Abstract
Purpose
This systematic review aimed to evaluate the effectiveness of the use of personal protective equipment (PPE) in closed environments, similar to waiting or exam rooms of healthcare facilities, in the face of exposure to a bioaerosol.
Methods
Combinations of words were selected for six electronic databases and for the gray literature. To consider the eligibility of the studies to be included/excluded, the acronym “PECOS” was used: humans and/or experimental models that simulate aerosol (Population); aerosol exposure and the use of masks/respirators (exposition/intervention); controlled or not controlled (comparison); effectiveness of PPE and the receiver exposure (outcomes); and randomized clinical studies or not, observational or laboratory simulation studies (Studies design).
Results
A total of 4820 references were retrieved by the search strategy. Thirty-five articles were selected for complete reading, of which 13 articles were included for qualitative synthesis. A surgical mask or N95 respirator reduced the risk of transmission, even over short distances. The use of masks, even those with less filtering power, when used by all individuals in the same environment is more effective in reducing risk than the use of respirators with high filtering power for only some of the individuals present.
Conclusion
The use of mask in closed environments is effective in reducing the risk of transmission and contagion of a contaminated bioaerosol, with greater effectiveness when these devices are used by the source and receiver, regardless of the equipment’s filtering power. (PROSPERO 2020 CRD 42020183759).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00420-021-01775-y.
Keywords: Aerosols, Personal protective equipment, Environments, Health
Introduction
The transmission of disease between individuals often occurs by the dispersion of droplets of saliva from an infected person through breathing, coughing, speaking or sneezing; in closed environments, this contaminated bioaerosol can be transmitted to another person through the respiratory viruses' entry points (eyes, mouth and/or nose) (Centers for Disease Control and Prevention 2016; Noti et al. 2012; Shiu et al. 2019).
SARS-CoV-2 has a high transmission rate and was classified as a pandemic in March 2020 (Faridi et al. 2020). Similar to SARS-CoV and the influenza virus, an individual can be infected with SARS-CoV-2 by direct contact with contaminated body fluids (Cegolon 2020; Chen et al. 2004; Larson and Liverman 2011). Studies suggest the use of personal protective equipment (PPE), such as surgical masks or N95 respirators, as an inhalation barrier (Loeb et al. 2009; Siegel et al. 2007) for air control and filtration (Larson and Liverman 2011; Lindsley et al. 2012; Noti et al. 2012).
Smaller aerosol particles are known to be easily inhaled and are capable of being transmitted from short to long range (Tellier 2019), in addition to being suspended in the air for a longer time than larger particles (Gralton et al. 2011). Droplets of larger diameter settle more quickly on surfaces, making them more difficult to inhale; however, these droplets have a greater potential to carry pathogens than smaller droplets, in addition to not penetrating deep into the respiratory tract (Tellier 2019).
Studies indicate that the forms of ventilation where frequent air circulation occurs in environments, such as waiting rooms for health services, are not effective for the total removal of particles generated by bioaerosols but contribute to a gradual decrease in concentration (Lindsley et al. 2012). Individuals are more exposed to this type of contamination indoors (Lindsley et al. 2012), and care must be taken to decrease the risk when these bioaerosols are dispersed in the environment.
Because COVID-19 is a disease in which the virus can be incubated for an average of 5–6 days before presenting any symptoms, the infected patient has the potential to transmit SARS-CoV-2 before symptoms develop (World Health Organization 2020a, b). The World Health Organization (WHO) recommends caution, especially in closed environments, where there may be a circulation of contaminants shed by people with COVID-19, symptomatic or not (World Health Organization, 2020a, b).
Some systematic reviews have addressed the effectiveness of different types of PPE (Bartoszko et al. 2020; Long et al. 2020; Offeddu et al. 2017; Smith et al. 2016; Verbeek et al. 2020); however, to date, none have addressed the effectiveness of this equipment concerning the risk of contamination in closed environments. Therefore, the aim of this systematic review was to analyze the effectiveness of the use of PPE in closed environments, in situations similar to waiting rooms or examination rooms of healthcare facilities, in the face of exposure to a bioaerosol.
Methods
Protocol and registration
The protocol for this systematic review was registered on the PROSPERO website (International prospective register of systematic review—Center for Reviews and Dissemination University of York)—CRD42020183759, and the review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist (Moher 2009).
Eligibility criteria
To consider the eligibility of the studies to be included/excluded from this review, the acronym “PECOS” was used.
Population (P)
Studies were included where the population consisted of humans (regardless of age, gender, or ethnicity) and/or experimental models (regardless of design) that simulate the aerosol generated by breathing, speaking, coughing, or sneezing. Animal studies were excluded.
Exposition (E)
In the case of observational studies, the study population must have been exposed to at least one type of aerosol generated by breathing, speaking, coughing, or sneezing, which may have been generated naturally (humans) or by simulators that resemble the natural production. In interventional studies, the population must have used masks/respirators (homemade, surgical, N95, FFP, FFP2, FFP3) or eye or facial protection as a form of intervention. In both cases, the intervention or exposure must have been carried out in a closed environment, which is physically similar to a health service environment, with the simultaneous presence of an aerosol generator (source) and at least one receiver that breathes in the generated aerosol. In addition, the permanence in the environment must have been transitory (in the same way as the waiting room and exams), excluding non-transitory environments (for example, families in the same residence, or similar situations), or when the study did not make it clear whether or not it was a closed environment. Studies were excluded if the population had not been exposed to the generated aerosol, or if the source and the receiver of the aerosol were not in the same environment at the same time.
Comparison (C)
No inclusion/exclusion criteria were adopted for the control group, and uncontrolled studies were also included.
Outcomes (O)
The outcomes of interest were as follows:
The effectiveness of PPE against exposure to an aerosol generated by speech, breathing, coughing or sneezing.
The dispersion and exposure of the receiver and the environment to this aerosol.
Studies that did not assess the outcome of interest were excluded.
Study design (S)
Randomized or non-randomized clinical studies, observational studies, and experimental laboratory simulation studies were included.
Experimental animal studies, reviews, letters, conference abstracts, expert opinions, or case reports were excluded. There were no exclusion criteria concerning the study language or publication dates.
