Abstract
Coronary microvascular dysfunction (CMD) encompasses several pathogenetic mechanisms involving coronary microcirculation and plays a major role in determining myocardial ischemia in patients with angina without obstructive coronary artery disease (CAD), as well as in several other conditions, including obstructive CAD, non-ischemic cardiomyopathies, Takotsubo syndrome and heart failure, especially the phenotype associated with preserved ejection fraction. Unfortunately, despite the identified pathophysiological and prognostic role of CMD in several conditions, to date, there is no specific treatment for CMD. Due to the emerging role of CMD as common denominator in different clinical phenotypes, additional research in this area is warranted in order to provide personalized treatments in this “garden variety” of patients. The purpose of this review is to describe the pathophysiological mechanisms of CMD and its mechanistic and prognostic role across different cardiovascular diseases. We will also discuss diagnostic modalities and the potential therapeutic strategies resulting from recent clinical studies.
Keywords: Coronary microvascular dysfunction, microvascular angina, angina, heart failure, HFpEF, ischemia, MINOCA, INOCA, microcirculation, provocative testing, coronary flow reserve, coronary spasm, ischemic heart disease, coronary flow reserve
Condesed abstract
Coronary microvascular dysfunction (CMD) is prevalent across several cardiovascular conditions. The emerging role of CMD as common denominator in different clinical entities, calls for addressing knowledge gaps for the management of these heterogeneous patients with evidence of CMD. We revise the pathophysiological mechanisms of CMD, its prognostic role across different cardiovascular diseases, the diagnostic modalities commonly used in clinical practice and the potential therapeutic strategies for optimal patient management.
1. Introduction
The term coronary microvascular dysfunction (CMD) has been used referring to the spectrum of structural and functional alterations at level of coronary microcirculation, leading to an impaired coronary blood flow (CBF) and ultimately resulting in myocardial ischemia(1,2). As a result of the growing knowledge of the physiological mechanisms of CMD and the new methods for its assessment, CMD is emerging as a major cause of myocardial ischemia in patients with angina without obstructive coronary artery disease (CAD) (“primary” microvascular angina) as well as in several other conditions, including obstructive CAD, primary cardiomyopathies, Takotsubo syndrome (TTS), and heart failure (HF), especially the phenotype with preserved ejection fraction (HFpEF)(3). The purpose of this narrative review is to describe the pathophysiological mechanisms of CMD and its mechanistic and prognostic role across different cardiovascular diseases (CVD). We will also discuss diagnostic modalities and potential therapeutic strategies.
2. Pathogenetic Mechanisms of Ischemia due to CMD
The coronary arterial system is composed of three uninterrupted compartments with decreasing size and distinct functions(4). The proximal compartment encloses the large epicardial coronary arteries, which have a capacitance function and offer little resistance to CBF; these vessels present a diameter that ranges from 5.0 to 0.5 mm with a three-layered wall. The intermediate compartment comprises pre-arteriolar vessels (500 to 100 µm diameter) that offer significant resistance to blood flow resulting in a measurable pressure drop along its development. It can be further distinguished in two territories: the proximal (500 to 150 µm), more responsive to changes in flow, and the distal (150 to 100 µm), more sensitive to pressure variations. In general, their vasomotor actions are not directly governed by diffusible myocardial metabolites. The distal compartment is represented by intramural arterioles, with diameter<100 µm, and with the function of matching myocardial blood supply with oxygen consumption. Arterioles are more responsive to changes in the intramyocardial concentration of metabolites and are mainly responsible for the metabolic regulation of CBF(5). Pre-arterioles, arterioles, and capillaries constitute the coronary microcirculation (Figure 1). The subset of disorders affecting the structure and function of the coronary microcirculation resulting in an inadequate coronary blood supply, is defined as CMD.
Figure 1: Coronary artery circulation and diagnostic tools for CMD assessment.
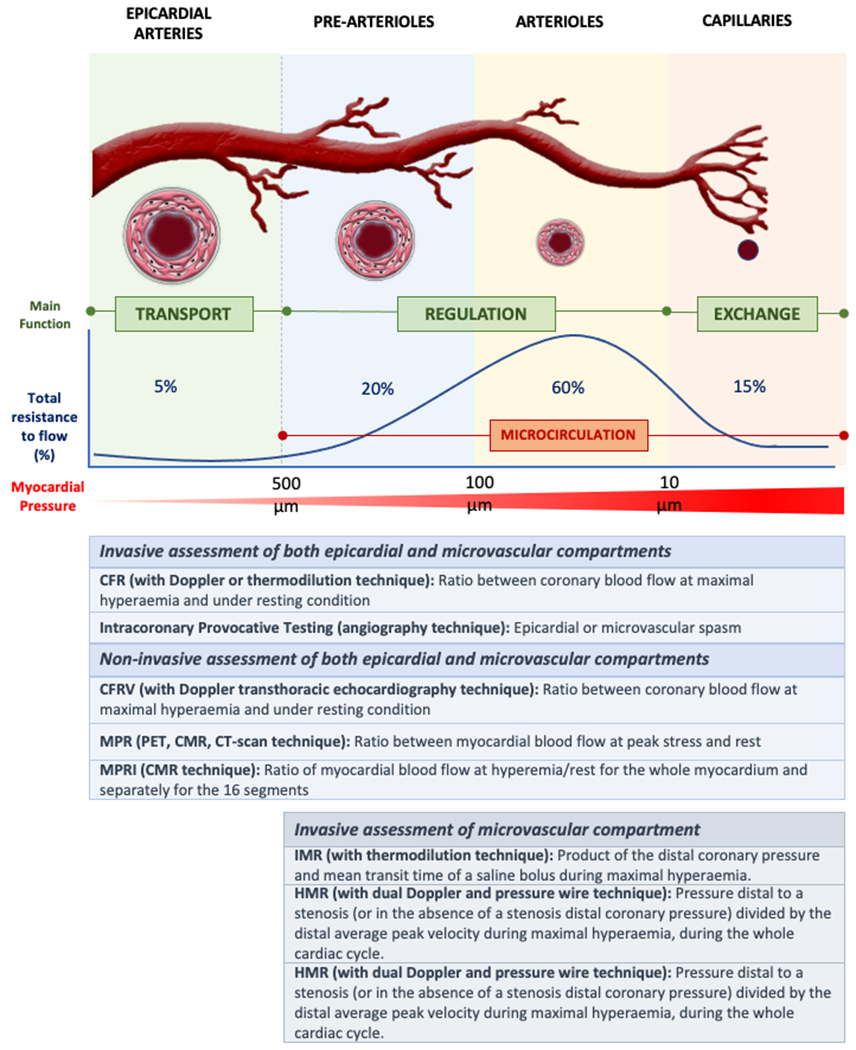
The coronary arterial system is composed of large conductive vessels and the microcirculation (prearterioles and arterioles). Different invasive and non-invasive modalities can be used to characterize CMD. Notably, using non-invasive techniques, the diagnosis of CMD should be made only after the exclusion of obstructive CAD.
Abbreviations:CFR:coronary flow reserve;; CFRV: coronary flow reserve velocity; CMR = cardiac magnetic resonance; CT: computed tomography; IMR: microcirculatory resistance index;HMR: hyperaemic microvascular resistance; MPR: myocardial perfusion reserve; MPRI: myocardial perfusion reserve index; PET: positron emission tomography;
Molecular Mechanisms
Although the mechanisms leading to CMD are not entirely clear, oxidative stress, caused by cellular reactive oxygen species (ROS) overproduction and accumulation, together with the consequent inflammatory response, are considered key pathogenic mechanisms driving the development of CMD(6). Endothelial cells play a crucial role in regulating vasomotor activity by releasing vasoactive substances such as vasodilator nitric oxide (NO) and vasoconstrictor endothelin-1 (ET-1). Nicotinamide adenine dinucleotide phosphate oxidases (Nox) isoforms and mitochondria represent major systems accounting for the regulation of ROS production(7). The activation of Nox leads to ROS production and triggers p66Shc phosphorylation and translocation within the mitochondria. In mammals, p66Shc is a pro-apoptotic protein that further enhances ROS generation by altering the mitochondrial bio-energetic properties(6,8,9). In turn, p66Shc activation stimulates the activity of Nox, thus generating a vicious cycle of ROS augmentation. In vitro and in vivo studies showed that an increased concentration of intracellular ROS promotes the transformation of NO in peroxynitrite radicals and uncouple the endothelial NO synthetase (eNOS), switching its activity from a NO to a ROS producing enzyme, leading to an impairment of NO-mediated vasodilation and an enhanced ET-1 vasoconstriction activity through activation of RhoA/Rho-kinase pathway(10,11). Furthermore, epigenetic modifications, commonly detected in ageing, further sustain oxidative stress by increasing ROS production, reducing the expression of antioxidant enzymes and promoting pro-inflammatory cytokine production through activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and expression of adhesion molecules(6).
The RhoA/Rho-kinase is another pathway strongly implicated in ROS synthesis, as well as in vascular smooth muscle cells (VSMCs) hypercontraction through the modulation of calcium sensitivity and phosphorylation of contractile myofilaments, regulating the force of smooth muscle contraction. It is therefore considered responsible for susceptibility of coronary vessels to spasm(11) and augments inflammation by inducing proinflammatory molecules both in VSMCs and endothelial cells.
Functional Alterations
CMD occurs as a consequence of functional alterations, structural alterations or from a combination of both (Figure 2). In particular, functional mechanisms responsible for CMD may be related to the presence of an impaired dilation (vasodilator abnormalities) and/or an increased constriction of coronary microvessels (microvascular spasm). Impaired vasodilation may be, in turn, caused by endothelium-dependent and/or endothelium-independent mechanisms(1–3,12). The reduced production and/or enhanced degradation of NO and other endothelial-derived relaxation factors result in a limited endothelial-mediated vasodilatory capacity. Of importance, the presence of CVD risk factors have been shown to induce endothelial dysfunction resulting in blunted CBF augmentation or vasoconstriction with frank reduction in CBF. Endothelium-independent mechanisms are less understood; attenuated vasodilator responses to papaverine, adenosine, or dipyridamole, suggest that endothelial-independent vasodilatation likely involves an impaired relaxation of VSMCs. Moreover, an increased release of vasoconstrictor agonists (i.e. ET-1), an enhanced susceptibility of VSMCs to normal vasoconstrictor stimuli and an abnormal increase in sympathetic activity, have also been questioned as potential mechanisms behind this phenomenon. Microvascular spasm is part of the spectrum of vasomotor abnormalities seen in patients with CMD and it appears to be closely linked to the presence of endothelial dysfunction with prevailing vasoactive tone. Of note, the functional responsiveness of the microcirculation can be influenced by several variables, such as heart rate, diastolic time, driving blood pressure and left ventricular inotropism(1–3,12).
