Graphical abstract
Keywords: Terminalia catappa, Acute toxicity, Sub-acute toxicity, Proinflammatory indices, Biochemical indices, Stress response
Highlights
-
•
Proinflammatory and stress response molecular biomarkers in addition to standard toxicological parameters outlined by OECD were investigated.
-
•
Terminalia catappa aqueous leaf extract (TCA) did not alter the appearance and behavioural responses of the animals.
-
•
Investigated biochemical, haematological, proinflammatory and stress response parameters remained unaltered in treated rats.
-
•
Evidence of mortality was not observed, suggesting the oral safety of TCA.
-
•
The toxicological stress response of TCA on heat shock proteins is reported for the first time.
Abstract
The present study was carried out to assess the oral safety, proinflammatory and stress response effect of Terminalia catappa aqueous leaf extract (TCA) in male Wistar rats. The acute and sub-acute oral toxicity of TCA was assessed using guidelines 423 and 407 of the Organisation for Economic Co-operation and Development (OECD), respectively. Signs of clinical toxicity, morbidity and mortality were observed. The biochemical, haematological, proinflammatory, stress response and histopathological indices were assessed. In the acute toxicity study, no sign of clinical toxicity, morbidity, and mortality was observed for TCA treatment, up to 5000 mg/kg bwt. However, in the sub-acute toxicity study, repeated daily TCA treatment significantly (p<0.05) altered the body weight gain, plasma alkaline phosphatase activity and albumin concentration. There were no obvious morphological and macroscopic alterations in the organs investigated. TCA appear not to elicit any proinflammatory, stress, systemic and organ toxic effect when utilised at the reported dose and time frame.
1. Introduction
Terminalia catappa is one of the numerous Terminalia species found in Nigeria. It is geographically distributed in the Southwest region as a fast-growing, semi-evergreen, tall tree with cracked bark, broad leaves and edible fruit. High content of flavonoids, saponins, tannins and phytosterols contribute to its high medicinal properties [1]. Among the reported pharmacological activities of various T. catappa extracts are antiaging, anticancer, antidiabetic, antimicrobial, wound healing, anti-inflammatory, antioxidant and hepatoprotection [2]. The sap of its young leaves are used in treating leprosy; the red leaves are reported to have antihelminthics properties while the seed kernel has aphrodisiac activity [3,4]. Ferulic, p-coumaric, protocatechuic acids, catechin, 2-prenylated benzoic acid, siringic, palmitic, vanillic, stearic and 3, 4, 40-tri-O-methyl ellagic acids are some of the identified bioactive compounds with numerous medicinal properties present in T. catappa [5,6].
Most developing countries still rely on traditional medicine, with heavy dependence on medicinal plants and natural products, for their health needs [7]. The presence of bioactive plant metabolites, affordability, accessibility and long history of use of medicinal plants are some of the reasons traditional medicine is preferred to modern medicine in developing countries [8]. A positive correlation between modern therapeutic active ingredients and the traditional use of their plant of origin has generated increased scientific interest in medicinal plants for diverse pharmacological and dietary properties [9]. In spite of the wide use of medicinal plants and natural products, their toxicity and pharmacologic actions are rarely tested. This underscores the necessity for safety evaluation and standardisation of herbal extracts. The need to incorporate molecular assessments of toxicity, in addition to the conventional toxicological parameters on testing of chemicals, was recently made by the Organisation for Economic Cooperation and Development (OECD) [10]. In view of the foregoing, this study evaluated the safety of Terminalia catappa aqueous leaf extract in male Wistar rats using proinflammatory and stress response molecular biomarkers in addition to the biochemical, haematological and histopathological indices.
2. Materials and methods
2.1. Collection, identification and preparation of plants
Leaves of T. catappa were obtained in November 2018 from Covenant University, Ota, Ogun State, Nigeria (6 ° 40′ 19.8768″ N, 3 ° 9′ 33.3432″ E). Dr. J.O. Popoola of the Department of Biological Sciences, Covenant University, authenticated the plant. For future reference, a voucher specimen (FHI 112775) was prepared and deposited in Forest Research Institute of Nigeria (FRIN), Ibadan, Nigeria. T. catappa aqueous leaf extract (TCA) was prepared as previously reported by Iheagwam et al. [11].
2.2. Experimental animals
Forty male albino Wistar rats (200–220 g) were used for this study; they were obtained from the National Institute of Medical Research (NIMR), Lagos, Nigeria. They were housed in the Department’s animal house with an alternating 12 h light/dark cycle at normal room temperature (23 ± 2 °C) and humidity (50 ± 5 %), and given access to food and water ad libitum all through the acclimatisation and experimental period. The research protocol (CHREC/031/2018) was approved by Covenant University Health Research Ethics Committee (CHREC) in accordance with the guidelines for the care and use of laboratory animals as contained in the Sub-Code for Research Involving Animals of the National Health Research Ethics Committee of Nigeria and documented by the National Institute of Health for the Care and Use of Laboratory Animals.
2.3. Acute oral toxicity study
Acute oral toxicity test was carried out according to the Organization for Economic Cooperation and Development (OECD) guidelines 423 with slight modification as described by Ogunlana et al. [12]. Briefly, twenty male Wistar rats were used for this study and were randomly divided into four groups (n = 5). The control group was orally administered with distilled water (the vehicle), while treatment groups received 1000, 2500 and 5000 mg/kg bwt of TCA. The animals were maintained on a standard diet for two weeks; body weight was measured at the beginning and the end of the experiment and weight gain was recorded. The vehicle and TCA were administered once; observations were made for signs of clinical toxicity, morbidity and mortality during the first 30 min, 1, 2, 4 and 6 h. after dosing and subsequently once daily over 14 days. The body weight of the animals during the experimental period was recorded. On the 14th day, after an overnight fast, the rats were anaesthetised using xylazine/ketamine mixture (5:50 mg/mL) and sacrificed by cardiac puncture.
2.4. Sub-acute toxicity study
Sub-acute toxicity study was carried out using OECD guideline 407 as described by Iheagwam et al. [11]. Vehicle and TCA were administered daily by gastric intubation repeatedly for twenty-eight (28) days. The rats were weighed before the commencement of treatment and weekly throughout the study. The rats were anaesthetised using xylazine/ketamine mixture (5:50 mg/mL) and sacrificed by cardiac puncture on the 28th day after an overnight fast.