Information sources and search strategy
Combinations of words and appropriate truncations have been adapted for the following electronic databases selected as the sources of information: PubMed/Medline, EMBASE, Latin American and Caribbean Literature in Health Sciences (LILACS), Web of Science, Scopus and Cochrane Library. Gray literature was also used as a source of information through Google Scholar, Proquest, and Open Gray (Online resource 1). All searches were performed on May 1, 2020. The references were managed using appropriate software (EndNote® X7 Thomson Reuters, Philadelphia, PA), and all duplicate studies were removed.
In addition to searching the electronic databases and gray literature, an expert (A.L.O.T) on the subject was also consulted via e-mail to verify any possible publications on the subject and to indicate any relevant articles that could be included.
Study selection
The selection of articles was carried out in two phases, with the total result of records retrieved by the search strategy divided between two pairs (C.M.A/F.M.G and A.G.D.S/O.G.F). To calibrate the selection of articles, the kappa coefficient of the agreement was calculated for each of the pairs. In the first phase, the titles and abstracts of all references retrieved by the search were independently reviewed. All articles that did not meet the eligibility criteria previously established were excluded at this stage. In the second phase, the full text of the articles selected in the first phase was independently read. Whenever there was some disagreement and the lack of consensus persisted even after discussion, a third reviewer (R.S.S) was involved in the final decision.
To protect the reading of references and guarantee independence and confidentiality in both phases, the Rayyan website was used (http://rayyan.qcri.org), where the reviewers were blinded in all evaluations and a member of the team (I.B.B), who did not participate in the selection, served as the moderator.
Data collection process
Two reviewers (B.L.C.L and G.M.C.R) independently collected information from the included studies, and this information was discussed with two other members of the team (J.S.N and F.M.G). The data collected consisted of characteristics of the study (author, year of publication, country, title and design of the study), characteristics of the exposure (the type of aerosol generated, contamination, particle size, room size, air humidity and room temperature, PPE used, expiration/breath rate and the distance between the source and the receiver), the results and conclusions. When data were missing or incomplete in the article, attempts were made to contact the authors to obtain relevant unpublished information.
Risk of bias in individual studies
An adaptation of the Simulation Research Evaluation Rubric (SRR) (17) tool was used to assess the risk of bias in the experimental studies using simulations. This tool includes 16 evaluation items, classified on a scale of 0 to 4 points (0 = unsatisfactory and 4 = excellent), resulting in a total score of 56 points in quantitative or qualitative studies and 64 points in studies with mixed methods. Due to the heterogeneity of the methodologies that could be included in this review, items that did not apply to the study design evaluated were judged as not applicable (NA). To facilitate visualization, the scores were transformed into percentages given by the ratio between the total score obtained and the total possible score. Based on quartiles within the rating scale of the score obtained, the studies were classified as having a high risk of bias (0–50%), moderate risk of bias (51–75%), and low risk of bias (76–100%).
Summary measures
Any outcome measures were considered, provided the study assessed the outcome of interest.
Results
Study selection
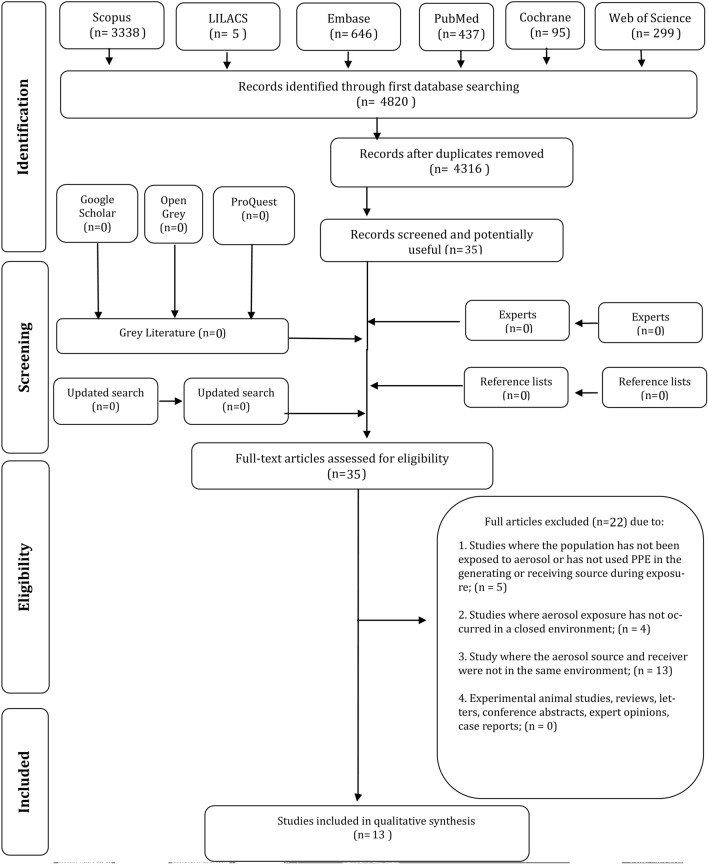
A total of 4820 references were retrieved by the search strategy from the six electronic databases, leaving 4316 after the removal of duplicate references. After reading the titles and abstracts (phase 1), a total of 35 articles were selected for complete reading (phase 2), after which 22 were excluded (Online resource 2), resulting in 13 articles included for the qualitative synthesis (Fig. 1). No additional articles were included from the reference lists, gray literature, or consultation with the expert. The value of the kappa coefficient of agreement was > 0.8 for both pairs, indicating excellent agreement.
Fig. 1.
Flow diagram of literature search and selection criteria
Study characteristics
Of the 13 studies included in this systematic review, all were published in the last 10 years (variation from 2010 to 2019) in English, and 9 were from the United States, 3 were from China, and 1 was from the United Kingdom.
All studies were classified as experimental studies; however, 10 studies used mannequins as a source and receiver simulator, 1 study used a mathematical model to predict the risk of transmission, 1 used a mixed model composed of simulator/mannequins and humans, and 1 study used only humans as the study population.
The areas of the rooms (m2) ranged from 2.35 m2 × 1.88 m in height to 10.24 m2 × 2.3 m. Two studies presented only the volume (m3), and it was not possible to define the exact area of each environment, as the individual measurements (width × length × height) of the environment were not provided; two studies did not describe the size of the environment. The temperature varied from 17 to 24 °C, and the humidity varied from 20 to 68%. The size and temperature of the environments, and the distance between the aerosol-generating source and the receiver, included in this review, are available in Table 1.
