To the Editor:
Hypersensitivity pneumonitis (HP) is an immune-mediated disease that manifests as interstitial lung disease (ILD) in susceptible individuals after exposure to an inducing factor (1).
Recently, two societally endorsed clinical practice guidelines for the diagnosis of HP were published; although they address somewhat different questions to inform their clinical recommendations, overall they are mutually complementary (2, 3). Both guidelines present criteria for establishing the diagnosis in a patient with ILD suspected to have HP, with a few differences, using combinations of several specific features to support or rule out disease.
Both documents’ diagnostic criteria are rooted in three domains: 1) exposure identification, 2) high-resolution computed tomography (HRCT), and 3) BAL lymphocytosis, which—in the case of the American Thoracic Society (ATS), Japanese Respiratory Society (JRS), and Asociación Latinoamericana del Tórax (ALAT) guideline—is reinforced by histopathology to increase diagnostic accuracy (definite diagnosis) (2).
The two guidelines present differing algorithmic approaches to arrive at a confident diagnosis, with strong considerations of diagnostic likelihood. In this study, we applied both diagnostic approaches to a well-characterized cohort of patients with HP to determine how the guidelines perform in a real-world setting and to identify differences in diagnostic confidence.
Methods
We applied the diagnostic criteria of both guidelines to 144 cases with a previously confirmed diagnosis of HP at the National Institute of Respiratory Diseases in Mexico. Patients with nonfibrotic and fibrotic HP were included, with the latter classified based on the presence of HRCT fibrosis. All patients had exposure information, HRCT results, and BAL cell profile. Antigen identification was uniformly determined using a systematic exposure history and the presence of specific circulating antibodies. Lymphocytosis was defined as the presence of lymphocytes ⩾30%, and 70% of patients (101/144) had BAL lymphocytosis. Eighteen percent of patients (26/144) also had histopathology confirmation. The diagnosis was established after review by our Multidisciplinary Discussion Team (MDT).
The ATS/JRS/ALAT guideline presents five grades of confidence: definite (>90% diagnostic likelihood), high confidence (80–89%), moderate confidence (70–79%), low confidence (51–69%), and HP considered not excluded (⩽50%). The American College of Chest Physicians (CHEST) guideline presents a four-tiered ontology: confident diagnosis (>90%), provisional high confidence (70–89%), provisional low confidence (51–69%), and unlikely (⩽50%, as previously proposed) (4). A chi-square test was used to compare the grades of confidence in both algorithms. This study was approved by the institutional ethics committee (C42-14).
Results
A total of 144 patients with HP (n = 44 nonfibrotic, n = 100 fibrotic) were included in the study, 85% female and 35% with unknown antigen exposure.
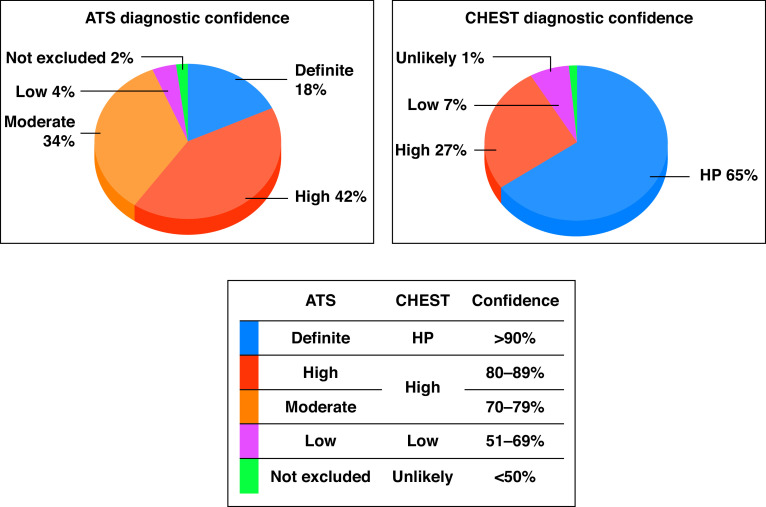
When we applied the diagnostic criteria from ATS/JRS/ALAT, only 26 biopsied patients (18%) could be classified as having definitive HP. By contrast, 94 patients (65%) were classified as having confident HP when the CHEST criteria were used (P < 0.0001).
