Abstract
1,5-diphenylpent-4-en-1-one derivatives were synthesised using the grindstone method with Cu(II)-tyrosinase used as a catalyst. This method showed a high yield under mild reaction conditions. The synthesised compounds were identified by FTIR, 1H NMR, 13C NMR, mass spectrometry, and elemental analysis. In this study, a total of 17 compounds (1a–1q) were synthesised, and their larvicidal and antifeedant activities were evaluated. Compound 1i (1-(5-oxo-1,5-diphenylpent-1-en-3-yl)-3-(3-phenylallylidene)thiourea) was notably more active (LD50: 28.5 µM) against Culex quinquefasciatus than permethrin(54.6 µM) and temephos(37.9 µM), whereas compound 1i at 100 µM caused 0% mortality in Oreochromis mossambicus within 24 h in an antifeedant screening, with ichthyotoxicity determined as the death ratio (%) at 24 h. Compounds 1a, 1e, 1f, 1j, and 1k were found to be highly toxic, whereas 1i was not toxic in antifeedant screening. Compound 1i was found to possess a high larvicidal activity against C. quinquefasciatus and was non-toxic to non-target aquatic species. Molecular docking studies also supported the finding that 1i is a potent larvicide with higher binding energy than the control (− 10.0 vs. − 7.6 kcal/mol) in the 3OGN protein. Lead molecules are important for their larvicidal properties and application as insecticides.
Subject terms: Chemical biology, Environmental chemistry, Green chemistry, Chemical synthesis
Introduction
In the broadest sense, human beings are part of nature; however, our activity is often understood and interpreted as a category that is unique and separate from the rest of the natural phenomena. It is both the legal and moral obligation of every human to protect planet Earth by undertaking activities that would prevent contamination of our planet and thereby protect it for future generations. For instance, as a scientist in chemical industries or academia, one could focus on protecting nature by employing green chemistry to produce various chemical and pharmaceutical active ingredients. Of the several green chemistry methodologies, the grindstone chemistry technique is a simple practice for the preparation of chemical compounds. Toda et al. developed a range of chemical reactions carried out by simply grinding or triturating the solids together1. We will now focus on Mannich reactions, which are a widely studied type of reaction in the organic and medicinal chemistry domains2.
Mushroom tyrosinase, which has a dinuclear copper active centre, catalyses the hydroxylation and subsequent oxidation reactions that convert phenol to the related ortho-quinone as well as the oxidation of catechol to quinone3–8. Tyrosinase, alongside catechol oxidase9 and hemocyanin10, belongs to the type 3 copper protein class. The dicopper core of this type-3 copper protein takes three redox forms3–8. The active core of the deoxy type [Cu(I)–Cu(I)] contains two cuprous ions, which attach dioxygen to produce the oxy form. Dioxygen bonds as a peroxide ion in the oxy form in the µ-ŋ2:ŋ2 side-on bridging mode [Cu(II)–O22−–Cu(II)]. The met type [Cu(II)–Cu(II)] signifies a condition wherein copper atoms only at the active site have been oxidised but have not been bound by dioxygen. The met type of tyrosinase is an enzymatic form wherein two cupric ions are bridged by one or two tiny ligands, along with water molecules or hydroxide ions, while the enzyme is at rest and acting as a catalyst.
Mannich-type reactions face significant challenges in terms of reaction time, reaction conditions, toxicity, catalyst requirements, and separation and determination of the purity of final product(s). Other challenges include synthetic methodologies such as ultrasound or microwave irradiation, the use of Lewis acids or bases, and the use of solubilizing agents or surfactant-type catalysts11. In addition, some of the known green trends in Mannich reactions consist of ball milling without solvents12, using ionic liquid mediums13, using ionic liquids reinforced with nanoparticles14, or applying enzymes under bio-catalytic conditions15,16. However, the present study focused on the grindstone green chemistry method in order to overcome the abovementioned challenges in the preparation of Mannich base derivatives.
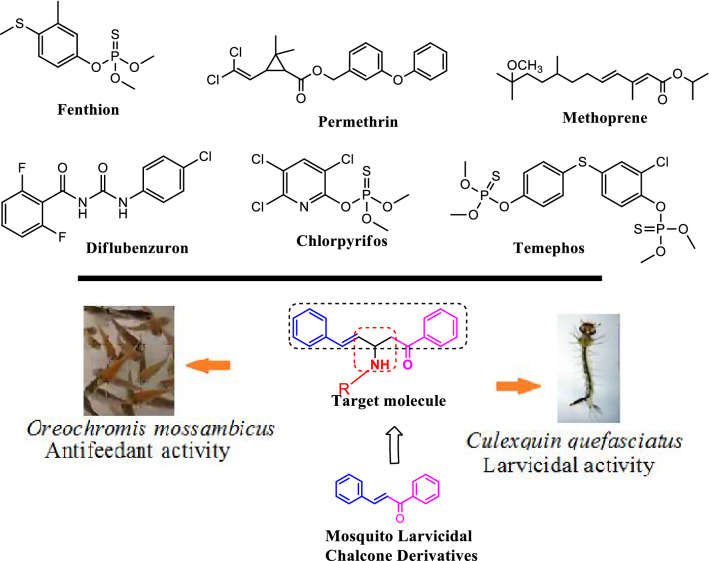
Mosquitoes are an important transmission vector for several diseases, particularly malaria17,18. These types of diseases have economic and social impacts worldwide. Among the mosquito species, Culex quinquefasciatus is particularly associated with various vector-spread diseases in several regions. Larvicides are insecticides designed to kill insects during their larval stage. Methoprene is an insect growth controller that prevents larvae from developing significantly beyond the pupa stage by interrupting their growth period. Methoprene is mildly toxic to a variety of crabs, shrimp, lobster, and crayfish and is extremely toxic to a variety of fish and aquatic herbivores; it tends to accumulate in fish tissues19. Olfaction plays an important role in many species and is linked to host-seeking, replication, predator recognition, and food detection20. Odorant-binding proteins (OBPs) aid signal transduction by transporting odorants to olfactory receptors21,22. Some example, consider previous reports, the ligand (5R,6S)-6-acetoxy-5-hexadecanolide23–25 was bound to OBP of the C. quinquefasciatus mosquito (PDB ID: 3OGN), it is best model for selection 1,5-diphenyl pent-4-en-1-one targets and molecular docking in this study.
The control of mosquitos presents a substantial challenge, and currently inhibitors such as permethrin26, organophosphates27, fenthion28,29, chlorpyrifos30–32, temephos33,34, diflubenzuron35 and methoprene36 are used; Fig. 1 details the compositions of these commercial insecticides. However, the use of chemical insecticides pose bigger challenges and various potential environmental problems, such as the widespread development of resistance and disruption of natural biological control systems37,38. These problems require overcoming new mosquito larvae inhibitors and improving green methodologies, which can be achieved through Mannich base condensation reactions.
Figure 1.
Synthetic marketable insecticides and our target molecule drawn by ChemDraw Ultra 12.0 Suite (PerkinElmer, USA).
Mannich base synthesis is one of the best tools for green synthesis, in this way preparation of target compound based on cinnamylacetophenone (1,5-diphenylpent-4-en-1-one) comparable to cinnamylphenone (1,3-diphenylprop-2-en-1-one (or) chalcone, Fig. 1), basically chalcone derivatives have mosquito larvicidal properties39. Some publications have investigated the environmental study of chalcones40 and 1,5-diphenylpent-4-en-1-one (cinnamylacetophenone)41. In general, chemical insecticides are the main agents used to reduce populations of vector mosquitoes42, even though their accessibility and use are limited by their toxicity to the environment and non-target organisms43,44 as well as the resistance of some mosquito species to them.
Chemically modified chalcones have been recently used to control insect populations; for instance, chalcone derivatives are toxic to Ae. aegypti first instar larvae and adults45 and Aedes albopictus larvae46. Some furan-chalcones are toxic to Culex quinquefasciatus larvae in the fourth stage of development47.
The current work was focused on the presence of alkenyl imine/β-amino ketones, particularly imines, which are frequently used in organic synthesis because of their high reactivity and the synthetic utility of the ensuing products48. Furthermore, β-amino ketones and their analogues have shown effective medicinal properties49,50. So that, current study was to determine novel water-soluble and nontoxic Mannich base 1,5-diphenylpent-4-en-1-one derivatives via grindstone green chemistry methodology that can be used to inhibit the second instar Culex mosquito larvae as a bio-indicator of aquatic pollution.
Results and discussion
Chemistry
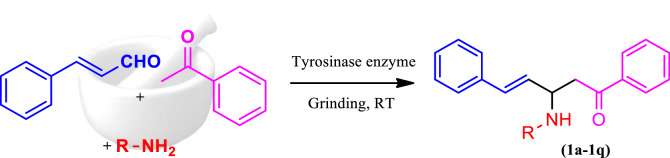
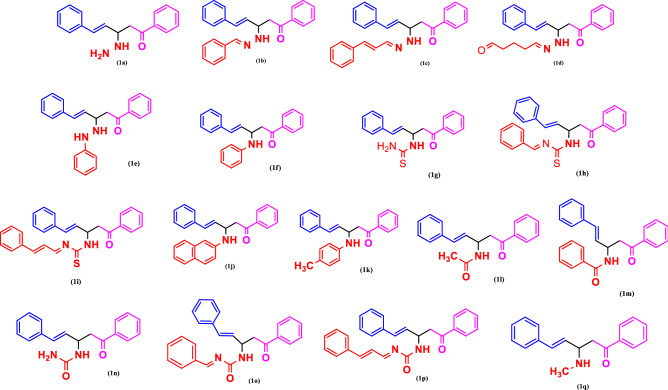
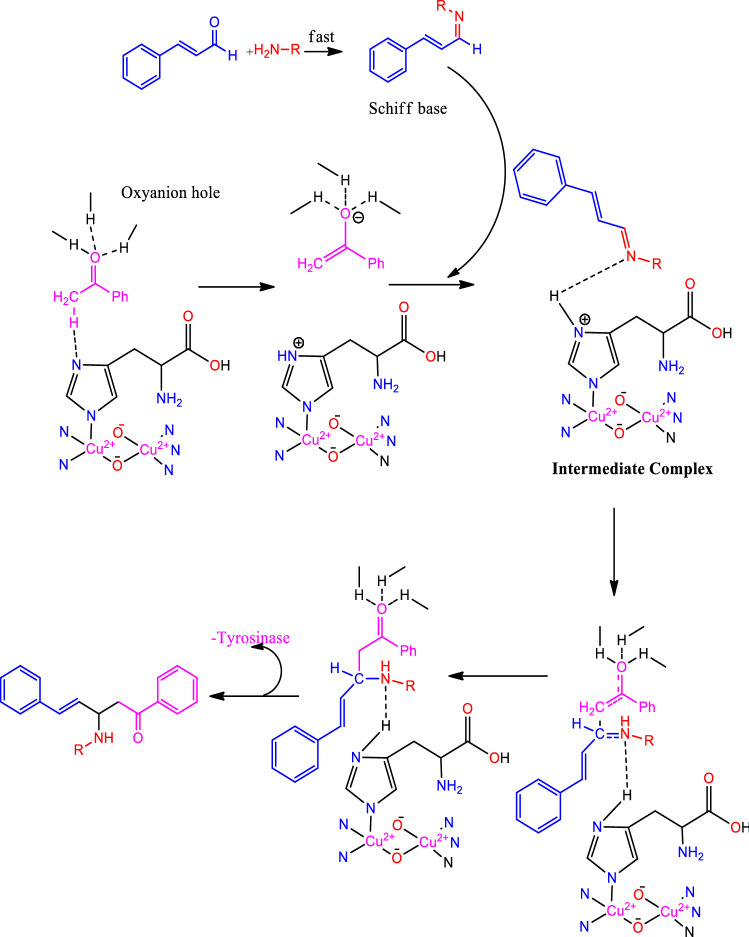
A one-pot multicomponent synthesis of the title compounds was achieved using the grindstone green chemistry method. A mixture of acetophenone, cinnamaldehyde, substituted amine, and a catalytic amount of Cu(II)-tyrosinase enzyme was ground together in a pestle mortar. This was then followed by purification via column chromatography, in order to obtain the title compounds (1a–1q). The synthetic route outline is shown in Scheme 1. The chemical structures of synthesized compounds (1a–1q) were represented in Fig. 2. The active site in hydrolases is often thought to be responsible for promiscuous catalysis51. We suggest a mechanism for the Cu(II)-tyrosinase-catalysed Mannich reaction, outlined in Scheme 2, by combining this perspective with our findings, as mentioned above. First, the aldehyde and amine can easily react to form the Schiff base, and the ketone is simultaneously pre-activated by Cu(II)-tyrosinase to produce the enolate anion. Second, with the aid of the His residue of Cu(II)-tyrosinase, the Schiff base may form an intermediate complex. The Mannich adduct is then freed from the oxyanion hole after a proton is moved from the Schiff base to the enolate anion to create a new carbon–carbon bond. The core steps in this enzymatic mechanism are the formation of the enolate anion and the intermediate complex. Copper-containing materials such as coppertriflate52, copperacetate53, copperbromide54, and copper nanoparticles55 play a vital role in Mannich base reactions. The one-pot multicomponent Mannich reaction was catalysed via various enzymes, such as trypsin56, lipase57, and protease58. In the present study, copper containing the Cu(II)-tyrosinase enzyme was used as a catalyst for the synthesis of N-Mannich base (1a–1q) derivatives.