Table 1.
Characteristics of included studies (n = 13)
| Author, year (country) | Study design | Type of aerosol generated (contamination) | Particle size | Room size—m2 (height) | Aerosol flow (source) | Breathing rate (Receiver) | Room temperature and humidity | Individual protection equipment | distance between generator and receiver | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adhikari et al. 2019 (United States of America) | Experimental laboratory study (mathematical modeling) | Cough (MERS-CoV) | Respirable droplets were modeled as aerosols with mean post evaporation diameters of 4 μm and 8 μm (for small and large respirable droplets) | 230m3 (description made only in cubic meters) | N/A | N/A | N/A | N95 respirator (source) | 1–2 m from the source | The results of the risk management assessment showed that increasing the rate of air ventilation was an effective measure to decrease the risk for other patients who share the environment, but not for people in close contact. For others in the same environment, the risk can be reduced by about 30% or 58% by increasing air ventilation. Wearing a mask was considered the most effective intervention measure to minimize the risk of infection, reducing an average of 89–97%, suggesting that all exposed groups should wear a mask as protection to minimize the associated risk of infection | Increasing the ventilation rate was considered an effective measure to reduce the risk of infection, but not for short distances. The use of a mask was considered more effective in reducing the risk of infection for exposed groups |
| Lindsley et al. 2014 (United States of America) | Experimental laboratory study (study with mannequin) | Cough (influenza strain A/WS/33—H1N1, ATCC VR-825) | Test aerosol had a count median diameter of 0.44 μm and a geometric standard deviation of 1.48 | 10.24m2 (2.3 m in height)* | The cough had a volume of 4.2 l and a peak flow rate of 11.4 L per second (l/sec) |
The breathing waveform was sinusoidal, with a flow rate of 32 L per minute (l/min) |
24◦C (SD 1.4◦C) / 21% (SD 5.9%) | FaceShield (receiver) | 0.46 m* and 1.83 m* | The amount of aerosol inhaled by the simulator, increasing the distance between the cough and breathing simulators and using a faceshield, reduced significantly (p < 0.001 and p = 0.009, respectively). The total amount of virus that was deposited in both simulators was significantly lower when using the faceshield (p = 0.001) | The use of faceshield can substantially reduce exposure to infectious particles present in the aerosol, and can reduce respiratory contamination. These devices are less effective against smaller particles, which can remain in the air for long periods. Thus faceshields can provide a complementary protection to the use of masks / respirators, however they should not be used as a substitute for respiratory protection |
| Lindsley et al. 2012 (United States of America) | Experimental laboratory study (study with mannequin) | Cough (no contamination) | Test aerosol had a count median diameter of 0.44 μm and a geometric standard deviation of 1.48 | 7.29m2 (2.4 m in height)* | The cough had a 2.1 L volume with a peak flow of 8.45 L/sec and a mean flow of 2.64 L/ sec | 32, 85 and 95 L/min | 24 °C (SD 1.4 °C) / 33% (SD 6%) | Different brands of N95 masks, surgical masks and a mask combination (source and/or receiver) | 1.83 m* | Immediate aftermath of a cough, the exposure is much higher when the worker is directly in the path of the cough plume. However, after 5 min, the aerosol concentrations at all locations are very similar (20% after 5 min and 15% after 10 min). Aerosol exposure is highest with no personal protective equipment, followed by surgical masks, and the least exposure is seen with N95 respirators. These differences are seen regardless of breathing rate and relative position of the coughing and breathing simulators | These results provide a better understanding of the exposure of workers to cough aerosols from patients and of the relative efficacy of different types of respiratory personal protective equipment, and they will assist investigators in providing research-based recommendations for effective respiratory protection strategies in health care settings |
| Booth et al. 2013 (United Kingdom) | Experimental laboratory study (study with mannequin) | Breathing (A-type influenza virus) | Dispersed aerosol covering a size range < 1 μm to > 200 μm, of which 50% were of a size < 60 μm and 15% > 100 μm | ND | ND | Inhalation/ exhalation rate of 40 L/min (stroke volume of 2.0 L 20 cycles/min) | ND | Surgical masks (receiver) | 0.7 m* | The influenza virus was recovered from behind (the breathing zone) of all tested surgical masks, i.e. the mask does not completely prevent the entry of the virus in breathing simulators. Most masks showed an average of 10 times reduction in exposure to viral infection in the face of a direct challenge | Surgical masks have shown limitations, although to some extent they have provided protection for the breathing source in the face of a viral challenge |
| Mansour et al. 2013 (United States of America) | Experimental laboratory study (study with mannequin) | Breathing (no contamination) | Approximately 90% of the particles were less than 2 μm, with an average of 0.95 μm (95% CI: +—0.119) | 2.35m2 (1,88 m in height)* | 10 L/min | Respiratory rate of 15 breaths/min, and duty cycle of 0.5 s | 20.5 C to 23.2 C and 23% to 68% | N95 respirator and an ear loop surgical mask (source and/or receiver) | 0.91 m* | When the source and the receiver did not use a mask, there was no protection, obtaining a simulated protection factor value of 1.363% (95% CI: 0.912–1.81%). When a N95 surgical mask or respirator was applied to the source, it resulted in a significant reduction in exposure, with protection values of 0.00637% (95% CI: 0.00009 –0.00038%) for surgical mask with natural adjustment around the ears, 0.00023% (95% CI: 0.00009 –0.00038%) for a well-sealed surgical mask (best fitted and adapted to the mannequin) and 0.00019% (95% CI: 0.00012–0, 00,027%) for the N95. On the other hand, only minimal and insignificant reductions (p > 0.05) were measured when a surgical mask or unsealed respirator (natural adjustment) was placed on the receiver, with values of 1.38% (95% CI: 0.992–1, 77%) for surgical mask with natural adjustment around the ears, 0.699% (CI: 0.0641–1.27%) for well-sealed surgical mask and 0.181% (95% CI: 0.106–0.256) for N95. At the source, the filtration of the N95 respirator averaged 77.06% (95% CI: 73.25–80.88%) compared to 37.03% (95% CI: 5.096–68.96%) for the mask well-sealed surgical mask, and 13.18% (95% CI: 7.476 18.89%) for the natural-fit surgical mask. Filtration at the receiver obtained an average of 92.28% (95% CI: 37.62– 146.9%) for N95, 48.83% (95% CI: 32.1–65.55%) for mask well-sealed surgical mask and 32.68% (95% CI: 22.36– 43.01%) for the natural-fit surgical mask | The adjustment and sealing of the masks significantly increased the protection effects of the source. A N95 respirator sealed at the receiver (better fit and seal) offered less protection when compared to any mask at the source. The control of the respiratory source can offer more protection to the health professional and potentially reduce the spread of aerosolized infections |
