Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is a malignant carcinoma with high rate of mortality. The current treatment is ineffective with poor survival time. Therefore, there is an urgent need for effective therapeutic drug regimens. The multi‐target tyrosine kinase inhibitor (TKI) anlotinib has been approved for treating non‐small cell lung cancer (NSCLC); however, the combined therapeutic regimen of anlotinib for ICC has not been investigated yet. This study aims to investigate the inhibitory effect of anlotinib and the mechanism of gemcitabine combination for ICC treatment.
Methods
Two ICC cell lines, HCCC‐9810 and RBE cells, were used in this study. Cell Counting Kit‐8 (CCK‐8) was used to study the cell viability, and flow cytometry (FCM) was used to evaluate the apoptosis and cell cycle arrest. Compusyn software was used to calculate the combination index (CI) of anlotinib and gemcitabine. The protein expression rate of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3 was detected by Western blotting.
Results
Our result showed that the anlotinib and gemcitabine combination significantly inhibits the growth of ICC cell lines. Compusyn software results showed that the combination regimen had an anti‐tumor synergistic effect. FCM results showed that it promoted apoptosis. Moreover, it increased the protein expression rate of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3. Finally, we found a synergistic anti‐tumor effect by increasing G0/G1 cell cycle arrest.
Conclusion
The combination of anlotinib and gemcitabine can increase the anti‐tumor effect and may be a potential therapeutic drug regimen in a clinical setting.
Keywords: anlotinib, apoptosis, cell cycle arrest, gemcitabine, intrahepatic cholangiocarcinoma, synergistic
Anlotinib combined with gemcitabine significantly increased the effects of gemcitabine on G0/G1 cell cycle arrest and promoted apoptosis of gemcitabine on G0/G1 phase for ICC cell lines. Therefore, anlotinib has a synergistic effect in the two‐drug combination regimen. It is possible to be used as an alternative treatment for ICC and has potential clinical application.
1. INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer, and the incidence is rapidly increasing worldwide. 1 , 2 Surgical resection is the only standard therapeutic option for ICC, with a 5‐year survival rate of 30%–35%. 3 However, the degree of ICC malignancy is high, with a poor prognosis. 4 The 5‐year survival rates are significantly low. 5 In addition to surgery, chemotherapeutic drugs, including gemcitabine, cisplatin, and fluorouracil, can be used to treat ICC, but the therapeutic effect observed in patients is not satisfactory. 6 Therefore, effective drug treatment and regimens are urgently needed.
Anlotinib is a highly effective, selective, ATP‐competitive small‐molecule tyrosine kinase inhibitor (TKI), which has been reported in several preclinical studies. 7 , 8 The multi‐target TKI anlotinib suppresses tumor angiogenesis and proliferation signaling. The main targets are vascular endothelial growth factor 1–3 (VEGFR1–3), fibroblast growth factor receptor 1–4 (FGFR1–4), platelet‐derived growth factor receptor (PDGFR), and c‐kit. 9 , 10 Anlotinib was approved as a third‐line treatment for locally advanced or metastatic non‐small cell lung cancer (NSCLC) in a randomized, double‐blind clinical trial, ALTER0303. 11 However, clinical practice has shown that a single drug small‐molecule TKI has a limited therapeutic effect, and combined chemotherapeutic drugs can significantly improve their anti‐tumor effect. 12 , 13
Currently, studies have shown that targeted drugs combined with chemotherapy drugs can significantly improve the efficacy of anti‐tumor therapy. 14 Due to the combination of targeted drugs and reduced dose of chemotherapeutic drugs, patients have better tolerance to anti‐tumor therapy and better treatment compliance, thus achieving the purpose of low‐toxicity and high‐efficiency anti‐tumor therapy. 15 According to this view, the hypothesis of this study is that gemcitabine is a cell cycle‐specific chemotherapy drug. 16 Anlotinib also showed cell cycle arrest efficacy, according to preliminary experiments. 17 Therefore, the hypothesis that anlotinib combined with gemcitabine can act on intrahepatic cholangiocarcinoma cell lines is proposed in this study.
There is a lack of studies reported on the action of anlotinib combined with gemcitabine in ICC; therefore, this study aims to investigate the anlotinib and gemcitabine combination in vitro on ICC cell lines. The synergistic effect of the two drugs was verified, and the mechanism was further explored. This study provides a new and effective treatment regimen for ICC. Therefore, the combined regimen has potential clinical value for the treatment of ICC.