Figure 2: Role of CMD in determining ischemia.
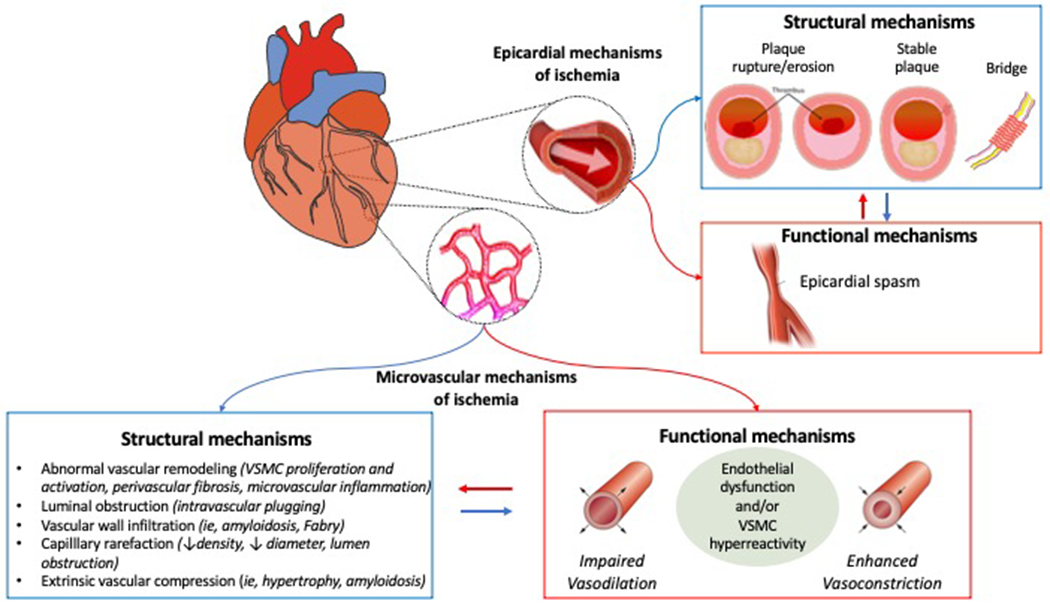
Ischemia may be cause by subtended by epicardial and/or microvascular structural and functional mechanisms. Epicardial causes determining ischemia include acute plaque disruption with lumen occlusion and epicardial coronary spasm, myocardial bridge, or progressive obstruction with vessel narrowing. CMD can result from an abnormal vasodilatory ability of the microvasculature, compressive external forces affecting the intramural microvessels or microvascular spasm.
Abbreviations:CAD:coronary artery disease;VSMC:vascular smooth muscle cells.
Structural Alterations
Along with functional disorders, CMD may also be a consequence of structural alterations, particularly in patients with risk factors for CAD or with underlying cardiomyopathies. Structural abnormalities associated with CMD are mainly represented by luminal narrowing of the intramural arterioles and capillaries, perivascular fibrosis and capillary rarefaction, often in the context of increased left ventricular mass(2). These phenomena are consistently documented in patients with hypertrophic cardiomyopathy (HCM) and hypertensive heart disease(13). In both of these conditions, the morphological changes observed are characterized by adverse remodelling of arterioles, resulting in medial wall thickening (mainly owing to smooth muscle hypertrophy and increased collagen deposition) and variable degrees of intimal thickening, causing altered coronary physiology and CBF(13). An important common feature to these conditions is the diffuse nature of ‘microvascular remodelling’, which extends to the whole left ventricle.
3. Assessment of CMD
Invasive and non-invasive techniques assessing the function and integrity of coronary microvasculature are required to evaluate the presence of CMD (Table 1 and Table 2).
Table 1.
Non-invasive tools for evaluation of CMD.
| Modality | Technique | Agent | Parameter | Diagnostic Threshold | Pro | Cons |
|---|---|---|---|---|---|---|
| Echocardiography | Pulsed-wave Doppler on the proximal LAD artery | Adenosine Dipyridamole Regadenoson | CFRV | CFRV <2 | • Inexpensive • No radiation exposure • No risks |
• Limited do LAD region • Extensive training • Technical pitfalls (poor acoustic window in obese, lung diseases) • Obstructive CAD need to be excluded • Very limited data with use in nonobstructive CAD |
| PET | Dynamic rest and vasodilator stress perfusion imaging | Adenosine Dipyridamole Regadenoson 13Nammonia. 82Rb |
MPR MBF | MPR <2 | • Gold standard for non-invasive assessment of coronary microvascular function • Global evaluation of microvascular function • Low radiation exposure |
• Limited availability • Limited spatial resolution • High costs • Obstructive CAD need to be excluded |
| CMR | Dynamic first-pass vasodilator stress and then rest perfusion images | Adenosine Dipyridamole Regadenoson Gadoliniun-based contrast agents |
MPR MBF MPRI | MPRI <2 | • No radiation exposure • Excellent spatial resolution • All coronary territories can be evaluated at the same time • Tissue characterization |
• High costs • Time-consuming • Poor patient compliance • Limited availability • Limited ability for absolute quantification of MBF • Obstructive CAD need to be excluded • Imaging artifacts • Contraindicated in patients with severe renal disease, claustrophobia, arrhythmias and implanted devices • Still under research investigation |
| CT scan | Dynamic first-pass vasodilator stress and then rest perfusion imaging | Adenosine Dipyridamole Regadenoson Iodine-based contrast agent |
MPR | MPR <2 | • Combination of coronary anatomy and coronary perfusion data • Evaluation of all coronary territories • CTA-derived FFR (FFRCT). |
• Radiation exposure • Risk of kidney disease • Overestimation of MBF (due to the vasodilatory effect of iodinated contrast) • Limited ability for absolute quantification of MBF • Still under research investigation |
Abbreviations: CAD: coronary artery disease: CFRV: coronary flow reserve velocity; CMR: cardiac magnetic resonance; CT: computed tomography; FFR: fractional flow reserve; LAD: left anterior descending artery; MBF: myocardial blood flow; MPR myocardial perfusion reserve;MPRI: myocardial perfusion reserve index; PET: Positron Emission Tomography
*MPR is preferred to CFR when is not calculated invasively.
Table 2:
Invasive tools for evaluation of CMD.
| Modality | Technique | Agent | Parameter | Diagnostic threshold | Pro | Cons |
|---|---|---|---|---|---|---|
| Coronary angiography | Dynamic passage of angiographic contrast | Iodine-contrast agent | TIMI flow TFC |
TIMI-2 TFC> 25 frames |
• Do not necessitate additional costs | • Do not provide information regarding the mechanism of CMD (impaired dilation vs microvascular spasm) • Semiquantitative parameter • Limited sensitivity • Usually calculated after coronary angiography |
| Intracoronary temperature-pressure wire | Estimate of coronary blood flow using bolus (calculating the mean transit time) or continuous thermodilution techniques (does not need pharmacological agents to induce hyperemia) | Adenosine Papaverine Saline solution | CFR IMR | CFR <2- 2.5 IMR >25 U |
• CFR and IMR allow a combined assessment impaired vasodilation and microvascular hyperconstrictive response • IMR is specific for microcirculation and is not affected by resting hemodynamic • HMR is independent of resting coronary • flow • FFR using the standard technique can be measured simultaneously |
• CFR does not distinguish between microvascular and epicardial disease • Cut-off values for IMR still debated • Worse correlation with PET than HMR |
| Intracoronary Doppler flow-pressure wire | Direct measurement of coronary peak flow velocity | Adenosine | CFR HMR | CFR < 2.5 HMR> 1.7 mmHg/cm per second |
• CFR and HMR allow a combined assessment impaired vasodilation and microvascular hyperconstrictive response • HMR is independent of resting coronary Flow FFR using the standard technique can be measured simultaneously |
• CFR does not distinguish between microvascular and epicardial disease • Cut-off values for HMR still debated |
| Intracoronary provocative testing | Intracoronary infusion of vasoactive agents | Acetylcholine Ergonovine | - | - | • Easy to assess • Evaluated at the time of coronary angiography • Do not necessitate additional equipment |
• Additional contrast and radiation • Do not provide direct evidence of microvascular spasm • Risk of arrhythmias • Lack of availability |
Abbreviations: CFR: coronary flow reserve; ECG: electrocardiography; FFR: fractional flow reserve; HMR: hyperaemic microvascular resistance; IMR: index of microvascular resistance; PET: Positron Emission Tomography;
Non-invasive Techniques
Different non-invasive modalities can be used to characterize CMD. Notably, using non-invasive techniques, the diagnosis of CMD should be made only after the exclusion of obstructive CAD using computed tomography coronary angiography (CTCA) or invasive coronary angiography. Additionally, non-invasive techniques do not directly assess the inclination of coronary arteries to spasm, but do only test the vasodilator capacity of VSMC, allowing the identification of impaired vasodilatory capacity of the microcirculation.
Transthoracic Doppler echocardiography (TTDE) allows to measure the maximal diastolic flow in the epicardial arteries during rest and adenosine/dipyridamole/regadenoson stress, known as coronary flow velocity ratio (CFVR)(14). It is often limited to left anterior descending artery (LAD) region, implicitly assuming that this is representative of global microvascular function. CFVR is a reliable measure of coronary microvascular function in absence of any epicardial flow limitation, and cut-off values ≤2-2.5 are commonly used as indicative for impaired coronary microvascular function(15). TTDE is feasible, relatively inexpensive and does not expose patients to radiations, however, it requires extensive training and is associated with many technical pitfalls(16). Contrast echocardiography can also be helpful, but is less commonly used in clinical practice.
Positron Emission Tomography (PET) is now considered the gold standard reference for non-invasive assessment of CMD, as it allows to measure myocardial perfusion reserve (MPR), evaluating all coronary territories at the same time by quantification of myocardial blood flow (MBF) at rest and during pharmacologically induced maximal hyperaemia (ratio of stress/rest MBF)(17). Presently, MBF can be quantified noninvasively using radionuclide imaging, cardiac magnetic resonance (CMR), or contrast echocardiography, but, PET is the mostly commonly used and validated technique. However, its use is restricted in clinical practice due to the limited availability and high cost(16).
CMR allows a simultaneous assessment of cardiac anatomy, morphology, functionality, and myocardial perfusion. CMR stress perfusion with vasodilator stress is becoming routinely used for the evaluation of myocardial ischemia in patients with suspected obstructive CAD, but has also diagnostic and prognostic value in patients with suspected CMD(18,19). As for PET, CMR allows the quantification of MBF at rest and during pharmacologically induced maximal hyperaemia. Semi-quantitative evaluation of the first-pass perfusion images can be used to calculate an indexed ratio of perfusion-time intensity curve upslopes as a measure of myocardial perfusion reserve index (MPRI) in response to vasodilator stress(20).
