Abstract
Aging is characterized by a progressive inability to maintain homeostasis, self-repair, renewal, performance, and fitness of different tissues throughout the lifespan. Senescence is occurring following enormous intracellular or extracellular stress stimuli. Cellular senescence serves as an antiproliferative process that causes permanent cell cycle arrest and restricts the lifespan. Senescent cells are characterized by terminal cell cycle arrest, enlarged lysosome, and DNA double-strand breaks as well as lipofuscin granularity, senescence-associated heterochromatin foci, and activation of DNA damage response. Curcumin, a hydrophobic polyphenol, is a bioactive chemical constituent of the rhizomes of Curcuma longa Linn (turmeric), which has been extensively used for the alleviation of various human disorders. In addition to its pleiotropic effects, curcumin has been suggested to have antiaging features. In this review, we summarized the therapeutic potential of curcumin in the prevention and delaying of the aging process.
1. Introduction
Aging is identified by a progressive inability to maintain homeostasis, self-repair, renewal, performance, and fitness of different tissues with advancing age [1]. The picture of aging is characterized by genetic and environmental factors ultimately leading to gradual but persistent reduction in cellular proliferation, abnormal oxygen metabolism, and structural instability [2]. A complex gene network contributes to organism lifespan by regulating several critical pathways including protein synthesis and catabolism, energy metabolism, redox balance, intracellular communication, DNA repair, inflammation, cellular senescence, and death [3]. The aging process also involves the vascular system. In this context, cell senescence involving either endothelial cells (ECs) or vascular smooth muscle cells (VSMCs) [4] determines structural and functional alterations resulting in development of endothelial dysfunction [5]. Previous researches identified several molecules and signaling pathways involved in the aging process: among them, growth hormone (GH)/insulin-like growth factor 1(IGF1)/forkhead box O (FOXO) pathway, target of rapamycin (TOR)/ribosomal S6 kinase (S6K), sirtuins (Sirts), p38 mitogen-activated protein kinase (MAPK), and AMP-activated protein kinase (AMPK) [6–8]. Despite many efforts in clarifying the biology of aging and its cellular and molecular mechanisms, standardized biomarkers and therapeutic targets are scarce. Only several senotherapeutics, agents which inhibit senescence (senomorphics) and selectively kill senescent cells (senolytics), have been proposed. Senolytics are drugs that particularly target senescent cells through promoting the apoptosis of senescence [9–11].
In this field of research, there is a growing interest towards the natural compound curcumin (CUR; diferuloylmethane), which is known as an active therapeutic compound against various human disorders owing to its numerous pharmacological actions [12–17]. In light of this, research groups worldwide are attempting to clarify biological pathways, pharmaceutical properties, and potential clinical application of CUR [18]. In this narrative review, we will summarize the therapeutic potential of CUR, especially focusing on prevention and delaying of the aging process.
2. Hallmarks of Aging
2.1. Oxidative Stress
A prooxidant environment certainly contributes to the aging process by sustaining oxidative modifications of cellular molecules [19–21]. Targets of oxidative stress (OS) include structural damage in cellular macromolecules such as nuclear and mitochondrial DNA, proteins, and lipids [22]. Nevertheless, the “free radical theory of aging” is no longer considered a primitive causal pathway. Free radicals and related oxidants are a subset of stressors with which all living beings must cope with over their lifespans. Rather, the concept of “defective adaptive homeostasis” better describes how aging organisms fail to dynamically expand the homeostatic range of stress defense and repair systems. Indeed, many signal transduction pathways contribute to best fit cellular response to a particular need.
2.2. Cellular Senescence
Cellular response to stressors includes three distinctive cellular processes: apoptosis, autophagy, and senescence [23–25]. The latter (from the latin term “senex”: growing old) occurs in response to enormous intracellular or extracellular stress stimuli [26]. Cellular senescence was firstly described by Hayflick and Moorhead [27] as an antiproliferative process leading to permanent cell cycle arrest lifespan reduction [25]. Such effect on the biological clock (Hayflick limit) is generally associated with progressive telomere attrition/dysfunction [28, 29], loss of proteostasis, induction of genes located in the INK4a/ARF locus [30], aberrant oncogene activation, DNA damage during cell division/replication, and apoptosis-resistance [31]. Leading mediators of cellular senescence include the p16INK4a/Rb and tumor suppressor p53/p21 CIP1/WAF1 families of cyclin-dependent kinase (CDK) [32]. Senescent cells endure futile growth, hypertrophy, and hyperfunctions, together with generation and release of inflammatory mediators named senescence-associated secretory phenotype (SASP) [33, 34]. SASP includes multiple inflammatory elements such as interleukin- (IL-) 6, IL-8, IL-1, tumor necrosis factor-α (TNF-α), nuclear factor kappa B (NF-κB), and growth factors like insulin-like growth factor- (IGF-) 1, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) [35, 36].
Alongside SASP, the core event in cellular senescence cell nucleus are the disturbances in DNA repair mechanisms, which determine DNA double-strand breaks senescence-associated heterochromatin foci (SAHF), terminal cell cycle arrest with resistance to apoptosis, and loss of regeneration/resilience [37]. Additional features include enlarged lysosomes, overexpression of senescence associated β-galactosidase (SA-β-gal), and lipofuscin granularity as well. A relevant feature of aging is chronic low grade inflammation, referred to as “inflammaging” which is the age-related inflammatory status, results from immunosenescence, as it is found to be associated with the majority of age-related diseases sharing an inflammatory basis [38]. Together with immunological elements, cellular senescence and the SASP are the major contributors to inflammaging.
That cellular senescence may have a causative role in organismal aging [39]. During aging, senescent cells are possibly persistent, activated by random molecular damage and related with the activation of a DNA damage response [40]. The collection of senescent cells in animal organs may be involved in the aging process through reducing the renewal competence of tissues [30] and/or via reforming the tissue structure and activity by secretion of matrix metalloproteinases, epithelial growth factors, and inflammatory mediators which could intrude with the tissue microenvironment [41]. Therefore, tissue homeostasis will be compromised which finally will result to aging.
2.3. Sirtuins
Sirtuins are NAD+-dependent deacetylases, ubiquitously distributed in either prokaryote or eukaryote cells [42]. In mammalians, 7 Sirt genes (Sirt1 to Sirt7) have been identified. Sirt1 belongs to the class III histone deacetylases (HDAC) with activity on various transcriptional factors (TFs), histones, and cytoplasmic proteins with acyl-lysine residues [43]. Antiaging properties of Sirt1 include the suppression of a typical senescent secretome through epigenetic gene modulation [44]. However, the antiaging effects of Sirt1 are far from being elucidated, potentially ranging from mitochondrial respiration to stress modulation, energy expenditure, and p53 deacetylation [37, 45].
