Abstract
Objectives
Soluble suppression of tumorigenicity 2 (sST2) is a member of the interleukin-1 receptor family. It is raised in various cardiovascular diseases, but its value in predicting disease severity or mortality outcomes has been controversial. Therefore, we conducted a systematic review and meta-analysis to determine whether sST2 levels differed between survivors and non-survivors of patients with cardiovascular diseases, and whether elevated sST2 levels correlated with adverse outcomes.
Methods
PubMed and Embase were searched until 23rd June 2021 for studies that evaluated the relationship between sST2 levels and cardiovascular disease severity or mortality.
Results
A total of 707 entries were retrieved from both databases, of which 14 studies were included in the final meta-analysis. In acute heart failure, sST2 levels did not differ between survivors and non-survivors (mean difference [MD]: 24.2 ± 13.0 ng/ml; P = 0.06; I2: 95%). Elevated sST2 levels tend to be associated with increased mortality risk (hazard ratio [HR]: 1.12, 95 %CI: 0.99–1.27, P = 0.07; I2: 88%). In chronic heart failure, sST2 levels were higher in non-survivors than in survivors (MD: 0.19 ± 0.04 ng/ml; P = 0.001; I2: 0%) and elevated levels were associated with increased mortality risk (HR: 1.64, 95% CI: 1.27–2.12, P < 0.001; I2: 82%). sST2 levels were significantly higher in severe disease compared to less severe disease (MD: 1.56 ± 0.46 ng/ml; P = 0.001; I2: 98%). Finally, in stable coronary artery disease, sST2 levels were higher in non-survivors than survivors (MD: 3.0 ± 1.1 ng/ml; P = 0.005; I2: 80%) and elevated levels were significantly associated with increased mortality risk (HR: 1.32, 95% CI: 1.04–1.68, P < 0.05; I2: 57%).
Conclusions
sST2 significantly predicts disease severity and mortality in cardiovascular disease and is a good predictor of mortality in patients with stable coronary artery disease and chronic heart failure.
Keywords: Soluble suppression of tumorigenicity 2, sST2, Severity, Mortality, Heart failure, Coronary artery disease
1. Introduction
The interleukin (IL)-1 receptor family is a family of receptors mediating the activities of specific members of the IL-1 family of ligands, which are important in both innate and adaptive immune response [1]. Suppression of Tumorigenicity 2 (ST2) is a member of the IL-1 receptor family and it consists of two important isoforms, namely, ST2 ligand (ST2L) and soluble ST2 (sST2) [2]. ST2L is a transmembrane receptor while sST2 is a soluble receptor that circulates in the bloodstream [2]. IL-33 is a functional ligand of ST2L receptor [3]. Like other ligands in the IL-1 family, the binding of IL-33 and ST2L on the inflammatory cell membrane activates subsequent intracellular signaling and mediates its pro-inflammatory action [4].
Apart from the role of ST2 in mediating inflammatory responses, the expression of ST2 is stimulated by cardiomyocyte stretch [5]. Mouse studies have shown that mice lacking the ST2 gene had increased cardiac fibrosis and cardiomyocyte cross-sectional area after transverse aortic constriction compared to mice with the ST2 gene [6]. The binding of IL-33 to ST2L exerts a protective effect against angiotensin II-driven adverse remodeling of the myocardium. sST2 acts as a decoy receptor and binds to IL-33, which reduces the amount of IL-33 available for interacting with ST2L [7]. Hence, the cardioprotective action of IL-33 is reduced when circulating sST2 levels are elevated [6].
Based on the above principles, sST2 has been widely investigated on its potential to become a prognostic biomarker for pulmonary hypertension [8], post-aortic valve replacement [9] and cardiovascular diseases (CVDs) [10]. Although some studies reported its predictive value for risk stratification in the context of CVDs, data on its value in predicting disease severity or mortality outcomes are inconclusive. Therefore, we conducted a systematic review and meta-analysis on the utility of sST2 in predicting disease severity or mortality outcomes in cardiovascular diseases, including acute heart failure, chronic heart failure, stable acute coronary syndrome and chest pain.
2. Methods
2.1. Search strategy, inclusion and exclusion criteria
This systematic review and meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. PubMed and EMBASE were searched for studies that investigated the relationship between soluble suppression of tumorigenicity-2 (sST2) levels and cardiovascular diseases using the following terms: [(Soluble suppression of tumorigenicity-2 OR sST2) and (severity OR mortality OR outcome)]. The databases were searched from inception to 23rd June 2021, with no language restrictions. The following inclusion criteria were applied: i) case-control, prospective or retrospective cohort studies in humans; ii) sST2 values were provided and related to disease severity or mortality in cardiovascular diseases; iii) the study assessed cardiovascular diseases. The following studies were excluded: i) did not assess cardiovascular diseases; ii) systematic reviews, meta-analyses and editorials; iii) absence of comparable data.
