Modern treatment algorithms for pulmonary arterial hypertension (PAH) using multiparametric risk stratification have improved outcomes for patients with PAH (1). Currently, treatment algorithms propose upfront triple combination therapy, including a parenteral prostacyclin, for high-risk patients, citing observational studies (2), and upfront dual oral combination therapy for the majority of low- and intermediate-risk patients based on the Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension (AMBITION) trial, which demonstrated a 50% relative risk reduction for time to clinical failure with combination therapy (3).
In this context, in this issue of the Journal, Boucly and colleagues (pp. 842–854) present a retrospective cohort study evaluating the association between initial treatment strategy and survival among patients with newly diagnosed PAH using the French PH Registry (4). The study included 1,611 patients, of whom 984, 551, and 76 were treated with an initial strategy of mono, dual, or triple therapy with a parenteral prostacyclin and were followed for a median of 32 months. The primary outcomes were overall survival and transplant-free survival. The triple therapy group was younger with fewer comorbidities but more severe PAH. Triple therapy was associated with improved survival (91% vs. 61%) and transplant-free survival (75% vs. 56% for monotherapy and 58% for dual combination) at 5 years, with improvements similarly seen in a propensity-matched cohort. After adjusting for mortality risk factors, triple therapy was associated with a >70% lower risk of death. Importantly, triple combination therapy was associated with improved survival in 1,135 intermediate-risk patients. Limitations of the study include the observational nature and likely selection bias in therapy choice.
Boucly and colleagues should be commended on this analysis with relevant implications for patients with newly diagnosed PAH. The study is the largest real-world cohort to support the overall treatment strategy from the 2018 World Symposium (2). It adds to the observational evidence for upfront combination therapy including a parenteral prostacyclin for high-risk patients. Notably, it provides the first evidence for the potential role of earlier triple combination therapy with a parenteral prostacyclin for intermediate-risk disease.
So, should all patients with newly diagnosed PAH be treated with upfront triple combination therapy? Perhaps not all; however, this work compels us to look closely at the intermediate-risk group. The majority of patients, 70% in the current study, fall into this broad category. Unfortunately, current risk assessment tools cannot differentiate responders or those likely to progress among this group regardless of treatment strategy. More precise risk stratification, potentially including parameters of right ventricular function, might inform more personalized treatment decisions. Until this becomes available, the evidence suggests that perhaps we should be more ambitious than AMBITION.
This begins with considering the downsides of not prescribing upfront triple therapy with a parenteral prostacyclin in intermediate-risk patients. A closer look at AMBITION highlights that a satisfactory clinical response was unacceptably low, occurring in only 39% of the combination arm (3). Real-world data suggest that a minority of patients treated with upfront oral combination therapy reach low-risk status (5). At the same time, there is ample evidence that the timing of therapies matter—treatments are more efficacious early (6), mortality is higher in incident versus prevalent patients (7), clinical worsening that may trigger escalation is associated with increased mortality (8), and, most disconcerting, the functional capacity of the placebo group in open-label extensions of randomized controlled trials (RCTs) that receive therapy just 12–16 weeks later never catch up (9).
With most patients not meeting treatment goals and recognizing that timing matters, the risks, uncertainties, and benefits of upfront triple combination therapy including a parenteral prostacyclin in intermediate-risk patients should be considered. The potential disadvantages include inconvenient delivery, side effects, complications such as infection, and cost. The uncertainties revolve around the absence of an RCT. The AMBITION and The Efficacy and Safety of Initial Triple versus Initial Dual Oral Combination Therapy in Patients with Newly Diagnosed Pulmonary Arterial Hypertension (TRITON) RCTs have provided the framework for combination oral therapy for low- and intermediate-risk patients. The landmark AMBITION trial found that upfront combination therapy with ambrisentan and tadalafil resulted in a 50% relative risk reduction for time to clinical failure compared with monotherapy, and is the cornerstone of the recommendation for upfront combination therapy for most patients with PAH (3). The TRITON trial, which has been previously reported in the form of an abstract, filled a critical gap showing that the addition of the oral prostacyclin agonist selexipeg to macitentan and tadalafil was not superior to initial dual therapy in decreasing pulmonary vascular resistance (10). However, these results are not generalizable to the more potent parenteral therapy combination strategy being evaluated in the present analysis. The evidence of benefit for parenteral prostacyclin in high-risk patients would make an RCT unethical in this group. Although an RCT of upfront parenteral prostacyclin combination therapy in intermediate-risk patients would be informative, it is likely not feasible for several reasons, including ethics in blinding to long-term administration of placebo through a central line, challenges that would arise with enrollment because patients would be unlikely to want to go on oral medications, and lack of funding. There are now several observational studies that have demonstrated a survival benefit of upfront triple combination therapy with a parenteral prostacyclin (11–13). Boucly and colleagues extend this observation to an intermediate-risk group. There has yet to be a study that showed worse outcomes with combination therapy, and upfront triple combination therapy allows for deescalation should there be an excellent response. Taken together, the treatment paradigm should evolve. Optimal medical therapy for intermediate-risk patients should include either upfront triple combination therapy with a parenteral prostacyclin or dual oral combination therapy with a rapid reassessment to ensure response (Figure 1).