Scheme 1.
Synthetic route of Mannich base derivative.
Figure 2.
Structures of synthesized Mannich base derivatives (1a–1q) drawn by ChemDraw Ultra 12.0 Suite (PerkinElmer, USA).
Scheme 2.
Proposed mechanism of Mannich base derivative formation.
Some of the previously reported compounds, such as compound 1l, were reported by β-acetamido ketones from cinnamaldehyde to react with acetophenone at room temperature, with L-proline used as a catalyst, to result in a yield of 75%. Another method was reported previously where N-substituted β-amino ketone derivatives had been produced by a one-pot multi-component process using copper(II)-phthalocyanine as a catalyst to result in an yield of 51%, which is comparable to the compound produced in the present work, which showed an 84% yield. Compound 1m was also reported previously; an imine derived from an α,β-unsaturated aldehyde was also related to the present high binaphthol-derived monophosphoric acids as organocatalysts for enantioselective carbon–carbon bond-forming reactions, thus resulting in a product yield of 81%; an 82% yield was obtained in this study. There is no enzymatic catalysis was involved in the synthesis of compounds 1l and 1m in the literature. In our study we utilized Cu(II)-tyrosinase as a catalyst for producing compounds 1l and 1m and also the compounds acquired with high yields comparing previous literatures.
The compound 1a was synthesised using the catalysts trypsin, lipase, protease, CuCl2.2H2O, and Cu(II)-tyrosinase with yields of 64%, 72%, 68%, 84%, and 92%, respectively. The use of the Cu(II)-tyrosinase enzyme green catalyst, instead of CuCl2.2H2O, increased the yield of the Mannich derivatives to 92% and reduced the reaction time. The optimisation of the reaction conditions and catalysts is presented in Table 1. The obtained compounds were analysed via FT-IR, 1H, and 13C NMR spectroscopy. The key assignments of the compounds showed significant bands at 3170.23–3176.54, 2595.45–2599.98, and 1710.68–1716.70 cm−1 in the IR spectrum, conforming to the –NH, –C=N, and –C=O groups, respectively. The 1H NMR showed signals at δ 8.03–9.70, 3.82–4.81 and 2.40–2.98 ppm, indicating –NH, 4-CH, and –CH2 protons, respectively. The 13C NMR showed peaks at δ 197.4–197.6, 48.4–59.2, and 48.0–50.6 ppm, which conforms to –C=O, –CH, and –CH2 atoms, respectively. Mass spectra and elemental analysis were used to determine the conformation of all these compounds.
Table 1.
Catalyst optimization for compound 1a.
| Entry | Catalyst | Yield (%) | Time (min) |
|---|---|---|---|
| 1 | No enzyme | 06 | 30 |
| 2 | Trypsin from bovine pancreas | 64 | 8 |
| 3 | Lipase from Candida antarctica | 72 | 12 |
| 4 | Protease from Streptomyces griseus | 68 | 10 |
| 5 | CuCl2·2H2O | 84 | 5 |
| 6 | Cu(II)-Tyrosinase from mushroom | 92 | 2 |
“In general, E-alkenyl imines are organized from the corresponding E-alkenyl aldehydes through imine precursors59–61. In this reaction, the carbon–carbon bond formation rate allows the isomerisation of the in situ generated E-alkenyl imine from E-alkenyl aldehydes with secondary amine and acetophenone, in the presence of 5 mol% of Cu(II)-tyrosinase catalysis to afford the corresponding Mannich adducts (1a-1q) in moderate to good yields with high E-selectivity”.
NOE NMR data (see Supplementary Material) clearly confirmed the stereochemistry of the E isomers of compounds 1a, and 1i. Thus, based on this study the spectroscopic characteristic downfield shift is observed for this pent-4-en-1-one proton in the E-isomer than in the Z-isomer.
Catalyst recovery studies
The recovered catalyst was recycled for at least 10 run times with a small defeat in catalytic action (Fig. 3). The decrease in catalytic action perceived through the reinforced catalyst on recycling might be owing to limited loss of basic locates or loss of catalyst surface area during regeneration/reaction. The values are displayed in Table 2.
Figure 3.
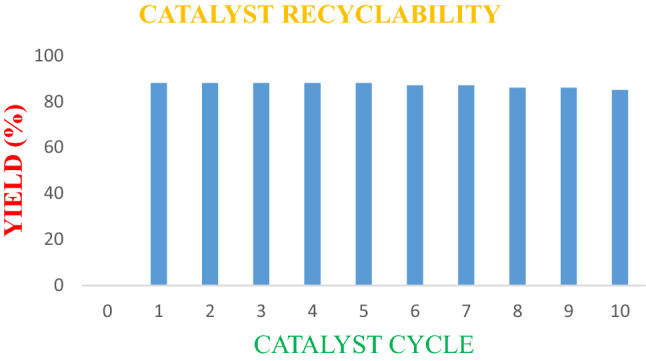
Catalyst recyclability avtivity of Cu(II)-tyrosinase enzyme drawn by Microfoft Office 2019 Suite.
Table 2.
Recyclability of Cu(II)-tyrosinase enzyme catalyst.
| Entry | Catalyst | Yield (%) |
|---|---|---|
| 1 | 1st use | 92 |
| 2 | 2nd use | 92 |
| 3 | 3rd use | 90 |
| 4 | 4th use | 90 |
| 5 | 5th use | 88 |
| 6 | 6th use | 87 |
| 7 | 7th use | 87 |
| 8 | 8th use | 86 |
| 9 | 9th use | 86 |
| 10 | 10th use | 85 |
Biological activity
A total of 17 compounds (1a–1q) were tested against second instar C. quinquefasciatus larvae, and the toxicity of the title compounds was assessed in the marine fish Oreochromis mossambicus. Toxicity was defined as the ratio of deaths (%) at 24 h. Structure–activity relationships showed that the final compounds contained 1,5-diphenylpent-4-en-1-one with different types of amines, thus exerting larvicidal and toxic effects based on the formation of the specific chemical composition.
Compound 1i showed a higher larvicidal activity than other compounds, with an LD50 of 28.5 µM, which was better than that of the controls temephos (LD50 of 37.9 µM)62 and permethrin (LD50 of 54.6 µM). The antifeedant induced 0% mortality even at LD50 > 100 µM, which was represented by no toxicity in water.
Compound 1a induced 80% mortality at 100 µM and its LD50 value was 223.0 µM, whereas the antifeedant induced 100% mortality at 100 µM and had a LD50 value of 49.5 µM. This suggests that the presence of the hydrazine group may be the reason for the observed antifeedant-induced 100% mortality, as evident from toxicity against O. mossambicus fingerlings within 15 min of screening.
Compounds 1f and 1j induced a mortality rate of 80% with LD50 values of 177.4 µM and 154.9 µM, respectively, in larvicidal screening whereas they induced 100% mortality in antifeedant screening. This suggests that the presence of aniline and naphthalen-2-amine groups may be the reason for the observed biological effects, respectively.
Compounds 1m and 1n induced a mortality rate of 80% with LD50 values of 159.8 µM and 190.9 µM, respectively, in larvicidal screening whereas they induced 0% mortality in antifeedant screening. This suggests that the presence of the benzamide and urea groups could be the reason for the respective observed biological effects.
Compounds 1d and 1o induced 0% mortality at 100 µM in both the larvicidal and antifeedant screening. This suggests that the presence of the 5-hydrazonopentanal and 1-benzylideneurea groups may be the reason for the observed biological effect as they exhibited no active or toxic behaviour.
The above analysis therefore indicates that compound li was significantly active in larvicidal screening and displayed low toxicity in antifeedant screening. The percentages of mortality and LD50 values are presented in Tables 3 and 4.
Table 3.
Larvicidal activity of compounds (1a–1q).
| Compounds | % of Mortality at 25 µM | % of Mortality at 50 µM | % of Mortality at 100 µM | LD50 (µM)a |
|---|---|---|---|---|
| 1a | 24.1 ± 0.2 | 43.2 ± 0.1 | 80.2 ± 0.2 | 223.0 ± 0.0 |
| 1b | 11.2 ± 0.2 | 27.1 ± 0.2 | 40.1 ± 0.1 | 282.1 ± 0.0 |
| 1c | 19.3 ± 0.4 | 26.3 ± 0.4 | 40.2 ± 0.6 | 262.8 ± 0.0 |
| 1d | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 286.9 ± 0.0 |
| 1e | 33.3 ± 0.1 | 48.3 ± 0.2 | 60.4 ± 0.2 | 193.3 ± 0.3 |
| 1f | 25.0 ± 0.2 | 44.1 ± 0.2 | 80.0 ± 0.2 | 177.4 ± 0.2 |
| 1g | 22.1 ± 0.2 | 34.2 ± 0.2 | 40.1 ± 0.3 | 322.1 ± 0.0 |
| 1h | 34.5 ± 0.2 | 47.9 ± 0.3 | 60.1 ± 0.2 | 165.1 ± 0.2 |
| 1i | 68.2 ± 0.4 | 88.2 ± 0.6 | 100 ± 0.0 | 28.5 ± 0.2 |
| 1j | 26.1 ± 0.2 | 44.5 ± 0.2 | 80.4 ± 0.3 | 154.9 ± 0.2 |
| 1k | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 292.8 ± 0.0 |
| 1l | 20.8 ± 0.1 | 20.8 ± 0.1 | 20.8 ± 0.1 | 340.8 ± 0.0 |
| 1m | 29.9 ± 0.3 | 42.3 ± 0.3 | 80.9 ± 0.3 | 159.8 ± 0.2 |
| 1n | 29.9 ± 0.2 | 43.6 ± 0.2 | 81.0 ± 0.2 | 190.9 ± 0.0 |
| 1o | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 261.4 ± 0.0 |
| 1p | 40.4 ± 0.1 | 40.4 ± 0.1 | 40.4 ± 0.1 | 244.8 ± 0.0 |
| 1q | 20.4 ± 0.2 | 20.4 ± 0.2 | 20.4 ± 0.2 | 376.8 ± 0.0 |
| Permethrin | 51.1 ± 1.0 | 76.3 ± 0.1 | 100 ± 0.0 | 54.6 ± 0.0 |
| Temephos | 56.1 ± 0.2 | 79.3 ± 0.2 | 100 ± 0.0 | 37.9 ± 0.0 |
Larvicidal activity model is used for the activity assays (second instar C. quinquefasciatus), one-day-old larvae were considered as 2nd instar.
aValues are mean ± SD (n = 3). Lethal Dose (LD50): the LD50 is one way to measure the short-term poisoning potential (acute toxicity) of a material.
Table 4.
