Abstract
A subdural hematoma (SDH) is a type of bleeding in which a collection of blood gathers between the inner layer of the dura mater and the arachnoid mater of the meninges surrounding the brain. Although most cases reported of subdural hematoma are due to traumatic brain injury, to the best of our knowledge this is a rare case of nontraumatic subdural hematoma. A 31 year-old-Male presented to the emergency department with a severe headache for 3 weeks, with the presence of oculomotor disorders. There is no history of major trauma, minor trauma or fights. Also, no history of hypertension, or Haematological diseases. Magnetic resonance imaging (MRI) showed bilateral subdural hematoma. The right-sided hematoma was treated conservatively and the Left-sided was treated by surgery. Follow-up for three months revealed no recurrence. Nontraumatic subdural hematoma is one of the challenging cases that neurosurgeons face. We presented a patient with idiopathic spontaneous subdural hematoma. Computed tomography (CT) is the first step for diagnosis and Magnetic resonance imaging (MRI) is considered an excellent imaging investigation to evaluate such patients. Symptomatic subdural hematomas require an emergent treatment by identifying and controlling sites of bleeding conservatively or by surgery. Subdural hematoma with no history of trauma should be treated emergently and evaluated strictly. Follow-up is essential in patients with neural symptoms. Massive symptomatic subdural hematoma (SDH) should be treated with surgery to control the bleeding.
Keywords: Subdural hematoma, Bilateral hematoma, Nontraumatic haemorrhage, A case report
Highlights
-
•
A subdural hematoma (SDH) is a collection of blood between the dura and the arachnoid membranes.
-
•
There are many causes of SDH like trauma, hematological diseases and hypertension.
-
•
Bilateral nontraumatic subdural hematoma is an extremely rare.
-
•
MRV and MRI and Computed tomography confirm the diagnosis.
1. Introduction and importance
A subdural hematoma (SDH) refers to a collection of blood between the dura and the arachnoid membranes. SDH is classified into three types: acute, sub-acute, and chronic. Acute SDH is usually caused by head injury [1].
A subdural hematoma can have various causes, including trauma, arteriovenous malformation, and the use of anticoagulation medications [2]. In rare cases, it can spontaneously occur in the absence of any pathology [3,4]., subdural haemorrhage can present with neurological signs similar to Brown-Séquard syndrome [5].
The identification of SDH has improved with the availability of different types of imaging and symptoms studies [6].
Magnetic resonance imaging (MRI) provides a relatively definitive diagnosis [7] with computed tomography and magnetic resonance venography.
If the neurological symptoms grow progressively debilitating with time, emergency surgery needs to be considered.
However, due to the consequence of bilateral SDH in our case, the right side was treated conservatively and the left side was treated by surgery considering the neurological symptoms and the imaging findings.
This case report has been reported in line with the SCARE criteria [8].
2. Case presentation
A 31-year-old male with a clear medical background was brought to the emergency department complaining of headaches for 3 weeks, with the presence of oculomotor disorders. The headache was severe, aggravated by laying down and slightly relieved by sitting and analgesia. The patient has no history of trauma. There is no history of projectile vomiting, convulsions, decreased mental abilities, weakness, or loss of consciousness. There is no history of any symptoms suggesting bleeding disorders, including easy bruising, heavy bleeding from small cuts, unexplained nosebleeds, or any heavy bleeding from any other sites of his body. There is no history of Fever or loss of appetite.
The systemic review was unremarkable. The patient is not diabetic, hypertensive, or asthmatic. The patient is not on any current medications. There is no history of a similar condition or previous hospitalization. There is no family history of a similar condition or any blood-related disorders. The patient is a smoker (less than 10 cigarettes per day) but quit one year ago. He is not an alcoholic, or in use of illicit drugs.
On presentation, the patient looked unwell. Not pale, jaundiced, or cyanosed. Vitally stable. Glasgow coma scale was 15/15. pupils were equally reactive bilaterally. The examination confirmed diplopia on his left eye. On the neurological assessment, the power is grade 5 in all limbs, and all muscle groups. The tone is normal in all limbs and all muscle groups. The reflexes were normal, and there was no sensory loss. Examination of cranial nerves was normal. Other systemic examinations revealed no abnormality.
