Highlights
-
•
Pesticide residues become part of our diet, having a lot of negative impacts on human health, but farmers are unaware.
-
•
It is difficult to completely remove the use of pesticides from the F&V supply chain, there was need of a strategy which could be used at household level.
-
•
Sonolytic ozonation (O3/US) is an innovative technique, never tested for its potential in removal of pesticides using a household machine.
-
•
Current documentation will contribute towards improving food safety.
Abbreviations: O3/US Sonolytic ozonation, ozone & ultrasound combined application, in an aqueous media; HPLC, High Performance Liquid Chromatography; MRLs, Maximum residual levels; US, Ultrasound; UV/H2O2, Ultraviolet rays/hydrogen peroxide
Keywords: Fruits and vegetables, Sonolytic ozonation, Pesticides degradation
Abstract
High pesticide residues in fresh produce is a serious food safety issue. This study was aimed at assessing the pesticides residues in some important vegetables and fruits marketed in Faisalabad, Pakistan and the impact of sonolytic ozonation (O3/US) treatment in removing these contaminants. From a short grower’s survey, five registered and mostly used pesticides (acetamiprid, carbendazim, imidacloprid, thiacloprid and thiamethoxam) were identified. A time optimization trial of O3/US application (05, 10 and 15 min) on okra, showed that 10 min treatment significantly reduced three identified chemicals (thiamethoxam 100 %, imidacloprid and thiacloprid 97.17 %), without any adverse effect on its quality. In follow up trial, five fresh vegetables (cauliflower, chillies, cucumber, spinach and tomato) three fresh fruits (grapes, guava and peach) collected from three markets of Faisalabad, were pooled together to have uniform samples. Vegetables and fruits were treated with O3/US for 10 and 6 min, respectively, along with control (simple tap wash) for determining the impacts on pesticides degradation. Samples were processed for extraction, clean up and analysis using HPLC-UV–Vis in isocratic mode. The data revealed the presence of five mentioned chemicals, with an accumulative mean residue of 9.006 and 1.921 µg/g in tested vegetables and fruits, respectively. After subjecting to O3/US, the accumulative chemical residues were reduced to 3.214 µg/g (64.313 %) and 1.064 (44.6 %) in treated vegetables and fruits respectively. Irrespective of fresh produce, the mean residues of thiamethoxam, imidachloprid, acetamiprid and thiachloprid and carbendazim were reduced by 99.3 %, 52.6 %, 65.2 %, 87.3 % and 72% respectively. It was concluded that sonolytic ozonation treatment was effective in significant reduction of pesticide residues from vegetables and fruits and thus can be employed as a good food safety practice at culinary level to reduce the associated health hazardous risks.
1. Introduction
Food security and food safety are emerging concerns globally because of increasing population and the requirement of producing food to feed the burgeoning population [1]. While the use of pesticides is playing an important role for increased yield of horticultural crops but the presence of their chemicals residues above maximum residues levels/limits (MRLs) significantly affect the human and animal health [2], [3], [4], [5]. Further, the use of pesticides on horticultural crops pollutes the environment as well as leave terminal degradation products in food inducing carcinogenic, teratogenic and immunosuppressive effect in human beings [6], [7], [8], [9], [10]. Evidence of higher pesticide residues were found in different commodities [3], [6], [11], which cause several health-hazardous issues [12], [13], [14], [15]. A study in India, gave critical data on the residue status of commonly used pesticides (chlorpyrifos and carbofuran), in some regularly utilized vegetables in Hyderabad indicating presence of conceivably toxic pesticides, linked to their high application, which needs to be controlled [16]. The use of pesticides beyond the limit has resulted in the accumulation of heavy amount of organophosphate and organochloride residues in fruits and vegetables; available for human consumption [15], [16], [17], [18].
Most commonly used pesticides are: parathion methyl and dimethoate (organophosphates), considered as genotoxic, since their consumption causes DNA damage in human lymphocytes [19]; residues of chlorpyrifos indirectly affects neurodevelopment [20] and is also considered carcinogenic for living beings [6], [21]. These residues vastly becoming the part of our food supply chain system [22] and adversely affects the human blood circulatory system [23] after consumption of fresh produce. It is assumed that neonicotinoids and carbendazim are extensively used against sucking insects and a range of fungal diseases respectively, in fruits and vegetables and their high residues have been reported in different countries [2], [15], [24], [25], [26], [27], [28], [29]. Several techniques have been researched to diminish pesticide residues in fruits and vegetables [30], [31], [32]. These techniques include thermal treatment, chlorination, cerium oxide, O3 + Chlorine and UV/H2O2 treatments [2], [33], [34], [35] etc. Ozone has also been used to decontaminate fresh produce in a gaseous form or in the combination with chlorine or O3/UV [30], [31], [36]. Ozone in combination with ultrasound waves (sonolysis and ozonation) has been tested to remove chemicals in wastewater [37], [38].Ozone microbubbles (500 ppm concentration for 10 min) has been used to decontaminate Fenitrothion in lettuce, cherry tomato and strawberry up to 33, 84 and 62 %, respectively [39]. Likewise raw cucumber when spiked in ultrasonic bath for 5, 10 and 20 min, showed significant reduction in trichlorfon (82.9 %), dimethoate (52.2 %), dichlorvos (49.8 %), fenitrothion (84.4 %) and chlorpyrifos (63.0 %) [40] with 20 min treatment.
However, previously very limited or almost no work has been done on degradation of pesticides residues of fresh produce with combined application of ozone and ultrasound (O3/US), both considered as ecofriendly techniques. Further, since it is hard to eliminate pesticides use in commercial production of vegetables and fruits or setting up mass scale chemical residues testing system in commercial supply chains, especially in developing countries, it warrants to explore certain options to reduce these chemicals, at terminal stage of supply chain (Kitchen level, before use). Therefore, this study was planned to evaluate the efficacy of combined application of ozone and ultrasound (O3/US) in reducing pesticide residues of selected fresh vegetables and fruits.
2. Material and methods
2.1. Study site
The study was carried out at Postharvest Research and Training Centre (PRTC), University of Agriculture (UAF), Faisalabad, Pakistan. Random samples of fresh vegetables (okra, spinach, tomato, cucumber, cauliflower and chillies) and fruits (peach, grapes and guava) were purchased from three local markets of Faisalabad, during their optimum time of availability. The analysis of pesticides was performed at Food Toxicology Laboratories, Nuclear Institute of Agriculture and Biology (NIAB) Faisalabad, Pakistan, and confirmation of pesticide residues was carried out at National Institute of Biology and Genetic Engineering (NIBGE), Faisalabad, Pakistan.
