Abstract
Type 2 diabetes mellitus (T2DM) with nonalcoholic fatty liver disease (NAFLD) is a pathological metabolic disease characterized by high ketone lipid based on abnormal lipid metabolism. Compared with patients with single T2DM or NAFLD, T2DM complicated with NAFLD has more complicated pathogenic factors and pathological processes. Hepatocellular carcinoma (HCC), the leading malignancy arising from cirrhosis, is the second most lethal cancer globally. The purpose of this study was to clarify the main risk factors of T2DM with NAFLD and HCC. There are many challenges in the diagnosis and treatment of T2DM patients with NAFLD and HCC. The current gold standard is to adjust treatment strategy, optimize metabolic control, and improve liver phenotype. It is necessary to identify further the risk factors driving the progression of T2DM with NAFLD and HCC and evaluate new therapeutic targets, in addition to exploring the syndromic forms of T2DM combined with NAFLD and providing a theoretical basis for early prevention, diagnosis, and treatment of the disease using traditional Chinese medicine (TCM).
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is prevalent in patients with type 2 diabetes mellitus (T2DM) [1]. Previous studies had shown that 50% of T2DM patients had NAFLD, while the incidence of NAFLD in obese diabetic patients is as high as 100% [2]. There is increasing evidence that patients with T2DM have a particularly high risk of developing nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and hepatocellular carcinoma (HCC) [3]. HCC is a major life-limiting factor in progressive fibrotic liver disease, mainly caused by a chronic viral infection, alcohol abuse, and nonalcoholic fatty liver disease [4].
In prospective studies, preexisting diabetes mellitus was an independent risk factor for NAFLD progression and liver-related mortality [5, 6]. Studies had shown that the existence of NAFLD predicted the development of T2DM [7]. A cross-sectional study of T2DM patients found that the prevalence of NAFLD identified by ultrasound was 69% [8]. In a Swedish cohort study, most NAFLD patients (78%) were diagnosed with diabetes or impaired glucose tolerance at follow-up [9]. In addition, the interaction of environmental and genetic factors can promote the progress of T2DM with NAFLD. NAFLD increased the incidence of T2DM. At the same time, T2DM can effectively accelerate the development of NAFLD to a more serious form. In most developed countries, NAFLD is currently the most common liver disease and a major risk factor for HCC [10]. One study showed that diabetes increases the risk of HCC [11]. Whether the interaction between diabetes and the etiology of cirrhosis affects the risk of liver cancer remains controversial.
Although significant progress has been made in discovering new targets and treating chronic liver disease in recent decades, most treatment methods have not achieved satisfactory results [12]. Traditional Chinese medicine (TCM) treatment of the disease has the advantages of stable curative effect, safety, being nontoxic, low price, and multitarget effect [13]. In particular, the TCM syndrome types of different diseases may suggest different TCM treatment schemes.
2. Epidemiology of T2DM
The International Diabetes Federation estimates that 371 million adults worldwide had diabetes [14]. In China, the prevalence of diabetes reached 11.6% in 2010, affecting about 113.9 million adults [15]. It is estimated that, by 2040, about 642 million people will have diabetes, and T2DM is the main type of diabetes [16]. T2DM had become a heavy burden of limited medical resources. Since 1980, the incidence rate and prevalence of T2DM in the world had increased two times, and they are still increasing [17]. It had been reported that the prevalence of T2DM in women was on the rise globally, which was more common in low-income countries where obesity and aging were seen as driving forces [18]. In the United States, about one-third of patients with T2DM are adolescents [19]. It is estimated that the prevalence of T2DM in the population above 20 years of age ranges from 6.6% to 7.0% in Spain and 6.3% in Midi-Pyrénées, while the estimated value in men in these three regions is about more than 2% [20].
3. Epidemiology of NAFLD
NAFLD can be divided into two categories: primary and secondary. Fatty liver associated with metabolic syndrome caused by excess nutrition and cryptogenic fatty liver belongs to the category of primary nonalcoholic fatty liver disease. Fatty liver caused by malnutrition, total parenteral nutrition, drug/environment, and industrial toxicosis belongs to the category of secondary nonalcoholic fatty liver disease. NAFLD refers to all kinds of liver diseases, such as nonalcoholic steatohepatitis (NASH), simple steatosis (NAFL), and fibrosis. The incidence rate of NAFLD is expected to increase worldwide with the increase of obesity and diabetes. Recently, studies concluded that the prevalence of NAFLD worldwide is 25.2%, and the prevalence of NAFLD in the US is expected to increase by 50% by 2030 [21, 22]. The prevalence of NAFLD in China was about 20% [23]. NAFLD patients had not only a risk of progressive liver disease but also a significantly increased risk of cancer death [24]. NAFLD is diagnosed when more than 5% of liver cells show fat accumulation or by histological or imaging evaluation [25]. The pathogenesis of NAFLD is complex and has not been fully elucidated.
4. Epidemiology of HCC
Liver cancer mainly refers to malignant tumors originating from hepatocytes, liver epithelium, or liver mesenchymal tissue. HCC is more specific, mainly hepatocellular carcinoma. The etiology of the two cancers is also slightly different. Hepatocellular carcinoma is mainly caused by hepatitis B and hepatitis C. HCC accounts for >80% of primary liver cancers worldwide [26]. HCC accounted for 72.7% of global deaths in 2015 [27]. In addition, the World Health Organization (WHO) estimates that more than 1 million patients are expected to die from liver cancer within the next 10 years [28]. The incidence of liver cancer varies geographically, with the majority of liver cancer cases occurring in less developed regions, such as East Asia (54.8% of cases) and Southeast Asia (10.8% of cases) [29]. From 2006 to 2017, the incidence of HCC increased by 2-3% per year, mainly due to viral cirrhosis and a high incidence of NAFLD [30].
