Abstract
This study elucidated potential genetic variants and QTLs associated with clinical mastitis incidence traits in Bos indicus breed, Sahiwal. Estimated breeding values for the traits (calculated using Bayesian inference) were used as pseudo-phenotypes for association with genome-wide SNPs and further QTL regions underlying the traits were identified. In all, 25 SNPs were found to be associated with the traits at the genome-wide suggestive threshold (p ≤ 5 × 10−4) and these SNPs were used to define QTL boundaries based on the linkage disequilibrium structure. A total of 16 QTLs were associated with the trait EBVs including seven each for clinical mastitis incidence (CMI) in first and second lactations and two for CMI in third lactation. Nine out of sixteen QTLs overlapped with the already reported QTLs for mastitis traits, whereas seven were adjudged as novel ones. Important candidates for clinical mastitis in the identified QTL regions included DNAJB9, ELMO1, ARHGAP26, NR3C1, CACNB2, RAB4A, GRB2, NUP85, SUMO2, RBPJ, and RAB33B genes. These findings shed light on the genetic architecture of the disease in Bos indicus, and present potential regions for fine mapping and downstream analysis in future.
Keywords: Clinical mastitis incidence, Genome-wide association study, Quantitative trait loci, Sahiwal
Introduction
Bovine mastitis is one of the most widespread and economically challenging diseases of the global dairy industry. The gravity of this disease can be dually projected in the form of huge economic losses incurred by dairy farmers (Halasa et al. 2007) and in spurring the menace of anti-microbial resistance in animals and humans (Sharma et al. 2018; Krömker and Leimbach 2017). Selection for genetic resistance against mastitis could be a big step in alleviating this crisis (Weigel and Shook 2018).
Generally, indicator traits like Somatic Cell Count (SCC) and the consequent Somatic Cell Score (SCS) are used to record Clinical Mastitis (CM) in the farm (Carlén et al. 2004). However, these may not present a true picture of the severity as the CM caused by environmental pathogens (of short duration) may go undetected by these measures. In this context, Clinical Mastitis Incidence (CMI) traits have been found to be critical due to their easy recording and high genetic correlation with subclinical mastitis incidence, as well (Heringstad et al. 2000; Koeck et al. 2010; Urioste et al. 2012). In fact, a selection index with CMI traits has been found to be almost as effective as the one with SCC or SCS (Shook 1993). Thus, recording and analysis of CMI data are indispensible to identify genetic variants conferring resistance against mastitis.
With the commercial availability of genotyping assays, Genome-wide Association studies (GWAS) are being performed to highlight the complex underlying genetic architecture of clinical mastitis resistance. Numerous genetic variants and QTL regions have also been identified in relation to CM (Meredith et al. 2012; Sahana et al. 2014; Tiezzi et al. 2015; Welderufael et al. 2018). QTL regions associated with CM phenotypes have been mapped to several Bos taurus autosomes (BTAs) including BTA 6, 13, 16, 19, and 20 (Sahana et al. 2014; Kurz et al. 2019). On the other hand, as a strategy for indirect selection against mastitis, QTLs related to immune response traits have also been highlighted (Thompson-Crispi et al. 2014). Additionally, QTL regions for udder conformation and milk production traits have also been used as a yardstick for mastitis resistance/susceptibility (Kurz et al. 2019) owing to their high genetic correlation with mastitis (Nash et al. 2000; Cai et al. 2020).
To further improve the accuracy of GWAS results, several studies have considered Estimated Breeding Values (EBVs) of mastitis-related traits as pseudo-phenotypes (Sahana et al. 2014; Thompson-Crispi et al. 2014; Wang et al. 2015). Comparison between the reliabilities of different pseudo-phenotypes for genomic predictions revealed that trait EBVs perform at par with and sometimes, even outperform de-regressed EBVs (dEBV) and daughter yield deviations (DYD) in both real as well as simulated datasets (Jiang et al. 2010; Gao et al. 2013). Thus, EBVs of binary CMI traits in first three lactations can serve as reliable predictors for screening animals for mastitis through GWAS (Johansson et al. 2006; Sahana et al. 2014).
With the intensification of the production systems and lack of appropriate weightage to the functional traits in the selection index, the problem of mastitis is on the rise in Bos indicus cattle, particularly Sahiwal which is one of the major milch cattle breed of India (Sentitula and Kumar 2012; Sinha et al. 2019). A plethora of literature is available regarding important variants, putative candidate genes and QTL regions for mastitis in Bos taurus breeds (Wang et al. 2015; Tiezzi et al. 2015; Kirsanova et al. 2020). However, to the best of our knowledge, there has been no report on genome-wide associations for CM traits in any of the Bos indicus breeds.
Keeping the above into consideration, this study identified SNPs, candidate genes and QTL regions associated with clinical mastitis incidence in first three lactations in Sahiwal cattle using trait EBVs as pseudo-phenotypes. These findings would pave the way for improved biological understanding of clinical mastitis in Bos indicus.
Materials and methods
Ethics statement
Handling and treatment of animals for blood collection were conducted in accordance with the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) (http://cpcsea.nic.in/Content/55_1_GUIDELINES.aspx) and with the approval of Institutional Animal Ethics Committee (IAEC) of ICAR-National Dairy Research Institute (Approval number: 44-IAEC-19-15).
Sampling design and recorded phenotypes
Two hundred and thirteen adult female Sahiwal cows reared in the herd of ICAR-National Dairy Research Institute, Karnal, Haryana, India comprised the sampled population. These animals were sired by 38 bulls and were born between the years 2003 and 2016. The phenotypes considered in the study included binary mastitis traits like CMI in first lactation (CMIF), CMI in second lactation (CMIS) and CMI in third lactation (CMIT).
Animals were categorized into CM-susceptible and CM-resistant individuals based on the clinical mastitis symptoms, antibiotic treatment history, California mastitis test results and bacteriological counts. Animals affected with subclinical form of the disease were not included in the study. The incidence rate of clinical mastitis in first, second, and third lactations were 36.54%, 37.67%, and 34.31%, respectively.
Variance estimation and estimated breeding values’ calculation
To mitigate the concerns of getting accurate EBVs in a small sample size, a Bayesian approach of variance estimation was relied on to get the reliable estimates of posterior additive variance by providing suitable parameter-expanded priors (Van de Schoot et al. 2015; Villemereuil 2019). Markov Chain Monte Carlo (MCMC) algorithm was executed to obtain the posterior distribution of the parameters using MCMCglmm package in R 3.6.2 (Hadfield 2010). Here, a Chi-square prior distribution was assumed for estimating additive variance and heritability where the residual variance was fixed to one and the parameter family was set to ordinal. Convergence of the chain was achieved by manipulation of criteria like number of iterations (3,000,000), burn-in period (30,000) and thinning interval (300), and was verified based on autocorrelation between lags (< 0.01 in each lag) and different diagnostic tests like Gelman–Rubin diagnostics (point estimate and Upper Confidence limits with respect to potential scale reduction factor was 1) and Heidel’s diagnostics (both stationarity test and halfwidth test passed for the random variables). Posterior mean and Highest Probable Density Interval (HPDI) of additive variance and heritability estimates were determined for each trait. The fixed non-genetic factors considered in the animal model included season of calving, period of calving, age group at first calving and level of milk production. Posterior mean EBV solutions for all three CMI traits were subsequently, used as pseudo-phenotypes in GWAS.
DNA isolation
Blood was collected from live animals (189) and DNA isolated following the standard phenol–chloroform protocol (Sambrook and Russel 2006), whereas for dead/sold animals (24), stored DNA was used and all the samples were sent for genotyping after fulfilling the requisite criteria for DNA quality as well as concentration (100–400 ng/µl).
SNP genotyping using customized IlluminaHD microarray
All the samples were genotyped using a customized version of Illumina’s BovineHD BeadChip called INDUSCHIP designed by National Dairy Development Board (NDDB), Anand, Gujarat, India (https://www.nddb.coop/services/animalbreeding/geneticimprovement/genomic). The assay comprises 54 K SNPs handpicked from Illumina’s BovineHD 770 K chip based on quality control (QC) criteria and has been tailor-made for genotyping indigenous cattle breeds (https://www.dairyknowledge.in/sites/default/files/genomic_selection.pdf).
Three randomly selected samples were genotyped twice so as to ascertain the accuracy and reliability of the genotyping panel. The concordance rate between the called genotypes for both the replicates in case of all three samples was more than 99.9%.