2.5. Tissue collection and preparation
The blood was collected from the heart and placed in heparin and ethylenediaminetetraacetic acid (EDTA) tubes, respectively, for biochemical and haematological analyses. The organs were harvested and rinsed with normal saline to eliminate blood contamination and dried by blotting. A sizeable portion of the liver was stored in Transgen RNAlater (Beijing TransGen Biotech Co., Ltd, China) for total RNA extraction, while another portion of the liver, kidney and spleen were kept in 10 % neutral buffered formal saline for macroscopic and histopathological assessment. Plasma was obtained from whole blood by centrifugation at 4000 rpm for 5 min. and stored at −20 °C (Haier Thermocool, BD-719R6) until they were required for analysis.
2.6. Blood analysis
The plasma was assayed for liver and kidney function parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, albumin (ALB), urea, creatinine (Crea), sodium (Na), calcium (Ca), bicarbonate (HCO3) and uric acid (UA) following the manufacturer’s instructions in the diagnostic kits (Randox Lab., England, UK). According to the manufacturers’ manual, plasma insulin concentration was assayed using an ELISA kit (Hangzhou Eastbiopharm Co. LTD, Hangzhou, China). Proinflammatory markers such as tumour necrosis factor-alpha (TNF-α) and interleukin VI (IL-6) were analysed according to procedures described in the ELISA kits (Solarbio Science and Technology, Beijing, China).
2.7. RNA extraction and RT-PCR analysis
The extraction of total hepatic RNA and reverse transcriptase-polymerase chain reaction (RT-PCR) for TNF-α, IL-6, heat shock protein 27 (HSP 27) and HSP 70 were carried according to the methodology of Stalin et al. [13] with slight modification. RT-PCR was performed using the Transgen EasyScript® one-step RT-PCR supermix (Beijing TransGen Biotech Co., Ltd, China) according to the manufacturer’s instruction. PCR amplification was carried out on C1000 thermal cycler (Bio-Rad, CA, USA) with initial denaturation at 95 °C for 5 min., denaturation at 95 °C for 30 s., annealing at 52 °C for IL-6 and TNF-α, 53 °C for HSP 27 and 50 °C for HSP 70 and β-actin for 30 s. and extension at 72 °C for 30 s., with a final extension at 72 °C for 7 min. for 40 cycles. The gene-specific primers below were used for the first-strand cDNA synthesis with β-actin serving as the internal control.
| TNF-α | 5′- ACGGCATGGATCTCAAAGAC-3′ (F) |
| 5′- CGGACTCCGCAAAGTCTAAG-3′ (R) | |
| IL-6 | 5′-ATTGTATGAACAGCGATGATGCAC-3′ (F) |
| 5′-CCAGGTAGAAACGGAACTCCAGA-3′ (R) | |
| HSP-27 | 5′- CCTGGAAGCCATCTTTGC-3′ (F) |
| 5′- CGTCGTTATTCGCCGTAC-3′ (R) | |
| HSP-70 | 5′- CGTTTGACGGAAGGTAAAT-3′ (F) |
| 5′- TCATCAGCGGGCTGTATC-3′ (R) | |
| β-actin | 5ʹ-TGTTGTCCCTGTATGCCTCT-3ʹ (F) |
| 5ʹ-TAATGTCACGCACGATTTCC-3ʹ (R) |
RT-PCR products were loaded onto a 1.5 % agarose gel, stained with ethidium bromide and subsequently visualised.
2.8. Haematological analysis
White blood cell count (WBC), lymphocyte, MID, granulocyte, haemoglobin (HB), red blood cell count (RBC), haematocrit (HCT), platelet count (PLT) and plateletcrit (PCT) were determined using an automated haematology analyser (Mindray Automated analyser BC 3200).
2.9. Histopathological analysis
Histopathological assessment of hepatic, renal and splenic tissues was carried out adopting the methodology described by Chinedu et al. [14].
2.10. Data analysis
Statistical analysis was done using IBM SPSS statistics 23 (IBM Inc.) with data expressed as mean ± standard error of the mean of five replicates. Data were subjected to one-way analysis of variance followed by Duncan multiple range test to determine significant differences at p<0.05.
3. Results
3.1. Acute toxicity
3.1.1. TCA single-dose treatment did not alter animal and organ weight
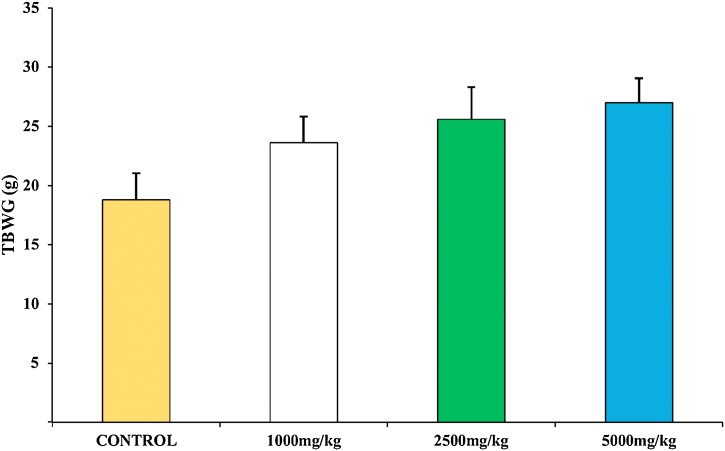
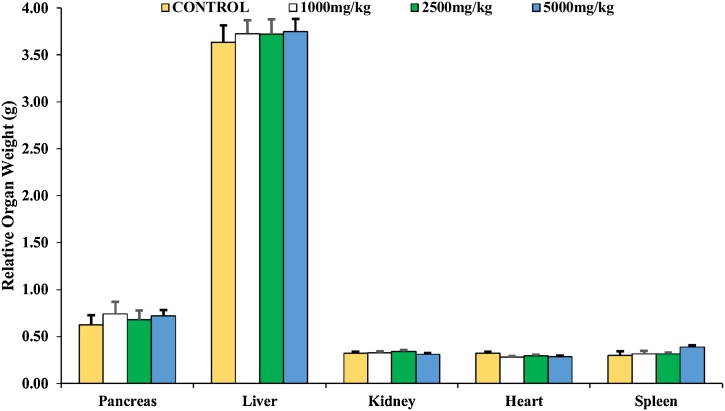
The experimental animals did not exhibit morbidity, toxicity, mortality and behavioural change (Table 1). The body weight of all animals in both control and the experimental group increased throughout the experiment. However, there was no observable (p>0.05) difference in the total body weight gained (TBWG) across all groups (Fig. 1). The relative weight of the liver, kidney, pancreas, heart and spleen in the experimental animals was not significantly (p>0.05) different from the control animals (Fig. 2).