| Noti et al. 2012 (China) | Experimental laboratory study (study with mannequin) | Cough (Influenza A) | 2.5–4 μm | 7.56m2 (2.40 m in height)* |
The cough had a 4.2-L volume with a peak flow of 16.9 L/s and a mean flow of 5.28 L/s |
Flow rate of 32 L/min | ND | A surgical mask and N95 respirator (receiver) | 1.83 m* | The sealed seal of a surgical mask on the face blocked the entry of 94.5% of the total virus and 94.8% of the infectious virus (n = 3). A hermetically sealed N95 respirator blocked 99.8% of the total virus and 99.6% of the infectious virus (n = 3). An ill-fitting respirator blocked 64.5% of the total virus and 66.5% of the infectious virus (n = 3). A naturally adjusted surgical mask blocked 68.5% of the total virus and 56.6% of the infectious virus (n = 2) | The results indicate that a poorly fitted respirator performs no better than a loosely fitting mask |
| Patel et al. 2016 (United States of America) | Experimental laboratory study (study with mannequin) | Breathing and cough (no contamination) | ND | 3.04m2 (1.90, in height)* |
1.5-L breaths generated by the pump, with a peak flow of 5.2 L/sec |
tidal volume 500 mL, respiratory rate of 15 breaths/min, and duty cycle of 50% | 21.0–22.8 °C and 33–58% | Surgical mask and N95 respirator (source and/or receiver) | 0.91 m* | The data for tidal breathing, for the room without air flow, the mask at the source was statistically (p < 0.05) superior for the adjusted surgical mask and the N95 respirator with or without vaseline seal. The only mask to provide significantly different results at the receiver was the N95 vaseline seal. The differences between the types of mask were significant, indicating that the main protection mechanism was filtration. Similar findings were observed in a hospital room, that is, with better filtration, the exposure was reduced with N95 with or without sealing at the Source or Receptor. In general, the exposure was lower when the respirator was at the source. Compared to tidal breathing, there are major differences in the magnitude and mechanisms of exposure. In general, for all environments, the mask at the Source was superior to the mask at the Receiver. The results were relatively insensitive to capture efficiency, that is, compared to an N95 at the receiver, the natural-fit surgical mask at the source was as effective (no airflow and hospital room) or more effective (pressure room negative) | Control of the source through surgical masks can be an important auxiliary defense against the spread of respiratory infections. The adjustment of the mask or respirator, in combination with the airflow patterns in a given environment, contribute significantly to the effectiveness of the source control |
| Blachere et al. 2018 (United States of America) | Experimental laboratory study (study with mannequin) | Breathing and Cough (influenza) | 0.1–30 μm | 10.24m2 (2.3 m in height)* | The volume of the coughs was either 2.1 l (peak flow of 8.45 L/s and mean flow of 2.64 L/s) or 4.2 l (peak flow 16.9 L/s and mean flow 5.28 L/s) | The breathing waveform was sinusoidal with a flow rate of 32 L/min | Humidity 20–22% | A surgical mask and N95 respirator (receiver) | 1.83 m* | The total number of viruses (infectious and non-infectious) extracted by mask (surgical mask or N95) was 18.9%. The extraction efficiency of the surgical mask was lower (9.9% ± 8.2 SD) than for N95s (20.6% ± 14.8 SD) | The study demonstrates the retention of infectivity in contaminated surgical masks and N95 respirators, in addition to providing information on the risk of exposure to aerosol, as it suggests that the virus trapped on the outside of the masks may represent a risk of transmission through indirect contact, especially when used for a long period of time |
| Xu et al. 2017 (China) | Experimental laboratory study (study with humans) | Breathing (No contamination) | 1.5 µm | Varied within the study | N/A | N/A | N/A | N95 masks and surgical mask (source) | ND | The presence of 5 people without wearing masks increased the concentration of bioaerosol by 107% in 30 min. When using N95 masks or surgical masks, increases in bioaerosol were observed by 81% and 31%, respectively, less compared to those without a mask | Bioaerosols emitted from the breath of infected individuals can be pathogenic. The use of a respirator can prevent humans from releasing bioaerosols (especially those that are pathogenic) into the environment, and efficiency depends on the type of respiration. Because of the difference in facial adjustment, it seems that surgical masks are better than the N95 mask in terms of preventing humans from releasing bioaerosol into the environment |
| Lai et al. 2012 (China) | Experimental laboratory study (study with mannequin) | Sneeze (no contamination) | The peak concentration obtained was for particle sizes of about 35 nm with geometric standard deviation of 1.81 | 5.17m2 (2.30 m in height)* | The on/off electrically modulated valve controlled the sneezing duration, governed by the LabVIEW program at 0.5, 1 and 2 s. A manual electrically modulated valve was used to control the flow rate of sneezing | The maximum breathing flow rate was set to 15 l per minute. Each cycle lasted 4 s, i.e., 2 s for inhalation and 2 s for exhalation | ND | Surgical masks in different sealing conditions (receiver) | 0.3* to 0.6 m* | The fully sealed mask offered the most protection, providing almost 100% degree of protection, while the normally fitted mask offered the least protection. When an artificial leak of 4 mm was created, the degree of protection reduced to a minimum of 80%. The adaptation of the face mask was found to have a significant influence on the degree of protection (p < 0.05). The distance between the source and the receiver also played a significant role in determining the degree of protection, the greater the distance, the greater the degree of protection. The higher the emission speed, the lower the degree of protection observed. In addition, the longer the duration of the emission, the lower the degree of protection, due to increased exposure. The results showed that the degree of protection of the masks is more influenced by the adaptation of the masks (sealing) than by the other parameters of speed, distance and duration (p < 0.05). The p-value of the distance was the smallest (p < 0.05) compared to the speed and duration in three different scenarios of adaptation of the mask. The ventilation of the environment also demonstrated an influence on the degree of protection, especially in the case of greater separation distances between the source and the receiver and lower emission speeds | It was observed that fully sealed facemasks provide the highest protection, while the least protective was the normal wearing. It was also observed that the reduction of exposure decreases with increasing emission velocity and emission duration, and with decreasing separation distance between source and susceptible manikins. The current results have important implications for public health as wearing facemasks has become a common protection measure |