Only SNPs present in BTA 1–29 were included for filtering of data based on certain criteria. The genotypic data were subjected to both sample as well as SNP QC using PLINK1.9 (www.cog-genomics.org/plink/1.9/; Chang et al. 2015)—samples with call rate (CR) less than 95%, SNPs with call rate less than 95%, minor allele frequency (MAF) less than 5% and p values for Hardy–Weinberg equilibrium (HWE) lesser than 1e−06 were excluded from the further analysis. Finally, 39,473 variants and 211 individuals passed the applied filters in QC criteria.
Genome-wide association using a compressed mixed linear model
A single-marker association was used to fit one SNP at a time to the trait EBVs using a compressed mixed linear model approach in GAPIT package in R (Zhang et al. 2010; Lipka et al. 2012). The model is described below
where y = vector of pseudo-phenotype or trait EBVs, 1µ = overall mean, b = allelic substitution effect of SNP on trait EBV, x = vector of SNP genotype, Z = incidence matrix connecting random additive polygenic effects to the corresponding EBV, a = vector of random additive polygenic effects (a ~ N (0, G)) which follows a normal distribution with mean 0 and G = genomic relationship matrix estimated from SNP data, = polygenic variance, and e = vector of random residual (e ~ N (0, I)) with I = identity matrix and being residual error variance.
To counter the problem of multiple comparisons, a genome-wide suggestive threshold of p ≤ 5 × 10−4 was considered significant for association. Significant associations for each trait were plotted in the form of Manhattan plots using package manhattanly (https://github.com/sahirbhatnagar/manhattanly/) in R.
Defining quantitative trait loci
A quantitative trait loci (QTL) was defined based on the local LD (linkage disequilibrium) structure, as per the approach of Meredith et al. (2013). Pairwise LD was calculated between the SNP adjudged as ‘significant’ (p ≤ 5 × 10−4) and the individual genotyped SNPs present within 60 kb (based on LD decay in our population) upstream and downstream distance using PLINK 1.9 (www.cog-genomics.org/plink/1.9/; Chang et al. 2015). SNPs with r2 ≥ 0.20 were considered a part of the QTL region and the farthest upstream and downstream SNPs were used to define QTL boundaries. QTLs comprising of a single SNP were not included in the analysis, whereas overlapping QTLs were merged to obtain a single QTL defined by the farthest upstream and downstream SNPs of the region (Kurz et al. 2019). Protein-coding genes lying within each QTL region were determined based on Bos taurus genome assembly build UMD3.1 using UCSC Genome browser (Zimin et al. 2009; https://genome.ucsc.edu/). Cattle QTLdb was also queried to find out whether the QTLs identified in this study overlapped with the previously reported ones for mastitis or associated traits like udder conformation traits and milk production, as of September 2020 (https://www.animalgenome.org/cgi-bin/QTLdb/BT/index).
Results
Posterior mean EBV estimates
The posterior mean variance and heritability estimates for the three CMI traits are presented in Table 1. In line with the expectations, the heritability of the binary threshold traits was found to be very low. Posterior mean estimated breeding value (EBV) estimates revealed that the mean EBV for CMIF was 0.0003, while it ranged from − 0.190 to 0.284. For CMIS, the mean EBV estimate was 0.010 and it ranged from − 0.202 to 0.321, whereas the mean EBV for CMIT was 0.008 and the range was from − 0.525 to 0.615.
Table 1.
Posterior means of additive genetic variance and heritability estimates for clinical mastitis incidence in first three lactations
| Trait | Posterior means | |
|---|---|---|
| Additive genetic variance | Heritability ± posterior standard deviation | |
| CMIF | 0.0009 | 0.0004 ± 0.001 |
| CMIS | 0.0010 | 0.0005 ± 0.001 |
| CMIT | 0.0009 | 0.0004 ± 0.001 |
Genome-wide associations with clinical mastitis trait EBVs
Genome-wide associated significant SNPs (p ≤ 5 × 10−4) and QTL regions associated with the CMI traits are elucidated in Tables 2 and 3. Additionally, most of the identified QTLs were found to be overlapping with already known QTLs for mastitis and associated pleiotropic traits like udder conformation and milk production (in Table 3). Manhattan plots showing the SNPs significant at genome-wide suggestive threshold are presented in Fig. 1.
Table 2.
SNPs significant for clinical mastitis incidence traits at genome-wide suggestive levels (p ≤ 5 × 10−4) of significance
| rs ID of SNP | BTA | Position | p value | MAF | Alleles |
|---|---|---|---|---|---|
| Clinical mastitis incidence in first lactation | |||||
| rs41568040 | 11 | 102,911,946 | 2.06E−05 | 0.08 | G/A |
| rs134553823 | 4 | 4,668,574 | 4.50E−05 | 0.38 | G/A |
| rs134448529 | 7 | 56,252,492 | 6.64E−05 | 0.45 | C/A |
| rs42350672 | 7 | 56,195,602 | 7.65E−05 | 0.49 | G/A |
| rs109412613 | 16 | 24,322,073 | 1.59E−04 | 0.08 | G/A |
| rs134473565 | 13 | 32,725,444 | 1.67E−04 | 0.39 | C/A |
| rs137812439 | 7 | 54,515,095 | 1.87E−04 | 0.43 | G/A |
| rs42351443 | 7 | 56,374,904 | 3.55E−04 | 0.37 | A/C |
| rs134482551 | 28 | 129,187 | 3.59E−04 | 0.38 | G/A |
| rs135788866 | 7 | 50,113,395 | 3.63E−04 | 0.43 | A/G |
| rs135954818 | 17 | 18,686,517 | 3.75E−04 | 0.06 | A/C |
| rs135390224 | 19 | 57,094,538 | 4.04E−04 | 0.41 | G/A |
| rs136175818 | 4 | 59,976,303 | 5.24E−04 | 0.43 | A/G |
| Clinical mastitis incidence in second lactation | |||||
| rs136667981 | 18 | 16,073,485 | 0.000195 | 0.14 | G/A |
| rs132917076 | 28 | 20,124,697 | 0.000234 | 0.35 | A/G |
| rs133288259 | 6 | 47,057,796 | 0.000261 | 0.46 | G/A |
| rs136722833 | 3 | 97,530,688 | 0.000265 | 0.28 | G/A |
| rs43349452 | 3 | 81,243,127 | 0.000399 | 0.40 | A/G |
| rs137090832 | 6 | 30,988,645 | 0.000404 | 0.26 | A/G |
| rs136214974 | 25 | 29,366,079 | 0.000447 | 0.38 | A/G |
| rs41624497 | 6 | 30,990,436 | 0.000463 | 0.26 | G/A |
| rs43710090 | 6 | 46,481,458 | 0.000523 | 0.49 | A/C |
| Clinical mastitis incidence in third lactation | |||||
| rs135185080 | 16 | 22,998,686 | 0.000161 | 0.44 | A/G |
| rs109440280 | 26 | 36,808,909 | 0.000200336 | 0.09 | G/A |
| rs136030221 | 16 | 24,568,849 | 0.00028003 | 0.48 | G/A |
Table 3.