2.2. Treatment
Experiment 1: Sonolytic ozonation time optimization trial
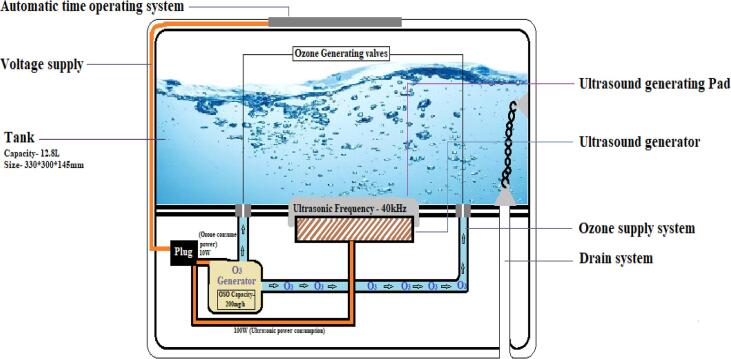
Among selected vegetables and fruits, okra (Abelmoschus esculentus) was identified having greater number of pesticide sprays (Table 1) and chosen for O3/US time optimization trial. Samples collected from three markets were pooled and divided into two halves. Half of the pooled sample of each produce was treated with combined application of ozone and ultrasound (O3 ≥ 0.3–0.4 mg.L−1 + 40 kHz US) (Sonolytic ozonation) for 5, 10 and 15 min, using a small O3/US Vegetable & Fruit Sterilizer unit (Model KD-6002, Guangdong GT Ultrasonic Co., Ltd., China) (Fig. 1), while remaining half samples were kept as untreated (simple tap water wash). Samples of approx. 1 kg (in triplicate) were air dried and then blended using high speed blender (Mix 2000, Braun Germany), to get homogenized samples and packed in zipper bag along with identification code and stored in freezer (-4 ℃) for further analysis (pesticide residues).
Table 1.
Background information related to selected fruits and vegetables from Faisalabad market.
| Commodity | Botanical name | Family | Area for market | *G. Avg. Edu. | *N. P. S |
|---|---|---|---|---|---|
| Okra | Abelmoschus esculentus | Malvaceae | PUA | Primary | 4 ± 1 |
| Spinach | Spinacia oleracea | Amaranthaceae | PUA | Secondary | 2 ± 1 |
| Tomato | Solanum lycopersicum | Solanaceae | PUA | Primary | 3 ± 1 |
| Cucumber | Cucumis sativus | Cucurbitaceae | PUA | Primary | 2 ± 1 |
| Cauliflower | Brassica oleracea | Brassicaceae | PUA | Secondary | 3 ± 1 |
| Chillies | Capsicum annuum | Solanaceae | PUA | Primary | 3 ± 1 |
| Grapes | Vitis vinifera | Vitaceae | DR | Secondary | 2 ± 1 |
| Guava | Psidium guajava | Myrtaceae | PUA | Primary | 2 ± 1 |
| Peach | Prunus persica | Rosaceae | DR | Primary | 2 ± 1 |
*PUA = Peri-urban area of Faisalabad.
*DR = Distant regions.
*G. Avg. Edu = Grower’s Average Education.
*N. P. S = Number of Pesticide Sprays.
Fig. 1.
KD-6002 working principle.
Experiment 2: Impact of sonolytic ozonation on pesticides degradation in selected vegetables and fruits
Based on the outcomes of experiment 1, literature review and O3/US impacts on microbial disinfestation and quality (results not shown), the selected vegetables (spinach, tomato, cucumber cauliflower and chillies) and fruits (peach, grapes and guava), were subjected to O3/US treatment for 10 min and 06 min, respectively, and samples were stored in freezer (−4 °C) as detailed above.
2.3. Solvent and reagent
Ethyl acetate, Methanol and acetonitrile HPLC grade were purchased from Sigma-Aldrich (MERCK group, St. Louis, Missouri, US). Sodium chloride, Sodium sulphate anhydrous were purchased (UNI-CHEM-Chemical Reagent), certified reference standard of acetamiprid, carbendazim, imidachloprid, thiacloprid, thiamethoxam (Dr. Ehrenstorfer, Augsburg, Germany). Stock solution of each standard was prepared in acetonitrile (1000 µg ml−1) and stored at 4◦C in refrigerator [41].
2.4. Sample preparation
Samples were taken out from the freezer and thawed at ambient conditions. After thawing, accurate weight (50 g) of each sample was taken in Erlen myer glass flask, added sodium chloride (5 %) and sodium sulfate anhydrous (20 %) along with 70 ml ethyl-acetate as extracting solvent. These flasks were shaken in a horizontal shaker (GFL shaker, Germany) at medium speed (50 rpm) for 1 h. The samples were taken out from shaker and filtered by whatsman no. 1 filter paper. The colored extracts were passed through the activated charcoal column and the filtrate was evaporated to dryness with rotary evaporator (011, Buchi, Switzerland) under reduced pressure. Then residues were dissolved in 2 ml ethyl-acetate, evaporated to dryness under nitrogen stream and re-dissolved the residue in 0.5 ml acetonitrile vortexed (Thermolyne, Switzerland) and analyzed. The sample was filtered through syringe filter (0.45 µm) and analyzed for the presence of pesticide residues using a High-Performance Liquid Chromatograph (HPLC) [42].
2.5. Pesticides detection and quantification
Pesticide residues analysis were performed by HPLC (LC-10, Shimadzu, Japan), equipped with UV visible detector SPD-10A, delivery pumps (LC-10AS), column oven CTO-10A, system controller unit SCL-10A, injector 20 µl (Reodyn, USA), and signal was received through Communication Bass Module (CBM-10A). The analytical column was of Discovery Supelco C18 (250×4.5 mm, 5 µm particle size) fixed at 246 nm wavelength the mobile phase was the mixture of acetonitrile, double distilled water and phosphoric acid maintaining the pH at 4.0. The flow rate of the mobile phase was maintained at 1.5 ml/min along with column temperature at 30 ○C. Certified reference standards were used to test HPLC performance parameters which showed linear behaviour with regression coefficient (R2 0.9979). The pesticide residue was confirmed by LCMS-MS at selective ion monitoring mode (SIM) QTL, Thermo, USA. [41].
2.6. Statistical analysis
Simple CDR was used to conduct the experiment, ANOVA was applied to evaluate the significance level (P ≤ 0.01) of treatments, using statistics 8.1 software (Statitix 8-Analytical software). LSD was used to reveal significance of treatment means (3 replications).