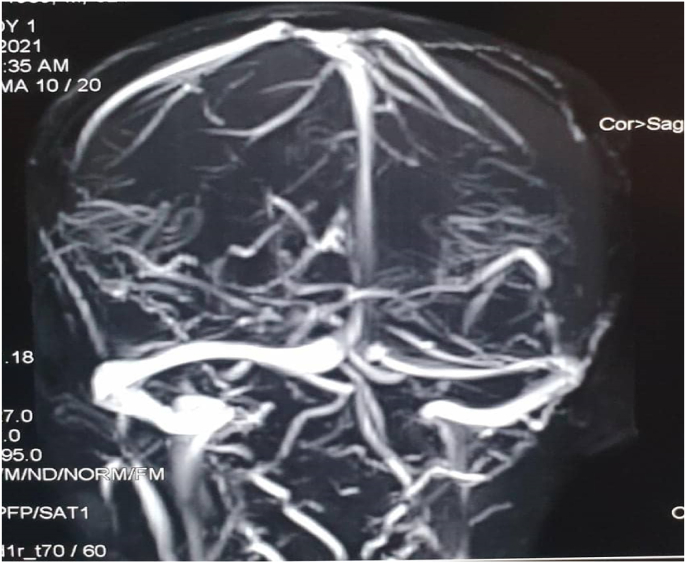
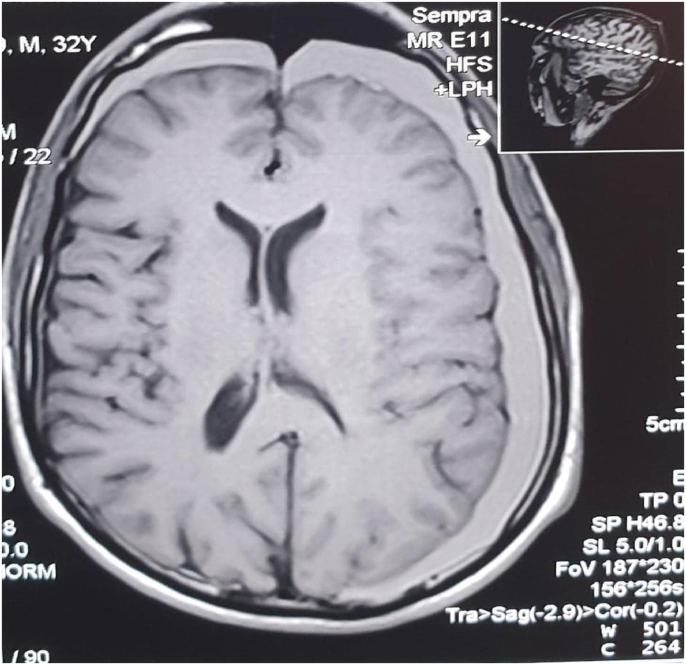
Regarding the investigations of this patient, Complete blood count values were within normal ranges. Platelets function tests were normal. Bleeding profile values are the following:[prothrombin time (PT) is 15s, and international normalized ratio (INR) is 1.2 (The patient is not on blood thinners or hormonal therapy). protien C is within normal concentration and activity. liver Function Test (LFT) is normal. All other routine investigations were normal. Computed tomography (CT), Magnetic resonance imaging (MRI), and magnetic resonance venography(MRV) for the brain were ordered for further investigations. Computed tomography was not available at the hospital for technical difficulties at the time of request; Therefore magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) was done directly. The magnetic resonance imaging MRI shows left fronto-tempro-parieto-occipital crescent shape subdural lesion of high signal in T1 and T2W1 it measures about (2 cm) in its maximum depth at the occipital region (Fig. 1). It is associated with mild mass effect and midline shift There is another similar right-sided lesion seen in the frontal region that measures about (1cm) in its maximum depth. The cerebrospinal fluid space is consistent with age. There is no space-occupying lesion, free cerebral palsy angles, normal brain stem, and cerebellum, and the rest is unremarkable. The magnetic resonance venography shows normal dural and venous sinuses (Fig. 2). There are no feeling defects.
Fig. 1.
Magnetic resonance imaging shows bilateral subdural hematoma.
Fig. 2.
Magnetic resonance venography pre-operative.