3. Curcumin
Due to their ubiquitous distribution in food, phytochemicals attract more attention because of their obvious safety. Accumulating evidences reported how phytochemicals that can extend lifespan also enhance wellness in different heterotrophic organisms [46–49]. The hydrophobic yellow polyphenol CUR is a bioactive chemical constituent of the rhizome of Curcuma longa Linn, extensively used in cooking as food coloring and preservative. CUR is the main chief ingredient of turmeric representing nearly 2–5% of the plant [50]. Toxicity studies claimed it is a safe compound agent even at high doses [51]. Concerning effectiveness, several lines of evidence highlighted a pleiotropic potential of CUR towards several human diseases, such as malignancies, skin and immune-related disorders, cardiovascular diseases, pulmonary and renal fibrosis, nonalcoholic fatty liver disease (NAFLD), fatigue, neuropathic pain, bone and muscle loss, neurodegenerative disease, ocular diseases, leprosy, osteoporosis, leishmaniosis, and HIV infection [52–57]. Pleiotropic functions of CUR mainly rely on the inhibition of IκB kinase (IKK) phosphorylation [58] and the consequent suppression of the nuclear translocation of the NF-κB p65 subunit [59]. As an alternative epigenetic modulator, CUR also enhances Sirt1 expression at both mRNA and protein levels, ultimately resulting in the suppression of histone acetyltransferase (HAT) activity and increased NAD+/NADH ratio [60, 61]. With the same mechanism, CUR modulated the expression of several types of microRNAs [62–65]. Through those mechanisms, CUR supplementation in human melanoma cells induces growth arrest in the G2/M phase and then apoptosis [66]. Other studies also reported that CUR may target oncogene expression, angiogenesis, invasion, and metastatic dissemination [67, 68] by interfering with several other intracellular pathways including hypoxia-inducible factor-1 α (HIF-1 α), mammalian sterile 20-like kinase 1 (MST1), enhancer of zeste homolog 2 (EZH2), platelet-derived growth factor (PDGF) receptor binding, Wnt/β-catenin, transforming growth factor beta (TGF-β), Sonic Hedgehog, Notch, and phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) cascade [69–71]. Alongside with antitumorigenic activity, CUR was also shown to induce antimicrobial, antioxidant, antiglycemic, antiseptic, and analgesic effects [72–74]. This “pleiotropic” potential may be ascribed to the potent metal-chelating effects of CUR, which include the scavenging of the superoxide anion, hydroxyl radical, singlet oxygen, and nitrogen dioxide [75, 76]. In line with this, other studies demonstrated that CUR may reduce levels of malondialdehyde (MDA), protein carbonyls, thiols, and nitrotyrosines. With regard to inflammation, CUR stimulates a xenobiotic response with upregulation of defense genes (e.g., phase II enzymes and hemeoxygenase-1 [HO-1]) [77] and suppression of proinflammatory transcription factors (e.g., activator protein-1 [AP1]) and cytokines (e.g., TNF-α, IL-1b, IL-6, IL-8, and monocyte chemotactic protein 1 [MCP-1]), signal transducer activator of transcription (STAT), peroxisome proliferator-activated receptor-γ (PPAR-γ), activating transcription factor 3 (ATF3), C/EBP homologous protein (CHOP), and the inducible inflammatory enzymes cyclooxygenase- (COX-) 2 and metalloproteinases [78].
Finally, as observed in human skin fibroblasts, CUR may activate cellular stress response by interacting with the thiol-disulfide redox system. Such stress determines a rise in cellular GSH amounts via HO-1 and nuclear factor E2-related factor 2 (NRF2) signaling [79], ultimately improving cellular antioxidant defenses [80, 81]. Moreover, several studies indicated that CUR and may be used as senolytic and anti-inflammatory agents for senescent cells [82, 83]. For instance, a CUR analog, EF24, promoted senescent cell apoptosis and showed protection effect against ionizing-stimulated senescent cells [83].
4. Effect of Curcumin on Aging/Longevity
4.1. Vascular Aging
Further enhancing a wide spectrum of activity, growing evidence indicates CUR as a promising antiaging agent (Table 1; Figure 1) [84, 85]. The effects of CUR feeding have been largely investigated in animal models, unanimously reporting a suppression of intermediated oxidative stress (e.g., lipoxygenases [LPO], MDA, lipofuscin granules, and NO) and inflammation [3, 86]. By chelating nitrogen dioxide (NO2), CUR administration in mice significantly attenuates nitric oxide- (NO-) associated vascular endothelial dysfunction and generation of advanced glycation end-products (AGEs), leading determinants of age-related large elastic artery stiffening [87]. As an additional mechanism, CUR fixes lysosomal membranes and reduces the function of lysosomal acid hydrolases, thus preventing the aberrant deposition of different connective tissue components in aging endothelium. A similar upgrade in endothelial function was also observed in postmenopausal women after eight weeks of treatment [88], whereas in elderly with diabetes and cardiomyopathy, CUR mitigated hypertrophy in the aging heart via suppression of p300, the global transcription activator [89]. Beneficial effects of CUR on vascular aging also concern the development of age-related macular degeneration (AMD), one of the most important causes of blindness in elderly [90, 91]. CUR remarkably increases the viability of retinal pigment epithelial cells (RPECs) modulating their proliferation apoptosis and OS [92]. Overall, those evidences suggest potential application of CUR as an innovative approach to AMD, as for other ocular diseases (e.g., ocular dryness, conjunctivitis, uveitis, pterygium, and glaucoma) [93]. Even CUR has been found to prevent the development of cataract in diabetic rats by decreasing AGE accumulation and serum LPO [94, 95]. Aging-associated cerebrovascular endothelial dysfunction with consequent chronic cerebral ischemia also plays a critical role in stroke, as well as in cerebral amyloid angiopathy, cognitive impairments, and neurodegenerative disorders [96–98]. One of the main pathological mechanisms behind this effect is the generation of ROS, due to the suppression of mitochondrial uncoupling protein 2 (UCP2) [99] and the downregulation of AMPK. CUR reverses those effects in cultured ECs, whereas in experimental models, prolonged CUR feeding decreased ROS generation and promoted cerebrovascular endothelium-dependent relaxation, finally leading to improved cerebrovascular function [100–103]. Neuroprotective effects of CUR due to UCP2 overexpression suppression especially target hippocampal neurogenesis in the CA1 area, thus affecting spatial learning and memory. CUR also prevents detrimental effects of chronic cerebral hypoperfusion by maintaining cholesterol homeostasis. CUR also contributes to maintain cholesterol homeostasis, otherwise upset by chronic cerebral ischemia. Indeed, CUR promotes cholesterol efflux through the ATP-binding cassette transporter A1 (ABCA1) and the pathway involving apoA-I and the liver X receptor (LXR)/retinoic X receptor (RXR) [104].
Table 1.
Antiaging effect of curcumin.