Antifeedant activity of compounds (1a–1q).
| Compounds | % of Mortality at 10 µM | % of Mortality at 25 µM | % of Mortality at 50 µM | % of Mortality at 100 µM | LD50 (µM)a |
|---|---|---|---|---|---|
| 1a | 33.3 ± 0.2 | 66.2 ± 0.0 | 88.2 ± 0.0 | 100 ± 0.0 | 49.5 ± 0.7 |
| 1b | 20.2 ± 0.3 | 20.2 ± 0.3 | 20.2 ± 0.3 | 20.2 ± 0.3 | 282.1 ± 0.0 |
| 1c | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 262.8 ± 0.0 |
| 1d | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 286.9 ± 0.0 |
| 1e | 31.3 ± 0.0 | 66.1 ± 0.0 | 82.2 ± 0.0 | 100 ± 0.0 | 47.8 ± 0.0 |
| 1f. | 41.2 ± 0.0 | 51.3 ± 0.0 | 72.2 ± 0.0 | 100 ± 0.0 | 64.4 ± 0.4 |
| 1 g | – | 5.2 ± 0.1 | 10.3 ± 0.1 | 20.6 ± 0.2 | 322.1 ± 0.0 |
| 1 h | 5.3 ± 0.1 | 20.2 ± 0.1 | 49.4 ± 0.1 | 60.4 ± 0.1 | 131.2 ± 0.8 |
| 1i | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 235.5 ± 0.0 |
| 1j | 42.2 ± 0.4 | 59.2 ± 0.3 | 88.2 ± 0.0 | 100 ± 0.0 | 26.7 ± 0.2 |
| 1 k | 33.1 ± 0.0 | 67.1 ± 0.74 | 87.9 ± 0.0 | 100 ± 0.0 | 40.4 ± 0.6 |
| 1 l | – | 5.2 ± 0.1 | 10.3 ± 0.1 | 20.2 ± 0.1 | 340.8 ± 0.0 |
| 1 m | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 281.3 ± 0.0 |
| 1n | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 339.7 ± 0.0 |
| 1o | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 261.4 ± 0.0 |
| 1p | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 244.8 ± 0.0 |
| 1q | – | 5.2 ± 0.1 | 10.3 ± 0.1 | 20.2 ± 1.0 | 376.8 ± 0.0 |
Antifeedant activity for the toxicity measurement against marine fish Oreochromis.
aValues are mean ± SD (n = 3). The LD50 is one way to measure the short-term poisoning potential (acute toxicity) of a material.
Culex quinquefasciatus larval growth regulation
To explore the impact of 1,5-diphenylpent-4-en-1-one formulations on C. quinquefasciatus larvae growth, metamorphosis, and production, we exposed the larvae to compound 1i for 72 h. Table 5 summarizes the effects of compound 1i impact on larval weight and growth inhibition. When subjected to 10 µM of compound 1i, the eclosion rate and time of the pupal and adult periods of administered C. quinquefasciatus is calculated, and the findings are seen in Table 6. Compound 1i had a growth-inhibition score of 41.36% and suppressed larval weight development. Furthermore, compound 1i had little effect on the duration of the adult and pupal periods, but it did result in a 55 percent eclosion rate. Compound 1i hindered the production and growth of C. quinquefasciatus larvae, according to these findings.
Table 5.
Compound 1i on the growth of Culex quinquefasciatus.
| Compound | Weight of larvae (mg) | Weight gain (mg) | Inhibition (%) | |
|---|---|---|---|---|
| 0 h | 72 h | |||
| 1ia | 100.3 ± 1.9 | 104.1 ± 0.2 | 3.9 ± 0.9 | 41.4 ± 2.8 |
| Controlb | 100.16 ± 0.3 | 106.7 ± 1.5 | 6.6 ± 1.4 | – |
aThe concentration of 1i was 10 µM.
bControl is not containing the compounds.
Table 6.
Analysis of progress of Culex quinquefasciatus growth.
| Compound | Duration of pupae (h) | Duration of adult (h) | Rate of eclosion (%) |
|---|---|---|---|
| 1ia | 68.1 ± 0.64 | 23.1 ± 1.36 | 55 ± 1.7 |
| Controlb | 65.5 ± 1.21 | 24.2 ± 0.82 | 80 ± 1.0 |
aThe concentration of 1i was 10 µM.
bControl is not containing the compounds.
Docking results
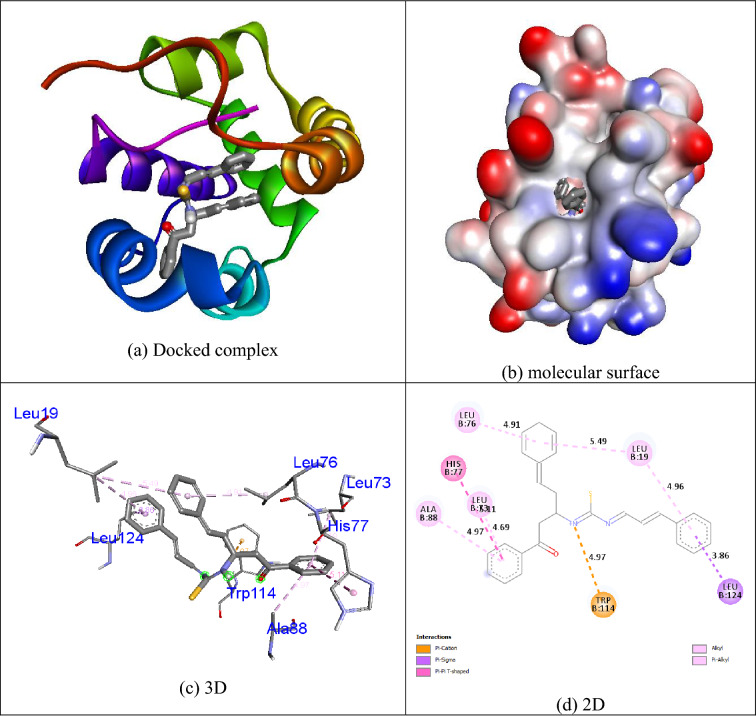
The Autodock Vina program was used to assess the docking behavior between compounds 1i, permethrin and temephos with the mosquito odorant binding protein (PDB ID: 3OGN). Compound 1i displayed more binding affinity (− 10.0 kcal/mol) than other compounds and permethrin (− 9.7 kcal/mol) and temephos (− 7.6 kcal/mol) with the mosquito odorant binding protein (PDB ID: 3OGN). Residues of the amino acids Leu19, Leu73, Leu76, His77, Ala78, Trp114, and Leu124 were tangled in hydrophobic connections. The interaction of compound 1i with mosquito odorant binding protein (PDB ID: 3OGN) is shown in Fig. 4. In the control permethrin, residues of the amino acids Leu15, Leu19, Phe59, Leu73, Leu76, His77, Leu80, Ala88, Met89, Gly92, His111, Trp114, Phe123, and Leu124 were tangled in hydrophobic connections.
Figure 4.
Molecular docking representation of ligand 1i within the active site of mosquito odorant binding protein (PDB ID: 3OGN). Chemical structures were drawn by ChemDraw Ultra 12.0 Suite (PerkinElmer, USA) and analyzed by the Discovery studio visualizer (BIOVIA Discovery studio 2019 Client).
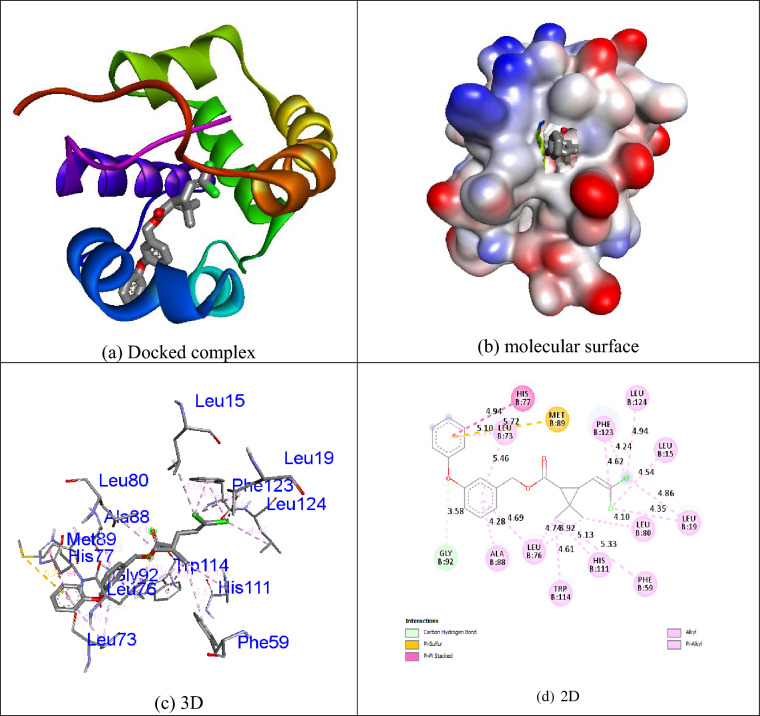
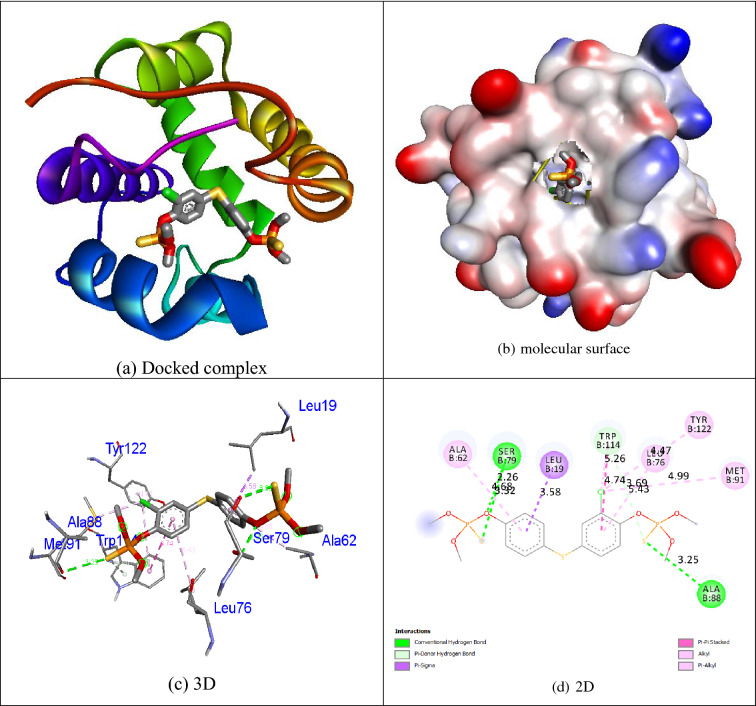
The positive control permethrin connected in the mosquito odorant binding protein (PDB ID: 3OGN) protein is shown in Fig. 5. The control temephos displayed three hydrogen bond interactions with the receptor mosquito odorant binding protein (PDB ID: 3OGN). The amino acid residue Ser79 showed two hydrogen bonds with temephos, with the bond lengths of 3.32 and 2.26 Å, and the amino acid residue Ala88 showed one hydrogen bond with temephos, with the bond length of 3.25 Å. Residues of the amino acids Leu19, Ala62, Leu76, Met91, Trp114, and Tyr122 were involved in hydrophobic contacts with the receptor. The interaction of the control temephos with the mosquito odorant binding protein (PDB ID: 3OGN) protein is shown in Fig. 6. The helix representation of inhibitor molecule docked into the receptor was shown in Figs. 4a, 5a, and 6a. The inhibitor molecule docked into the binding pocket of the receptor was shown in Figs. 4b, 5b, and 6b. The 3D representation of inhibitor molecule docked into the receptor was shown in Figs. 4c, 5c, and 6c. The 2D representation molecule docked with receptor was shown in Figs. 4d, 5d, and 6d. The results show that compound 1i possesses comparable inhibition abilities relative to the controls permethrin and temephos. The results are listed in Table 7.
Figure 5.