3. Results
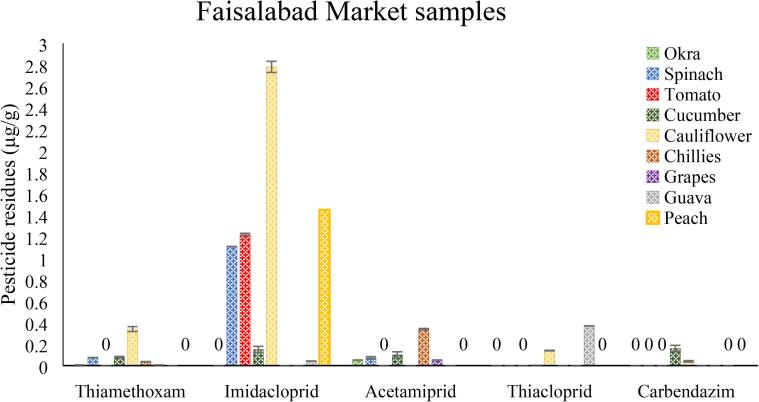
This study comprised of two phases. In first phase, a short survey of growers having reference vegetables (okra, spinach, tomato, cucumber, cauliflower and chillies) and fruits (guava, grapes and peaches) was conducted. Growers were contacted to get some background information, especially about the use of pesticides on reference crops. Brief information is presented in Table 1, which showed that majority of the farmers have limited education and were using 2–3 sprays (on the average) of pesticides on reference crops. Maximum number of sprays (approx. 4 ± 1) was done on okra crop. The pesticides commonly used included four neonicotinoids (neuro- active insecticides) named thiamethoxam, imidacloprid, acetamiprid and thiacloprid, while fifth was benzimidazoles (fungicide) known as carbendazim (Fig. 2).
Fig. 2.
Pesticides residues in untreated (control) samples of different vegetables (okra, spinach, tomato, cucumber, cauliflower and chillies) and fruits (grapes, guava and peach) and collected from different Faisalabad markets, Vertical bars represent ± SEM (n = 3).
During the second phase, two experiments on sonolytic ozonation effects on pesticides degradation in selected vegetables and fruits were performed, and results are discussed as below.
Experiment 1. Sonolytic ozonation time optimization trial.
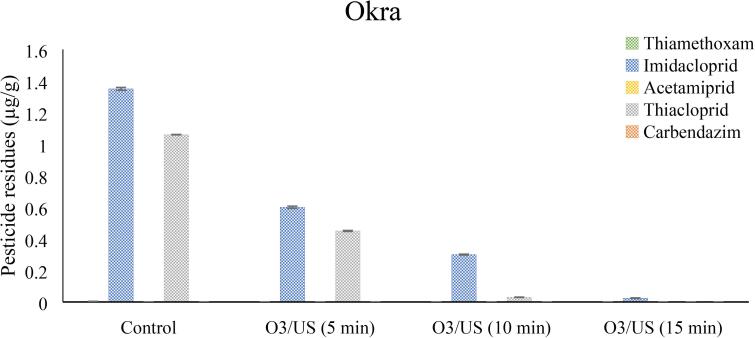
Statistical analysis of data pertaining to pesticides residues in okra samples revealed that in control samples (tap water), three pesticides were detected out of the five targeted, with accumulated chemical residues load of 2.241 µg/g. the three chemicals in ranked order were imidacloprid (1.35 ± 0.009 µg/g), thiacloprid (1.06 ± 0.0012 µg/g) and thiamethoxam (0.004 ± 0.0001 µg/g). There was significant reduction of pesticide residues in samples treated with O3/US and the impact increased with increasing time of application (Fig. 3). Combine application of O3/US for 5 min, resulted in 100 % reduction in thiamethoxam, 55.56% reduction in imidacloprid and 57% reduction in thiacloprid. O3/US 10 min further reduced chemical residues (Thiamethoxam 100 %: Thiacloprid 97.17 %: Imidacloprid 77.78 %). With 15 min ozone and ultrasound treatment, there was 100% reduction in thiamethoxam and thiacloprid, while 98.23 % in imidacloprid, however, 15 min treated okra showed brown patches once kept at refrigerate conditions. Therefore, 10 min treatment time was considered as optimum.
Fig. 3.
Potential evaluation of sonolytic ozonation (O3/US) 5, 10 and 15 min treatment on okra (Abelmoschus esculentus) in pesticides residue reduction. Vertical bars represent ± SEM (n = 3).
Experiment 2. Impact of Sonolytic ozonation on pesticides degradation in selected vegetables and fruits
Vegetables and fruits samples were treated with O3/US (10 min for vegetables and 6 min for fruits), as explained in materials and methods. HPLC analysis showed that all untreated fruits and vegetable samples were contaminated with indicated pesticides (Table 2), as simple tap washing of fruits and vegetables was not enough to remove pesticides residues. Among detected chemicals the highest residues were quantified for imidacloprid, while cauliflower (3.3 µg/g), and peach (1.45 µg/g) had maximum accumulative pesticides load, among tested vegetables and fruits respectively.
Table 2.
Selected registered pesticides found in reference fruits and vegetables randomly collected from Faisalabad markets.
| Properties | Thiamethoxam | Imidacloprid | Acetamiprid | Thiacloprid | Carbendazim |
|---|---|---|---|---|---|
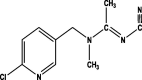
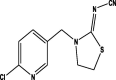
| Formula | C8H10ClN5O3S | C9H10ClN5O2 | C10H11ClN4 | C10H9ClN4S | C9H9N3O2 |
| Mol. Weight (mol/g) | 291.71 | 266.66 | 222.67 | 252.72 | 191.19 |
| Structure | 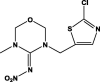 |
 |
 |
 |
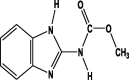 |
| Group | Neonicotinoid | Neonicotinoid | Neonicotinoid | Neonicotinoid | Benzimidazoles |
| Detected in Horticultural crops | Okra Spinach Cucumber Cauliflower Chillies Grapes |
Okra Tomato Spinach Cucumber Cauliflower Guava Peach |
Spinach Cucumber Chillies Grapes |
Okra Cucumber Cauliflower Guava |
Cucumber Cauliflower |
*Results obtained by HPLC and confirmed by LCMS.