5. Main Risk Factors of T2DM with NAFLD
5.1. Genetic Factors
TM6SF2rs 58542926 mutation was closely related to NAFLD, age, body mass index (BMI), and T2DM [31]. TM6SF2 is located in ER and Golgi complex and has the function of mobilizing neutral lipids for VLDL assembly. In the absence of lipid droplets, lipids accumulate in the droplets [32]. However, the assessment of insulin resistance (IR) or oral glucose tolerance test did not reduce in the TM6SF2 gene mutation vector [33]. Therefore, the mutation may not be associated with IR.
Not only is PNPLA 3 gene mutation related to NAFLD, but also it has a slightly increased risk of T2DM [34, 35]. In fact, the expression of PNPLA 3 is directly regulated by the insulin regulatory transcription factor sterol regulatory element-binding protein 1c (SREBP-1c). In the case of obesity and IR, the accumulation of pathogenic PNPLA 3 mutation products aggravates liver steatosis, inflammation, and cirrhosis [36].
Adiponectin (HMW) is an adipocytokine and insulin-sensitive substance, which plays an essential role in the pathogenesis of diabetes mellitus and NAFLD [37]. HMWrs 266729 polymorphism is associated with an increased risk of NAFLD patients [38]. Studies on different populations showed that HMW gene polymorphism affected the development of NAFLD [39, 40]. There was a significant correlation between rs1501299 and NAFLD in some female diabetic patients in Japan [41]. HMW is considered a potential biomarker for the detection and prediction of NAFLD complicated with T2DM [42]. Lu et al. found that the mutation frequency of LEPR nucleotide 3057 G > A (rs1805096) was 76.0% in 104 T2DM patients with NAFLD. The results suggested that LEPR gene G3057 A (rs1805096) polymorphism may be involved in NAFLD by regulating lipid metabolism and affecting insulin sensitivity in patients with T2DM [43].
5.2. Insulin Resistance
The close relationship between NAFLD and T2DM is that they have common pathogenesis, namely, IR [44]. IR refers to the decrease of tissue response to insulin [45]. The pathogenesis of NAFLD is described as the “multiple hit hypothesis.” IR plays a central role in the first attack, resulting in an imbalance between factors that promote liver fat accumulation and factors that prevent fatty acid accumulation [46, 47]. The steady-state model assessment value of β cell function and the decreased value of β cell function in patients of T2DM with NAFLD were higher than those in patients without NAFLD, including IR of liver and adipocytes [48]. Therefore, NAFLD often coexists with T2DM.
Swollen and inflamed visceral adipose tissue is likely to trigger various factors that may be correlated with the development of IR and NAFLD, such as inflammatory adipocytokines and free fatty acids [49]. The interaction between hepatic steatosis and IR establishes a circle to promote the development of T2DM and NAFLD. In addition, glucose cotransporter 2 can promote renal reabsorption of glucose and reduce urinary glucose excretion by increasing blood glucose and body weight (BW), thus aggravating IR in T2DM and NAFLD patients [50]. This relationship between T2DM, IR, and NAFLD is believed to be due to insulin being delivered directly to the portal vein after secretion in the same way as glucose absorbed. IR plays a crucial role in the pathogenesis of T2DM with NAFLD. Therefore, insulin sensitizer is considered as an effective treatment.
5.3. Lifestyle
A multicenter clinical trial involving 5145 overweight adults with T2DM showed that, after 12 months of intensive lifestyle intervention, steatosis and NAFLD were significantly reduced, and weight loss was at least 7% [51]. Recent randomized controlled trials (n = 154) had again shown that lifestyle intervention could effectively alleviate NAFLD in nonobese and obese patients [52]. Numerous studies have shown that developing a reasonable exercise plan is significant for alleviating T2DM and NAFLD. Reasonable exercise plays an important role in controlling the blood sugar and blood lipids of patients and can significantly improve the therapeutic effect of T2DM or NAFLD.
The relationship between diet and T2DM with NAFLD is very complex. Excess of total energy intake can lead to obesity by changing the energy balance. A high carbohydrate diet (50% to 65% of carbohydrate calories) is associated with IR and obesity [53]. All of these are risk factors for damaging NAFLD phenotype and increasing IR [25]. A review study evaluated the effects of probiotics and synbiotics on obesity, T2DM, and NAFLD [54]. The beneficial effects of probiotics and synbiotics improved liver function and metabolic parameters in NAFLD patients.
Lower frequency and level of physical activity and being sedentary for a long time were associated with IR, T2DM, and NAFLD. Sedentary behavior is associated with chronic low-grade inflammation and can lead to obesity [55]. Exercise management can prevent or delay the progress of T2DM [56]. Among large numbers of middle-aged Korean people, being sedentary and reduced physical activity are positively correlated with the prevalence of NAFLD, which supports the importance of increasing physical activity to promoting physical activity [57, 58].
5.4. Obesity
Obesity is a chronic metabolic disease, which is mainly characterized by excessive accumulation of fat and overweight. The current research showed that the causes of obesity are diverse, and the main reasons are divided into congenital factors and exogenous factors. Studies have shown that the congenital factors of obesity are mainly genetic factors, while the exogenous factors are mainly excessive diet, lack of exercise, or pathological obesity.