Molecular docking representation of ligand permethrin within the active site of mosquito odorant binding protein (PDB ID: 3OGN). Chemical structures were drawn by ChemDraw Ultra 12.0 Suite (PerkinElmer, USA) and analyzed by the Discovery studio visualizer (BIOVIA Discovery studio 2019 Client).
Figure 6.
Molecular docking representation of ligand temephos within the active site of mosquito odorant binding protein (PDB ID: 3OGN). Chemical structures were drawn by ChemDraw Ultra 12.0 Suite (PerkinElmer, USA) and analyzed by the Discovery studio visualizer (BIOVIA Discovery studio 2019 Client).
Table 7.
Molecular docking interaction of compounds (1a–1q) and control Temephos, Permethrin.
| Compounds | Mosquito odorant-binding protein 3OGN | ||
|---|---|---|---|
| Binding affinity (kcal/mol) | No. of H-bonds | H-bonding residues | |
| 1a | − 9.0 | 2 | His121, Phe123 |
| 1b | − 9.7 | 0 | – |
| 1c | − 9.0 | 0 | – |
| 1d | − 8.8 | 0 | – |
| 1e | − 9.7 | 1 | Phe123 |
| 1f | − 9.6 | 0 | – |
| 1g | − 8.3 | 0 | – |
| 1h | − 9.3 | 0 | – |
| 1i | − 10.0 | 0 | – |
| 1j | − 9.8 | 0 | – |
| 1k | − 9.8 | 0 | – |
| 1l | − 8.9 | 0 | – |
| 1m | − 9.8 | 0 | – |
| 1n | − 8.8 | 0 | – |
| 1o | − 9.5 | 0 | – |
| 1p | − 9.2 | 0 | – |
| 1q | − 8.3 | 0 | – |
| Temephos | − 7.6 | 3 | Ser79, Ala88 |
| Permethrin | − 9.7 | 0 | – |
MD simulation analysis
The protein–ligand complex structure of ligand 1i with 3OGN stability was carried out by Molecular Dynamics (MD) simulation method using Gromacs. Root Mean Square Deviation (RMSD) plot is an important to know the stability of the complex structure. From the analysis of values of RMSD plot, the values from 4.5 to 10 ns shows that the structure was stable because Cα backbone of protein was not fluctuated more (Fig. 7).
Figure 7.
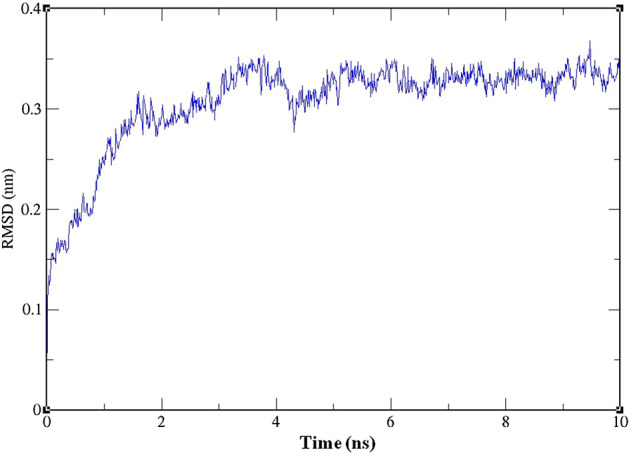
Graphical representation of Time vs. RMSD map for Protein after ligand fit to the protein during molecular dynamics simulation. XMgrace (Version 5.1. 19) tool was used to prepare the graphs (Turner, Land-Margin Research, & Technology, 2005).
Root Mean Square Fluctuation (RMSF) is an important analysis to characterize the protein residues throughout the simulation time period. From the RMSF analysis, the protein residues other than C terminal were not fluctuated more, especially the residues which were interacted by the ligand Leu 73, Leu 76, His 77, Ala 88, Trp 114 and Leu 124 were within the range of 0.3 nm (Fig. 8).
Figure 8.
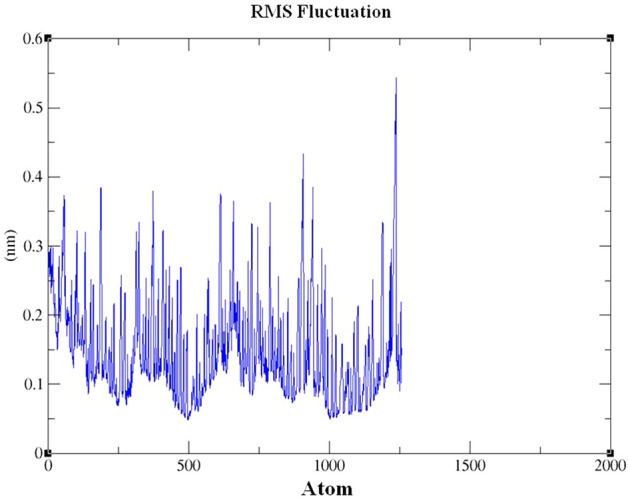
Graphical representation of RMS Fluctuation map during molecular dynamics simulation. XMgrace (Version 5.1. 19) tool was used to prepare the graphs (Turner, Land-Margin Research, & Technology, 2005).
The hydrogen bond interaction between the protein 3OGN and ligand 1i was formed during the period of simulation. 3 hydrogen bonds and pi–pi interaction were formed between the docked complex structures during different nano seconds of simulation system (Fig. 9).
Figure 9.
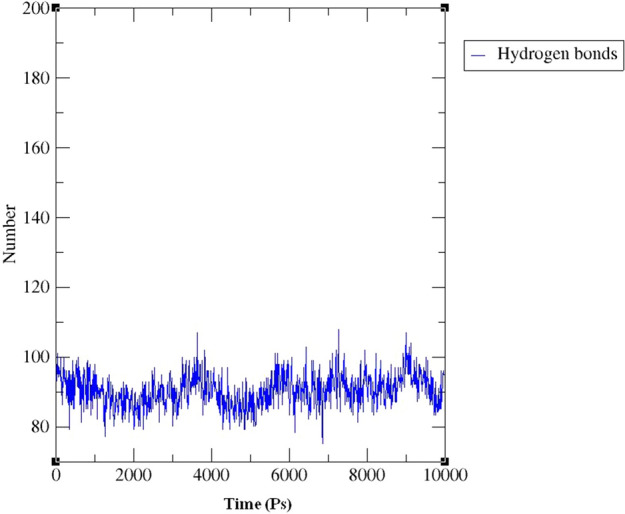
The hydrogen bond interaction between the protein 3OGN and compound 1i. XMgrace (Version 5.1. 19) tool was used to prepare the graphs (Turner, Land-Margin Research, & Technology, 2005).
The radius of gyration value of complex structure of protein 3OGN bounded with the ligand 1i shows that the ligand causes an alteration of the protein microenvironment. The radius started with 1.36 nm and it is decreased upto 1.33 nm at 6 ns and finally it is increased to 1.34 nm at the 10 ns (Fig. 10).
Figure 10.
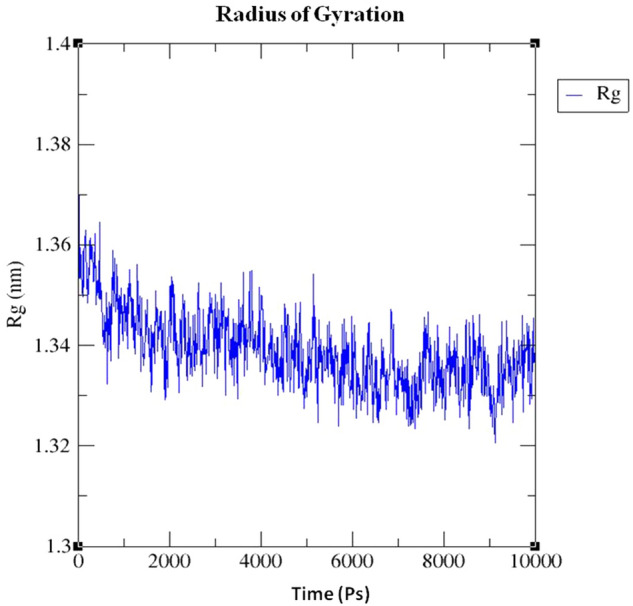
Radius of gyration value of complex structure of protein 3OGN bounded with the compound 1i. XMgrace (Version 5.1. 19) tool was used to prepare the graphs (Turner, Land-Margin Research, & Technology, 2005).
From this MD simulation analysis, the compound ligand 1i is stable with the respective of protein and it has good interaction with the important residues of protein. Hence, this compound may suggest to good inhibitor against the 3OGN protein.
Materials and methods
Chemistry
Thermo scientific Nicolet iS5 FTIR (4000–400 cm−1) was used for analysis of all compounds. Bruker DRX-300 MHz, 75 MHz was used for the analysis of 1H and 13C NMR spectra. An elemental analyzer (model Vario EL III) was used to analyze elements (C, H, N, and S) percentage (%). Mass spectra were recorded by Perkin Elmer GCMS model Clarus SQ8 (EI).
General procedure for the synthesis of compounds (1a–1q)
A reaction mixture made up of cinnamaldehyde (0.01 mol, 1.32 mL), acetophenone (0.01 mol, 1.20 mL), substituted amine (0.01 mol) and Cu(II)-tyrosinase enzyme (0.5 g) was mixed in a mortar and ground at RT. Then 2 mL of 50 mM potassium phosphate buffer (pH 6.0) was added and filtered to recover the catalyst. The final filtered solid material was separated using column chromatography (Ethyl acetate4:hexane6). The same method was followed when mixing compounds 1b–1q.
3-Hydrazinyl-1,5-diphenylpent-4-en-1-one (1a)
White solid; mp: 110–112 °C; Yield: 92%; Water solubility: 0.11 mM/mL; IR(KBr) ν: 3171.48, 3065.51, 3041.02, 1715.02, 1624.53 cm−1; 1H NMR (300 MHz): δ 9.20 (s, 1H), 8.84 (s, 2H, NH2), 7.97–7.96 (dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar-ring), 7.63–7.60 (d, J = 6.21 Hz, 1H, Ar-ring), 7.53–7.51 (dd, J = 7.30 Hz, J = 7.34 Hz, 2H, Ar-ring), 7.41–7.37 (dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar-ring), 7.34 (d, J = 6.22 Hz, 1H, Ar-ring), 7.21 (dd, J = 7.30 Hz, J = 7.35 Hz, 2H, Ar-ring), 6.56–6.51 (d, J = 6.22 Hz, 1H, CH), 6.19–6.14 (d, J = 6.22 Hz, 1H), 3.84–3.80 (m, 1H), 2.94–2.91 (d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.4 (1C), 136.7, 133.1, 128.8, 128.6 (6C, Ph ring), 136.4, 128.6, 128.5, 127.9 (6C, Ar ring), 133.4 (1C), 128.4 (1C), 59.2 (1C), 48.0 (1C); EIMS (m/z): 267.15 (M+,18%); Anal. Calcd. for C17H18N2O: C, 76.66; H, 6.81; N, 10.52%; found: C, 76.68; H, 6.80; N, 10.51%.
3-(2-Benzylidenehydrazinyl)-1,5-diphenylpent-4-en-1-one (1b)
Greenish solid; mp:145–148 °C; Yield: 86%; Water solubility: 0.06 mM/mL; IR(KBr) ν: 3176.51 (NH), 3072.50, 3032.32, 2596.43, 1716.08, 1623.43; 1H NMR(300 MHz,): δ 9.21(s,1H), 8.36(s,1H,–CH), 7.97–9.94(dd, J = 7.33 Hz, J = 7.37 Hz), 7.86–7.81 (dd, J = 7.33 Hz, J = 7.37 Hz), 7.63–7.60(d, J = 6.21 Hz, 1H, Ph), 7.55–7.53(dd, J = 7.31 Hz, J = 7.34 Hz, 2H), 7.50–7.47(m, 3H, Ar ring), 7.40–7.38(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.34–7.31(d, J = 6.21 Hz, 1H, Ar ring), 7.20–7.17(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.58–6.54 (d, 1H, J = 6.21 Hz, CH), 6.18–6.14(d, J = 6.21 Hz, 1H, CH), 3.80–3.76(m, 1H, CH), 2.95–2.92(d, J = 6.21 Hz, 2H, CH2); 13C NMR (75 MHz): 197.6 (1C), 143.3 (1C), 136.6, 133.0, 128.7, 128.5(6C, Ph ring), 136.5, 128.7, 128.6, 128.0(6C, Ar ring), 134.4(1C), 133.7, 131.0, 129.2, 128.8 (6C, Ph ring), 128.5(1C), 55.1(1C), 48.5(1C); EIMS(m/z) 355.18 (M+, 26%); Anal. Calcd. for C24H22N2O: C, 81.33; H, 6.26; N, 7.90%; found: C, 81.31; H, 6.27; N, 7.91%.
