Abstract
Introduction
Coccidioidomycosis is a fungal infection endemic in the southwestern United States (US). Primary pulmonary coccidioidomycosis (PPC) is a leading cause of community-acquired pneumonia (CAP) in this region, although its diagnosis is often delayed, leading to lag in antifungal treatment and subsequent morbidity. The impact of early empiric antifungal therapy as part of treatment for CAP in endemic areas on clinical outcomes is unknown.
Methods
Phase IV randomized, double-blind, placebo-controlled trial in individuals aged 18 years or older with CAP who met all eligibility criteria in Coccidioides endemic regions in the US. Eligible participants with CAP were randomized to receive either fluconazole (400 mg daily) or matching placebo for 42 days and were subsequently monitored for clinical resolution of their illness.
Objectives
The primary objective was to assess the clinical response of early empiric antifungal therapy with fluconazole through Day 22 in subjects with PPC who were adherent to the study intervention. Secondary objectives included: assessments of the impact of early empiric antifungal therapy with fluconazole through Day 22 and 43 in subjects with PPC regardless of adherence, comparisons of the clinical response and its individual components over time by treatment group in subjects with PPC, assessments of days lost from work or school, hospitalization, and all-cause mortality.
Discussion
This trial was halted early due to slow enrollment (72 participants in one year, 33 received fluconazole and 39 received placebo). Of those enrolled, eight (11%) met the study definition of PPC. The study design and challenges are discussed.
Keywords: Coccidioidomycosis, Community-acquired pneumonia, Valley fever
Highlights
-
•
Clinical impact of early antifungal therapy for pneumonia in Coccidioides endemic regions is unknown.
-
•
We designed a phase IV trial in adults with community-acquired pneumonia in regions endemic for Coccidioides.
-
•
Trial was halted early due to slow enrollment and low prevalence of coccidioidomycosis in the enrollment population.
-
•
Lost to follow-up and treatment discontinuation were common in this trial.
Abbreviations
- AEs
adverse events
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- CAP
community acquired pneumonia
- CAR
Clinical Agents Repository
- CF
complement fixation
- dL
deciliter
- DMID
Division of Microbiology and Infectious Diseases
- DSMB
data safety monitoring board
- EIA
enzyme linked immunoassay
- FLEET-Valley Fever
Fluconazole as Early Empiric Treatment of Coccidioidomycosis Pneumonia (Valley Fever)
- IDCF
immunodiffusion complement fixation
- IDTP
immunodiffusion tube precipitin
- ITT
intent-to-treat
- IU
international unit
- mcg
microgram
- MCS
mental component summary
- mg
milligram
- mITT
modified intent-to-treat
- NIAID
National Institute of Allergy and Infectious Diseases
- PPC
primary pulmonary coccidioidomycosis
- PCS
physical component summary
- ULN
upper limit of normal
- SAEs
serious adverse events
- Sands-PPC
Study to Assess the Prevalence and Outcomes of Primary Pulmonary Coccidioidomycosis
- VTEU
Vaccine and Treatment Evaluation Unit
1. Introduction
Coccidioidomycosis is caused by the dimorphic fungal pathogens Coccidioides posadasii and C. immitis, which primarily live in the soil of the Southwestern United States. Primary pulmonary coccidioidomycosis (PPC) occurs after inhalation of fungal spores aerosolized from the soil. The presentation of PPC ranges from a non-specific, self-limited febrile illness to disseminated infection including meningitis. A significant proportion of patients diagnosed with PPC experience a protracted disease course with prolonged symptoms lasting on average 18 weeks [2].
Previous studies have documented the morbidity and cost associated with coccidioidomycosis. Up to 82% of patients with PPC miss work due to their illness [2]. Approximately 40% of patients diagnosed with PPC are hospitalized at a cost of almost $50,000 per hospital stay [16]. The Valley Fever 2017 Annual Report notes that the total in-patient charges for Arizona residents hospitalized with the primary diagnosis of coccidioidomycosis was $50.6 million [15].
Testing for coccidioidomycosis at the time of CAP presentation is uncommon and the diagnosis of PPC is frequently delayed. Early diagnosis is also hampered by poor diagnostic test sensitivity and specificity [3,12,14]. Furthermore, diagnostic testing often occurs after a patient fails antibacterial therapy for CAP. Delay in diagnostic testing for coccidioidomycosis leads to a further lag in recognition of disease and when appropriate, antifungal treatment [6]. Once diagnosed, some providers elect to not treat PPC as patients’ symptoms may resolve without therapy. Other providers may choose to prescribe antifungal therapy, such as fluconazole, particularly in persons at risk of developing severe disease. The Infectious Diseases Society of America (IDSA) provides guidelines for decision making on treatment, but these recommendations are not based on randomized trials [7].
The FLEET-Valley Fever trial aimed to determine whether there is benefit to early empiric treatment for coccidioidomycosis in patients with CAP in communities with a high incidence of coccidioidomycosis (NCT02663674). This trial enrolled patients in endemic areas who presented with CAP to evaluate the efficacy of early empiric fluconazole therapy. However, it was stopped early due to slow enrollment and a concern for lack of feasibility to enroll a sufficient number of subjects with PPC. The purpose of this publication is to share the study design and the descriptive data analysis on those subjects that were enrolled.
2. Methods
This clinical trial was carried out in accordance with the Declaration of Helsinki and approved by each participating site's institutional review board. Informed consent was obtained from each study subject, and subject privacy was observed.
2.1. Study objectives
The primary objective of this clinical trial was to assess the clinical response of early empiric antifungal therapy with fluconazole through Day 22 in subjects with coccidioidomycosis pneumonia who were adherent to the study intervention (fluconazole or placebo). The secondary objectives were to assess the clinical response of early empiric antifungal therapy with fluconazole through Days 22 and 43 in subjects with PPC regardless of adherence to the study intervention, to compare the clinical response by treatment group in subjects with PPC, to assess the impact of early fluconazole therapy on days lost from work or school, mortality, and responses to the SF-12v2 and PROMIS Item Bank v2.0 - Ability to Participate in Social Roles and Activities - Short Form 4a in subjects with PPC, and to assess whether early empiric antifungal therapy with fluconazole at Day 22 was non-inferior to placebo as defined by clinical response at Day 22 in all randomized subjects, regardless of PPC status or adherence with study intervention. Among multiple exploratory objects, one was to assess the adherence to long term self-administration of daily fluconazole or placebo in a clinical trial setting using self-reported adherence and pill count, and a second was to assess medication adherence in the active fluconazole arm by measuring serum fluconazole levels.
2.2. Study design
This trial was a Phase IV randomized, double-blind, placebo-controlled study of individuals aged 18 years or older with CAP in Coccidioides endemic regions. The planned accrual was 1000 subjects with CAP. This study was designed to provide data on the effectiveness of early antifungal treatment (fluconazole 400 mg [mg] per day) versus placebo in subjects with PPC. Study sites included healthcare facilities in Phoenix, Tucson, and Scottsdale, AZ and Bakersfield, Lancaster, and Fresno, CA where the prevalence of coccidioidomycosis among cases of CAP was reported to be 20% or greater.