The incidence rate of obesity and its metabolic complications worldwide had risen sharply in recent years. Obesity is an important risk factor for NAFLD and T2DM and may provide a common link through IR [59]. Recent studies had shown that obesity (whether peripheral or central obesity) usually preceded NAFLD, and NAFLD preceded the development of T2DM [60]. Obesity is closely related to adipose tissue dysfunction in NAFLD patients, which may accelerate IR and pancreatic β cell dysfunction [61]. To a large extent, IR in obese patients is the result of adipose tissue inflammation and adipocyte regulation disorder [62]. Weight loss has a significant effect on T2DM with NAFLD, and the weight loss is mainly due to the reduction of fat mass, especially visceral fat, rather than skeletal muscle mass [63]. Bariatric surgery is an effective method to treat obesity, which has been proved to significantly improve or even cure diabetes and improve the histological characteristics of NAFLD [64].
In addition, a large number of studies have shown that obesity is closely related to the intestinal flora. The change of intestinal microbiota composition has been considered an effective therapy to regulate obesity [65].
5.5. Others
The data showed that NAFLD and diabetes were related to the decrease of CYP3A4 activity in the liver [66]. In human studies, low plasma adiponectin levels are associated with an increased risk of T2DM, and low adiponectin levels are an independent risk factor for NAFLD [67]. In addition, LDL-c, FPG, BMI, FINS, TC, and HOMA-IR were also risk factors of T2DM with NAFLD [44]. In 146 T2DM patients with NAFLD, multivariate analysis showed that dyslipidemia, elevated LDL, HbA1c, and diastolic blood pressure were risk factors [68]. In addition, human and animal intestines are occupied by a variety of microorganisms. These microorganisms play a key role in maintaining intestinal function and regulating host immune response and chronic diseases such as obesity, diabetes, and NAFLD [69–71].
6. Main Risk Factors of HCC
Major risk factors contributing to the rise in HCC include high prevalence of HBV and HCV infection, followed by an increased incidence of alcohol abuse, obesity, NAFLD, and uncontrolled type 2 diabetes [10]. In areas with high incidence, 80% of HCC patients test positive for hepatitis B surface antigen (HBsAg) in serum [72]. Moreover, 10–20% of patients with hepatitis B can develop HCC without cirrhosis [73]. Hepatitis C virus (HCV) infection is also a major risk factor for HCC, which leads to a 5- to 20-fold risk of HCC [74]. Indeed, persistent cellular stress, repeated necrosis, and compensatory regeneration of cells, as well as chronic inflammation, lead to cellular senescence and mutagenesis, ultimately leading to hepatocarcinogenesis [75]. The mechanism of NAFLD-induced HCC is not fully understood, and there is no way to prevent NAFLD patients from progressing to HCC [76]. T2DM is a risk factor for NAFLD and increases HCC incidence two- to threefold [77]. NAFLD HCC patients have increased levels of IL-13, which can activate myeloid-derived suppressor cells and promote tumor progression by suppressing tumor immunity [78]. Another mechanism underlying NAFLD HCC is PNPLA3 gene polymorphism, possibly related to by enhancing inflammatory signaling [79]. Table 1 compiles the principal observational investigations and meta-analyses analyzing the association between T2DM and the risk of HCC.
Table 1.
Studies which have evaluated the association between type 2 diabetes and risk of HCC.
| Study | Study characteristics | Diabetes diagnosis | Covariate adjustment considered | Main findings |
|---|---|---|---|---|
| Huo et al., Eur J Gastroenterol Hepatol 2003; 15 : 1203-8 | Prospective study: 239 HCC patients (16.3% of whom had DM). Mean follow-up: 2.6 years | Fasting glucose ≥126 mg/dL or 2-hour postload glucose ≥200 mg/dL, or past history | Age, sex, tumor size, anti-HCV-Ab positivity, HBeAg-positivity, cirrhosis, alcohol intake, alpha-fetoprotein, albumin, bilirubin | DM did not affect long-term survival in HCV-related HCC but was a recurrence-independent prognostic factor for HBV-related HCC |
| Coughlin et al., Am J Epidemiol 2004; 159 : 1160-7 | Population cohort study: 467,922 men and 588,321 women without history of cancer at baseline. Mean follow-up: 16 years | Self-reported | BMI | DM was associated with increased risk of incident HCC only in men |
| El-Serag et al., Gastroenterology 2004; 126 : 460-8 | Prospective study: 73,643 patients with DM and 650,620 patients without DM. Mean follow-up: 5 years | Self-reported | Alcoholic liver disease, viral chronic hepatitis, demographic variables | DM was associated with an increased risk of incident HCC. DM carried the highest risk among patients with a follow-up longer than 10 years |
| Davilla et al., Gut 2005; 54 : 533-9 | Population-based case-control study: 2,061 HCC patients (of whom 43% with DM) and 6,183 noncancer controls (of whom 19% with DM) | Electronic register | Age, sex, race, HCV, HBV, alcoholic liver disease, and hemochromatosis | DM was associated with a nearly threefold increased risk of HCC |