Quality assessment was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS). The point score system evaluated the categories of 1) study participant selection, 2) comparability of the results, and 3) quality of the outcomes. The following characteristics were assessed: a) representativeness of the exposed cohort; b) selection of the non-exposed cohort; c) ascertainment of exposure; d) demonstration that outcome of interest was not present at the start of study; e) comparability of cohorts on the basis of the design or analysis; f) assessment of outcomes; g) follow-up period sufficiently long for outcomes to occur; and h) adequacy of follow-up of cohorts. Each criteria met contributed to a point in the scale, which varied from zero to nine points: Study quality was deemed poor if<5 points, fair if 5 to 7 points, and optimal if 8 or more points. The details of the NOS quality assessment are shown in Supplementary Table 1.
2.2. Data extraction and statistical analysis
Data extraction was performed with a pre-specified spreadsheet in Microsoft Excel. All publications identified were assessed for compliance with the inclusion criteria. In this meta-analysis, the extracted data elements consisted of: surname of first author, publication year, study design, follow-up duration, sample size, gender, age, and cut-off point for sST2 levels. Two reviewers (CI and KSL) independently reviewed each included study and disagreements were resolved by adjudication with input from a third reviewer (GT).
Mean differences in sST2 levels between survivors and non-survivors were extracted from each study and subsequently pooled in our meta-analysis. For the relationship between sST2 and mortality, multivariate adjusted hazard ratios (HR) with 95% confidence interval (CI) were extracted and analyzed for each study. When values from multivariate analysis were not available, those from univariate analysis were used. When the latter were not provided, raw data where available were used to calculate unadjusted risk estimates.
Heterogeneity across studies was determined using Cochran's Q value, which is the weighted sum of squared differences between individual study effects and the pooled effect across studies; and the I2 statistic from the standard chi-square test, which describes the percentage of the variability in the effect estimates resulting from heterogeneity. A value of I2 > 50% was considered to reflect significant statistical heterogeneity. The random-effects model using the inverse variance heterogeneity method was used if I2 > 50%. To locate sources of heterogeneity, sensitivity analysis was performed by excluding one study at a time. Subgroup analyses based on different disease conditions and different endpoints were performed. Funnel plots showing standard errors or precision against the logarithms of the odds ratio were constructed. The Begg and Mazumdar rank correlation test and Egger’s test were used to assess for possible publication bias.
3. Results
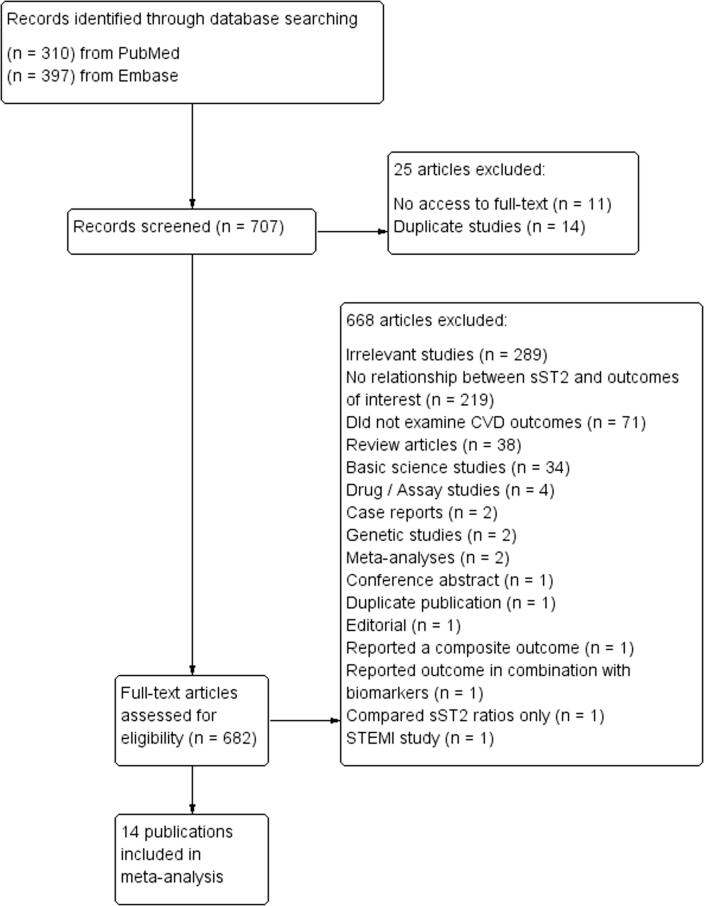
The flow diagram detailing the search strategy and study selection process is shown in Fig. 1. A total of 310 and 397 entries were retrieved from PubMed and Embase, of which 14 studies were included in the final meta-analysis [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. The baseline characteristics of included studies were listed in Table 1. All studies were prospective in design. The predictive value of sST2 was examined in the following conditions: acute heart failure (n = 5), chronic heart failure (n = 6) and stable coronary artery disease (n = 3).
Fig. 1.
PRISMA flow chart of the study selection process.
Table 1.