| Compound | Animal model | Effect | Reference |
|---|---|---|---|
| Curcumin | (i) Aged female Wistar rats | (i) Decreasing the MDA and LPO levels in brain tissue | [109] |
| Curcumin (20, 40, and 80 μM) | (i) Aging RPE cells | (i) Improvement of cell viability (ii) Reducing the apoptosis and OS (iii) Decreasing the expression of apoptosis-related proteins and OS biomarkers |
[92] |
| Curcumin (0.2%) | (i) Male Sprague Dawley rats (ii) UCP2 knockout (UCP2-/-) (iii) Matched wild-type mice |
(i) Restoring the impaired cerebrovascular endothelium-dependent vasorelaxation (ii) Promoting eNOS and AMPK phosphorylation (iii) Overexpression of UCP2 and reduction of ROS generation |
[103] |
| Curcumin (0.2%) | (i) Male C57BL/6N mice | (i) Ameliorates age-associated large elastic artery stiffening (ii) Improvement of NO-mediated vascular endothelial dysfunction (iii) Oxidative stress (iv) Decreasing the collagen I and AGEs in the arterial wall |
[87] |
| Curcumin (100 μM) | (i) Wild-type Canton-S flies | (i) Protective effect against radiation damage (ii) Decrement of the amount of protein carbonylation and γH2Ax foci |
[142] |
| Curcumin (100, 200, and 400 mg/kg BW) | (i) Female Wistar albino rats | (i) Increased the NO and MDA levels | [3] |
| Curcumin (50 mg/kg) | (i) Adult and aging male C57BL/6 mice | (i) Modulation of hippocampal redox status (ii) Restoring aging-related loss of synapse input specificity of HFS-LTP |
[110] |
| Curcumin (50 and 100 mg/kg) | (i) Male Sprague Dawley rats | (i) Improving the spatial learning and memory (ii) Alleviating pathological change (iii) Reduction of the level of MDA (iv) Increment of the activity of SOD (v) Inducing HO-1 protein expression (vi) Increasing the protein levels of UCP2 (vii) Inhibiting OS induced by ischemia |
[167] |
| PE859 | (i) SAMP8 | (i) Inhibition of Aβ aggregation (ii) Amelioration of cognitive dysfunction (iii) Decrements of the amount of aggregated Aβ and tau |
[158] |
| Curcumin (5 to 100 μM) | (i) HUVECs | (i) Mitigated the H2O2-induced endothelial premature senescence (ii) Decrements of population of senescence-related β-galactosidase-positive cells (iii) Motivating cell division (iv) Dwindling RNA amplification of senescence-related protein p21, OS, and apoptosis (v) Induction of the expression of the phosphorylation of eNOS (vi) Increments of the amount of NO (vii) Stimulation of the transcription, translation, and enzymatic activity of Sirt1 |
[159] |
| Piperine (12 mg/kg)+curcumin (40 mg/kg) | (i) Adult male Wistar rats | (i) Improvement of spatial memory and serotoninergic signaling (ii) Decrements of OS and lipofuscin deposition (iii) Higher hippocampal volume (iv) Hippocampal neuroprotection (v) Promotion of cognition (vi) Inhibition of senescence by the free radical quenching |
[153] |
| Curcumin (50 mg/kg) | (i) SAMP8 mice | (i) Narrowing the hippocampal SOD activities (ii) Elevation of the amount of p-CaMKII in the stratum lucidum of hippocampal CA3 and p-NMDAR1 in the hippocampal membrane |
[156] |
| Curcumin (0 to 500 mM) | (i) Two strains of Drosophila (Canton-S and Ives flies) | (i) Protection against oxidative stress (ii) Improvement in locomotion (iii) Modulating the expression of different aging-associated genes, including mth, Thor, InR, and JNK |
[139] |
| Curcumin (0 to 200 mM) | (i) Normal-lived Ra strain (Drosophila) | (i) Induction of an extended longevity phenotype (ii) Slowing the aging rate (iii) Increases the adult animal's geotactic activity |
[144] |
| Curcumin (0.5 to 1.0 mg/g of diet) | (i) Oregon-R strain (Drosophila) | Overexpression of Mn-SOD and CuZn-SOD genes (i) Downexpression of age-associated genes (dInR, ATTD, Def, CecB, and DptB) (ii) Modulating the gene expression of SOD (iii) Decrements of MDA and LPO |
[143] |
| Galantamine (5 mg/kg) and curcumin (15 and 30 mg/kg) | (i) Old male LACA mice | (i) Postponing aging process (ii) Improving cognitive functions, locomotor activity, and antioxidation (iii) Decrements of acetylcholine esterase activity (iv)Restoring the mitochondrial enzyme complex execution |
[154] |
| Curcumin | (i) Transgenic Drosophila | (i) Increments of amyloid fibril conversion by decreasing the prefibrillar/oligomeric species of Aβ | [148] |
| Curcumin and disulfiram/gram of media | (i) Male D. melanogaster | (ii) Promotion of SOD activity | [168] |
Figure 1.
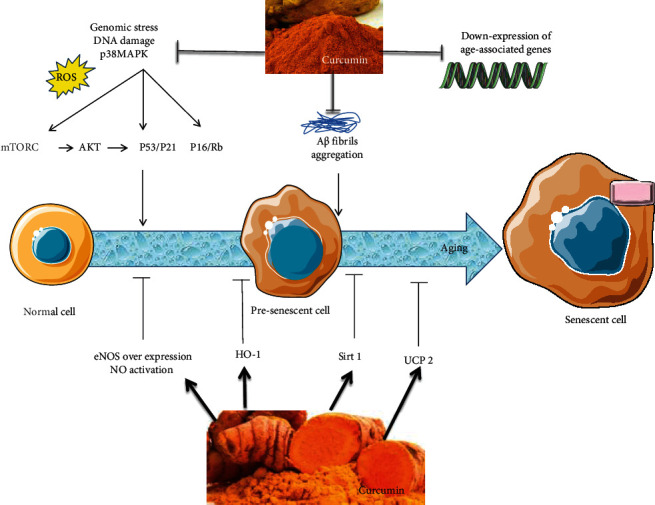
Mechanisms by which curcumin modulate aging process and senescence. Curcumin inhibited OS-stimulated p38MAPK activation, Aβ fibril aggregation, and expression of age-associated genes (dInR, ATTD, Def, CecB, DptB, mth, thor, InR, and JNK), although curcumin induced eNOS, NO, Sirt1, HO-1, and UCP2 expression. Curcumin also mitigates the SASP and its aging-induction consequences of senescent cell. Abbreviations: Aβ: amyloid-β; eNOS: endothelial nitric-oxide synthase; HO-1: hemeoxygenase-1; mTORC 1: mammalian/mechanistic target of rapamycin complex 1; NO: nitric oxide; ROS: reactive oxygen species; SASP: senescence-associated secretory phenotype; Sirt: sirtuins; UCP2: uncoupling protein 2.
4.2. Cognitive Impairments
With similar mechanisms, the reduction of circulating antioxidants is tightly associated with memory loss and cognitive impairment in the elderly [105]. It is then not surprising that CUR has been reported to improve neuropsychological functions. CUR has several inhibitory effects on combining aging and Alzheimer's disease pathophysiology, such as the suppression of amyloid precursor protein (APP) and Aβ synthesis and the overexpression of ApoE and Nrf2 gene, as well as the prohibition of p-mTOR and p-NF-κB [106, 107]. CUR prevents D-gal-induced brain aging and cognitive impairment through increments of antioxidant enzymes and inhibition of apoptosis [108]. Beneficial effects of CUR on mental abilities and functional capacities are associated with a LPO reduction in brain tissue [109], especially in the hippocampal area. CUR improves the redox state in this area and prevents the decline of hippocampal long-term potentiation by maintaining synapse input specificity [110, 111]. Recently, Olesen et al. described that the dysfunction of synaptic mitochondria of the hippocampus causing memory loss during aging. They showed that curcumin feeding significantly improved integration and activity of the synaptic mitochondrial of the hippocampus, inhibiting mitochondrial swelling and enhancing the production of synapses surrounding the mitochondria in mice [112].
4.3. Evidence from Experimental Models
4.3.1. Study of Longevity in Drosophila melanogaster and Caenorhabditis elegans
Drosophila melanogaster (D. melanogaster) and Caenorhabditis elegans (C. elegans) are widely recognized models for the study of aging processes [113]. In particular, D. melanogaster represented a paradigm of experimental gerontology during the last century [114–118] because of its complex biology and the ease of rearing and housing as well [119, 120]. More recently, in 1983, Klass isolated the first long-lived mutants of C. elegans [121], which rose to become a promising model for aging investigations due to the small size, anatomical simplicity, small genome, short life cycle, and inexpensive laboratory manipulation [122]. In C. elegans, longevity is widely determined by the expression of the Age-1 gene [123, 124]. As one of the main elements in the insulin/insulin-like growth factor-1 signaling (IIS) axis, Age-1 is a subunit of phosphoinositide 3-kinase (PI3K), which suppresses DAF-16 action [123–125]. Suppression of the IIS pathway activates the downstream gene DAF-16, which in turn promotes the transcription of genes associated with longevity, metabolism, and response to cellular stress [126–128]. In line, increased lifespan may also be obtained through TOR inhibition, another DAF-16 suppressor [129, 130]. By sharing the same downstream signaling of DAF-16, also the FOXO3 A gene is involved in lifespan extension, cell growth, and stress response through a direct activity on DNA repair and transcription involving p21/p53 and β-catenin pathways [131–133]. Noteworthy, FOXO has a multistep regulation involving not only IGF-1 but also NAD+/Sirt1, 5′AMPK, and OS, all known as aging genes [134]. Due to these similarity with human beings, C. elegans became a genetic model organism already in 1965. Multiple pharmacological interventions have been found to prolong the survival of D. melanogaster and C. elegans [135–137]. Also, CUR was shown to increase the fecundity, reproductive lifespan, and child viability of D. melanogaster [85]. It has been shown that CUR supplementation at the larval stage of D. melanogaster elevated the developmental duration and longevity of adult Drosophila possibly through epigenetic programming of the pace of life [138].