1,5-Diphenyl-3-(2-(3-phenylallylidene)hydrazinyl)pent-4-en-1-one (1c)
Light green powder; mp: 148–150 °C; Yield: 88%; Water solubility: 0.14 mM/mL; IR(KBr) ν 3176.50, 3073.51, 3031.30, 2595.45, 1714.08, 1624.40 cm−1; 1H NMR(300 MHz,): δ 9.26(s, 1H, NH), 7.95–7.91(dd, J = 7.33 Hz, J = 7.37 Hz), 7.63–7.60–7.58(d, J = 6.21 Hz, 1H), 7.57–7.54(dd, J = 7.33 Hz, J = 7.37 Hz, 2H), 7.53–7.50(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ph), 7.50(s, 1H, CH), 7.40–7.37(dd, J = 7.33 Hz, J = 7.37 Hz, 4H, Ar ring), 7.36–7.33 (d, J = 6.21 Hz, 2H, Ar-ring), 7.24–7.21(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.54–6.52(d, J = 6.21 Hz, 2H, CH), 6.17–6.12(d, J = 6.21 Hz, 2H, CH), 3.78–3.74(m, 1H), 2.92–2.89 (d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.2 (1C), 137.2 (1C), 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 128.5, 127.8 (6C, Ar ring), 135.2, 128.6, 128.5, 127.9(6C, Ph ring), 133.9, 133.7, 128.2, 125.3, 56.2, 48.5; EIMS(m/z): 381.19(M+, 28%); Anal. Calcd. for C26H24N2O: C, 82.07; H, 6.36; N, 7.36%; found: C, 82.05; H, 6.37; N, 7.37%.
5-(2-(5-Oxo-1,5-diphenylpent-1-en-3-yl)hydrazono)pentanal (1d)
White powder; mp: 126–129 °C; Yield: 85%; Water solubility: 0.08 mM/mL; IR(KBr)ν :3176.54, 3073.50, 3031.32, 2595.48, 1714.18, 1624.45; 1H NMR (300 MHz,): δ 9.70(s, 1H, CH), 9.24(s, 1H), 7.97–7.94(dd, J = 7.33 Hz,J = 7.37 Hz, 2H), 7.60–7.57(d, J = 6.21 Hz,1H), 7.53–7.50(dd, J = 7.31 Hz, J = 7.33 Hz, 2H), 7.42–7.37(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.34–7.31(d, J = 6.21 Hz, 1H, Ar ring), 7.21(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.97(s, 1H, CH), 6.56–6.51(d, J = 6.21 Hz, 1H), 6.16–6.13(1H, d, J = 6.21 Hz, CH), 3.85–3.82(m, 1H, CH), 2.93–2.88 (d, J = 6.21 Hz, 2H), 2.42–2.36(m, 2H), 1.82–1.74(m, 2H), 1.53–1.49 (m, 2H); 13C NMR(75 MHz,): 202.2, 197.4, 158.3, 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 28.5, 127.9(6C, Ar ring), 134.7, 134.1, 127.9, 56.1, 48.5, 43.3, 25.9(1C); EIMS(m/z): 349.19(M+, 24%); Anal.Calcd.for C22H24N2O2: C, 75.83; H, 6.94; N, 8.04%; found: C, 75.80; H, 6.96; N, 8.06%.
1,5-Diphenyl-3-(2-phenylhydrazinyl)pent-4-en-1-one (1e)
White powder; mp: 143–145 °C; Yield: 88%; Water solubility: 0.20 mM/mL; IR(KBr) ν: 3176.52, 3073.50, 3031.28, 1714.10, 1624.38 cm−1; 1H NMR(300 MHz,): δ 9.22 (s, 1H), 9.16(s, 1H), 7.97(dd, J = 7.34 Hz, J = 7.38 Hz, 2H, Ph), 7.65(d, J = 6.21 Hz, 1H), 7.55–7.53(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.38–7.34(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.35–7.32(dd, J = 7.31 Hz, J = 7.35 Hz, Ph),7.32–7.30(d, J = 6.21 Hz, 1H, Ar-ring), 7.21–7.19(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar-ring), 7.02–6.98(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ph), 6.88–6.86 (d, J = 6.21 Hz, 1H, Ar-ring), 6.56–6.54(d, J = 6.22 Hz, 1H), 6.17–6.15(d, J = 6.21 Hz, 1H), 3.84–3.79(m, 1H), 2.95–2.92(d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.4(1C), 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 128.5, 127.8 (6C, Ar ring), 151.0, 129.2, 122.8, 113.2 (6C, Ph ring), 134.2, 127.9, 56.6, 48.3; EIMS(m/z): 343.18 (M+, 25%); Anal. Calcd. for C23H22N2O: C, 80.67; H, 6.48; N, 8.18%; found: C, 80.65; H, 6.47; N, 8.19%.
1,5-Diphenyl-3-(phenylamino)pent-4-en-1-one (1f)
Yellow powder; mp: 101–103 °C; Yield: 86%; Water solubility: 0.16 mM/mL; IR(KBr) ν: 3176.53,3072.50, 3030.28, 1715.10, 1623.38; 1H NMR (300 MHz,): δ 9.26(s, 1H, NH), 7.97–7.95(dd, J = 7.33 Hz, J = 7.37 Hz, 2H), 7.67–7.63(d, J = 6.21 Hz, 1H), 7.53–7.51(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.44–7.41(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.35–7.30 (d, J = 6.21 Hz, 1H, Ar-ring), 7.28–7.23(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar-ring), 7.25–7.19(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ph), 6.83–6.80(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ph), 6.74–6.71(d, J = 6.21 Hz, 1H, Ar ring), 6.56–6.54 (d, J = 6.20 Hz, 1H, CH), 6.19–6.17(d, J = 6.21 Hz, 1H), 3.84–3.79(m, 1H, -CH), 2.90–2.87(d, J = 6.21 Hz); 13C NMR(75 MHz,): 197.4(1C), 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 128.5, 127.9 (6C, Ar ring), 147.6, 129.5, 120.8, 119.7 (6C, Ph ring), 133.1, 127.7, 57.2, 50.5; EIMS(m/z): 328.17 (M+, 25%); Anal. Calcd. for C23H21NO: C, 84.37; H, 6.46; N, 4.28%; found: C, 84.30; H, 6.49; N, 4.30%.
1-(5-Oxo-1,5-diphenylpent-1-en-3-yl)thiourea (1g)
Green solid; mp: 139–141 °C; Yield: 91%; Water solubility: 0.24 mM/mL; IR(KBr) ν: 3176.51, 3072.74, 3029.32, 1712.18, 1625.45; 1H NMR (300 MHz,) δ 9.22(1H, s, NH), 8.52(s, 2H, NH2), 7.97–7.94(dd, J = 7.33 Hz, J = 7.37 Hz, 2H), 7.63–7.61(d, J = 6.21 Hz, 1H, Ph), 7.55–7.50(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ph), 7.40–7.36(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.33–7.30(1H, d, J = 6.21 Hz, Ar ring), 7.23–7.19(dd, 2H, J = 7.31 Hz, J = 7.35 Hz, Ar ring), 6.56–6.54(d, J = 6.22 Hz, 1H), 6.19–6.17(d, J = 6.21 Hz, 1H), 3.82–3.79(m, 1H), 2.98–2.96 (d, J = 6.20 Hz, 2H); 13C NMR(75 MHz,): 197.4(1C), 182.0(1C), 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 28.5, 127.9 (6C, Ar ring), 134.2, 128.2, 55.6, 50.6; EI-MS(m/z) 311.12 (M+, 19%); Anal. Calcd. for C18H18N2OS: C, 69.65; H, 5.84; N, 9.02%; found: C, 69.68; H, 5.85; N, 9.06%.
1-benzylidene-3-(5-oxo-1,5-diphenylpent-1-en-3-yl)thiourea (1h)
Brown powder; mp: 111–114 °C; Yield: 80%; Water solubility: 0.40 mM/mL; IR(KBr)ν: 3175.53, 3070.50, 3032.28, 2597.48, 1714.10,1624.38; 1H NMR(300 MHz) δ 9.47(s,1H), 9.26(s,1H), 7.97–7.94(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar-ring), 7.86–7.84(dd, J = 7.31 Hz, J = 7.35 Hz, 2H,), 7.63–7.59(d, 1H, J = 6.21 Hz, Ar-ring), 7.53–7.51(2H, dd, J = 7.31 Hz, J = 7.35 Hz Ph), 7.50–7.44(3H, m, Ar-ring), 7.40–7.37(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.35–7.32(d, J = 6.21 Hz, 1H, Ar ring), 7.26–7.24(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.56–6.54(d, J = 6.20 Hz, 1H), 6.19–6.17(d, J = 6.21 Hz, 1H), 3.84–3.82(m, 1H), 2.94–2.92(d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.4(1C), 182.0(1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6, 128.5, 127.9(6C, Ar ring), 135.2, 134.4, 116.1, 20.6 (6C, Ph ring), 134.6, 128.1, 55.6, 50.1, 14.4; EIMS(m/z): 399.15(M+,27%); Anal. Calcd. for C25H22N2OS: C, 75.35; H, 5.56; N, 7.03%; found: C, 75.30; H, 5.60; N, 7.04%.
1-(5-Oxo-1,5-diphenylpent-1-en-3-yl)-3-(3-phenylallylidene)thiourea (1i)
Light yellow powder; mp: 276–279 °C; Yield: 87%; Water solubility: 0.10 mM/mL; IR(KBr) ν: 3174.23, 3069.30, 3031.68, 2598.98, 1715.70, 1626.38; 1H NMR (300 MHz,): δ 9.26(s, 1H), 7.98–9.96(dd, J = 7.33 Hz, J = 7.37 Hz, 2H), 7.65–7.63 (d, J = 6.21 Hz, 1H), 7.62–7.59(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.56–7.54(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.52(s, 1H), 7.42–7.39 (dd, J = 7.33 Hz, J = 7.37 Hz, 4H,Ar ring), 7.31–7.27(d, J = 6.21 Hz, 2H, Ar ring), 7.26–7.24(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 7.22–7.18(d, J = 6.21 Hz, 1H, CH), 6.81–6.79(d, J = 6.23 Hz, 1H), 6.56–6.52(d, J = 6.21 Hz, 1H), 6.19–6.17(d, J = 6.21 Hz, 1H, CH), 3.84–3.79(m, 1H), 2.94–6.92(d, J = 6.21 Hz, 2H); 13C NMR(75 MHz,): 197.4 (1C), 189.3(1C), 163.7, 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 128.5, 127.9(6C, Ar ring), 135.2, 134.4, 116.1, 20.6(6C, Ph ring), 134.6, 132.9, 128.3, 119.9, 55.9, 50.6; EIMS(m/z) 425.16 (M+, 30%); Anal. Calcd. for C27H24N2OS: C, 76.38; H, 5.70; N, 6.60%; found: C, 76.30; H, 5.74; N, 6.62%.