Myocardial first-pass dynamic computed tomography (CT) scan, similarly to CMR, allows a (semi-) quantitative assessment of MBF and MPR(21). Studies on perfusion CT in the setting of CMD assessment are still sparse and, in contrast to CMR, this method is associated with substantial radiation. Nevertheless, CT imaging represents a promising technique that allows to combine CTCA and CT perfusion for the exclusion of epicardial CAD and assessment of microvascular function within one diagnostic tool(16).
Invasive Techniques
Coronary angiography is an invasive test that allows to exclude the presence of obstructive CAD. Moreover, the dynamic passage of angiographic contrast also provides information on coronary microcirculation. The “coronary slow-flow phenomenon” is an angiographic entity characterized by the delayed opacification of the distal coronary vasculature in the presence of non-obstructive CAD, and is considered a surrogate for the presence of CMD. The criteria used to define the coronary slow-flow varies among studies, some utilizing the thrombolysis in myocardial infarction (i.e. TIMI-2 flow) and others using the corrected TIMI frame count (CTFC>25 frames)(15).
A comprehensive invasive functional assessment of coronary microcirculation requires the investigation of the vasodilator as well as the vasoconstrictor microvascular response. For this purpose, coronary flow reserve (CFR) and microvascular resistance measurements by intracoronary Doppler or thermodilution along with intracoronary provocative testing have been introduced to obtain a combined assessment and diagnostic distinction of an impaired vasodilation of the microvasculature as well as an increased microvascular hyperconstrictive response.
The vasodilator capacity of the coronary microcirculation is usually assessed by calculating the CFR as the ratio of maximum hyperaemic to basal coronary flow velocity (with adenosine, being the most used vasodilator for hyperaemia)(1,2,16). It can be assessed by measuring coronary blood flow velocity by an intracoronary Doppler flow wire placed in the distal part of the studied coronary artery (usually the LAD artery) or using a temperature sensor-tipped guidewire. The thermodilution technique estimates CFR indirectly via the saline bolus transit time, both of which are surrogates of true CBF. CFR can be reduced in patients with epicardial CAD as well as in patients with CMD who have an impaired hyperaemic microvascular dilatory response, and therefore CFR can only serve as indicator of microvascular function in patients with unobstructed coronary arteries since it does not distinguish between microvascular and epicardial CAD. CFR is considered normal when values are >2.5(1,2,16). The thermodilution-based index of microvascular resistances (IMR) has been introduced to selectively test microvascular dilatory function and is defined as the product of the distal coronary pressure and mean transit time of a saline bolus during maximal hyperaemia (using adenosine or papaverine)(22). IMR is independent on epicardial vascular function and reproducible(22,23). Alternatively, hyperaemic microvascular resistance (hMR) incorporates Doppler flow velocity to estimate flow instead of thermodilution(24).
The diagnosis of a coronary vasospasm at the epicardial and/or microvascular level requires an intracoronary provocative test with pharmacologic vasoactive agents(25). Microvascular spasm is defined as the concomitance of ischemic electrocardiographic changes along with reproduction of the typical symptoms (i.e., chest pain) without angiographic evidence of epicardial spasm(26,27). In clinical practice, the most commonly used vasoactive provocative agents are acetylcholine (ACh) and ergonovine. ACh stimulates NO release from endothelium to vasodilate and directly induce VSMCs contractility. Dependent on endothelial integrity and VSMC reactivity, the net effect of intracoronary ACh administration is either vasodilatation (healthy endothelium) or coronary spasm(26).
4. CMD Across Different CVD
CMD is prevalent across different CVD, and may have a pathophysiological and a prognostic role in specific populations (Central Illustration).
Central Illustration: Role of CMD across different cardiovascular diseases.
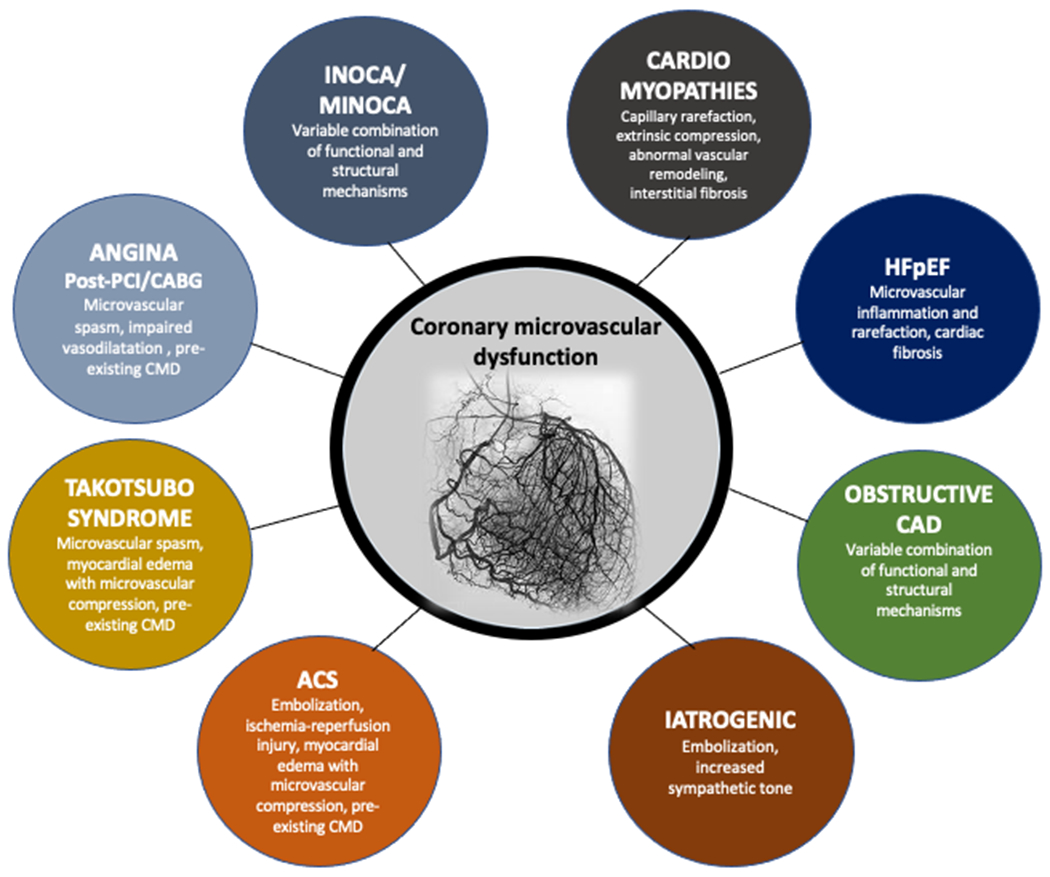
CMD plays a major role in determining myocardial ischemia in many cardiovascular conditions, including angina with and without obstructive CAD, myocardial infarction, non-ischemic cardiomyopathies, Takotsubo syndrome and heart failure (especially HFpEF). Many molecular, functional and structural mechanisms may be involved and are related to the underlying disease.
Abbreviations:ACS:acute coronary syndrome;CABG:coronary artery bypass graft;CAD:coronary artery disease;HFpEF:HF with preserved ejection fraction;INOCA:ischemia with non-obstructive coronary arteries;MINOCA;myocardial infarction with non-obstructive coronary arteries;PCI:percutaneous coronary intervention.
CMD in Patients without Obstructive CAD, Myocardial Diseases and Valvular Heart Diseases
Ischemia with non-obstructive CAD (INOCA)
CMD, alone or in combination with CAD, is one of the mechanisms responsible for myocardial ischemia and symptoms in INOCA (Ischemia with non-obstructive CAD) and can result in the clinical picture of primary microvascular angina (MVA), in which CMD is the principal alteration causing symptoms(15). MVA represents up to 40% of patients presenting with signs and symptoms of myocardial ischemia with normal or near-normal (<50%) coronary arteries at coronary angiography(1,15). The COVADIS (Coronary Vasomotor Disorders) study group proposed the following diagnostic criteria for MVA: signs and symptoms of myocardial ischemia, reduced CFR or microvascular spasm, and documented myocardial ischemia, which is not triggered by obstructive CAD but by functional or structural abnormalities at the site of the coronary microcirculation(Figure 3)(15). Of importance, in this subset of patients, the presence of CMD has also prognostic implications. Indeed, data from the National Heart, Lung, and Blood Institute–sponsored WISE (Women’s Ischemia Syndrome Evaluation) study suggested that there is a worse prognosis in patients with INOCA, and low CFR was a robust independent predictor of MACE(28). These findings were then extended and a reduced CFR (CFR<2) was found as a powerful incremental predictor of MACE in both women and men(2). Moreover, along with an adverse cardiovascular prognosis, INOCA patients have also a poor physical functioning and a reduced quality of life(30). Recently, the CORonary MICrovascular Angina (CorMicA) trial provided proof-of-concept clinical evidence that a strategy of adjunctive invasive testing for disorders of coronary function linked with stratified medical therapy led to improvements in patient outcomes, including reduction in angina severity and better quality of life(31).
Figure 3: Assessment of CMD in a patient with microvascular angina.