Characteristics of the studies included in this meta-analysis.
| First author / Year | Population | Country | Method of sST2 detection | sST2 cut-off value (ng/ml) | Sample size (n) | Mean age (years) | Males (n[%]) | Follow-up (months) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Jin, 2017 | Acute heart failure | China | Enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Abingdon, UK) | 36 | 287 | 60.5 | 181 (63.1%) | 12 | [11] |
| Manzano-Fernández, 2012 | Acute heart failure | Spain | Presage → ST2 (Critical Diagnostics, USA) | 76 | 72 | 69 | 47 (65.3%) | 25 | [12] |
| Manzano-Fernández, 2011 | Acute heart failure | United States, Austria, and Spain | ELISA (Medical and Biological Laboratories, USA) | 0.53 | 447 | 73 | 290 (64.9%) | 12 | [13] |
| Pascual-Figal, 2011 | Acute heart failure | Spain | Presage → ST2 (Critical Diagnostics, USA) | 65 | 107 | 72 | 47 (43.9%) | 25 | [14] |
| Mueller, 2008 | Acute heart failure | Austria | BEP® 2000 instrument (Dade Behring); sandwich double monoclonal antibody ELISA (Medical and Biological Laboratories, USA) | 700 | 137 | – | – | – | [15] |
| Sinning, 2017 | Chronic heart failure | Germany | Presage → ST2 (Critical Diagnostics, San Diego, California) | – | 5000 | 56 | 2540 (50.8%) | 88 | [18] |
| Gül, 2017 | Chronic heart failure | Turkey | Presage → ST2 Assay (Critical Diagnostics, USA) | 30 | 130 | 67 | 90 (69.2%) | 12 | [16] |
| Wojtczak-Soska, 2014 | Chronic heart failure | Poland | Sandwich ELISA kit (Medical and Biological Laboratories, Japan) | 0.34 | 167 | 63 | 139 (83.2%) | 12 | [20] |
| Sobczak, 2014 | Chronic heart failure | Poland | Sandwich monoclonal ELISA kits (Medical and Biological Laboratories, USA) | 0.30 | 145 | 62 | 120 (82.8%) | 12 | [21] |
| Zhang, 2014 | Chronic heart failure | China | ELISA in a microtiter plate format (Critical Diagnostics, USA) | – | 1528 | 58 | 1075 (70.4%) | 8 | [19] |
| Scott, 2011 | Chronic heart failure | United Kingdom | ELISA and methylacridinium ester- labelled streptavidin on an MLX plate luminometer (Dynex Technologies Ltd, UK) | – | 156 | 69 | 132 (84.6%) | 15 | [17] |
| Pfetsch, 2017 | Stable coronary artery disease | Germany | ELISA (Critical Diagnostics, USA) | 35 | 1081 | 59 | 915 (84.6%) | 156 | [22] |
| Dieplinger, 2014 | Stable coronary artery disease | Germany | BEP® 2000 instrument (Siemens Healthcare Diagnostics) with the PresageΦST2 sandwich immunoassay assay (Critical Diagnostics, USA) | 25 | 1345 | 65 | 1008 (74.9%) | 118 | [24] |
| Demyanets, 2014 | Stable coronary artery disease | Austria | ELISA (R&D Systems, USA) | – | 373 | 64 | 279 (74.8%) | 43 | [23] |
3.1. sST2 and mortality in acute heart failure
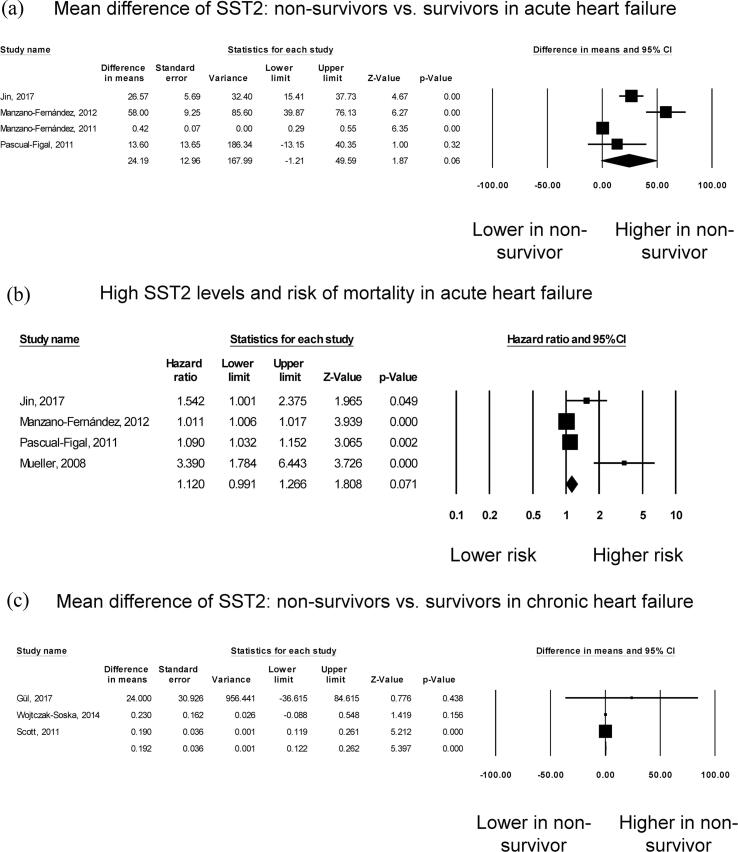
A total of 1050 patients (62% male, mean age 68 years; mean follow-up duration of 14 months) from five studies assessing acute heart failure were analysed [11], [12], [13], [14], [15]. Five studies compared sST2 levels between survivors and non-survivors in acute heart failure, but one study was excluded from the meta-analysis because it did not provide any measure of dispersion [15]. Three studies reported significantly higher sST2 levels in non-survivors than in survivors, whereas one study found no significant difference (Fig. 2A). Pooled meta-analysis showed a mean difference of 24.2 ng/ml (standard error: 13.0 ng/ml) but this did not quite reach statistical significance (P = 0.06). I2 took a value of 95%, indicating the presence of substantial heterogeneity. The funnel plot of standard error against mean difference is shown in Supplementary Figure 1. Begg and Mazumdar rank correlation suggested no significant publication bias (Kendal’s Tau value 2.0, P > 0.05). Egger’s test demonstrated no significant asymmetry (intercept 4.0, t-value 2.5; P > 0.05). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 2).
