Abstract
Digitalization of any manufacture industry is a key step in any progress of the production process. The process of digitalization includes both increased use of robotics, automatization solutions and computerization, thereby allowing to reduce costs, to improve efficiency and productivity, and to be flexible to changes. Pharmaceutical Industry (PI) has however been resistant to digitalization, mainly due to fair experience and complexity of the entailed development and manufacture processes. Nevertheless, there is a clear need to digitalize PI as the demand in both traditional and new drugs is constantly growing. Contract Development Manufacture Organizations (CDMOs) have a special digitalizing challenge. Digitalization of PI, and CDMO precisely, should be tightly related to the main aspects of Good Manufacture Practice (GMP), and, to succeed in PI digitalizing requires constant focus on GMP. Close collaboration with constantly changing stakeholders is another important factor which should be in focus during digitalization of CDMO. This paper represents an overview over the main aspects of CDMO digitalization and discusses both the opportunities and challenges of the process, focusing on the practical solutions for successive digital implementation.
Keywords: Pharmaceutical industry, Contract development manufacture organization, Digitalization, Process improvements
Abbreviations: AIDS, Acquired Immune Deficiency Syndrome; CDMO, Contract Development and Manufacturing Organization; EMA, European Medicines Agency; EU, European Union; FDA, Food and Drug Administration; GMP, Good Manufacturing Practice; ITA., International Trade Administration; MHRA, Medicines and Healthcare Products Regulatory Agency; PAI, Pre-Approval Inspections; PI, Pharmaceutical Industry; TDM, Traditional Drug Manufacturing; USD, United States Dollars
Graphical abstract
Highlights
-
•
Nowadays Pharmaceutical Industry needs digitalization.
-
•
Manufacture digitalization is necessary for profitability, progress, and quality.
-
•
Good Manufacture Practice should be in focus under industrial digitalization.
-
•
To succeed with digitalization both opportunities and challenges should be in focus.
1. Background
1.1. Introduction
The pharmaceutical industry (PI) is one of the fastest-growing economic sectors with worldwide sales of more than $1228.45 billion last year in 2020. Since 2017, the pharmaceutical market has grown at the rapid annual rate of 5.8%. Worldwide revenue in the pharmaceutical market was 1143 billion US dollars in the year of 2017 and it will cross 1462 billion US dollars in 2021 (Crawley, 2012). According to International Trade Administration (ITA), “The research, development, manufacture, and marketing of medicines and biologicals for human or veterinary use” are at the heart of the industry (Henkel, Innovationsmanagement et al.). To understand and predict consumer demand and increase supply chain efficiency, digitalization is the utilization of information shared via systems integration, connected devices, and much more.
The industry 4.0 of the Pharmaceutical Industry will in the future contribute toward an intelligent automation technology and may support augmented manufacturing, such as a personalized medicine, additive manufacturing, localized 3D printing of treatments etc. (Reinhardt et al., 2021; Hariry et al., 2021).
In the wake of Covid-19, digitalized technology is more important than ever in allowing firms in all sectors to improve performance through better manufacturing productivity, stronger competitive skills, more accurate planning and forecasting, and financial sustainability (Faraj et al., 2021). Together with this, the old product-oriented business model is being challenged by patent expirations, increasing customers demand, rising competition, and rising pricing pressures. The industries are now being shaped by digital transformation, as digital services beyond the product are being integrated into the range of offers. The face of healthcare is changing thanks to digitization, and ‘connected health’ has the potential to benefit all stakeholders by attaining the ‘triple aim’ of providing a better care experience, increasing health outcomes, and lowering per capita costs (Iglehart, 2014; Fecha, 2017).
High-profit margins, high risk, rigorous rules, long and investment-intensive R&D periods, and significant marketing are all characteristics of the pharmaceutical sector (Scherer, 2000). In the PI, digitization and data analytics can help reduce the high amounts of downtime that pharmaceutical plants are prone to experience (Anthony Jnr and Abbas Petersen, 2021). Machine-to-machine communication and machine-learning artificial intelligence enable seamless procedures, automatic corrective actions, and predictive maintenance via the Internet of Things (Ngamvichaikit, 2021). Since the pharmaceutical manufacturing environment is tightly controlled and highly sensitive, the tiniest mistakes can have life-changing consequences for patients along with a severe business, legal, and reputational impact on the manufacturer (Sehlstedt et al., 2016). For example, a worldwide pharmaceutical manufacturer had to recall almost half a million tablets a few years ago due to packaging and human-monitoring irregularities in the production plant. Digitalization and automation are now ensuring that companies reduce similar mistakes in the future, resulting in the decrease of financial and reputation damage (Kitson et al., 2018). To avoid data-transfer concerns between units, some of the pharmaceutical organizations have introduced digital sensors and robotics and invested in high-availability computing technology. This has resulted in a completely automated production line that makes it much easier to maintain cleanroom procedures, to capture and manage electronic batch records, and analysis of process performance (using root-cause analysis) to find and implement changes. Demand-supply Management is also substantially improved as a result of digital information integration up and down the supply chain (Zhou, 2013). With pharmaceutical business undergoing significant transformations, pharmaceutical companies are still in an experimental phase when it comes to offering digital services beyond traditional products (Parida et al., 2019).
In the PI, digitalization can be extremely beneficial to both small and large firms (Lakshmi and Patel, 2020). For example, using digitalization to develop counterfeit-proof pharmaceuticals with trackable serial numbers through the supply chain should ensure quality while satisfying forthcoming serialization regulations (Anderson, 2018) (Rosenbaum et al., 2017). Pharmaceutical firms can also embrace digitization to fulfill the predicted rise in demand from global markets. They may use digitalization to comply with regulations, uncover manufacturing efficiencies to reduce costs, and interact with suppliers and distributors more swiftly using cloud-based information exchanges (Kumar and Panigrahi, 2014). Furthermore, automation, smart sensors, social media, and health applications may be used to track medicine compliance and forecast demand across regions, allowing for real-time manufacture (van Velthoven et al., 2019).
Unlike other businesses, the healthcare industry is also struggling to deliver digital tools to end-users (Chilukuri et al., 2014). When we come toward Digital transformation we found that it has transformed business models in a number of health industries as well. (Lakshmi and Patel, 2020). However, the pharmaceutical sector has a history of being reluctant to adapt to new technology and embrace digital solutions. This is why the acceptance of digital services in the PI, has progressed relatively slowly (Lee et al., 2019). However, with the Covid-19 pandemic posing unprecedented concerns and continued threat, digitization seems to be the best way to ensure that everyone has access to safe medicines (Ayati et al., 2020).
It is clear, that PI needs to implement digitalization tools. Digitalization is necessary to continue to deliver medical products in accordance with the growing demand of a constantly changing world and population. Although other sectors show successful digitalization experiences, PI has very limited and delayed digitalization experience. This paper, therefore, aims to review the principles of successful digitalization that can be applied to PI. Focus of the paper will be directed to Contract Development and Manufacturing Organizations (CDMOs). General aspects of Good Manufacturing Practice (GMP) in PI are also included and discussed as GMP is a key element in implementation of industrial digitalization.
This is primarily an introductory review article focusing on GMP and processes that are important when it comes to digitizing the pharmaceutical industry. As an introductory article, it will not go into depth on various technologies.
1.2. Good manufacturing practice (GMP) within pharmaceuticals
GMP stands for Good Manufacturing Practice Regulations, which are implemented by regulatory authorities in each country to govern permission and licensing (Cramer, 2006). These regulations allow medicine, medical device, food, and blood makers, processors, and packagers to take proactive actions to guarantee that their goods are safe and effective (Beri and Wolton). GMP standards demand a quality-oriented approach to manufacturing, allowing businesses to reduce or eliminate contamination, mix-ups, and errors. As a result, the buyer is protected against selecting a product that is ineffective or even harmful (Patel and Chotai, 2011). In addition, GMP systems also specify a set of quality based operations controls, like management systems, operating procedures, reliable testing, quality raw materials, detection, and also the investigation of deviation (Villa, 1984), (Sarvari et al., 2020), (Villa, 1984). Recordkeeping, staff qualifications, sanitation, equipment verification, cleanliness, and process validation are all covered under GMP rules (Patel and Chotai, 2008).
1.2.1. Enforcement of GMP
The US Food and Drug Administration (FDA) now has 34 final guideline documents for GMP in the pharmaceutical business, which cover process validation, data integrity, and a wide range of other areas. The FDA defines current GMP as systems that provide proper design, monitoring, and control over manufacturing processes and facilities in the PI and other FDA-regulated industries (Organization, 2011). These systems are intended to assist organizations in ensuring the identification, strength, purity, and quality of drug items (Rangarajan, 2015) (Harris, 2010). While GMP inspections are carried out by National Regulatory Agencies within the European Union, the European Medicines Agency (EMA) oversees inspections to ensure that these standards are followed and are significant players in standardising GMP activities across the European Union (EU). GMP must be followed by any manufacturer of pharmaceuticals for the EU market, regardless of where they are based in the world. The Health Products and Food Branch Inspectorate oversee GMPs in Canada, while the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom conducts GMP inspections. Routine GMP inspections are conducted by each inspectorate to guarantee that drug items are manufactured safely and correctly. The FDA has also begun inspecting Chinese pharmaceutical production plants to guarantee that GMP requirements are being followed. In addition, many national bodies across the world conduct routine GMP inspections to verify that drug products are manufactured safely and correctly. Many countries also conduct pre-approval inspections (PAI) for GMP compliance before the marketing authorisation of a new medicine.