Table 1.
Effect of T. catappa aqueous extract single-dose treatment on some animal appearance and behavioural responses in the acute toxicity study.
| Observation | Control | 1000 mg/kg | 2500 mg/kg | 5000 mg/kg |
|---|---|---|---|---|
| Temperature | Normal | Normal | Normal | Normal |
| Eye colour change | Nil | Nil | Nil | Nil |
| Food intake | No effect | No effect | No effect | No effect |
| General physique | Normal | Normal | Normal | Normal |
| Coma | Nil | Nil | Nil | Nil |
| Breathing difficulty | Nil | Nil | Nil | Nil |
| Tremor | Nil | Nil | Nil | Nil |
| Diarrhoea | Nil | Nil | Nil | Nil |
| Drowsiness | Nil | Nil | Nil | Nil |
| Sedation | Nil | Nil | Nil | Nil |
| Death | Alive | Alive | Alive | Alive |
Fig. 1.
Effect of T. catappa aqueous extract single-dose treatment on the total body weight gain in the acute toxicological assessment. Bars were expressed as mean ± SEM (n = 5). Bars are not significantly different at p<0.05. TBWG: total body weight gained.
Fig. 2.
Effect of T. catappa aqueous extract single-dose treatment on the relative organ weight in the acute toxicological assessment. Bars were expressed as mean ± SEM (n = 5). Bars are not significantly different at p<0.05.
3.1.2. Effect of TCA single-dose treatment on liver function
There was no significant (p>0.05) change in the plasma levels of ALB, ALP, BIL and TP in the treatment groups compared with the control. Treatment with TCA significantly (p<0.05) reduced plasma ALT and AST activities when compared with the control (Table 2).
Table 2.
Effect of T. catappa aqueous extract single-dose treatment on some biochemical and proinflammatory parameters in the acute toxicity study.
| Parameters | Control | 1000 mg/kg | 2500 mg/kg | 5000 mg/kg |
|---|---|---|---|---|
| ALT (U/I) | 8.55 ± 2.57a | 5.82 ± 1.82b | 5.26 ± 1.42b | 1.97 ± 0.74c |
| AST (U/I) | 29.39 ± 6.97b | 25.57 ± 2.58b | 25.32 ± 13.61b | 15.11 ± 6.14a |
| ALP (U/I) | 523.30 ± 10.35a | 547.50 ± 9.20a | 505.06 ± 22.20a | 536.50 ± 7.87a |
| ALB (g/L) | 36.14 ± 1.61a | 33.10 ± 0.79a | 32.36 ± 2.14a | 34.20 ± 1.06a |
| BIL (mg/dL) | 0.69 ± 0.35a | 1.25 ± 0.87a | 0.95 ± 0.40a | 1.45 ± 0.95a |
| TP (mg/mL) | 70.03 ± 5.82a | 72.11 ± 6.32a | 77.83 ± 3.28a | 74.57 ± 7.24a |
| Urea (mg/dL) | 32.68 ± 2.55a | 37.64 ± 2.10a | 34.47 ± 2.00a | 29.78 ± 2.00a |
| Crea (mg/dL) | 0.56 ± 0.04a | 0.39 ± 0.11a | 0.48 ± 0.05a | 0.61 ± 0.09a |
| Na (mEQ /L) | 91.13 ± 5. 84a | 95.53 ± 6.78a | 92.27 ± 8.43a | 91.19 ± 7.21a |
| Ca (mg/dL) | 8.94 ± 1.47a | 9.32 ± 0.89a | 9.43 ± 1.15a | 8.97 ± 0.57a |
| HCO3(mmol/L) | 33.77 ± 4.02a | 29.78 ± 2.74a | 31.15 ± 3.61a | 28.88 ± 2.87a |
| UA (mg/dL) | 9.52 ± 0.94a | 8.02 ± 0.12a | 7.71 ± 1.74a | 9.31 ± 0.98a |
| IL-6 (pg/mL) | 28.38 ± 6.77a | 20.63 ± 4.13a | 25.84 ± 7.31a | 28.00 ± 4.21a |
| TNF-α (pg/mL) | 9.37 ± 1.56a | 11.22 ± 2.60a | 13.11 ± 2.59a | 8.88 ± 1.24a |
| Insulin (mIU/L) | 18.41 ± 1.03a | 19.22 ± 1.74a | 16.97 ± 2.16a | 18.66 ± 1.47a |
Data are presented as mean ± SEM (n=5). Values with different superscripts (a.b) across a row are significantly different at p<0.05. ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; ALB: albumin; BIL: bilirubin; Crea: creatinine; HCO3: bicarbonate; UA: uric acid; TP: total protein; IL-6: interleukin VI; TNF-α: tumour necrosis factor-alpha.
3.1.3. TCA single-dose treatment does not alter kidney function, proinflammatory and haematology biomarkers
Data obtained from Table 2 revealed that treatment with TCA had no significant (p>0.05) effect on plasma urea, Crea, Na, Ca, HCO3 and UA levels compared with the control group. However, groups treated with 5000 mg/kg bwt had a significantly lower plasma urea concentration than the control group. Likewise, in plasma TNF-α, IL-6 and insulin, TCA treatment did not significantly (p>0.05) change their concentration when compared with the control group (Table 2). There was also no significant (p>0.05) difference between the WBC, lymphocytes, MID, granulocytes, HB, RBC, HCT, PLT and PCT count of the control and treatment groups (Table 3).
Table 3.