Figure 1.
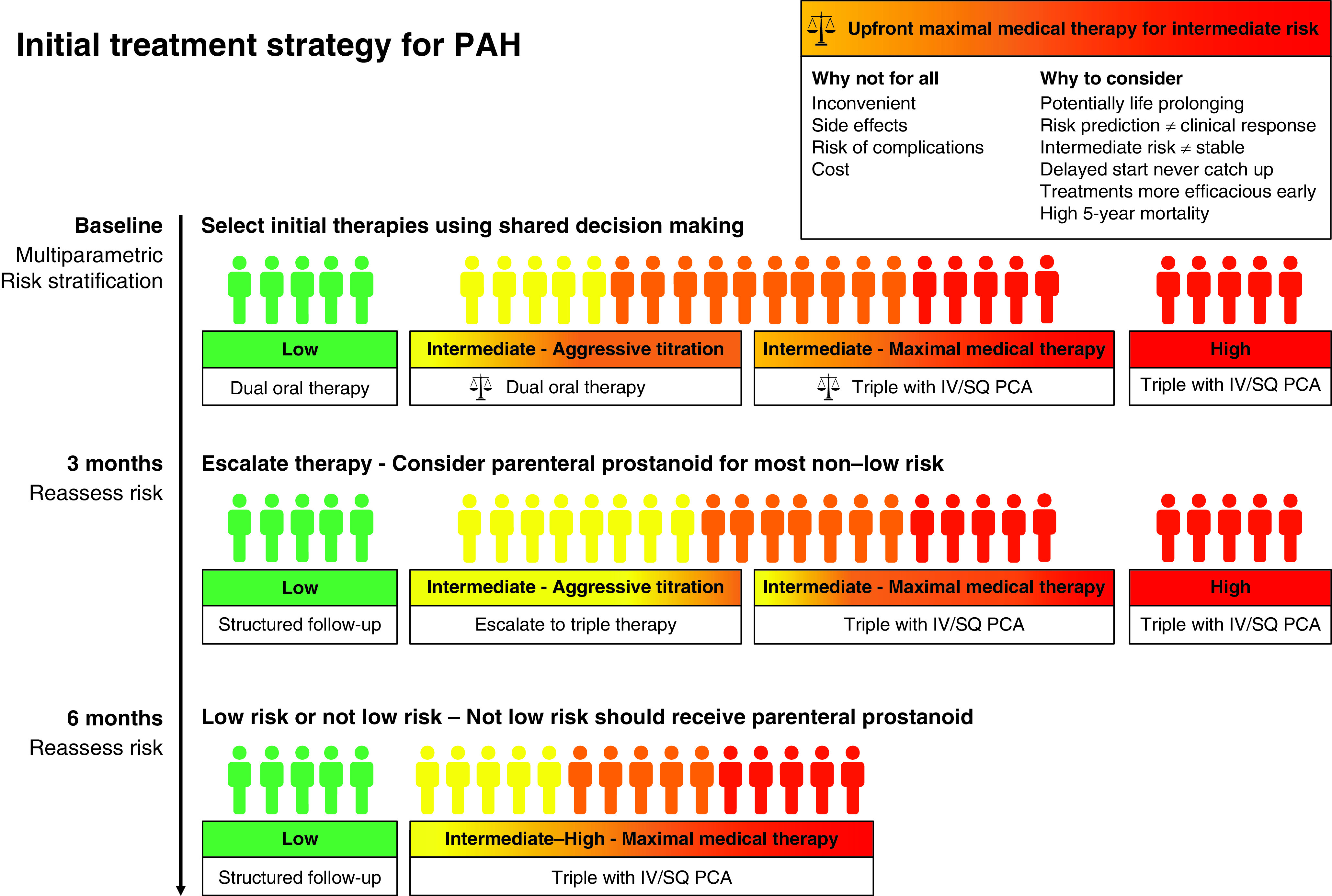
Initial treatment strategy for pulmonary arterial hypertension. Intermediate-risk patients can be treated with an initial treatment strategy of either maximal medical therapy with triple combination therapy, including a parenteral prostacyclin, or aggressive titration with dual oral therapy. Selection of a treatment strategy requires shared decision-making. The risks, uncertainties, and potential benefits of maximal medical therapy with upfront triple combination therapy including a parenteral prostacyclin should be discussed at the time of diagnosis. There should be a plan for a rapid reassessment of response at 3 months if an aggressive titration strategy is chosen. IV = intravenous; PAH = pulmonary arterial hypertension; PCA = prostacyclin analog; SQ = subcutaneous.