Overall, among all samples, mostly detected pesticides were found beyond the safe level of MRLs (EU, 2019). Thiamethoxam was found in spinach, cucumber, cauliflower, chillies, and grapes, among them its residues were found above the MRLs standards except in grapes. Imidacloprid was detected in spinach, tomato, cucumber, cauliflower, guava and peach, beyond MRLs standards in all reference crops. Likewise, acetamiprid was also found in spinach, cucumber, chillies and grapes, above MRLs standards. Thiacloprid was found in cucumber, cauliflower and guava, while among these commodities except guava others were found above the MRLs standards. Carbendazim was found in cucumber and cauliflower, and both were found above the MRLs standards (EU, 2019) (Table 2).
Ozone and ultrasound (O3/US) applied in an aqueous media as a sterilized wash (discharging ≥ 0.3–0.4 mg.L−1 ozone and 40 kHz ultrasound waves) for 10 and 6 min for vegetables and fruits, respectively showed significant reduction in pesticide residues (Table 3).
Table 3.
Pesticide residues in reference fruits & vegetables.
| Group | Commodity | Pesticides detected | Control (µg/g ± S.E) | Treated (µg/g ± S.E) | Reduction (%) | T.A.P Control (µg g) | T.A.P. Treated (µg/g) |
|---|---|---|---|---|---|---|---|
| Vegetable | Okra | Thiamethoxam | 0.004 ± 0.0001 | 0 | 100 | 2.414 | 0.330b |
| Imidacloprid | 1.35 ± 0.009 | 0.3 | 77.78 | ||||
| Acetamiprid | 0 | 0 | 0 | ||||
| Thiacloprid | 1.06 ± 0.0012 | 0.03 | 0 | ||||
| Carbendazim | 0 | 0 | 97.17 | ||||
| Spinach | Thiamethoxam | 0.075 ± 0.001 | 0 | 100 | 0.943 | 0.122 a | |
| Imidacloprid | 1.108 ± 0.002 | 0.072 ± 0.002 | 91.21951 | ||||
| Acetamiprid | 0.02 ± 0.012 | 0 | 100 | ||||
| Thiacloprid | 0 | 0 | 0 | ||||
| Carbendazim | 0 | 0 | 0 | ||||
| Tomato | Thiamethoxam | 0 | 0 | 0 | 1.22 | 0.93 e | |
| Imidacloprid | 1.22 ± 0.012 | 0.93 ± 0.006 | 23.77049 | ||||
| Acetamiprid | 0 | 0 | 0 | ||||
| Thiacloprid | 0 | 0 | 0 | ||||
| Carbendazim | 0 | 0 | 0 | ||||
| Cucumber | Thiamethoxam | 0.08 ± 0.006 | 0 | 100 | 0.493 | 0.122b | |
| Imidacloprid | 0.15 ± 0.029 | 0.068 ± 0.002 | 54.67 | ||||
| Acetamiprid | 0.1 ± 0.029 | 0.028 ± 0.001 | 72 | ||||
| Thiacloprid | 0.003 ± 0.001 | 0 | 100 | ||||
| Carbendazim | 0.16 ± 0.029 | 0.026 ± 0.009 | 83.75 | ||||
| Cauliflower | Thiamethoxam | 0.34 ± 0.023 | 0 | 100 | 3.33 | 1.61c | |
| Imidacloprid | 2.78 ± 0.052 | 1.55 ± 0.058 | 44.24 | ||||
| Acetamiprid | 0 | 0 | 0 | ||||
| Thiacloprid | 0.14 ± 0.003 | 0.03 ± 0.001 | 78.57 | ||||
| Carbendazim | 0.04 ± 0.007 | 0.03 ± 0.003 | 25 | ||||
| Chillies | Thiamethoxam | 0.036 ± 0.002 | 0 | 100 | 0.376 | 0.15c | |
| Imidacloprid | 0 | 0 | 0 | ||||
| Acetamiprid | 0.34 ± 0.006 | 0.15 ± 0.003 | 55.88235 | ||||
| Thiacloprid | 0 | 0 | 0 | ||||
| Carbendazim | 0 | 0 | 0 | ||||
| Fruits | Grapes | Thiamethoxam | 0.006 ± 0.0002 | 0.004 ± 0.0005 | 33.33 | 0.058 | 0.004 a |
| Imidacloprid | 0 | 0 | 0 | ||||
| Acetamiprid | 0.052 ± 0.001 | 0 | 100 | ||||
| Thiacloprid | 0 | 0 | 0 | ||||
| Carbendazim | 0 | 0 | 0 | ||||
| Guava | Thiamethoxam | 0 | 0 | 0 | |||
| Imidacloprid | 0.043 ± 0.001 | 0 | 100 | 0.413 | 0.14c | ||
| Acetamiprid | 0 | 0 | 0 | ||||
| Thiacloprid | 0.37 ± 0.001 | 0.14 ± 0.003 | 62.16 | ||||
| Carbendazim | 0 | 0 | 0 | ||||
| Peach | Thiamethoxam | 0 | 0 | 0 | |||
| Imidacloprid | 1.45 ± 0.006 | 0.92 ± 0.005 | 36.55 | 1.45 | 0.92 d | ||
| Acetamiprid | 0 | 0 | 0 | ||||
| Thiacloprid | 0 | 0 | 0 | ||||
| Carbendazim | 0 | 0 | 0 |
T.A.P = Total amount of Pesticide.
= more than 90 % reduced.
= more than 70 % reduced.
= more than 50 % reduced.
= more than 30 % reduced.
= less than 30% reduced.