3-(Naphthalen-2-ylamino)-1,5-diphenylpent-4-en-1-one (1j)
Dark yellow colour; mp: 101–104 °C; Yield: 88%; Water solubility: 0.32 mM/mL; IR(KBr) ν: 3174.63, 3069.70, 3031.48, 1715.50, 1626.48 cm−1; 1H NMR (300 MHz,): δ 9.26(s, 1H, NH), 7.97–7.94 (dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ph), 7.88–7.84 (d, J = 6.21 Hz, 1H, Napthyl), 7.83–7.81(d, J = 6.21 Hz, 1H, Napthyl), 7.77–7.74(d, J = 6.21 Hz, 1H, Napthyl), 7.49–7.45 (d, J = 6.21 Hz, 1H, Napthyl), 7.45–7.41 (d, J = 6.21 Hz, 1H, Napthyl), 7.50 -7.48(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Naphthyl), 7.63–7.59(d, J = 6.23 Hz, 1H, Ph), 7.53–750(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar-ring), 7.42–7.40 (dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar ring), 7.35–7.33(d, J = 6.21 Hz, 1H, Ar-ring), 7.25–7.21(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar, ring), 6.56 -6.54(d, J = 6.21 Hz, 1H, CH), 6.19–6.17(d, J = 6.21 Hz, 1H), 3.84–3.81(m, 1H), 2.90–2.87(d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.4(1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6, 128.5, 127.9(6C, Ar ring), 146.0, 133.7, 129.0, 126.8, 126.5, 125.3, 124.6, 121.4, 118.1, 104.5(10C, Naphthyl ring), 134.4, 128.1, 57.2, 50.5; EI-MS(m/z) 378.18 (M+, 29%); Anal. Calcd. for C27H23NO: C, 85.91; H, 6.14; N, 3.71%; found: C, 85.90; H, 6.10; N, 3.76%.
1,5-Diphenyl-3-(p-tolylamino)pent-4-en-1-one (1k)
White powder; mp: 72–74 °C; Yield: 85%; Water solubility: 0.26 mM/mL; IR(KBr) ν: 3173.23, 3068.30, 3030.68, 1714.70, 1625.38; 1H NMR (300 MHz): δ 9.28(s, 1H), 7.50(s, 1H, –CH), 7.97–7.96(dd, J = 7.35 Hz, J = 7.39 Hz, 2H, Ph), 7.64(d, J = 6.21 Hz, 1H), 7.53(dd, J = 7.31 Hz, J = 7.34 Hz, 2H), 7.39(dd, J = 7.33 Hz, J = 7.37 Hz, 4H, Ar-ring), 7.33–7.31(d, J = 6.21 Hz, 2H, Ar-ring), 7.24–7.20(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar-ring), 7.22–7.18(d, J = 6.21 Hz,1H), 7.01–6.98 (dd, J = 7.31 Hz, J = 7.35 Hz, 1H, Ph), 6.85–6.84(1H, d, J = 6.21 Hz), 2.90–2.87 (d, J = 6.21 Hz, 2H), 2.34(s, 3H); 13C NMR (75 MHz,): 197.4(1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6, 128.5, 127.9 (6C, Ar ring), 144.6, 129.8, 129.6, 113.4(6C, 4-CH3-Ph ring), 134.5, 128.6, 55.2, 50.6, 21.3; EIMS(m/z) 342.18(M+, 26%); Anal. Calcd. for C24H23NO: C,84.42; H, 6.79; N, 4.10%; found: C, 84.30; H, 6.89; N, 4.12%.
N-(5-oxo-1,5-diphenylpent-1-en-3-yl)acetamide (1l)
Pale yellow powder; mp: 122–124 °C; Yield: 84%; Water solubility: 0.15 mM/mL; IR(KBr)ν: 3170.23, 3065.30, 3027.68, 1711.70, 1622.38; 1H NMR (300 MHz,): δ 8.05(s, 1H, NH), 7.95–7.92 (dd, J = 7.31 Hz, J = 7.36 Hz, 2H, Ph), 7.65–7.64(d, J = 6.21 Hz, 1H), 7.54–7.50(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar-ring), 7.38–7.34(dd, J = 7.31 Hz, J = 7.35 Hz, 1H, Ar ring), 7.31–7.28(d, J = 6.21 Hz, 2H, Ar-ring), 7.25–7.21(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.56–6.53(d, J = 6.21 Hz, 1H, CH), 6.17–6.15(d, J = 6.21 Hz, 1H), 4.81–4.78 (m, 1H), 2.94–2.91(d, J = 6.21 Hz,2H), 1.84 (s, 3H); 13C NMR (75 MHz,): 197.4(1C), 170.7(1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6, 128.5, 127.9(6C, Ar ring), 134.1, 127.9, 48.4, 50.4, 23.7; EIMS(m/z) 294.14 (M+, 20%); Anal. Calcd. for C19H19NO2: C, 77.79; H, 6.53; N, 4.77%; found: C, 77.80; H, 6.51; N, 4.75%.
N-(5-oxo-1,5-diphenylpent-1-en-3-yl)benzamide (1m)
Brown powder; mp: 205–208 °C; Yield: 82%; Water solubility: 0.34 mM/mL; IR(KBr) ν: 3172.21, 3063.28, 3025.66, 1710.68, 1620.36; 1H NMR (300 MHz): δ 8.41(s, 1H, NH), 8.03–7.96(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar-ring), 7.97(dd, J = 7.32 Hz, J = 7.34 Hz, 2H), 7.70–7.67(1H, d, J = 6.21 Hz, Ar ring), 7.63–7.60(3H, m, Phenyl), 7.53–7.50(dd, J = 7.31 Hz, J = 7.33 Hz, 2H), 7.42–7.38 (dd, J = 7.33 Hz, J = 7.37 Hz, 4H, Ar ring), 7.33–7.30 (1H, d, J = 6.21 Hz, Ph), 6.51–6.49(d, J = 6.21 Hz, 1H), 6.19–6.17(d, J = 6.21 Hz, 1H), 4.81–4.78(1H, m,–CH), 2.98–2.95(d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.4(1C), 167.5(1C), 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 128.5, 127.9 (6C, Ar ring), 134.2, 132.1, 128.8, 127.5 (6C, Ph ring), 135.1, 127.9, 49.2, 50.4; EIMS(m/z): 356.16 (M+, 26%); Anal. Calcd. for C24H21NO2: C, 81.10; H, 5.96; N, 3.94%; found: C, 80.10; H, 5.92; N, 4.04%.
1-(5-Oxo-1,5-diphenylpent-1-en-3-yl)urea (1n)
Pale green powder; mp: 260- 262 °C; Yield: 82%; Water solubility: 0.40 mM/mL IR (KBr) ν: 3173.21, 3064.28, 3026.66, 1711.68, 1621.36; 1H NMR (300 MHz,): δ 9.22(s, 1H, NH), 8.83(s, 2H, NH2), 7.97–7.94 (dd, J = 7.33 Hz, J = 7.37 Hz, 2H), 7.64–7.59 (m, 1H, Phenyl), 7.55–7.53(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.40–7.37(dd, J = 7.35 Hz, J = 7.33 Hz, 1H, Ar ring), 7.34–7.31(d, J = 6.21 Hz, 2H, Ar ring), 7.23–7.18(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.56–6.54(d, J = 6.20 Hz, 1H), 6.17–6.15(d, J = 6.21 Hz, 1H), 4.81–4.78 (m, 1H), 2.94–2.91 (d, J = 6.21 Hz, 2H); 13C NMR(75 MHz,): 197.4 (1C), 162.7 (1C), 136.7, 133.1, 128.8, 128.6, (6C, Ph ring), 136.4, 128.6, 128.5, 127.9(6C, Ar ring), 133.9, 128.9, 50.5, 49.9; EIMS(m/z): 295.14 (M+, 19%); Anal. Calcd. for C18H18N2O2: C, 73.45; H, 6.16; N, 9.52%; found: C, 73.40; H, 6.17; N, 9.54%.
1-Benzylidene-3-(5-oxo-1,5-diphenylpent-1-en-3-yl)urea (1o)
Green solid; mp: 132–135 °C; Yield: 80%; Water solubility: 0.18 mM/mL; IR(KBr) νJ: 3174.23, 3069.30, 3031.68, 2598.98, 1715.70, 1626.38; 1H NMR (300 MHz) δ 9.48(s, 1H), 8.06 (s, 1H), 7.97(dd, J = 7.33 Hz, = 7.37 Hz, 2H), 7.85–7.53(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Phenyl), 7.60–7.57 (dd, J = 7.31 Hz, J = 7.35 Hz, 1H), 7.63–7.60 (d, J = 6.21 Hz, 1H, Phenyl), 7.55–7.52(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.52 (m, 2H, Ph), 7.40–7.37(dd, J = 7.35 Hz, J = 7.38 Hz, 1H, Ar ring), 7.35–7.31(d, J = 6.21 Hz, 2H, Ar ring), 7.27–7.23(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 6.56–6.54 (d, J = 6.21 Hz, 1H), 6.19–6.16(d, J = 6.22 Hz, 1H), 4.81–4.79 (m, 1H), 2.94 (d, J = 6.21 Hz, 2H); 13C NMR (75 MHz): 197.4(1C), 164.5 (1C), 163.7 (1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6, 128.5, 127.9 (6C, Ar ring), 133.7, 131.0, 129.2, 128.8(6C, Ph ring), 133.8, 127.7, 50.8, 49.9; EIMS(m/z): 383.17 (M+, 28%); Anal. Calcd. for C25H22N2O2: C, 78.51; H, 5.80; N, 7.32%; found: C, 78.50; H, 5.82; N, 7.31%.
1-(5-Oxo-1,5-diphenylpent-1-en-3-yl)-3-(3-phenylallylidene)urea (1p)
White greenish powder; mp: 145–148 °C; Yield: 89%; Water solubility: 0.52 mM/mL; IR(KBr) ν: 3175.23,3070.30,3032.68, 2599.98, 1716.70, 1627.38; 1H NMR (300 MHz,): δ 8.04(s, 1H), 7.50(s, 1H), 7.96–7.93(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar-ring), 7.60–7.54(dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar-ring), 7.64–7.59(d, J = 6.21 Hz,1H, Ph), 7.54–7.51(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.42–7.39(dd, J = 7.33 Hz, J = 7.37 Hz, 4H, Ar-ring), 7.32–7.28(d, J = 6.21 Hz, 2H, Ar ring), 7.28–7.25 (dd, J = 7.31 Hz, J = 7.35 Hz, 2H, Ar ring), 7.24–7.21(d, J = 6.21 Hz, 1H, CH), 6.85–6.83 (d, J = 6.21 Hz, 1H), 6.54–6.51 (d, J = 6.21 Hz, 1H, CH), 6.17–6.13(d, J = 6.21 Hz, 1H, CH), 4.81–4.78 (m, 1H), 2.94–2.92 (d, J = 6.21 Hz, 2H); 13C NMR (75 MHz,): 197.4(1C), 164.5(1C), 163.7 (1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6, 128.5, 127.9(6C, Ar ring), 135.2, 134.4, 116.1, 20.6(6C, Ph ring), 134.1, 133.5, 128.5, 119.9, 50.8, 49.9; EI-MS: 409.19 (M+, 29%); Elemental analysis: Anal. Calcd. for C27H24N2O2: C, 79.39; H, 5.92; N, 6.86%; found: C, 79.30; H, 5.96; N, 6.91%.
3-(Methylamino)-1,5-diphenylpent-4-en-1-one (1q)
Light yellow powder; mp: 84–88 °C; Yield: 86%; Water solubility: 0.46 mM/mL; IR(KBr) ν: 3173.21, 3064.28, 3026.66, 1711.68, 1621.36; 1H NMR(300 MHz) δ 9.26(s, 1H, NH), 7.97–7.94(dd, J = 7.31 Hz, J = 7.36 Hz, 2H, Ph), 7.68–7.62 (m,1H,Ar-ring), 7.51–7.48(dd, J = 7.31 Hz, J = 7.35 Hz, 2H), 7.41–7.37(dd, J = 7.33 Hz, J = 7.37 Hz, 2H, Ar-ring), 7.31–7.29(d, J = 6.21 Hz, 2H, Ar ring), 7.25–7.19(dd, J = 7.31 Hz, J = 7.35 Hz, 1H, Ar ring), 6.56–6.54 (d, J = 6.21 Hz, 1H, CH), 6.19–6.17(d, J = 6.20 Hz, 1H), 3.84–3.81 (m, 1H, –CH), 3.36(s, 3H), 2.79–2.77(d, J = 6.20 Hz, 2H); 13C NMR (75 MHz): 197.4(1C), 136.7, 133.1, 128.8, 128.6(6C, Ph ring), 136.4, 128.6,128.5, 127.9(6C, Ar-ring), 134.6 (1C), 127.5, 57.1, 50.3, 23.1; EIMS(m/z): 266.15 (M+, 19%); Anal. Calcd. for C18H19NO: C, 81.47; H, 7.22; N, 5.28%; found: C, 81.40; H, 7.25; N, 5.32%.