| He et al. 2013 (United States of America) | Experimental laboratory study (study with mannequin) | Breathing (no contamination) | ≤ 100 nm | 24.3m3 (description made only in cubic meters) | ND | Cyclic breathing flows with MIF rates of 15, 30, 55 and 85 L/min and breathing frequencies of 10, 15, 20, 25 and 30 breaths/min were examined | 17–22 ℃ and 30–60% | N95 filtering facepiece respirator and a surgical mask (receiver) | ND | The penetration of the N95 filter increased with the increase in the average inspiratory flow rate (p < 0.05). The effect of the respiratory rate on the penetration of the filter was complex and strongly dependent on the particle size and the average inspiratory flow rate. As expected, the N95 respirator had a filter penetration below 5% for any particle size, respiratory rate and average inspiratory flow rate. The Total Inward Leakage of N95 increased as the particle size increased, regardless of the average inspiratory flow rate and respiratory rate (p < 0.05). Compared to the N95, the surgical mask had much greater penetration (p < 0.05), and may not offer the filter efficiency expected at higher respiratory flows. The total penetration of particles in the surgical mask was about 10 times greater than the penetration in N95. The data suggest that the tested surgical mask may not be able to provide substantial protection against the aerosol particle range up to ~ 500 nm, in any relevant combination of respiratory rate and flow rate | For the N95 FFR and SM tested, filter penetration was significantly affected by particle size and respiratory flow rate, while the effect of respiratory rate on filter penetration was generally less pronounced and less important from a practical point of view, especially for lower mean inspiratory flow rates. For N95 and surgical mask, total penetration increased with increasing particle size. The surgical mask produced much higher values of filter penetration than the N95. The results suggest that the surgical mask may not be able to provide substantial protection against aerosol particles up to ~ 500 nm |
| Drewry III et al. 2018 (Unites States of America) | Experimental laboratory study (study with cough simulator and humans) | Cough (Influenza) | < 5 nm | ND | 5 L/min | N/A | ND | ND | The coughing device was placed near the patient (did not specify distance) | During patient care, potentially infectious particles are introduced into the exchange area, possibly due to new aerosolization during the PPE exchange process or by opening the room door. The number of particles and the amount of new aerosolization depend in part on the activities of the healthcare professional and the patient before the exchange, and on the exchange procedures themselves. This information can inform changes in protocols that can be tested empirically to further minimize the health professional's risk of exposure | These preliminary data and this method will increase healthcare worker and healthcare system safety, mitigate healthcare worker fears about the risk of treating patients with highly infectious diseases, and raise the overall quality of care for patients in high-containment environments |
| Diaz and Smaldone 2010 (United States of America) | Experimental laboratory study (study with mannequin) | Breathing (no contamination) |
95% of the particles were less than 2 μm, with an average of 1.046 mm (95% CI: 0.984–1.11) |
2.08m2 (1.83 m in height)* | 10 L/min |
Respiratory rate of 15 breaths/min, and duty cycle of 0.5 |
20 °C to 22 °C and 21% to 26% | NIOSH approved N95 respirator and an ear loop surgical mask (source and/or receiver) | 0.91 m* | In the presence of chamber air exchange, applying a mask on the source (primarily deflection) resulted in significant reduction (P < 0.05) in exposure to the receiver (sWPF170-320). Applying either a surgical mask or N95 respirator at the source resulted in significant reductions in exposure and corresponding simulated workplace protection factor of 172 to 317. Applying either surgical mask or respirator to the receiver (without a perfect seal) did not significantly reduce exposure from that of no masks (simulated workplace protection factor of 1.37–2.21). At the source, N95 resulted in significantly greater filtration averaging 35.7% (95% CI: 27.7–43.7) in comparison with surgical mask tightly fit 14.8% (95% CI: 10.1–19.6) and surgical mask loosely fit 6.07% (95% CI: 5.43 6.72). However, significantly greater filtration with the N95 did not result in a significant reduction in exposure compared with surgical mask tightly fit or surgical mask loosely fit, suggesting deflection was the dominant factor | Mask filtration, applied either at the source or the receiver, does not play a significant role in reducing exposure to the recipient unless a respirator is physically sealed to the face of the source. Deflection of exhaled particles, such as can be achieved with a surgical mask worn at the source, achieves far greater levels of protection than an N95 respirator on the recipient |
*Transformation of measures and calculation made by the researcher
ND not described
N/A not applicable
The types of masks evaluated in the studies were surgical masks, N95 respirators, face shields, and the combination of an N95 respirator and a surgical mask.
The bioaerosols evaluated in the included studies came from breathing, coughing, and sneezing, but no study evaluated speech-generated aerosols. Most studies used uncontaminated aerosol as the suspension to be dispersed, five studies evaluated influenza virus and only one study used MERS-CoV. The size of the evaluated particles ranged from 0.1 to > 200 µm. The distance from the aerosol-generating source to the receiver ranged from 0.3 to 2 m. The full description of the included studies is available in Table 1.