CUR-mediated increased longevity was observed in two distinctive strains of D. melanogaster (Canton-S and Ivies flies) as a result of the delayed expression of aging genes (e.g., methuselah (mth), thor, insulin receptor [InR], and c-jun N-terminal kinase [JNK]), improved locomotion, and chemoprevention as well [139]. CUR was also shown to reduce OS, DNA damage, and number of mutagenic phenotypes induced via high-dose ionizing irradiation. These effects may be ascribed to ROS scavenging and transcriptional regulation of OS-related genes, which mainly involves γH2Ax, a histone protein belonging to the H2A family and involved in DNA damage response [140–142]. Also, in vivo experiments on CUR-fed diets (0.5 and 1.0 mg/g of diet) were effective in extending the average lifespan in both females (6.2% and 25.8%, respectively) and males (15.5% and 12.6%, respectively), and this effect could be more likely attributed to the overexpression of Mn-SOD and CuZn-SOD genes and the downregulation of aging genes associated with the TOR pathway including Drosophila insulin receptor (dInR), attacin-D (ATTD), defensin (Def), cecropin B (CecB), and diptericin B (DptB) genes [143, 144]. Also, in C. elegans, CUR effectively improves lifespan and aging by lowering intracellular ROS and lipofuscin. The effects of CUR on C. elegans longevity are manifested by body size and pharyngeal pumping rate but not reproduction ability. Further studies revealed that the long-lived phenotype induced by CUR was maintained in mev-1 and daf-16 mutants but lost in osr-1, sek-1, skn-1, unc-43, mek-1, sir-2.1, and age-1 ones [145]. This evidence indicates that CUR would exert its effects independently of the Age-1-DAF-16 pathway but rather through other constituents of the IIS pathway. With regard to cognitive impairment, the in vivo experiment demonstrated that CUR can improve learning and memory also reducing Aβ plaque formation in the context of Alzheimer disease (AD) [146]. D. melanogaster is a promising animal model for research in AD [147]. By increasing amyloid fibril conversion, CUR reduces the generation of prefibrillar/oligomeric species of Aβ, ultimately protecting against neurotoxicity [148]. The human β-amyloid precursor cleavage enzyme (BACE-1) is another critical enzyme targeted by CUR [149, 150] in the D. melanogaster model of AD [150].
4.3.2. Studies of Cell Senescence: Evidence from Mice and Rats
High doses of CUR (2.5-10 μM) were shown to trigger senescence in cancer and vascular cells [151]. On the other hand, low doses of CUR (0.1 and 1 μM) failed to prevent early senescence in doxorubicin-treated (VSMC) and even slightly accelerated replicative senescence in endothelial cells [152]. It is therefore evident how the antiaging effect of CUR does not rely on delayed cellular senescence. As reported by Banji et al., CUR (40 mg/kg) and piperine (12 mg/kg), especially when combined, counteract D-gal-induced senescence in male Wistar rats by targeting OS and lipofuscin deposition, finally leading to higher hippocampal volume and function with improved spatial memory and serotoninergic signaling [153]. Another study even reported how long-time CUR therapy may progressively reverse cognitive dysfunction in D-gal-induced senescent mice by delaying the aging process and improving cognitive functions and locomotor activity, as well as restoring the mitochondrial enzyme complex function [154]. In a recent study, CUR supplementation rejuvenates senescence-associated changes in thymus among D-gal-induced senescent mice through promotion of proliferating cells, preventing cells from apoptosis, and enhancing the transcription of the autoimmune regulator (Aire) [155].
CUR feeding (50 mg/kg) was also tested in senescence-accelerated mouse prone (SAMP) mice resulting in increased hippocampal SOD activity as well as upregulation of p-calcium/calmodulin-dependent kinase II (p-CaMKII) in the stratum lucidum and p-N-methyl-D-aspartate receptor subunit 1 (p-NMDAR1) in the hippocampal membrane [156]. Noteworthy, clinical benefits of the CUR analogue PE859 have been recently reported and associated with reduction of Aβ and tau aggregates in the mouse brain [157, 158]. Overall, these findings suggest a role of CUR in improving cognitive difficulties and the expression of hippocampal plasticity-associated proteins. With regard to vascular function, CUR administration significantly mitigated premature senescence in HUVECs, characterized by a reduction of senescence-related β-galactosidase-positive cells, cell division, levels of senescence-related protein p21 RNA, OS, and apoptosis. CUR is also associated with enhanced eNOS phosphorylation and NO generation, in addition to upregulating Sirt1 transcription, translation, and enzymatic activity [159]. In light of these mechanisms, diets containing tetrahydrocurcumin (THC), the main metabolite of CUR, were demonstrated to significantly extend mean lifespan in male C57BL/6 mice [160], whereas bisdemethoxycurcumin administration delayed the OS-caused premature senescence via Sirt1/AMPK cascade activation [161]. As recently demonstrated, Sirt1 signaling also mediates the anti-inflammatory effects of CUR in C57BL/6 mice fed with high fat diet [162] in addition to improved myocardial structure and function in streptozocin-induced diabetic mice fed with THC (120 mg/kg/d) [163]. Even more recently, it has been hypothesized that the antiaging effect of CUR may rely on the control of core clock genes on which Sirt1 belongs alongside rBmal1, rCry1, rCry2, rPer1, rPer2, and rRev-erba. CUR treatment in middle aged male Wistar rats restored the phase and daily pulse of rCry1, rCry2, rPer1, and rPer2 as in the young, whereas only rPer1 and partly rBmal1, rCry1, and rCry2 were restored in the old ones [164]. Moreover, it has been shown that CUR mitigated mouse ovarian aging, upgraded embryonic development, promoted oocyte maturation and fertilization via improvement of ovarian hormones, and elevated the amounts of SIRT1 and 3 genes as well as attenuation of aging-associated oxidative stress and cell death [165]. Besides, CUR can reduce oxidative stress, inflammation status, and lipofuscin deposition in aged rat liver [166].
5. Conclusion
Aging and senescence are complex processes leading to organ dysfunction. Despite being permanent, delaying the occurrence of these processes is a reliable target, and CUR might be a promising candidate for this purpose. Nevertheless, evidence from clinical studies on the long-term effects of CUR on age-related pathological events remains largely understudied. While several strategies to enhance the systemic bioavailability of CUR have been suggested, the effects of long-term therapy with such bioavailability-boosted CUR preparations is not fully known, and increased concentrations may even lead to opposite results. Pleiotropic benefits of CUR supplementation involve the control of aging genes, OS, and inflammation in both the vascular system and the central nervous system. Further studies are warranted to clarify the mechanisms of CUR function for potential clinical application.
Abbreviations
- Aβ:
Amyloid-β
- AGEs:
Advanced glycation end-products
- ATTD:
Attacin-D
- CecB:
Cecropin B
- Def:
Defensin
- dInR:
Drosophila insulin receptor
- DptB:
Diptericin B
- HO-1:
Hemeoxygenase-1
- HFS:
High-frequency stimulation
- H2O2:
Hydrogen peroxide
- HUVECs:
Human umbilical vein endothelial cells
- GSH:
Glutathione
- LTP:
Long-term potentiation
- MDA:
Malondialdehyde
- NO:
Nitric oxide
- RPE:
Retinal pigment epithelial
- p-CaMKII:
p-Calcium/calmodulin-dependent kinase II
- SAMP8:
p-N-Methyl-D-aspartate receptor subunit 1 (p-NMDAR1), senescence-accelerated mouse prone 8
- SOD:
Superoxide dismutase
- UCP2:
Uncoupling protein 2.
Conflicts of Interest
The authors declare that no competing interests exist.