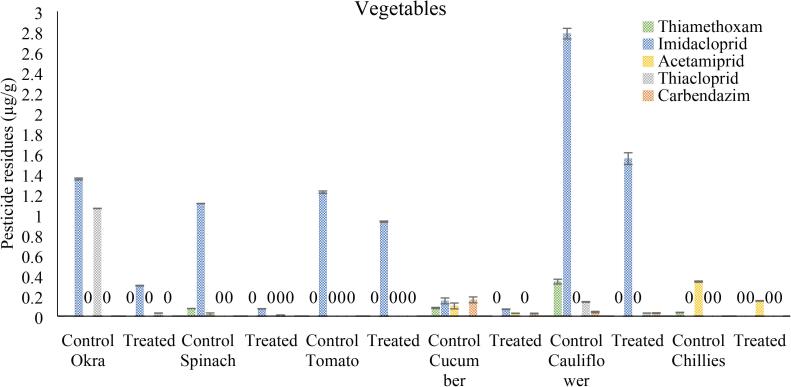
Among vegetables, control and okra residues results were obtained from Exp. 1, in which overall detected pesticides in control samples were 2.414 and O3/US 10 min reduces 86.33%, while in spinach samples, thiamethoxam, imidacloprid and acetamiprid were detected in homogenized market samples with residues of 0.075 ± 0.001, 1.108 ± 0.002 and 0.02 ± 0.012 µg/g, respectively. While after treatment with O3/US for 10 min, significant reduction was observed in thiamethoxam, imidacloprid and acetamiprid concentration up to 100, 91.2 and 100 % respectively. In tomato, imidacloprid was detected in representative homogenized market samples with mean chemical residues of 1.22 ± 0.012 µg/g and when treated with the O3/US for 10 min, there was significant reduction (27.77 %) in chemicals (pesticide degradation) to the level of 0.93 µg/g. In cucumber, thiamethoxam, imidacloprid, acetamiprid, thiacloprid and carbendazim were detected in homogenized market samples with chemical residues of 0.08 ± 0.006, 0.15 ± 0.029, 0.1 ± 0.029, 0.003 ± 0.001 and 0.16 ± 0.029 µg/g, respectively. While significant reduction was observed in thiamethoxam, imidacloprid, acetamiprid, thiacloprid and carbendazim concentration up to 100, 54.67, 72 100 and 83.75 % respectively, after treatment with O3/US for 10 min. In cauliflower, thiamethoxam, imidacloprid, thiacloprid and carbendazim were detected in homogenized market samples with the concentration of 0.34 ± 0.023, 2.78 ± 0.052, 0.14 ± 0.003 and 0.04 ± 0.007 µg/g respectively. While O3/US treatment for 10 min resulted in significant reduction in thiamethoxam, imidacloprid, thiacloprid and carbendazim concentration up to 100, 44.25, 78.57 and 25% respectively. In case of chillies, thiamethoxam, and acetamiprid were detected in homogenized market samples with residues level of 0.036 ± 0.002 and 0.34 ± 0.006 µg/g, respectively. While significant reduction was observed in thiamethoxam and acetamiprid concentration up to 100 and 55.9% respectively, when treated with ozone and ultrasound (O3/US) for 6 min (Fig. 4).
Fig. 4.
Pesticides residues in vegetables (okra, spinach, tomato, cucumber, cauliflower and chillies) samples collected from different Faisalabad markets compared with the standards of Thiamethoxam, Imidacloprid, Acetamiprid, Thiacloprid and Carbendazim after treated with ozone and ultrasound (O3/US) combined 10-minute treatment, Vertical bars represent ± SEM (n = 3).
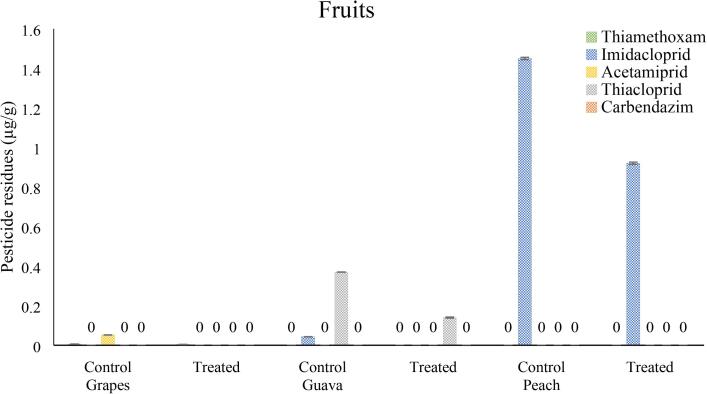
Among fruits, in grapes samples were found contaminated with thiamethoxam, and acetamiprid up to 0.006 ± 0.0002 and 0.052 ± 0.001 µg/g respectively. While significant reduction was observed in thiamethoxam and acetamiprid concentration up to 33.33 and 100 % respectively after treatment with O3/US for 6 min. In guava samples, imidacloprid and thiacloprid were detected with respective quantities of 0.043 ± 0.001 and 0.37 ± 0.001 µg/g, respectively. While after treatment with O3/US for 6 min, significant reduction was observed in imidacloprid and thiacloprid concentration up to 100, and 62.16 % respectively. Peach samples were also found highly contaminated with imidacloprid (1.45 ± 0.006 µg/g), while samples treated with O3/US for 6 min had 36.55 % reduction when compared with untreated control samples (Fig. 5).
Fig. 5.
Pesticides residues in fruits (grapes, guava and peach) samples collected from different Faisalabad markets after treatment with ozone and ultrasound (O3/US) 6 min, Vertical bars represent ± SEM (n = 3).
4. Discussion
While the use of pesticides is almost indispensable in commercial fruits and vegetables supply chains, its non-judicious application has become a food safety concern, globally, and more especially in developing countries. Our results showed that pooled samples of fruits and vegetables collected from Faisalabad market were contaminated with various pesticides more or less beyond the MRLs standards (EU, 2019) [43], and it is well established that pesticide residues beyond MRLs level adversely affect human health [15], [25], [26], [28], [44]. Irrespective of the tested fresh produce, maximum pesticides residues were quantified for imidacloprid, which is a commonly used insecticide for controlling sucking insects. Imidacloprid belongs to neonicotinoid group, which also includes thiamethoxam, acetamiprid and thiacloprid, detected in tested fruits and vegetables. Previous local research reports also showed that different fruits and vegetables of Punjab market were found contaminated with different pesticides [9], [10]. Akhtar et al. [45] reported pesticides (bifenthrin, difenoconazole, paraquat, diomethomorph, deltamethrin and imidacloprid) contamination in selected fruits and vegetables from Lahore market including guava, eggplant and round gourd and argued that these pesticides were not removed from their surfaces by simple tap water washing.