QTL regions identified for clinical mastitis incidence traits and their overlap with the previously reported QTLs for mastitis and other traits
| BTA | QTL start (bp) | QTL end (bp) | QTL length | No. of SNPs | −log10 (p value) for significant SNP | No. of genes | Genes | Traits for which QTLs reported | Reported QTL ID | Reported QTL position (bp) |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical mastitis incidence in first lactation | ||||||||||
| 4 | 4,090,824 | 4,668,574 | 577,750 | 5 | 4.35 | 1 | COBL |
Udder swelling score Milk yield |
106,571 106,683 |
3,827,792–6,359,338 2,845,004–4,898,788 |
| 7 | 56,140,368 | 56,464,491 | 324,123 | 9 | 4.18 | 2 |
ARHGAP26 NR3C1 |
Milk yield Milk beta-casein percentage |
10,291 21,539 |
25,254,399–64,995,512 20,669–56,958,148 |
| 13 | 32,725,444 | 33,601,894 | 876,450 | 8 | 3.78 | 4 |
SLC39A12 CACNB2 NSUN6 EPC1 |
Teat length SCS |
1386 4983 |
8,230,392–76,386,569 33,021,666–53,561,417 |
| 28 | 26,638 | 583,883 | 557,245 | 7 | 3.44 | 3 |
RAB4A ACTA1 NUP133 |
None | ||
| 17 | 18,528,356 | 18,686,517 | 158,161 | 2 | 3.43 | 2 |
RAB33B NAA15 |
Udder height | 1612 | 17,139,346–25,083,645 |
| 19 | 56,499,004 | 57,152,368 | 653,364 | 3 | 3.39 | 26 |
ITGB4 SAP30BP RECQL5 SMIM6 SMIM5 LLGL2 TSEN54 CASKIN2 GRB2 SLC25A19 MIF4GD MRPS7 GGA3 NUP85 SUMO2 JPT1 NT5C ARMC7 ATP5PD MRPL58 CDR2L HID1 OTOP3 OTOP2 USH1G FADS6 |
Clinical mastitis SCS |
57,308 10,446 |
57,101,615–57,198,154 677,484–58,947,524 |
| 4 | 59,020,173 | 60,733,009 | 1,712,836 | 13 | 3.28 | 5 |
ATRAID RNF167 DNAJB9 THAP5 ELMO1 |
SCS | 13,230 | 58,983,223–59,192,073 |
| Clinical mastitis incidence in second lactation | ||||||||||
| 18 | 15,946,472 | 16,073,485 | 127,013 | 3 | 3.71 | 0 | None |
SCS SCS |
3554 18,469 |
4,992,421–18,045,667 11,438,802–46,178,647 |
| 28 | 20,124,697 | 21,117,323 | 992,626 | 4 | 3.63 | 0 | None |
Udder height Udder composite index Milk yield |
1661 1663 2691 |
14,153,160–25,145,146 14,153,160–25,145,146 14,153,160–25,145,146 |
| 6 | 46,481,458 | 47,954,669 | 1,473,211 | 7 | 3.58 | 7 |
ANAPC4 SLC34A2 SEL1L3 SMIM20 RBPJ CCKAR TBC1D19 |
SCS Udder height Clinical mastitis Clinical mastitis Milk yield Milk yield Milk yield Milk yield Milk yield |
10,439 28,171 19,009 19,010 174,420 25,429 25,430 156,710 2512 |
4,380,012–65,653,453 46,834,289–46,834,365 46,178,647–52,998,234 46,178,647–52,998,234 23,227,144–76,844,549 46,834,289–46,834,365 46,834,289–46,834,365 47,132,494–47,132,534 39,094,762–50,534,735 |
| 3 | 96,827,079 | 97,854,341 | 1,027,262 | 8 | 3.58 | 0 | None | Milk yield | 175,933 | 97,690,467–97,690,507 |
| 3 | 81,094,906 | 82,106,296 | 1,011,390 | 5 | 3.39 | 1 | UBE2U |
SCS Somatic cell count |
2764 4619 |
81,616,554–81,817,752 81,616,554–81,817,752 |
| 6 | 30,988,645 | 30,990,436 | 1791 | 2 | 3.39 | 0 | None |
Clinical mastitis Udder swelling score SCS |
2493 106,577 10,439 |
9,283,340–33,227,425 30,805,204–31,600,938 4,380,012–65,653,453 |
| 25 | 29,227,859 | 29,855,993 | 628,134 | 7 | 3.35 | 2 |
CALN1 GALNT17 |
Milk yield Milk yield Milk yield Immunoglobulin G level Immunoglobulin G level |
161,652 1538 2591 66,230 66,234 |
29,715,968–29,716,008 27,460,729–39,618,958 27,460,729–35,322,340 21,632,493–30,033,432 21,632,493–30,033,432 |
| Clinical mastitis incidence in third lactation | ||||||||||
| 16 | 22,774,111 | 23,127,031 | 352,920 | 6 | 3.79 | 0 | None |
Milk yield Milk yield Teat length SCS |
177,953 177,856 1608 10,185 |
22,637,210–24,244,162 22,778,918–24,293,750 12,209,667–26,166,559 22,717,984–22,875,548 |
| 16 | 24,271,694 | 24,996,912 | 725,218 | 7 | 3.55 | 6 |
BPNT1 IARS2 RAB3GAP2 MARK1 C16H1orf115 MTARC2 |
Milk yield Teat length SCS SCS |
177,856 1608 48,237 6221 |
22,778,918–24,293,750 12,209,667–26,166,559 24,848,848–24,848,888 24,512,141–24,669,705 |
Fig. 1.
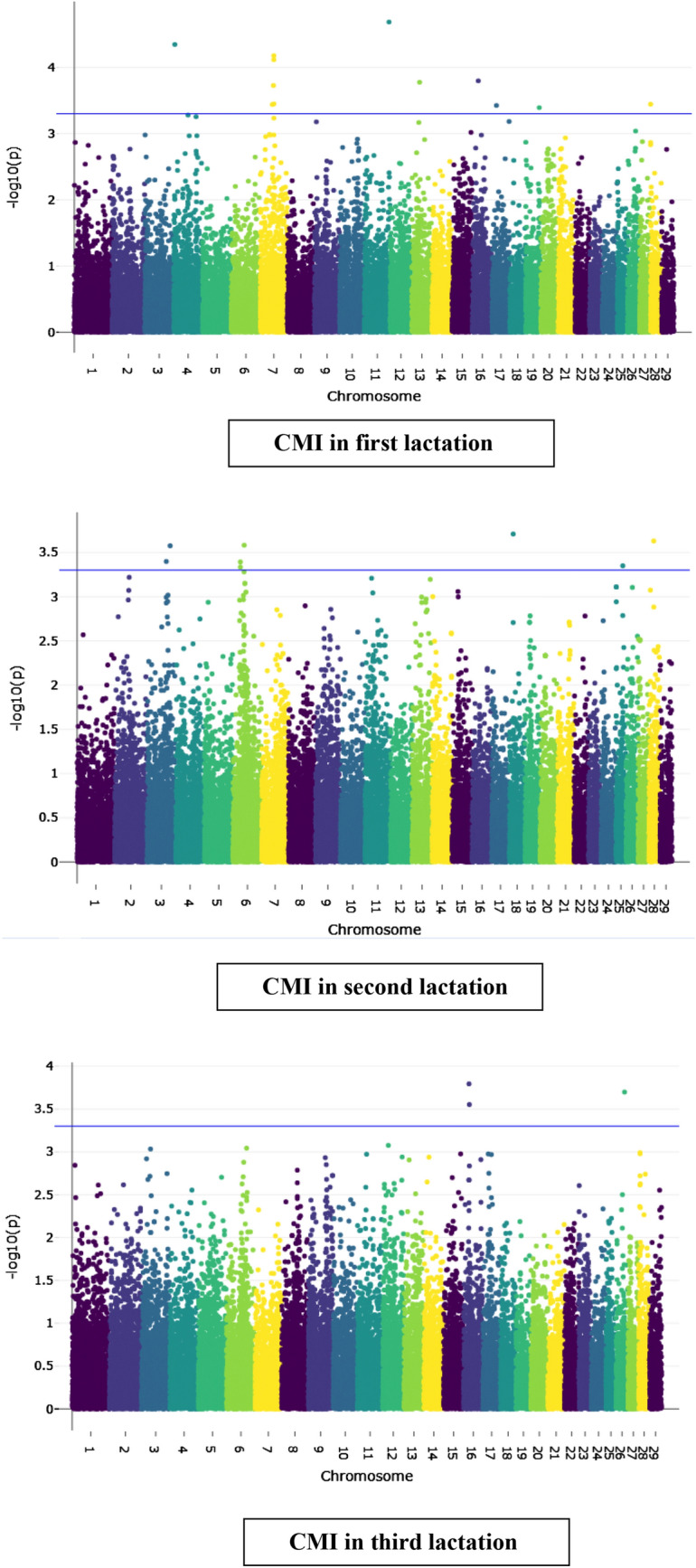
Manhattan plots of genome-wide associations for clinical mastitis incidence traits in Sahiwal cattle
Clinical mastitis incidence in first lactation (CMIF)
A total of seven QTLs identified to be having significant association with CMIF were cross-referenced to already known QTLs in the cattle QTLdb (https://www.animalgenome.org/cgi-bin/QTLdb/BT/index) and three were found to be a part of previously identified larger QTL regions for CM traits. The most significant SNP rs41568040 for the trait was identified on BTA11, but it was not included in the QTL analysis as none of the genotyped SNPs was in LD with the former. However, this SNP is located within AK8 gene which is a positional candidate gene for abomasal displacement in cattle (Mömke et al. 2013) which, subsequently, may result due to concurrent mastitis (Karatzias et al. 2013).
The strongest association signal for the trait was identified on BTA4 (4.0–4.6 Mb) encompassing five significant SNPs at genome-wide suggestive threshold. Only one protein-coding gene COBL (Cordon-bleu WH2 repeat protein) was annotated to this region. Another QTL identified on the same chromosome (59.0–60.7 Mb) revealed important genes associated with immune response and inflammation like DNAJB9 (DNAJ Heat Shock Protein Family Member B9) and ELMO1 (Engulfment and cell motility 1) (Fortune et al. 2015; Márquez et al. 2018). Additionally, this region overlaps with a previously known QTL region for SCS.