References
- 1.Hung C.-W., Chen Y.-C., Hsieh W.-L., Chiou S.-H., Kao C.-L. Ageing and neurodegenerative diseases. Ageing Research Reviews . 2010;9:S36–S46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Van Voorhies W. A., Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proceedings of the National Academy of Sciences . 1999;96(20):11399–11403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shailaja M., Damodara Gowda K. M., Vishakh K., Suchetha Kumari N. Anti-aging role of curcumin by modulating the inflammatory markers in albino Wistar rats. Journal of the National Medical Association . 2017;109(1):9–13. doi: 10.1016/j.jnma.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Minamino T., Komuro I. Vascular cell Senescence. Circulation Research . 2007;100(1):15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta E. G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease Enterprises. Circulation . 2003;107(1):139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L., Partridge L., Longo V. D. Extending healthy life span—from yeast to humans. Science . 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haigis M. C., Sinclair D. A. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol Mech Dis . 2010;5(1):253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenyon C. J. The genetics of ageing. Nature . 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrmann-Stroissnigg H., Ling Y. Y., Zhao J., et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nature Communications . 2017;8(1) doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M., Pirtskhalava T., Farr J. N., et al. Senolytics improve physical function and increase lifespan in old age. Nature Medicine . 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickson L. J., Langhi Prata L. G. P., Bobart S. A., et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus quercetin in individuals with diabetic kidney disease. eBioMedicine . 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghandadi M., Sahebkar A. Curcumin: an effective inhibitor of interleukin-6. Current Pharmaceutical Design . 2017;23(6):921–931. doi: 10.2174/1381612822666161006151605. [DOI] [PubMed] [Google Scholar]
- 13.Ghasemi F., Shafiee M., Banikazemi Z., et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathology Research and Practice . 2019;215(10):p. 152556. doi: 10.1016/j.prp.2019.152556. [DOI] [PubMed] [Google Scholar]
- 14.Mortezaee K., Salehi E., Mirtavoos-mahyari H., et al. Mechanisms of apoptosis modulation by curcumin: implications for cancer therapy. Journal of Cellular Physiology . 2019;234(8):12537–12550. doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 15.Panahi Y., Khalili N., Sahebi E., et al. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Research . 2018;68(7):403–409. doi: 10.1055/s-0044-101752. [DOI] [PubMed] [Google Scholar]
- 16.Mohajeri M., Bianconi V., Ávila-Rodriguez M. F., et al. Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacological Research . 2020;156:p. 104765. doi: 10.1016/j.phrs.2020.104765. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghian M., Rahmani S., Jamialahmadi T., Johnston T. P., Sahebkar A. The effect of oral curcumin supplementation on health-related quality of life: a systematic review and meta-analysis of randomized controlled trials. Journal of Affective Disorders . 2021;278:627–636. doi: 10.1016/j.jad.2020.09.091. [DOI] [PubMed] [Google Scholar]
- 18.Hewlings S., Kalman D. Curcumin: a review of its’ effects on human health. Food . 2017;6(10):p. 92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M., Zhu P., Fujino M., et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. International Journal of Molecular Sciences . 2016;17(12):p. 2078. doi: 10.3390/ijms17122078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellezza I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Frontiers in Pharmacology . 2018;9 doi: 10.3389/fphar.2018.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrera G., Pizzimenti S., Daga M., et al. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants . 2018;7(8):p. 102. doi: 10.3390/antiox7080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ighodaro O., Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine . 2018;54(4):287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 23.Vicencio J. M., Galluzzi L., Tajeddine N., et al. Senescence, apoptosis or autophagy? Gerontology . 2008;54(2):92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- 24.Faragher R. G., McArdle A., Willows A., Ostler E. L. Senescence in the aging process. F1000Research . 2017;6 doi: 10.12688/f1000research.10903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagi S., Tsubouchi H., Miura A., Matsuo A., Matsumoto N., Nakazato M. The impacts of cellular senescence in elderly pneumonia and in age-related lung diseases that increase the risk of respiratory infections. International journal of molecular sciences. . 2017;18(3):p. 503. doi: 10.3390/ijms18030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooten N. N., Evans M. K. Techniques to induce and quantify cellular senescence. JoVE (Journal of Visualized Experiments) . 2017;123, article e55533 doi: 10.3791/55533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayflick L., Moorhead P. S. The serial cultivation of human diploid cell strains. Experimental cell research. . 1961;25(3):585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 28.von Figura G., Hartmann D., Song Z., Rudolph K. L. Role of telomere dysfunction in aging and its detection by biomarkers. Journal of molecular medicine. . 2009;87(12):1165–1171. doi: 10.1007/s00109-009-0509-5. [DOI] [PubMed] [Google Scholar]
- 29.Karlseder J., Smogorzewska A., de Lange T. Senescence induced by altered telomere state, not telomere loss. Science . 2002;295(5564):2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]
- 30.Kim W. Y., Sharpless N. E. The Regulation of _INK4_ / _ARF_ in Cancer and Aging. Cell . 2006;127(2):265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang C., Jurk D., Maddick M., Nelson G., Martin-Ruiz C., Von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell . 2009;8(3):311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 32.Herbig U., Jobling W. A., Chen B. P., Chen D. J., Sedivy J. M. Telomere Shortening Triggers Senescence of Human Cells through a Pathway Involving ATM, p53, and p21CIP1, but Not p16INK4a. Molecular Cell . 2004;14(4):501–513. doi: 10.1016/S1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 33.Blagosklonny M. V. Hypoxia, MTOR and autophagy. Autophagy . 2013;9(2):260–262. doi: 10.4161/auto.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coppé J.-P., Patil C. K., Rodier F., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS biology. . 2008;6(12, article e301) doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pahl H. L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene . 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 36.Demaria M., Ohtani N., Youssef S. A., et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell . 2014;31(6):722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabowska W., Sikora E., Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology . 2017;18(4):447–476. doi: 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prattichizzo F., De Nigris V., La Sala L., Procopio A. D., Olivieri F., Ceriello A. “Inflammaging” as a druggable target: a senescence-associated secretory phenotype—centered view of type 2 diabetes. Oxidative medicine and cellular longevity. . 2016;2016, article 1810327:p. 10. doi: 10.1155/2016/1810327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J.-H., Hales C. N., Ozanne S. E. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic acids research. . 2007;35(22):7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz-Espin D., Serrano M. Cellular senescence: from physiology to pathology. Nature reviews Molecular cell biology . 2014;15(7):482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 41.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell . 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 42.North B. J., Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome biology . 2004;5(5):p. 224. doi: 10.1186/gb-2004-5-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu T., Liu P. Y., Marshall G. M. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer research . 2009;69(5):1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa T., Iwai M., Aoki S., et al. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS One . 2015;10(1, article e0116480) doi: 10.1371/journal.pone.0116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantó C., Auwerx J. Caloric restriction, SIRT1 and longevity. Trends in Endocrinology & Metabolism . 2009;20(7):325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harikumar K. B., Aggarwal B. B. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle . 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 47.Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative medicine and cellular longevity . 2012;2012 doi: 10.1155/2012/914273.914273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adrian M., Jeandet P., Veneau J., Weston L. A., Bessis R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. Journal of Chemical Ecology . 1997;23(7):1689–1702. doi: 10.1023/B:JOEC.0000006444.79951.75. [DOI] [Google Scholar]