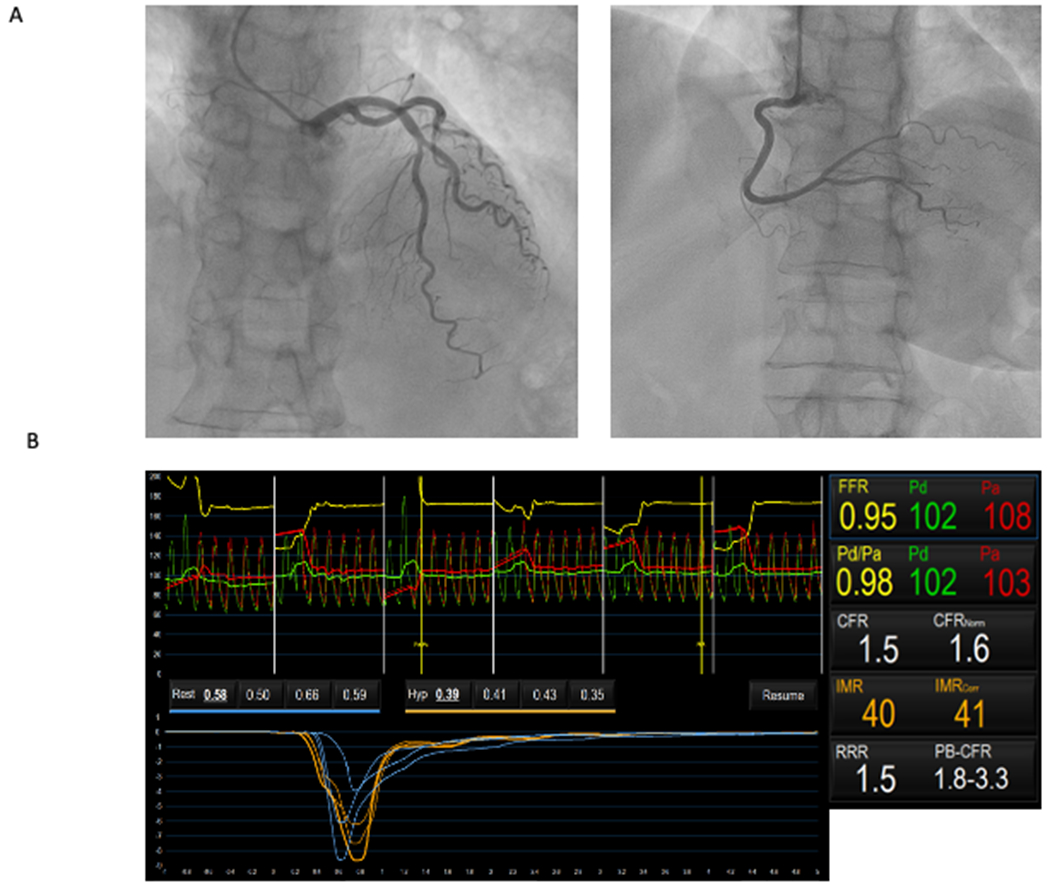
Patient with effort angina and positive non-invasive stress testing. Coronary angiography documented an angiographically normal right coronary artery (right) and intermediate coronary stenosis on mid-left anterior descending artery (left) without haemodynamic significance (FFR 0.95) (Panel A). Microvascular function measured using a pressure wire coupled with thermodilution advanced into the distal part of the left anterior descending artery, at rest and during adenosine-induced maximal hyperaemia, demonstrated an impaired coronary microvascular function (CFR 1.5 and IMR 40) (Panel B). Intracoronary provocative test with acetylcholine on left anterior descending artery was negative for epicardial and/or microvascular spasm, suggesting a mechanism of CMD due to impaired vasodilation.
Abbreviations:CFR:coronary flow reserve;FFR:fractional flow reserve;IMR:index of microcirculatory resistance.
Myocardial infarction with non-obstructive CAD (MINOCA)
Although CMD typically presents as stable ischemic heart disease, it is also considered a potential cause of myocardial infarction with non-obstructive CAD. The most common microvascular causes of MINOCA are the oblitaration of coronary microcirculation linked to thromboembolism (from left heart, paradox embolism due to dx-sn shunt or hereditary thrombophilic disorders) or microvascular spasm(32,33). In fact, while impaired vasodilation is the most common identifiable mechanism for symptoms in patients with effort angina, microvascular spasm is usually associated with angina at rest, and sometimes with an acute presentation as MINOCA(27). However, limitated studies evaluated the role of CMD in these patients. MINOCA remains often an underdiagnosed and untreated condition, associated with a significant incidence of adverse events as well as impaired quality of life(34).
Takotsubo Syndrome
CMD may also be involved in the pathogenesis of TTS(35,36). Sympathetic over-reactivity following a stressful event may trigger a local myocardial spillover of catecholamines resulting in an acute microvascular coronary vasoconstriction and myocardial stunning. Accordingly, clinical studies using serial Doppler transthoracic echocardiography or PET scan demonstrated a reduced microvascular blood flow and CFR in the acute phase of TTS(37,38). CMD is reversible in most patients with TTS in which a perfusion defect was observed in the dysfunctional segments with transient improvements after intracoronary adenosine infusion and with complete recover at 1 month of follow-up(39). Of importance, the degree of CMD may have also prognostic implications, as patients with TTS presenting with coronary slow-flow at coronary angiography have a worse clinical presentation and a poor long-term clinical outcome(40).
Angina Post PCI or coronary artery bypass (CABG)
CMD may also play a role in determining recurrent or persistent angina even in patients treated with effective PCI or CABG. The mechanisms underlying angina in these scenarios are complex, multifactorial and involve both structural and functional causes(41). The presence of pre-existing CMD along with a predisposition to microvascular spasm and/or impaired vasodilatation may account for a significant proportion of cases. Indeed, the drug eluted by the stent after PCI has been shown to trigger vasoconstrictor disorders probably enhancing a pre-existing endothelial dysfunction(41). Importantly, a study by Ong et al. demonstrated that approximately half of patients undergoing coronary angiography for recurrent angina after PCI despite patent coronary arteries, have functional causes of myocardial ischemia due to microvascular or epicardial coronary spasm detected by ACh provocative test(42).
CMD in Patients with Obstructive CAD
Chronic Coronary Syndrome
In patients with obstructive CAD, CMD may coexist and determine myocardial ischemia in regions supplied by arteries without stenosis as well as synergistically contribute to magnify myocardial ischemia in regions with epicardial flow limitation(43). In fact, in patients with stable obstructive CAD, preserved coronary arteriolar vasodilator capacity in conjunction with the development of collateral flow may serve to prevent the occurrence of stress-induced myocardial ischemia(44–46). Furthermore, chronic adaptation of coronary microcirculation to a low perfusion pressure distally to a stenosis may negatively influence microvascular remodelling and capacity of maximal vasodilation after restoration of a normal basal CBF, with a variable time being required for coronary microcirculation to resume its normal vasomotility(47). At the same time, in the presence of CMD, fractional flow reserve may potentially underestimate the severity of an epicardial stenosis. This may also explain the discordance between lesion severity and the extent and severity of myocardial ischaemia. Of importance, the presence of CMD among patients with obstructive CAD has also prognostic implications. Indeed, in patients undergoing elective PCI, an intracoronary Doppler-derived CFR<2.5 after PCI predicted recurrence of angina or ischemia within one month(48). Moreover, a reduced CFR was also associated with an adverse outcome in terms of cardiovascular death and readmission for HF independently of the presence of angiographic CAD, and modified the effect of early revascularization, such that only patients with severly reduced CFR appeared to benefit from revascularization (in particular with CABG), underscoring the morbidity associated with CMD(49).
Atherosclerotic Acute Coronary Syndrome (ACS)
In patients with ACS, an acute CMD, namely microvascular obstruction (MVO), may occur(50). In particular, MVO refers to the inability to reperfuse the coronary microcirculation in a previously ischemic region, despite opening of the epicardial vessel. Pathogenic mechanisms underlying MVO are multiple and interacting, including distal atherothrombotic embolization, ischemia-reperfusion injury with endothelial cells death along with cardiomyocyte death, and myocardial oedema and/or inflammation leading to microvascular compression ab-extrinseco. Extensive and severe MVO is associated with intramyocardial haemorrhage, an irreversible process of extravasation of erythrocytes due to capillary destruction(50). Of note, in patients with STEMI, the occurrence of MVO (as assessed by different non-invasive modalities) has consistently been found to be a predictor of adverse events, with higher incidence of left ventricular remodelling, HF, and death(50). A recent meta-analysis showed that patients with an IMR>41 after primary PCI were more likely to have MVO at CMR(51). In addition, a high IMR (IMR>40) measured at the time of primary PCI for ST elevation acute myocardial infraction (STEMI) predicts longer term clinical outcomes such as death and rehospitalization for HF(52).
CMD in Patients with Myocardial Diseases and Valvular Heart Diseases
CMD in HFpEF
HFpEF is an umbrella term encompassing a clinical heterogenous syndrome characterized by classic symptoms and signs of HF despite a normal or near-normal EF(53). Comorbidities, such as obesity, hypertension, metabolic syndrome and type 2 diabetes mellitus (DM), are extremely common and, in fact, independent risk factors for HFpEF(53–55). Diastolic dysfunction, the key hemodynamic feature of HFpEF, is a multifaceted process of adverse LV remodeling, cardiometabolic dysfunction and extracellular fibrosis. In animal models and patients with HFpEF, limited endothelial-dependent NO bioavailability has shown to promote proliferation of fibroblasts and myofibroblasts and affects energy-dependent cardiomyocyte relaxation through the hypophosphorylation of the cytoskeletal protein titin(56). Data from endomyocardial biopsy samples from patients with HFpEF showed that an inflamed microvascular endothelium allows monocytes’ migration and transforming growth factor (TGF)-β release that promotes the differentiation of fibroblasts into myofibroblasts, and collagen production and cross-linking(55,57). Furthermore, this pro-inflammatory and pro-oxidative state, may render the dysfunctional coronary microvasculature more vulnerable to repeat episodes of myocardial ischemia and micro-infarcts leading to interstitial fibrosis, shift in substrate metabolism and reduced systolic reserve, ultimately leading to HFpEF in humans(57). Beyond the diastolic dysfunction, recently HFpEF has been reconceptualized more as a systemic disease, not limited at myocardium and characterized by systemic multiorgan inflammation and microvascular dysfunction(58). The systemic multimorbidity driven pro-inflammatory milieu may promote microvascular inflammation and rarefaction, and cardiac and extracardiac fibrosis(59–61). Whereas CMD exists also in HF with reduced EF (HFrEF), it is postulated to play a dominant role in the pathophysiology and outcomes of HFpEF(62,63). In a small prospective observational study, mean CFR was significantly lower and mean IMR was significantly higher in the HFpEF population compared with the control population, with over one-third of the HFpEF cohort exhibiting overt CMD, the latter associated with worse outcomes in terms of death or HF hospitalization at follow-up(64,65). The prevalence of CMD was the confirmed in a large series of patients with HFpEF undergoing direct intracoronary haemodynamic measurements using Doppler wire, the majority of which displayed CMD caused by both endothelium-dependent (an increase in CBF ≤ 0% in response to ACh) and independent mechanisms (CFR ≤ 2.5)(62). Recently, the multi-national PRevalence Of MIcrovascular dySfunction in Heart Failure with Preserved Ejection Fraction (PROMIS-HFpEF) study showed that 75% of HFpEF patients have CMD (defined, using Doppler echocardiography, as CFR<2.5) and a prespecified exploratory analysis found that CMD was also independently associated with cardiovascular and HF-related events at follow-up(66,67). However, remains to be determined whether CMD is the “primum movens” leading to ventricular remodeling/diastolic dysfunction and HFpEF, or alternatively, this myocardial remodeling proper of HFpEF may secondarily lead to CMD. Previous studies suggested that women with CMD often have left ventricular diastolic dysfunction and are at increased risk of developing HFpEF(68). Recently, Taqueti at al. showed that the presence of both CMD and diastolic dysfunctions was associated with a markedly increased (>5-fold) risk of HFpEF hospitalization(69). Moreover, recently has been shown that exercise intolerance symptoms not only recognize reduced cardiac output as leading cause of limitation, but underscored the importance of peripheral contribution with impaired lung function and skeletal muscle diffusion capacity on the delineation of this clinical scenario. This capillarity alteration is probably linked to a systemic microvascular deregulation and inflammation, that progressively reveal to be the leitmotif of this tangled continuum(59).