In the PI, the purpose of GMP is to reduce any hazards associated with pharmaceutical manufacturing that cannot be avoided by testing the finished product (Haleem et al., 2015). The main risks are: incorrect containers or labels (patient receives wrong medicine), unexpected and undesired contamination of products (which can damage health or even lead to death), and too much, or insufficient quantity, of an ingredient (which can offer adverse effect or poor treatment), (Abhinaya et al., 2019).
GMP in pharmaceutical production also covers every area of production, from raw materials, facilities, and equipment to employee training and personal hygiene (Abou-El-Enein et al., 2013), (Taylor, 2008). The GMP system demands that processes required for production and testing are clearly defined, reviewed, validated and documented (Ohannesian and Streeter, 2001; Padilla-Zakour, 2009). These also ensure that personnel and materials are well suited for the production of biological products like vaccines and pharmaceuticals (Nally, 2016). Even if there is a Quality Control Laboratory, GMP is still required because good quality must be integrated into the manufacturing process to prevent those errors that cannot be eliminated through final product quality control (Doherty and Kettler, 2005). It is impossible to ensure that every unit of medicine is of the same quality as the laboratory-tested units without GMP (Organization, W. H, 2007a, Organization, W. H, 2007b), (Peng and Abdul Karim, 2013).
GMP is a globally recognized acronym for the regulation and management of pharmaceutical product manufacturing and quality control testing. Everyone in the PI should be familiar with the need of GMP (Kamble et al., 2020). From a health and financial standpoint, low-quality pharmaceuticals can be disastrous for both patients and governments (Del Ciello, 2005). GMP may help reduce losses and waste, as well as safeguard both the company, and the consumer, from foodborne illness (Patel and Chotai, 2008), (Abedellah et al., 2016).
Since the pharmaceutical sector has a responsibility to maintain a safe and sufficient supply of products, GMP must be taken into account from the beginning of pharmaceutical engineering and consulting projects (Woodcock, 2004), (Joseph, 2000). Furthermore, GMP can aid in the expansion of pharmaceutical export potential. Most countries only allow the import and sale of pharmaceuticals that have been produced in accordance with globally recognized GMP standards (Jerez, 2020) and the number of such countries is increasing. Investing in GMP involves investing in high-quality pharmaceuticals. It lowers prices, reduces hazards, and improves the global medication standard (Taylor, 2008).
Products testing by GMP is mostly done on a small sample of a batch (for example, a medicine manufacturer may test 50 tablets from a batch of 1 million tablets) so consequently, the majority of the batch will be used for patients instead of being destroyed by testing. It is critical that medications are made in accordance with the GMP requirements to ensure that quality is embedded into the design and manufacturing process at every stage (Jain and Jain, 2017).
The consequences of GMP infractions vary depending on the nature of the infractions and the medications involved (Kumar and Jha, 2019). A medicine made in violation of GMP may nevertheless match the drug's listed criteria, and the chance of it being harmful or ineffective is low (Banker et al., 2002). As a result, recommendations from the FDA and other regulatory agencies will be tailored to the situation, and health care providers will be the most qualified to weigh the risks and benefits and make the best decision for their patients (Organization, W. H, 2007a, Organization, W. H, 2007b). Regulatory actions against companies with insufficient GMP are frequently taken to prevent the release of potentially dangerous or ineffective medications. However, FDA regulatory action is only taken in exceptional circumstances to prevent the distribution or manufacture of illegal products.
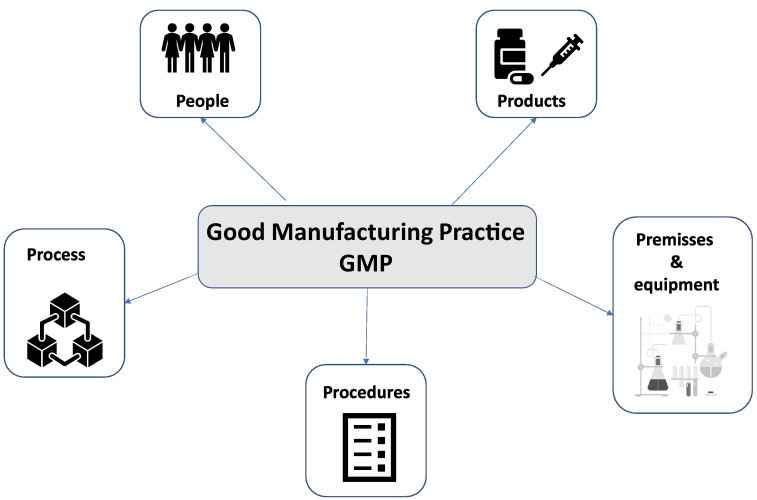
1.2.2. The five principles of GMP in pharmaceutical industry
The Five Principles (5Ps) of GMP in the PI refer to those five key elements which assist to ensure the best and consistent quality, as well as safety, of products. The 5Ps of GMP is a good way of thinking about the major compliance areas (Andraski and Novack, 1996).
The 5Ps of GMP includes People, Process, Procedures, Premises and Equipment and Products. It is known, to succeed with GMP these five parameters must be in focus within the industry. The 5Ps of GMP must be included in any discussion on Best Practices. This is the backbone of most successful manufacturing enterprises because, without perfect tolerance of the 5Ps, your business is effectively non-existent (George, 2012). GMP is designed in such a way that it aids in the reduction of the risks connected with the production of pharmaceutical products, particularly those risks that cannot be avoided after the finished products have been consumed (Lee et al., 2010). Pharmaceutical companies and enterprises that make consumables are the most affected by GMP and 5Ps (Tomić et al., 2010).
The 5Ps of GMP are schematically presented in Fig. 1 and discussed below.
Fig. 1.
Five principles of Good Manufacturing Practice, schematical overview.
1.2.2.1. People
Everyone participating in the production of medicine must have a clear understanding of their responsibilities. Employees must be trained, and their performance must be evaluated, to follow the process. Manufacturers must assess training methods on a regular basis to ensure that they remain effective in maintaining that staff are properly taught and competent (Organization, W. H, 2007a, Organization, W. H, 2007b). Without the people who manage its operations, a manufacturing company is nothing. This is an essential component. People are at the heart of any organization, and they must be trained in accordance with the company's desired output (Organization, W. H, 2007a, Organization, W. H, 2007b).
To make work more effective and rapid, roles and tasks are allocated. Each person is assigned specific responsibility according to his capability, knowledge, and experience. This is at the top of the list since the other principles are meaningless without it (Velagaleti et al., 2002).
1.2.2.2. Process
A process is a collection of interconnected actions that transform inputs into outputs. It is a sequence set of well-organized activities which need to be followed properly to gain maximum output and benefits. Different strategies and operations can be chosen for speeding up the process of manufacturing practices which result in production of larger quantity of product within a shorter period. However, thorough studies and inspections should be made early about the credibility and potential of newly adopted strategies in this regard so that quality must not be compromised. There must be complete trust that no deviation or any kind of contamination occurred during the manufacturing process (Gouveia et al., 2015) The need for repeatable precision is therefore critical in pharmaceutical manufacture. Therefore, manufacturing processes must be precisely defined through consistency and documentation. This has been shown to aid in the unfettered flow of manufacturing throughout time. Critical steps in the production process must be recognized, and control procedures must be flexible enough to alter as needed (Gad, 2008). By processes, we mean good documentation and the recording of everything that happens in the organization, as well as adequate reports of deviations when they occur and any other vital processes that need to be tracked (Organization, W. H, 2007a, Organization, W. H, 2007b). So, processes should be well-documented, transparent, and consistent, and they should be made available to all personnel. Regular evaluations should be undertaken to ensure that all employees are following existing practices and meeting the organization's requirements. These timely evaluations will ensure the product safety and reduce the chances of contamination. Even if there will be any error in the actual process and requirements, it will be detected early, resulting in less spoilage of product which will be valuable for the organization.
1.2.2.3. Procedures
A procedure is a set of instructions for carrying out a process or a component of a process to obtain a consistent result. Any manufacturing company's employees must follow the rules and procedures in place to ensure that it runs smoothly (Joseph, 2000). If a batch has a problem and needs to be recalled, routinely documenting data at crucial stages of manufacture will make determining the root of any fault or non-conformance in quality much easier (Zacharia and Mentzer, 2004).
Both processes and procedures must be created and recorded to provide total clarity on what a company must do and how it must fulfill the required standards in its operations. All procedures must be clearly spelt out and followed (Schaufelberger et al., 1991). It is necessary to investigate any deviations from the regular procedure (Phelps and Madhavan, 2017).
1.2.2.4. Premises and equipment
Any building or other structure, including any machinery, apparatus, engineering systems, or other objects that are physically affixed and integrated to the building or structure, is described as a «premises» while machines and medical gadgets used to help, prevent, cure, or monitor a person's health or illness are referred to as “equipment.”