| Inoue et al., Arch Intern Med 2006; 166 : 1871-7 | Prospective study: 97,771 Japanese adult individuals followed up for cancer incidence over 5 years. At baseline, 4.7% of them had DM | Self-reported | Age, study area, BMI, prior cardiovascular disease, smoking, alcohol intake, leisure-time physical activity, green vegetable intake, coffee intake | DM was associated with increased risk of total cancer and cancer in specific sites, including HCC |
| El-Serag et al. Clin Gastroenterol Hepatol 2006; 4 : 369-80 | Meta-analysis: a total of 26 studies (of which 13 were case-control studies and 13 were cohort studies), inclusive of approximately 3 million individuals | Self-reported | Alcohol intake, chronic viral hepatitis, diet, BMI | Among 13 cohort studies, DM was associated with an increased risk of HCC |
| Kawamura et al., J Gastroenterol Hepatol 2008; 23 : 1739-46 | Prospective study: 40 consecutive HCC patients (with HCC associated with non-B, non-C hepatitis) and later underwent surgical resection or radiofrequency ablation. Prevalence of DM was 45%. Mean follow-up: 5 years | Fasting glucose ≥126 mg/dL or past history | Age, sex, dyslipidemia, smoking, alcohol intake, history of blood transfusion, state of liver disease (chronic hepatitis or cirrhosis), AST, albumin, bilirubin, alpha-fetoprotein, prothrombin time, tumor size, multiplicity, hypervascularity, and portal vein invasion of HCC | DM was a significant predictor of tumor recurrence after potentially curative therapy for HCC |
| Donadon et al., World J Gastroenterol 2009; 15 : 2506-11 | Case-control study: 465 HCC patients, 618 with cirrhosis, and 490 control subjects. The prevalence of DM was 31.2% in HCC, 23.3% in cirrhotic patients, and 12.7% in control group | Self-reported | Age, sex, BMI, alcohol abuse, HBV, and HCV | DM was an independent risk factor for HCC. Among male patients with DM, there was a positive association of HCC with insulin/sulphonylurea treatment and an inverse association with metformin |
| Hassan et al., Cancer 2010; 116 : 1938-46 | Hospital-based case-control study: 420 patients with HCC and 1,104 healthy controls. The prevalence of DM was 33.3% in patients with HCC and 10.4% in controls | Self-reported | Age, sex, race, educational level, smoking, alcohol intake, HCV, HBV, family history of cancer | DM increased the risk of HCC. Treatments with sulfonylureas or insulin were associated with higher HCC risk, whereas treatments with metformin or glitazones were associated with lower HCC risk |
| Hense et al., Diabetol Metab Syndr 2011; 3 : 15 | Community-based study: 26,742 DM patients, who were 40 to 79 years old and resided in the Muenster district. Mean follow-up: 3.3 years | Self-reported | Sex, diabetes duration, BMI, insulin treatment | Risk of any incident cancer in DM was increased, in particular for HCC. Insulin therapy was related to higher cancer risk, while metformin was not |
| Johnson et al., Diabetologia 2011; 54 : 2263-71 | Population-based retrospective cohort study: 185,100 individuals with DM and 185,100 without DM, matched by sex and age. Mean follow-up: 10 years | Electronic register | Age, sex, socioeconomic status, number of physician visits, year of diagnosis | DM was associated with increased risk of selected cancers, including HCC |
| Li et al., Int J Canc 2012; 131 : 1197-202 | Hospital-based case-control study: 1,105 patients with HBV-related HCC and 5,170 patients with chronic HBV. The whole prevalence of DM was 6.7% | Fasting glucose ≥126 mg/dL or past history | Age, family history of HCC, city of residence, HBV-Ag, and cirrhosis | DM was associated with increased risk of HCC, only in women |
| Wang et al., Int J Cancer 2012; 130 : 1639-48 | Meta-analysis: a total of 25 cohort studies, enrolling 1,283,112 persons. Mean follow-up: 8.8 years | Self-report, medical records | Geographic location, alcohol intake, history of cirrhosis, or HBV and HCV infections | DM was associated with increased risk of incident HCC and higher HCC mortality. Longer diabetes duration and use of sulfonylureas or insulin were associated with increased risk of HCC. Metformin treatment was protective |
| Wang et al., Diabetes Metab Res Rev 2012; 28 : 109-22 | Meta-analysis: 17 case-control studies (a total of nearly 6,000 HCC cases and 74,000 controls) and 32 cohort studies (a total of nearly 6,500,000 individuals) | Self-report, medical records | BMI, prior hepatitis, cirrhosis, alcohol intake, smoking, treatment, duration of diabetes | The combined risk estimate of all studies showed a significant increased risk of HCC among DM individuals. In addition, meta-analysis of 7 cohort studies found a significant increased risk of HCC mortality for individuals with DM compared to those without |
| Lai et al., Am J Gastroenterol 2012; 107 : 46-52 | Population-based cohort study: 19,349 newly diagnosed DM patients and 77,396 control subjects without DM. Mean follow-up: 5 years | Electronic register | Age, sex, cirrhosis, alcoholic liver damage, viral hepatitis | DM was associated with increased risk of incident HCC. Use of metformin or glitazones was associated with reduced HCC risk |
| Schlesinger et al., Ann Oncol 2013; 24 : 2449-55 | Community-based cohort study: 363,426 participants, after excluding those with cancer at baseline. Mean follow-up: 8.5 years | Self-reported | Age, sex, center, education level, smoking, alcohol intake, BMI, waist-to-height ratio | DM was independently associated with higher risk of incident HCC and biliary tract cancer. HCC risk was higher in those treated with insulin. Results were similar in HCV/HBV-negative individuals |