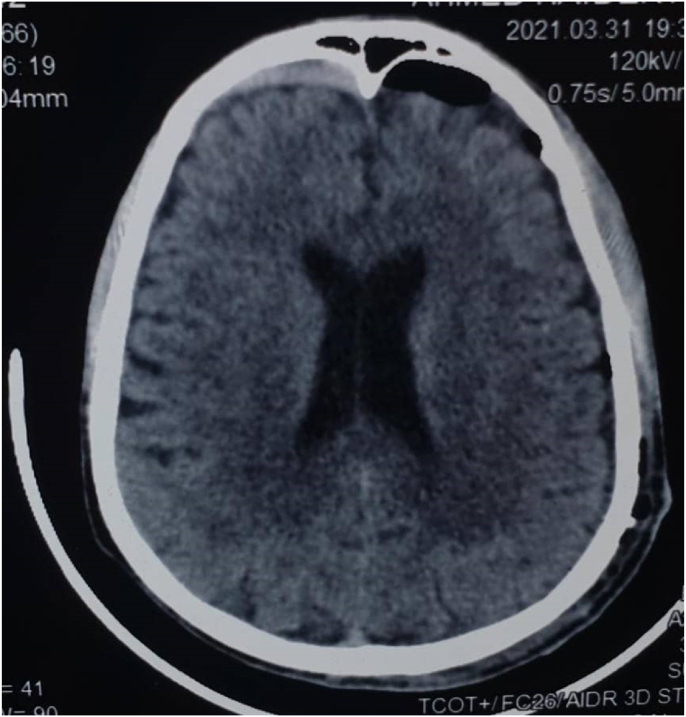
After subdural hematoma is diagnosed and confirmed, the decision is to be made whether the patient is for conservative treatment or surgical intervention. The indications for surgical intervention are the following: The patient is experiencing symptoms, or/and Midline shift more than 5 mm, or/and thickness more than 1 cm on imaging. Therefore patient was planned for surgical intervention (Burr holes evacuation) for the left-sided subdural hematoma; as it satisfies the criteria for surgical intervention. The patient was operated on the 30th of March 2021. The operation was performed by two neurosurgeons; A specialist and a senior resident in the presence of a senior anaesthesia resident and a scrub nurse. The operation took 90 minutes. The patient received general anaesthesia and the operation went smoothly, with no intra-operative complications. A drain was placed and connected to a collecting system. There were no post-operative complications. The patient did not need ICU (intensive care unit) admission. Post-operatively, the patient was managed by analgesia, antibiotics (Samixone), dexamethasone, and pantoprazole. The drain was removed after 3 days from the operation after achieving 50 ml in 24 hours (350ml in total). The right-sided subdural hematoma was planned to be treated conservatively; as it was very small, and the mass the left-sided hematoma was the reason of the patient's symptoms, and the findings on the MRI image. The patient was discharged a few days later in good condition. A follow-up period of 3 months revealed no reoccurrence. The post-operative computed tomography scan shows a Burr hole noted on the left side, there is a left-sided pneumocephalus with minimal residual SDH (Fig. 3). The pneumocephalus is small, and considered a usual post-operative finding that doesn't need active management. No significant mass effect or midline shift. Normal brain parenchyma. The previous lesion right frontal SDH is not with significant effect. The rest is unremarkable.
Fig. 3.
Computed tomography post-operative.
3. Clinical discussion
Spontaneous subdural hematoma is an extremely rare case, especially in healthy young patients. A previous literature review found only 22 cases of acute spontaneous subdural hematoma in patients younger than 40 years old [9]. The first case was reported by Munro in 1943 [10].
The clinical presentation mainly depends on the location of the lesion and the rate at which it develops.
Symptoms of chronic subdural hematoma are often insidious including headaches, decreased level of consciousness, difficulty with gait or balance, cognitive dysfunction or memory loss, personality change, and motor weakness. However in this case the patient had had severe headaches and double vision.
Risk factors of spontaneous subdural hematoma are hypertension, vascular abnormalities such as arteriovenous malformation (AVM) and aneurysm, neoplasm specially meningioma, infections including meningitis and tuberculosis, substance abuse (alcoholism and cocaine), and coagulopathies [11].
The diagnosis is established by CT-Scan. Further radiological imaging, laboratory, or investigations such as MRI, MRV, Angiography, and coagulation profile may help to reveal the underlying cause of spontaneous SDH.
The treatment of spontaneous subdural hematoma is similar to that of subdural hematoma caused by trauma, but the underlying cause must be if possible sought and treated. Symptomatic and/or > 1cm thick SDH, surgical evacuation is the treatment of choice. For subacute to a chronic subdural hematoma, burr-holes evacuation is usually adequate. For acute SDH, a craniotomy is usually required and should expose the Sylvian fissure to identify the bleeding point.