- 49.Murakami A. Modulation of protein quality control systems by food phytochemicals. Journal of clinical biochemistry and nutrition . 2013;52(3):215–217. doi: 10.3164/jcbn.12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapakos G., Youreva V., Srivastava A. K. Cardiovascular Protection by Curcumin: Molecular Aspects . 2012;49(5):306–315. [PubMed] [Google Scholar]
- 51.Soleimani V., Sahebkar A., Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytotherapy Research . 2018;32(6):985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- 52.Pescosolido N., Giannotti R., Plateroti A. M., Pascarella A., Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Medica . 2014;80(4):249–254. doi: 10.1055/s-0033-1351074. [DOI] [PubMed] [Google Scholar]
- 53.Saleheen D., Ali S. A., Ashfaq K., Siddiqui A. A., Agha A., Yasinzai M. M. Latent activity of curcumin against leishmaniasis in vitro. Biological and Pharmaceutical Bulletin. . 2002;25(3):386–389. doi: 10.1248/bpb.25.386. [DOI] [PubMed] [Google Scholar]
- 54.Gomes C. D., Alegrio L. V., Leon L., Araújo C. Synthetic derivatives of curcumin and their activity against Leishmania amazonensis. Arzneimittel-Forschung. . 2002;52(2):120–124. [PubMed] [Google Scholar]
- 55.Jordan W., Drew C. Curcumin--a natural herb with anti-HIV activity. Journal of the National Medical Association . 1996;88(6):p. 333. [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z., Xue J., Shen T., Mu S., Fu Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. International journal of molecular medicine . 2016;37(2):329–338. doi: 10.3892/ijmm.2015.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koide T., Nose M., Ogihara Y., Yabu Y., Ohta N. Leishmanicidal effect of curcumin in vitro. Biological and Pharmaceutical Bulletin . 2002;25(1):131–133. doi: 10.1248/bpb.25.131. [DOI] [PubMed] [Google Scholar]
- 58.Singh S., Aggarwal B. B. Activation of Transcription Factor NF-κB Is Suppressed by Curcumin (Diferuloylmethane) (∗) Journal of Biological Chemistry . 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 59.Kim G.-Y., Kim K.-H., Lee S.-H., et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. The Journal of Immunology . 2005;174(12):8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 60.Zendedel E., Butler A. E., Atkin S. L., Sahebkar A. Impact of curcumin on sirtuins: a review. Journal of Cellular Biochemistry . 2018;119(12):10291–10300. doi: 10.1002/jcb.27371. [DOI] [PubMed] [Google Scholar]
- 61.Morimoto T., Sunagawa Y., Kawamura T., et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. The Journal of clinical investigation. . 2008;118(3):868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta S. C., Kismali G., Aggarwal B. B. Curcumin, a component of turmeric: from farm to pharmacy. BioFactors . 2013;39(1):2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 63.Boyanapalli S. S., Kong A.-N. T. Curcumin, the king of spices: epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Current pharmacology reports. . 2015;1(2):129–139. doi: 10.1007/s40495-015-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Remely M., Lovrecic L., de la Garza A. L., et al. Therapeutic perspectives of epigenetically active nutrients. British journal of pharmacology . 2015;172(11):2756–2768. doi: 10.1111/bph.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reuter S., Gupta S. C., Park B., Goel A., Aggarwal B. B. Epigenetic changes induced by curcumin and other natural compounds. Genes & nutrition. . 2011;6(2):93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng M., Ekmekcioglu S., Walch E. T., Tang C.-H., Grimm E. A. Inhibition of nuclear factor-κB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma research . 2004;14(3):165–171. doi: 10.1097/01.cmr.0000129374.76399.19. [DOI] [PubMed] [Google Scholar]
- 67.Schaaf C., Shan B., Buchfelder M., et al. Curcumin acts as anti-tumorigenic and hormone-suppressive agent in murine and human pituitary tumour cells in vitro and in vivo. Endocrine-related cancer . 2009;16(4):1339–1350. doi: 10.1677/ERC-09-0129. [DOI] [PubMed] [Google Scholar]
- 68.Hamzehzadeh L., Atkin S. L., Majeed M., Butler A. E., Sahebkar A. The versatile role of curcumin in cancer prevention and treatment: a focus on PI3K/AKT pathway. Journal of cellular physiology . 2018;233(10):6530–6537. doi: 10.1002/jcp.26620. [DOI] [PubMed] [Google Scholar]
- 69.Ramasamy T. S., Ayob A. Z., Myint H. H. L., Thiagarajah S., Amini F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer cell international . 2015;15(1):p. 96. doi: 10.1186/s12935-015-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bao B., Ali S., Banerjee S., et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer research . 2012;72(1):335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seo J. H., Jeong K. J., Oh W. J., et al. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: their inhibition by curcumin. Cancer letters . 2010;288(1):50–56. doi: 10.1016/j.canlet.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Menon V. P., Sudheer A. R. The molecular targets and therapeutic uses of curcumin in health and disease . Springer; 2007. Antioxidant and anti-inflammatory properties of curcumin; pp. 105–125. [DOI] [PubMed] [Google Scholar]
- 73.Han Y. K., Lee S. H., Jeong H. J., Kim M. S., Yoon M. H., Kim W. M. Analgesic effects of intrathecal curcumin in the rat formalin test. The Korean journal of pain . 2012;25(1):1–6. doi: 10.3344/kjp.2012.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Lu Z., Wu H., Lv F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. International journal of food microbiology . 2009;136(1):71–74. doi: 10.1016/j.ijfoodmicro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Daniel S., Limson J. L., Dairam A., Watkins G. M., Daya S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. Journal of inorganic biochemistry . 2004;98(2):266–275. doi: 10.1016/j.jinorgbio.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Bielak-Zmijewska A., Grabowska W., Ciolko A., et al. The role of curcumin in the modulation of ageing. International journal of molecular sciences. . 2019;20(5):p. 1239. doi: 10.3390/ijms20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balogun E., Hoque M., Gong P., et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochemical Journal . 2003;371(3):887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu C. T., Yao Q.-y., Xu B.-l., Wang J.-y., Zhou C.-h., Zhang S.-c. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: modulation of high-mobility group box 1, toll-like receptor 4 and 2 expression. Food and chemical toxicology . 2012;50(9):3343–3351. doi: 10.1016/j.fct.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 79.Lima C. F., Pereira-Wilson C., Rattan S. I. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: relevance for anti-aging intervention. Molecular nutrition & food research . 2011;55(3):430–442. doi: 10.1002/mnfr.201000221. [DOI] [PubMed] [Google Scholar]
- 80.Mein J. R., James D. R., Lakkanna S. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: perspectives in maintaining the antioxidant activity of vitamins A, C, and E. Frontiers in genetics . 2012;3:p. 7. doi: 10.3389/fgene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rojo A. I., Medina-Campos O. N., Rada P., et al. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: role of glycogen synthase kinase-3. Free Radical Biology and Medicine . 2012;52(2):473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Cherif H., Bisson D. G., Jarzem P., Weber M., Ouellet J. A., Haglund L. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. Journal of Clinical Medicine . 2019;8(4):p. 433. doi: 10.3390/jcm8040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li W., He Y., Zhang R., Zheng G., Zhou D. The curcumin analog EF24 is a novel senolytic agent. Aging . 2019;11(2):771–782. doi: 10.18632/aging.101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fadus M. C., Lau C., Bikhchandani J., Lynch H. T. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of traditional and complementary medicine . 2017;7(3):339–346. doi: 10.1016/j.jtcme.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandrashekara K., Popli S., Shakarad M. Curcumin enhances parental reproductive lifespan and progeny viability in Drosophila melanogaster. Age . 2014;36(5):p. 9702. doi: 10.1007/s11357-014-9702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarvalkar P., Walvekar M., Bhopale L. Antioxidative effect of curcumin (Curcuma longa) on lipid peroxidation and lipofuscinogenesis in submandibular gland of D-galactose-induced aging male mice. Journal of Medicinal Plants Research . 2011;5(20):5191–5193. [Google Scholar]