Risk of bias within the studies
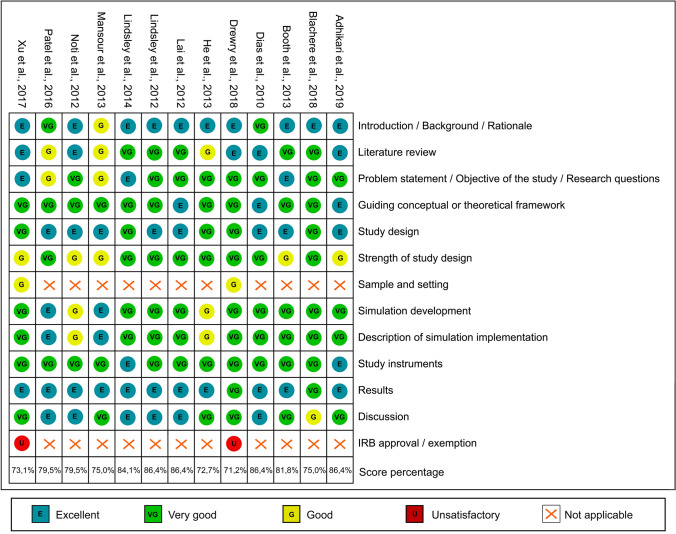
Of the 13 articles included, 5 were classified as having a moderate risk of bias, and 8 studies were classified as having a low risk of bias. None of the included studies had a score of < 70% in the evaluation (Fig. 2).
Fig. 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Results of individual studies
The only study that evaluated the contaminated aerosol from a virus of the Coronaviridae family was carried out by Adhikari et al. 2019. They predicted the risk of transmission of MERS-CoV in a closed environment where there was movement of several people (healthcare professionals and visitors) in front of an infected patient and observed that an increase in the ventilation rate (air exchange) was an effective measure to reduce the risk for individuals present in the same environment but not for groups exposed to an air route of a short distance. Thus, the N95 respirator was indispensable for reducing the risk of transmission over short distances (Adhikari et al. 2019).
In an environment of 27 m3, the presence of 5 people without a mask increased the concentration of bioaerosol by 107% within 30 min, and this increase was dependent on the duration people stayed in the environment. However, in this same scenario (environment of 27 m3 occupied by five people), when using an N95 respirator or a surgical mask, the level of bioaerosol increased only 81% and 31%, respectively. Although N95 respirators promote greater air filtration, surgical masks appeared to be more effective in decreasing the release of bioaerosol, a difference mainly due to the adjustment and sealing of the mask to the source’s face (Xu et al. 2017). The filtering level of the mask, whether used by the source or the receiver, did not play a significant role in reducing exposure, unless the PPE was physically well sealed on the face of the source (Diaz and Smaldone 2010). The greater the adjustment and sealing of the mask to the face, the greater the degree of protection that was provided (Lai et al. 2012), mainly concerning the fit of the mask on the aerosol-generating source (Mansour and Smaldone 2013). Surgical masks and ill-fitting N95 respirators are not effective in filtering aerosols contaminated by infectious viruses (Noti et al. 2012).
Another result to be highlighted is that the use of a surgical mask by the aerosol-generating source during breathing reached a level of protection in the receiver much higher than that of an N95 respirator (Diaz and Smaldone 2010; Mansour and Smaldone 2013). The N95 respirator had a filtration level of approximately 95% of the aerosol, but the reduction in exposure by the mask control at its source was significantly greater than the N95 filtering capacity in reducing exposure (Mansour and Smaldone 2013). On the other hand, Patel et al. 2016, observed that the biggest difference with the control of the source with the use of a mask or respirator (even unsealed) was in the aerosol generated by coughing, but when the aerosol was generated by breathing, source control was comparable to or greater than the mask or respirator protection on the receiver (Patel et al. 2016).
The risk of contamination in closed environments was related both to the direct transmission between the aerosol and the receiver and to the indirect transmission through contact with contaminated PPEs, which is a source of infectivity retention in environments with the presence of a contaminated source (Blachere et al. 2018). In the study by Drewry et al. 2018, it was observed that transmission can occur not only near the source of the infected aerosol but also during the disposal of PPE (Drewry et al. 2018).
The aerosol generated by coughing can disperse in a plume capable of moving through a closed environment and expose people present in the environment to a highly concentrated aerosol; however, after a few minutes, a wide range of aerosol particle sizes disperse, reaching everyone in the environment (Lindsley et al. 2012). For both surgical masks and N95 respirators, the penetration of the aerosol into the filter was significantly affected by the size of the inhaled particle and the respiratory flow (He et al. 2013). Well-sealed N95 respirators provided good protection for the cough aerosol with particles of all sizes; on the other hand, surgical masks did not provide adequate protection against small particles present in cough aerosols, even when well-sealed (Lindsley et al. 2012), not being able to provide substantial protection against aerosol particles up to approximately 500 nm in size, in any combination of flow and respiratory rate (He et al. 2013).
The only study that evaluated the effectiveness of the face shield against exposure to a cough aerosol in a closed environment was carried out by Lindsley et al. 2014 (Lindsley et al. 2014). It was observed that the use of the face shield substantially reduced the exposure to the contaminated aerosol (96% reduction in the period immediately after the cough) and protected the respirator PPE used (97% protection). However, face shields were less effective against smaller particles that can remain in the air for long periods and can disperse around the face shield to be inhaled. Thus, the face shield is useful as a complement; however, it should not be used as a substitute for respiratory protection.
No study has evaluated the effectiveness of using cloth masks in closed environments.
It was not possible to quantify the risk of publication bias. However, a database with other languages, such as Portuguese and Spanish, was used (Lilacs), in addition to a broader search in the gray literature, to reduce the probability of this error occurring.
Discussion
Social distancing and hand hygiene have been the main WHO recommendations to prevent the spread of SARS-CoV-2; however, these measures do not prevent infection from inhaling small droplets exhaled by an infected person that can travel a distance of dozens of meters, which is a significant route of transmission indoors (Morawska and Cao 2020). It is known that SARS-CoV-2 is transmitted through contaminated fluids from the upper airways of infected people, with or without symptoms, and can spread through close contact (World Health Organization 2020a; b). The WHO suggests the use of PPE as one of the measures to prevent the spread of the disease (World Health Organization 2020a, b); however, the types of PPE are still widely discussed in terms of their effectiveness due to the wide variety of materials offered and the inefficient sealing to the face. Therefore, this systematic review proposed to address the effectiveness of masks/respirators (homemade, surgical, N95, FFP, FFP2, FFP3) or eye or facial protection, against the bioaerosol generated by individuals contaminated by infectious diseases (symptomatic or asymptomatic) in closed environments, such as the waiting rooms or exam rooms of healthcare facilities, and to extrapolate these results to the current COVID-19 pandemic. The results of the qualitative synthesis of the present review demonstrate that PPE is effective in these situations; however, the results are influenced by ventilation, number of people and length of stay in the environment, the distance between the source and the receiver and the level of filtering and sealing of the mask or respirator.