Manufacturing organizations should strive to build their facilities and equipment in a way that allows for proper cleaning and prevents cross-contamination. Premises should be designed, located, constructed, maintained, and adapted to best suit the operations to be carried out.
All the facilities and equipment must have properly documented cleaning processes. Cross-contamination prevention measures must be in place, as well as written calibration instructions (Krekora, 2008). These needs must be considered when designing a facility, and equipment must be calibrated and evaluated on a regular basis to ensure it is suitable for its purpose and produces consistent results. Apart from routine inspections of equipment and machinery, sanitation inspections must be carried out. (Aghayan et al., 2016). Equipment must be thoroughly washed, cleaned, and dried on scheduled basis and this equipment should not pose any harm to products.
Additionally, the maintenance area needs to be separated from the production area. Similarly, storage areas must have sufficient capacity for storage of different products in well organized and orderly manner. All required conditions must be fulfilled, for example, humidity, temperature, continuous electricity supply, pipe fitting, ventilation. Harmful, highly reactive, and radioactive material, and dangerous medicines should be stored in a safe and secure place.
1.2.2.5. Products
All the mentioned above principles are meant to support the quality and consistency of the final products that consumers will utilize. It's also an end-to-end obligation, ensuring that commodities entering and leaving the facility are in good condition and handled properly (Render et al., 2005). Manufacturers, for example, must have requirements for the raw materials and components that they utilize (Kapoor, Vyas et al.). There must be repeatable methods for research, development, manufacturing, processing, packaging, sampling, testing, status control, and record-keeping (Chan et al., 2005).
This carries the weight of the problem that a manufacturing firm is attempting to solve. This requirement prompted the study and the allocation of time, money, human, and other resources (Jaiganesh and Sudhahar, 2013). As a result, a manufacturing company's inability to translate what the mind envisioned into a physical product that actually meets the desired requirement represents a serious threat to the company's long-term viability and integrity (Nayereh et al., 2012). As a result, requirements for raw materials, components, intermediates, and finished products are required (Sharp, 2004). Seek out the most efficient and hygienic methods for manufacturing, packaging, sampling, testing, maintaining stability records, and monitoring status (Karmacharya, 2014).
If the 5 Ps are followed correctly in the manufacturing of pharmaceuticals and other consumables, they can reduce the danger of not just cross-contamination and errors but also increase the availability of high-quality products (Peng and Abdul Karim, 2013). The consistency of strategy and technique in the manufacture of drugs is critical in ensuring that users can trust that their medicines contain the proper chemicals in the proper amounts to have the desired effect. We are frequently advised that quality cannot be retroactively ‘tested into’ pharmaceuticals. Instead, in the large-scale production of medications, the only way to ensure quality and consistency is by the rigorous execution of standardized procedures by properly trained and accountable staff (Vugigi et al., 2019). Thus, the 5 Ps of GMP should be in focus under implementation of digitalization processes.
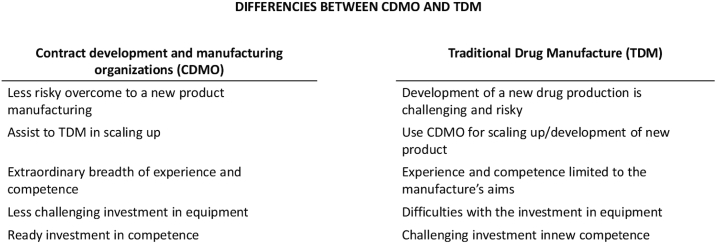
1.3. Contract development and manufacturing organization (CDMO) vs. traditional drug manufacturing (TDM)
A contract development and manufacturing Organization (CDMO) is a corporation that provides drug development and manufacturing services to the pharmaceutical sector. CDMOs and pharmaceutical corporations collaborate to outsource medication development and manufacture. CDMOs that provide full service can handle all aspects of drug development and manufacture, as well as work with clients who want to outsource specific parts of their process. It all depends on the requirements of each client. Services which are offered by CDMO comprised of pre-formulation and formulation development, method development and stability studies, materials for Pre-clinical, Phase I, and last stage clinical trials, formal stability and scale-up, commercial production, and registration batches, serialization, and shipment, etc.
CDMO differs from traditional drug manufacturer (TDM) and must be considered with respect on those special differences with TDM when GMP and digitalization process are under development and implementation. Briefly, the main aspects of differences between TDM and CDMO are presented on Fig. 2. Some of the aspects are discussed below as well.
Fig. 2.
Main different aspects between Contract Development and Manufacture Organizations (CDMO) and TDM.
A full-service CDMO has an extraordinary depth and breadth of experience and competence than a traditional drug manufacturer (TDM). CDMOs invest in talented researchers, chemists, and development specialists because their core competency is their Company. After all, those that can give pharmaceutical businesses knowledge, oversight, and innovation will be the most successful.
Equipment is another area where major CDMOs are investing. From the perspective of the TDM, pharmaceutical businesses have a difficult dilemma when it comes to internal drug research and production when they want to expand capabilities, introduce a new drug, or manufacture at a different rate. After all, those choices all entail significant financial investments. Outsourcing to a CDMO, on the other hand, allows businesses to access large amounts of equipment and facilities without incurring the costs of ownership.
CDMOs are also well-known for their capacity to assist pharmaceutical businesses in scaling up. For pharma companies, changes in production volume or the inclusion of a new drug variety can be exceedingly risky. When they join with a CDMO, however, those decisions are far less risky, and they come with shorter lead times. Pharmaceutical development and production outsourcing allow firms of all sizes to grow. Pharmaceutical companies of all sizes can operate more leanly and efficiently, knowing that drug development and manufacture would not break their budgets.
Further information and discussion about digitization process in pharmaceutical industries is mainly directed to digitization in CDMOs, although it can be applied to TDM as well.
2. The digitization process in contract development manufacturing organizations (CDMO)
We are certain to witness the expected improvement in productivity with a digital transformation strategy, robust levels of digital diffusion, and personnel up-skilling. From the top of the corporate ladder to the bottom, now is the time to embrace digitization more than ever. After all, it is essential to our economy. During digitization and digital transformation, your company will need to develop cybersecurity, artificial intelligence, and other strategies. To manage these technologies, you'll need a current skill pool that can put them to work as soon as they arrive. Up-skilling and training can make a huge difference in the game. To increase productivity, it is vital that all businesses, not just a few, adopt digitalization and technology. Businesses that do not choose to accept emerging technology will be impacted by the digital transformation. In fact, these businesses may be left behind because of the consequences. To make a significant effect, true digitization will need to embrace all industries and businesses. This means that huge organizations will have to face their technological demons head-on and devise a strategy to address their adoption challenges. To stay competitive, small, and mid-sized firms will need to start implementing technology. Companies will need to devise a productivity strategy that encompasses the digital transformation of their business model, as well as their entire sector and value chain. Every necessary change, every training strategy, and every move toward digitization are all part of a bigger digital transformation strategy. Businesses that change their strategy and invest in digital transformation increase their productivity through revenue growth and return on digital investment.
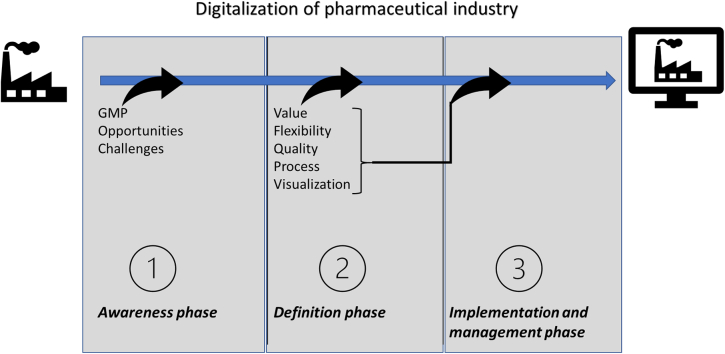
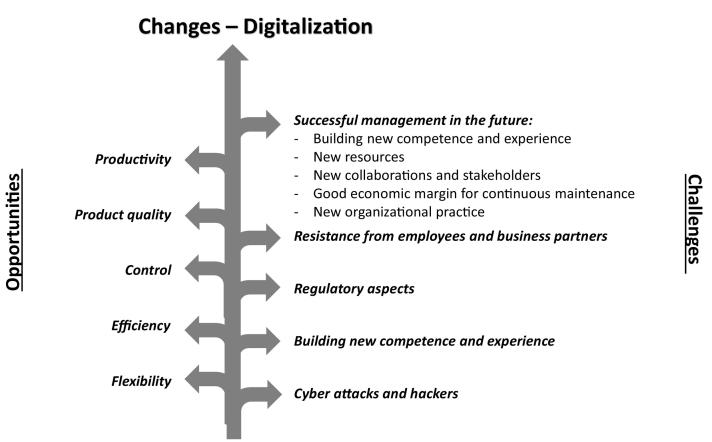
As with everything in life, everything that connects with changes also gives opportunities and challenges for an organization. The biggest challenge and perhaps the mistake that happens today is that managers see change as a transitory process. Change is not something that is transient it is a constant process in any business. It is something that a business must continually manage to navigate and deal with if they are to survive over time. Digitalizationis largely about changing and renewing services, processes and working methods. And it brings both opportunities and challenges. To be able to handle challenges correctly, it is necessary to foresee them, thereby being prepared. Opportunities and challenges related to digitalization are therefore discussed here (Fig. 3). Solutions to some general digitalization challenges are also presented.