| Zheng et al., PLoS One 2013; 8:e84776 | Hospital-based retrospective case-control study: 1,568 participants of whom 716 patients were diagnosed with benign liver diseases and 852 patients were diagnosed with HCC. The prevalence of DM was 7.6% | Fasting glucose ≥126 mg/dL or 2-hour postload glucose ≥200 mg/dL, HbA1c ≥ 6.5% | Age, sex, HBV and HCV infections, cirrhosis, gallstone disease, cholinesterase, alkaline phosphatase | DM was associated with increased risk of HCC. However, there was a significant interaction between DM and HBV on HCC occurrence |
| Koh et al., Br J Cancer 2013; 108 : 1182-8 | Community-based cohort study: 63,257 middle-aged and older individuals. The prevalence of DM was 8.6%. Mean follow-up: 14 years | Self-reported | Age, sex, BMI, recruitment year, education level, smoking, alcohol intake, consumption of coffee and tea | DM was associated with an increased risk of incident nonviral HCC |
| Miele et al., Gastroenterol Res Pract 2015; 2015 : 570356 | Hospital-based case-control study: 224 HCC patients and 389 controls. The prevalence of DM was 19.7% | Self-reported | Age, sex, smoking, alcohol intake | DM was associated with increased risk of HCC. Treatment with any glucose-lowering drugs was not associated with increased HCC risk |
| Adami et al., J Natl Cancer Inst 1996; 88 : 1472-7 | Hospital-based cohort: 153,852 patients with DM. Follow-up: from 1 to 24 years | Hospital discharge diagnosis | None | DM was associated with increased risk of incident HCC |
| La Vecchia et al., Int J Cancer 1997; 73 : 204-7 | Case-control study: 428 HCC cases, 59 with gallbladder and bile duct cancers, and 1,502 control subjects from hospital | Self-reported | Age, sex, area of residence, education level, alcohol intake, BMI, smoking, history of chronic hepatitis and cirrhosis, family history of liver cancer | DM was associated with increased risk of incident HCC |
7. TCM Syndrome Types of T2DM with NAFLD
In recent years, TCM and its extracts have been considered a new potential source of therapeutic drugs for preventing and treating fatty liver disease [80]. According to modern TCM theory, type 2 diabetes belongs to the category of diabetes. There are many problems, such as dryness and heat injury, qi and yin deficiency, liver qi and yin deficiency, liver failure, spleen failure, liver blood deficiency, and spleen stomach heat accumulation. Therefore, it can be divided into eight types: stomach heat syndrome, lung dryness syndrome, spleen qi deficiency syndrome, lung qi deficiency syndrome, yin and yang deficiency syndrome, kidney yin deficiency syndrome, blood stasis syndrome, and phlegm retention syndrome.
Statistical analysis showed that spleen deficiency syndrome was the main syndrome type in T2DM with the NAFLD group [81]. This has also been confirmed in other studies, and phlegm is one of the main syndrome characteristics [82]. There are also studies suggesting that, in T2DM patients with NAFLD, the proportion of damp-heat accumulation is the highest, followed by yin deficiency heat [83]. Additionally, studies also found that damp-heat trapped spleen syndrome and qi and yin deficiency syndrome are the most important syndrome types [84]. Correct evaluation of TCM syndrome types is helpful to improve the clinical effect of TCM combined with general therapy in the treatment of T2DM with NAFLD.
8. Conclusion and Future Prospect
At present, T2DM with NAFLD is considered as a multifactorial disease with genetic and environmental factors. IR is considered as a key risk factor for the occurrence and development of T2DM with NAFLD. IR in the peripheral tissue and liver is one of the causes of this condition, leading to the increase of circulating glucose and lipid substrates for lipid accumulation in the liver. Changing diet structure is beneficial to delay the progression of T2DM with NAFLD. It supports more extensive application of traditional Chinese medicine, Chinese patent medicine, and acupuncture physiotherapy, which provides theoretical support for the clinical application of traditional Chinese medicine therapy. However, there are still many deficiencies in the treatment of TCM. Therefore, further research and clinical verification are needed (Figure 1).
Figure 1.
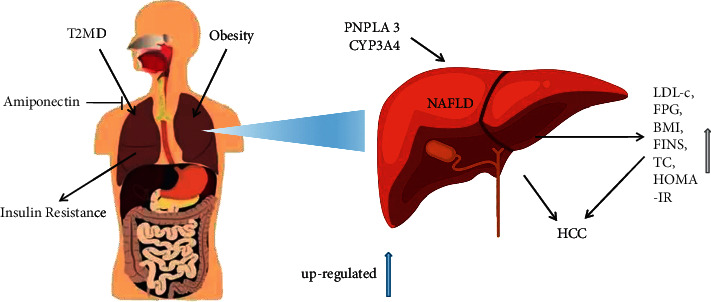
Biological mechanisms linking type 2 diabetes mellitus and NAFLD.
Prevention and treatment of viral hepatitis and NAFLD were vital factors in reducing the global burden of liver cancer. Implementation of screening for viral hepatitis and surveillance for hepatocellular carcinoma in high-risk patients are essential to improve current poor outcomes for patients with HCC. However, a better understanding of risk factors for liver cancer is required for developing new effective regimens and improving the efficacy of the existing therapies. [85]
Acknowledgments
This work was supported by the Research Project of Hebei Administration of Traditional Chinese Medicine (no. 2018479) and Project Support of Yuansong Wang Heritage Studio of Famous Traditional Chinese Medicine in Hebei Province.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Yueying Qi and Decong Ran have contributed equally to this work.