SARS-CoV-2 can remain viable and infectious in aerosols for hours and on surfaces for days (van Doremalen et al. 2020) and is closely related phylogenetically to its predecessor, SARS-CoV-1 (89% similarity) (Jalava 2020). Due to its similarity, the hypothesis that SARS-CoV-2 also spreads through the air is strengthened (Morawska and Cao 2020). Normal expiratory activities can result in aerosol generation with an 80–90% distribution of particle size < 1 µm (Morawska 2009). Expiratory droplets can carry pathogens and thus transmit diseases through the air and can be easily inhaled even after being generated (Leung et al. 2020; Lindsley et al. 2014). The results of the present review indicate that in these situations, increasing the ventilation of the environment is considered an effective measure to reduce the risk of exposure to pathogens present in contaminated bioaerosols, but it is not effective for groups exposed to a short-distance air route (Adhikari et al. 2019). Thus, in busy environments such as waiting rooms for health services, even if well ventilated, the use of masks is essential to reduce the risk of transmission.
The results of the present review indicate that the use of a surgical mask by the source of the aerosol reaches a higher level of protection than the use of the N95 respirator by the receiver (Diaz and Smaldone 2010; Mansour and Smaldone 2013; Patel et al 2016). These data suggest that traditional surgical masks are useful in preventing the transmission of respiratory diseases when applied at the source of the infected aerosol, significantly reducing the exposure of pathogens, functioning as an inhalation barrier; however, in regard to respiratory protection equipment, there are still doubts about which is the best type to be used for this purpose (Patel et al. 2016). In an environment of 27 m3 occupied by five people, although the N95 respirator promotes greater filtration, surgical masks seemed to be more effective in reducing the release of bioaerosol, a difference mainly due to the adjustment and sealing of the mask to the face of the source (Xu et al. 2017). The contribution of asymptomatic exposure to SARS-CoV-2 cases can play an important role in transmission, even in individuals who are not coughing or sneezing (Anderson et al. 2020), representing a challenge in infection control (Al-Tawfiq 2020). Because of this, during the current pandemic, the use of masks with less filtering power by all people present in the environment is more effective in reducing the risk of exposure than the use of respirators with high filtering power for only a portion of the people in the environment.
Although the mechanism for SAR-CoV-2 transmission through the eyes is not clearly understood, eye protection is recommended not only for all healthcare professionals but also for people in the risk group (Napoli et al. 2020). The qualitative synthesis of this review points to a greater risk of transmission over short distances, in which case the use of masks is essential. Face shields seem to substantially reduce exposure to a contaminated aerosol; however, they are less effective when exposed to smaller particles, so they are useful as a complement to masks and respirators (Lindsley et al. 2014). Given these results, it is indicated that health professionals working in close contact and people in the high-risk group use face shields as a way to complement the protection against a contaminated bioaerosol, not to eliminate the need to use a mask/respirator.
It is known that the size, speed, shape, and physical properties of the particles are factors taken into account when making masks. Even if the chosen material offers a good aerosol barrier, if it does not have a good seal on the face, then its use would not provide much protection to the individual (Davies et al. 2013). The N95 respirator showed greater filtering power, with a filtration level of approximately 95% of the aerosol particles; on the other hand, surgical masks did not provide adequate protection against small particles (Lindsley et al. 2012; Mansour and Smaldone 2013). The filtering level of the mask, whether used by the source or receiver, plays a significant role in reducing exposure only if the mask is well sealed (Diaz and Smaldone 2010). Based on these results, the provision of information sources (whether printed or digital) to patients regarding the best adjustments of the mask to the face and the importance of sealing the mask is a measure that can be taken to increase the effectiveness of this equipment in closed environments.
Despite the robustness of this review generated by the qualitative synthesis of 13 articles, some limitations should be noted. Most of these studies are experimental simulations using mannequins; therefore, the data should be viewed with caution, as their results are extrapolation to what would happen to humans. Additionally, the methodological heterogeneity of the studies prevented a quantitative analysis on the topic. It is suggested that further studies in humans be carried out, with control of the confounding variables (ventilation, number of people and length of stay in the environment, the distance between the source and the receiver, level of filtering and sealing of the mask or respirator, temperature, and humidity) to generate even more robust scientific evidence. On the other hand, the data in this review provide important information for the control of transmission, in the midst of the COVID-19 pandemic, in addition to the possibility of extrapolating these findings to any closed environment.
Conclusion
The use of mask in closed environments is effective in reducing the risk of transmission and contagion of a contaminated bioaerosol when the mask is well sealed, with greater effectiveness when these types of equipment are used by the source and receiver of the bioaerosol, regardless of the power of filtration of the equipment. Ventilation of the environment can also be performed as an auxiliary measure to reduce the risk; however, ventilation is not effective for preventing the spread of aerosols over a short distance.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Database search strategy (DOC 50 KB)
Supplementary file2 Excluded articles and reasons for exclusion (n = 22) (DOCX 19 KB)
Author contributions
CA, OG, FG, contributed to conception, design, data acquisition, drafted and critically revised the manuscript; AG and BL, contributed to design, data analysis, drafted and critically revised the manuscript; IB, contributed to design, data acquisition, drafted and critically revised manuscript; JS, contributed to conception, data interpretation, drafted and critically revised manuscript; GR, contributed to conception, data analysis, drafted and critically revised manuscript; BZ, contributed to design, data analysis and interpretation, drafted and critically revised manuscript; RS, contributed to conception and design, data acquisition, analysis and interpretation, drafted and critically revised manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Funding
None.
Declarations
Conflict of interest
None to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cristiano Miranda de Araujo, Email: cristiano.araujo@utp.br.
Odilon Guariza-Filho, Email: odilongfilho@gmail.com.
Flavio Magno Gonçalves, Email: flaviomagno93@yahoo.com.br.
Isabela Bittencourt Basso, Email: isabelabbasso@hotmail.com.
Angela Graciela Deliga Schroder, Email: dra.angela@digitalface.com.br.
Bianca L. Cavalcante-Leão, Email: bianca.leao@utp.br
Glória Cortz Ravazzi, Email: gcortz87@hotmail.com.