CMD and Diabetic Cardiomyopathy
In patients with DM, the evidence of myocardial dysfunction in absence of apparent pathological substrates for cardiac damage (e.g. arterial hypertension, CAD, valvular abnormalities) has been classically defined as ‘diabetic cardiomyopathy’(70). However, the presence of several concomitant and confounding factors such as CAD, aging and comorbidities, led to abandoning the use of the term “diabetic cardiomyopathy” and promoting the use of HFrEF and HFpEF as separate entities.
Impaired insulin signalling, hyperglycaemia/glucotoxicity and lipotoxicity are considered major pathophysiological drivers of DM-related diastolic dysfunction. These factors, as aforementioned, increase oxidative stress and contribute to create the proinflammatory substrate promoting microvascular dysfunction and HFpEF phenotype. Data from prospective and retrospective studies suggest that DM-HFpEF is associated with multimorbidity, poor functional status, lower exercise capacity, increased markers of inflammation, fibrosis, endothelial dysfunction, worse congestion and increased morbidity and mortality supporting the existence of a distinct, high-risk, DM-HFpEF phenotype(71,72).
HFrEF phenotype is the result of sarcomere disappearance, cardiomyocyte cell death followed by the extensive replacement fibrosis. Cell death is the principal mechanism caused by oxidative stress, tissue hypoxia induced by microvascular rarefaction, advanced glycation end-products deposition (AGEs) and autoimmunity(70). The microvascular damage in DM patients is not only related to AGEs deposition, vascular inflammation and reduction in NO production, but also by a direct effect of the disease itself, which depletes endothelial cells and reduces capillary surface area(59).
CMD in Patients with Aortic Stenosis
Up to 40% of patients with aortic stenosis (AS) experience angina that occurs frequently in the absence of epicardial CAD. The appearance of angina greatly increases the risk of sudden death, and when symptoms appear, the only effective treatment is surgical or transcatheter aortic valve replacement (TAVR). Progression of AS is accompanied by discrepancies between blood supply and metabolic demand, with failing of the compensatory mechanisms because of structural and mechanical effects on the ventricle and coronary circulation(73). These patients have reduced MBF, impaired CFR and reduce exercise capacity(74). A complex array of abnormalities in myocardial remodeling, coronary microvascular function and pressure gradients are responsible for the distortion of coronary flow and symptoms developing, including: i) reduced diastolic time of coronary filling (because of prolonged systole); ii) increased diastolic filling pressure (compressing the endocardium and leading to a selective hypoperfusion of the subendocardium); iii) delayed peak systolic forward flow and reduced velocity time integral; iv) disrupted backward expansion wave; v) capillary rarefaction, arteriolar remodeling and perivascular fibrosis; vi) reversal of normal endocardial-epicardial blood flow ratio at rest resulting in subendocardial ischemia; vii) low coronary perfusion pressure due to Venturi effect when compared with intra-cavitary pressure; viii) increased intramyocardial systolic pressure and delay in myocardial relaxation at the end of systole (which further reduces time of coronary filling and perfusion)(73). Of interest, one study found that low CFR was the only independent predictor of future cardiovascular events in AS patients(74). Percutaneous and surgical aortic valve replacement are associated with the restoration of myocardial perfusion and contractility and improved microcirculatory function by reducing LV wall stress(75) as showed by the reduced hMR values after TAVR(76).
CMD in Patients with Infiltrative Heart Diseases
Anderson-Fabry disease (AFD) is caused by an X-linked inherited deficiency of lysosomal a-galactosidase-A, determining a multiorgan glycosphingolipid deposition with renal, cardiac and cerebrovascular damage. CMD has emerged as an important feature of AFD-associated cardiomyopathy and accounts for the considerable presence of angina in absence of obstructive CAD (Figure 4)(77–79). Myocyte hypertrophy, replacement fibrosis, hypertrophy and proliferation of VSMCs and endothelial cells, narrowing intramural arteries, all contribute to raise coronary vascular resistance and increase myocardial oxygen demand(78–80). Moreover, albeit the typical hypertrophic phenotype is almost exclusively present in males, Tomberli et al. found that even females without hypertrophy had a significant degree of CMD as assessed by PET scanning, which could even represents an early sign of the disease(81). Recent findings have suggested that subtle abnormalities of microvascular function characterize a pre-hypertrophy phenotype and pre-detectable storage phase of cardiac involvement, providing a promising window for precocious therapeutic interventions(82).
Figure 4: Evidence of CMD in a patient with Anderson-Fabry Disease Cardiomyopathy.
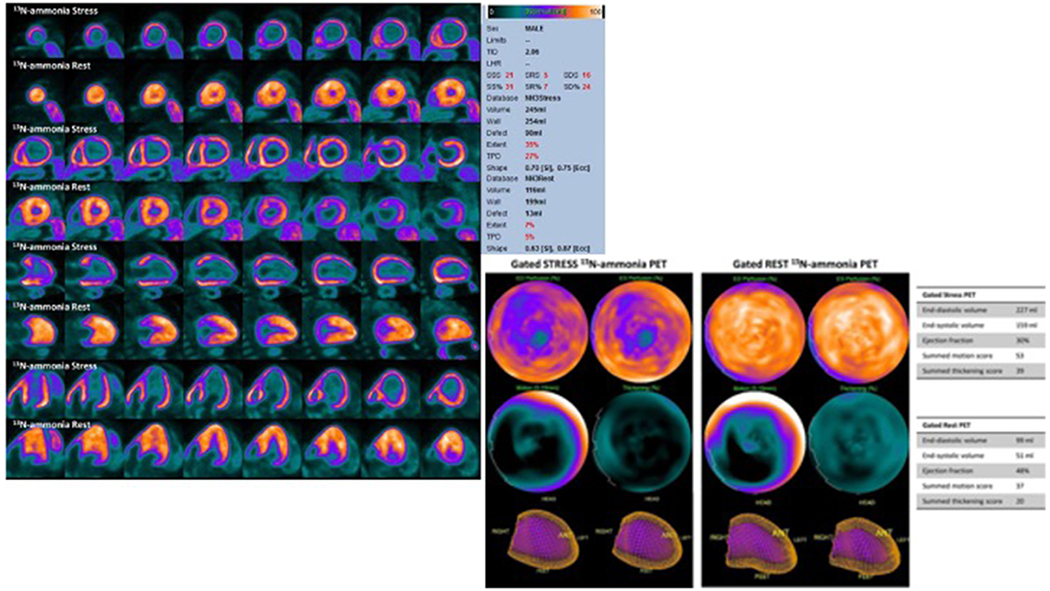
13N-labelled ammonia cardiac PET images show no significant perfusion defects at rest with a severe global subendocardial ischemia after pharmacological stress (Panel A). Cardiac CT-scan was previously performed to rule out obstructive CAD as the cause of angina. PET show abnormal left ventricular wall motions as well as a significant reduction in left ventricle ejection fraction from rest to peak stress (Panel B). Reproduced from Circulation, Cardiovascular Imaging (78).
Cardiac amyloidosis is characterized by the extracellular deposition of insoluble fibrils composed of misfolded proteins. Pathogenetic mechanisms of CMD in amyloidosis include structural changes of intramyocardial arteries (infiltration and thickening of the vascular wall with vessel lumen obstruction), functional abnormalities related to the imbalance of autonomic regulation and endothelial dysfunction, and extravascular factors (perivascular and interstitial amyloid deposits) resulting in increased left mass and extramural compression, decreased left ventricular compliance, increased diastolic filling pressure(82). In the past decades, few studies have investigated CMD in a limited number of patients with amyloidosis complaining of chest pain. Al Suwaidi et al. demonstrated that patients with systemic amyloidosis can present with angina pectoris before the typical systemic manifestations of amyloidosis and this presentation was associated with CFR abnormalities measured through intracoronary Doppler wire(83). Other clinical studies have confirmed a reduction of CFR in patients with amyloidosis and angina non-invasively, resorting to contrast echocardiography, PET and CMR(82). Hitherto, no available study has investigated neither the prognostic impact of CMD severity, nor the effects of specific therapies on microcirculation and symptoms control.
Sarcoidosis is a multisystem noncaseating granulomatous disease. Up to 25% of patients with sarcoidosis have myocardial involvement, although only 5% of patients show clinical manifestations of cardiac disease; angina is a frequent complaint in sarcoidosis, while only a limited fraction of patients has obstructive CAD. Many studies, using non-invasive methods, demonstrated reduced CFR in patients with sarcoidosis, however the underlying putative mechanisms are not well understood(84).
CMD in Patients with Hypertrophic Cardiomyopathy
The hallmark of HCM is the massive hypertrophy, which is generally asymmetric and develops independently of hemodynamic or systemic triggers. Along with cardiomyocyte hypertrophy, HCM phenotype involves a complex interplay of myocyte disarray, interstitial fibrosis with thickened fibres encasing myocytes, mitral valve and sub-valvular abnormalities, and coronary microvascular remodeling. These structural abnormalities are considered the most relevant substrate of CMD that, in the presence of increased oxygen demand, ultimately exposes to recurrent myocardial ischaemia and its sequelae(13). Several studies have shown that CFR is more blunted in the subendocardium and the more hypertrophied areas, but is also impaired in the non-hypertrophied ones, in line with the evidence of a widespread remodeling of intramural arterioles at autopsy(85–87). CMD over time may also lead to recurrent ischaemia and myocyte death leading to foci of replacement fibrosis, as showed by the presence of areas of late gadolinium enhancement at CMR. Furthermore, CMD assessed by PET, has been shown to be a long-term predictor of adverse remodeling, systolic dysfunction, clinical deterioration and death in HCM(85–87).
CMD in Patients with Dilated Cardiomyopathy (DCM)
Previous studies have identified myocardial perfusion abnormalities in patients with DCM with moderate or severe adverse remodeling. Myocardial ischaemia attributable to CMD is an independent contributor in the progression of the disease(88,89). However, further work is required to elucidate the temporal relationship between perfusion abnormalities and ventricular dysfunction in DCM. The degree of CMD has been shown to be an independent predictor of CVD events and is associated with increased risk of death and further progression of HF(88).