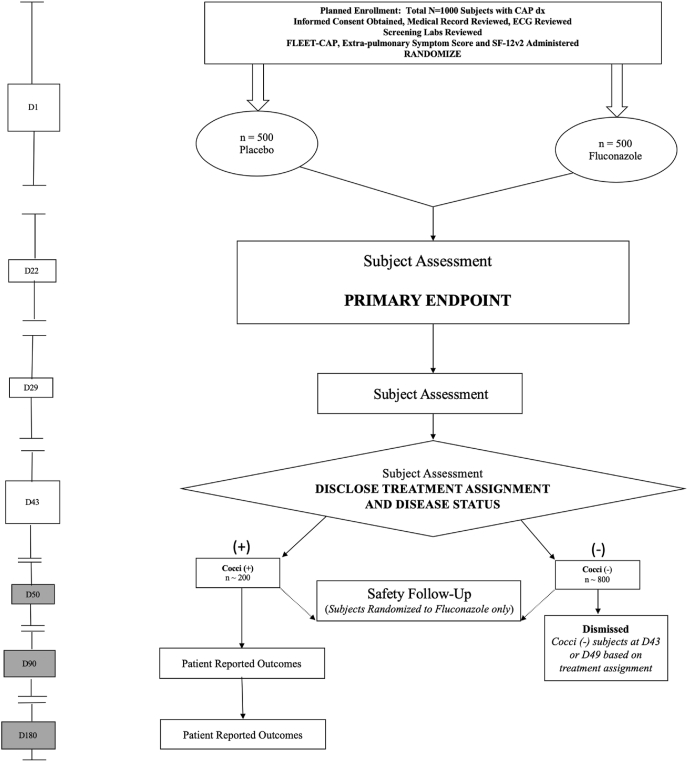
Patients diagnosed clinically with CAP and prescribed antibiotic therapy by their healthcare provider were concurrently randomized to receive either fluconazole 400 mg/day or placebo for 42 days. Blood work for serologic determination of coccidioidomycosis infection was drawn at the time of randomization (Day 1) and again on Days 22, 29, and 43. On Day 43, subjects were unblinded and informed of their coccidioidomycosis status based on serologic results at Days 1, 22, and 29 and their treatment assignment. Those who tested positive for coccidioidomycosis were referred to a healthcare provider with the results of their serologic testing and continued in the study with follow-up telephone visits on Days 90 and 180. Patients who received placebo and were coccidioidomycosis-negative completed the study after the Day 43 visit. Patients who received fluconazole and had negative testing for coccidioidomycosis remained in the study for an additional 6 days past unblinding (Day 49) for safety follow-up. Fig. 1 depicts a schematic of the study design.
Fig. 1.
Schematic of study design.
The protocol and informed consent form were reviewed and approved by the Duke University Health System and respective clinical sites’ Institutional Review Boards. Informed consent was obtained from all trial participants and the privacy rights of participants were continuously observed. The study was registered under ClinicalTrials.gov identifier NCT02663674.
2.3. Study outcome measures
The primary study outcome was the proportion of subjects who achieved a clinical response at Day 22 in each treatment group. This primary analysis was performed in the coccidioidomycosis-positive per-protocol population of subjects who met the case definition of coccidioidomycosis pneumonia and were adherent to the intervention. Adherence was defined as taking at least 80% of the study medication (fluconazole or placebo) as determined by self-report and pill count at the day 22 visit. Clinical response was defined as at least a 50% reduction in composite FLEET CAP score from baseline. The FLEET CAP is a scoring system designed from a compilation of multiple published clinical scores to assess clinical response (Table 1) [1,2,4,9,10].
Table 1.
Modified scoring system for evaluating treatment response in early coccidioidal pneumonia (FLEET CAP score)a.
| Community-Acquired Pneumonia (CAP) SYMPTOMS | Points | Proposed Definition |
|---|---|---|
| bCough | 0 | No coughing, unaware of coughing or cough only now and then |
| 1 | Occasional coughing (less than hourly) | |
| 2 | Frequent coughing (one or more times per hour), interferes with sleep | |
| 3 | Almost constant coughing (never free of cough or need to cough), makes sleep almost impossible | |
| cFatigue | 0 | Absent |
| 1 | Minimal interference with physical function, no interference with carrying out duties and responsibilities | |
| 2 | Interference with carrying out duties and responsibilities | |
| 3 | Prevents usual work, school, family or social interactions | |
| Chest pain | 0 | Absent |
| 1 | Noticeable only when coughing | |
| 2 | Noticeable during deep breaths or when coughing | |
| 3 | Almost constant, present even when resting, without cough | |
| bDyspnea (Shortness of breath) | 0 | None, unaware of any difficulty |
| 1 | Noticeable during strenuous activity | |
| 2 | Noticeable during light activity, or when washing or dressing | |
| 3 | Almost constant, present even when resting | |
| bSputum production | 0 | None, unaware of any difficulty or rarely caused problem |
| 1 | Noticeable as a problem | |
| 2 | Causes a great deal of inconvenience | |
| 3 | An almost constant problem | |
| Night sweats | 0 | Absent |
| 1 | Bed clothing (e.g., pajamas) damp | |
| 2 | Bedding wet and requires change of bedding or clothing | |
| Fever | 0 | Less than 37.8°Celsius |
| 1 | 37.8°–38.5°Celsius | |
| 2 | 38.6–39.5° Celsius | |
| 3 | Greater than 39.5° Celsius | |
| Hypoxia | 0 | SpO2 greater than or equal to 96% on Room Air |
| 1 | 96% > SpO2 ≥89% on Room Air | |
| 2 | SpO2 < 89% on Room Air | |
| Total Score | ||
The secondary study outcomes for each treatment group were: 1) proportion of subjects who achieved a clinical response at Day 22 and 43; 2) the mean, median, and quartiles of the FLEET CAP score and it's components at Days 22, 29, and 43; 3) the number of days of school or work missed after the start of the treatment through Day 43; 4) the mean, median, and quartiles for the mental component summary (MCS) and physical component summary (PCS) scores of the SF-12v2 instrument and the responses to the individual items of the PROMIS Item Bank v2.0 - Ability to Participate in Social Roles and Activities - Short Form 4a at Days 22, 29, 43, 90 and 180; 5) the incidence rates of all-cause mortality after the start of treatment and through Day 43; and 6) the proportion of subjects who achieved a clinical response at Day 22 among all randomized subjects, regardless of coccidioidomycosis status or adherence to study drug.
2.4. Case definition
The case definition of coccidioidomycosis pneumonia was met if the subject was diagnosed with CAP at the time of enrollment as determined by the referring health care provider and met one of the following serologic criteria:
-
1.