Fig. 3.
Opportunities and challenges related to digitalization process, changing process in organization.
2.1. Opportunities
In manufacturing, there is often worry about machines replacing workers, but the conversation should truly focus on how they will collaborate for the best results. It means that making technology more accessible to manufacturers will enhance the productivity of their workers who interface with computers and software, allowing you to get the most out of your production operations and labeling in a smart factory. The focus of the discussion should be on the understanding of human-computer symbiosis, or the mutually beneficial relationship between technology and humans. There should be efforts on how machines and software can smartly and physically increase the productivity of systems to be greater than either human or machine productivity alone.
Pharmaceutical firms are subject to rigorous rules and standards and must also carefully secure the integrity of their data. Finding an effective, reliable means to do so will help these organizations to enhance their production and product quality. The use of a digitization strategy can help to monitor, regulate, and optimise production processes, increasing efficiency and productivity. Following, are different business aspects which can be improved by implementation of digitization regarding GMP (Lakshmi and Patel, 2020) (Plumb, 2005) (Trienekens and Zuurbier, 2008).
2.1.1. Productivity
Pharmaceutical manufacturing has increased its global reach in recent decades. Demand for domestic and imported pharmaceutical items is increasing as economies grow and health-care supply and insurance mechanisms expand. Not only is demand rising, but so the diversity of pharmaceutical needs is rising too, as emerging nations increasingly cope with non-communicable diseases that are widespread in affluent countries. Diabetes and hypertension are examples of such disorders, while communicable diseases such as acquired immune deficiency syndrome (AIDS), malaria, and tuberculosis continue to plague many emerging economies. Furthermore, people are living longer, with figures indicating that the global population over 65 years old will rise. Another aspect of manufacturing quality is the capacity to continuously produce the product in sufficient quantities and at a fast enough rate to ensure that supply satisfies demand over long periods of time (Awad et al., 2018a, Awad et al., 2018b). This is particularly true in the PI, where the medication is often life-saving and continuous access is essential.
This scenario demands innovative approaches to counter these new challenges in the production sector. Digitization offers new and more efficient ways to meet up with this increased production demand by improving manufacturing process and making them faster while maintaining the product quality.
2.1.2. Product quality
Pharmaceutical companies must collect data on their procedures to ensure product quality. Many businesses, on the other hand, continue to rely on paper documentation, which makes data gathering and management more susceptible to errors. This information also takes more time to process when recorded on paper. The use of digitization technologies can help to reduce the risk of these errors and increase the efficiency of data collection. Pharmaceutical firms can collect data from a variety of sources, standardize it, and analyse it by applying digitization techniques. It generates simple reports that aid in quality assurance and are available immediately after a batch is completed (Awad et al., 2018a, Awad et al., 2018b; Capel et al., 2018; Patidar et al., 2018).
An automatic alert can also be sent to quality management whenever a recorded value falls outside of the expected range. Furthermore, when data is kept in the cloud, all employees have access to the most up-to-date information, independent of whatever internet-connected device they are using (Yacuzzi et al., 2004). Since the procedure itself does not alter, it is also effective for validation. When GMP is digitised, the user receives precise instructions for each activity, such as safety instructions or recommended user actions, which help to prevent errors.
To summarize, switching to digitalization can improve quality control efficiency and reliability. Initial results are available during the production process, and reports and compliance documentation are ready as soon as a batch is completed, all thanks to the usage of digitization technology (Shah, 2004).
2.1.3. Control
Few sectors have as stringent quality standards as the PI. Every step of the process, from product creation to packaging labelling, to cleaning the equipment, must be meticulously monitored.
The batch process, in which items are created in batches rather than continuously, is widespread in the pharmaceutical sector. While this has advantages, it also means that one minor error can damage an entire batch. A faulty batch implies a lot of money, time, and raw resources are squandered. If the manufacturer fails to detect a faulty batch, the consequences can be severe, resulting in serious bodily injuries, penalties, and lawsuits, as well as a significant loss of reputation. Effective batch control is necessary to avoid these, and other, harmful occurrences. The usage of digitization in this circumstance will allow you to easily control the process and optimise production (Lexchin et al., 2003) (Basu, Gupta et al.).
Digitization also aids in streamlining the packaging phase of your manufacturing process. Digitization technologies can control, monitor, and visualise your process during packing, just like they can during batch manufacturing, making it easier to identify ways of improving it. You may also access data remotely, allowing you to keep an eye on, and work on, your process from wherever you are.
Digitalization of control processes plays one of the key roles in profitability of the pharma-business. Reduction in the use of raw materials, human resources and time, as well as reduced risk for final products of low quality due to digitally automatized control steps, directly increase the profitability.
2.1.4. Efficiency
Control is related to efficiency and efficiency relates to how much time, money, and materials a company needs to create a certain amount of output. As a result, efficiency and production are inextricably intertwined. Using digitalization, you will be able to collect data throughout your entire facility, giving you a clear image of operational efficiency. You can utilize the data and reports you collect to identify areas where your procedures and facility could be improved. You can also use it to automate certain aspects of your procedures. You can accomplish the same amount of work with less manual input, time, and resources if you implement digitization. Operators, for example, can receive immediate information of problems and promptly remedy them via automated alarms (Alloghani et al., 2018; Steinwandter et al., 2019) (Gbadegeshin, 2019).
Also, because of the norms and standards that the pharmaceutical sector must follow, validation is an important element of the process. In this sector as well, digitization increases efficiency. It comes with full compliance as standard and allows you to update or improve your processes without having to revalidate them, saving your time and money (Arden et al., 2021).
2.1.5. Flexibility
The capacity to have a flexible manufacturing process will certainly allow for production of novel medications and vaccines to happen faster than with a traditional fixed production process. When a new medication is added to a fixed process, it must either be added to existing infrastructure, or a new facility must be developed. Flexible manufacturing would enable the production of a new drug in a facility that uses single-use materials for processing, allowing for the by-passing of cleaning validation of current equipment (Hurter et al., 2013). Because these facilities are so flexible, time is saved during the manufacturing process, allowing the drug or vaccine to reach the market faster. The patient is thought to benefit immediately from the acceleration in production process (Bennett and Lemoine, 2014).
Aside from facilities, technologies like continuous manufacturing enable manufacturing to be adjusted based on patient demands. Similarly, the adoption of automation, robotics and single-use isolators would directly address patient safety. Patient adherence, or the ability to increase medication compliance, may also be enabled through flexible manufacturing. In a fixed production facility and process, for example, a dose is determined by the batch record and does not allow for process modification (Smith D et al. 2018). A more flexible facility, on the other hand, enables for modifications in the drug product production process, such as adjusting the dose in a vial or modifying the drug product's image (example, dose variation using 3D printing). As a result, alternative therapeutic representations, doses, and durations might have a direct impact on patient demands. When these technologies are integrated across scales, they will result in a paradigm shift in how development and production are done in the future. Overall, implementing digitised technologies in the pharmaceutical production sector would provide for patient benefits in the future (Jamroz et al., 2018).
2.2. Challenges
Pharmaceutical companies that wish to digitize their supply chains and operations do face difficult issues (Fig. 3).
Even as technology continues to rapidly change, they must build solutions not just within their own four walls but in collaboration with numerous external partners throughout the supply chain (Sarkis et al., 2021a, Sarkis et al., 2021b). Businesses will need to be extremely transparent and overcome deeply embedded ways of working, as well as resistance from organizations and employees who are unduly habituated to decades-old methods. Furthermore, there is a lot of hype to cut through as well as hazards to manage(Chowdary and George, 2012). Regulatory aspects provide another degree of complexity, as they include not only new requirements for businesses but also a changing environment in which regulators must learn new things. On this path, there are several critical success criteria. Companies must first gain a thorough understanding of the ecosystem and its changing technology (Parekh et al., 2016).
Pharmaceutical companies must also have the necessary resources, such as a cross-functional team of experts and the necessary funds to make the necessary investments. If players are to develop fully integrated end-to-end supply chain solutions, they must collaborate with partners(Patel et al., 2013). And, most importantly, businesses must acknowledge that this is a journey that will include prioritizing a few projects, including an experimental mindset and learning by doing. At the same time, Pharma businesses will have to deal with a number of significant risks and concerns. One of top concern when it comes to digitised operations is cybersecurity (Sokolov, 2020).
Like the financial sector, which continues to be a target of cyber-attacks, pharma executives are concerned about hackers gaining access to digital assets, physical assets, and machinery. Similarly, maintaining safe cloud transfers of sensitive data (such as demand, supply, price, and contact information) is a fundamental requirement that cannot be overstated (Srai et al., 2015). In addition, the industry will require some level of standardization to assure compatibility among different systems and devices, given the large diversity of technology and rapid development cycles. Policymakers and industry associations, in particular, may assist with these difficulties by advocating for common industrial standards and efficient data security and data protection policies (Stanić, 2019
2.3. Implementation of digitization
The pharmaceutical sector will be impacted by technological improvements. Industrial productions are linked with current information and communication technology as part of the digitalization process, allowing for a basically self-organized manufacturing process and the acquisition of valuable, usable data (Rantanen and Khinast, 2015). Pharma and biotech companies must plan forward for the future, taking into account technical improvements such as digitalization (Awad et al., 2018a, Awad et al., 2018b). Their partners, such as CDMOs, must also keep up with these advances. They have the potential to be involved in their customer's processes at the ground level and become a valued partner by doing so early in the process (Hunt, 2006).