High/moderate confidence (which in both guidelines included 70–89% likelihood, although these are separated in the ATS/JRS/ALAT guideline) was found in 109 patients (76%) by the ATS/JRS/ALAT criteria compared with 38 patients (27%) by the CHEST criteria (P < 0.0001). When confident/definite HP and high/moderate confidence were considered together, 135 patients were classified as such using the ATS/JRS/ALAT criteria and 132 patients using the CHEST criteria. No difference was found in the number of patients classified as low confidence or unlikely HP between the two guidelines (Figure 1).
Figure 1.
Pie chart showing the distribution of the 144 patients included in the study according to the categories established in each guideline. ATS = American Thoracic Society; CHEST = American College of Chest Physicians; HP = hypersensitivity pneumonitis.
Discussion
HP has a heterogeneous clinical presentation and diverse radiological/morphological patterns that may mimic other inflammatory and fibrotic ILD. Importantly, fibrotic HP should be considered in the differential diagnosis for patients consulting for a fibrotic ILD, mainly idiopathic pulmonary fibrosis (IPF) (1–3, 5). Additionally, because of the frequent absence of identification of the source of antigen exposure (6, 7), it is relevant to have diagnostic criteria and algorithms that allow for a more precise diagnosis when a patient with newly detected ILD is evaluated.
The ATS/JRS/ALAT guideline presents five diagnostic confidence categories separating high (80–89%) and moderate (70–79%) confidence, whereas the CHEST guideline unifies both likelihoods as “provisional high confidence” (70–89%) and includes “unlikely,” which is not considered in the ATS/JRS/ALAT guideline.
Our findings indicate that the principal difference between the two guidelines is that the ATS/JRS/ALAT guideline is more restrictive in determining a definitive diagnosis, yielding markedly fewer confident diagnoses. This occurred because the algorithm requires morphology even in clinical scenarios considering an antigen-exposed patient with typical HRCT and BAL lymphocytosis. The emphasis on biopsy is so great that indeterminate histopathology transforms a high-confidence diagnosis into a definitive diagnosis, and a moderate-confidence diagnosis into a high-confidence diagnosis, even with an unidentified antigen. However, in real life, the percentage of patients with ILD who are biopsied is low. In this setting, the CHEST algorithm is less stringent, supporting a confident diagnosis considering only these three domains, without the need for biopsy. Moreover, the CHEST guideline supports an HP diagnosis even if the patient has only a combination of typical HRCT and positive exposure. Similarly, a provisional high-confidence diagnosis of fibrotic HP can be established in a patient with indeterminate HRCT pattern fibrosis without other features suggestive of HP, if antigen exposure is identified and demonstrates BAL lymphocytosis. For example, a patient with an identified antigen, indeterminate HRCT pattern, and BAL lymphocytosis would only achieve a low-confidence provisional diagnosis of HP according to the ATS/JRS/ALAT criteria but a provisional high-confidence diagnosis according to the CHEST criteria.
Additionally, CHEST criteria classify using provisional high-confidence, a combination that may result in only 70% (the lower limit of) probability, whereas the ATS/JRS/ALAT guideline separates high confidence from moderate confidence (Table 1). This approach is supported by IPF data demonstrating that a majority of clinicians would initiate IPF-specific therapy at a threshold of 70% confidence (8). Finally, both sets of diagnostic criteria are equally appropriate to determine which patients have a low likelihood of having or are unlikely to have HP, opening a quick window to search for an alternative diagnosis.
Table 1.
HP Diagnostic Confidence in Both Guidelines
| Degree of Diagnostic Confidence | ATS/JRS/ALAT (n = 144) [n (%)] | CHEST (n = 144) [n (%)] | P Value |
|---|---|---|---|
| Definite/HP | 26 (18) | 94 (65) | <0.0001 |
| High/moderate* | 109 (76) | 38 (27) | <0.0001 |
| High | 60 (42) | 38 (27) | 0.008 |
| Moderate | 49 (34) | — | — |
| Low | 6 (4) | 10 (7) | 0.44 |
| Not excluded/unlikely | 3 (2) | 2 (1) | 1.0 |
Definition of abbreviations: ALAT = Asociación Latinoamericana del Tórax; ATS = American Thoracic Society; CHEST = American College of Chest Physicians; HP = hypersensitivity pneumonitis; JRS = Japanese Respiratory Society.