- 87.Fleenor B. S., Sindler A. L., Marvi N. K., et al. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Experimental gerontology. . 2013;48(2):269–276. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akazawa N., Choi Y., Miyaki A., et al. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutrition research . 2012;32(10):795–799. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Wongcharoen W., Phrommintikul A. The protective role of curcumin in cardiovascular diseases. International journal of cardiology . 2009;133(2):145–151. doi: 10.1016/j.ijcard.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 90.Bressler N. M. Age-related macular degeneration is the leading cause of blindness. Journal of the American Medical Association . 2004;291(15):1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 91.Zhuang M., Shao J., Tan C., Yao Y. Effects of transthyretin on biological behavior of retinal pigment epithelial cells and retinal microvascular epithelial cells. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology . 2016;52(11):856–860. doi: 10.3760/cma.j.issn.0412-4081.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Zhu W., Wu Y., Meng Y.-F., et al. Effect of curcumin on aging retinal pigment epithelial cells. Drug design, development and therapy . 2015;9:p. 5337. doi: 10.2147/DDDT.S84979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sundar Dhilip Kumar S., Houreld N., Abrahamse H. Therapeutic potential and recent advances of curcumin in the treatment of aging-associated diseases. Molecules . 2018;23(4):p. 835. doi: 10.3390/molecules23040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar P. A., Suryanarayana P., Reddy P. Y., Reddy G. B. Modulation of alpha-crystallin chaperone activity in diabetic rat lens by curcumin. Molecular Vision . 2005;11:561–568. [PubMed] [Google Scholar]
- 95.Suryanarayana P., Saraswat M., Mrudula T., Krishna T. P., Krishnaswamy K., Reddy G. B. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investigative ophthalmology & visual science . 2005;46(6):2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 96.Chen R.-L., Balami J. S., Esiri M. M., Chen L.-K., Buchan A. M. Ischemic stroke in the elderly: an overview of evidence. Nature Reviews Neurology . 2010;6(5):256–265. doi: 10.1038/nrneurol.2010.36. [DOI] [PubMed] [Google Scholar]
- 97.Shin H. K., Jones P. B., Garcia-Alloza M., et al. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain . 2007;130(9):2310–2319. doi: 10.1093/brain/awm156. [DOI] [PubMed] [Google Scholar]
- 98.Vasilevko V., Passos G., Quiring D., et al. Aging and cerebrovascular dysfunction: contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Annals of the New York Academy of Sciences. . 2010;1207(1):58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fridell Y.-W. C., Sánchez-Blanco A., Silvia B. A., Helfand S. L. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell metabolism . 2005;1(2):145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Winder W., Holmes B., Rubink D., Jensen E., Chen M., Holloszy J. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of Applied Physiology . 2000;88(6):2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 101.Reznick R. M., Zong H., Li J., et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell metabolism . 2007;5(2):151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing research reviews . 2012;11(2):230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Pu Y., Zhang H., Wang P., et al. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cellular Physiology and Biochemistry. . 2013;32(5):1167–1177. doi: 10.1159/000354516. [DOI] [PubMed] [Google Scholar]
- 104.Tian M., Zhang X., Wang L., Li Y. Curcumin induces ABCA1 expression and apolipoprotein A-I-Mediated cholesterol transmembrane in the chronic cerebral hypoperfusion aging rats. The American journal of Chinese medicine . 2013;41(5):1027–1042. doi: 10.1142/S0192415X13500699. [DOI] [PubMed] [Google Scholar]
- 105.Reeta K., Mehla J., Gupta Y. K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain research . 2009;1301:52–60. doi: 10.1016/j.brainres.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 106.Su I.-J., Chang H.-Y., Wang H.-C., Tsai K.-J. A curcumin analog exhibits multiple biologic effects on the pathogenesis of Alzheimer’s disease and improves behavior, inflammation, and β-amyloid accumulation in a mouse model. International journal of molecular sciences . 2020;21(15):p. 5459. doi: 10.3390/ijms21155459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benameur T., Soleti R., Panaro M. A., et al. Curcumin as prospective anti-aging natural compound: focus on brain. Molecules . 2021;26(16):p. 4794. doi: 10.3390/molecules26164794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee J., Kim Y. S., Kim E., Kim Y., Kim Y. Curcumin and hesperetin attenuate D-galactose-induced brain senescencein vitroandin vivo. Nutrition Research and Practice . 2020;14(5):438–452. doi: 10.4162/nrp.2020.14.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Belviranlı M., Okudan N., Atalık K., Öz M. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology . 2013;14(2):187–196. doi: 10.1007/s10522-013-9422-y. [DOI] [PubMed] [Google Scholar]
- 110.Cheng Y.-F., Guo L., Xie Y.-S., et al. Curcumin rescues aging-related loss of hippocampal synapse input specificity of long term potentiation in mice. Neurochemical Research . 2013;38(1):98–107. doi: 10.1007/s11064-012-0894-y. [DOI] [PubMed] [Google Scholar]
- 111.Taghizadeh M., Talaei S. A., Djazayeri A., Salami M. Vitamin D supplementation restores suppressed synaptic plasticity in Alzheimer's disease. Nutritional neuroscience . 2014;17(4):172–177. doi: 10.1179/1476830513Y.0000000080. [DOI] [PubMed] [Google Scholar]
- 112.Olesen M. A., Torres A. K., Jara C., Murphy M. P., Tapia-Rojas C. Premature synaptic mitochondrial dysfunction in the hippocampus during aging contributes to memory loss. Redox Biology . 2020;34:p. 101558. doi: 10.1016/j.redox.2020.101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grotewiel M. S., Martin I., Bhandari P., Cook-Wiens E. Functional senescence in _Drosophila melanogaster_. Ageing research reviews . 2005;4(3):372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 114.Libert S., Zwiener J., Chu X., VanVoorhies W., Roman G., Pletcher S. D. Regulation of Drosophila life span by olfaction and food-derived odors. Science . 2007;315(5815):1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 115.Toivonen J. M., Partridge L. Endocrine regulation of aging and reproduction in _Drosophila_. Molecular and cellular endocrinology . 2009;299(1):39–50. doi: 10.1016/j.mce.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 116.Giannakou M. E., Partridge L. Role of insulin-like signalling in _Drosophila_ lifespan. Trends in biochemical sciences. . 2007;32(4):180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 117.Demontis F., Perrimon N. FOXO/4E-BP Signaling in _Drosophila_ Muscles Regulates Organism-wide Proteostasis during Aging. Cell . 2010;143(5):813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karpac J., Younger A., Jasper H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Developmental cell . 2011;20(6):841–854. doi: 10.1016/j.devcel.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Osterwalder T., Yoon K. S., White B. H., Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proceedings of the National Academy of Sciences. . 2001;98(22):12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee T., Luo L. Mosaic analysis with a repressible cell marker (MARCM) for _Drosophila_ neural development. Trends in neurosciences . 2001;24(5):251–254. doi: 10.1016/S0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 121.Klass M. R. A method for the isolation of longevity mutants in the nematode _Caenorhabditis elegans_ and initial results. Mechanisms of ageing and development . 1983;22(3-4):279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- 122.Taormina G., Ferrante F., Vieni S., Grassi N., Russo A., Mirisola M. G. Longevity: lesson from model organisms. Genes . 2019;10(7):p. 518. doi: 10.3390/genes10070518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friedman D. B., Johnson T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics . 1988;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Friedman D. B., Johnson T. E. Three mutants that extend both mean and maximum life span of the nematode, Caenorhabditis elegans, define the age-1 gene. Journal of gerontology . 1988;43(4):B102–B109. doi: 10.1093/geronj/43.4.B102. [DOI] [PubMed] [Google Scholar]