Iatrogenic CMD
Iatrogenic CMD may occur after percutaneous or surgical coronary revascularization, limiting their clinical benefit. In patients undergoing PCI the mechanisms underlying CMD are distal embolization of plaque material during the stenting procedure or functional alterations, which may overalp a pre-existent CMD. Ischemia due to balloon inflations and the stretch of the artery may in fact elicit a reflex sympathetic increase of α-adrenergic constrictor tone in the microcirculation(90). This complication often results in troponin elevation which occurs in one-third of patients and is associated with an increased risk of subsequent major CVD events, death, myocardial infarction and re-PCI. In patients with stable CAD undergoing elective PCI, IMR (IMR≥25) measured immediately after PCI predicts adverse events independently of fractional flow reserve, indicating the prognostic importance of CMD despite successful epicardial coronary revascularization(91). In patients undergoing CABG, a number of factors can influence microvascular function, including cardioplegia, extracorporeal circulation, periprocedural ischemia and inflammatory response. Similarly, the prognostic impact of myocardial damage following CABG is clinically relevant as the one following PCI, suggesting that patient prognosis is eventually driven by the extent of necrosis regardless of the mechanisms responsible for its occurrence(92). A potential explanation is that myocardial damage might lead to increased mortality as a result of electrical instability or of persistent myocardial ischemia associated with CMD.
Furthermore, CMD is also common in heart transplant recipients with cardiac allograft vasculopathy (CAV). Of importance, CMD has been shown to be independently associated with the onset of epicardial CAV and associated with a higher risk of death, regardless of CAV onset(93).
5. Targeting CMD
To date, there are no specific therapeutic strategies targeting CMD validated by large scale randomized clinical trials and therefore treatment of patients with CMD should be targeted on the risk factors and specific phenotypic presentation.
Physical training, smoking cessation and intentional weight loss (in obese patients) have consistently been shown to be beneficial in improving CFR, exercise capacity, cardiorespiratory fitness and CVD outcomes(94–97) (Table 3).
Table 3:
Non-pharmacological treatments targeting CMD.
| Class of intervention | Study design | N° of patients | Major inclusion criteria | Primary endpoints | Main findings | Ref |
|---|---|---|---|---|---|---|
| Aerobic interval training and weight loss |
CUT-IT trial, Randomized clinical trial (12 weeks aerobic interval training or low energy diet). |
70 | Obese patients with stable CAD | CFR assessed by transthoracic Doppler echocardiography on the LAD. | ↑ CFR after aerobic interval training and after low energy diet without significant between-group difference. | 95 |
| Smoking cessation |
Rooks et al. Twins Heart Stud. Investigation of psychological, behavioural and biological risk factors for subclinical cardiovascular disease using twins. |
360 | Smoking and non-smoking middle aged male twins | CFR assessed as response to adenosine with PET. |
CFR was significantly lower in smokers compared to non-smokers (p<0.01). | 96 |
| Exercise training |
Hambrecht R. et al,
Randomized clinical trial (exercise-training group n=10 and control group n=9). |
19 | Obstructive CAD and a noncritical stenosis in another coronary vessel with signs of endothelial dysfunction (defined as constriction or no change in response to acetylcholine). | Changes in vascular diameter in response to the intracoronary infusion of increasing doses of acetylcholine. | ↑ endothelium-dependent vasodilatation with exercise training (P<0.01). | 97 |
Abbreviations: CFR: Coronary flow reserve; ECG: electrocardiogram; LAD: left anterior descending artery.
In patients with high levels of low density lipoprotein-cholesterol or at high CVD risk profile, statin therapy exerts significant off-target effects as consequence of their anti-inflammatory and anti-oxidant properties and previous studies have confirmed its efficacy in improving CFR(98).
Third-generation beta-blockers, such as nebivolol and carvedilol, and dihydropyridine-type calcium channel blockers have been shown to modify endothelial function along with their effect on reducing myocardial oxygen demand and increase diastolic perfusion time(99,100). Beta-blockers are the cornerstone of therapy in HFrEF patients; they have been also shown to improve angina symptoms in MVA patients with effort-induced angina and evidence of increased adrenergic activity and are considered as first line therapy in this patients’ population(99,100). However, currently there is no benefit evidence for using of beta-blockers in patients with HFpEF(101). Non-dihydropyridine calcium-channel are effective in patients with MVA often triggered by microvascular spasm, as well as in patients with documented epicardial coronary spasm(102).
The large TREND study(103) provided evidence that angiotensin converting enzyme (ACE) inhibitors, in particular quinapril, was able to reverse endothelial dysfunction, and these beneficial effects have been replicated by several other studies involving angiotensin II receptor blockers. ACE inhibitors and angiotensin II receptor blockers are the first line therapy in patients with HFrEF(100) but failed to improve overall morbidity or mortality in patients with HFpEF(101). In patients with INOCA, enalapril improved CFR as well as exercise parameters in patients with MVA in a cohort of 20 Taiwanese patients compared with placebo(104). In another randomized study of women with INOCA and CMD, quinapril treatment improved CFR in response to adenosine as well as angina pectoris symptoms after 16 weeks of treatment, when compared with placebo(105).
Sacubitril-valsartan has been approved by the United States Food and Drug Administration for patients with symptomatic chronic HFrEF to reduce CVD mortality amd hospitalization for HF compared to enalapril(106). Conversely, sacubitril-valsartan failed to meet a statistical difference on the primary composite endpoint (total HF hospitalizations and cardiovascular death) in the HFpEF population when compared to valsartan(107). The ongoing PRISTINE-HF Study (Study of Sacubitril/ValsarTan on MyocardIal OxygenatioN and Fibrosis in Heart Failure with Preserved Ejection Fraction) will investigate the effects of this drug on microvascular function and ischemia, as assessed by oxygen sensitive-CMR at rest and after stress(NCT04128891).
The effects of several other anti-anginal drugs, such as ivabradine, ranolazine, nicorandil and trimetazidine, all cooperating in reducing oxygen demand, have been investigated in patients with CMD(102). Ranolazine has been extensively used in small cohort randomized clinical trials, with contrasting efficacy results in terms of CFR, symptom control and stress test metrics(108,109). Ivabradine improved angina in patients with MVA without changing in coronary microvascular function, suggesting that symptomatic improvement could be attributed to heart rate-lowering effect. However, it also showed to improve CFR in patients with stable CAD, even after heart rate correction suggesting a potential role on microvascular function(102).
Nitrates have shown inconclusive effects in patients with CMD in multiple studies, probably burdened by bias in patient selection and inclusion criteria. However, short-acting nitrates can useful to treat anginal attacks (especially in those patients with abnormal vasodilator reserve), but often they are only partially effective(102,108).
Phosphodiesterase Type-5 inhibitors, such as sildenafil, enhances the effect of NO by inhibiting the breakdown of cyclic guanosine monophosphate favouring smooth muscle relaxation. The positive effects of Sildenafil in CMD were firstly investigated by Denardo et al. who demonstrated a significant increase in CFR after the administration of the drug in patients with impaired microvascular function(110).
Fasudil, a specific Rho-kinase inhibitor, is highly effective in preventing ACh-induced coronary spasm and consequent myocardial ischaemia in both patients with epicardial and microvascular spasm(111,112) (Table 4).
Table 4:
Upcoming pharmacotherapies for treating CMD.
| Drug Compound | Pharmacological Class | Beneficial mechanisms in CMD |
|---|---|---|
| Fasudil | Rho-kinase inhibitor | Rho-kinase pathway is involved in endothelial dysfunction, vascular smooth muscle hypercontraction, vasospasm and inflammatory cell accumulation in blood vessel adventitia |
| Atrasentan Darusentan Zibotentan Sitaxentan |
Endothelin receptor antagonists | ET-1 contributes to coronary endothelial dysfunction |
| Dichloroacetate | Inhibitor of all isoforms of PDK kinase. | Activation of PDK maintains myocardial ATP and glycogen, improving hypoxic tolerance and reversing microvascular dysfunction |
| Amiodarone Dronedarone |
Cardiac K+ channel blockers | Potent coronary vasodilation, anti-remodeling and myocardial protectant actions |
| Colchicine Anakinra Rilonacept Canakinumab Tocilizumab |
Tubulin Inhibitor IL-1r antagonist IL-1 Trap IL1 β Inhibitor IL-6 Inhibitor |
Block coronary endothelial dysfunction Anti-ischemic, anti-atherosclerosis actions |
| Empaglifozin Dapaglifozin Canaglifozin |
SGLT2-channel inhibitors | Block coronary endothelial dysfunction Probable pleiotropic effects |
| Evelocumab Alirocumab |
PCSK-9 inhibitors | Improvement in lipidic profile Reduction of ROS production Block coronary endothelial dysfunction |
| NPY receptor inhibitors | NPY is a potent vasoconstrictor and inhibitor of cardiac vagal activity inducing transient ischaemia by microvascular constriction NPY has a role in adverse differentiation of mesenchymal stem cells |
|
| TT-10 | Fluorine substituent | Promotion of cardiomyocyte proliferation, with anti-apoptotic and antioxidant effects in vivo and in vitro |
| Vorinostat | Histone deacetylase inhibitor | Prevention of NF-kβ activation, eNOS uncoupling, and vascular oxidative stress |
| Apabetalone | Epigenetic regulator targeting bromo-domain and extra-terminal proteins | Modulation of vascular inflammation |
| Bone-marrow-derived CD34+ cells | Release of growth and differentiation factors Promotion of neovascularization and endogenous repair of damaged myocardium Improvement of bioenergetics Prevention of fibrosis |
|
Abbreviations: ATP= Adenosine triphosphate; CD= Cluster of Differentiation; ET-1= endothelin-1; IL=interleukin; NF-kB= nuclear factor kappa-light-chain-enhancer of activated B cells; NPY= Neuropeptide Y; PCSK-9= Proprotein convertase subtilisin/kexin type 9; PDK: pyruvate dehydrogenase kinases ROS: reactive oxygen species; SGLT-2= Sodium–glucose cotransporter 2.
In patients with MVA, the findings of elevated circulating concentrations of ET-1 led to conducting two small trials with ET-1 receptor antagonists (atrasentan and darusentan; NCT00271492; NCT00738049) with favourable profile in patients with MVA. However, following neutral results from phase III trials relating to their primary indications they were no more investigated for application in MVA. A randomized controlled trial in patients with MVA with Zibotentan (NCT04097314), a potent inhibitor of the ETA receptor with no “off-target” binding to the ETB receptor, is now ongoing. (Table 4).
Recently, two classes of drugs, the sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1-receptor antagonists (GLP1-RA) have consistently demonstrated to reduce rates of major adverse CVD events in subjects with DM at high risk for CVD (113). SGLT2i and GLP1-RA could exert their effects by targeting several mediators of macro- and micro-vascular pathophysiology, such as cytokines and inflammation mediators, oxidative stress-induced endothelial dysfunction and vascular smooth muscle cell proliferation, all aetiological features of CMD(113). These data are further supported by the results recently published by Adingupu et al.(114), who investigated in a pre-diabetic mice model, the effects of SGLT2i on coronary microvascular function and cardiac contractility, showing improvement in CFVR and fractional area change. The encouraging results by the EMPA-REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) led to focus the effects of empaglifozin on CMD. It has been shown that empagliflozin directly restores the beneficial effect of cardiac microvascular endothelial cell on cardiomyocyte function, suggesting its potential role to improve CMD(115). Ongoing clinical trials are examining the effects of SGLT2i in HFpEF (EMPEROR-PRESERVED Trial NCT0305795;DELIVER Trial NCT03619213), which may recognize CMD as pathological substrate (Table 4).