The subject was positive for any two serologic tests at any time point from Day 1 through Day 29. The two positive results could but did not necessarily have to be from the same assay or the same time point. The assays considered for this criteria were: Immunodiffusion Tube Precipitin (IDTP) (IgM) and Immunodiffusion Complement Fixation (IDCF) (IgG); Enzyme linked immunoassay testing (EIA-IgM and EIA-IgG); OR
-
2.
The subject was negative for anti-Coccidioides antibody by immunodiffusion assay (or EIA-IgG) at Day 1 and seroconverted to positive for anti-Coccidioides antibody by immunodiffusion assay (or EIA-IgG) at any time point after Day 1 through Day 29; OR
-
3.
The subject was negative or indeterminate for anti-Coccidioides antibody by Complement Fixation (CF) assay on Day 1 and demonstrated a titer of greater than or equal to two by CF assay at any point after Day 1 through Day 29; OR
-
4.
The subject was positive for anti-Coccidioides antibody by CF assay on Day 1 and demonstrated a rise of greater than or equal to a two-fold dilution in CF titer compared to baseline at any time point after Day 1 through Day 29.
2.5. Alternative case definition for exploratory analyses
An alternate case definition of coccidioidomycosis pneumonia utilizing different serologic criteria was also proposed for exploratory analyses. Subjects who met at least one of the following serologic criteria were considered cases per the alternative case definition:
-
1.
Subject was negative for anti-Coccidioides antibody by CF assay on Day 1, and demonstrated a titer of greater than or equal to two by CF assay at both Days 22 and Day 29
-
2.
Subject was negative for anti-Coccidioides antibody by complement fixation (CF) assay on Day 1, and demonstrated a titer of greater than or equal to 4 by CF assay at either Day 22 or Day 29
-
3.
Subject was negative for anti-Coccidioides antibody by IDCF (IgG) on Day 1, and had a positive IDCF test on either Day 22 or Day 29
-
4.
Subject was negative for anti-Coccidioides antibody by IDTP (IgM) on Day 1, and had a positive IDTP test on either Day 22 or Day 29
-
5.
Subject was negative for anti-Coccidioides antibody by enzyme linked immunoassay testing (EIA-IgM) on Day 1, and had a positive EIA-IgM at both Days 22 and Day 29
-
6.
Subject was negative for anti-Coccidioides antibody by enzyme linked immunoassay testing (EIA-IgG) on Day 1, and had a positive EIA-IgG at both Days 22 and Day 29.
2.6. Sample size
The primary analysis intended to compare the proportion of subjects achieving clinical response at Day 22 among efficacy evaluable subjects as defined as subjects in the per protocol analysis population who met the case definition of coccidioidomycosis pneumonia, were adherent with the intervention, and had coccidioidal serology data available at the Day 1 and 22 visits. A per protocol design was choosen over intent-to-treat (ITT) to get the best assessment as to whether fluconazole had an effect in a coccidioidomycosis-positive population who was deemed adherent to fluconazole or placebo. This was a post-marketing study that was not looking for a label change. Thus, the goal was to identify the cleanest population to assess the outcome and to ensure adequate power for that group.
For sample size determination, the following assumptions were made: the cumulative clinical response rate at Day 22 would be 0.20 in the placebo arm and 0.40 in the fluconazole arm, 10% of subjects would be censored due to non-compliance or competing risk of death over the 22-day period and the time to drop-out and time to event would follow independent exponential distributions. Under these assumptions, a sample size of 100 subjects with PPC per treatment arm in the per protocol analysis population was determined sufficient for 88% power for a two-sided level 0.05 two-sample test and comparing the Kaplan Meier estimates of the cumulative proportion of clinical responses at 22 days.
We estimated that 20% of the enrolled population would be eligible for the per protocol analysis population. Therefore, to reach the per protocol analysis population sample size of 200 subjects with PPC, it would be necessary to enroll at least 1000 subjects with CAP.
2.7. Analysis populations
The primary efficacy analysis was specified for the Coccidioides-positive per protocol analysis population. The primary analysis was specified as such to ascertain the best estimate of fluconazole efficacy in subjects with PPC who were deemed adherent to study medication. However, additional secondary analyses were planned in the all randomized per protocol population, all randomized and Coccidioides-positive ITT and modified intent-to-treat (mITT) populations. The ITT population included all randomized subjects. The Coccidioides-positive mITT population included all randomized subjects who met the case definition of coccidioidomycosis pneumonia and took at least one dose of study medication. The all randomized mITT population included all randomized subjects who took at least one dose of study medication regardless of coccidioidomycosis status. The safety analysis population included all subjects who took at least one dose of study medication.
2.8. Study procedures
2.8.1. Participant eligibility
Subjects eligible to participate in the study had to meet all of the following inclusion criteria:
-
1.
Aged ≥18 years and presenting for clinical care in coccidioidomycosis endemic areas
-
2.
Have a health care provider who has decided to treat CAP with antibacterials
-
3.
Able to take and tolerate oral antibacterials/antifungals
-
4.
Able to understand the study and provide informed consent
-
5.
Willing and able to comply with study procedures and complete study visits
-
6.
Willing to allow access to medical records, and medical records were available to the study team
-
7.
Able to receive the first dose of study drug within 72 h of presentation for care
-
8.
Able to swallow large pills
-
9.
Sexually active female subjects were required to be of non-childbearing potential or, if of childbearing potential, were required to use a highly effective method of birth control
-
10.
Non-pregnant female subjects of childbearing potential were required to have a negative pregnancy test within 24 h prior to enrollment
-
11.
Subjects receiving any of the drugs reported to have manageable drug interactions with fluconazole were allowed to be enrolled based on PI clinical judgment
Subjects eligible to participate in the study could not meet any of the following exclusion criteria:
-
1.
Have recently received an experimental agent within 30 days of enrollment or participating in or plan to participate in a study involving an experimental agent while in the active drug administration phase of this study
-
2.
Have hospital-acquired pneumonia
-
3.
Have microbiologically- or serologically-confirmed past infection with coccidioidomycosis (including pathologic diagnosis)
-
4.Have a clinical diagnosis of coccidioidal infection that is of sufficient certainty as to
- exclude the need for antibacterial therapy
-
5.
Have a history of systemic antibacterial treatment for CAP occurring within the four weeks prior to enrollment
-
6.
Have a history of systemic antifungal treatment within the four weeks prior to enrollment (single dose of fluconazole for vulvovaginitis was allowed)
-
7.
Have long-term immunosuppressive use of high-dose glucocorticoids or high-dose inhaled steroids taken within four weeks prior to enrollment (see full protocol for definition of long-term and high-dose)
-
8.
Have confirmed or suspected immunosuppression as a result of an underlying illness or primary immunodeficiency
-
9.
Have a history of bone marrow or solid organ transplant
-
10.
Have poorly controlled HIV-infection (see full protocol for definition of poorly controlled)
-
11.
Have a current diagnosis of active liver disease including abnormal baseline liver function tests as defined as: total bilirubin greater than or equal to 3.0 mg/dL AND either aspartate aminotransferase (AST) greater than or equal to 135 IU/L OR alanine amino transferase (ALT) greater than or equal to 150 IU/L
-
12.