References
- 1.Adams L. A., Anstee Q. M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut . 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 2.Chitturi S., Wong V. W.-S., Farrell G. Nonalcoholic fatty liver in Asia: firmly entrenched and rapidly gaining ground. Journal of Gastroenterology and Hepatology . 2011;26(1):163–172. doi: 10.1111/j.1440-1746.2010.06548.x. [DOI] [PubMed] [Google Scholar]
- 3.Leite N. C., Villela-Nogueira C. A., Cardoso C. R., Salles G. F. Non-alcoholic fatty liver disease and diabetes: from physiopathological interplay to diagnosis and treatment. World Journal of Gastroenterology . 2014;20(26):8377–8392. doi: 10.3748/wjg.v20.i26.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higashi T., Friedman S. L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Advanced Drug Delivery Reviews . 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z. M., Gramlich T., Matteoni C. A., Boparai N., McCullough A. J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clinical Gastroenterology and Hepatology . 2004;2(3):262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 6.Porepa L., Ray J. G., Sanchez-Romeu P., Booth G. L. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. Canadian Medical Association Journal . 2010;182(11):E526–E531. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Annals of Medicine . 2011;43(8):617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 8.Leite N. C., Salles G. F., Araujo A. L. E., Villela-Nogueira C. A., Cardoso C. R. L. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver International . 2009;29(1):113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 9.Ekstedt M., Franzén L. E., Mathiesen U. L., et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology . 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 10.Yang J. D., Hainaut P., Gores G. J., Amadou A., Plymoth A., Roberts L. R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nature Reviews Gastroenterology & Hepatology . 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J. D., Mohamed H. A., Cvinar J. L., Gores G. J., Roberts L. R., Kim R. W. Diabetes mellitus heightens the risk of hepatocellular carcinoma except in patients with hepatitis C cirrhosis. American Journal of Gastroenterology . 2016;111(11):1573–1580. doi: 10.1038/ajg.2016.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B. E. Treatment of chronic liver diseases with traditional Chinese medicine. Journal of Gastroenterology and Hepatology . 2000;15:E67–E70. doi: 10.1046/j.1440-1746.2000.02100.x. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M., Hayashi A., Taguchi T., et al. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non‐alcoholic fatty liver disease. Journal of Diabetes Investigation . 2019;10(4):1004–1011. doi: 10.1111/jdi.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Y., Zhang M., Zhao J., et al. Association of the hypertriglyceridemic waist phenotype and type 2 diabetes mellitus among adults in China. Journal of Diabetes Investigation . 2016;7(5):689–694. doi: 10.1111/jdi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y., Wang L., He J., et al. Prevalence and control of diabetes in Chinese adults. Journal of the American Medical Association . 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 16.Oguntibeju O. O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. International journal of physiology, pathophysiology and pharmacology . 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Bashier A., Bin Hussain A., Abdelgadir E., Alawadi F., Sabbour H., Chilton R. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetology & Metabolic Syndrome . 2019;11(1):p. 80. doi: 10.1186/s13098-019-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roglic G. Diabetes in women: the global perspective. International Journal of Gynaecology & Obstetrics . 2009;104:S11–S13. doi: 10.1016/j.ijgo.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Silink M. Childhood diabetes: a global perspective. Hormone Research . 2002;57(Suppl 1):1–5. doi: 10.1159/000053304. [DOI] [PubMed] [Google Scholar]
- 20.Moulis G., Ibañez B., Palmaro A., et al. Cross-national health care database utilization between Spain and France: results from the EPICHRONIC study assessing the prevalence of type 2 diabetes mellitus. Clinical Epidemiology . 2018;10:863–874. doi: 10.2147/clep.s151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology . 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 22.Fleischman M. W., Budoff M., Zeb I., Li D., Foster T. NAFLD prevalence differs among hispanic subgroups: the Multi-Ethnic Study of Atherosclerosis. World Journal of Gastroenterology . 2014;20(17):4987–4993. doi: 10.3748/wjg.v20.i17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Xue J., Chen P., Chen L., Yan S., Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. Journal of Gastroenterology and Hepatology . 2014;29(1):42–51. doi: 10.1111/jgh.12428. [DOI] [PubMed] [Google Scholar]
- 24.Calzadilla Bertot L., Adams L. A. The natural course of non-alcoholic fatty liver disease. International Journal of Molecular Sciences . 2016;17(5) doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Chiara F., Ureta Checcllo C., Ramon Azcon J. High protein diet and metabolic plasticity in non-alcoholic fatty liver disease: myths and truths. Nutrients . 2019;11(12) doi: 10.3390/nu11122985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Serag H. B., Rudolph K. L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology . 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 27.Sarin S. K., Kumar M., Eslam M., et al. Liver diseases in the asia-pacific region: a lancet gastroenterology and hepatology commission. The Lancet Gastroenterology and Hepatology . 2020;5(2):167–228. doi: 10.1016/s2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kole C., Charalampakis N., Tsakatikas S., et al. Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers . 2020;12(10) doi: 10.3390/cancers12102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z., Xie H., Hu M., et al. Recent progress in treatment of hepatocellular carcinoma. American journal of cancer research . 2020;10(9):2993–3036. [PMC free article] [PubMed] [Google Scholar]