2. MATERIALS AND METHODS
2.1. Reagents and cell culture
The human ICC cell lines, including HCCC‐9810 and RBE cells, were obtained from the Cell Resource Center, Peking Union Medical College, Dongcheng District, Beijing, China (The headquarters for the National Infrastructure of Cell Line Resource, NSTI). Cells were grown in RPMI 1640 with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin‐streptomycin solution. They were cultured at 37°C humidified atmosphere with 5% CO2. Anlotinib was provided by Chia Tai Tianqing Pharmaceutical Group Co., Ltd. The compounds were dissolved in dimethyl sulfoxide (DMSO) to 4.2 × 103 μM as a stock solution and stored at −20°C for in vitro studies and diluted with the medium before each assay. Cleaved caspase‐3, caspase‐3, cleaved PARP, PARP, and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology.
2.2. Cytotoxicity assay
The HCCC‐9810 and RBE cells were cultured in 96‐well plates at a density of 5000 cells per well. Cells were harvested at indicated times and stained with Trypan blue (Sigma Chemical Co.), according to the manufacturer's instructions for the Cell Counting Kit‐8 (CCK‐8; Dojindo Laboratories). The cells were incubated for 24 h and then with or without anlotinib and/or gemcitabine at different concentrations for 24, 48, and 72 h. The cells were incubated with CCK‐8 working solution for 1–2 h at 37°C. The resulting absorbance was measured at 450 nm using a multifunctional enzyme‐linked immunosorbent assay (ELISA) instrument (Thermo).
2.3. Cell cycle and apoptosis analyses
HCCC‐9810 and RBE cells were collected at a final concentration of 4 × 105 cells/ml in 6‐well plates. For the cell cycle assays, the cells were digested by trypsin, washed with PBS, and fixed with 70% alcohol overnight. Subsequently, the cells were stained by FXCycle TMPI/RNase Staining Solution (Thermo Fisher Scientific), incubated for 30 min at room temperature in the dark. For the apoptosis assays, the cells were stained with annexin V‐FITC and propidium iodide (PI) (Thermo Fisher Scientific). After harvesting the cells, FCM was used for evaluating the cell cycle and apoptosis. All assays were performed in triplicate.
2.4. Western blotting
Western blotting was performed as previously described. 17 Briefly, the cells were lysed with radioimmunoprecipitation (RIPA) lysis buffer (Beyotime Biotechnology) containing protease and phosphatase inhibitor cocktails (Beyotime Biotechnology). The protein extracts were resolved using sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and then transferred to polyvinylidene difluoride membranes (PVDF) (MilliporeSigma) for Western blotting. PVDF membranes were incubated with primary antibodies (cleaved PARP, PARP, cleaved caspase‐3, and caspase‐3 at 1:1000), washed with Tris‐buffered saline with Tween (TBST), followed by secondary antibodies (at 1:5000), and conjugated with horseradish peroxidase. Afterward, PVDF membranes were scanned and photographed using a Bio‐Rad imager (Bio‐Rad). Three independent assays were performed.
2.5. Statistical analysis
All experiments were performed in triplicate. Statistical analysis was performed by SPSS version 18.0 software (IBM Corporation). Quantitative data are shown as the mean ±SD. Significant differences between groups were assessed by one‐way ANOVA with the least significant difference (LSD) test. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Anlotinib inhibits the proliferation of ICC cell lines in vitro
We used different concentrations of anlotinib (0, 0.5, 2, 8, 32, and 128 μM) and added them to human ICC cell lines (HCCC‐9810 and RBE) for 24, 48, and 72 h. Then, we detected the ability to inhibit growth by a CCK‐8 assay. As shown in Figure 1A, the inhibition of HCCC‐9810 and RBE cell lines was significantly increased with the increase in anlotinib concentration, and the growth of the ICC cell lines was also inhibited with time. Consequently, anlotinib inhibited the growth of ICC cell lines in a dose‐ and time‐dependent manner. The IC50 values of 24, 48, and 72 h of anlotinib treatment were 45.51 μM, 21.33 μM, and 8.13 μM in HCCC‐9810 cell lines and 49.37 μM, 7.18 μM, and 4.67 μM in RBE cell lines, respectively (Table 1).
FIGURE 1.