Be on dialysis or have a creatinine of greater than or equal to 2.0 mg/dL or estimated creatinine clearance less than or equal to 50 mL/min
-
13.
Have history of hypokalemia, defined as less than 3.5 mEQ/L, on more than one occasion during the four weeks prior to enrollment
-
14.
Have history of cardiovascular disease with increased risk for torsades de pointes (see full protocol for full definition)
-
15.
Have a marked baseline prolongation of the QT/QTc interval defined as a QTc interval greater than 450 ms (ms) for male subjects or greater than 470 ms for female subjects
-
16.
Be a pregnant or lactating female
-
17.
Have a history of azole intolerance or allergy
-
18.
Taking medications that are contraindicated with concurrent use of fluconazole
-
19.
Have a positive point-of-care HIV test at Day 1 visit consistent with new HIV diagnosis
-
20.
Individuals for whom study participation would not be in their best interest, as determined by the clinical investigator
2.8.2. Randomization, blinding, and study drug administration
Using a centralized randomization system, eligible subjects were randomly assigned to one of two groups (1:1 ratio) to receive either 42 days of placebo or 400 mg/day fluconazole capsule (see Supplemental Appendix with full protocol). The randomization code was prepared by statisticians at the Statistical and Data Coordinating Center and sent to the Division of Microbiology and Infectious Diseases (DMID) Clinical Agents Repository where the study product was labeled with a randomization code, which links to the treatment assignment. Once consented and determined to be eligible for the trial, the subject was assigned the next available assignment in sequence of the product available at the site. The randomization code of the distributed study product was entered into Advantage EDCSM (Electronic Data Capture System) upon enrolling the subject in the trial. Subjects, investigators, study personnel performing any study-related assessments following study drug administration, and laboratory personnel performing antibody assays were blinded to group assignment until all study assessments through the Day 43 visit were completed. On Day 43, subjects were informed of their coccidioidomycosis status (positive or negative) and their treatment group assignment. Subjects remaining in the study remained unblinded for the duration of their study participation.
All study drugs (fluconazole and placebo) were acquired through the DMID Clinical Agents Repository (CAR, Fisher BioServices). For fluconazole, each gelatin capsule contained two 100 mg fluconazole tablets and microcrystalline cellulose for overfill. Placebo was supplied as matching gelatin capsules containing microcrystalline cellulose only. To maintain blinding, the gelatin capsules were the same size, weight, and color as the capsules containing fluconazole tablets.
2.8.3. Clinical evaluations
A schedule of study procedures and evaluations is depicted in Table 2. Complete medical history was obtained by review of the medical record and interview of subjects during the screening visit. Subjects were queried regarding any history of significant medical disorders including any allergies, cancer, immunodeficiency, psychiatric illness, substance abuse, and cardiovascular or autoimmune disease. On Days 22, 29, and 43, an interim medical history was obtained by interview of the subjects noting changes since the previous clinic visit or contact. Medication history included a review of all current medications and medications taken within 30 days prior to signing the informed consent form through Day 43 or early termination, whichever occurred first (more details in Supplemental Appendix).
Table 2.
Schedule of study procedures and evaluations.
| Study Visit Number |
V 01f |
V 02f |
V 03f |
V 04f |
V 05h |
V 06i |
V 07i |
Early Termination or Unscheduled Visit |
|---|---|---|---|---|---|---|---|---|
| Study Day |
Day 1 |
Day 22 |
Day 29 |
Day 43 |
Day 49 |
Day 90 |
Day 180 |
|
| – | -2d to +1d | -2d to +1d | -1d to +3d | -1d to +2d | ±7d | ±7d | ||
| Obtain Informed Consent∞ | X | |||||||
| Collect Demographics and Employment/School Enrollment Status | X | |||||||
| Review Eligibility Criteria | X | X | X | |||||
| Medical Record Review |
X | X | X | X | X | X | X | |
| Concomitant Medications | X | X | X | X | Xd | |||
| Obtain Medical Historye | X | X | X | X | Xd | |||
| Vital Signs (Temp, BP, Pulse, Resp, O2 Sat, Weight) | X | X | X | X | Xd | |||
| Height | X | |||||||
| Resting 12-Lead ECG | Xm | Xc | ||||||
| Physical Examinationa | Xm | X | X | X | Xd | |||
| FLEET CAP score Extra-Pulmonary Symptom Score |
X X |
X X |
X X |
X X |
X | X | Xd X |
|
| SF-12v2 PROMIS Short Form |
X | X | X | X | X | X | X | |
| Days Missed from School or Work | X | X | X | Xd | ||||
| Pregnancy Testk | X | X | X | X | Xd | |||
| Venous Blood Collection for Safety Labsg | Xζ | X | X | X | Xd | |||
| POC HIV-1 Antibody Test | X | |||||||
| Venous Blood Collection for Coccidioidal serologies | X | X | X | X | Xd | |||
| Future Use Samples: Bloodl | X | X | X | X | Xd | |||
| Future Use Samples: Urine, Nasopharyngeal Swab, and Throat Swabl |
X | |||||||
| Enroll/Randomize | X | |||||||
| Dispense Study Product | X | X | ||||||
| Obtain Cocci Status | Xj | |||||||
| Reveal Treatment Status | Xj | Xd,j | ||||||
| Pill Count and Adherence Interview | X | X | X | Xd | ||||
| Therapeutic Drug Monitoring (Fluconazole) | X | X | Xd | |||||
| SAE Assessment | X | X | X | X | X | Xd | ||
| Refer to Health Care Provider for Follow-Upb | X | X | Xd |
∞Prior to study procedures.
Clinician must be licensed to make medical diagnoses. Full examination to be performed on Day 1, thereafter, perform a targeted physical examination if indicated based on review of complete medical history.
Referrals to heath care provider can be made at various time points. See study schedule for details.
If termination occurs prior to Day 15.
If termination occurs prior to Day 43.
Complete medical history by medical record review and interview of subjects to be obtained on Day 1 and interim medical history by interview and medical record review of subjects to be obtained at follow-up visits.
Indicates visits during blinded phase where the schedule is the same for all participants (all In Person).
Includes Hepatic function panel (AST, ALT, alkaline phosphatase and total bilirubin), BUN, Creatinine.
Safety follow up for those randomized to fluconazole.
Follow-up (phone call) for all subjects who meet the protocol defined case definition as coccidioidomycosis positive. Patient reported outcomes (SF-12v2, PROMIS short form) and Extra-Pulmonary Symptom Scores will be performed, but FLEET-CAP will not.
Revealing the subject's treatment status and disease status should be done only after all study related assessments are completed. Disease status will be revealed if known based on review of available serologies.
Urine for early termination. Serum or urine for all other visits. Only required for women of childbearing potential.
Only required for subjects that have consented to additional sample collection for future use.
