R agonism decreases adrenocortical steroidogenesis in basal and stimulated states [14, 15]. If AM6545 increases corticosterone via an isolated effect on the adrenal gland, there would be no effect of AM6545 on ACTH concentrations. Therefore, we determined plasma ACTH concentrations in male and female mice treated with vehicle or AM6545 (5mg/kg) (Figure 1c). AM6545 treatment significantly increased circulating ACTH concentrations in male mice. The effect in female mice was dependent on an ACTH value of approximately 1000pg/ml that was inside the standard curve for the assay, was not associated with any identifiable experimental abnormalities, and is potentially an interesting observation. AM6545 effects may be sexually dimorphic, but further experiments would be necessary to test that hypothesis. While these data do not rule out adrenal effects of AM6545, they suggest that AM6545 acts at the pituitary to increase ACTH release. CB1R expression has been demonstrated in the pituitary [16]; and CB1R signaling has been shown to dampen ACTH release from isolated pituitary cells [17]. This hypothesis is consistent with the low brain bioavailability of AM6545 (the plasma/brain ratio of AM6545 is 33 compared to 1.2 for rimonabant) since the anterior pituitary is outside of the blood brain barrier (BBB) [2].
Despite the high affinity of AM6545 for mouse and rat CB1R (Ki = 1.7 – 3.3nM), previous reports suggest that AM6545 can reduce food intake in rodents via a non-CB1R mechanism [2, 18]. To explore the role of CB1R in the actions of AM6545 on the HPA axis, we examined its effects during restraint in CB1R null mice (Figure 1d). We found that AM6545 increased the corticosterone response to stress in both CB1R null males and females, while the effects of rimonabant were absent in CB1R null mice (Figure 1e), in accord with earlier reports.
The adverse effect profile of AM6545 and other peripherally restricted compounds seems to be more benign given the data presently available. AM6545-induced changes that would suggest a CNS mediated mechanism have thus far been negative for cannabinoid withdrawal [8], catalepsy, ambulation, hypomotility, hypothermia, anxiogenic [2], hedonic substance palatability, or conditioned gaping [18], which would suggest low concentrations in limbic, cortical, subcortical or brainstem structures. Interestingly, i.p. AM6545 did not substitute for rimonabant in a discriminative drinking aversion study in rats [4], but intravenous AM6545 did produce rimonabant-like effects in a discriminative-stimulus study in squirrel monkeys [19]. It is unclear whether study design, species, administration route, CNS concentrations or a peripherally-mediated mechanism, for example the gut-brain axis [20], is responsible for the differences between these studies.
In summary, we found that, like rimonabant, AM6545 increases stress-induced HPA axis activation. The increase in corticosterone is, at least partly, due to elevated ACTH concentrations, which highlights that the BBB does not isolate the pituitary (or potentially other circumventricular regions) from AM6545. Despite the previously published literature suggesting that AM6545 should not have effects on the hypothalamus or higher brain regions, our data do not rule out actions in these areas. Peripherally restricted drugs are a major advancement worthy of enthusiasm; however, even in the absence of pituitary effects, alterations of peripheral pathways (for example of the adrenal glands or autonomic nervous system) could result in short- or long-term effects. It is our hope that other newly developed peripherally restricted drugs are tested to assess their effects at these peripheral sites. Effects at various peripheral sites do not necessarily preclude the successful clinical use of compounds with limited brain penetrance for the treatment of obesity or other disorders.
Acknowledgements
The authors would like to thank Alexandros Makriyannis for his generosity in providing AM6545 for these studies.
Funding
This work was supported by NIDA grants R21 DA022439 and R01 DA026996. CJR was partially supported by T32 GM080202 and T32 GM089586. Partial funding was provided by the Research and Education Program, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin.
Footnotes
Research Resource Identifiers (RRID)
Software: GraphPad Prism v8 RRID:SCR_002798
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Nissen SE, et al. , Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA, 2008. 299(13): p. 1547–60. [DOI] [PubMed] [Google Scholar]
- 2.Tam J, et al. , Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. Journal of Clinical Investigation, 2010. 120(8): p. 2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randall PA, et al. , The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacology Biochemistry and Behavior, 2010. 97(1): p. 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Järbe TUC, et al. , Central mediation and differential blockade by cannabinergics of the discriminative stimulus effects of the cannabinoid CB1 receptor antagonist rimonabant in rats. Psychopharmacology, 2011. 216(3): p. 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon MR, et al. , Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J, 2014. 28(12): p. 5361–75. [DOI] [PubMed] [Google Scholar]
- 6.Bowles NP, et al. , A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc Natl Acad Sci U S A, 2015. 112(1): p. 285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argueta DA and Dipatrizio NV, Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiology & Behavior, 2017. 171: p. 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai S, et al. , Cannabinoid withdrawal in mice: inverse agonist vs neutral antagonist. Psychopharmacology, 2015. 232(15): p. 2751–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillard CJ, Beatka M, and Sarvaideo J, Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr Physiol, 2016. 7(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S, et al. , Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology, 2004. 145(12): p. 5431–8. [DOI] [PubMed] [Google Scholar]
- 11.Hill MN, et al. , Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci, 2011. 31(29): p. 10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan B, Hillard CJ, and Liu QS, Endocannabinoid Signaling Mediates Cocaine-Induced Inhibitory Synaptic Plasticity in Midbrain Dopamine Neurons. 2008. 28(6): p. 1385–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim MM, et al. , Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. 2003. 100(18): p. 10529–10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surkin PN, et al. , Pharmacological augmentation of endocannabinoid signaling reduces the neuroendocrine response to stress. Psychoneuroendocrinology, 2018. 87: p. 131–140. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler CG, et al. , Expression and function of endocannabinoid receptors in the human adrenal cortex. Horm Metab Res, 2010. 42(2): p. 88–92. [DOI] [PubMed] [Google Scholar]
- 16.Wenger T, Fernandez-Ruiz JJ, and Ramos JA, Immunocytochemical demonstration of CB1 cannabinoid receptors in the anterior lobe of the pituitary gland. J Neuroendocrinol, 1999. 11(11): p. 873–8. [DOI] [PubMed] [Google Scholar]
- 17.Cota D, et al. , Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology, 2007. 148(4): p. 1574–81. [DOI] [PubMed] [Google Scholar]
- 18.Cluny NL, et al. , A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol, 2010. 161(3): p. 629–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kangas BD, et al. , Cannabinoid Antagonist Drug Discrimination in Nonhuman Primates. Journal of Pharmacology and Experimental Therapeutics, 2019: p. jpet.119.261818. [DOI] [PMC free article] [PubMed]
- 20.Argueta DA, et al. , Cannabinoid CB1 Receptors Inhibit Gut-Brain Satiation Signaling in Diet-Induced Obesity. Frontiers in Physiology, 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]