Fig. 2.
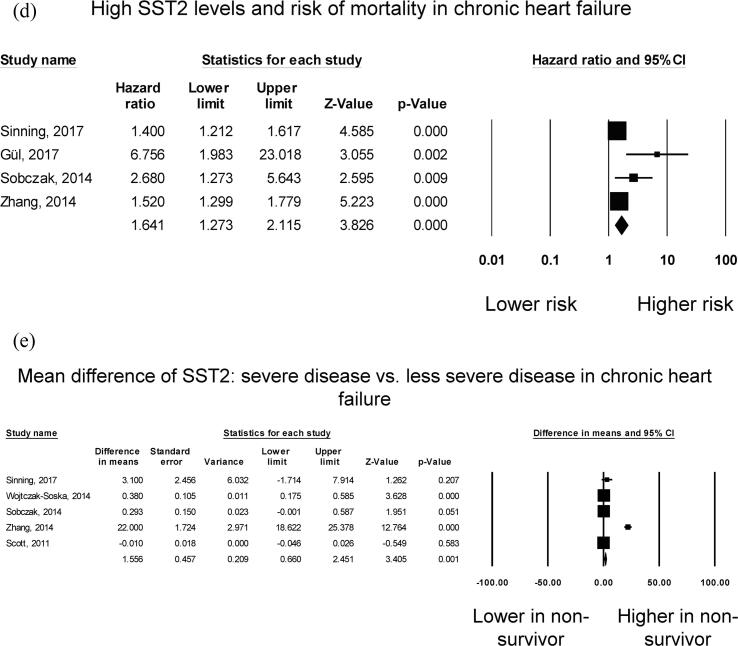
Mean difference in sST2 between non-survivors and survivors in acute heart failure (A). High sST2 and mortality risk in acute heart failure (B). Mean difference in sST2 between non-survivors and survivors in chronic heart failure (C). High sST2 and mortality risk in acute heart failure (D). Mean difference in sST2 between severe and non-severe disease in chronic heart failure (E).
Four studies examined the risk of mortality with increased sST2 in acute heart failure (Fig. 2B). Elevated levels of sST2 increased the risk of all-cause mortality by 12% but this value was not statistically significant (HR: 1.12, 95% CI: 0.99 to 1.26, p = 0.07). I2 took a value of 88%, indicating substantial heterogeneity. The funnel plot is shown in Supplementary Figure 3. Begg and Mazumdar rank correlation suggested no significant publication bias (Kendal’s Tau value 0.4, P > 0.05). Egger’s test demonstrated significant asymmetry (intercept 3.6, t-value 4.6; P < 0.05). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 4).
3.2. sST2 and mortality in chronic heart failure
A total of 7126 patients (57% male, mean age 57 years; mean follow-up duration of 64 months) from six studies for chronic heart failure were analysed [16], [17], [18], [19], [20], [21]. Three studies compared sST2 levels between survivors and non-survivors in chronic heart failure. Of these, one study demonstrated significantly higher sST2 levels in non-survivors than in survivors, whereas the other two studies found no significant difference (Fig. 2C). Nevertheless, pooled results showed a mean difference of 0.19 ng/ml (standard error: 0.04 ng/ml; P < 0.001). I2 took a value of 0%, indicating absence of heterogeneity. The funnel plot of standard error against mean difference is shown in Supplementary Figure 5. Begg and Mazumdar rank correlation suggested no significant publication bias (Kendal’s Tau value 1.0, P > 0.05). Egger’s test demonstrated no significant asymmetry (intercept 0.6, t-value 2.8; P > 0.05). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 6).
Four studies examined the risk of mortality with increased sST2 in chronic heart failure, all of which reported significant associations (Fig. 2D). Our meta-analysis showed that elevated levels of sST2 significantly increased the risk of all-cause mortality by 64% (HR: 1.64, 95% CI: 1.27 to 2.12, p < 0.001). I2 took a value of 82%, indicating the presence of substantial heterogeneity. The funnel plot for standard error against mean difference is shown in Supplementary Figure 7. Begg and Mazumdar rank correlation suggested significant publication bias (Kendal’s Tau value 1.0, P < 0.05). Egger’s test demonstrated significant asymmetry (intercept 3.0, t-value 6.1; P < 0.01). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 8).