For patients referred to study with existing standard of care safety labs, physical exam, and/or ECG within the past 72 h and are available to the study team at the time of screening, these SOC activities can serve as the screening activities and do not require repeating. (laboratory tests are required to have the same normal range as the study site).
At the screening visit, a physical examination was performed by a clinician licensed to make medical diagnoses (Supplemental Appendix). Assessments of any specific signs or symptoms reported by the subject were required. Targeted physical exams were performed on Days 22, 29, and 43 if indicated based on the subject's interim medical history. Changes were classified as new, worsened, or improved from those at screening. A resting 12-lead ECG was performed at the screening visit. The FLEET CAP score was completed by study staff on Days 1, 22, 29, and 43. The Extra-Pulmonary Symptom Score was used to rate arthralgia, rash, and headache and was completed by the subject on Days 1, 22, 29, and 43. For subjects who were coccidioidomycosis positive, the score was also collected on Days 90 and 180. The SF-12v2, a questionnaire measuring functional health and well-being from the study subject's perspective and the PROMIS Ability to Participate in Social Roles and Activities - Short Form 4a which uses four questions to measure the subject's ability to participate in social roles and activities in the context of family, friends, leisure, and work was completed by the subject on study Days 1, 22, 29, and 43 and for those who were coccidioidomycosis positive, the questionnaires were also collected on Days 90 and 180. At all study visits after study Day 1 until Day 43, subjects were queried about time missed from school or work.
Study product adherence was assessed on study Days 22, 29, and 43. Adherence was assessed by pill count and subject self-report. Subjects were asked to bring their study product containers to each study visit and were interviewed at each visit on medication adherence. Pill count, if available, took precedence over subject report. To be considered adherent at a visit, the subjects were required to have taken at least 80% of their expected pills.
2.8.4. Laboratory evaluations
Clinical screening laboratory parameters were evaluated on Day 1 to confirm study eligibility prior to receipt of study drug and included hepatic function panel (aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and total bilirubin), blood urea nitrogen (BUN), creatinine, and HIV-1 antibody testing. A urine or serum pregnancy test was performed in women of child-bearing potential. A screening 12-lead EKG was also completed prior to dosing.
Assays to determine Coccidioides antibodies were performed at ARUP laboratories. Venous blood samples were collected for complement fixation, qualitative IDCF and IDTP and enzyme-linked immunoassays for both IgM and IgG on Days 1, 22, 29, and 43. All serological testing for a single participant from Day 1 through Day 29 were batched and run concurrently to eliminate confounding due to test run variability.
Fluconazole blood levels were measured on Day 22 and Day 43 in all participants and were performed at ARUP laboratories.
2.8.5. Safety assessments
Clinical safety laboratory parameters were evaluated on Days 22, 29 and 43 including hepatic function panel, BUN, creatinine, and pregnancy test (urine or serum) for women of child-bearing potential. These evaluations were performed by the local or site laboratory with same day results. Due to the well-established safety profile of fluconazole, non-serious adverse events (AEs) were not reported in this study. Serious adverse events (SAEs) were followed from the time of enrollment through Day 43 for those receiving placebo and Day 49 for those receiving fluconazole. For this protocol only the following were considered as SAEs: 1) congenital anomalies or birth defects or 2) any condition that met the regulatory definition of a SAE and was unexpected (per Principal Investigator) and was suspected to be directly caused by the study drug (e.g., anaphylaxis, Stevens-Johnson syndrome, on exfoliative dermatitis).
Safety oversight was conducted by a Data and Safety Monitoring Board (DSMB) whose members were separate and independent of study personnel participating in the trial and did not have scientific, financial or other conflict of interest related to the study. The DSMB met nine times before the study was ended to review safety and study feasibility.
2.8.6. Individual halting rules
Study drug was to be discontinued for an individual subject for the following: the AST or ALT was five times the upper limit of normal (ULN); the total bilirubin was 1.5 times ULN; the liver enzymes exceeded the following values (AST ≥225 IU/L or ALT ≥250 IU/L); AST or ALT was three times ULN accompanied by total bilirubin elevation 1.5 times ULN; QTc prolongation >500 ms (ms) or >60 ms above baseline measurement; torsades de pointes; unexplained syncope, or seizure in non-epileptic patients.
2.9. Statistical analysis
Baseline and demographic characteristics and reasons for study drug discontinuation were summarized by treatment group. For both continuous and categorical variables appropriate summary statistics were applied. For continuous variables, descriptive statistics included the median and range. For categorical variables, descriptive statistics included counts and percentages per category.
The primary efficacy analysis intended to explore the difference in the proportion of subjects with an occurrence of clinical response at Day 22 between the fluconazole and placebo treatment groups. Subjects without a clinical response at Day 22 were coded as treatment failures while subjects with a clinical response at Day 22 were coded as a clinical response if the outcome measure definition was met. Descriptive analyses were performed for the primary endpoint. The number of clinical responses observed were tabulated by time point, including and beyond Day 22, and treatment group. Tabular summaries were prepared for the composite efficacy scores (FLEET CAP, Extra-Pulmonary Symptom Score, health and mental composite scores based on SF-12v2, PROMIS score) and their subcomponents across all assessment time points. The number of coccidioidomycosis cases in each treatment arm were tabulated among all randomized subjects.
This study planned to enroll 200 subjects evaluable for efficacy, with 100 subjects in each of the 2 study groups. However, this study was stopped early due to very low enrollment rates, and only 72 subjects were enrolled out of 1000 planned per protocol, and 6 subjects were evaluable for efficacy out of 200 planned per protocol. Due to the small sample size, no formal hypothesis tests were performed. After the decision to stop the trial early, no additional interim analyses were performed (see Supplemental Appendix with full protocol for additional information regarding the original statistical analysis plan).
3. Results
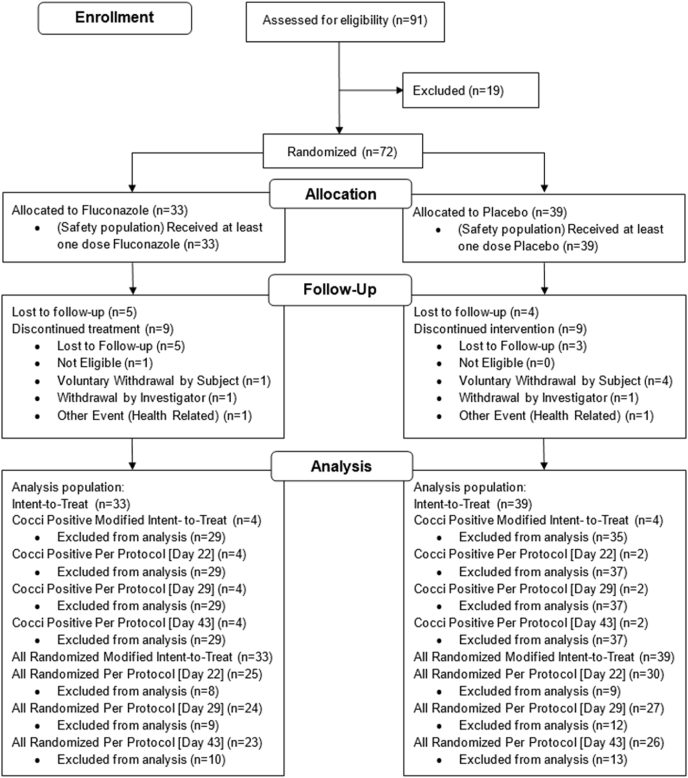
Trial enrollment occurred at eight clinical sites, which were phased in over the course of a 27-month period, starting in March of 2016. During this time, 91 participants were consented and screened for enrollment and 72 were randomized; 33 to fluconazole and 39 to placebo (Fig. 2). All 72 randomized subjects received at least one dose of study medication. Overall, 17 subjects were lost to follow-up and 18 discontinued treatment. Table 3 depicts the reasons for study drug discontinuation.