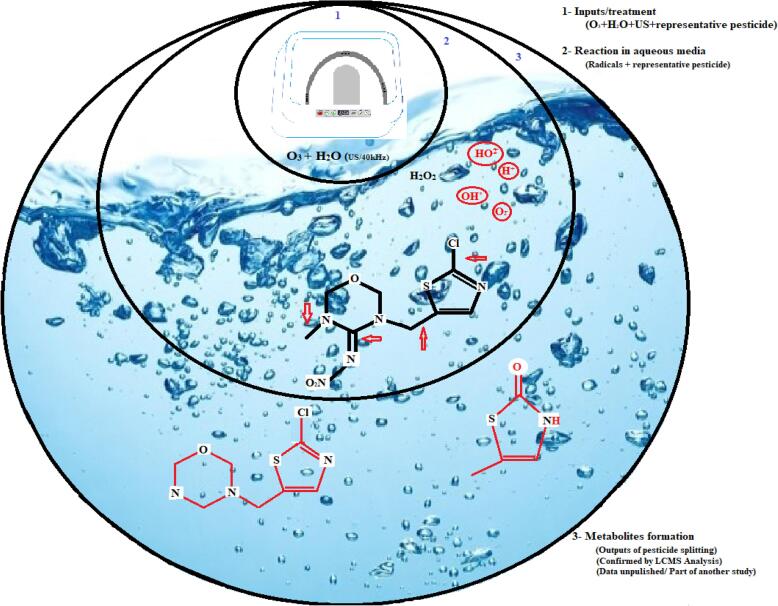
Our results showed that combined application of O3/US significantly reduced the pesticides residues in tested fruits and vegetables. However, the effectiveness of O3/US treatment varied with time of application (Expt. 1), type of fresh produce, nature of chemical and its pre-treatment level (Expt. 2) in the produce. It is well known that ozone and ozone generated radicals have ability to react with other elements like nitrogen, sulphur and chlorine compounds etc. so it can break the C−N, C−S and C−Cl bonding present in pesticide compounds, ozone decay in water proceed to form OH– and OH◦ when react with other elements forms nitrogen oxides (NO, NO2), sulphur di- and tri-oxide and other hydroxyl compounds like hydrogen sulphide, hydrogen chlorides [46] (Fig. 6). Some earlier findings support the current results as, Chanrattanayothin et al. (2019) reported that gaseous ozonation have ability to degrade cypermethrin and dicofol residue when applied on dried basil leave. It is evident that when UV/H2O2 produce hydroxyl ions (OH–) in an aqueous media, it can reduce pesticide residues by degrading its structure into metabolites [38].
Fig. 6.
Fate of representative pesticide in an aqueous media in the presence of highly reactive radicals formed in the result of sonolytic ozonation reaction.
Chen et al. [47] stated that ozone discharge switched immediately to hydroxyl radicals (⋅OH) and the method found effective to decontaminate water from Imidacloprid at pH 6.02–8.64. O3/US also produce hydroxyl ions in an aqueous media and working on the same principle as UV/H2O2 can degrade the pesticides in fresh produce. A key point of consideration is that, with sonolytic ozonation treatment, the overall reduction in pesticides residues load varied among crops: Vegetables (O3/US.10 min) (86.33 % in okra, 94 % in spinach, 75.3 % in cucumber, 60.1 % in chillies, 51.21 % in cauliflower and 23.8 % in tomato) and Fruit (O3/US.6 min) (93.1 % reduction in grapes, 66.1 % in guava and 36.6 % reduced in peach). Likewise, degradation of chemicals irrespective of crops also varied: Thiamethoxam (99.3 %), thiacloprid (87.3 %), carbendazim (72 %), acetamiprid (65.23 %) and imidacloprid (52.6 %).
The reason for this difference could be due to nature of chemical, applied quantity/time, chemical residue status and produce structure/nature of tissues. Still, the study clearly demonstrates that irrespective of fresh produce (vegetables or fruits) or pesticides, the sonolytic ozonation (O3/US) treatment in range of 6–10 min reduced the pesticides residues by more than half (mean 60.85 %; ranged 100–24 %), compared to the simple water washing samples, thereby, reducing associated health hazard risks to consumers.
Another point of consideration is that while O3/US at extended treatment time (15 min) reduced pesticides residues to maximum, it also had some qualitative issues in okra (brown patches), spinach (discoloration of leaves), and cauliflower (black spots on curds, data not shown) etc, when the treated samples were stored in refrigerator (4 °C) for 14 days. Now, these symptoms could be attributed to an interaction effect of O3/US treatment and low temperature during storage, since the control samples under same storage condition did not show such symptoms. This implicates that optimum treatment time of O3/US in also be considered from the perspective the way product is to be utilized after treatment (immediately used/cooked/eaten after treatment or to be stored in fridge for few days).
5. Conclusion
Different vegetables (okra, spinach, tomato, cucumber, cauliflower and chillies) and fruits (grapes, guava and peach) and collected from Faisalabad market were found contaminated with pesticides, with MRLs mostly above the CODEX standards; vegetables (mean residues 9.006 µg/g) being comparatively more contaminated than fruits (mean residues 1.921 µg/g). Sonolytic ozonation (O3/US) for 10 and 6 min in vegetables and fruits, respectively was found effective in significant reduction of pesticides residues varying from 100 % to 24 % depending upon the chemical and crop. On an average, O3/US treatment for 10 and 6 min in vegetables and fruits respectively reduced pesticides residues by 60.85 % in vegetables and 44.61 % in fruits compared with tap washed samples. So, based on the experimental outcome, sonolytic ozonation (O3/US) can be considered as an effective food safety intervention at household level, to significantly reduce pesticides contamination in fresh produce and the associated health risks to household consumers. The future studies should include other fruits and vegetables, and with extended treatment timing for produce with thick and waxy cuticle.
CRediT authorship contribution statement
Zarghona Siddique: Responsible of experiment as a researcher (analysis, data compiling, statistical analysis) and author of paper (Write paper from own Ph.D. research). Aman Ullah Malik: The experiment was conducted under kind supervision of Prof. Dr. Aman Ullah Malik. Principle investigator of TDF-017 project funded by Higher Education Commission. (Conceptualization, Write up Finalizing). Muhammad Rafique Asi: HPLC analysis were performed under kind supervision of Muhammad Rafique Asi, in his lab. (Research expert). Muhammad Inam-ur-Raheem: Paper setting was done with his kind assistance. Muhammad Iqbal: Helps in conducting survey. Muhammad Abdullah: Paper Setting and Write up.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors acknowledge the financial support of Higher Education Commission (HEC), Pakistan under Technology Development Fund Program (TDF-017 project).