Effect of T. catappa aqueous extract single-dose treatment on haematological parameters in acute toxicity study.
| Parameter | Control | 1000 mg/kg | 2500 mg/kg | 5000 mg/kg |
|---|---|---|---|---|
| WBC (x1012/L) | 6.01 ± 0.90 | 6.83 ± 0.65 | 6.63 ± 0.43 | 6.29 ± 0.60 |
| Lymphocytes (x109/L) | 1.84 ± 0.28 | 1.82 ± 0.47 | 1.69 ± 0.39 | 1.98 ± 0.20 |
| MID (x109/L) | 2.28 ± 0.45 | 1.80 ± 0.57 | 2.76 ± 0.98 | 2.58 ± 0.46 |
| Granulocytes (x109/L) | 3.14 ± 0.26 | 2.79 ± 1.16 | 2.96 ± 0.56 | 2.64 ± 1.05 |
| HB (g/dL) | 27.85 ± 5.85 | 28.17 ± 3.04 | 26.99 ± 2.44 | 24.76 ± 9.76 |
| RBC (x107/L) | 7.93 ± 0.98 | 7.86 ± 0.94 | 7.64 ± 0.84 | 7.46 ± 0.73 |
| HCT (%) | 54.86 ± 3.07 | 52.28 ± 8.03 | 59.48 ± 4.41 | 55.88 ± 3.11 |
| PLT (x1012/L) | 577.89 ± 25.37 | 563.62 ± 20.94 | 578.67 ± 29.01 | 567.20 ± 24.95 |
| PCT (%) | 0.62 ± 0.09 | 0.59 ± 0.06 | 0.61 ± 0.06 | 0.60 ± 0.11 |
Data are presented as mean ± SEM (n=5). Values without superscripts across a row are not significantly different at p<0.05. MID: Medium ranged monocytes, eosinophils, basophils, blasts and other precursor white cells; PCT: plateletcrit.
3.1.4. Effect of T. catappa aqueous extract single-dose treatment on organ pathology
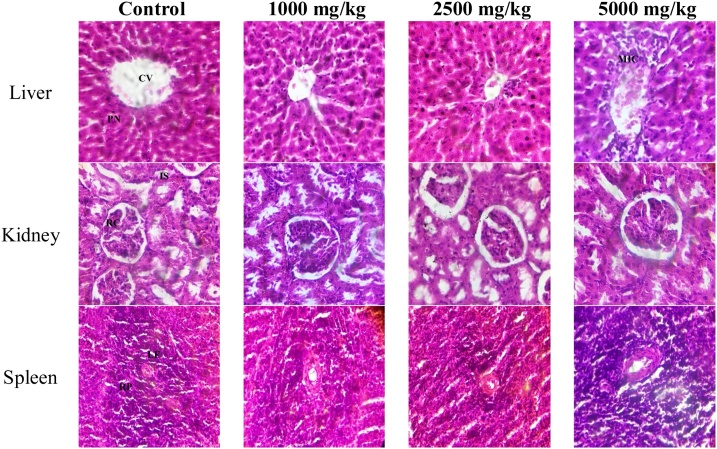
Histopathology of hepatic tissues revealed distinct centriole and hepatocytes with pyknotic nucleus and well-fenestrated sinusoids in control and TCA treatment groups. However, hepatic tissues of 5000 mg/kg bwt treatment groups revealed the centriole wall with slight inflammatory cells surrounding it. Histopathology of renal tissues revealed visible renal corpuscle and interstitial space and tubules across all groups. Histopathology of spleen tissues revealed prominent lymphoid follicles with centrally to eccentrically located blood vessels. The follicles consist of aggregates of lymphocytes. The red pulps are prominent and show a normal configuration across all groups (Plate 1 ).
Plate 1.
Histological photomicrograph (H&E stain ×400) of rat tissues in acute toxicity study. CV: centriole; PN: pyknotic nucleus; MIC: mild inflammatory cells; RC: renal corpuscle; IS: interstitial space; LF: lymphoid follicles; RP: red pulps.
3.2. Sub-acute (28-day) toxicity
3.2.1. Effect of sub-acute 28-day TCA treatment on animal and organ weight
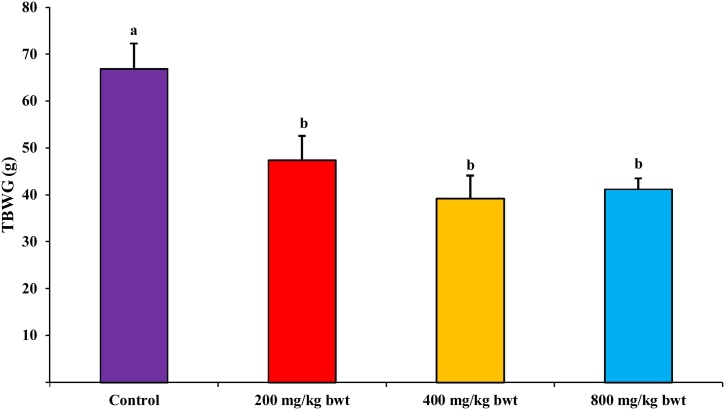
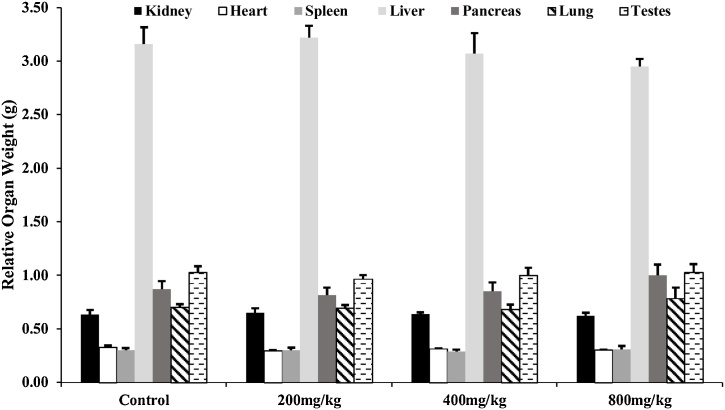
Fig. 3 shows that the body weight of all animals in the control and experimental groups increased all through the experiment. There was a significant (p<0.05) decrease in TBWG of the treatment group when compared with the control group. At the same time, there was no significant (p>0.05) difference in the relative weight of the liver, kidney, pancreas, heart, lung, testes and spleen between the experimental and control animals (Fig. 4).
Fig. 3.
Effect of T. catappa aqueous extract 28-day repeated dose treatment on total body weight gained in the sub-acute toxicological assessment. Bars were expressed as mean ± SEM (n=5). Bars with different superscripts (a.b) are significantly different at p<0.05. TBWG: total body weight gained.
Fig. 4.
Effect of T. catappa aqueous extract 28-day repeated dose treatment on relative organ weight in the sub-acute toxicological assessment. Bars were expressed as mean ± SEM (n = 5). Bars across the groups are not significantly different at p<0.05.