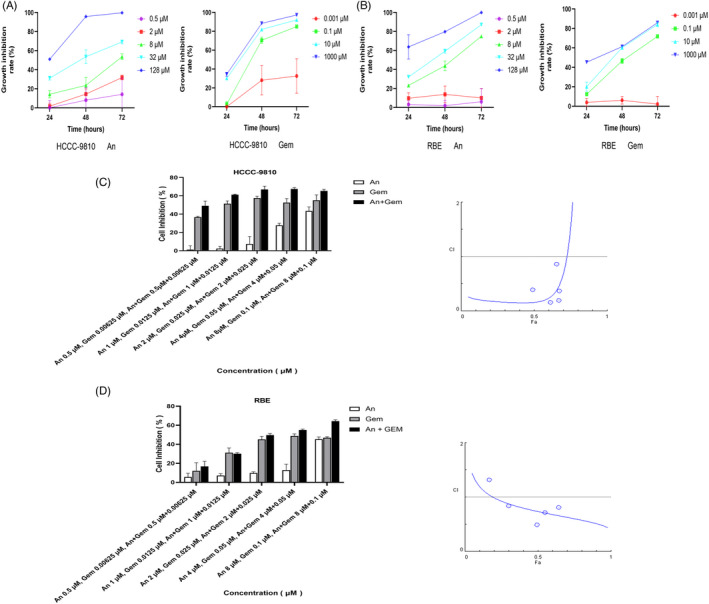
Anlotinib and gemcitabine inhibit cell growth of ICC cell lines in vitro. (A and B) HCCC‐9810 and RBE cells were treated with anlotinib or gemcitabine at the indicated concentrations for 24, 48, and 72 h. The CCK‐8 assay measured the growth inhibition rate. (C and D) HCCC‐9810 and RBE cell lines were treated with the combination of anlotinib and gemcitabine for 48 h by constant dilution, and then the growth inhibition rate of ICC cell lines was detected by CCK‐8. The CI of the two drugs was calculated by Compusyn software, and the value of CI less than 1.0 indicated a synergistic effect (n = 3). Con: control; An: anlotinib; Gem: gemcitabine
TABLE 1.
Half‐maximal inhibitive concentration (IC50) values of HCCC‐9810 and RBE cell lines were time‐ and dose‐dependent after treatment with different concentrations of anlotinib
| Cell lines | HCCC−9810 | RBE |
|---|---|---|
| IC50 24 h (95% CI) |
45.51 μM (95% CI 38.18–56.16) |
49.37 μM (95% CI 39.25–66.96) |
| IC50 48 h (95% CI) |
21.33 μM (95% CI 17.7–26.11) |
7.18 μM (95% CI 5.30–9.73) |
| IC50 72 h (95% CI) |
8.13 μM (95% CI 6.50–10.09) |
4.67 μM (95% CI 4.05–5.40) |
3.2. Gemcitabine inhibits the growth of ICC cell lines in vitro
We used different concentrations of gemcitabine (0, 0.001, 0.1, 10, and 1000 μM) and added them to human ICC cell lines (HCCC‐9810 and RBE) for 24, 48, and 72 h, and the ability to inhibit growth was detected by a CCK‐8 assay. As shown in Figure 1B, the inhibition of HCCC‐9810 and RBE cell lines was significantly increased with the increase in gemcitabine concentration, and the growth of the ICC cell lines was also inhibited with time. Consequently, gemcitabine inhibited the growth of ICC cell lines in a dose‐ and time‐dependent manner. The IC50 values of 24, 48, and 72 h of gemcitabine treatment were 3277862 μM, 2.08 μM, and 0.28 μM in HCCC‐9810 cell lines and 153735 μM, 4.92 μM, and 0.03 μM in RBE cell lines, respectively (Table 2). We found that HCCC‐9810 and RBE cell lines were resistant to gemcitabine treatment for 24 h. Therefore, we chose 48 h as the incubation time for the follow‐up experiments.
TABLE 2.
IC50 values of HCCC‐9810 and RBE cell lines were time‐ and dose‐dependent after treatment with different concentrations of gemcitabine
| Cell lines | HCCC−9810 | RBE |
|---|---|---|
| IC50 24 h (95% CI) |
3277862 μM (95% CI 168851–537181042) |
153735 μM (95% CI 12688–13674180) |
| IC50 48 h (95% CI) |
2.08 μM (95% CI 1.08–4.16) |
4.92 μM (95% CI 1.62–17.34) |
| IC50 72 h (95% CI) |
0.28 μM (95% CI 0.14–0.56) |
0.03 μM (95% CI 0.02–0.07) |
3.3. Anlotinib combined with gemcitabine had synergistic anti‐tumor effects
The response to chemotherapy against ICC is not satisfactory; therefore, we demonstrated whether anlotinib could increase the anti‐tumor effect of gemcitabine on ICC. Anlotinib and gemcitabine were diluted in equal proportions. Anlotinib significantly inhibited growth of ICC cell lines when administered at very low concentration (2 μM), a concentration at which it fails to affect the viability of more than 80% of ICC cell lines. However, gemcitabine inhibited growth of ICC cell lines when administered at low concentration (0.025 μM). It restrains the viability of approximately 40% to 50% of HCCC‐9810 cell lines and less than 40% of RBE. Furthermore, the results of the CI of anlotinib combined with gemcitabine, which was performed with Compusyn software, showed that the CI of anlotinib (2 μM) and gemcitabine (0.025 μM) was less than 1.0. It was also shown that the combination of the two drugs at those concentrations had a synergistic effect in HCCC‐9810 and RBE cell lines, as shown in Figure 1C and D. Therefore, the above concentrations were selected for the experimentation in the current study. The molar ratio of anlotinib:gemcitabine was 80:1. The utility of the two‐drug combination is additive when CI = 1.0, synergistic when CI <1.0, and antagonistic when CI >1.0.