Fig. 2.
Consort flow diagram.
Table 3.
Reasons for study drug discontinuation.
| Reason Drug Discontinuation | Fluconazole (N = 33) | Placebo (N = 39) | All Subjects (N = 72) |
|---|---|---|---|
| Lost to Follow-up (%) | 10 (30) | 7 (18) | 17 (24) |
| Voluntary Withdrawal by Subject (%) | |||
| Unable to Return for Follow-up | 0 | 4 (10) | 4 (6) |
| Moved Away for Work | 2 (6) | 0 | 2 (3) |
| Decrease in Sleep | 0 | 2 (5) | 2 (3) |
| Worried about Long-Term Side Effects | 0 | 2 (5) | 2 (3) |
| Withdrawal by Investigator (%) | |||
| Unblinded for Treatment for Coccidioidomycosis | 0 | 2 (5) | 2 (3) |
| Patient-Reported Syncopal Event | 2 (6) | 0 | 2 (3) |
| Health-Related Event (%) | |||
| Atrial Fibrillation | 0 | 2 (5) | 3 (4) |
| Pruritis | 1 (3) | 0 | 1 (1) |
| Deemed Ineligible after Enrollment (due QTc> 450 ms) (%) | 1 (3) | 0 | 1 (1) |
For the overall study population, the proportion of males and females was equal (50% females, 50% males) (Table 4). Subjects were non-Hispanic (51%) or Hispanic or Latino (49%), and most were white (79%). The mean age was 49.8 years (range: 19–79 years). The distribution of ethnicity, age, race, sex and PPC case definition were similar across treatment groups. Eight (11%) subjects met the case definition of PPC.
Table 4.
Patient demographics by treatment group.
| Characteristic | Fluconazole (N = 33) | Placebo (N = 39) | All Subjects (N = 72) |
|---|---|---|---|
| Age, Median Years (Range) | 52 (21–79) | 50 (19–71) | 51 (19–79) |
| Female Gender, n (%) | 18 (55) | 18 (46) | 36 (50) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 17 (52) | 20 (51) | 37 (51) |
| Hispanic or Latino | 16 (48) | 19 (49) | 35 (49) |
| Unknown or Not Reported | 0 | 0 | 0 |
| Race, n (%) | |||
| American Indian or Alaska Native | 1 (3) | 0 | 1 (1) |
| Asian | 0 | 2 (5) | 2 (3) |
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 |
| Black or African American | 4 (12) | 4 (10) | 8 (11) |
| White | 26 (79) | 31 (79) | 57 (79) |
| Multi-Racial | 1 (3) | 1 (3) | 2 (3) |
| Unknown | 1 (3) | 1 (3) | 2 (3) |
| History of Diabetes Mellitus, n (%) | 7 (21) | 6 (15) | 13 (18) |
| History of Asthma/COPD, n (%) | 5 (15) | 11 (28) | 16 (22) |
| Tobacco Use, n (%) | 7 (21) | 7 (18) | 14 (19) |
| Coccidioidomycosis Status (%) | |||
| Coccidioides-Positive | 4 (12) | 4 (10) | 8 (11) |
| Coccidioides-Negative | 29 (88) | 35 (90) | 64 (89) |
N= Number of subjects in the ITT Population (all randomized subjects).
Sixty-two of the 72 enrolled subjects completed the visit at Day 22 and of those, 55 (89%) were adherent to study treatment (Fig. 2). Fifty-seven of the 72 enrolled subjects completed the visit at Day 29, and 52 (91%) were adherent to study treatment. Fifty-six of the 72 enrolled subjects completed the visit at Day 43, and of those, 50 (89%) were adherent to study treatment. In the fluconazole treatment arm, the mean fluconazole concentration at Day 22 was 19.1 mg/L and 24 out of 27 subjects (88.9%) had a detectable fluconazole level. At Day 43, the mean drug concentration was 16.8 mg/L and 20 out of 25 subjects (80%) had detectable fluconazole level. In the placebo treatment arm, the mean drug concentration was less than 1.0 mg/L and no subject had detectable a fluconazole level at any visit.
Among enrolled subjects who took at least one dose of study treatment (ITT and mITT populations), there were eight (11%) subjects that met the primary case definition and three (4%) that met the alternative case definition. In the Coccidioides-positive per protocol population, there were six (8%) subjects that met the primary case definition and were adherent to treatment, and three (4%) that met the alternative case definition and were adherent to treatment.
Among the eight subjects that met the primary case definition, there were three subjects with detectable antibody by immunodiffusion at Day 1 and four subjects at Day 22 and 29. Among the three subjects that met the alternative case definition, there was one subject with detectable antibody by immunodiffusion at Day 1 and three subjects at Day 22 and 29. The median fold change in antibody from baseline to Day 22 and 29 was negligible (all were close to 1.0) among primary cases at both visits. Among alternative cases, the median fold-rise was 1.67 for EIA-IgM at both visits and 7.67 and 10.0 for EIA-IgG on Days 22 and 29, respectively.
Due to slow enrollment, this study was terminated early, and the efficacy outcomes were underpowered. There were insufficient subjects with PPC to provide results related to response to therapy. For the ITT population there were larger numbers. At Day 22, in the ITT population 42 (68%) were responders: 20 (74%) responders in the fluconazole and 22 (63%) responders in the placebo group. At Day 43 in the ITT population, 53 (96%) were responders: 25 (100%) responders in the fluconazole and 28 (93%) responders in the placebo group. Thus, there was a high response rate in both treatment arms. In the ITT population, the total FLEET CAP score across all subjects decreased over time, from a mean 7.92 at baseline to a mean 1.44 at Day 43. A similar trend over time was observed within each treatment group. In the ITT population (and mITT), subjects receiving fluconazole missed on average 1 day of work or school by Day 22 and didn't miss any work or school days after Day 22. In the ITT population (and mITT), placebo group subjects missed on average 2.3 days of work or school by Day 22, 0.9 days from Day 22 to Day 29 and 1.5 days from Day 29 to Day 43. Similar results were observed in the all randomized per protocol population.