3.2.2. Effect of T. catappa aqueous extract 28-day repeated dose treatment on liver function
Table 4 showed no significant (p>0.05) difference in plasma AST activity and BIL concentration between experimental and control groups. A dose-dependent significant (p<0.05) increase in plasma ALP activity and ALB concentration was observed in the experimental group compared with the control group. The reverse was the case in plasma ALT activity where a dose-dependent significant (p<0.05) decrease was observed in all treatment groups compared with the control group.
Table 4.
Effect of T. catappa aqueous extract 28-day repeated dose treatment on some biochemical and proinflammatory parameters in the sub-acute toxicity study.
| Parameters | Control | 200 mg/kg | 400 mg/kg | 800 mg/kg |
|---|---|---|---|---|
| ALT (U/I) | 5.97 ± 0.46a | 4.94 ± 1.82b | 4.33 ± 1.42b | 1.86 ± 0.74c |
| AST (U/I) | 28.52 ± 5.10a | 42.98 ± 4.86a | 43.71 ± 7.88a | 38.96 ± 4.29a |
| ALP (U/I) | 226.14 ± 35.76a | 296.81 ± 6.76b | 267.14 ± 12.56b | 335.55 ± 35.45c |
| ALB (g/L) | 35.26 ± 2.32a | 36.53 ± 2.42a | 40.62 ± 2.22b | 43.52 ± 1.75c |
| BIL (mg/dL) | 0.94 ± 0.11a | 0.90 ± 0.17a | 0.86 ± 0.24a | 0.92 ± 0.23a |
| TP (mg/mL) | 63.61 ± 2.03a | 62.80 ± 1.54a | 66.11 ± 1.35a | 64.41 ± 2.07a |
| Urea (mg/dL) | 30.31 ± 3.59a | 27.46 ± 3.66a | 27.28 ± 1.77a | 28.19 ± 0.61a |
| Crea (mg/dL) | 0.57 ± 0.19a | 0.60 ± 0.13a | 0.63 ± 0.16a | 0.81 ± 0.01a |
| Na (mEQ /L) | 91.11 ± 6.32a | 89.33 ± 12.53a | 90.28 ± 13.89a | 95.18 ± 4.65a |
| Ca (mg/dL) | 8.34 ± 1.87a | 8.94 ± 1.98a | 8.08 ± 0.53a | 8.55 ± 1.89a |
| HCO3(mmol/L) | 36.65 ± 4.55a | 34.32 ± 5.54a | 34.67 ± 5.26a | 34.22 ± 4.55a |
| UA (mg/dL) | 12.95 ± 2.33a | 11.32 ± 0.71a | 14.17 ± 2.99a | 16.63 ± 3.39a |
| IL-6 (pg/mL) | 25.71 ± 3.47a | 27.22 ± 4.70a | 22.39 ± 6.88a | 20.75 ± 5.61a |
| TNF-α (pg/mL) | 28.34 ± 1.87a | 28.94 ± 1.98a | 30.08 ± 7.53a | 25.55 ± 4.89a |
| Insulin (mIU/L) | 15.35 ± 5.03a | 18.92 ± 6.14a | 15.67 ± 3.12a | 13.66 ± 4.24a |
Data are presented as mean ± SEM (n=5). Values with different superscripts (a.b) across a row are significantly different at p<0.05. ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; ALB: albumin; BIL: bilirubin; Crea: creatinine; HCO3: bicarbonate; UA: uric acid; TP: total protein; IL-6: interleukin VI; TNF-α: tumour necrosis factor-alpha.
3.2.3. T. catappa aqueous extract 28-day repeated dose treatment does not alter kidney function, proinflammatory and haematology biomarkers
Data obtained from Table 4 revealed treatment with TCA had no significant (p>0.05) effect on plasma urea, Crea, Na, Ca, HCO3, UA, TP, TNF-α, IL-6 and insulin levels when compared with the control group. There was also no significant (p>0.05) change in the WBC, lymphocytes, MID, granulocytes, HB, RBC, HCT, PLT and PCT count of the treatment groups when compared with the control animals (Table 5).
Table 5.
Effect of T. catappa aqueous extract 28-day repeated dose treatment on haematological parameters in sub-acute toxicity study.
| Parameter | Control | 200 mg/kg | 400 mg/kg | 800 mg/kg |
|---|---|---|---|---|
| WBC (x1012/L) | 6.11 ± 0.78 | 6.19 ± 0.36 | 6.03 ± 0.43 | 6.06 ± 0.60 |
| Lymphocytes (x109/L) | 1.04 ± 0.19 | 1.12 ± 0.53 | 1.09 ± 0.61 | 1.07 ± 0.88 |
| MID (x109/L) | 2.28 ± 0.45 | 1.80 ± 0.57 | 2.76 ± 0.98 | 2.58 ± 0.46 |
| Granulocytes (x109/L) | 1.74 ± 0.26 | 2.62 ± 1.16 | 1.46 ± 0.56 | 2.64 ± 1.05 |
| HB (g/dL) | 24.65 ± 1.43 | 23.17 ± 1.64 | 26.99 ± 2.44 | 24.76 ± 1.72 |
| RBC (x107/L) | 7.43 ± 0.87 | 7.58 ± 0.32 | 7.30 ± 0.58 | 7.45 ± 0.45 |
| HCT (%) | 44.26 ± 3.07 | 42.38 ± 8.16 | 49.68 ± 6.41 | 50.00 ± 7.11 |
| PLT (x1012/L) | 571.40 ± 45.37 | 593.62 ± 69.90 | 588.67 ± 27.45 | 607.20 ± 54.55 |
| PCT (%) | 0.61 ± 0.05 | 0.63 ± 0.07 | 0.56 ± 0.09 | 0.64 ± 0.03 |
Data are represented as mean ± SEM (n=5). Values without superscripts across a row are not significantly different at p<0.05. MID: Medium ranged monocytes, eosinophils, basophils, blasts and other precursor white cells; PCT: plateletcrit.