Five studies examined the relationship between sST2 levels and disease severity in chronic heart failure. Of these, four studies reported significantly higher sST2 levels in severe disease than in less severe disease, whereas the remaining study found no significant difference (Fig. 2E). Nevertheless, pooled meta-analysis showed a mean difference of 1.56 ng/ml (standard error: 0.46 ng/ml; P = 0.001). I2 took a value of 98%, indicating the presence of substantial heterogeneity. The funnel plot of standard error against mean difference is shown in Supplementary Figure 9. Begg and Mazumdar rank correlation suggested no significant publication bias (Kendal’s Tau value 0.2, P > 0.05). Egger’s test demonstrated no significant asymmetry (intercept 5.5, t-value 2.0; P > 0.05). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 10).
3.3. sST2 and mortality in stable coronary artery disease
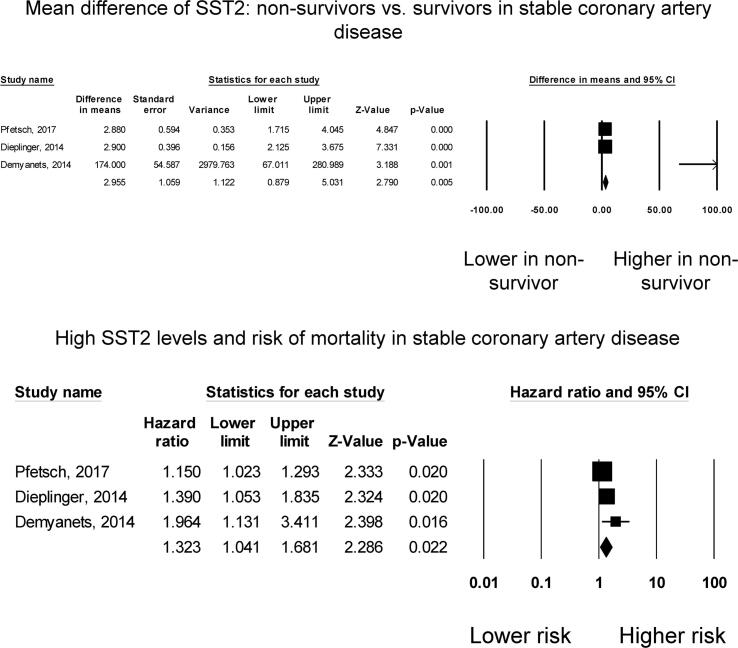
A total of 2799 patients (79% male, mean age 63 years; mean follow-up duration of 122 months) from three studies on stable coronary artery disease were included [22], [23], [24]. Three studies compared sST2 levels between survivors and non-survivors in stable coronary artery disease. All reported significantly higher sST2 levels in non-survivors than in survivors (Fig. 3A). Pooled meta-analysis showed a mean difference of 2.96 ng/ml (standard error: 1.06 ng/ml; P = 0.005). I2 took a value of 80%, indicating the presence of substantial heterogeneity. A funnel plot of standard error against mean difference is shown in Supplementary Figure 11. Begg and Mazumdar rank correlation suggested no significant publication bias (Kendal’s Tau value 0.3, P > 0.05). Egger’s test demonstrated no significant asymmetry (intercept 2.9, t-value 3.4; P > 0.05). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 12).
Fig. 3.
Mean difference in sST2 between non-survivors and survivors in stable coronary artery disease (A). High sST2 and mortality risk in stable coronary artery disease (B). Mean difference in sST2 between non-survivors and survivors in patients with dyspnoea or chest pain (C). High sST2 and mortality risk in patients with dyspnoea or chest pain (D).
Three studies examined the relationship between mortality risk with sST2 levels in stable coronary artery disease, all of which demonstrated significant associations (Fig. 3B). Our meta-analysis showed that elevated levels of sST2 significantly increased the risk of all-cause mortality by 32% (HR: 1.32, 95% CI: 1.04 to 1.68, p < 0.05). I2 took a value of 57%, indicating the presence of moderate heterogeneity. The funnel plot is shown in Supplementary Figures 13. Begg and Mazumdar rank correlation suggested no significant publication bias (Kendal’s Tau value 1.0, P > 0.05). Egger’s test demonstrated significant asymmetry (intercept 2.4, t-value 48; P < 0.05). Sensitivity analysis excluding one study at a time did not significantly affect the pooled estimate (Supplementary Figure 14).
4. Discussion
The main findings of this systematic review and meta-analysis are that elevated levels of sST2 significantly predicted the severity of chronic heart failure and the risk of mortality in chronic heart failure (HR: 1.64, 95% CI: 1.27 to 2.12, p < 0.001) and stable coronary artery disease (HR: 1.32, 95% CI: 1.04 to 1.68, p < 0.05).