Two genes—ARHGAP26 (Rho GTPase-Activating protein 26) and NR3C1 (Nuclear Receptor Subfamily 3 Group C Member 1)—were found on an important QTL on BTA7 (56.1–56.4 Mb). The identified QTL region is a small component of a larger known QTL for traits like milk yield and beta-casein percentage in milk, both of which may have indirect associations with mastitis.
On BTA13 (32.7–33.6 Mb), a QTL region found significant for the trait overlapped the region for two previously known QTLs for teat length and SCS. This region comprised four genes, two of which SLC39A12 (Solute Carrier Family 39 Member 12) and CACNB2 (Calcium channel voltage-dependent subunit beta 2) have crucial underpinnings in determining immune response.
Additionally, a QTL identified on BTA28 located at 0.02–0.5 Mb was found to be a novel one as far as mastitis phenotypes are concerned and harbored RAB4A (Ras-related protein RAB4A), ACTA1 (Actin alpha 1, skeletal muscle) and NUP133 (Nucleoporin 133) genes. Another QTL on BTA17 (18.5–18.6 Mb) encompassed an important gene RAB33B (Member RAS Oncogene Family) which plays a key role in immunomodulation by facilitating autophagosome formation (Jiang et al. 2019).
A significant association signal on BTA19 located at 56.4–57.1 Mb comprised of 26 genes and overlapped with previously identified QTL regions for clinical mastitis and SCS. Important immune-related genes in the region include GRB2 (Growth Factor Receptor-Bound Protein 2), SLC25A19 (Solute Carrier Family 25 Member 19), NUP85 (Nucleoporin 85), SUMO2 (Small Ubiquitin like Modifier 2), and CDR2L (Cerebellar Degeneration Related Protein Like 2) genes.
Clinical mastitis incidence in second lactation (CMIS)
Out of the seven identified QTL regions for the trait, none of the protein-coding genes could be assigned to four (including the two most significant ones) of these regions. However, three of these four QTLs—BTA18 (15.9–15.0 Mb), BTA28 (20.1–21.1 Mb) and BTA6 (30.98–30.99 Mb) overlapped with already identified QTLs for mastitis and udder traits whereas the fourth one BTA3 (96.8–97.8 Mb) was in convergence with a QTL for milk yield.
On BTA6, another QTL was identified to be having an association with the CMIS which extended from 46.4 to 47.9 Mb and this region included two genes—SLC34A2 (Solute Carrier Family 34 Member 2) and RBPJ (Recombination Signal Binding Protein for Immunoglobulin Kappa J Region). Another significant association signal was detected on chromosome 3 in the region between 81.0 and 82.1 Mb in which one protein-coding gene UBE2U (Ubiquitin Conjugating Enzyme E2 U) was traced. Though this gene could not be linked with inflammatory or immune response directly, the QTL region was part of a previously identified larger region significant for somatic cell count as well as SCS. Finally, a QTL region was identified on BTA25 (29.2–29.8 Mb) in which seven SNPs reached the genome-wide suggestive threshold and this region was found to harbor a number of QTLs for milk yield and immunoglobulin-G levels, as well.
Clinical mastitis incidence in third lactation (CMIT)
Only two QTLs were identified for the trait CMIT and both were located on chromosome 16. Both the identified QTLs overlapped with the known QTLs for SCS, teat length as well as milk yield. While the QTL region mapped at 22.7–23.1 Mb could not be annotated to any of the protein-coding genes, the other region (24.2–24.9 Mb) comprised of six genes including IARS2 (Isoleucyl-TRNA Synthetase 2, Mitochondrial) and MARK1 (Microtubule Affinity Regulating Kinase 1) genes. Documented evidence exists for involvement of both the genes in tumorigenesis and immunity (Ye et al. 2014; Di et al. 2019).
Discussion
This study was undertaken to identify variants and QTLs associated with CMI traits in first three lactations in Bos indicus breed Sahiwal using trait EBVs as pseudo-phenotype. Since CMI is a binary threshold trait requiring a large sample size to reveal any meaningful associations (due to very low heritability), we resorted to the use of trait EBVs as dependent variables so as to make the trait continuous and to reduce the limitation of small sample size (Guo et al. 2010; Gao et al. 2013) to some extent. Moreover, we adopted a Bayesian approach for additive genetic variance estimation by incorporating prior information into the BLUP animal model. The advantages of this approach are that it provides inferences in the form of posterior distributions and more effectively captures the uncertainty around estimates using robust sampling algorithms like MCMC. Additionally, advanced knowledge about a trait could be added as an input in the form of prior information to more efficiently fit the models (Villemereuil 2019). However, we are completely aware of the fact that small sample size is still a hindrance in Bayesian inference as the inflated values of posterior standard deviation and widened highest probability density intervals reveal. Furthermore, the low EBV accuracies found in the studied population indicate that the findings should be read with caution keeping the limitation of small sample size in mind. Nevertheless, the study provides a unique peek into the genomic background of clinical mastitis in Bos indicus.
In line with the expectations, the posterior mean heritability estimates for all three binary CMI traits were found to be very low. However, several workers have reported higher estimates than our findings (Heringstad et al. 2004; Zwald et al. 2006) which may be due to comparatively larger sample sizes and the different genetic structure of populations considered in those studies.
All the samples were genotyped using a customized version of Illumina’s high-density chip designed by NDDB, Anand, India. Customized DNA chips are a viable cost-effective alternative to genotype animals using pre-selected population-specific SNPs (Boichard et al. 2018). INDUSCHIP v2 was used in this study by evaluating QC parameters obtained by genotyping representative samples of almost all the major Indian cattle breeds. It was an improvement over the earlier version (INDUSCHIP v1) in the sense that the major LD gaps were covered and various ancestry-informative and disease-informative SNPs were added (https://www.dairyknowledge.in/sites/default/files/genomic_selection.pdf). Additionally, to validate the technical robustness and accuracy of the genotyping panel, three samples were randomly selected and were genotyped twice. 99.9% similarity in the called genotypes for both the replicates of the same individual vindicated our stand that this customized chip was suitable for conducting genomic studies in the aforementioned population.
A compressed mixed linear model was used to unveil genome-wide associations for the traits. In this model, random polygenic component accounts for population stratification and known as well as cryptic relatedness, thus preventing type-I errors to a certain extent (Schmid and Bennewitz 2017). However, polygenic inheritance of a trait may also be influenced by sample size, heritability, LD structure, and the number of causal variants, all of which may lead to inflation of test statistics (Yang et al. 2011). Thus, p value threshold for significance needs to be flexible considering MAF, sample size, and LD thresholds used in the study (Fadista et al. 2016). Furthermore, the limited power of QTL detection in a small sample size necessitates consideration of a suggestive threshold for significance (Qanbari 2020). In this study, we opted for a suggestive threshold of p ≤ 5 × 10−4 to determine significance.
In all, we identified a total of 16 QTLs associated with the trait EBVs for CMI traits encompassing eleven Bos taurus autosomes, viz., 3, 4, 6, 7, 13, 16, 17, 18, 19, 25, and 28. Nine out of sixteen identified QTLs overlapped with the already reported potential regions for mastitis. Interestingly, chromosomes 6, 13, 16, and 19 have been fine-mapped earlier as potential regions for CM traits (Sahana et al. 2014).
Two QTLs present on BTA4 were found to overlap previously identified regions for udder swelling and SCS. Also, one of the regions closer to the identified QTL between 59.0 and 60.7 Mb including DNAJB9 and ELMO1 genes has been previously assigned to mastitis phenotypes (Tal-Stein et al. 2010). The second region between 4.0 and 4.6 Mb on BTA4 which also included the topmost QTL for the most significant SNP could be considered as a novel one related to the disease.
DNAJB9 gene facilitates the synthesis of a heat shock protein which acts as a tumor suppressor (Lee et al. 2015), whereas ELMO1 is a putative candidate gene for mastitis resistance which helps in phagocytosis of apoptotic cells by causing cytoskeletal rearrangements (Das et al. 2015; Cai et al. 2018). An important QTL was identified on BTA7 harboring ARHGAP26 and NR3C1 genes which was around 7 Mb farther from the earlier identified QTLs for clinical mastitis (Marete et al. 2018). ARHGAP26 gene has documented role in tumor suppression, particularly in ovarian cell proliferation and migration in humans (Chen et al. 2019). NR3C1 gene is also a potential candidate gene for mastitis as it encodes for glucocorticoid receptors which, consequently, regulate neutrophil migration and cytokine production during periparturient period, thus determining susceptibility/resistance to mastitis (Preisler et al. 2000; Burton and Erskine 2003). It is possible that higher SNP density may result in overlap of this QTL with the one identified in our study.