The coronary microcirculation may also represent an important therapeutic target in patients presenting with myocardial infarction(50). In STEMI patients, no definite therapies have consistently been shown to be effective in improving MVO(4). Future research efforts should be directed to evaluate the potential benefits of therapies aimed at improving coronary microcirculation in high-risk subgroups (i.e. those with evidences high microvascular resistances). Furthermore, the current guidelines do not address the issue about the management of patients with MINOCA due to the lack of randomized control trials so far in this patient population(33). The “one-size-fits-all” approach uniformly applied to patients presenting with MI-CAD should be replaced by a personalized therapeutic approach guided the underlying pathophysiological mechanism responsible for the clinical presentation. Finally, CMD has been demonstrated to play a pathogenetic role in most myocardial diseases. Therefore, future studies specifically assessing the effect of therapy on CMD across different CVD are warranted.
6. Conclusion
CMD represents a combination of structural and functional abnormalities affecting the coronary microcirculation. It is prevalent across a broad spectrum of CVD risk factors and diseases. CMD significantly contributes to determine the clinical manifestations in all these conditions and has a significant impact on prognosis. The limited knowledge about CMD across different cardiovascular diseases, precludes providing clarity concerning the underlying pathophysiological mechanisms. This extends also to therapy, where, to date, no treatment specifically targets CMD. However, due to the emerging role of CMD as a key player in different clinical phenotypes, additional research in this area is warranted in order to fill knowledge gaps and provide personalized treatments in this “garden variety” of patients.
Highlights:
Coronary microvascular dysfunction (CMD) plays a pathophysiological role in various cardiovascular disease states, influencing ischemic manifestations, symptoms and prognosis, but molecular, functional and structural mechanisms have not been clarified.
Various non-invasive and invasive techniques can be used to evaluate CMD in clinical practice, each of which has strengths and limitations.
Better understanding of the physiopathology of CMD is needed to develop therapeutic strategies that ameliorate angina, and improve long-term outcomes.
Acknowledgements
We thank Dr. A.M. Leone for his help in preparing Figure 3.
Funding
Dr Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association and by the Clinical and Translational Science Awards Program UL1TR002649 from National Institutes of Health to Virginia Commonwealth University. The remaining authors have nothing to disclose.
Abbreviations:
- ACh
acetylcholine
- AFD
Anderson-Fabry disease
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CBF
coronary blood flow
- CFR
coronary flow reserve
- CMD
coronary microvascular dysfunction
- CMR
Cardiac magnetic resonance
- CTCA
tomography coronary angiography
- CVD
cardiovascular diseases
- CVFR
coronary flow velocity ratio
- DCM
dilated cardiomyopathy
- ET-1
endothelin-1
- GLP1-RA
glucagon-like peptide 1-receptor antagonists
- HCM
hypertrophic cardiomyopathy
- HFpEF
Heart failure with preserved ejection fraction
- hMR
hyperemic microvascular resistance
- IMR
microvascular resistances
- INOCA
Ischemia with non-obstructive CAD
- LAD
left anterior descenging artery
- MBF
myocardial blood flow
- MINOCA
myocardial infarction with non-obstructive CAD
- MPR
myocardial perfusion reserve
- PCI
percutaneous coronary intervention
- PET
Positron Emission Tomography
- ROS
reactive oxygen species
- SGLT2i
sodium-glucose cotransporter 2 inhibitors
- TTDE
Transthoracic Doppler echocardiography
- VSMCs
vascular smooth muscle cells
Footnotes
Conflict of interests: none.
References
- 1.Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of Ischemic Heart Disease. Circulation. 2018;138(14):1463–1480. [DOI] [PubMed] [Google Scholar]
- 2.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. [DOI] [PubMed] [Google Scholar]
- 3.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomanek RJ. Structure–Function of the Coronary Hierarchy: Coronary Vasculature: New York: Springer; 2013:59–81. [Google Scholar]
- 5.Deussen A, Ohanyan V, Jannasch A, Yin L, Chilian W. Mechanisms of metabolic coronary flow regulation. J Mol Cell Cardiol. 2012;52(4):794–801. [DOI] [PubMed] [Google Scholar]
- 6.Masi S, Rizzoni D, Taddei S, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. 2020;ehaa857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med. 2017;109:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francia P, delli Gatti C, Bachschmid M, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110(18):2889–2895. [DOI] [PubMed] [Google Scholar]
- 9.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–313. [DOI] [PubMed] [Google Scholar]
- 10.Magenta A, Greco S, Capogrossi MC, Gaetano C, Martelli F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. Biomed Res Int. 2014;2014:193095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc Res. 2017;113(11):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pries AR, Badimon L, Bugiardini R, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015;36(45):3134–3146. [DOI] [PubMed] [Google Scholar]
- 13.Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res. 2020;116(4):806–816. [DOI] [PubMed] [Google Scholar]
- 14.Vegsundvåg J, Holte E, Wiseth R, Hegbom K, Hole T. Coronary flow velocity reserve in the three main coronary arteries assessed with transthoracic Doppler: a comparative study with quantitative coronary angiography. J Am Soc Echocardiogr. 2011;24(7):758–767. [DOI] [PubMed] [Google Scholar]
- 15.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 16.Ong P, Safdar B, Seitz A, Hubert A, Beltrame JF, Prescott E. Diagnosis of coronary microvascular dysfunction in the clinic. Cardiovasc Res. 2020;116(4):841–855. [DOI] [PubMed] [Google Scholar]
- 17.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6):623–640. [DOI] [PubMed] [Google Scholar]
- 18.Kotecha T, Martinez-Naharro A, Boldrini M, et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation Against Invasive Coronary Physiology. JACC Cardiovasc Imaging. 2019;12(10):1958–1969. doi: 10.1016/j.jcmg.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4): 10.1161/CIRCIMAGING.114.002481 e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larghat AM, Maredia N, Biglands J, et al. Reproducibility of first-pass cardiovascular magnetic resonance myocardial perfusion. J Magn Reson Imaging. 2013;37(4):865–874. [DOI] [PubMed] [Google Scholar]
- 21.Branch KR, Haley RD, Bittencourt MS, Patel AR, Hulten E, Blankstein R. Myocardial computed tomography perfusion. Cardiovasc Diagn Ther. 2017;7(5):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 23.Mangiacapra F, Peace AJ, Di Serafino L, Pyxaras SA, Bartunek J, Wyffels E, Heyndrickx GR, Wijns W, De Bruyne B, Barbato E. Intracoronary EnalaPrilat to Reduce MICROvascular Damage During Percutaneous Coronary Intervention (ProMicro) study. J Am Coll Cardiol 2013;61:615–621. [DOI] [PubMed] [Google Scholar]
- 24.Xaplanteris P, Fournier S, Keulards DCJ, Adjedj J, Ciccarelli G, Milkas A, Pellicano M, Van’t Veer M, Barbato E, Pijls NHJ, De Bruyne B. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance: Feasibility, Safety, and Reproducibility in Humans. Circ Cardiovasc Interv 2018;11(3):e006194. [DOI] [PubMed] [Google Scholar]
- 25.Montone RA, Meucci MC, De Vita A, Lanza GA, Niccoli G. Coronary provocative tests in the catheterization laboratory: Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis. 2020;318:14–21. [DOI] [PubMed] [Google Scholar]
- 26.Zaya M, Mehta PK, Merz CN. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol 2014;63(2):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montone RA, Niccoli G, Fracassi F, et al. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39(2):91–98. [DOI] [PubMed] [Google Scholar]
- 28.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27(12):1408–1415. [DOI] [PubMed] [Google Scholar]
- 31.Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841–2855. [DOI] [PubMed] [Google Scholar]
- 32.Mohri M, Koyanagi M, Egashira K, et al. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351(9110):1165–1169. [DOI] [PubMed] [Google Scholar]
- 33.Del Buono MG, Montone RA, Iannaccone G, et al. Diagnostic work-up and therapeutic implications in MINOCA: need for a personalized approach. Future Cardiol. 2020; 10.2217/fca-2020-0052. [DOI] [PubMed] [Google Scholar]
- 34.Choo EH, Chang K, Lee KY, et al. Prognosis and Predictors of Mortality in Patients Suffering Myocardial Infarction With Non-Obstructive Coronary Arteries. J Am Heart Assoc. 2019;8(14):e011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(16):1955–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135(24):2426–2441. [DOI] [PubMed] [Google Scholar]
- 37.Meimoun P, Malaquin D, Sayah S, et al. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr. 2008;21(1):72–77. [DOI] [PubMed] [Google Scholar]
- 38.Feola M, Chauvie S, Rosso GL, Biggi A, Ribichini F, Bobbio M. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: a myocardial PET study. J Nucl Cardiol. 2008;15(6):811–817. [DOI] [PubMed] [Google Scholar]
- 39.Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31(11):1319–1327. [DOI] [PubMed] [Google Scholar]
- 40.Montone RA, Galiuto L, Meucci MC, et al. Coronary slow flow is associated with a worse clinical outcome in patients with Takotsubo syndrome. Heart. 2020;106(12):923–930. [DOI] [PubMed] [Google Scholar]
- 41.Mangiacapra F, Del Buono MG, Abbate A, et al. Role of endothelial dysfunction in determining angina after percutaneous coronary intervention: Learning from pathophysiology to optimize treatment. Prog Cardiovasc Dis. 2020;63(3):233–242. [DOI] [PubMed] [Google Scholar]
- 42.Ong P, Athanasiadis A, Perne A, et al. Coronary vasomotor abnormalities in patients with stable angina after successful stent implantation but without in-stent restenosis. Clin Res Cardiol. 2014;103(1):11–19. [DOI] [PubMed] [Google Scholar]
- 43.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato A, Hiroe M, Tamura M, et al. Quantitative measures of coronary stenosis severity by 64-Slice CT angiography and relation to physiologic significance of perfusion in nonobese patients: comparison with stress myocardial perfusion imaging. J Nucl Med. 2008;49(4):564–572. [DOI] [PubMed] [Google Scholar]
- 45.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330(25):1782–1788. [DOI] [PubMed] [Google Scholar]
- 46.Di Carli M, Czernin J, Hoh CK, et al. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation. 1995;91(7):1944–1951. [DOI] [PubMed] [Google Scholar]
- 47.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Delayed recovery of coronary resistive vessel function after coronary angioplasty. J Am Coll Cardiol. 1993;21(3):612–621. [DOI] [PubMed] [Google Scholar]
- 48.Serruys PW, di Mario C, Piek J, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation. 1997;96(10):3369–3377. [DOI] [PubMed] [Google Scholar]