For safety assessments, there were no subjects with an elevated hepatic function test or QTc prolongation in any treatment group. No deaths or SAEs were reported. No pregnancies or new HIV diagnoses occurred during this study. Three patients discontinued the study drug due to health-related events, which were not thought to be related to the study drug (one with pruritis in the fluconazole group and two with atrial fibrillation in the placebo group).
4. Discussion
The FLEET-Valley Fever study was a randomized, double-blind, placebo-controlled trial designed to determine if early fluconazole treatment at the time of CAP presentation, in coccidioidomycosis endemic regions of the US, could improve clinical symptoms and long-term outcome of patients with PPC. This trial was halted early due to slow enrollment and determination by the DSMB that the feasibility for achieving full accrual was low. Furthermore, the low incidence of PPC in those enrolled raised further concerns that full accrual may still result in an underpowered study. Several factors that resulted from the early treatment intervention impacted timely enrollment including: 1) stringent protocol enrollment criteria including short time to enroll from CAP diagnosis; 2) Requirement of treating CAP as part of the study which led to many issues with drug-drug interactions with fluconazole; 3) the willingness of participants to enroll in a clinical study when they were acutely ill but not hospitalized and which randomized them to treatment for a disease for which they had not yet received a diagnosis; and 4) presence of associated signs such as classic skin manifestations seen in acute pulmonary coccioiodomycosis that dissuaded experts from enrolling patients into the trial.
Acute pulmonary coccidioidomycosis results in significant morbidity for some patients and can result in loss of productivity in school and/or work. There remain significant barriers to the diagnosis, treatment and management of this infection, which is highly prevalent in the southwestern US [8]. There is not a single effective diagnostic test to establish a clinical diagnosis of coccidioidomycosis early in the course of infection. The sensitivity and specificity of currently available diagnostic tests are not sufficient. In addition, it is not fully understood whether a single diagnostic test is sufficient to identify and/or differentiate the different presentations and stages (acute vs. chronic, primary vs. disseminated) of coccidioidomycosis. The benefit of antifungal treatment for acute coccidioidomycosis is not clear, and current guidelines recommend monitoring patients with newly diagnosed, uncomplicated PPC and only treating patients with debilitating disease or those with extensive pulmonary involvement, concurrent diabetes, extra-pulmonary soft tissue infection, otherwise frail because of age or comorbidities, or of African or Filipino ancestry [7]. The FLEET-VF study intended to address these gaps in knowledge and provide high quality evidence to providers who manage this infection regularly. However, the randomization of subjects to fluconazole resulted in multiple barriers to enrollment as described above. Yet the need to understand the prevalence of disease and the benefit of early therapy in PPC remains.
While the FLEET-VF study ended early and thus is not powered for comparisons, there is still an opportunity to learn from those subjects who were enrolled. Lost to follow-up and treatment discontinuation was high in this cohort (24% and 25% of subjects, respectively). The prevalence of coccidioidomycosis, based on the study definition, was less than 20%, which was significantly below the target prevalence threshold for the study population. There are multiple potential reasons for the lower than expected prevalence seen in the enrolled population. The target number of CAP to enroll was 1000 to achieve a coccidioidomycosis prevalence of 20%, thus with early discontinuation of the study, it is not clear whether coccidioidomycosis prevalence would have met 20% as has been previously estimated for the regions represented in the study. In addition, the focus of most sites on enrolling from emergency departments may have impacted the prevalence as patients presenting to emergency departments are a self-selected cohort and are often more sick, which may not be selective for PPC.
Overall, adherence to study medication was good among subjects retained in the study, with approximately 90% adherence reported at each on study visit. Fluconazole levels confirmed adherence in the active treatment arm and suggested a decline in adherence over the on-treatment study period. Tolerance and safety of fluconazole was good with no increase in liver transaminases and no SAEs. This is in contrast to a prior single center study noted that 51.6% of patients taking fluconazole for greater than or equal to 28 days for proven or probable coccidioidomycosis discontinued therapy due to adverse effects from fluconazole [5]. Therefore, future study is needed to better assess the tolerability and safety of fluconazole for long-term treatment of coccidiomycosis.
The number of subjects meeting primary or alternate case diagnoses were few. Yet, differences in kinetics of antibody titers between the two groups highlights the challenges with diagnosis, especially when trying to differentiate early or acute infection versus prior exposure or subacute/chronic infection. Lastly, in the ITT and mITT groups, there was evidence of high treatment response and improvement in FLEET CAP scores, regardless of treatment arm.
With a goal of filling crucial knowledge gaps in the diagnosis, treatment and management of early coccidioidomycosis, we have designed a new study titled: An Observational Study to Assess the Prevalence and Outcomes of Primary Pulmonary Coccidioidomycosis in Persons Aged >14 years Presenting with Community Acquired Pneumonia (CAP) in Endemic Areas (SAnds-PPC) (NCT03908632). This observational study is actively recruiting subjects from 6 of the prior 8 sites with the addition of one new site and has a new primary objective: to assess the prevalence of PPC in subjects with CAP in coccidioidomycosis endemic areas. Secondary objectives include: 1) In subjects with CAP in coccidioidomycosis endemic areas, describe the practice of empiric antifungal treatment of subjects with CAP; and 2) describe the practice of antifungal treatment of subjects with PPC and compare the outcomes of antifungal therapy vs. no therapy for PPC. This observational study has a multi-step design. Step 1 enrolls subjects aged 14 and older recently diagnosed with CAP in high endemicity areas. Step 1 is a low-risk study with short-term follow-up; patients complete step 1 when they meet the serologic criteria for PPC or after 28 days when they fail to seroconvert their repeat serology. This design was felt to provide the best feasibility to enroll the required 1000 subjects to reliably estimate the prevalence of PPC in patients diagnosed with CAP in endemic regions. Step 2 is also a low-risk study with long-term follow-up; patients who meet serologic criteria for PPC in step 1 move to step 2 (part of step 1 consent), which observes patients through their disease course, recording treatment decisions made by their clinical providers as part of local standard of care as well as clinical, laboratory, and functional assessments. New subjects can also enter the study directly into step 2 if they have been diagnosed with PPC within the past 14 days outside of the study and if onset of symptoms are no earlier than 7 weeks prior to enrollment. This step design was included to ensure we met the goal accrual of 200 subjects with PPC in case the sero-conversion rate in step 1 was less than 20%. Follow-up for subjects in step 2 includes clinical visits on Days 29 and 85, and two phone follow-ups on days 180, and 365. Blood samples for coccidioidal serologies are collected on Days 1 and 22 of step 1 and Days 29 and 85 of step 2. Subjects in step 2 who develop persistent or disseminated disease also have blood collected on Days 180 and 365.