In this case, the left-sided subdural hematoma was evacuated by burr hole trephination and the right-sided was treated conservatively.
Follow-up is very important to detect any bleeding after surgical repair or any of the complications of surgery. In our case After 3 months follow up the patient had no symptoms and there was no reoccurrence.
4. Conclusion
Nontraumatic subdural hematoma is a rare entity that presents gradually progressive neurological symptoms with an emphasis on the absence of any previous pathological or traumatic precedents, and this is something special in our case. A patient who has a subdural hematoma in the brain should be followed up. Once the diagnosis is confirmed, SDH must be evacuated throughout craniotomy, burr hole trephination or treated conservatively.
Ethical approval
This case reports didn't require review by Ethics committee, The National Ribat University hospital/Ribat/Sudan.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Tasneem Mohamed: contributed in study concept and design, data collection, and writing the paper.
Sarya Swed: contributed in data interpretation and writing the paper.
Ahmad Al-Mouakeh:contributed in data interpretation and writing the paper.
Bisher Sawaf:contributed in reviewing the paper.
Consent for publication
Written informed consent was obtained from the patient for publication of these two case reports and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Registration of research studies
Not applicable.
Guarantor
Sarya Swed.
Declaration of competing interest
All authors declared no conflict of interest.
Acknowledgement
We would like to say thanks to Mr. Ahmed Nabhan for his great efforts. Mr. Nabhan saved neither time nor effort in editing the English language of this article by checking the spelling, grammar, and syntax.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102907.
Contributor Information
Tasneem Mohamed, Email: Tas.mohamed95@gmail.com.
Sarya Swed, Email: saryaswed1@gmail.com.
Ahmad Al-Mouakeh, Email: Dr.ahmad.mouakeh97@gmail.com.
Bisher Sawaf, Email: bishersawaf.94@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Miller J.D., Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J. Neurosurg. 2014;120(6):1378–1384. doi: 10.3171/2013.10.JNS13272. [PubMed] [CrossRef] [Google Scholar] [Ref list] [DOI] [PubMed] [Google Scholar]
- 2.De Beer M.H., Eysink Smeets M.M., Koppen H. Spontaneous spinal subdural hematoma. Neurol. 2017;22(1):34–39. doi: 10.1097/NRL.0000000000000100. [PubMed] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto H., Miki T., Miyaji Y. Spontaneous spinal epidural hematoma with hemiparesis mimicking acute cerebral infarction: two case reports. J Spinal Cord Med. 2012;35(4):262–266. doi: 10.1179/2045772312Y.0000000014. ([PMC free article] [PubMed] [Google Scholar]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyriakides A.E., Lalam R.K., El Masry W.S. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine (Phila Pa 1976. 2007;32(21):E619–E622. doi: 10.1097/BRS.0b013e318154c618. ([PubMed] [Google Scholar]) [DOI] [PubMed] [Google Scholar]
- 5.Rascón-Ramírez F., Avecillas-Chasín J.M., Trondin A., Arredondo M.J. Brown-Séquard syndrome and cervical post-traumatic subarachnoid hematoma. Neurocirugia. 2018;29(4):209–212. doi: 10.1016/j.neucir.2017.09.002. [PubMed] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 6.de Beer M.H., Eysink Smeets M.M., Koppen H. Spontaneous spinal subdural hematoma. Neurol. 2017;22:34–39. doi: 10.1097/NRL.0000000000000100. [PubMed] [Google Scholar] [Ref list] [DOI] [PubMed] [Google Scholar]
- 7.Kreppel D., Antoniadis G., Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg. Rev. 2003;26:1–49. doi: 10.1007/s10143-002-0224-y. [PubMed] [Google Scholar] [Ref list] [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Coombs J.B., Coombs B.L., Chin E.J. Acute spontaneous subdural hematoma in a middle-aged adult: case report and review of the literature. J. Emerg. Med. 2014 Sep;47(3):e63–e68. doi: 10.1016/j.jemermed.2014.04.026. Epub 2014 Jun 7. PMID: 24915743. [DOI] [PubMed] [Google Scholar]
- 10.Munro D. The diagnosis and treatment of subdural hematoma. A report of sixty-two cases. N. Engl. J. Med. 1934;210:1145–1160. [Google Scholar]
- 11.Hesselbrock R., Sawaya R. Means ED. Acute spontaneous subdural hematoma. Surg. Neurol. 1984;21:363–366. doi: 10.1016/0090-3019(84)90115-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.