- 30.Li S., Saviano A., Erstad D. J., et al. Risk factors, pathogenesis, and strategies for hepatocellular carcinoma prevention: emphasis on secondary prevention and its translational challenges. Journal of Clinical Medicine . 2020;9(12) doi: 10.3390/jcm9123817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y.-L., Reeves H. L., Burt A. D., et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nature Communications . 2014;5(1):p. 4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smagris E., Gilyard S., BasuRay S., Cohen J. C., Hobbs H. H. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. Journal of Biological Chemistry . 2016;291(20):10659–10676. doi: 10.1074/jbc.m116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Llauradó G., Orešič M., Hyötyläinen T., Orho-Melander M., Yki-Järvinen H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. Journal of Hepatology . 2015;62(3):657–663. doi: 10.1016/j.jhep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Romeo S., Kozlitina J., Xing C., et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics . 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dongiovanni P., Stender S., Pietrelli A., et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. Journal of Internal Medicine . 2018;283(4):356–370. doi: 10.1111/joim.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., He S., Li J. Z., et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proceedings of the National Academy of Sciences . 2010;107(17):7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P.-W., Hsieh C.-J., Psang L.-C., et al. Fatty liver and chronic inflammation in Chinese adults. Diabetes Research and Clinical Practice . 2008;81(2):202–208. doi: 10.1016/j.diabres.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh C.-J., Wang P. W., Hu T. H. Association of adiponectin gene polymorphism with nonalcoholic fatty liver disease in Taiwanese patients with type 2 diabetes. PloS One . 2015;10(6) doi: 10.1371/journal.pone.0127521.e0127521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z. L., Xia B., Shrestha U., et al. Correlation between adiponectin polymorphisms and non-alcoholic fatty liver disease with or without metabolic syndrome in Chinese population. Journal of Endocrinological Investigation . 2008;31(12):1086–1091. doi: 10.1007/bf03345657. [DOI] [PubMed] [Google Scholar]
- 40.Hashemi M., Hanafi Bojd H., Eskandari Nasab E., et al. Association of adiponectin rs1501299 and rs266729 gene polymorphisms with nonalcoholic fatty liver disease. Hepatitis Monthly . 2013;13(5) doi: 10.5812/hepatmon.9527.e9527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokushige K., Hashimoto E., Noto H., et al. Influence of adiponectin gene polymorphisms in Japanese patients with non-alcoholic fatty liver disease. Journal of Gastroenterology . 2009;44(9):976–982. doi: 10.1007/s00535-009-0085-z. [DOI] [PubMed] [Google Scholar]
- 42.Leite N. C., Salles G. F., Cardoso C. R. L., Villela-Nogueira C. A. Serum biomarkers in type 2 diabetic patients with non-alcoholic steatohepatitis and advanced fibrosis. Hepatology Research . 2013;43(5):508–515. doi: 10.1111/j.1872-034x.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 43.Lu H., Sun J., Sun L., Shu X., Xu Y., Xie D. Polymorphism of human leptin receptor gene is associated with type 2 diabetic patients complicated with non-alcoholic fatty liver disease in China. Journal of Gastroenterology and Hepatology . 2009;24(2):228–232. doi: 10.1111/j.1440-1746.2008.05544.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z., Wang J., Wang H. Correlation of blood glucose, serum chemerin and insulin resistance with NAFLD in patients with type 2 diabetes mellitus. Experimental and therapeutic medicine . 2018;15(3):2936–2940. doi: 10.3892/etm.2018.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung U., Choi M.-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International Journal of Molecular Sciences . 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day C. P., James O. F. W. Steatohepatitis: a tale of two “hits”? Gastroenterology . 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 47.Targher G., Day C. P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. New England Journal of Medicine . 2010;363(14):1341–1350. doi: 10.1056/nejmra0912063. [DOI] [PubMed] [Google Scholar]
- 48.Simoes I. C. M., Janikiewicz J., Bauer J., et al. Fat and sugar-A dangerous duet. A comparative review on metabolic remodeling in rodent models of nonalcoholic fatty liver disease. Nutrients . 2019;11(12) doi: 10.3390/nu11122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoelson S. E., Herrero L., Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology . 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 50.Arase Y., Shiraishi K., Anzai K., et al. Effect of sodium glucose Co-transporter 2 inhibitors on liver fat mass and body composition in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus. Clinical Drug Investigation . 2019;39(7):631–641. doi: 10.1007/s40261-019-00785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazo M., Solga S. F., Horska A., et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care . 2010;33(10):2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong V. W.-S., Wong G. L.-H., Chan R. S.-M., et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. Journal of Hepatology . 2018;69(6):1349–1356. doi: 10.1016/j.jhep.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Grundy S. M., Abate N., Chandalia M. Diet composition and the metabolic syndrome: what is the optimal fat intake? Americas Journal of Medicine . 2002;113(Suppl 9B):25S–9S. doi: 10.1016/s0002-9343(01)00988-3. [DOI] [PubMed] [Google Scholar]