To get a successful implementation, one must focus on the following parameters and aspects. As discussed below.
2.3.1. Focus on value
The term “digitalization” covers a wide range of topics and can occur in many different areas of a CDMO. Different digitalization efforts, such as data analytics software in the field of R&D, are not directly related to the value chain. To acquire a thorough picture of the topic of digitalization, it is necessary to divide it into distinct divisions (Macdonald, 2021a, Macdonald, 2021b). Digitalization can be as basic as replacing a paper book with a tablet at a visual inspection workstation. Instead of documenting their inspection results in a paper book, employees would use the tablet to enter them immediately into the enterprise resource planning system (Ganesh et al., 2020). It could also be a more complicated procedure, such as digitally developing formerly paper-based operations into the new software. For example, in a laboratory, the transfer of measured data that was previously done in an analog method could be linked to a digital process. The data would be sent automatically from the measuring equipment to the software, which would store and process it for further processing (Volgina, 2021). Machine learning, artificial intelligence, blockchain, and big data are examples of new trends and technology covered by digitalization. While these technologies have a lot of potentials, it's wise to employ them only when they're really necessary (Mendenhall and Kontny, 2010). Projects should be chosen based on whether they will improve quality and efficiency and hence provide value to the company and all relevant partners, including customers and suppliers, rather than on their duration and complexity (Mackey and Nayyar, 2017).
2.3.2. Maintenance of flexibility
While standardization can assist a CDMO in minimizing overall process complexity, flexibility is required to meet the needs of specific clients. For example, when handling multiple filling processes based on a customer's requirements, paper-based processes can be adaptable. However, because it seeks to standardize operations in order to handle them in the system, the usage of a digital system may limit flexibility (Ganesh, 2020). Customers of a CDMO, for example, utilize several wordings in their manufacturing specifications (Pandya and Shah, 2013). Different wordings result in many process variants that must be documented in the digital system if they are not standardized (Chen et al., 2020). The usage of predetermined text modules that cover all relevant components would meet both standardization and flexibility in this scenario. While digitalization will not prohibit a CDMO from remaining flexible, it is important to consider flexibility while designing systems (Iezzi, 2014).
2.3.3. Prioritize the quality
Quality is the first and primary need in the realm of high-value injectable products. Patients are being injected with the same medications that a CDMO creates for its pharma and biotech customers, so this should come as no surprise. (Doig and Jones, 2016). One way to get there is to look at multiple single pharmaceutical production steps as a whole, with a focus on the entire value chain and potentially the entire supply chain up to the application because one of the main benefits of pharma digitization is to improve the quality of business processes and make them both safer and more efficient (Closs, 2014).
Digitalization, on the other hand, is unlikely to be completed in a single step and must be viewed as a whole program. The process steps within an organization become increasingly connected as a result of several subprojects (Alagarsamy et al., 2019). This presents unique hurdles for a corporation, particularly in the pharmaceutical industry. Even minor adjustments must be reviewed since processes are well-established and recognized by regulatory agencies. When these procedures are successfully performed, however, there is great potential for ever-higher quality and increased efficiency (Demesmaeker, Kopec et al.).
2.3.4. Standardization of process
In the pharmaceutical sector, one of the most significant advantages of digitization is that it makes documentation processes safer and more efficient. CDMOs differ from their pharmaceutical and biotech customers by their very nature, and these distinctions can be particularly obvious when it comes to digitalization. An example is the use of laboratory testing equipment. Customers, not the CDMO, choose what laboratory testing equipment needs to be used for a specific particular analysis (Coyle and Nguyen, 2020). A TDM can utilize one type of equipment, while a CDMO may require multiple types of equipment for the same type of testing, depending on the clients' preferences (Macdonald, 2021a, Macdonald, 2021b). The ensuing range of test techniques makes connecting to the internet and designing digital processes more difficult. Furthermore, CDMOs typically have a wide customer base with a variety of goods, resulting in a variety of process variations, such as different documentation processes (May, 2021). Adoption of these various paper-based documentation processes one-to-one in the digital system would result in significant system reliability and, as a response, a loss of efficiency (Faridi and Malik, 2020). As a result, it's critical to harmonize and standardize procedures before employing software to digitalize them.
Digitalization is rarely accomplished in a single project. Rather, it is addressed as a comprehensive program with multiple sub-projects. When it comes to procedures, these sub-projects are frequently interdependent and have some ties (Kumar et al., 2020). As a result, it's crucial to figure out if there are any project dependencies or even potential synergies. A process's digitization may have an impact on other projects. Furthermore, determining the ownership of corporate processes may not be as straightforward as one might expect (Chircu et al., 2017). As a result, to digitalize processes successfully, it is critical to identify cross-divisional activities and specify their ownership.
2.3.5. Visual inspection as an example of digitalization
The procedures must adhere to several other regulations and documentation requirements. Every single filled unit purchased by the customer is submitted to a final visual check after the production process. Following compounding and filling, and before further packaging operations, a quality check of the aseptically prefilled syringes, vials, and cartridges is performed separately. Let us understand this with an example of Vetter Pharma Company. Several hundred employees of Vetter Pharma company are involved with this inspection on a daily basis (Macdonald, 2021a, Macdonald, 2021b). For many years, the inspection findings and their evaluations were kept on paper and then analyzed using an SAP (System Application and Products) system. Each year, SAP printed around 60,000 manufacturing standards for the Visual Inspection crew to use in documenting their inspections. The results were then manually inputted into the batch analysis and SAP system by the shift coordinator (Demyanenko et al., 2016).
Staff might be classified as digital or non-digital natives when it comes to accepting and using digitalization (Belhamel, 2019), (Patidar et al., 2018), (Chawla et al., 2016), (Parekh et al., 2016). Other types of stakeholders, such as Management, affected employees, the workers' council, and both advocates and critics of the project, should be considered as well (Macdonald, 2021a, Macdonald, 2021b). Organizations should build a specialized transformation team as early as possible, ideally as part of the business strategy.
2.4. Implementation solutions
2.4.1. Challenges 1: Managing higher complexity
Digitalization has the potential to significantly improve the quality of critical industrial processes like documentation and materials management, making them safer, more efficient, and consistent. However, CDMOs must overcome higher complexity in several important areas, than their pharmaceutical industry counterparts, in order to gain these benefits (McWilliams et al., 2018). While TDM may only need one type of equipment for a certain investigation, CDMOs may require a large number to meet client preferences. This results in a plethora of nuances in test methods and processes that must be considered. Similarly, the diverse nature of CDMO customers and products results in a plethora of process variations. It can be difficult to try to digitize each process for each product for each consumer (Pleitt et al., 2019). In the pharmaceutical sector, digitalization is not a single project but rather a multi-project initiative with several interconnected sub-projects (Edwards, 2010). For CDMOs, digitization of one process is likely to have an impact on a number of other initiatives (Bravo and de Carvalho, 2013).
2.4.1.1. Solution
Smart CDMOs are actively working to harmonize and standardize cross-divisional processes to promote software-based digitization. Processes that can be aligned and consolidated, with clear ownership definitions. These firms' project managers are also proactively mapping out any dependencies — as well as potential synergies — among their numerous workflows and projects.
2.4.2. Challenges 2: Staying flexible by building adaptability into the digitalized system
While process uniformity is required for digitization in the pharmaceutical business, CDMOs must nevertheless meet the unique needs of each customer. For example, various clients' production specifications frequently utilize various terminology and taxonomies, resulting in many process variants that must be accounted for during production (Sarkis et al., 2021a, Sarkis et al., 2021b).
2.4.3. Solution
As this scenario demonstrates, digitalization does not imply flexibility. This quality must be a primary consideration when CDMOs construct systems to manage their processes.
Predefined text modules that cover all major components of product specifications, for example, can assist achieve both standardization and flexibility criteria in the example stated above.
2.4.4. Challenges 3: Getting the people on board
Digital transformation can have a direct influence on your pharmaceutical company's workforce (Pandya and Shah, 2013). Teams and individuals may not accept the new method if the process is not adequately explained, or concerns are not taken seriously. Many factors might influence a company's perceptions of the value and appeal of digitalization. Some of these factors include educational level, generational gap, different types of stakeholders, and the company's mission statements.
2.4.4.1. Solution
A digital task force that is well-trained and focused can be a valuable option to tackle this challenge. This task force can work together to do a thorough stakeholder analysis, handle consumer complaints and expectations, and ensure a smooth-running culture and workplace throughout the digitization process (Bieri and AG, 2017).