- 49.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niccoli G, Montone RA, Ibanez B, et al. Optimized Treatment of ST-Elevation Myocardial Infarction. Circ Res. 2019;125(2):245–258. [DOI] [PubMed] [Google Scholar]
- 51.Bulluck H, Foin N, Cabrera-Fuentes HA, et al. Index of Microvascular Resistance and Microvascular Obstruction in Patients With Acute Myocardial Infarction [published correction appears in JACC Cardiovasc Interv. 2017 Feb 13;10(3):313]. JACC Cardiovasc Interv. 2016;9(20):2172–2174. [DOI] [PubMed] [Google Scholar]
- 52.Fearon WF, Low AF, Yong AS, et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127(24):2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2017. 2;376(9):897. [DOI] [PubMed] [Google Scholar]
- 54.Carbone S, Lavie CJ, Elagizi A, Arena R, Ventura HO. The Impact of Obesity in Heart Failure. Heart Fail Clin. 2020;16(1):71–80. [DOI] [PubMed] [Google Scholar]
- 55.Kirkman DL, Bohmke N, Billingsley HE, Carbone S. Sarcopenic Obesity in Heart Failure With Preserved Ejection Fraction. Front Endocrinol (Lausanne). 2020;11:558271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borbély A, Falcao-Pires I, van Heerebeek L, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104(6):780–786. [DOI] [PubMed] [Google Scholar]
- 57.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131(6):550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. [DOI] [PubMed] [Google Scholar]
- 59.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137(2):148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Del Buono MG, Arena R, Borlaug BA, et al. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(17):2209–2225. [DOI] [PubMed] [Google Scholar]
- 61.Crea F, Bairey Merz CN, Beltrame JF, et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38(7):473–477. [DOI] [PubMed] [Google Scholar]
- 62.Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):432–441. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad A, Corban MT, Toya T, et al. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2020; 10.1002/ejhf.2010. [DOI] [PubMed] [Google Scholar]
- 64.Allan T, Dryer K, Fearon WF, Shah SJ, Blair JEA. Coronary Microvascular Dysfunction and Clinical Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. J Card Fail. 2019;25(10):843–845. [DOI] [PubMed] [Google Scholar]
- 65.Dryer K, Gajjar M, Narang N, et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;314(5):H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39(37):3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hage C, Svedlund S, Saraste A, et al. Association of Coronary Microvascular Dysfunction With Heart Failure Hospitalizations and Mortality in Heart Failure With Preserved Ejection Fraction: A Follow-up in the PROMIS-HFpEF Study. J Card Fail. 2020;26(11):1016–1021. [DOI] [PubMed] [Google Scholar]
- 68.Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg?. Eur Heart J. 2018;39(10):850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39(10):840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murtaza G, Virk HUH, Khalid M, et al. Diabetic cardiomyopathy - A comprehensive updated review. Prog Cardiovasc Dis. 2019;62(4):315–326. [DOI] [PubMed] [Google Scholar]
- 71.Lindman BR, Dávila-Román VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64(6):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and Echocardiographic Characteristics and Cardiovascular Outcomes According to Diabetes Status in Patients With Heart Failure and Preserved Ejection Fraction: A Report From the I-Preserve Trial (Irbesartan in Heart Failure With Preserved Ejection Fraction). Circulation. 2017;135(8):724–735. [DOI] [PubMed] [Google Scholar]
- 73.McConkey HZR, Marber M, Chiribiri A, Pibarot P, Redwood SR, Prendergast BD. Coronary Microcirculation in Aortic Stenosis. Circ Cardiovasc Interv. 2019;12(8):e007547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nemes A, Balázs E, Csanády M, Forster T. Long-term prognostic role of coronary flow velocity reserve in patients with aortic valve stenosis - insights from the SZEGED Study. Clin Physiol Funct Imaging. 2009;29(6):447–452. [DOI] [PubMed] [Google Scholar]
- 75.Beyerbacht HP, Lamb HJ, van Der Laarse A, et al. Aortic valve replacement in patients with aortic valve stenosis improves myocardial metabolism and diastolic function. Radiology. 2001;219(3):637–643. [DOI] [PubMed] [Google Scholar]
- 76.Wiegerinck EM, van de Hoef TP, Rolandi MC, et al. Impact of Aortic Valve Stenosis on Coronary Hemodynamics and the Instantaneous Effect of Transcatheter Aortic Valve Implantation. Circ Cardiovasc Interv. 2015;8(8):e002443. [DOI] [PubMed] [Google Scholar]
- 77.Chimenti C, Morgante E, Tanzilli G, et al. Angina in fabry disease reflects coronary small vessel disease. Circ Heart Fail. 2008;1(3):161–169. [DOI] [PubMed] [Google Scholar]
- 78.Graziani F, Lillo R, Panaioli E, et al. Massive Coronary Microvascular Dysfunction in Severe Anderson-Fabry Disease Cardiomyopathy. Circ Cardiovasc Imaging. 2019;12(6):e009104. [DOI] [PubMed] [Google Scholar]
- 79.Elliott PM, Kindler H, Shah JS, et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart. 2006;92(3):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Augusto JB, Johner N, Shah D, et al. The myocardial phenotype of Fabry disease pre-hypertrophy and pre-detectable storage. Eur Heart J Cardiovasc Imaging. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomberli B, Cecchi F, Sciagrà R, et al. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson-Fabry disease. Eur J Heart Fail. 2013;15(12):1363–1373. [DOI] [PubMed] [Google Scholar]
- 82.Dorbala S, Vangala D, Bruyere J Jr, et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail. 2014;2(4):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al Suwaidi J, Velianou JL, Gertz MA, et al. Systemic amyloidosis presenting with angina pectoris. Ann Intern Med. 1999;131(11):838–841. [DOI] [PubMed] [Google Scholar]
- 84.Kul S, Kutlu GA, Guvenc TS, et al. Coronary flow reserve is reduced in sarcoidosis. Atherosclerosis. 2017;264:115–121. [DOI] [PubMed] [Google Scholar]
- 85.Camici P, Chiriatti G, Lorenzoni R, et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: a study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1991;17(4):879–886. [DOI] [PubMed] [Google Scholar]
- 86.Olivotto I, Cecchi F, Gistri R, et al. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47(5):1043–1048. [DOI] [PubMed] [Google Scholar]
- 87.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349(11):1027–1035. [DOI] [PubMed] [Google Scholar]
- 88.Neglia D, Parodi O, Gallopin M, et al. Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. A quantitative assessment by positron emission tomography. Circulation. 1995;92(4):796–804. [DOI] [PubMed] [Google Scholar]
- 89.Koutalas E, Kanoupakis E, Vardas P. Sudden cardiac death in non-ischemic dilated cardiomyopathy: a critical appraisal of existing and potential risk stratification tools. Int J Cardiol. 2013;167(2):335–341. [DOI] [PubMed] [Google Scholar]
- 90.Cuisset T, Hamilos M, Melikian N, et al. Direct stenting for stable angina pectoris is associated with reduced periprocedural microcirculatory injury compared with stenting after pre-dilation. J Am Coll Cardiol. 2008;51(11):1060–1065. [DOI] [PubMed] [Google Scholar]
- 91.Nishi T, Murai T, Ciccarelli G, et al. Prognostic Value of Coronary Microvascular Function Measured Immediately After Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study. Circ Cardiovasc Interv. 2019;12(9):e007889. [DOI] [PubMed] [Google Scholar]
- 92.Gahl B, Göber V, Odutayo A, et al. Prognostic Value of Early Postoperative Troponin T in Patients Undergoing Coronary Artery Bypass Grafting. J Am Heart Assoc. 2018;7(5):e007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tona F, Osto E, Famoso G, et al. Coronary microvascular dysfunction correlates with the new onset of cardiac allograft vasculopathy in heart transplant patients with normal coronary angiography. Am J Transplant. 2015;15(5):1400–1406. [DOI] [PubMed] [Google Scholar]
- 94.Carbone S, Del Buono MG, Ozemek C, Lavie CJ. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 2019;62(4):327–333. [DOI] [PubMed] [Google Scholar]
- 95.Olsen RH, Pedersen LR, Jürs A, Snoer M, Haugaard SB, Prescott E. A randomised trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int J Cardiol. 2015;185:229–235. [DOI] [PubMed] [Google Scholar]
- 96.Rooks C, Faber T, Votaw J, Veledar E, Goldberg J, Raggi P, Quyyumi AA, Bremner JD, Vaccarino V. Effects of smoking on coronary microcirculatory function: a twin study. Atherosclerosis. 2011;215(2):500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283(23):3095–3101. [DOI] [PubMed] [Google Scholar]
- 98.Eshtehardi P, McDaniel MC, Dhawan SS, et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J Invasive Cardiol. 2012;24(10):522–529. [PubMed] [Google Scholar]
- 99.Padro T, Manfrini O, Bugiardini R, et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc Res. 2020;116(4):741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121(21):2317–2325. [DOI] [PubMed] [Google Scholar]
- 101.Del Buono MG, Iannaccone G, Scacciavillani R, et al. Heart failure with preserved ejection fraction diagnosis and treatment: An updated review of the evidence. Prog Cardiovasc Dis. 2020;63(5):570–584. [DOI] [PubMed] [Google Scholar]
- 102.Ong P, Athanasiadis A, Sechtem U. Pharmacotherapy for coronary microvascular dysfunction. Eur Heart J Cardiovasc Pharmacother. 2015;1(1):65–71. [DOI] [PubMed] [Google Scholar]
- 103.Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94(3):258–265. [DOI] [PubMed] [Google Scholar]
- 104.Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90(9):974–982. [DOI] [PubMed] [Google Scholar]
- 105.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162(4):678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381(17):1609–1620. [DOI] [PubMed] [Google Scholar]
- 108.Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(21):2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maier LS, Layug B, Karwatowska-Prokopczuk E, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1(2):115–122. [DOI] [PubMed] [Google Scholar]
- 110.Denardo SJ, Wen X, Handberg EM, et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women’s Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol. 2011;34(8):483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41(1):15–19. [DOI] [PubMed] [Google Scholar]
- 112.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105(13):1545–1547. [DOI] [PubMed] [Google Scholar]
- 113.Carbone S, Dixon DL, Buckley LF, Abbate A. Glucose-Lowering Therapies for Cardiovascular Risk Reduction in Type 2 Diabetes Mellitus: State-of-the-Art Review. Mayo Clin Proc. 2018;93(11):1629–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adingupu DD, Göpel SO, Grönros J, et al. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob−/− mice. Cardiovasc Diabetol. 2019;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Juni RP, Kuster DWD, Goebel M, et al. Cardiac Microvascular Endothelial Enhancement of Cardiomyocyte Function Is Impaired by Inflammation and Restored by Empagliflozin. JACC Basic Transl Sci. 2019;4(5):575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]