3.2.4. T. catappa aqueous extract 28-day repeated dose does not affect hepatic mRNA expression of proinflammation and stress response indicators
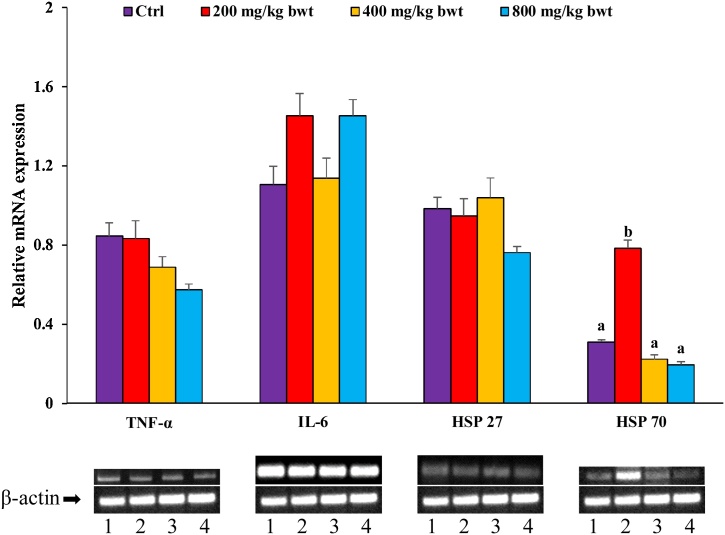
From Fig. 5, there was no significant (p>0.05) alteration in the hepatic mRNA expression of TNF-α, IL-6, HSP 27 and HSP 70 of TCA treatment groups compared with the control. However, at 200 mg/kg bwt, TCA significantly (p<0.05) upregulated HSP 70 expression compared with the control group.
Fig. 5.
Effect of T. catappa aqueous extract 28−day repeated dose treatment on TNF-α, IL-6, HSP 27 and HSP 70 in the sub-acute toxicological assessment. Bars were expressed as mean ± SEM (n=5). Bars without superscripts for each gene are not significantly different, while those with different superscripts (a.b) are significantly different at p<0.05. TNF-α: tumour necrosis factor-alpha; IL-6: interleukin six; HSP: heat shock protein.
3.2.5. Effect of T. catappa aqueous extract 28-day repeated dose treatment on organ pathology
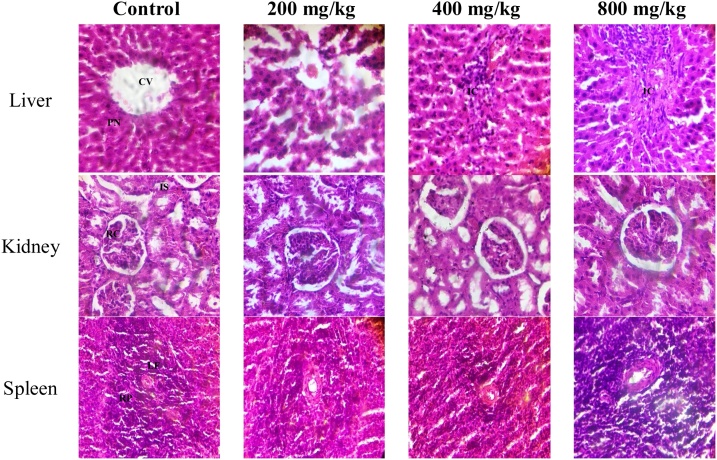
Histopathology of hepatic tissues revealed distinct centriole and hepatocytes with pyknotic nucleus and well-fenestrated sinusoids in control. Slight diffusion of inflammatory cells was common in 400 and 800 mg/kg TCA-treated animals. For renal tissues, visible renal corpuscle and tubules were observed across all groups. Histopathology of spleen tissues revealed prominent lymphoid follicles with centrally to eccentrically located blood vessels consisting of aggregates of lymphocytes and red pulps across all groups (Plate 2 ).
Plate 2.
Histological photomicrograph (H&E stain ×400) of rat tissues in sub-acute toxicity study. CV: centriole; PN: pyknotic nucleus; IC: Inflammatory cells; RC: renal corpuscle; IS: interstitial space; LF: lymphoid follicles; RP: red pulps.
4. Discussion
From the acute toxicity study, no morbidity or mortality was observed across the treatment groups; the biochemical, haematological and histopathological analyses also did not indicate any adverse effect at the doses used. The LD50 value of TCA was evaluated to be higher than 5000 mg/kg bwt, suggesting that it is practically non-toxic as classified by OECD [15]. This finding corroborates the reports of various Terminalia species, extracted with different solvents, having LD50 classified as practically non-toxic (≥ 2000–5000 mg/kg bwt) [11,[16], [17], [18]]. The sub-acute study showed that the 28-day repeated dose treatment of TCA in experimental animals did not elicit clinical signs of morbidity, toxicity, or mortality across all the treatment groups, thus validating TCA’s non-toxic effect at the tested doses over the period of study. Organ and body weight changes are important indicators in accessing the tested compound or medicinal plant’s adverse effect and the overall health condition of the experimental animals [19,20]. The significant reduction in TBWG of the TCA administered group on continuous exposure could be due to linoleic acid and n-hexadecanoic acid, which cause satiety effects [21,22]. Despite the continuous administration and gradual accumulation of TCA in the experimental animals’ body and tissue, the inability to change various organ weights indicates that it exerted no pathological and physiological toxicity on the organs [15,23,24]. This may suggest TCA’s oral safety, buttressing the acute toxicity study [18,25,26]. Though a significant increase in ALP and ALB was observed after the daily dosage of TCA for 28 days, it does not infer any sign of toxicity as an increase in these parameters alone is not sufficient to suggest hepatotoxicity [27]. Bilirubin clearance and biliary obstruction have also been linked with increased ALP. Since total plasma bilirubin was not elevated, the increase might not be associated with biliary obstruction, suggesting toxicity is unlikely. The reduction of ALT may suggest that TCA possess hepatoprotective properties [14]. AST and ALT are enzymes synthesised by the liver; their plasma activity is used to evaluate hepatocyte cellular integrity, while albumin, bilirubin and protein concentration are metabolic biomarkers used to measure hepatic tissue functionality, further signifying full functionality of the liver after TCA treatment [15]. Plasma glucose and protein are also important markers for assessing the liver’s metabolic functionality and synthetic capacity [25]. This study reflected no significant effect of TCA on these parameters, suggesting its relatively non-toxic effect. It can also be inferred that TCA does not hamper both synthetic and metabolic hepatic function [26]. The plasma concentration of creatinine, uric acid, urea, sodium and bicarbonate are classic biomarkers for detecting renal dysfunction [28]. Since there was no significant alteration of these biomarkers in the experimental animals, it can be inferred that TCA does not elicit renal toxicity at the experimental dosage and time frame. Assessing various haematological indices is useful in determining the physiological and pathological state of the animals [28]. Exposure to chemical substances originating from plants can lead to acute inflammatory responses. IL-6 and TNF-α are the chief inflammatory mediators that instigate reactive oxygen species generation, which leads to a myriad of systemic complications [29]. TCA’s inability to alter these proinflammatory cytokines would suggest it does not activate inflammatory responses. A herbal formulation in Nigeria was similarly found not to alter the expression of these proinflammatory mediators, thus, corroborating our finding [10]. Heat shock proteins (HSPs) expedite the proper folding and function of proteins. During stress incidence, there is an increase in HSP 27 and 70 mRNA expression and the amount of cytosolic unfolded proteins [30]. The increase in HSP 70 could be triggered as a response to external stimulus and may not be caused by toxicity [31]. It could also be regarded as toxicologically irrelevant since it was inconsistent with the result of all other assessed toxicological parameters. The toxicological stress response of TCA on HSPs is being reported for the first time to the best of our knowledge. An induction of haematological alterations in animals by extracts or substances usually infers a high risk of toxicity [32]. The insignificant alteration of haematological parameters corroborates the biochemical results, indicating that TCA elicits “no observed adverse effect level” (NOAEL). Evaluation of tissue histopathological architecture is considered the gold standard of toxicity testing for examining the effect of extracts and substances on vital metabolic organs [33]. The normal macroscopic architecture, presence of preserved colour and texture with an absence of infiltrative or degenerative lesions in the tissues suggests that TCA did not cause organ injuries. Nonetheless, the observed inflammation in some tissues may be as a result of immune response to the accumulated interaction of the extract with the various organs [14]. These findings further support our suggestion that TCA did not elicit systemic and organic toxicity in the animals at the doses tested in the experimental time frame.