Bianca Simone Zeigelboim, Email: bianca.zeigelboim@utp.br.
José Stechman-Neto, Email: stechman1@gmail.com.
Rosane Sampaio Santos, Email: rosane.santos2@utp.br.
References
- Adhikari U, Chabrelie A, Weir M, Boehnke K, McKenzie E, Ikner L, Wang M, Wang Q, Young K, Haas CN, Rose J, Mitchell J. A case study evaluating the risk of infection from middle eastern respiratory syndrome coronavirus (MERS-CoV) in a hospital setting through bioaerosols. Risk Anal. 2019;39:2608–2624. doi: 10.1111/risa.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA. Asymptomatic coronavirus infection: MERS-CoV and SARS CoV-2 (COVID-19) Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EL, Turnham P, Griffin JR, Clarke CC. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40:902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020 doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachere FM, Lindsley WG, McMillen CM, Beezhold DH, Fisher EM, Shaffer RE, Noti JD. Assessment of influenza virus exposure and recovery from contaminated surgical masks and N95 respirators. J Virol Methods. 2018;260:98–106. doi: 10.1016/j.jviromet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Clayton M, Crook B, Gawn JM. Effectiveness of surgical masks against influenza bioaerosols. J Hosp Infect. 2013;84(1):22–26. doi: 10.1016/j.jhin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Cegolon L. Investigating hypothiocyanite against SARS-CoV-2. Int J Hyg Environ Health. 2020;227:113520. doi: 10.1016/j.ijheh.2020.113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for disease control and prevention (2016) Prevention strategies for seasonal influenza in healthcare settings. Guidelines and Recommendations. CDCP. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed 28 May 2020
- Chen YC, Huang LM, Chan CC, Su CP, Chang SC, Chang YY, Chen ML, Hung CC, Chen WJ, Lin FY, Lee YT, National Taiwan University, H SARS in hospital emergency room. Emerg Infect Dis. 2004;10:782–788. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz KT, Smaldone GC. Quantifying exposure risk: surgical masks and respirators. Am J Infect Control. 2010;38:501–508. doi: 10.1016/j.ajic.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Drewry DG, 3rd, Sauer LM, Shaw-Saliba K, Therkorn J, Rainwater-Lovett K, Pilholski T, Garibaldi BT. Identifying potential provider and environmental contamination on a clinical biocontainment unit using aerosolized pathogen simulants. Health Secur. 2018;16:83–91. doi: 10.1089/hs.2017.0064. [DOI] [PubMed] [Google Scholar]
- Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Jandaghi NZS, Sadeghniiat K, Nabizadeh R, Yunesian M, Momeniha F, Mokamel A, Hassanvand MS, MokhtariAzad T. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Reponen T, McKay RT, Grinshpun SA. Effect of particle size on the performance of an N95 filtering facepiece respirator and a surgical mask at various breathing conditions. Aerosol Sci Technol. 2013;47:1180–1187. doi: 10.1080/02786826.2013.829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalava K. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. 2020;226:113490. doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AC, Poon CK, Cheung AC. Effectiveness of facemasks to reduce exposure hazards for airborne infections among general populations. J R Soc Interface. 2012;9:938–948. doi: 10.1098/rsif.2011.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EL, Liverman CT (eds) (2011) Institute of medicine (US) committee on personal protective equipment for healthcare personnel to prevent transmission of pandemic influenza and other viral respiratory infections: Current research issues. Preventing transmission of pandemic influenza and other viral respiratory diseases: Personal protective equipment for healthcare personnel: Update 2010. Washington (DC): National Academies Press (US), PMID: 24983058 [PubMed]
- Leung NHL, Chu DKW, Shiu EYC, Chan KH, McDevitt JJ, Hau BJP, Yen HL, Li Y, Ip DKM, Peiris JSM, Seto WH, Leung GM, Milton DK, Cowling BJ. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, King WP, Thewlis RE, Reynolds JS, Panday K, Cao G, Szalajda JV. Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room. J Occup Environ Hyg. 2012;9:681–690. doi: 10.1080/15459624.2012.725986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11:509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M, Dafoe N, Mahony J, John M, Sarabia A, Glavin V, Webby R, Smieja M, Earn DJ, Chong S, Webb A, Walter SD. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- Long Y, Hu T, Liu L, Chen R, Guo Q, Yang L, Cheng Y, Huang J, Du L. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. 2020 doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour MM, Smaldone GC. Respiratory source control versus receiver protection: impact of facemask fit. J Aerosol Med Pulm Drug Deliv. 2013;26:131–137. doi: 10.1089/jamp.2012.0998. [DOI] [PubMed] [Google Scholar]
- Moher DA, JenniferAltman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2009;62:7. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Morawska L, Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska LJG, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40(43):256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [Google Scholar]
- Napoli PE, Nioi M, d'Aloja E, Fossarello M. The ocular surface and the coronavirus disease 2019: does a dual ‘ocular route’ exist? J Clin Med. 2020 doi: 10.3390/jcm9051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti JD, Lindsley WG, Blachere FM, Cao G, Kashon ML, Thewlis RE, McMillen CM, King WP, Szalajda JV, Beezhold DH. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis. 2012;54:1569–1577. doi: 10.1093/cid/cis237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RB, Skaria SD, Mansour MM, Smaldone GC. Respiratory source control using a surgical mask: an in vitro study. J Occup Environ Hyg. 2016;13:569–576. doi: 10.1080/15459624.2015.1043050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ. 2016;188:567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, Tikka C, Ruotsalainen JH, Kilinc Balci FS. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2020;4:CD011621. doi: 10.1002/14651858.CD011621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, e.a., (2020) Modes of transmission of virus causing COVID 19: implications for IPC precaution recommendations. WHO Scientific brief. Available at: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed 28 May 2020
- World Health Organization (2020) Advice on the use of masks in the context of COVID-19: interim guidance; WHO. Available at: https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak. Accessed 28 May 2020
- Xu C, Wu C-Y, Yao M. Fluorescent bioaerosol particles resulting from human occupancy with and without respirators. Aerosol Air Quality Res. 2017;17:198–208. doi: 10.4209/aaqr.2016.09.0400. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Database search strategy (DOC 50 KB)
Supplementary file2 Excluded articles and reasons for exclusion (n = 22) (DOCX 19 KB)