In conclusion, Boucly and colleagues add to the evidence for upfront triple combination therapy with a parenteral prostacyclin for patients with PAH. In an ideal world, this study would set the stage for an RCT of combination therapy including parental prostacyclin therapy in intermediate-risk patients with PAH, which is unfortunately unlikely to be feasible. Short of this and until risk stratification improves, the treatment pathway that likely offers the highest probability of long-term survival for most intermediate-risk patients with PAH is triple combination therapy with a parenteral prostacyclin; whether it is started at the time of diagnosis depends on the patient’s ambitions.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202107-1625ED on August 17, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lajoie AC, Lauzière G, Lega JC, Lacasse Y, Martin S, Simard S, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 2. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 4. Boucly A, Savale L, Jaïs X, Bauer F, Bergot E, Bertoletti L, et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;204:842–854. doi: 10.1164/rccm.202009-3698OC. [DOI] [PubMed] [Google Scholar]
- 5. Badagliacca R, D’Alto M, Ghio S, Argiento P, Bellomo V, Brunetti ND, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;203:484–492. doi: 10.1164/rccm.202004-1006OC. [DOI] [PubMed] [Google Scholar]
- 6. Gaine S, Sitbon O, Channick RN, Chin KM, Sauter R, Galiè N, et al. Relationship between time from diagnosis and morbidity/mortality in pulmonary arterial hypertension: results from the phase III GRIPHON study. Chest. 2021;160:277–286. doi: 10.1016/j.chest.2021.01.066. [DOI] [PubMed] [Google Scholar]
- 7. Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, et al. French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 8. McLaughlin VV, Hoeper MM, Channick RN, Chin KM, Delcroix M, Gaine S, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol. 2018;71:752–763. doi: 10.1016/j.jacc.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 9. Vizza CD, Badagliacca R, Messick CR, Rao Y, Nelsen AC, Benza RL. The impact of delayed treatment on 6-minute walk distance test in patients with pulmonary arterial hypertension: a meta-analysis. Int J Cardiol. 2018;254:299–301. doi: 10.1016/j.ijcard.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 10. Sitbon O, Doelberg M, Gibbs JSSR, Hoeper MM, Martin N, Mathai SC, et al. Efficacy and safety of initial triple oral versus initial double oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension (PAH): results of the randomized controlled TRITON study [abstract] Am J Respir Crit Care Med. 2020;201:A2928. [Google Scholar]
- 11. Kemp K, Savale L, O’Callaghan DS, Jaïs X, Montani D, Humbert M, et al. Usefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study. J Heart Lung Transplant. 2012;31:150–158. doi: 10.1016/j.healun.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 12. Sitbon O, Jaïs X, Savale L, Cottin V, Bergot E, Macari EA, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014;43:1691–1697. doi: 10.1183/09031936.00116313. [DOI] [PubMed] [Google Scholar]
- 13. D’Alto M, Badagliacca R, Argiento P, Romeo E, Farro A, Papa S, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest. 2020;157:376–383. doi: 10.1016/j.chest.2019.09.009. [DOI] [PubMed] [Google Scholar]