References
- 1.Rehman S.U., Predotova M., Ahmad Khan I., Schlecht E., Buerkert A. Socio-economic characterization of integrated cropping system in urban and peri-urban agriculture of faisalabad, pakistan. J. Agric. Rural Dev. Trop. Subtrop. 2013;114:133–143. [Google Scholar]
- 2.Bempah C.K., Agyekum A.A., Akuamoa F., Frimpong S., Buah-Kwofie A. Dietary exposure to chlorinated pesticide residues in fruits and vegetables from Ghanaian markets. J. Food Compos. Anal. 2016;46:103–113. doi: 10.1016/j.jfca.2015.12.001. [DOI] [Google Scholar]
- 3.Mac Loughlin T.M., Peluso M.L., Etchegoyen M.A., Alonso L.L., de Castro M.C., Percudani M.C., Marino D.J.G. Pesticide residues in fruits and vegetables of the argentine domestic market: Occurrence and quality. Food Control. 2018;93:129–138. doi: 10.1016/j.foodcont.2018.05.041. [DOI] [Google Scholar]
- 4.Mebdoua S., Lazali M., Ounane S.M., Tellah S., Nabi F., Ounane G. Evaluation of pesticide residues in fruits and vegetables from Algeria. Food Addit. Contam. Part B Surveill. 2017;10(2):91–98. doi: 10.1080/19393210.2016.1278047. [DOI] [PubMed] [Google Scholar]
- 5.Parrilla Vázquez P., Ferrer C., Martínez Bueno M.J., Fernández-Alba A.R. Pesticide residues in spices and herbs: Sample preparation methods and determination by chromatographic techniques, TrAC - Trends Anal. Chem. 2019;115:13–22. doi: 10.1016/j.trac.2019.03.022. [DOI] [Google Scholar]
- 6.Khan M.I., Shoukat M.A., Cheema S.A., Arif H.N., Niazi N.K., Azam M., Bashir S., Ashraf I., Qadri R. Use, contamination and exposure of pesticides in pakistan: A review, Pakistan. J. Agric. Sci. 2020;57:131–149. doi: 10.21162/PAKJAS/20.7437. [DOI] [Google Scholar]
- 7.Latif Y., Sherazi S.T.H., Bhanger M.I., Nizamani S. Evaluation of Pesticide Residues in Human Blood Samples of Agro Professionals and Non-Agro Professionals. Am. J. Anal. Chem. 2012;03(08):587–595. doi: 10.4236/ajac.2012.38077. [DOI] [Google Scholar]
- 8.Osman R.W., Munguia P., Zajac R.N. Ecological thresholds in marine communities: Theory, experiments and management. Mar. Ecol. Prog. Ser. 2010;413:185–187. doi: 10.3354/meps08765. [DOI] [Google Scholar]
- 9.Qamar A., Asi R., Iqbal M., Nazir A., Arif K. Survey of residual pesticides in various fresh fruit crops: A case study, Polish. J. Environ. Stud. 2017;26:2703–2710. doi: 10.15244/pjoes/73801. [DOI] [Google Scholar]
- 10.Randhawa M.A., Zaman Abid Q.U., Anjum F.M., Shakoor Chaudhary A., Sajid M.W., Khalil A.A. Organo-chlorine pesticide residues in okra and brinjal collected from peri-urban areas of big cities of Punjab-Pakistan, Pakistan. J. Agric. Sci. 2016;53(02):425–430. [Google Scholar]
- 11.Yuan Y., Chen C., Zheng C., Wang X., Yang G., Wang Q., Zhang Z. Residue of chlorpyrifos and cypermethrin in vegetables and probabilistic exposure assessment for consumers in Zhejiang Province, China. Food Control. 2014;36(1):63–68. doi: 10.1016/j.foodcont.2013.08.008. [DOI] [Google Scholar]
- 12.Bhandari G., Atreya K., Yang X., Fan L., Geissen V. Factors affecting pesticide safety behaviour: The perceptions of Nepalese farmers and retailers. Sci. Total Environ. 2018;631–632:1560–1571. doi: 10.1016/j.scitotenv.2018.03.144. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari G., Zomer P., Atreya K., Mol H.G.J., Yang X., Geissen V. Pesticide residues in Nepalese vegetables and potential health risks. Environ. Res. 2019;172:511–521. doi: 10.1016/j.envres.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Damalas C.A., Eleftherohorinos I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health. 2011;8:1402–1419. doi: 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumari D., John S. Health risk assessment of pesticide residues in fruits and vegetables from farms and markets of Western Indian Himalayan region. Chemosphere. 2019;224:162–167. doi: 10.1016/j.chemosphere.2019.02.091. [DOI] [PubMed] [Google Scholar]
- 16.Latif Y., Sherazi S.T.H., Bhanger M.I. Assessment of pesticide residues in commonly used vegetables in Hyderabad, Pakistan. Ecotoxicol. Environ. Saf. 2011;74(8):2299–2303. doi: 10.1016/j.ecoenv.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Kumari B., Madan V.K., Kathpal T.S. Monitoring of pesticide residues in fruits. Environ. Monit. Assess. 2006;123(1-3):407–412. doi: 10.1007/s10661-006-1493-7. [DOI] [PubMed] [Google Scholar]
- 18.Latif Y., Sherazi S.T.H., Bhanger M.I. Monitoring of Pesticide Residues in Commonly Used Fruits in Hyderabad Region, Pakistan. Am. J. Anal. Chem. 2011;02(08):46–52. doi: 10.4236/ajac.2011.228123. [DOI] [PubMed] [Google Scholar]
- 19.Ündeğer Ü., Başaran N. Effects of pesticides on human peripheral lymphocytes in vitro: Induction of DNA damage. Arch. Toxicol. 2005;79(3):169–176. doi: 10.1007/s00204-004-0616-6. [DOI] [PubMed] [Google Scholar]
- 20.Eaton D.L., Daroff R.B., Autrup H., Bridges J., Buffler P., Costa L.G., Coyle J., McKhann G., Mobley W.C., Nadel L., Neubert D., Schulte-Hermann R., Spencer P.S. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008;38:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- 21.Kaur R., Mavi G.K., Raghav S., Khan I. Pesticides Classification and its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2019;8(03):1889–1897. [Google Scholar]
- 22.Bempah C.K., Donkor A., Yeboah P.O., Dubey B., Osei-Fosu P. A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chem. 2011;128(4):1058–1065. doi: 10.1016/j.foodchem.2011.04.013. [DOI] [Google Scholar]
- 23.Soomro A.M., Seehar G.M., Bhanger M.I., Channa N.A. Pesticides in the Blood Samples of Spray-workers at Agriculture Environment: The Toxicological Evaluation, Pakistan J. Anal. Environ. Chem. 2008;9:32–37. [Google Scholar]
- 24.Anwar T., Ahmad I., Tahir S. Determination of pesticide residues in fruits of Nawabshah district, Sindh, Pakistan. Pakistan J. Bot. 2011;43:1133–1139. [Google Scholar]