Lund et al. (2008) reported a larger QTL region (33.0–53.6 Mb) on BTA13 for mastitis based on microsatellite analysis. The region identified on BTA13 for CMIF could be considered to be a part of this larger QTL for the trait. Furthermore, genome-wide significant SNPs identified for clinical mastitis incidence traits were located in the known QTL for teat length (Sahana et al. 2014). In our study, the genes identified in this region included SLC39A12 and CACNB2. SLC39A12 acts as a transporter of zinc which, subsequently, plays an important role in modulating host immunity (Prasad 2008). Also, the gene has been found to be differentially expressed in mammary gland following E. coli mastitis, thus consolidating its potential role as a part of innate immune response (Rinaldi et al. 2010). On the other hand, CACNB2 gene encodes for a regulatory subunit of Ca2+ channel which impairs T-cell development in the thymus and determines the immune response against clinical mastitis of viral etiology (Jha et al. 2015; Wellenberg et al. 2002).
Two novel QTLs were reported in this study on BTA28 extending from 0.02 to 0.5 Mb and 20.1–21.1 Mb and harboring RAB4A, ACTA1 and NUP133 genes. RAB4A gene regulates CD4+ T-cell recycling and plays a role in mounting an effective immune response against HIV infection (Nagy et al. 2006), though a variant inhabiting the gene has been found to be associated with chronic mastitis phenotypes, as well (Siebert 2017). While ACTA1 is a house-keeping gene involved in synthesizing alpha-actin protein for muscle contraction, evidence exists for its involvement in mastitis in caprines (Pisanu et al. 2020). Nucleoporin genes have been implicated in several human diseases and overexpression of NUP133 (Nucleoporin 133) gene has been associated with breast cancer (Nofrini et al. 2016).
Udder conformation traits are known to have high genetic correlation with mastitis incidence and susceptibility (Singh et al. 2014). On BTA17, we found a QTL region which was associated with udder height (Ashwell et al. 2005), thus hinting on its involvement in genetic resistance to mastitis. RAB33B gene in this region has been found to be differentially expressed in bovine mammary tissues challenged with mastitis (Zhang et al. 2018), thus consolidating its link with the disease.
The QTL region identified on BTA19 overlapped with already reported regions for clinical mastitis and SCS and included important immune-regulatory genes like GRB2, SLC25A19, NUP85, SUMO2, and CDR2L. GRB2 (Growth Factor Receptor-Bound Protein 2) gene helps in the synthesis of an adaptor protein serving critical functions in both B as well as T lymphocytes (Radtke et al. 2016; Vanshylla et al. 2018). SLC25A19 (Solute Carrier Family 25 Member 19) gene is purported to be regulating breast cancer in humans due to its role in thiamine transport (Zastre et al. 2013). NUP85 (Nucleoporin 85) gene which encodes for a protein that interacts with the cytokine receptors, thus promoting chemotaxis in leucocytes and generating an immune response (Toda et al. 2014). SUMO2 (Small Ubiquitin like Modifier 2) gene (along with SUMO1 and SUMO3) is involved in regulating innate immune response of the body against viral as well as bacterial attack (Adorisio et al. 2017; Crowl and Stetson 2018), whereas CDR2L (Cerebellar Degeneration Related Protein Like 2) is a putative gene for breast cancer and ovarian tumor in humans (Schubert et al. 2014) due to the presence of CDR2L antibodies in the affected patients (Eichler et al. 2013). Sahana et al. (2014) reported a putative candidate SNP at 55,296,191 bp for SCC on the same chromosome close to the QTL region identified in our study.
Identified QTL on BTA18 was part of the two large previously reported QTLs for SCS (Muncie et al. 2006), whereas a novel QTL was reported on BTA25 for CMIS.
The reviewed literature shows that chromosome 6 contains one of the highest numbers of significant associations with mastitis traits and it has a key role in determining genetic resistance against the disease (Meredith et al. 2013; Sahana et al. 2014; Olsen et al. 2016). We identified two QTLs—a larger one (46.4–47.9 Mb) and a small one (30.98–30.99 Mb) on BTA6—both of which were found to be overlapping with already identified QTLs for udder traits, SCS, as well as clinical mastitis and encompassed potential candidate genes like SLC34A2 and RBPJ. SLC34A2 is involved in phosphate transport, and has been identified as a potential candidate for mastitis phenotypes based on GWAS, differential expression and proteome studies (Zhang et al. 2015; Chen et al. 2015; Oliveira et al. 2019). On the other hand, RBPJ is a key gene in notch signaling which regulates the inflammatory process of macrophages (Xu et al. 2012) and has also been found to be differentially methylated in mastitis-affected cows, thus stressing on its relevance as a candidate gene (Ju et al. 2020). A nearby QTL region (56.5–56.6 Mb) distinct from the present study was reported by Wu et al. (2015) which revealed similar putative genes as in our findings. Similarly, out of the two QTLs identified on BTA3, the one mapped between 96.8 and 97.8 Mb was found to be a novel one, whereas another overlapped with a known QTL for SCC as well as SCR (Schrooten et al. 2000).
Finally, we report two QTLs associated with mastitis phenotypes on BTA16 which had known overlap with QTLs for SCS. Though Ogorevc et al. (2009) indicated the same chromosome to be least important with respect to mastitis, Cai et al. (2018) concluded the association signals on BTA16 to be contributing a substantial proportion of genetic variance to the mastitis traits.
This study is among the first to identify genome-wide associations and potential QTLs associated with CMI traits in Bos indicus breed, Sahiwal. Though the power of this study was reduced due to small sample size, Bayesian approach was pursued to improve the accuracy of phenotypes. Also, the reliability of the results could be strengthened by the fact that most of the identified potential QTL regions overlapped with already reported QTLs for mastitis as well as its genetically correlated traits like udder conformation traits and milk production. Sixteen identified QTLs, including seven novel ones, may be genetically regulating the resistance to CM in Bos indicus cattle, which needs to be further validated through fine-mapping as well as functional studies.
Conclusions
Sixteen QTLs were identified for their association with clinical mastitis incidence traits in Sahiwal cattle. Most of the QTL regions overlapped with previously annotated QTLs for mastitis traits whereas seven were reported to be novel ones. A host of candidate genes were mapped in relation to the identified QTL regions which had major roles in regulating immune response and immunity including DNAJB9, ELMO1, CACNB2, GRB2, NUP85, SUMO2, and RBPJ genes in the overlapped QTL regions and ARHGAP26, NR3C1, RAB4A, and RAB33B genes in the novel QTL regions. This study is among the foremost to study the genomic variants and QTLs underlying clinical mastitis in Bos indicus and provides interesting evidence to facilitate research in the area of genetic resistance against mastitis in Sahiwal cattle.
Acknowledgements
The authors are grateful to Director, National Dairy Research Institute (NDRI) for providing necessary support and staff of Livestock Health complex and Livestock Research Centre for facilitating sample collection and data recording. We are indebted to National Dairy Development Board (NDDB), Anand, Gujarat, for genotyping the samples free of cost, as a part of MoU between NDRI and NDDB. The first author is also thankful to Dr. S.K. Onteru for helping in the interpretation of results and Indian Council of Medical Research (ICMR) for providing Junior Research Fellowship (JRF).
Author contribution
AK and SMD conceived and designed the study, AK and NN performed the statistical analysis, NN and SKN helped in laboratory work and genotyping of samples, VSR and VY facilitated data curation, and AK and SMD wrote the paper.
Funding
No financial grant was received to carry out this study.
Data availability
The phenotypic as well as genotypic data used in the present study are available with the https://doi.org/10.6084/m9.figshare.16570587 and would be made accessible based on individual requests to the corresponding author after due consideration.
Declarations
Conflict of interests
All the authors declare that they have no conflict of interest.
Contributor Information
Aneet Kour, Email: aneetk25@gmail.com.
Sitangsu Mohan Deb, Email: sm_deb@yahoo.com.
Nilesh Nayee, Email: nileshn@nddb.coop.
Varinder Singh Raina, Email: varinderraina07@gmail.com.
Vandana Yadav, Email: dryadavvandana@gmail.com.
Saket Kumar Niranjan, Email: saketniranjan@gmail.com.