ATS/JRS/ALAT guideline separates high confidence (80–89%) from moderate confidence (70–79%), whereas the CHEST guideline unifies these under the category provisional high confidence (70–89%).
In summary, despite the fact that both guidelines use similar domains for the diagnosis of HP, the agreement between them was low for the definitive/high-confidence diagnosis among patients included in this study. Our study has some limitations, mainly that it was performed in only one center (albeit one with recognized experience in ILD), and our findings may not be generalizable to all centers. Prospective studies with large and diverse cohorts of patients suspected to have HP are necessary to test the performance of each approach to accurately establish HP diagnoses and inform future versions of these guidelines.
The questions remains as to whether we are going to overdiagnose HP with the CHEST algorithm (patient’s respiratory problem is mistakenly attributed to HP when another ILD is responsible) or underdiagnose HP by applying the ATS/JRS/ALAT algorithm (the clinician attributes the patient’s findings to another ILD). In either situation, the inaccurate identification of fibrotic HP instead of IPF may have significant therapeutic consequences, with the initiation of antiinflammatory/immunosuppressive drugs instead of antifibrotic therapy or vice versa. Furthermore, it remains to be shown whether a 70% confidence level is sufficient to initiate disease-appropriate management for patients with suspected HP, and whether the pursuit of higher confidence positively impacts patient outcomes or introduces unnecessary risk to the patient.
Certainly, this situation illustrates the importance of MDT engagement—which both guidelines emphasize as relevant—to improve diagnostic accuracy. We hope that this recommendation will encourage the formation of MDTs as standard of care for HP diagnosis. Future work should test the performance of these guidelines to differentiate HP from other ILDs in diverse and prospective cohorts, with an additional focus on developing robust studies that can answer the population, intervention, comparator, and outcome (PICO) questions stated in each guideline.
Acknowledgments
Acknowledgment
The authors thank the radiologist Fortunato Juárez for help with the HRCT readings.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202105-1091LE on July 7, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Costabel U, Miyazaki Y, Pardo A, Koschel D, Bonella F, Spagnolo P, et al. Hypersensitivity pneumonitis. Nat Rev Dis Primers. 2020;6:65. doi: 10.1038/s41572-020-0191-z. [DOI] [PubMed] [Google Scholar]
- 2. Raghu G, Remy-Jardin M, Ryerson CJ, Myers JL, Kreuter M, Vasakova M, et al. Diagnosis of hypersensitivity pneumonitis in adults: an official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2020;202:e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández Pérez ER, Travis WD, Lynch DA, Brown KK, Johannson KA, Selman M, et al. Executive summary: diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report Chest 2021160595–615.. [DOI] [PubMed] [Google Scholar]
- 4. Ryerson CJ, Corte TJ, Lee JS, Richeldi L, Walsh SLF, Myers JL, et al. A standardized diagnostic ontology for fibrotic interstitial lung disease: an International Working Group perspective. Am J Respir Crit Care Med. 2017;196:1249–1254. doi: 10.1164/rccm.201702-0400PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasakova M, Morell F, Walsh S, Leslie K, Raghu G. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med. 2017;196:680–689. doi: 10.1164/rccm.201611-2201PP. [DOI] [PubMed] [Google Scholar]
- 6. Fernández Pérez ER, Swigris JJ, Forssén AV, Tourin O, Solomon JJ, Huie TJ, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144:1644–1651. doi: 10.1378/chest.12-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johannson KA, Barnes H, Bellanger AP, Dalphin JC, Fernández Pérez ER, Flaherty KR, et al. Exposure assessment tools for hypersensitivity pneumonitis. an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2020;17:1501–1509. doi: 10.1513/AnnalsATS.202008-942ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh SLF, Lederer DJ, Ryerson CJ, Kolb M, Maher TM, Nusser R, et al. Diagnostic likelihood thresholds that define a working diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200:1146–1153. doi: 10.1164/rccm.201903-0493OC. [DOI] [PubMed] [Google Scholar]