While this observational study does not randomize subjects to early antifungal treatment versus placebo, it describes the standard practice and approach to treatment and management of patients with PPC. Local practice patterns vary across the clinical enrollment sites, creating the potential to assess differences in outcome while controlling for subject and location-specific variables. Although observational data is at risk for bias, including selection bias and confounding, this is a prospective, multi-center study where data collection is uniform. The study collects data on disease severity and excludes patients who would otherwise meet guideline criteria for antifungal therapy, thus focusing specifically on the population of patients where the benefit of early antifungal therapy is unclear. Therefore, this study is well designed to address prevalence of PPC in endemic regions in people with CAP, to describe the kinetics of coccidioidal serologies in patients with CAP and to assess response to antifungal treatment in patients with mild disease.
In conclusion, here we present the study design and final descriptive data from the FLEET-Valley Fever study, the first randomized, double-blind, placebo-controlled trial of fluconazole in PPC. Due to the complexity of the study design, the extensive drug-drug interactions of fluconazole, and the chronic co-morbidities of the recruitment populations, and lack of willingness for some to participate at the clinical sites, this study was ended early due to slow enrollment and lack of feasibility to meet accrual goals. Due to the ongoing need to address the outstanding questions in the diagnosis, treatment, and management of PPC, a new two-step observational study protocol is now actively enrolling at 6 sites. While there is risk of bias in the observational study design, the feasibility is significantly improved, and we believe the primary and secondary objectives will help improve the care and health of persons with coccidioidomycosis.
Funding
This project was supported by the Division of Microbiology and Infectious Diseases (DMID), National Institute of Allergy and Infectious Diseases (NIAID) of NIH through the Vaccine and Treatment Evaluation Units (VTEU), and the US Department of Health and Human Services, United States, under contract HHS (Duke University HHSN272201300017I).
Declaration of competing interest
Julia A. Messina, MD, MHS, MSc: Nothing to report.
Eileen K. Maziarz, MD: Nothing to report.
John Galgiani, MD: Nothing to report.
Jonathan T. Truong, MD: Nothing to report.
Aung K. Htoo, MD: Nothing to report.
Arash Heidari, MD: Nothing to report.
Royce H. Johnson, MD: Nothing to report.
Aneesh T. Narang, MD: Nothing to report.
Fabria M. Donovan, MD, PhD: Nothing to report.
Marion Ewell, ScD: Nothing to report.
Antonino Catanzaro, MD: Nothing to report.
George R. Thompson III, MD: Nothing to report.
Neil M. Ampel, MD: Nothing to report.
John R. Perfect, MD: reports research grants and consultation from the following: Astellas, Pfizer, Merck, Amplyx, Scynexis, Matinas, F2G, Appili and Minnetronix.
Susanna Naggie, MD, MHS: Nothing to report.
Emmanuel B. Walter, MD, MPH: has served as an investigator for clinical trials funded by Moderna and Pfizer.
Acknowledgements
We would like to thank all site staff, principal investigators and co-investigators for their dedication to this study. We would like to recognized the Data Safety and Monitoring Board, Richard B. Pollard, MD, Wendy Keitel, MD, Richard J. Hamill, MD, and Peter B. Imrey, PhD. for their sage advice.
References
- 1.Anstead G.M., Graybill J.R. Coccidioidomycosis. Infect. Dis. Clin. 2006;20(3):621–643. doi: 10.1016/j.idc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Blair J.E., Chang Y.H., Cheng M.R., Vaszar L.T., Vikram H.R., Orenstein R.…Parish J.M. Characteristics of patients with mild to moderate primary pulmonary coccidioidomycosis. Emerg. Infect. Dis. 2014;20(6):983–990. doi: 10.3201/eid2006.131842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair J.E., Mendoza N., Force S., Chang Y.H., Grys T.E. Clinical specificity of the enzyme immunoassay test for coccidioidomycosis varies according to the reason for its performance. Clin. Vaccine Immunol. 2013;20(1):95–98. doi: 10.1128/CVI.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catanzaro A., Galgiani J.N., Levine B.E., Sharkey-Mathis P.K., Fierer J., Stevens D.A.…Cloud G. Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am. J. Med. 1995;98(3):249–256. doi: 10.1016/s0002-9343(99)80371-4. [DOI] [PubMed] [Google Scholar]
- 5.Davis M.R., Nguyen M.H., Donnelley M.A., Thompson G.R., Iii Tolerability of long-term fluconazole therapy. J. Antimicrob. Chemother. 2019;74(3):768–771. doi: 10.1093/jac/dky501. [DOI] [PubMed] [Google Scholar]
- 6.Donovan F.M., Wightman P., Zong Y., Gabe L., Majeed A., Ynosencio T.…Galgiani J.N. Delays in coccidioidomycosis diagnosis and associated healthcare utilization, Tucson, Arizona, USA. Emerg. Infect. Dis. 2019;25(9):1745–1747. doi: 10.3201/eid2509.190023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galgiani J.N., Ampel N.M., Blair J.E., Catanzaro A., Geertsma F., Hoover S.E.…Theodore N. 2016 infectious diseases society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin. Infect. Dis. 2016;63(6):e112–146. doi: 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 8.Galgiani J.N., Blair J.E., Ampel N.M., Thompson G.R. Treatment for early, uncomplicated coccidioidomycosis: what is success? Clin. Infect. Dis. 2020;70(9):2008–2012. doi: 10.1093/cid/ciz933. [DOI] [PubMed] [Google Scholar]
- 9.Galgiani J.N., Catanzaro A., Cloud G.A., Johnson R.H., Williams P.L., Mirels L.F., Rinaldi M.G. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann. Intern. Med. 2000;133(9):676–686. doi: 10.7326/0003-4819-133-9-200011070-00009. [DOI] [PubMed] [Google Scholar]
- 10.Graybill J.R., Stevens D.A., Galgiani J.N., Dismukes W.E., Cloud G.A. Itraconazole treatment of coccidioidomycosis. NAIAD mycoses study group. Am. J. Med. 1990;89(3):282–290. doi: 10.1016/0002-9343(90)90339-f. [DOI] [PubMed] [Google Scholar]
- 11.Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 12.Kuberski T., Herrig J., Pappagianis D. False-positive IgM serology in coccidioidomycosis. J. Clin. Microbiol. 2010;48(6):2047–2049. doi: 10.1128/JCM.01843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leidy N.K., Rennard S.I., Schmier J., Jones M.K., Goldman M. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest. 2003;124(6):2182–2191. doi: 10.1378/chest.124.6.2182. [DOI] [PubMed] [Google Scholar]
- 14.Lindsley M.D., Ahn Y., McCotter O., Gade L., Hurst S.F., Brandt M.E.…Litvintseva A.P. Evaluation of the specificity of two enzyme immunoassays for coccidioidomycosis by using sera from a region of endemicity and a region of nonendemicity. Clin. Vaccine Immunol. 2015;22(10):1090–1095. doi: 10.1128/CVI.00375-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Services A.D.o.H. 2017. Valley Fever 2017 Annual Report. [Google Scholar]
- 16.Tsang C.A., Anderson S.M., Imholte S.B., Erhart L.M., Chen S., Park B.J.…Sunenshine R.H. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007-2008. Emerg. Infect. Dis. 2010;16(11):1738–1744. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]