References
- Adorisio S, Fierabracci A, Muscari I, Liberati AM, Ayroldi E, Migliorati G, Thuy TT, Riccardi C, Delfino DV. SUMO proteins: guardians of immune system. J Autoimmun. 2017;84:21–28. doi: 10.1016/j.jaut.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Ashwell MS, Heyen DW, Weller JI, Ron M, Sonstegard TS, Van Tassell CP, Lewin HA. Detection of quantitative trait loci influencing conformation traits and calving ease in Holstein–Friesian cattle. J Dairy Sci. 2005;88:4111–4119. doi: 10.3168/jds.S0022-0302(05)73095-2. [DOI] [PubMed] [Google Scholar]
- Boichard D, Boussaha M, Capitan A, Rocha D, Hozé C, Sanchez MP, Tribout T, Letaief R, Croiseau P, Grohs C, et al. Experience from large scale use of the EuroGenomics custom SNP chip in cattle. Proc World Congr Genet Appl Livest Prod. 2018;11:675. [Google Scholar]
- Burton JL, Erskine RJ. Immunity and mastitis. Some new ideas for an old disease. Vet Clin N Am Food Anim Pract. 2003;19:1. doi: 10.1016/s0749-0720(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Cai Z, Guldbrandtsen B, Lund MS, Sahana G. Prioritizing candidate genes post-GWAS using multiple sources of data for mastitis resistance in dairy cattle. BMC Genom. 2018;19:656. doi: 10.1186/s12864-018-5050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Dusza M, Guldbrandtsen B, Lund MS, Sahana G. Distinguishing pleiotropy from linked QTL between milk production traits and mastitis resistance in Nordic Holstein cattle. Genet Sel Evol. 2020;52:19. doi: 10.1186/s12711-020-00538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlén E, Strandberg E, Roth A. Genetic parameters for clinical mastitis, somatic cell score, and production in the first three lactations of Swedish Holstein cows. J Dairy Sci. 2004;87(9):3062–3070. doi: 10.3168/jds.S0022-0302(04)73439-6. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Cheng Z, Zhang S, Werling D, Cheng Z. Combining genome wide association studies and differential gene expression data analyses identifies candidate genes affecting mastitis caused by two different pathogens in the dairy cow. Open J Anim Sci. 2015;5:358–393. doi: 10.4236/ojas.2015.54040. [DOI] [Google Scholar]
- Chen X, Chen S, Li Y, Gao Y, Huang S, Li H, Zhu Y. SMURF1-mediated ubiquitination of ARHGAP26 promotes ovarian cancer cell invasion and migration. Exp Mol Med. 2019;51(4):1–2. doi: 10.1038/s12276-019-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl JT, Stetson DB. SUMO2 and SUMO3 redundantly prevent a noncanonical type I interferon response. Proc Natl Acad Sci. 2018;115(26):6798–6803. doi: 10.1073/pnas.1802114115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sarkar A, Choudhury SS, Owen KA, Castillo V, Fox S, Eckmann L, Elliott MR, Casanova JE, Ernst PB. ELMO1 has an essential role in the internalization of Salmonella Typhimurium into enteric macrophages that impacts disease outcome. Cell Mol Gastroenterol Hepatol. 2015;1(3):311–324. doi: 10.1016/j.jcmgh.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Jin X, Ma H, Wang R, Cong S, Tian C, Liu J, Zhao M, Li R, Wang K. The oncogene IARS2 promotes non-small cell lung cancer tumorigenesis by activating the AKT/MTOR pathway. Front Oncol. 2019;9:393. doi: 10.3389/fonc.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler TW, Totland C, Haugen M, Qvale TH, Mazengia K, Storstein A, Haukanes BI, Vedeler CA. CDR2L antibodies: a new player in paraneoplastic cerebellar degeneration. PLoS One. 2013;8(6):e66002. doi: 10.1371/journal.pone.0066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadista J, Manning AK, Florez JC, Groop L. The (in)famous GWAS P-value threshold revisited and updated for low-frequency variants. Eur J Hum Genet. 2016;24(8):1202–1205. doi: 10.1038/ejhg.2015.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune M, Guo H, Burren O, Schofield E, Walker NM, Ban M, Sawcer SJ, Bowes J, Worthington J, Barton A, Eyre S, Todd JA, Wallace C. Statistical colocalization of genetic risk variants for related autoimmune diseases in the context of common controls. Nat Genet. 2015;47:839–846. doi: 10.1038/ng.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Lund MS, Zhang Y, Su G. Accuracy of genomic prediction using different models and response variables in the Nordic Red cattle population. J Anim Breed Genet. 2013;130(5):333–340. doi: 10.1111/jbg.12039. [DOI] [PubMed] [Google Scholar]
- Guo G, Lund MS, Zhang Y, Su G. Comparison between genomic predictions using daughter yield deviation and conventional estimated breeding value as response variables. J Anim Breed Genet. 2010;127(6):423–432. doi: 10.1111/j.1439-0388.2010.00878.x. [DOI] [PubMed] [Google Scholar]
- Hadfield JD (2010) MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J Stat Softw 33(2):1–22. http://www.jstatsoft.org/v33/i02/
- Halasa T, Huijps K, Østerås O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Vet Q. 2007;29:18–31. doi: 10.1080/01652176.2007.9695224. [DOI] [PubMed] [Google Scholar]
- Heringstad B, Klemetsdal G, Ruane J. Selection for mastitis resistance in dairy cattle: a review with focus on the situation in the Nordic countries. Livest Prod Sci. 2000;64:95–106. doi: 10.1016/S0301-6226(99)00128-1. [DOI] [Google Scholar]
- Heringstad B, Chang YM, Gianola D, Klemetsdal G. Multivariate threshold model analysis of clinical mastitis in multiparous Norwegian Dairy Cattle. J Dairy Sci. 2004;87(9):3038–3046. doi: 10.3168/jds.S0022-0302(04)73436-0. [DOI] [PubMed] [Google Scholar]
- http://cpcsea.nic.in/Content/55_1_GUIDELINES.aspx. Accessed on 08 Apr 2020
- https://genome.ucsc.edu/. Accessed 10 Apr 2020
- https://github.com/sahirbhatnagar/manhattanly/. Accessed 10 Apr 2020
- https://www.animalgenome.org/cgi-bin/QTLdb/BT/index. Accessed 01 Sept 2020
- https://www.dairyknowledge.in/sites/default/files/genomic_selection.pdf. Accessed 09 Apr 2020
- https://www.nddb.coop/services/animalbreeding/geneticimprovement/genomic. Accessed 09 Apr 2020
- Jha A, Singh AK, Weissgerber P, Freichel M, Flockerzi V, Flavell RA, Jha MK. Essential roles for Cavβ2 and Cav1 channels in thymocyte development and T cell homeostasis. Sci Signal. 2015;8(399):103. doi: 10.1126/scisignal.aac7538. [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu J, Sun D, Ma P, Ding X, Yu Y, Zhang Q. Genome wide association studies for milk production traits in Chinese Holstein population. PLoS One. 2010;5(10):e13661. doi: 10.1371/journal.pone.0013661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Tan Y, Wang H, Peng L, Chen HT, Meng XJ, Li LL, Liu Y, Li WF, Shan H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18:17. doi: 10.1186/s12943-019-0944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K, Eriksson S, Pösö J, Toivonen M, Nielsen US, Eriksson JA, Aamand GP. Genetic evaluation of udder health traits for Denmark, Finland and Sweden. Interbull Bull. 2006;35:92–96. [Google Scholar]
- Ju Z, Jiang Q, Wang J, Wang X, Yang C, Sun Y, Zhang Y, Wang C, Gao Y, Wei X, Hou M, Huang J. Genome-wide methylation and transcriptome of blood neutrophils reveal the roles of DNA methylation in affecting transcription of protein-coding genes and miRNAs in E. coli-infected mastitis cows. BMC Genom. 2020;21:102. doi: 10.1186/s12864-020-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzias H, Karatzia M, Panagiotis K. Occurrence, etiology and prevention of abomasal displacement in dairy. Cattle: domestication, diseases and the environment. New York: Nova Science Publishers Inc.; 2013. pp. 127–138. [Google Scholar]
- Kirsanova E, Heringstad B, Lewandowska-Sabat A, Olsaker I. Identification of candidate genes affecting chronic subclinical mastitis in Norwegian Red cattle: combining genome-wide association study, topologically associated domains and pathway enrichment analysis. Anim Genet. 2020;51(1):22–31. doi: 10.1111/age.12886. [DOI] [PubMed] [Google Scholar]