- 125.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell . 2005;120(4):449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 126.Lee S. S., Kennedy S., Tolonen A. C., Ruvkun G. DAF-16 target genes that ControlC. elegansLife-Span and metabolism. science . 2003;300(5619):644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 127.Murphy C. T., McCarroll S. A., Bargmann C. I., et al. Genes that act downstream of DAF-16 to influence the lifespan of _Caenorhabditis elegans_. Nature . 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 128.Tatar M., Bartke A., Antebi A. The endocrine regulation of aging by insulin-like signals. Science . 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 129.Kaeberlein M., Powers R. W., Steffen K. K., Westman E. A., Hu D., Dang N. Regulation of yeast replicative life span by TOR and Sch 9 in response to nutrients. Science . 2005;310(5751):1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 130.Harrison D. E., Strong R., Sharp Z. D., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature . 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y., Wang W.-J., Cao H., et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Human molecular genetics. . 2009;18(24):4897–4904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anselmi C. V., Malovini A., Roncarati R., et al. Association of theFOXO3ALocus with extreme longevity in a southern Italian centenarian study. Rejuvenation research . 2009;12(2):95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- 133.Soerensen M., Dato S., Christensen K., et al. Replication of an association of variation in the FOXO3A gene with human longevity using both case–control and longitudinal data. Aging Cell . 2010;9(6):1010–1017. doi: 10.1111/j.1474-9726.2010.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. science . 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 135.Lithgow G. J., Gill M. S., Olsen A., Sampayo J. N. Pharmacological intervention in invertebrate aging. Age . 2005;27(3):213–223. doi: 10.1007/s11357-005-3625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang H.-L., Benzer S., Min K.-T. Life extension in Drosophila by feeding a drug. Proceedings of the National Academy of Sciences . 2002;99(2):838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jafari M., Felgner J. S., Bussel I. I., et al. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation research . 2007;10(4):587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- 138.Pisaruk A., Koshel N., Mekhova L., Zabuga O., Ivanov S. Influense of curcumin on lifespan if it is applied at the larval stage of Drosophila melanogaster. Aging and longevity . 2020;1(2):89–96. doi: 10.47855//jal9020-2020-2-5. [DOI] [Google Scholar]
- 139.Lee K.-S., Lee B.-S., Semnani S., et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes inDrosophila melanogaster. Rejuvenation Research . 2010;13(5):561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 140.Huang T.-S., Lee S.-C., Lin J.-K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proceedings of the National Academy of Sciences. . 1991;88(12):5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Korutla L., Kumar R. Inhibitory effect of curcumin on epidermal growth factor receptor kinase activity in A431 cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research . 1994;1224(3):597–600. doi: 10.1016/0167-4889(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 142.Seong K. M., Yu M., Lee K.-S., Park S., Jin Y. W., Min K.-J. Curcumin mitigates accelerated aging after irradiation in Drosophila by reducing oxidative stress. Bio Med research international . 2015;2015:1–8. doi: 10.1155/2015/425380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen L.-R., Xiao F., Yuan P., et al. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age . 2013;35(4):1133–1142. doi: 10.1007/s11357-012-9438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soh J.-W., Marowsky N., Nichols T. J., et al. Curcumin is an early-acting stage-specific inducer of extended functional longevity in _Drosophila_. Experimental gerontology . 2013;48(2):229–239. doi: 10.1016/j.exger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 145.Liao V. H.-C., Yu C.-W., Chu Y.-J., Li W.-H., Hsieh Y.-C., Wang T.-T. Curcumin-mediated lifespan extension in _Caenorhabditis elegans_. Mechanisms of ageing and development. . 2011;132(10):480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 146.Kim J. E., Shrestha A. C., Kim H. S., et al. WS-5 Extract of Curcuma longa, Chaenomeles sinensis, and Zingiber officinale Contains Anti-AChE Compounds and Improves -Amyloid-Induced Memory Impairment in Mice. Evidence-Based Complementary and Alternative Medicine . 2019;2019:16. doi: 10.1155/2019/5160293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wittmann C. W., Wszolek M. F., Shulman J. M., Salvaterra P. M., Lewis J., Hutton M. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science . 2001;293(5530):711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 148.Caesar I., Jonson M., Nilsson K. P. R., Thor S., Hammarström P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS One . 2012;7(2, article e31424) doi: 10.1371/journal.pone.0031424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hardy J., Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. science . 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 150.Suh Y.-H., Checler F. Amyloid precursor protein, presenilins, and alpha -Synuclein: molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacological Reviews . 2002;54(3):469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- 151.Grabowska W., Kucharewicz K., Wnuk M., et al. Curcumin induces senescence of primary human cells building the vasculature in a DNA damage and ATM-independent manner. Age . 2015;37(1) doi: 10.1007/s11357-014-9744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grabowska W., Suszek M., Wnuk M., et al. Curcumin elevates sirtuin level but does not postponein vitrosenescence of human cells building the vasculature. Oncotarget . 2016;7(15):19201–19213. doi: 10.18632/oncotarget.8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Banji D., Banji O. J., Dasaroju S., Annamalai A. Piperine and curcumin exhibit synergism in attenuating D-galactose induced senescence in rats. European journal of pharmacology . 2013;703(1-3):91–99. doi: 10.1016/j.ejphar.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 154.Kumar A., Prakash A., Dogra S. Protective effect of curcumin (Curcuma longa) againstd-galactose-induced senescence in mice. Journal of Asian natural products research . 2011;13(1):42–55. doi: 10.1080/10286020.2010.544253. [DOI] [PubMed] [Google Scholar]
- 155.Li J.-h., Wei T.-t., Guo L., et al. Curcumin protects thymus against D-galactose-induced senescence in mice. Naunyn-Schmiedeberg's Archives of Pharmacology . 2021;394(2):411–420. doi: 10.1007/s00210-020-01945-8. [DOI] [PubMed] [Google Scholar]
- 156.Sun C. Y., Qi S. S., Zhou P., et al. Neurobiological and pharmacological validity of curcumin in ameliorating memory performance of senescence-accelerated mice. Pharmacology Biochemistry and Behavior . 2013;105:76–82. doi: 10.1016/j.pbb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 157.Iijima K., Liu H.-P., Chiang A.-S., Hearn S. A., Konsolaki M., Zhong Y. Dissecting the pathological effects of human A 40 and A 42 in Drosophila: a potential model for Alzheimer's disease. Proceedings of the National Academy of Sciences . 2004;101(17):6623–6628. doi: 10.1073/pnas.0400895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Okuda M., Fujita Y., Hijikuro I., et al. PE859, a novel curcumin derivative, inhibits amyloid-β and tau aggregation, and ameliorates cognitive dysfunction in senescence-accelerated mouse prone 8. Journal of Alzheimer's Disease . 2017;59(1):313–328. doi: 10.3233/JAD-161017. [DOI] [PubMed] [Google Scholar]
- 159.Sun Y., Hu X., Hu G., Xu C., Jiang H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biological and Pharmaceutical Bulletin . 2015;38(8):1134–1141. doi: 10.1248/bpb.b15-00012. [DOI] [PubMed] [Google Scholar]
- 160.Kitani K., Osawa T., Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology . 2007;8(5):567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- 161.Li Y.-B., Zhong Z.-F., Chen M.-W., et al. Bisdemethoxycurcumin Increases Sirt1 to Antagonize t-BHP-Induced Premature Senescence in WI38 Fibroblast Cells. Evidence-Based Complementary and Alternative Medicine. . 2013;2013, article 851714:1–9. doi: 10.1155/2013/851714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takano K., Tatebe J., Washizawa N., Morita T. Curcumin inhibits age-related vascular changes in aged mice fed a high-fat diet. Nutrients . 2018;10(10):p. 1476. doi: 10.3390/nu10101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li K., Zhai M., Jiang L., et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxidative medicine and cellular longevity . 2019;2019 doi: 10.1155/2019/6746907.6746907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kukkemane K., Jagota A. Therapeutic effects of curcumin on age-induced alterations in daily rhythms of clock genes and Sirt 1 expression in the SCN of male Wistar rats. Biogerontology . 2019;20(4):405–419. doi: 10.1007/s10522-018-09794-y. [DOI] [PubMed] [Google Scholar]
- 165.Azami S. H., Nazarian H., Abdollahifar M. A., Eini F., Farsani M. A., Novin M. G. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reproduction, Fertility and Development . 2020;32(3):292–303. doi: 10.1071/RD18472. [DOI] [PubMed] [Google Scholar]
- 166.Selim A. M., Nooh M. M., El-Sawalhi M. M., Ismail N. A. Amelioration of age-related alterations in rat liver: Effects of curcumin C3 complex, Astragalus membranaceus and blueberry. Experimental Gerontology . 2020;137:p. 110982. doi: 10.1016/j.exger.2020.110982. [DOI] [PubMed] [Google Scholar]
- 167.Liu L., Zhang P., Li Y., Yu G. Curcumin protects brain from oxidative stress through inducing expression of UCP2 in chronic cerebral hypoperfusion aging-rats. Molecular neurodegeneration . 2012;7(1) [Google Scholar]
- 168.Suckow B. K., Suckow M. A. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. International journal of biomedical science: IJBS . 2006;2(4):p. 402. [PMC free article] [PubMed] [Google Scholar]