Biological activities
Larvicidal activity
Larvicidal activity assessed to control the breed of mosquitoes at their larval stage by using chemical compounds as larvicides. Test compounds were deviated in various concentrations of 10, 25, 50 and 100 µM according to a method described previously16. Mortality caused by the compounds was assessed as ratios (%) of the numbers of dead vs. live larvae. The LD50 values were calculated using probit analysis.
Antifeedant activity
Antifeedant activity was evaluated to study the effect of larvicides against non-target aquatic species. The antifeedant activity was screened via 10, 25, 50 and 100 µM concentrations of the tested samples and evaluated for marine fingerlings (O. mossambicus). Mortality caused by the compounds was assessed as ratios (%) of the numbers of dead vs. live fingerlings. Table 4 summarizes the results. The method followed was described previously16.
Larval growth inhibition and regulation
The regulation and inhibition of larval growth in C. quinquefasciatus by compound 1i (10 µM) were analysed via the water-immersion method63.
Molecular docking
Preparation of ligands
The ligand molecules (1a–1q) were drawn via Chemdraw 12.0 and energy was minimized by using the MM2 force field in Chem3Dpro software. The ligand molecules were then saved in Protein Data Bank (PDB) format and further used for molecular docking studies.
Preparation of receptor
The 3D crystal structure of mosquito odorant binding protein (PDB ID: 3OGN) was downloaded from Protein Data Bank. The water molecules and inbound co-crystallized ligands were removed from the receptor using the Discovery Studio 2019 program. The receptor was energy minimized via the SWISS PDB Viewer program. The receptor was then used for molecular docking evaluation.
Identification of binding pocket
The binding pocket of the target protein was recognized by using inbound co-crystallized ligands via the Discovery Studio 2019 Program. Residues of the amino acids Tyr10, Leu15, Leu19, Leu73, Leu80, Met84, Ile87, Ala88, Met91, His111, Trp114, His121, and Phe123 were situated in the binding pocket.
Docking
The interaction of binding modes between compounds 1a–1q, permethrin, temephos (see Supplementary Material) and the mosquito odorant binding protein was assessed using molecular docking studies via Autodock vina 1.1.2. software64. The selection of docking grid box was based on the active amino acid residues situated on the binding pocket. The search grid of the 3OGN protein was stable with the dimensions sizes x: 22, y: 20, and z: 22 with center_x: 18.681, y: 49.66, and z: 11.409, with a spacing of 1.0 Å65. The value of exhaustiveness was set to 8 and the interactions were visually examined using the Pymol and Discovery studio 2019 programs.
Molecular dynamics simulations
Gromacs 2020.1 version was used to carry out the Molecular dynamics simulation for docked complex structure of ligand 1i with protein 3OGN to understand the stability of the docked complexes. Ligand topology was generated using PRODRG server and it is combined with protein topology for making complex topology, the system was generated using force field GROMOS 43a1, solvated using a single point charge (SPC) water model. The system was framed by cubic box with a distance of 2 nm from the box to the surface of the protein.
The necessary ions were further added in order to neutralize the systems. The docked complex energy was minimized by energy minimization process using steepest descent algorithm, for each simulation, 50,000 steps were used for energy minimization. The LINCS algorithm was used to constrained the bond lengths and the electrostatics computed by PME method. NVT and NPT ensembles were used to equilibrating the systems for each 100 ps. The V-rescale thermostat was used for equilibration with a reference temperature of 300 K. Finally, the production MD run was approved for 10 ns with a time-step of 2 fs. Docked complex structure coordinates were hoarded every 10 ps and used for further analysis. The result was analysed through the RMSD, RMSF, gyration, hydrogen bonds plots and Xmgrace software was used for plotting graphs.
Statistical analysis
The LD50 values was calculated based on at least three independent assessments and the standard deviations (SD) were calculated using Microsoft Excel.
Conclusions
In this study, we identified the most effective and easily prepared active larvicidal Mannich base synthesis derivatives using the grindstone method using Cu(II)-tyrosinase as a catalyst, which is economical and leads to good coating and high yield. These compounds were investigated for their use as larvicides against Culex quinquefasciatus and for their toxicity against non-target aquatic species through ichthyotoxic activity. A total of 17 compounds were screened, and compound 1i was found to be the most active (LD50 = 12.09 µM) against Culex quinquefasciatus compared to Permethrin (LD50 = 54.6 µM). The compound 1i was highly active compared to Permethrin > 10 differences compared with standard permethrin and also compound 1i induced 0% mortality within 24 h against Oreochromis mossambicus in an antifeedant screening. Molecular docking was carried out with all compounds 1a–1q and the controls temephos and permethrin against the 3OGN protein, and the resulting docking score was the best for compound li. In conclusion, our results indicate that compound li is the most effective insecticide and that the compounds outlined in this paper may serve as a prospective foundation for emerging ecologically significant bioactive compounds as well as eco-friendly pesticides and biopharmaceuticals.
Supplementary Information
Acknowledgements
This work was funded by Researchers Supporting Project number (RSP-2021/27), King Saud University, Riyadh, Saudi Arabia.
Author contributions
C.S. Synthesis of compounds and docking result analysis; D.A. Design the biological experiment; S.A. Methodology of biological activity analysis; G.R. Biological data analysis Molecular dynamics simulation studies; R.S. chemical data analysis; A.I. Investigation total work chemistry and Biology. All authors were contributing through writing—original draft.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98281-5.
References
- 1.Toda F, Tanaka K, Sekikawa A. Host–guest complex formation by a solid–solid reaction. J. Chem. Soc. Chem. Commun. 1987;4:279–280. doi: 10.1039/C39870000279. [DOI] [Google Scholar]
- 2.Mannich C. Eine synthese von β-ketonbasen. Arch. Pharm. 1917;255:261–276. doi: 10.1002/ardp.19172550217. [DOI] [Google Scholar]
- 3.Sanchez-Ferrer A, Rodriguez-Lopez J, Garcia-Canovas F, Garcia-Carmona F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta. 1995;1247:1–11. doi: 10.1016/0167-4838(94)00204-T. [DOI] [PubMed] [Google Scholar]
- 4.Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem. Rev. 1996;96:2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 5.Solomon EI, Chen P, Metz M, Lee SK, Palmer A. Oxygen binding, activation, and reduction to water by copper proteins. Angew. Chem. Int. Ed. Engl. 2001;40:4570–4590. doi: 10.1002/1521-3773(20011217)40:24<4570. [DOI] [PubMed] [Google Scholar]
- 6.Rolff M, Schottenheim J, Decker H, Tuczek F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: Molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011;40:4077–4098. doi: 10.1039/C0CS00202J. [DOI] [PubMed] [Google Scholar]
- 7.Quist DA, Diaz DE, Liu JJ, Karlin KD. Activation of dioxygen by copper metalloproteins and insights from model complexes. J. Biol. Inorg. Chem. 2017;22:253–288. doi: 10.1007/s00775-016-1415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann JD, Herzigkeit B, Jurgeleit R, Tuczek F. Small-molecule models of tyrosinase: From ligand hydroxylation to catalytic monooxygenation of external substrates. Coord. Chem. Rev. 2017;334:54–66. doi: 10.1016/j.ccr.2016.07.009. [DOI] [Google Scholar]
- 9.Gerdemann C, Eicken C, Krebs B. The crystal structure of catechol oxidase: New insight into the function of type-3 copper proteins. Acc. Chem. Res. 2002;35:183–191. doi: 10.1021/ar990019a. [DOI] [PubMed] [Google Scholar]
- 10.van Holde KE, Miller KI. Hemocyanins. Adv. Protein Chem. 1994;47:1–81. doi: 10.1016/S0065-3233(08)60545-8. [DOI] [PubMed] [Google Scholar]
- 11.Manabe K, Kobayashi S. Mannich-type reactions of aldehydes, amines, and ketones in a colloidal dispersion system created by a Bronsted Acid-Surfactant-combined catalyst in water. Org. Lett. 1999;1:1965–1967. doi: 10.3390/insects7020025. [DOI] [Google Scholar]
- 12.Jing-Bo Y, Gang P, Zhi-Jiang J, Zi-Kun H, Wei-Ke S. Mechano- chemical oxidative Mannich reaction: evaluation of chemical and mechanical parameters for the mild and chemoselective coupling of N-tert- butoxycarbonyltetra- hydroquinolines and ketones. Eur. J. Org. Chem. 2016;22:5340–5344. doi: 10.1002/ejoc.201600987. [DOI] [Google Scholar]
- 13.Khanna G, Aggarwal K, Khuran JL. An efficient and confluent approach for the synthesis of novel 3,4-dihydro- 2H-naphtho [2,3-e] [1,3]oxazine-5,10-dione derivatives by a three component reaction in ionic liquid. RSC Adv. 2015;5:46448–46454. doi: 10.1039/C5RA06169E. [DOI] [Google Scholar]
- 14.Ghomi JS, Zahedi S. Novel ionic liquid supported on Fe3O4 nanoparticles and its application as a catalyst in Mannich reaction under ultrasonic irradiation. Ultrason Sonochem. 2017;34:916–923. doi: 10.1016/j.ultsonch.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Ling-Ling W, Yang X, Da-Cheng Y, Zhi G, Yan-Hong H. Bio-catalytic asymmetric Mannich reaction of ketimines using wheat germ lipase. Catal. Sci. Technol. 2016;6:3963–3970. doi: 10.1039/C5CY01923K. [DOI] [Google Scholar]
- 16.Abdel-Fattah Mostafa A, Sathish Kumar C, Al-Askar AA, Sayed SRM, Surendra Kumar R, Idhayadhulla A. Synthesis of novel benzopyran- connected pyrimidine and pyrazole derivatives via a green method using Cu(II)-tyrosinase enzyme catalyst as potential larvicidal, antifeedant activities. RSC Adv. 2019;9:25533–25543. doi: 10.1039/C9RA04496E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georges K, Jayaprakasam B, Dalavoy SS, Nair MG. Pestmanaging activities of plant extracts and anthraquinones from Cassia nigricans from Burkina Faso. Bioresour. Technol. 2008;99:2037–2045. doi: 10.1016/j.biortech.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Govindarajan M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac. J. Trop. Med. 2010;3:874–887. doi: 10.1016/S1995-7645(10)60210-6. [DOI] [Google Scholar]
- 19.Methoprene: General Fact Sheet. National Pesticide Information Center. Accessed 12 Feb 2016.