5. Conclusion
In conclusion, T. catappa aqueous leaf extract can be adduced as a category five substance and considered practically non-toxic according to OECD criteria under its Globally Harmonised System (GHS) for chemical substances and mixtures classification. When utilised at the reported dose and time frame, the extract may not likely elicit any proinflammatory, stress, systemic and organ toxic effect.
Funding
The authors did not receive support from any organisation for the submitted work.
Author contributions
Conceptualisation: FNI, OOO and SNC; Methodology: FNI; Formal analysis and investigation: FNI, COO, OCD and BEA; Writing - original draft preparation: FNI; Writing - review and editing: All authors; Resources: FNI and OOO; Supervision: FNI, OOO and SNC.
Conflict of Interest
The authors declare no conflict of interest.
Data availability
All data generated or analysed during this study are included in this published article
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We acknowledge Covenant University Center for Research Innovation and Development (CUCRID) for funding the article’s processing charge.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Iheagwam F.N., Dania O.E., Michael-Onuoha H.C., Ogunlana O.O., Chinedu S.N. Alternative Medicine. IntechOpen; 2020. Antidiabetic activities of Terminalia species in Nigeria. [DOI] [Google Scholar]
- 2.Anand A.V., Divya N., Kotti P.P. An updated review of Terminalia catappa. Pharmacogn. Rev. 2015;9(18):93–98. doi: 10.4103/0973-7847.162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratnasooriya W., Dharmasiri M. Effects of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J. Androl. 2000;2(3):213–219. [PubMed] [Google Scholar]
- 4.Seema J., Meeta B., Meenaksh B., Manjushree M. Comparative study of young and mature leaves of Terminalia catappa for evaluation of physico-chemical, pharmacognostical and phytochemical analysis. Int. J. Life Sci. 2015;(A4):12–20. [Google Scholar]
- 5.Baratelli T.D.G., Gomes A.C., Wessjohann L.A., Kuster R.M., Simas N.K. Phytochemical and allelopathic studies of Terminalia catappa L.(Combretaceae) Biochem. Syst. Ecol. 2012;41:119–125. [Google Scholar]
- 6.Chukwuma E. Antioxidative activity of the almond leaves (Terminalia Catappa) Int. J. Nurs. Midwife Health Relat. Cases. 2015;1(2):29–40. [Google Scholar]
- 7.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013;10(5):210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asase A., Kokubun T., Grayer R., Kite G., Simmonds M., Oteng-Yeboah A., Odamtten G. Chemical constituents and antimicrobial activity of medicinal plants from Ghana: cassia sieberiana, Haematostaphis barteri, Mitragyna inermis and Pseudocedrela kotschyi. Phytother. Res. 2008;22(8):1013–1016. doi: 10.1002/ptr.2392. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S., Zaidi S., Chaturvedi A., Srivastava R., Dwivedi P., Shukla R. Search for a herbal medicine: anti-asthmatic activity of methanolic extract of Curcuma longa. J. Pharmacogn. Phytochem. 2015;3:59–72. [Google Scholar]
- 10.Adebayo A.H., Ashano E.E., Yakubu O.F., Okubena O. Pro-inflammatory and toxicological evaluation of Hepacare® in mice. J. Taibah Univ. Med. Sci. 2017;12(4):313–323. doi: 10.1016/j.jtumed.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iheagwam F.N., Okeke C.O., DeCampos O.C., Okere D.U., Ogunlana O.O., Chinedu S.N. Safety evaluation of Terminalia catappa Linn (Combretaceae) aqueous leaf extract: sub-acute cardio-toxicopathological studies in albino Wistar rats. J. Phys. Conf. Ser. 2019;1299(1):012109. [Google Scholar]
- 12.Ogunlana O.O., Ogunlana O.E., Adeneye A.A., Udo-Chijioke O.A.C., Dare-Olipede T.I., Olagunju J.A., Akindahunsi A.A. Evaluation of the toxicological profile of the leaves and young twigs of Caesalpinia bonduc (Linn.) ROXB. Afr. J. Tradit. Complement. Altern. Med. 2013;10(6):504–512. doi: 10.4314/ajtcam.v10i6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stalin A., Stephen S., Rajiv G., Balakrishna K., Ignacimuthu S., Al-dhabi N.A. Hypoglycemic activity of 6-bromoembelin and vilangin in high-fat diet fed-streptozotocin-induced type 2 diabetic rats and molecular docking studies. Life Sci. 2016;153:100–117. doi: 10.1016/j.lfs.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Chinedu S.N., Iheagwam F.N., Anichebem C.J., Ogunnaike G.B., Emiloju O.C. Antioxidant and biochemical evaluation of Thaumatococcus daniellii seeds in rat. J. Biol. Sci. 2017;17(8):381–387. [Google Scholar]
- 15.Ugwah-Oguejiofor C.J., Okoli C.O., Ugwah M.O., Umaru M.L., Ogbulie C.S., Mshelia H.E., Umar M., Njan A.A. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon. 2019;5(1):e01179. doi: 10.1016/j.heliyon.2019.e01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arjariya S., Nema N., Tiwari S. Investigate the toxicological effect on aqueous extract of Terminalia catappa Linn. in rat. Int. J. Res. Dev. Pharm. Life Sci. 2013;2:596–601. [Google Scholar]