2.4.5. Challenge 4: Knowing the right option for digitalization purpose
In the pharmaceutical sector, digitalization encompasses a wide range of technologies used in practically every aspect of a CDMO, but the type of innovation pursued by a CDMO should always be one that adds value (Kane, 2012). While Machine Learning, Blockchain, Artificial Intelligence, and Big Data all have enormous potential, embracing them only for the sake of innovation might be an expensive error (Fekih and Lahami, 2020; Paul et al., 2020).
2.4.5.1. Solution
CDMOs that are proactive, focus on projects that will improve quality and efficiency. Begin by identifying low-hanging fruit or little tasks that will yield immediate results. For example, substituting a data-entry book with a tablet at a visual inspection workstation is straightforward to learn and adds value through efficiency. Identify projects that will need more effort but will provide significantly greater benefit while these activities are underway (Pack et al., 2009). Completely revamping a traditionally paper-based, manual data-transfer system, for example, is significantly more difficult. However, having a system that delivers data from laboratory measuring equipment to storage and processing software automatically may be well worth the effort (Iezzi, 2014).
3. Conclusions
It is known that digitalization in manufacturing practice is an important part in future development of both technology, business, and economy. However, PI has been resistant to digital implementation thereby leading to very slow progress within digitalization in pharmaceutical sector.
Digitalization in PI can bring several advantages like reduced production costs, improved quality reduced capacity restrictions. Most pharmaceutical businesses have been reluctant to implement digital manufacturing techniques because they were concerned that their systems, data, and people were not ready. However, many firms have realized that waiting is not an option and have begun experimenting with digitization. While the use of a digital platform can improve processes in a variety of ways, including data collection, real-time sharing of trial results, and the capacity to track various aspects of productions.
Pharmaceutical firms and CDMOs have a lot in common when it come to digitization. However, a CDMO's unique problems include high complexity due to a wide range of operations, as well as digitalization of operations while keeping flexibility. There will challenges in managing new initiatives resulting from digitalization as well the challenges of keeping the staff on board in terms of new propositions. There will be a need to train the people, keep them aware of new initiatives, and encouraging them to give the maximum output. A CDMO's ability to deal with these difficulties depends on their experience. When done correctly, digitization improves the quality of a CDMO's goods and processes. Digitalization in the pharmaceutical industry, when implemented right, can improve product and process quality. For CDMOs, this means navigating complexity without sacrificing flexibility for our clients, ensuring a smooth transition for all employees, and enhancing the entire value chain. Modern CDMOs can stimulate intimate interactions among diverse professionals by integrating all services at one location, resulting in a more agile approach to pharmaceutical development.
Authorship and conflict of interest statement
There are no conflicts of interest statement in this study. The study was performed after own initiative of and by , Dr. Glenn A. Hole, (Phd in Management). Dr. Hole is Thought leader in Business & Digital Transformation and Associate Professor in Digital Transformation @ Molde University College.
Credit author statement
Author contribution to the manuscript has been as followed:
Glenn Hole, article idea, academic draft for article, active writer.
Ian McFalone-Shaw, responsible for academic English expression, correct sentence structure, active proof-reader, assistance with references and abbreviation, active rewriting of structure.
Anastasia Hole, structure and models, active correction for academic build up, visualization of ideas and thoughts, active rewriting of structure.
Declaration of Competing Interest
None.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abedellah A., Noordin M., Zaki A.A. Pharmaceutical good manufacturing practice regulatory affairs in Sudan: continuous debate between regulatory authority and manufacturers. Pharmaceut. Reg. Affairs. 2016;5(166):2. [Google Scholar]
- Abhinaya N., Thunga G., Muddukrishna B., Pai R., Shenoy U.R., Khan S., Pai K.G. A research on effective management of manufacturing defects to avoid product recalls: a challenge to pharmaceutical industry. Res. J. Pharma. Technol. 2019;12(12):6124–6132. [Google Scholar]
- Abou-El-Enein M., Römhild A., Kaiser D., Beier C., Bauer G., Volk H.-D., Reinke P. Good Manufacturing Practices (GMP.) manufacturing of advanced therapy medicinal products: a novel tailored model for optimizing performance and estimating costs. Cytotherapy. 2013;15(3):362–383. doi: 10.1016/j.jcyt.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Aghayan H.R., Arjmand B., Burger S.R. GMP. facilities for clinical cell therapy product manufacturing: a brief review of requirements and design considerations. Perinatal Tissue-Derived Stem Cells. 2016:215–227. [Google Scholar]
- Alagarsamy S., Kandasamy R., Subbiah L., Palanisamy S. 2019. Applications of Internet of Things in Pharmaceutical Industry. Available at SSRN 3441099. [Google Scholar]
- Alloghani M., Al-Jumeily D., Hussain A., Aljaaf A.J., Mustafina J., Petrov E. 2018 11th International Conference on Developments in eSystems Engineering (DeSE) IEEE; 2018. Healthcare services innovations based on the state of the art technology trend industry 4.0. [Google Scholar]
- Anderson S. The digitization of the Pharmaceutical Historian archive. Pharm. Hist. 2018;48:2. [Google Scholar]
- Andraski J.C., Novack R.A. Marketing logistics value: managing the 5 P’s. J. Bus. Logist. 1996;17(1):23. [Google Scholar]
- Anthony B., Jnr., Abbas Petersen S. Examining the digitalisation of virtual enterprises amidst the COVID-19 pandemic: a systematic and meta-analysis. Enterprise Inform. Syst. 2021;15(5):617–650. [Google Scholar]
- Arden N.S., Fisher A.C., Tyner K., Lawrence X.Y., Lee S.L., Kopcha M. Industry 4.0 for pharmaceutical manufacturing: preparing for the smart factories of the future. Int. J. Pharm. 2021:120554. doi: 10.1016/j.ijpharm.2021.120554. [DOI] [PubMed] [Google Scholar]
- Awad A., Trenfield S.J., Gaisford S., Basit A.W. 3D printed medicines: a new branch of digital healthcare. Int. J. Pharm. 2018;548(1):586–596. doi: 10.1016/j.ijpharm.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Awad A., Trenfield S.J., Goyanes A., Gaisford S., Basit A.W. Reshaping drug development using 3D printing. Drug Discov. Today. 2018;23(8):1547–1555. doi: 10.1016/j.drudis.2018.05.025. [DOI] [PubMed] [Google Scholar]
- Ayati N., Saiyarsarai P., Nikfar S. Short and long term impacts of COVID-19 on the pharmaceutical sector. DARU J. Pharmaceut. Sci. 2020;28(2):799–805. doi: 10.1007/s40199-020-00358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G.S., Siepmann J., Rhodes C. C.R.C. Press; 2002. Modern Pharmaceutics. [Google Scholar]
- Belhamel C. The new challenges in the pharmaceutical industry, what strategy to face them? Sanofi experience. Studies. 2019;5(2) [Google Scholar]
- Bennett N., Lemoine J. What VUCA really means for you. Harv. Bus. Rev. 2014;92(1–2) [Google Scholar]
- Bieri C., Ag K.P. Pharma manufacturing. Contract. 2017;3:4. [Google Scholar]
- Bravo A.M.S., de Carvalho J.C. Understanding pharmaceutical sustainable supply chains–a case study application. Independent J. Manag. Prod. 2013;4(1):228–247. [Google Scholar]
- Capel A.J., Rimington R.P., Lewis M.P., Christie S.D. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018;2(12):422–436. [Google Scholar]
- Chan J.S., Chow Y.Y., Cheung W.C. A road map to good manufacturing practice. Quality Manag. A New Era, World Sci. 2005:38–50. [Google Scholar]
- Chawla V., Singh M.P., Kumar M. Product recall: a commentary on rising incidences. Pharm. Res. 2016;1(2):32–35. [Google Scholar]
- Chen Y., Yang O., Sampat C., Bhalode P., Ramachandran R., Ierapetritou M. Digital twins in pharmaceutical and biopharmaceutical manufacturing: a literature review. Processes. 2020;8(9):1088. [Google Scholar]
- Chilukuri S., Rosenberg R., Van Kuiken S. McKinsey & Company; 2014. A Digital Prescription for Pharma Companies; pp. 1–6. [Google Scholar]
- Chircu A.M., Sultanow E., Sözer L.D. A reference architecture for digitalization in the pharmaceutical industry. INFORMATIK. 2017;2017 [Google Scholar]