- 25.Lozowicka B., Kaczynski P., Paritova A.Y., Kuzembekova G.B., Abzhalieva A.B., Sarsembayeva N.B., Alihan K. Pesticide residues in grain from Kazakhstan and potential health risks associated with exposure to detected pesticides. Food Chem. Toxicol. 2014;64:238–248. doi: 10.1016/j.fct.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Mittal S., Kaur G., Vishwakarma G.S. Effects of Environmental Pesticides on the Health of Rural Communities in the Malwa Region of Punjab, India: A Review. Hum. Ecol. Risk Assess. 2014;20(2):366–387. doi: 10.1080/10807039.2013.788972. [DOI] [Google Scholar]
- 27.Lozowicka B., Abzeitova E., Sagitov A., Kaczynski P., Toleubayev K., Li A. Studies of pesticide residues in tomatoes and cucumbers from Kazakhstan and the associated health risks. Environ. Monit. Assess. 2015;187(10) doi: 10.1007/s10661-015-4818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammad A.M.A., Elaziz A., Ahmed S., Abdelbagi A.O. Knowledge, Attitudes and Practices of Farmers Towards Pesticides Use and Handling in Greenhouse Farms, Sudan. Int. J. Resarch - Granthaalayah. 2018;6:520–534. doi: 10.5281/zenodo.1465024. [DOI] [Google Scholar]
- 29.Sheikh Removal of Pesticide Residues from Okra Vegetable through Traditional Processing. J. Basic Appl. Sci. 2012;8:79–84. doi: 10.6000/1927-5129.2012.08.01.23. [DOI] [Google Scholar]
- 30.Li Y., Wang X., Yang H., Wang X., Xie Y. Oxidation of isoprothiolane by ozone and chlorine: Reaction kinetics and mechanism. Chemosphere. 2019;232:516–525. doi: 10.1016/j.chemosphere.2019.03.179. [DOI] [PubMed] [Google Scholar]
- 31.Rokbani O., Fattouch S., Chakir A., Roth E. Heterogeneous oxidation of two triazole pesticides (diniconazole and tebuconazole) by OH-radicals and ozone. Sci. Total Environ. 2019;694:133745. doi: 10.1016/j.scitotenv.2019.133745. [DOI] [PubMed] [Google Scholar]
- 32.Šojić D., Despotović V., Orčić D., Szabó E., Arany E., Armaković S., Illés E., Gajda-Schrantz K., Dombi A., Alapi T., Sajben-Nagy E., Palágyi A., Vágvölgyi C., Manczinger L., Bjelica L., Abramović B. Degradation of thiamethoxam and metoprolol by UV, O 3 and UV/O 3 hybrid processes: Kinetics, degradation intermediates and toxicity. J. Hydrol. 2012;472–473:314–327. doi: 10.1016/j.jhydrol.2012.09.038. [DOI] [Google Scholar]
- 33.Chitravathi K., Chauhan O.P., Raju P.S., Madhukar N. Efficacy of Aqueous Ozone and Chlorine in Combination with Passive Modified Atmosphere Packaging on the Postharvest Shelf-Life Extension of Green Chillies (Capsicum annuum L.) Food Bioprocess Technol. 2015;8(6):1386–1392. doi: 10.1007/s11947-015-1511-2. [DOI] [Google Scholar]
- 34.Meireles A., Giaouris E., Simões M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016;82:71–85. doi: 10.1016/j.foodres.2016.01.021. [DOI] [Google Scholar]
- 35.Ye B., Chen Z., Li X., Liu J., Wu Q., Yang C., Hu H., Wang Ronghe. Inhibition of bromate formation by reduced graphene oxide supported cerium dioxide during ozonation of bromide-containing water. Front. Environ. Sci. Eng. 2019;13(6) doi: 10.1007/s11783-019-1170-z. [DOI] [Google Scholar]
- 36.Chanrattanayothin P., Peng-Ont D., Masa-Ad A., Warisson T., Nirunsin R., Sintuya H. Degradation of Cypermethrin and Dicofol Pesticides Residue in Dried Basil Leave by Gaseous Ozone Fumigation. Ozone Sci. Eng. 2020;42(5):469–476. doi: 10.1080/01919512.2019.1708699. [DOI] [Google Scholar]
- 37.Fraiese A., Naddeo V., Prado M., Cesaro A., Zarra T., Liu H., Belgiorno V., Ii B.J.F.P. Removal of Emerging Contaminants in Wastewater by Sonolysis, Photocatalysis and Ozonation. Glob. NEST Int. J. 2018;21:98–105. doi: 10.30955/gnj.002625. [DOI] [Google Scholar]
- 38.Malakootian M., Shahesmaeili A., Faraji M., Amiri H., Silva Martinez S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. Prot. 2020;134:292–307. doi: 10.1016/j.psep.2019.12.004. [DOI] [Google Scholar]
- 39.Ikeura H., Kobayashi F., Tamaki M. Removal of residual pesticide, fenitrothion, in vegetables by using ozone microbubbles generated by different methods. J. Food Eng. 2011;103(3):345–349. doi: 10.1016/j.jfoodeng.2010.11.002. [DOI] [Google Scholar]
- 40.Liang Y., Wang W., Shen Y., Liu Y., Liu X.J. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chem. 2012;133(3):636–640. doi: 10.1016/j.foodchem.2012.01.016. [DOI] [Google Scholar]
- 41.Baig S.A., Akhtera N.A., Ashfaq M., Asi M.R. Determination of the Organophosphorus Pesticide in Vegetables by High-Performance Liquid Chromatography. Environ. Sci. 2009;6:513–519. [Google Scholar]
- 42.Siddique Z., Malik A.U., Asi M.R., Anwar R., Inam Ur Raheem M. Sonolytic-ozonation technology for sanitizing microbial contaminants and pesticide residues from spinach (Spinacia oleracea L.) leaves, at household level. Environ. Sci. Pollut. Res. 2021;28(38):52913–52924. doi: 10.1007/s11356-021-14203-y. [DOI] [PubMed] [Google Scholar]
- 43.EU, European Union (EU) pesticides database. Pesticide Residues MRLs. Directorate General for Health & Consumers., (2019). https://ec.europe.eu/food/plant/pesticides/eu-pesticides-database/public/?event=download.MRL.
- 44.M. Shafi, M. Imran, M. Sarwar, S. Kalsoom, H. Mujahid, A Study of Pesticide Residues in Different Fruits Collected from Different Fruit Markets of Lahore , Punjab, (2014).
- 45.Akhtar S., Yaqub G., Hamid A., Afzal Z., Asghar S. Determination of Pesticide Residues in Selected Vegetables and Fruits From A Local Market of Lahore, Pakistan. Curr. World Environ. 2018;13(2):242–250. [Google Scholar]
- 46.Brodowska A.J., Nowak A., Śmigielski K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018;58(13):2176–2201. doi: 10.1080/10408398.2017.1308313. [DOI] [PubMed] [Google Scholar]
- 47.Chen S., Deng J., Deng Y., Gao Naiyun. Influencing factors and kinetic studies of imidacloprid degradation by ozonation. Environ. Technol. (United Kingdom) 2019;40(16):2127–2134. doi: 10.1080/09593330.2018.1439105. [DOI] [PubMed] [Google Scholar]