Novel biomarkers have emerged and played an important role in assessing and monitoring the risk of patients with cardiovascular events in recent years. For example, natriuretic peptides (NPs) are produced in response to myocardial stress; mid-regional pro-adrenomedullin (MR-proADM) is related to global stress; and ST2 reflects ventricular fibrosis and remodeling [25]. Moreover, cancer antigen-125 has been identified as a promising marker for predicting fluid overload and guiding heart failure treatment as well as predicting atrial fibrillation risk [26], [27]. Thus, biomarkers have been developed as a tool to provide additional clinical information for assessing the progression and prognosis of heart failure and other cardiovascular diseases.
Circulating sST2 levels are increased in response to inflammatory diseases and heart diseases [28]. In a mouse study, it was shown that sST2 was released in cultured myocytes upon mechanical stress and increased in blood concentration following myocardial infarction [5]. sST2 is expressed on macrovascular and microvascular endothelial cells [29] and secreted by cardiomyocytes and fibroblasts when under mechanical stress [30]. Cytokines from damaged tissues are believed to activate neighboring cells to produce sST2, which in turn prevents an uncontrolled inflammatory response [31].
The cardioprotective IL-33/ST2L signaling pathway prevents the myocardium from maladaptive hypertrophy, fibrosis and cardiomyocytes apoptosis, reducing cardiac dysfunction and improving survival [6]. Since sST2 functions as a soluble decoy receptor of IL-33, it attenuates the protective effects of the IL-33/ST2L signaling pathway and works in a dose dependent manner [13]. sST2 was also identified to promote the processes of healing, myocardial fibrosis and cardiac remodeling [31].
Inflammation has been recognized to play an important role in the pathogenesis of different cardiovascular diseases. Higher concentrations of sST2 were associated with disease progression and predicted prognosis. As its concentration is less prone to haemodynamic fluctuations, it may mirror the progress of myocardial remodeling [32]. Since ventricular remodeling is often involved in various kinds of heart failure, the modulating role of sST2 in the IL-33/ST2L signaling pathway reflects its potential value as a biomarker for heart failure. In addition to its role as a biomarker of myocardial fibrosis and remodeling, multiple studies have suggested sST2 to be one of the most powerful prognostic biomarkers in both acute and chronic heart failure [11], [33]. The relationship of sST2 concentrations and mortality is reportedly independent of other relevant clinical and biochemical parameters [34]. There appears to be a dose–response relationship between sST2 concentration and such risk, consistent with the notion that its molecular action in IL-33 signaling is itself dose-dependent [13].
5. Strengths and limitations
This study has many strengths. Firstly, all included studies had a prospective design, so our results are more accurate and less prone to biases. Secondly, most of the included studies used multivariate analysis, reducing the influence of confounders. However, several limitations should be acknowledged. There was a high degree of heterogeneity observed. This may be due to differences in quantification assays used for sST2 and country of origin. Despite the promising predictive value of sST2 in predicting mortality, we did not define a specific cut-off value due to the heterogeneity among studies and selection criteria. Whilst the goal of this meta-analysis was to examine possible associations between sST2 levels and mortality in different cardiovascular conditions, it did not aim to provide risk quantification. Moreover, it did not examine sensitivities and specificities of sST2, which would be important for assessing its diagnostic ability. Future studies can perform meta-analysis of these statistical measures to explore this further. Therefore, the value of sST2 should be interpreted in the clinical context of the patient in conjunction with other laboratory test results for the purposes of risk stratification and deciding appropriate management.
6. Conclusion
High sST2 levels predict mortality in chronic heart failure and in stable coronary heart disease. It therefore provides incremental value for risk stratification purposes in these patient populations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81970270, 81570298 to TL).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100887.
Contributor Information
Gary Tse, Email: gary.tse@kmms.ac.uk.