- 54.Saez-Lara M. J., Robles-Sanchez C., Ruiz-Ojeda F. J., Plaza-Diaz J., Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. International Journal of Molecular Sciences . 2016;17(6) doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton M. T., Hamilton D. G., Zderic T. W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes . 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 56.Wang B., Mu X.-L., Zhao J., et al. Effects of lifestyle interventions on rural patients with type 2 diabetes mellitus. World Journal of Diabetes . 2020;11(6):261–268. doi: 10.4239/wjd.v11.i6.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snowling N. J., Hopkins W. G. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care . 2006;29(11):2518–2527. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 58.Ryu S., Chang Y., Jung H.-S., et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. Journal of Hepatology . 2015;63(5):1229–1237. doi: 10.1016/j.jhep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Hui E., Xu A., Bo Yang H., Lam K. S. L. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: role of adipokines. Journal of Diabetes Investigation . 2013;4(5):413–425. doi: 10.1111/jdi.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maliakkal B. J. Pathogenesis of non-alcoholic fatty liver disease and implications on cardiovascular outcomes in liver transplantation. Translational Gastroenterology and Hepatology . 2020;5:p. 36. doi: 10.21037/tgh.2019.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age? World Journal of Gastroenterology . 2014;20(27):9072–9089. doi: 10.3748/wjg.v20.i27.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tilg H., Moschen A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology . 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 63.Perdomo C. M., Frühbeck G., Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients . 2019;11(3) doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hazlehurst J. M., Woods C., Marjot T., Cobbold J. F., Tomlinson J. W. Non-alcoholic fatty liver disease and diabetes. Metabolism . 2016;65(8):1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duranti S., Ferrario C., van Sinderen D., Ventura M., Turroni F. Obesity and microbiota: an example of an intricate relationship. Genes & Nutrition . 2017;12(1):p. 18. doi: 10.1186/s12263-017-0566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jamwal R., de la Monte S. M., Ogasawara K., Adusumalli S., Barlock B. B., Akhlaghi F. Nonalcoholic fatty liver disease and diabetes are associated with decreased CYP3A4 protein expression and activity in human liver. Molecular Pharmaceutics . 2018;15(7):2621–2632. doi: 10.1021/acs.molpharmaceut.8b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marino L., Jornayvaz F. R. Endocrine causes of nonalcoholic fatty liver disease. World Journal of Gastroenterology . 2015;21(39):11053–11076. doi: 10.3748/wjg.v21.i39.11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butt A. S., Hamid S., Haider Z., et al. Nonalcoholic fatty liver diseases among recently diagnosed patients with diabetes mellitus and risk factors. Euroasian Journal of Hepato-Gastroenterology . 2019;9(1):9–13. doi: 10.5005/jp-journals-10018-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitocco D., Di Leo M., Tartaglione L., et al. The role of gut microbiota in mediating obesity and diabetes mellitus. European Review for Medical and Pharmacological Sciences . 2020;24(3):1548–1562. doi: 10.26355/eurrev_202002_20213. [DOI] [PubMed] [Google Scholar]
- 70.Mokkala K., Houttu N., Cansev T., Laitinen K. Interactions of dietary fat with the gut microbiota: evaluation of mechanisms and metabolic consequences. Clinical Nutrition . 2020;39(4):994–1018. doi: 10.1016/j.clnu.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Ji Y., Yin Y., Sun L., Zhang W. The molecular and mechanistic insights based on gut-liver Axis: nutritional target for non-alcoholic fatty liver disease (NAFLD) improvement. International Journal of Molecular Sciences . 2020;21(9) doi: 10.3390/ijms21093066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu Y.-S., Chien R.-N., Yeh C.-T., et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology . 2002;35(6):1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 73.Yang J. D., Kim W. R., Coelho R., et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clinical Gastroenterology and Hepatology . 2011;9(1):64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dash S., Aydin Y., Widmer K. E., Nayak L. Hepatocellular carcinoma mechanisms associated with chronic HCV infection and the impact of direct-acting antiviral treatment. Journal of Hepatocellular Carcinoma . 2020;7:45–76. doi: 10.2147/jhc.s221187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giraud J., Chalopin D., Blanc J.-F., Saleh M. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Frontiers in Immunology . 2021;12 doi: 10.3389/fimmu.2021.655697.655697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujiwara N., Friedman S. L., Goossens N., Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. Journal of Hepatology . 2018;68(3):526–549. doi: 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massarweh N. N., El-Serag H. B. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control : Journal of the Moffitt Cancer Center . 2017;24(3) doi: 10.1177/1073274817729245.1073274817729245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ponziani F. R., Bhoori S., Castelli C., et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology . 2019;69(1):107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 79.Friedrich K., Wannhoff A., Kattner S., et al. PNPLA3 in end-stage liver disease: alcohol consumption, hepatocellular carcinoma development, and transplantation-free survival. Journal of Gastroenterology and Hepatology . 2014;29(7):1477–1484. doi: 10.1111/jgh.12540. [DOI] [PubMed] [Google Scholar]
- 80.Panyod S., Sheen L.-Y. Beneficial effects of Chinese herbs in the treatment of fatty liver diseases. Journal of Traditional and Complementary Medicine . 2020;10(3):260–267. doi: 10.1016/j.jtcme.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C., Pang J. TCM clinical research progress of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Mass technology . 2019;21(02):61–64. [Google Scholar]
- 82.Liu X. Correlation Between TCM Syndrome Types and Islet β Cell Function in Newly Diagnosed Type 2 Diabetes Mellitus Patients with Nonalcoholic Fatty Liver Disease . Lanzhou, China: Traditional Chinese Medicine University of Gansu; 2017. [Google Scholar]
- 83.Zhang Z., Wu J., Qi Y. Clinical study on main risk factors and TCM syndrome types of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Modern distance education of traditional Chinese medicine in China . 2019;17(11):48–50. [Google Scholar]
- 84.Lu Y. Analysis of the Difference and Risk Factors of Insulin Resistance in Different TCM Syndrome Types of Type 2 Diabetes Mellitus with Nonalcoholic Fatty Liver Disease . Hangzhou, China: Chinese Medical University of Zhejiang; 2013. [Google Scholar]
- 85.Gao T., Yan L., Wang Y. Analysis of common TCM syndromes and nonalcoholic fatty liver disease and related risk factors in newly diagnosed type 2 diabetes mellitus. Journal of Traditional Chinese Medicine in Liaoning . 2011;38(02):200–202. [Google Scholar]