- 20.Bazaes A, Olivares J, Schmachtenberg O. Properties, projections, and tuning of teleost olfactory receptor neurons. J. Chem. Ecol. 2013;39:451–464. doi: 10.1007/s10886-013-0268-1. [DOI] [PubMed] [Google Scholar]
- 21.De March CA, Golebiowski JA. A computational microscope focused on the sense of smell. Biochimie. 2014;107:3–10. doi: 10.1016/j.biochi.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Pechlaner M, Oostenbrink C. Multiple binding poses in the hydrophobic cavity of bee odorant binding protein AmelOBP14. J. Chem. Inform. Model. 2015;55:2633–2643. doi: 10.1021/acs.jcim.5b00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leal WS, Barbosa R, Xu W, Ishida Y, Syed Z, Latte N, Chen AM, Morgan TI, Cornel AJ, Furtado A. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE. 2008;3(8):e3045. doi: 10.1371/journal.pone.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Brito NF, Moreira MF, Melo CA. A look inside odorant-binding proteins In insect chemoreception. J. Insect Physiolog. 2016;95:51–65. doi: 10.1016/j.jinsphys.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Chu WT, Zhang JL, Zheng QC, et al. Constant pH molecular dynamics (CpHMD) and molecular docking studies of CquiOBP1 pH-induced ligand releasing mechanism. J. Mol. Model. 2013;19:1301–1309. doi: 10.1007/s00894-012-1680-0. [DOI] [PubMed] [Google Scholar]
- 26.Agnieszka C, Iga HI, Inga D, Joanna B, Marcin W, Anna C, Dorota O. Current research on the safety of pyrethroids used as insecticides. Medicina. 2018;54:61. doi: 10.3390/medicina54040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Kaushik G, Villarreal-Chiu JF. Scenario of organophosphate pollution and toxicity in India: A review. Environ. Sci. Pollut. Res. 2016;23:9480–9949. doi: 10.1007/s11356-016-6294-0. [DOI] [PubMed] [Google Scholar]
- 28.Peiris HTR, Hemingway J. Effect of fenthion treatment on larval densities of insecticide- resistant Culex quinquefasciatus in an urban area of Sri Lanka. Med. Vet. Entomol. 1996;10:283–287. doi: 10.1111/j.1365-2915.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 29.Amel IO, Manal AH. Curcumin mitigates fenthion-induced testicular toxicity in rats: Histopathological and immunohistochemical study. Afr. Zool. 2017;52:3–4. doi: 10.1080/15627020.2017.1396194. [DOI] [Google Scholar]
- 30.Mense SM, Sengupta A, Lan C, Zhou M, Bentsman G, Volsky DJ, Whyatt RM, Perera FP, Zhang L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol. Sci. 2006;93:125–135. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- 31.Mie A, Rudén C, Grandjean P. Safety of Safety Evaluation of Pesticides: Developmental neurotoxicity of chlorpyrifos and chlorpyrifos-methyl. Environ. Health. 2018;17:77. doi: 10.1186/s12940-018-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, et al. Toxicity assessment of chlorpyrifos-degrading fungal bio-composites and their environmental risks. Sci. Rep. 2018;8:2152. doi: 10.1038/s41598-018-20265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce RH, Henry MS, Kelly D, Kozlowski W. Hazard assessment of temephos applied to a southwest Florida, USA, salt marsh community. Environ. Toxicol. Chem. 2000;19:501–507. doi: 10.1002/etc.5620190232. [DOI] [Google Scholar]
- 34.Pinkney AE, McGowan PC, Murphy DR, Lowe TP, Sparling DW, Ferrington LC. Effects of the mosquito larvicides temephos and methoprene on insect populations in experimental ponds. Environ. Toxicol. Chem. 2000;19:678–684. doi: 10.1002/etc.5620190320. [DOI] [Google Scholar]
- 35.Wade A, Lin CH, Kurkul C, Regan ER, Johnson RM. Combined toxicity of insecticides and fungicides applied to California almond orchards to honey bee larvae and adults. Insects. 2019;10:20. doi: 10.3390/insects10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu SS, Arthur FH, Vangundy D, Phillips TW. Combination of methoprene and controlled aeration to manage insects in stored wheat. Insects. 2016;7:25. doi: 10.3390/insects7020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park IK, Shin SC, Kim CS, Lee HJ, Choi WS, Ahn YJ. Larvicidal activity of lignans identified in Phryma leptostachya Var. asiatica roots against tree mosquito species. J. Agric. Food. Chem. 2005;53:969–972. doi: 10.1021/jf048208h. [DOI] [PubMed] [Google Scholar]
- 38.Yang JY, Cho KS, Chung NH, Kim CH, Suh JW, Lee HS. Constituents of volatile compounds derived from Melaleuca alternifolia leaf oil and acaricidal toxicities against house dust mites. J. Korean Soc. App. Biol. Chem. 2013;56:91–94. doi: 10.1007/s13765-012-2195-1. [DOI] [Google Scholar]
- 39.Pasquale G, Romanelli GP, Autino JC, García J, Ortiz EV, Duchowicz PR. Quantitative structure−activity relationships of mosquito larvicidal chalcone derivatives gustavo pasquale. J. Agric. Food. Chem. 2012;60:692–697. doi: 10.1021/jf203374r. [DOI] [PubMed] [Google Scholar]
- 40.Targanski SK, Sousa JR, de Pádua GMS, de Sousa JM, Vieirac LCC, Soares MA. Larvicidal activity of substituted chalcones against Aedes aegypti (Diptera: Culicidae) and non-target organisms. Pest Manag. Sci. 2020;77:325–334. doi: 10.1002/ps.6021. [DOI] [PubMed] [Google Scholar]
- 41.Schafer EW, Jr, Bowles WA., Jr Acute oral toxicity and repellency of 933 chemicals to house and deer mice. Arch. Environ. Contain. Toxicol. 1985;14:111–129. doi: 10.1007/BF01055769. [DOI] [PubMed] [Google Scholar]
- 42.Bowman LR, Donegan S, McCall PJ. Is dengue vector control deficient in effectiveness or evidence? Systematic review and metaanalysis. PLoS Negl. Trop. Dis. 2016;10(1):e0004551–e4624. doi: 10.1371/journal.pntd.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nkya TE, Akhouayri I, Kisinza W, David JP. Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects. Insect. Biochem. Mol. Biol. 2013 doi: 10.1016/j.ibmb.2012.10.006407-416. [DOI] [PubMed] [Google Scholar]
- 44.Jayaraj R, Megha P, Sreedev P. Review article: Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016;9:90–100. doi: 10.1515/intox-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross AD, Tabanca N, Islam R, Ali A, Khan IA, Kaplancikli ZA, Altintop MD, Ozdemir A, Bloomquist JR. Toxicity and synergistic activities of chalcones against Aedes aegypti (Diptera: Culicidae) and Drosophila melanogaster (Diptera: Drosophilidae) J. Med. Entomol. 2018;54:382–386. doi: 10.1093/jme/tjw183. [DOI] [PubMed] [Google Scholar]
- 46.Lee SH, Choi JY, Lee BR, Fang Y, Kim JH, Park DH, Park MG, Woo RM, Kim WJ, Je YH. Insect growth regulatory and larvicidal activity of chalcones against Aedes albopictus. Entomol. Res. 2018;48:55–59. doi: 10.1111/1748-5967.12288. [DOI] [Google Scholar]
- 47.Satyavani SR, Kanjilal S, Rao MS, Prasad RBN, Murthy USN. Synthesis and mosquito larvicidal activity of furanochalcones and furanoflavonoids analogous to karanjin. Med. Chem. Res. 2015;24:842–850. doi: 10.1007/s00044-014-1160-4. [DOI] [Google Scholar]
- 48.Vesely J, Rios R. Enantioselective methodologies using N-carbamoyl-imines. Chem. Soc. Rev. 2014;43:611–630. doi: 10.1039/C3CS60321K. [DOI] [PubMed] [Google Scholar]
- 49.Gul HI, Demirtas A, Ucar G, Taslimi P, Gulcin I. Synthesis of Mannich bases by two different methods and evaluation of their acetylcholine esterase and carbonic anhydrase inhibitory activities. Lett. Drug Design Discov. 2017;14:573–580. doi: 10.2174/1570180814666161128120612. [DOI] [Google Scholar]
- 50.Sivakumar KK, Rajasekaran A, Senthilkumar P, Wattamwar PP. Conventional and microwave assisted synthesis of pyrazolone Mannich bases possessing anti-inflammatory, analgesic, ulcerogenic effect and antimicrobial properties. Bioorg. Med. Chem. Lett. 2014;24:2940–2944. doi: 10.1016/j.bmcl.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 51.Branneby C, Carlqvist P, Hult K, Brinck T, Berglund P. Aldol additions with mutant lipase: Analysis by experiments and theoretical calculations. J Mol. Catal. B. 2004;31:123–12838. doi: 10.1016/j.molcatb.2004.08.005. [DOI] [Google Scholar]
- 52.Dindulkar SD, Puranik VG, Jeong YT. Supported copper triflate as an efficient catalytic system for the synthesis of highly functionalized 2-naphthol Mannich bases under solvent free condition. Tetrahedron Lett. 2012;53:4376–4380. doi: 10.1016/j.tetlet.2012.06.022. [DOI] [Google Scholar]
- 53.Zhang HJ, Xie YC, Yin L. Copper(I)-catalyzed asymmetric decarboxylative Mannich reaction enabled by acidic activation of 2H-azirines. Nat. Commun. 2019;10:1699. doi: 10.1038/s41467-019-09750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh SK, Chandna N, Jain N. N-Mannich bases of aromatic heterocyclic amides: Synthesis via copper-catalyzed aerobic cross-dehydrogenative coupling under ambient conditions. Org. Lett. 2017;19:1322–1325. doi: 10.1021/acs.orglett.7b00125. [DOI] [PubMed] [Google Scholar]
- 55.Kidwai M, Mishra NK, Bansal V, Kumar A, Mozumdar S. Novel one-pot Cu-nanoparticles-catalyzed Mannich reaction. Tetrahedron Lett. 2009;50:1355–1358. doi: 10.1016/j.tetlet.2009.01.031. [DOI] [Google Scholar]
- 56.Chai SJ, Lai YF, Zheng H, Zhang PF. A novel trypsin-catalyzed three-component Mannich reaction. Helv. Chim. Acta. 2010;93:2231–2236. doi: 10.1002/hlca.201000063. [DOI] [Google Scholar]
- 57.Li K, He T, Li C, Feng XW, Wang N, Yu XQ. Lipase-catalysed direct Mannich reaction in water: utilization of biocatalytic promiscuity for C-C bond formation in a “one- pot” synthesis. Green. Chem. 2009;11:777–779. doi: 10.1039/B817524A. [DOI] [Google Scholar]
- 58.Xue Y, Li LP, He YH, et al. Protease-catalysed direct asymmetric mannich reaction in organic solvent. Sci. Rep. 2012;2:761. doi: 10.1038/srep00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kano T, Sakamoto R, Akakura M, Maruoka K. Stereocontrolled synthesis of vicinal diamines by organocatalytic asymmetric mannich reaction of N-protected aminoacetaldehydes: Formal synthesis of (−)-Agelastatin A. J. Am. Chem. Soc. 2012;134:7516–7520. doi: 10.1021/ja301120z. [DOI] [PubMed] [Google Scholar]
- 60.Arteaga FA, Liu Z, Brewitz L, Chen J, Sun B, Kumagai N, Shibasaki M. Direct catalytic asymmetric mannich-type reaction of alkylamides. Org. Lett. 2016;18:2391–2394. doi: 10.1021/acs.orglett.6b00879. [DOI] [PubMed] [Google Scholar]
- 61.Kanazawa AM, Denis JN, Greene AE. Highly stereocontrolled and efficient preparation of the protected, esterification-ready docetaxel (taxotere) side chain. J. Org. Chem. 1994;59:1238–1240. doi: 10.1021/jo00085a004. [DOI] [Google Scholar]
- 62.Chen CD, Lee HL, Chan CK, Ang CL, Azahari AH, Lau KW, Sofian-Azirun M. Laboratory bioefficacy of nine commercial formulations of temephos against larvae of Aedes aegypti (L.), Aedes albopictus Skuse and Culex quinquefasciatus Say. Trop. Biomed. 2009;26:360–365. [PubMed] [Google Scholar]
- 63.Song GP, Hu DK, Tian H, Li YS, Cao YS, Jin HW, Cui ZN. Synthesis and larvicidal activity of novel thenoylhydrazide derivatives. Sci. Rep. 2016;6:22977–22990. doi: 10.1038/srep22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abreu RMV, Froufe HJC, Queiroz MRP, Isabel CFR, Ferreira ICFR. Selective flexibility of side-chain residues improves VEGFR-2 docking score using AutoDock Vina. Chem. Biol. Drug. Des. 2012;79:530–534. doi: 10.1111/j.1747-0285.2011.01313.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.