- Chowdary B.V., George D. Improvement of manufacturing operations at a pharmaceutical company: a lean manufacturing approach. J. Manuf. Technol. Manag. 2012 [Google Scholar]
- Closs S. Quality by design: working with your contract manufacturer. Chem. Today. 2014;32(4) [Google Scholar]
- Coyle D., Nguyen D. No plant, no problem? Factoryless manufacturing, economic measurement and national manufacturing policies. Rev. Int. Polit. Econ. 2020:1–21. [Google Scholar]
- Cramer M.M. C.R.C. Press; 2006. Food Plant Sanitation: Design, Maintenance, and Good Manufacturing Practices. [Google Scholar]
- Crawley M.J. John Wiley & Sons; 2012. The R Book. [Google Scholar]
- Del Ciello R. Good Design Practices for GMP. Pharmaceutical Facilities, C.R.C. Press; 2005. Current good manufacturing practices; pp. 49–66. [Google Scholar]
- Demyanenko V.G., Demyanenko D.V., Breusova S.V., Baranova I.I., Karpenko L.A. Effect of the material of primary packaging containers on providing of visual inspection of pharmaceutical products. Scripta Sci. Pharmaceut. 2016;3(1):60–72. [Google Scholar]
- Doherty S.J., Kettler C.N. On-line applications in the pharmaceutical industry. Anal. Technol. 2005;329 [Google Scholar]
- Doig A., Jones S. Bioprocess International; 2016. From C.M.O. to CDMO: opportunities for specializing and innovation. Tuesday, May 17: 2016. [Google Scholar]
- Edwards A. 2010. Manufacturing the Future. Integrated collaboration betwee n C.M.O.s and Sponsors. [Google Scholar]
- Faraj S., Renno W., Bhardwaj A. Unto the breach: what the COVID-19 pandemic exposes about digitalization. Inf. Organ. 2021;31(1):100337. [Google Scholar]
- Faridi M.R., Malik A. Digital transformation in supply chain, challenges and opportunities in S.M.E.s: a case study of Al-Rumman Pharma. Emerald Emerg. Markets Case Stud. 2020 [Google Scholar]
- Fecha P.M.S. Universidade de Coimbra; 2017. The Return of the Investment of the Digital Channels in Pharmaceutical Industry. [Google Scholar]
- Fekih R.B., Lahami M. International Conference on Smart Homes and Health Telematics. Springer; 2020. Application of blockchain technology in healthcare: a comprehensive study. [Google Scholar]
- Gad S.C. John Wiley & Sons; 2008. Pharmaceutical Manufacturing Handbook: Regulations and Quality. [Google Scholar]
- Ganesh S. Purdue University Graduate School; 2020. Continuous Pharmaceutical Manufacturing: Systems Integration for Process Operations Management. [Google Scholar]
- Ganesh S., Su Q., Nagy Z., Reklaitis G. Smart Manufacturing. Elsevier; 2020. Advancing smart manufacturing in the pharmaceutical industry; pp. 21–57. [Google Scholar]
- Gbadegeshin S.A. The effect of digitalization on the commercialization process of high-Technology companies in the life sciences industry. Technol. Innov. Manag. Rev. 2019;9(1) [Google Scholar]
- George B.V.C.D. Improvement of manufacturing operations at a pharmaceutical company. A lean manufacturing approach. J. Manuf. Technol. Manag. 2012;23(1):56–75. [Google Scholar]
- Gouveia B.G., Rijo P., Gonçalo T.S., Reis C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioal. Sci. 2015;7(2):87. doi: 10.4103/0975-7406.154424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem R.M., Salem M.Y., Fatahallah F.A., Abdelfattah L.E. Quality in the pharmaceutical industry–a literature review. Saudi Pharmaceut. J. 2015;23(5):463–469. doi: 10.1016/j.jsps.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariry R.E., Barenji R.V., Paradkar A. Handbook of Smart Materias Technologies and Devices. 2021. From industry 4.0 to pharma 4.0; pp. 1–22. [Google Scholar]
- Harris J.R. 2010. “Good Manufacturing Practices (GMP.) and Related FDA. Guidelines.” Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing; pp. 1–42. [Google Scholar]
- Hunt J. The evolution of pharmacy in Britain. Pharm. Hist. 2006;36(2 Suppl):S3–S6. [PubMed] [Google Scholar]
- Hurter P., Thomas H., Nadig D., Emiabata-Smith D., Paone A. Implementing continuous manufacturing to streamline and accelerate drug development. AAPS Newsmag. 2013;16:15–19. [Google Scholar]
- Iezzi D. B.M.C. proceedings, BioMed Central; 2014. Contract Development and Manufacturing Organizations (CDMO): Are they Needed in Brazil. [Google Scholar]
- Iglehart J.K. 2014. Connected Health: Emerging Disruptive Technologies. [DOI] [PubMed] [Google Scholar]
- Jaiganesh V., Sudhahar J.C. Sketching out the hidden lean management principles in the pharmaceutical manufacturing. Int. J. Sci. Res. Publ. 2013;3(2):1–12. [Google Scholar]
- Jain S.K., Jain R.K. Evolution of GMP. In pharmaceutical industry. Res. J. Pharma. Technol. 2017;10(2):601–606. [Google Scholar]
- Jamroz W., Szafraniec J., Kurek M., Jachowicz R. 3D printing in pharmaceutical and medical applications – recent achievements and challenges. Pharm. Res. 2018;35(9):176. doi: 10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerez C.I. 2020. Effective Strategic Decision-Making Strategies for Plant Managers in Pharmaceutical and Medical Device Manufacturing in Modern Day Puerto Rico: A Qualitative Case Study. [Google Scholar]
- Joseph D.N. C.R.C. Press; 2000. Good Manufacturing Practices for Pharmaceuticals: A Plan for Total Quality Control from Manufacturer to Consumer. [Google Scholar]
- Kamble S., Gunasekaran A., Dhone N.C. Industry 4.0 and lean manufacturing practices for sustainable organisational performance in Indian manufacturing companies. Int. J. Prod. Res. 2020;58(5):1319–1337. [Google Scholar]
- Kane A. Quality by design: a contract organization’s perspective. Pharm. Technol. 2012;36(8):s20–s26. [Google Scholar]
- Karmacharya J.B. Good manufacturing practices (GMP.) for medicinal products. Prom. Pharmaceut. 2014:101–148. [Google Scholar]
- Kitson P.J., Marie G., Francoia J.-P., Zalesskiy S.S., Sigerson R.C., Mathieson J.S., Cronin L. Digitization of multistep organic synthesis in reactionware for on-demand pharmaceuticals. Science. 2018;359(6373):314–319. doi: 10.1126/science.aao3466. [DOI] [PubMed] [Google Scholar]
- Krekora M. Kluwer Law International B.V; 2008. Contract Manufacturing of Medicines. [Google Scholar]
- Kumar N., Jha A. Application of principles of supply chain management to the pharmaceutical good transportation practices. Int. J. Pharm. Healthc. Mark. 2019 [Google Scholar]
- Kumar L., Panigrahi C. Communication with doctors: empowering Pharma field force with modern marketing techniques. Asian J. Manag. Res. 2014;5(2) [Google Scholar]
- Kumar S.H., Talasila D., Gowrav M., Gangadharappa H. Adaptations of pharma 4.0 from industry 4.0. Drug Invention Today. 2020;14(3) [Google Scholar]
- Lakshmi B., Patel S. 2020. Digital Marketing in Pharmaceutical Industry–An Overview and Assessment. [Google Scholar]
- Lee F.S., Wang X., Fu P.P. 18 Quality assurance and safety protection of traditional Chinese herbs as dietary supplements. Funct. Foods East. 2010;431 [Google Scholar]
- Lee J., Cameron I., Hassall M. Improving process safety: what roles for Digitalization and Industry 4.0? Process. Saf. Environ. Prot. 2019;132:325–339. [Google Scholar]
- Lexchin J., Bero L.A., Djulbegovic B., Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. bmj. 2003;326(7400):1167–1170. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G.J. CDMOs embrace industry 4.0 to satisfy customer demand: the digitalization of bioprocessing technology isn’t happening for its own sake, but to ensure that outsourced manufacturing tasks, however diverse, will be part of highly integrated processes. Genetic Eng. Biotechnol. News. 2021;41(4):43–45. [Google Scholar]
- Macdonald G.J. CDMOs go digital to gain a competitive edge: CDMOs are investing in digital manufacturing technology in the face of growing competition and rising demand for process intensification. Genetic Eng. Biotechnol. News. 2021;41(6):64–67. [Google Scholar]
- Mackey T.K., Nayyar G. A review of existing and emerging digital technologies to combat the global trade in fake medicines. Expert Opin. Drug Saf. 2017;16(5):587–602. doi: 10.1080/14740338.2017.1313227. [DOI] [PubMed] [Google Scholar]
- May M. Adaptability of CDMOs Sorely Tested by COVID-19: surviving and thriving though COVID-19-imposed rigors, contract development and manufacturing organizations are confident of meeting future challenges. Genetic Eng. Biotechnol. News. 2021;41(6):60–62. [Google Scholar]
- McWilliams J.C., Allian A.D., Opalka S.M., May S.A., Journet M., Braden T.M. The evolving state of continuous processing in pharmaceutical API manufacturing: a survey of pharmaceutical companies and contract manufacturing organizations. Org. Process. Res. Dev. 2018;22(9):1143–1166. [Google Scholar]
- Mendenhall D.W., Kontny M.J. 2010. CDMO Industry Update. [Google Scholar]
- Nally J.D. C.R.C. Press; 2016. Good Manufacturing Practices for Pharmaceuticals. [Google Scholar]