- 17.Das N., Goshwami D., Hasan M.S., Raihan S.Z. Evaluation of acute and subacute toxicity induced by methanol extract of Terminalia citrina leaves in Sprague Dawley rats. J. Acute Dis. 2015;4(4):316–321. [Google Scholar]
- 18.Beserra A.M.S., Vilegas W., Tangerina M.M.P., Ascêncio S.D., Soares I.M., Pavan E., Damazo A.S., Ribeiro R.V., de Oliveira Martins D.T. Chemical characterisation and toxicity assessment in vitro and in vivo of the hydroethanolic extract of Terminalia argentea Mart. Leaves. J. Ethnopharmacol. 2018;227:56–68. doi: 10.1016/j.jep.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Dongmo O.L.M., Epoh N.J., Tadjoua H.T., Yousuf S., Telefo P.B., Tapondjou L.A., Choudhary M.I. Acute and sub-acute toxicity of the aqueous extract from the stem bark of Tetrapleura tetrapteura Taub.(Fabaceae) in mice and rats. J. Ethnopharmacol. 2019;236:42–49. doi: 10.1016/j.jep.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Wu M.X., Ma X.J., Shi J.L., Wang S.N., Zheng Z.Q., Guo J.Y. Acute and sub-acute oral toxicity studies of the aqueous extract from radix, radix with cortex and cortex of Psammosilene tunicoides in mice and rats. J. Ethnopharmacol. 2018;213:199–209. doi: 10.1016/j.jep.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Tucci S.A. Phytochemicals in the control of human appetite and body weight. Pharmaceuticals. 2010;3(3):748–763. doi: 10.3390/ph3030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iheagwam F.N., Israel E.N., Kayode K.O., De Campos O.C., Ogunlana O.O., Chinedu S.N. GC-MS analysis and inhibitory evaluation of Terminalia catappa leaf extracts on major enzymes linked to diabetes. Evid. Based Complement. Altern. Med. 2019;2019 doi: 10.1155/2019/6316231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bariweni M.W., Yibala O.I., Ozolua R.I. Toxicological studies on the aqueous leaf extract of Pavetta crassipes (K. Schum) in rodents. J. Pharm. Pharmacogn. Res. 2018;6(1):1–16. [Google Scholar]
- 24.Unuofin J.O., Otunola G.A., Afolayan A.J. Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia less. in Wistar rats. J. Integr. Med. 2018;16(5):335–341. doi: 10.1016/j.joim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Sabiu S., O’Neill F.H., Ashafa A.O.T. Toxicopathological evaluation of a 28-day repeated dose administration of Zea mays L.(Poaceae), stigma maydis aqueous extract on key metabolic markers of Wistar rats. Trans. R. Soc. South Afr. 2017;72(3):225–233. [Google Scholar]
- 26.Taher M.A., Tadros L.K., Dawood D.H. Phytochemical constituents, antioxidant activity and safety evaluation of Kei-apple fruit (Dovyalis caffra) Food Chem. 2018;265:144–151. doi: 10.1016/j.foodchem.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim M.B., Sowemimo A.A., Sofidiya M.O., Badmos K.B., Fageyinbo M.S., Abdulkareem F.B., Odukoya O.A. Sub-acute and chronic toxicity profiles of Markhamia tomentosa ethanolic leaf extract in rats. J. Ethnopharmacol. 2016;193:68–75. doi: 10.1016/j.jep.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Clemente M., Miguel M.D., Felipe K.B., Gribner C., Moura P.F., Rigoni A.G.R., Fernandes L.C., Carvalho J.L.S., Hartmann I., Piltz M.T., Henneberg R. Acute and sub-acute oral toxicity studies of standardised extract of Nasturtium officinale in Wistar rats. Regul. Toxicol. Pharmacol. 2019;108:10443. doi: 10.1016/j.yrtph.2019.104443. [DOI] [PubMed] [Google Scholar]
- 29.Ni Z., Guo L., Liu F., Olatunji O.J., Yin M. Allium tuberosum alleviates diabetic nephropathy by supressing hyperglycemia-induced oxidative stress and inflammation in high fat diet/streptozotocin treated rats. Biomed. Pharmacother. 2019;12 doi: 10.1016/j.biopha.2019.108678. [DOI] [PubMed] [Google Scholar]
- 30.McConnell J.R., McAlpine S.R. Heat shock proteins 27, 40, and 70 as combinational and dual therapeutic cancer targets. Bioorg. Med. Chem. Lett. 2013;23(7):1923–1928. doi: 10.1016/j.bmcl.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waheed R., El Asely A.M., Bakery H., El-Shawarby R., Abuo-Salem M., Abdel-Aleem N., Malhat F., Khafaga A., Abdeen A. Thermal stress accelerates mercury chloride toxicity in Oreochromis niloticus via up-regulation of mercury bioaccumulation and HSP70 mRNA expression. Sci. Total Environ. 2020;718 doi: 10.1016/j.scitotenv.2020.137326. [DOI] [PubMed] [Google Scholar]
- 32.Wolff F.R., Broering M.F., Jurcevic J.D., Zermiani T., Bramorski A., de Carvalho Vitorino J., Malheiros A., Santin J.R. Safety assessment of Piper cernuumVell. (Piperaceae) leaves extract: Acute, sub-acute toxicity and genotoxicity studies. J. Ethnopharmacol. 2019;230:109–116. doi: 10.1016/j.jep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 33.Kumar K., Sharma S., Kumar A., Bhardwaj P., Barhwal K., Hota S.K. Acute and sub-acute toxicological evaluation of lyophilised Nymphaea x rubra Roxb. ex Andrews rhizome extract. Regul. Toxicol. Pharmacol. 2017;88:12–21. doi: 10.1016/j.yrtph.2017.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article