- Koeck A, Heringstad B, Egger-Danner C, Fuerst C, Winter P. Genetic analysis of clinical mastitis and somatic cell count traits in Austrian Fleckvieh cows. J Dairy Sci. 2010;93(12):5987–5995. doi: 10.3168/jds.2010-3451. [DOI] [PubMed] [Google Scholar]
- Krömker V, Leimbach S. Mastitis treatment—reduction in antibiotic usage in dairy cows. Reprod Dom Anim. 2017;52(Suppl. 3):21–29. doi: 10.1111/rda.13032. [DOI] [PubMed] [Google Scholar]
- Kurz P, Yang Z, Weiss RB, Wilson DJ, Rood KA, Liu GE, Wang Z. A genome-wide association study for mastitis resistance in phenotypically well-characterized Holstein dairy cattle using a selective genotyping approach. Immunogenetics. 2019;71(1):35–47. doi: 10.1007/s00251-018-1088-9. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim JM, Kim KH, Heo JI, Kwak SJ, Han JA. Genotoxic stress/p53-induced DNAJB9 inhibits the pro-apoptotic function of p53. Cell Death Differ. 2015;22(1):86–95. doi: 10.1038/cdd.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, Gore MA, Buckler ES, Zhang Z. GAPIT: genome association and prediction integrated tool. Bioinformatics. 2012;28(18):2397–2399. doi: 10.1093/bioinformatics/bts444. [DOI] [PubMed] [Google Scholar]
- Lund MS, Guldbrandtsen B, Buitenhuis AJ, Thomsen B, Bendixen C. Detection of quantitative trait loci in Danish Holstein cattle affecting clinical mastitis, somatic cell score, udder conformation traits, and assessment of associated effects on milk yield. J Dairy Sci. 2008;91(10):4028–4036. doi: 10.3168/jds.2007-0290. [DOI] [PubMed] [Google Scholar]
- Marete A, Sahana G, Fritz S, Lefebvre R, Barbat A, Lund MS, Guldbrandtsen B, Boichard D. Genome-wide association study for milking speed in French Holstein cows. J Dairy Sci. 2018;101(7):6205–6219. doi: 10.3168/jds.2017-14067. [DOI] [PubMed] [Google Scholar]
- Márquez A, Kerick M, Zhernakova A, Gutierrez-Achury J, Chen WM, Onengut-Gumuscu S, González-Álvaro I, Rodriguez-Rodriguez L, Rios-Fernández R, González-Gay MA, Coeliac Disease Immunochip Consortium, Rheumatoid Arthritis Consortium International for Immunochip (RACI), International Scleroderma Group, Type 1 Diabetes Genetics Consortium. Mayes MD, RayChaudhari S, Rich SS, Wijmenga C, Martin J. Meta-analysis of Immunochip data of four autoimmune diseases reveals novel single disease and cross-phenotype associations. Genome Med. 2018;10:97. doi: 10.1186/s13073-018-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith BK, Kearney FJ, Finlay EK, Bradley DG, Fahey AG, Berry DP, Lynn DJ. Genome-wide associations for milk production and somatic cell score in Holstein–Friesian cattle in Ireland. BMC Genet. 2012;13:21. doi: 10.1186/1471-2156-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith BK, Berry DP, Kearney F, Finlay EK, Fahey AG, Bradley DG, Lynn DJ. A genome-wide association study for somatic cell score using the Illumina high-density bovine beadchip identifies several novel QTL potentially related to mastitis susceptibility. Front Genet. 2013;4:229. doi: 10.3389/fgene.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mömke S, Sickinger M, Lichtner P, Doll K, Rehage J, Distl O. Genome-wide association analysis identifies loci for left-sided displacement of the abomasum in German Holstein cattle. J Dairy Sci. 2013;96(6):3959–3964. doi: 10.3168/jds.2012-5679. [DOI] [PubMed] [Google Scholar]
- Muncie SA, Cassady JP, Ashwell MS. Refinement of quantitative trait loci on bovine chromosome 18 affecting health and reproduction in US Holsteins. Anim Genet. 2006;37(3):273–275. doi: 10.1111/j.1365-2052.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- Nagy G, Ward J, Mosser DD, Koncz A, Gergely P, Jr, Stancato C, Qian Y, Fernandez D, Niland B, Grossman CE, et al. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J Biol Chem. 2006;281:34574–34591. doi: 10.1074/jbc.m606301200. [DOI] [PubMed] [Google Scholar]
- Nash DL, Rogers GW, Cooper JB, Hargrove GL, Keown JF, Hansen LB. Heritability of clinical mastitis incidence and relationships with sire transmitting abilities for somatic cell score, udder type traits, productive life, and protein yield. J Dairy Sci. 2000;83(10):2350–2360. doi: 10.3168/jds.S0022-0302(00)75123-X. [DOI] [PubMed] [Google Scholar]
- Nofrini V, Giacomo DD, Mecucci C. Nucleoporin genes in human diseases. Eur J Hum Genet. 2016;24(10):1388–1395. doi: 10.1038/ejhg.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogorevc J, Kunej T, Razpet A, Dovc P. Database of cattle candidate genes and genetic markers for milk production and mastitis. Anim Genet. 2009;40(6):832–851. doi: 10.1111/j.1365-2052.2009.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira HR, Lourenco D, Masuda Y, Misztal I, Tsuruta S, Jamrozik J, Brito LF, Silva FF, Cant JP, Schenkel FS. Single-step genome-wide association for longitudinal traits of Canadian Ayrshire, Holstein, and Jersey dairy cattle. J Dairy Sci. 2019;102(11):9995–10011. doi: 10.3168/jds.2019-16821. [DOI] [PubMed] [Google Scholar]
- Olsen HG, Knutsen TM, Lewandowska-Sabat AM, Grove H, Nome T, Svendsen M, Arnyasi M, Sodeland M, Sundsaasen KK, Dahl SR, et al. Fine mapping of a QTL on bovine chromosome 6 using imputed full sequence data suggests a key role for the group-specific component (GC) gene in clinical mastitis and milk production. Genet Sel Evol. 2016;48(1):79. doi: 10.1186/s12711-016-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanu S, Cacciotto C, Pagnozzi D, Uzzau S, Pollera C, Penati M, Bronzo V, Addis MF. Impact of Staphylococcus aureus infection on the late lactation goat milk proteome: new perspectives for monitoring and understanding mastitis in dairy goats. J Proteom. 2020;221:103763. doi: 10.1016/j.jprot.2020.103763. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc in human health: effect of Zinc on immune cells. Mol Med. 2008;14(5–6):353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler MT, Weber PS, Tempelman RJ, Erskine RJ, Hunt H, Burton JL. Glucocorticoid receptor down-regulation in neutrophils of periparturient cows. Am J Vet Res. 2000;61:14–19. doi: 10.2460/ajvr.2000.61.14. [DOI] [PubMed] [Google Scholar]
- Qanbari S. On the extent of linkage disequilibrium in the genome of farm animals. Front Genet. 2020;10:1304. doi: 10.3389/fgene.2019.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke D, Lacher SM, Szumilas N, Sandrock L, Ackermann J, Nitschke L, Zinser E. Grb2 is important for T cell development, Th cell differentiation, and induction of experimental autoimmune encephalomyelitis. J Immunol. 2016;196(7):2995–3005. doi: 10.4049/jimmunol.1501764M. [DOI] [PubMed] [Google Scholar]
- Rinaldi M, Li RW, Bannerman DD, Daniels KM, Evock-Clover C, Silva MV, Paape MJ, Van Ryssen B, Burvenich C, Capuco AV. A sentinel function for teat tissues in dairy cows: dominant innate immune response elements define early response to E. coli mastitis. Funct Integr Genom. 2010;10(1):21–38. doi: 10.1007/s10142-009-0133-z. [DOI] [PubMed] [Google Scholar]
- Rupp R, Boichard D. Genetics of resistance to mastitis in dairy cattle. Vet Res. 2003;34(5):671–688. doi: 10.1051/vetres:2003020. [DOI] [PubMed] [Google Scholar]
- Sahana G, Guldbrandtsen B, Thomsen B, Holm LE, Panitz F, Brøndum RF, Bendixen C, Lund MS. Genome-wide association study using high-density single nucleotide polymorphism arrays and whole-genome sequences for clinical mastitis traits in dairy cattle. J Dairy Sci. 2014;97(11):7258–7275. doi: 10.3168/jds.2014-8141. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Rapid isolation of yeast DNA. CSH Protoc. 2006 doi: 10.1101/pdb.prot4039. [DOI] [PubMed] [Google Scholar]
- Schmid M, Bennewitz J. Invited review: genome-wide association analysis for quantitative traits in livestock—a selective review of statistical models and experimental designs. Arch Anim Breed. 2017;60:335–346. doi: 10.5194/aab-60-335-2017. [DOI] [Google Scholar]