- Nayereh N., Jordi B., Vesal T. Vol. 3. 2012. Good Manufacturing Practice: A New Approach for the 21st Century; pp. 37–44. Управління, економіка та забезпечення якості в фармації. [Google Scholar]
- Ngamvichaikit A. Leveraging design thinking for pharmaceutical digital marketing. Asian J. Business Res. Vol. 2021;11(1) [Google Scholar]
- Ohannesian L., Streeter A. C.R.C. Press; 2001. Handbook of Pharmaceutical Analysis. [Google Scholar]
- Organization, W. H . Good Manufacturing Practices and Inspection. World Health Organization; 2007. Quality assurance of pharmaceuticals: A compendium of guidelines and related materials. [Google Scholar]
- Organization, W. H . World Health Organization; 2007. WHO Guidelines on Good Manufacturing Practices (GMP.) for Herbal Medicines. [Google Scholar]
- Organization, W. H WHO expert committee on specifications for pharmaceutical preparations. World Health Organ. Tech. Rep. Ser. 2011;961:1. [PubMed] [Google Scholar]
- Pack B.W., Stithit S., Ray L., Chen W., Zheng J.Y., Hwang R. Developing Solid Oral Dosage Forms. Elsevier; 2009. Clinical supplies manufacture: strategy, good manufacturing process considerations, and cleaning validation; pp. 577–597. [Google Scholar]
- Padilla-Zakour O.I. Good manufacturing practices. Microbiol. Safe Foods. 2009:395–414. [Google Scholar]
- Pandya E.J., Shah K. Contract manufacturing in pharma industry. Pharma Sci. Monitor. 2013;4(3) [Google Scholar]
- Parekh D., Kapupara P., Shah K. Digital pharmaceutical marketing: a review. Res. J. Pharm. Technol. 2016;9(1):108. [Google Scholar]
- Parida V., Sjödin D., Reim W. Multidisciplinary Digital Publishing Institute; 2019. Reviewing Literature on Digitalization, Business Model Innovation, and Sustainable Industry: Past Achievements and Future Promises. [Google Scholar]
- Patel K., Chotai N. Pharmaceutical GMP.: past, present, and future–a review. Die Pharmazie-An Int. J. Pharmaceut. Sci. 2008;63(4):251–255. [PubMed] [Google Scholar]
- Patel K., Chotai N. Documentation and records: harmonized GMP. Requirements. J. Young Pharma. 2011;3(2):138–150. doi: 10.4103/0975-1483.80303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H., Parmar S., Patel B. A comprehensive review on Quality by Design (QbD) in pharmaceuticals. Development. 2013;4:5. [Google Scholar]
- Patidar A., Vinchurkar K., Balekar N. 2018. Digitalisation in Pharmacy, International. [Google Scholar]
- Paul D., Sanap G., Shenoy S., Kalyane D., Kalia K., Tekade R.K. Drug Discovery Today; 2020. Artificial Intelligence in Drug Discovery and Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K., Abdul Karim R. Good manufacturing practices for halal pharmaceuticals. Phamaceut. Eng. 2013;33(4):493–501. [Google Scholar]
- Phelps C.E., Madhavan G. Using multicriteria approaches to assess the value of health care. Value Health. 2017;20(2):251–255. doi: 10.1016/j.jval.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Pleitt K., Somasundaram B., Johnson B., Shave E., Lua L.H. Evaluation of process simulation as a decisional tool for biopharmaceutical contract development and manufacturing organizations. Biochem. Eng. J. 2019;150:107252. [Google Scholar]
- Plumb K. Continuous processing in the pharmaceutical industry: changing the mind set. Chem. Eng. Res. Des. 2005;83(6):730–738. [Google Scholar]
- Rangarajan A. The FDA. and worldwide current good manufacturing practices and quality system requirements: guidebook for finished pharmaceuticals. Qual. Prog. 2015;48(4):60. [Google Scholar]
- Rantanen J., Khinast J. The future of pharmaceutical manufacturing sciences. J. Pharm. Sci. 2015;104(11):3612–3638. doi: 10.1002/jps.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt I.C., Oliveira J.C., Ring D.T. Industry 4.0 and the future of the pharmaceutical industry. Pharm. Eng. 2021 [Google Scholar]
- Render N., Greenwood D., Edge J. Association of Researchers in Construction Management; 2005. The Other GMP.: Good Manufacturing Practice and its Importance in the Validation of Constructed Pharmaceutical Facilities. [Google Scholar]
- Rosenbaum M.S., Ramírez G.C., Edwards K., Kim J., Campbell J.M., Bickle M.C. The digitization of health care retailing. J. Res. Interact. Mark. 2017 [Google Scholar]
- Sarkis M., Bernardi A., Shah N., Papathanasiou M. 2021. Emerging Challenges and Opportunities in Pharmaceutical Manufacturing and Distribution. Processes 2021, 9, 457, s Note: MDPI stays neutral with regard to jurisdictional claims in published …. [Google Scholar]
- Sarkis M., Bernardi A., Shah N., Papathanasiou M.M. Emerging challenges and opportunities in pharmaceutical manufacturing and distribution. Processes. 2021;9(3):457. [Google Scholar]
- Sarvari M., Alavi-Moghadam S., Larijani B., Rezazadeh I., Arjmand B. Biomedical Product Development: Bench to Bedside. Springer; 2020. Principles of good manufacturing practice; pp. 61–68. [Google Scholar]
- Schaufelberger D.E., Koleck M.P., Beutler J.A., Vatakis A.M., Alvarado A.B., Andrews P., Marzo L., Muschik G., Roach J., Ross J.T. The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J. Nat. Prod. 1991;54(5):1265–1270. doi: 10.1021/np50077a004. [DOI] [PubMed] [Google Scholar]
- Scherer F.M. The pharmaceutical industry. Handb. Health Econ. 2000;1:1297–1336. [Google Scholar]
- Sehlstedt U., Bohlin N., de Maré F., Beetz R. Embracing digital health in the pharmaceutical industry. Int. J. Healthcare Manag. 2016;9(3):145–148. [Google Scholar]
- Shah N. Pharmaceutical supply chains: key issues and strategies for optimisation. Comput. Chem. Eng. 2004;28(6–7):929–941. [Google Scholar]
- Sharp J. C.R.C. Press; 2004. Good Pharmaceutical Manufacturing Practice: Rationale and Compliance. [Google Scholar]
- Sokolov M. Decision making and risk management in biopharmaceutical engineering—opportunities in the age of covid-19 and digitalization. Ind. Eng. Chem. Res. 2020;59(40):17587–17592. doi: 10.1021/acs.iecr.0c02994. [DOI] [PubMed] [Google Scholar]
- Srai J.S., Harrington T., Alinaghian L., Phillips M. Evaluating the potential for the continuous processing of pharmaceutical products—a supply network perspective. Chem. Eng. Process. Process Intensif. 2015;97:248–258. [Google Scholar]
- Stanić M. FINIZ 2019-Digitization and Smart Financial Reporting. 2019. Contemporary marketing in pharmacy with the focus on the E-Pharmacy concept; pp. 78–84. [Google Scholar]
- Steinwandter V., Borchert D., Herwig C. Data science tools and applications on the way to Pharma 4.0. Drug Discov. Today. 2019;24(9):1795–1805. doi: 10.1016/j.drudis.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Taylor P. Pharmaceutical excipients: where now for GMP. R.A.J. Pharma. 2008;19(12):815–818. [Google Scholar]
- Tomić S., Sučić A., Martinac A. Good manufacturing practice: the role of local manufacturers and competent authorities. Arch. Ind. Hyg. Toxicol. 2010;61(4):425–436. doi: 10.2478/10004-1254-61-2010-2035. [DOI] [PubMed] [Google Scholar]
- Trienekens J., Zuurbier P. Quality and safety standards in the food industry, developments and challenges. Int. J. Prod. Econ. 2008;113(1):107–122. [Google Scholar]
- van Velthoven M.H., Cordon C., Challagalla G. Digitization of healthcare organizations: the digital health landscape and information theory. Int. J. Med. Inform. 2019;124:49–57. doi: 10.1016/j.ijmedinf.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Velagaleti R., Burns P.K., Gill M., Prothro J. Impact of current good manufacturing practices and emission regulations and guidances on the discharge of pharmaceutical chemicals into the environment from manufacturing, use, and disposal. Environ. Health Perspect. 2002;110(3):213–220. doi: 10.1289/ehp.02110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa M. Safety and Efficacy of Radiopharmaceuticals. Springer; 1984. Production Design—GMP. Industry; pp. 107–116. [Google Scholar]
- Volgina N.A. Pharmaceutical value chain: opportunities for outsourcing. RUDN J. Econ. 2021;29(1):150–163. [Google Scholar]
- Vugigi S., Thoithi G., Ogaji J., Onuonga S. Good manufacturing practices in the Kenyan pharmaceutical industry and impact of facility upgrading on domestic and international sales. East Central African J. Pharmaceut. Sci. 2019;22(3):77–84. [Google Scholar]
- Woodcock J. The concept of pharmaceutical quality. Am. Pharmaceut. Rev. 2004;7(6):10–15. [Google Scholar]
- Yacuzzi E.A., Martín F., Vignola G., Mayochi V., Tollio D. Serie Documentos de Trabajo. 2004. The sources of quality in the pharmaceutical industry (No. 284) [Google Scholar]
- Zacharia Z.G., Mentzer J.T. Logistics salience in a changing environment. J. Bus. Logist. 2004;25(1):187–210. [Google Scholar]
- Zhou J. Digitalization and intelligentization of manufacturing industry. Adv. Manuf. 2013;1(1):1–7. [Google Scholar]