Tong Liu, Email: liutongdoc@126.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Boraschi D., Tagliabue A. The interleukin-1 receptor family. Semin. Immunol. 2013;25(6):394–407. doi: 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Iwahana H., Yanagisawa K., Ito-Kosaka A., Kuroiwa K., Tago K., Komatsu N., Katashima R., Itakura M., Tominaga S.-I. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur. J. Biochem. 1999;264(2):397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D.M., Bazan J.F., Kastelein R.A. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Kakkar R., Lee R.T. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008;7(10):827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg E.O., Shimpo M., De Keulenaer G.W., MacGillivray C., Tominaga S.-I., Solomon S.D., Rouleau J.-L., Lee R.T. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanada S., Hakuno D., Higgins L.J., Schreiter E.R., McKenzie A.N.J., Lee R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Invest. 2007;117(6):1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Hammel M., He Y., Tainer J.A., Jeng U.-S., Zhang L., Wang S., Wang X. Structural insights into the interaction of IL-33 with its receptors. Proc. Natl. Acad. Sci. U S A. 2013;110(37):14918–14923. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.K.S. Luk, C. Ip, M.Q. Gong, S.H. Wong, W.K. Wu, M. Dong, G.P. Li, K.P. Chan, Y.M. Du, T. Liu, M.C. Wong, D.S.C. Hui, G. Tse, N. International Health Informatics Study, A meta-analysis of soluble suppression of tumorigenicity 2 (sST2) and clinical outcomes in pulmonary hypertension, J. Geriatr. Cardiol. 14(12) (2017) 766–771. [DOI] [PMC free article] [PubMed]
- 9.Tse Gary, Ip Christina, Luk King Sum, Gong Mengqi, Ting Yan Yee, Lakhani Ishan, Bazoukis George, Li Guangping, Letsas Konstantinos P, Dong Mei, Liu Tong, Wong Martin C S. Prognostic value of soluble ST2 postaortic valve replacement: a meta-analysis. Heart Asia. 2018;10(1):e010980. doi: 10.1136/heartasia-2017-010980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh R.H., Seliger S.L., Christenson R., Gottdiener J.S., Psaty B.M., deFilippi C.R. Soluble ST2 for Prediction of Heart Failure and Cardiovascular Death in an Elderly, Community-Dwelling Population. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2016;5(8) doi: 10.1161/JAHA.115.003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Mengchao, Wei Siqi, Gao Rongrong, Wang Kai, Xu Xuejuan, Yao Wenming, Zhang Haifeng, Zhou Yanli, Xu Dongjie, Zhou Fang, Li Xinli. Predictors of Long-Term Mortality in Patients With Acute Heart Failure. Int. Heart. J. 2017;58(3):409–415. doi: 10.1536/ihj.16-219. [DOI] [PubMed] [Google Scholar]
- 12.Manzano-Fernández S., Januzzi J.L., Pastor-Pérez F.J., Bonaque-González J.C., Boronat-Garcia M., Pascual-Figal D.A., Montalban-Larrea S., Navarro-Peñalver M., Andreu-Cayuelas J.M., Valdés M. Serial monitoring of soluble interleukin family member ST2 in patients with acutely decompensated heart failure. Cardiology. 2012;122(3):158–166. doi: 10.1159/000338800. [DOI] [PubMed] [Google Scholar]
- 13.Manzano-Fernández Sergio, Mueller Thomas, Pascual-Figal Domingo, Truong Quynh A., Januzzi James Louis. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am. J. Cardiol. 2011;107(2):259–267. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual-Figal D.A., Manzano-Fernandez S., Boronat M., Casas T., Garrido I.P., Bonaque J.C., Pastor-Perez F., Valdes M., Januzzi J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011;13(7):718–725. doi: 10.1093/eurjhf/hfr047. [DOI] [PubMed] [Google Scholar]
- 15.Mueller T., Dieplinger B., Gegenhuber A., Poelz W., Pacher R., Haltmayer M. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin. Chem. 2008;54(4):752–756. doi: 10.1373/clinchem.2007.096560. [DOI] [PubMed] [Google Scholar]
- 16.Gul I., Yucel O., Zararsiz A., Demirpence O., Yucel H., Zorlu A., Yilmaz M.B. Prognostic role of soluble suppression of tumorigenicity-2 on cardiovascular mortality in outpatients with heart failure. Anatol. J. Cardiol. 2017;18(3):200–205. doi: 10.14744/AnatolJCardiol.2017.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott P.A., Townsend P.A., Ng L.L., Zeb M., Harris S., Roderick P.J., Curzen N.P., Morgan J.M. Defining potential to benefit from implantable cardioverter defibrillator therapy: the role of biomarkers. Europace. 2011;13(10):1419–1427. doi: 10.1093/europace/eur147. [DOI] [PubMed] [Google Scholar]
- 18.Sinning Christoph, Kempf Tibor, Schwarzl Michael, Lanfermann Simon, Ojeda Francisco, Schnabel Renate B., Zengin Elvin, Wild Philipp S., Lackner Karl-J., Munzel Thomas, Blankenberg Stefan, Wollert Kai C., Zeller Tanja, Westermann Dirk. Biomarkers for characterization of heart failure - Distinction of heart failure with preserved and reduced ejection fraction. Int. J. Cardiol. 2017;227:272–277. doi: 10.1016/j.ijcard.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Rongcheng, Zhang Yuhui, Zhang Jian, An Tao, Huang Yan, Guo Xiao, Januzzi James L., Cappola Thomas P., Yin Shijie, Wang Yunhong, Zhou Qiong, Zou Changhong, Ji Shiming, Lv Rong, Bachschmid Markus Michael. The prognostic value of plasma soluble ST2 in hospitalized Chinese patients with heart failure. PLoS One. 2014;9(10):e110976. doi: 10.1371/journal.pone.0110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojtczak-Soska K., Sakowicz A., Pietrucha T., Lelonek M. Soluble ST2 protein in the short-term prognosis after hospitalisation in chronic systolic heart failure. Kardiol. Pol. 2014;72(8):725–734. doi: 10.5603/KP.a2014.0085. [DOI] [PubMed] [Google Scholar]