- Schrooten C, Bovenhuis H, Coppieters W, Van Arendonk JA. Whole genome scan to detect quantitative trait loci for conformation and functional traits in dairy cattle. J Dairy Sci. 2000;83(4):795–806. doi: 10.3168/jds.S0022-0302(00)74942-3. [DOI] [PubMed] [Google Scholar]
- Schubert M, Panja D, Haugen M, Bramham CR, Vedeler CA. Paraneoplastic CDR2 and CDR2L antibodies affect Purkinje cell calcium homeostasis. Acta Neuropathol. 2014;128:835–852. doi: 10.1007/s00401-014-1351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentitula YBR, Kumar R. Incidence of staphylococci and streptococci during winter in mastitic milk of Sahiwal cow and Murrah buffaloes. Indian J Microbiol. 2012;52(2):153–159. doi: 10.1007/s12088-011-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Rokana N, Chandra M, Singh BP, Gulhane RD, Gill J, Ray P, Puniya AK, Panwar H. Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front Vet Sci. 2018;4:237. doi: 10.3389/fvets.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook GE. Genetic improvement of mastitis through selection on somatic cell count. Vet Clin N Am Food Anim Pract. 1993;9(3):563–581. doi: 10.1016/s0749-0720(15)30622-8. [DOI] [PubMed] [Google Scholar]
- Siebert LJ (2017) Identifying genome associations with unique mastitis phenotypes in response to intramammary Streptococcus uberis challenge. Ph.D. Dissertation, University of Tennessee, Tennessee, US. https://trace.tennessee.edu/utk_graddiss/4424
- Singh RS, Bansal BK, Gupta DK. Udder health in relation to udder and teat morphometry in Holstein Friesian × Sahiwal crossbred dairy cows. Trop Anim Health Prod. 2014;46:93–98. doi: 10.1007/s11250-013-0454-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Sinha B, Kumari R, Vineeth MR, Verma A, Gupta ID. Effect of season, stage of lactation, parity and levels of milk production on incidence of clinical mastitis in Karan Fries and Sahiwal cattle. Biol Rhythm Res. 2019 doi: 10.1080/09291016.2019.1621064. [DOI] [Google Scholar]
- Tal-Stein R, Fontanesi L, Dolezal M, Scotti E, Bagnato A, Russo V, Canavesi F, Friedmann A, Soller M, Lipkin E. A genome scan for quantitative trait loci affecting milk somatic cell score in Israeli and Italian Holstein cows by means of selective DNA pooling with single- and multiple-marker mapping. J Dairy Sci. 2010;93(10):4913–4927. doi: 10.3168/jds.2010-3254. [DOI] [PubMed] [Google Scholar]
- Thompson-Crispi KA, Sargolzaei M, Ventura R, Abo-Ismail M, Miglior F, Schenkel F, Mallard BA. A genome-wide association study of immune response traits in Canadian Holstein cattle. BMC Genom. 2014;15(1):559. doi: 10.1186/1471-2164-15-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiezzi F, Parker-Gaddis KL, Cole JB, Clay JS, Maltecca C. A genome-wide association study for clinical mastitis in first parity US Holstein cows using single-step approach and genomic matrix re-weighting procedure. PLoS One. 2015;10(2):e0114919. doi: 10.1371/journal.pone.0114919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda E, Terashima Y, Esaki K, Yoshinaga S, Sugihara M, Kofuku Y, Shimada I, Suwa M, Kanegasaki S, Terasawa H, Matsushima K. Identification of a binding element for the cytoplasmic regulator FROUNT in the membrane-proximal C-terminal region of chemokine receptors CCR2 and CCR5. Biochem J. 2014;457(2):313–322. doi: 10.1042/BJ20130827. [DOI] [PubMed] [Google Scholar]
- Urioste JI, Franzén J, Windig JJ, Strandberg E. Genetic relationships among mastitis and alternative somatic cell count traits in the first 3 lactations of Swedish Holsteins. J Dairy Sci. 2012;95(6):3428–3434. doi: 10.3168/jds.2011-4739. [DOI] [PubMed] [Google Scholar]
- Van de Schoot R, Broere JJ, Perryck KH, Zwijnenburgm MZ, Loey NEV. Analyzing small data sets using Bayesian estimation: the case of posttraumatic stress symptoms following mechanical ventilation in burn survivors. Eur J Psychotraumatol. 2015 doi: 10.3402/ejpt.v6.25216.10.3402/ejpt.v6.25216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanshylla K, Bartsch C, Hitzing C, Krümpelmann L, Wienands J, Engels N. Grb2 and GRAP connect the B cell antigen receptor to Erk MAP kinase activation in human B cells. Sci Rep. 2018;8:4244. doi: 10.1038/s41598-018-22544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemereuil P. On the relevance of Bayesian statistics and MCMC for animal models. J Anim Breed Genet. 2019;136(5):339–340. doi: 10.1111/jbg.12426. [DOI] [PubMed] [Google Scholar]
- Wang X, Ma P, Liu J, Zhang Q, Zhang Y, Ding X, Jiang L, Wang Y, Zhang Y, Sun D, Zhang S, Su G, Yu Y. Genome-wide association study in Chinese Holstein cows reveal two candidate genes for somatic cell score as an indicator for mastitis susceptibility. BMC Genet. 2015;16:111. doi: 10.1186/s12863-015-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel KA, Shook GE. Genetic selection for mastitis resistance. Vet Clin N Am Food Anim Pract. 2018;34(3):457–472. doi: 10.1016/j.cvfa.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Welderufael BG, Løvendahl P, de Koning DJ, Janss LLG, Fikse WF. Genome-wide association study for susceptibility to and recoverability from mastitis in Danish Holstein cows. Front Genet. 2018;9:141. doi: 10.3389/fgene.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenberg GJ, van der Poel WH, Van Oirschot JT. Viral infections and bovine mastitis: a review. Vet Microbiol. 2002;88(1):27–45. doi: 10.1016/s0378-1135(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Wu X, Lund MS, Sahana G, Guldbrandtsen B, Sun D, Zhang Q, Su G. Association analysis for udder health based on SNP-panel and sequence data in Danish Holsteins. Genet Sel Evol. 2015;47:50. doi: 10.1186/s12711-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.cog-genomics.org/plink/1.9/ Purcell S, Chang C PLINK 1.9. Accessed 09 Apr 2020
- Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13(7):642–650. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weedon MN, Purcell S, Lettre G, Estrada K, Willer CJ, Smith AV, Ingelsson E, O’Connell JR, Mangino M, et al. Genomic inflation factors under polygenic inheritance. Eur J Hum Genet. 2011;19:807–812. doi: 10.1038/ejhg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastre JA, Hanberry BS, Sweet RL, McGinnis AC, Venuti KR, Bartlett MG, Govindarajan R. Up-regulation of vitamin B1 homeostasis genes in breast cancer. J Nutr Biochem. 2013;24(9):1616–1624. doi: 10.1016/j.jnutbio.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ersoz E, Lai C, Todhunter RJ, Tiwari HK, Gore MA, Bradbury PJ, Yu J, Arnett DK, Ordovas JM, et al. Mixed linear model approach adapted for genome-wide association studies. Nat Genet. 2010;42:355–360. doi: 10.1038/ng.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Boeren S, Van Hooijdonk ACM, Vervoort JM, Hettinga KA. A proteomic perspective on the changes in milk proteins due to high somatic cell count. J Dairy Sci. 2015;98:5339–5351. doi: 10.3168/jds.2014-9279. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jiang H, Fan Y, Chen Z, Li M, Mao Y, Karrow NA, Loor JJ, Moore S, Yang Z. Transcriptomics and iTRAQ-proteomics analyses of bovine mammary tissue with Streptococcus agalactiae-induced mastitis. J Agric Food Chem. 2018;66(42):11188–11196. doi: 10.1021/acs.jafc.8b02386. [DOI] [PubMed] [Google Scholar]
- Zimin AV, Delcher AL, Florea L, Kelley DR, Schatz MC, Puiu D, Hanrahan F, Pertea G, Van Tassell CP, Sonstegard TS, et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10(4):R42–R72. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwald NR, Weigel KA, Chang YM, Welper RD, Clay JS. Genetic analysis of clinical mastitis data from on-farm management software using threshold models. J Dairy Sci. 2006;89:330–336. doi: 10.3168/jds.S0022-0302(06)72098-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The phenotypic as well as genotypic data used in the present study are available with the https://doi.org/10.6084/m9.figshare.16570587 and would be made accessible based on individual requests to the corresponding author after due consideration.