- 21.Sobczak S., Wojtczak-Soska K., Ciurus T., Sakowicz A., Pietrucha T., Lelonek M. Single sST2 protein measurement predicts adverse outcomes at 1-year follow-up in patients with chronic heart failure. Polskie Archiwum Medycyny Wewnetrznej. 2014;124(9):452–458. doi: 10.20452/pamw.2403. [DOI] [PubMed] [Google Scholar]
- 22.Pfetsch V., Sanin V., Jaensch A., Dallmeier D., Mons U., Brenner H., Koenig W., Rothenbacher D. Increased Plasma Concentrations of Soluble ST2 Independently Predict Mortality but not Cardiovascular Events in Stable Coronary Heart Disease Patients: 13-Year Follow-up of the KAROLA Study. Cardiovasc. Drugs Ther. 2017;31(2):167–177. doi: 10.1007/s10557-017-6718-1. [DOI] [PubMed] [Google Scholar]
- 23.Demyanets Svitlana, Speidl Walter S., Tentzeris Ioannis, Jarai Rudolf, Katsaros Katharina M., Farhan Serdar, Krychtiuk Konstantin A., Wonnerth Anna, Weiss Thomas W., Huber Kurt, Wojta Johann, Ahrens Ingo. Soluble ST2 and interleukin-33 levels in coronary artery disease: relation to disease activity and adverse outcome. PLoS One. 2014;9(4):e95055. doi: 10.1371/journal.pone.0095055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieplinger B., Egger M., Haltmayer M., Kleber M.E., Scharnagl H., Silbernagel G., de Boer R.A., Maerz W., Mueller T. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin. Chem. 2014;60(3):530–540. doi: 10.1373/clinchem.2013.209858. [DOI] [PubMed] [Google Scholar]
- 25.Mebazaa Alexandre, Di Somma Salvatore, Maisel Alan S., Bayes-Genis Antoni. ST2 and multimarker testing in acute decompensated heart failure. Am. J. Cardiol. 2015;115(7):38B–43B. doi: 10.1016/j.amjcard.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Li Ka Hou Christien, Gong Mengqi, Li Guangping, Baranchuk Adrian, Liu Tong, Wong Martin C S, Jesuthasan Aaron, Lai Rachel W C, Lai Jenny Chi Ling, Lee Alex Pui Wai, Bayés-Genis Antoni, de la Espriella Rafael, Sanchis Juan, Wu William K K, Tse Gary, Nuñez Julio. International Health Informatics Study, Cancer antigen-125 and outcomes in acute heart failure: a systematic review and meta-analysis. Heart Asia. 2018;10(2):e011044. doi: 10.1136/heartasia-2018-011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung Angel, Gong Mengqi, Bellanti Roberto, Ali-Hasan-Al-Saegh Sadeq, Li Guangping, Roig Eulàlia, Núñez Julio, Stamos Thomas D, Yilmaz Mehmet Birhan, Hakki Kaya, Wu William K K, Wong Sunny Hei, Wong Wing Tak, Bazoukis George, Lampropoulos Konstantinos, Tse Lah Ah, Zhao Jichao, Lip Gregory Y H, Baranchuk Adrian, Wong Martin C S, Liu Tong, Tse Gary. Cancer antigen-125 and risk of atrial fibrillation: a systematic review and meta-analysis. Heart Asia. 2018;10(1):e010970. doi: 10.1136/heartasia-2017-010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller T., Dieplinger B. The Presage((R)) ST2 Assay: analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev. Mol. Diagn. 2013;13(1):13–30. doi: 10.1586/erm.12.128. [DOI] [PubMed] [Google Scholar]
- 29.Barbarash Olga, Gruzdeva Olga, Uchasova Evgenya, Dyleva Yulia, Belik Ekaterina, Akbasheva Olga, Karetnikova Victoria, Shilov Aleksandr. Prognostic Value of Soluble ST2 During Hospitalization for ST-Segment Elevation Myocardial Infarction. Ann. Lab. Med. 2016;36(4):313–319. doi: 10.3343/alm.2016.36.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M. Mildner, A. Storka, M. Lichtenauer, V. Mlitz, M. Ghannadan, K. Hoetzenecker, S. Nickl, B. Dome, E. Tschachler, H.J. Ankersmit, Primary sources and immunological prerequisites for sST2 secretion in humans, Cardiovasc Res 87(4) (2010) 769–777. [DOI] [PubMed]
- 31.Weir R.A., Miller A.M., Murphy G.E., Clements S., Steedman T., Connell J.M., McInnes I.B., Dargie H.J., McMurray J.J. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2010;55(3):243–250. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Bartunek Jozef, Delrue Leen, Van Durme Frederik, Muller Olivier, Casselman Filip, De Wiest Bart, Croes Romaric, Verstreken Sofie, Goethals Marc, de Raedt Herbert, Sarma Jaydeep, Joseph Lija, Vanderheyden Marc, Weinberg Ellen O. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J. Am. Coll. Cardiol. 2008;52(25):2166–2174. doi: 10.1016/j.jacc.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg Ellen O., Shimpo Masahisa, Hurwitz Shelley, Tominaga Shin-ichi, Rouleau Jean-Lucien, Lee Richard T. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107(5):721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 34.J.A. Vilchez, M. Perez-Cuellar, F. Marin, P. Gallego, S. Manzano-Fernandez, M. Valdes, V. Vicente, J.A. Noguera-Velasco, G.Y. Lip, J. Ordonez-Llanos, V. Roldan, sST2 levels are associated with all-cause mortality in anticoagulated patients with atrial fibrillation, Eur. J. Clin. Invest. 45(9) (2015) 899–905. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.