3.4. The combination of anlotinib and gemcitabine synergistically promoted apoptosis
FCM was used to detect the effect of anlotinib (2 μM) combined with gemcitabine (0.025 μM) on ICC cell lines. In HCCC‐9810 and RBE cell lines, apoptosis was significantly increased after combined administration (p < 0.05; Figure 2A–D). Furthermore, the protein expression rate of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3 were analyzed by Western blotting. Our results suggested that the protein expression rates of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3 in the combined treated cell lines were significantly up‐regulated (p < 0.05; Figure 2E and F).
FIGURE 2.
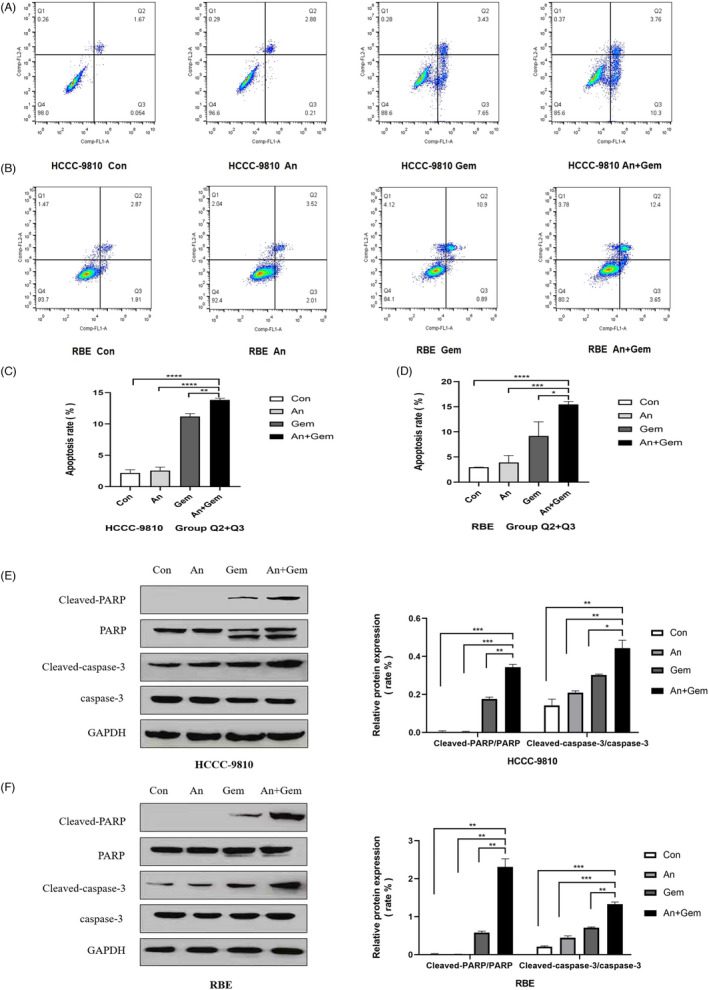
Anlotinib and gemcitabine combination promoted apoptosis of ICC cell lines, and the protein expression rates of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3 were up‐regulated. (A, B, C and D) The ICC cell lines were treated with anlotinib (2 μM) and gemcitabine (0.025 μM) for 48 h. FCM showed that combining the two drugs significantly promoted apoptosis of ICC cell lines compared with the anlotinib and gemcitabine monotherapy. (E and F) Western blotting confirmed that the protein expression rate of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3 protein in the combined group was up‐regulated. (n = 3, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001)
3.5. The combination of anlotinib and gemcitabine resulted in G0/G1 phase cell cycle arrest synergistically
Previous studies have shown that gemcitabine can cause S phase cell cycle arrest, and under certain conditions, it prevents the G1 phase transition to S phase. Blockchain extension also exerts its anti‐tumor activity through a series of other cellular mechanisms on DNA synthesis. 18 , 19 Anlotinib is a multi‐target drug; however, the purpose of this study was to investigate the anti‐tumor mechanism of anlotinib combined with gemcitabine. To determine whether the inhibitory effect of anlotinib combined with gemcitabine on cell growth inhibition was due to cell cycle arrest, we analyzed the cell cycle distribution of these cells using FCM with PI/RNase staining. Our results showed that anlotinib (2 μM) combined with gemcitabine (0.025 μM) significantly increased the percentage of cells arrested at the G0/ G1 phase (p < 0.05, Figure 3A–D). Taken together, these results indicate that anlotinib has a synergistic effect when combined with gemcitabine.
FIGURE 3.
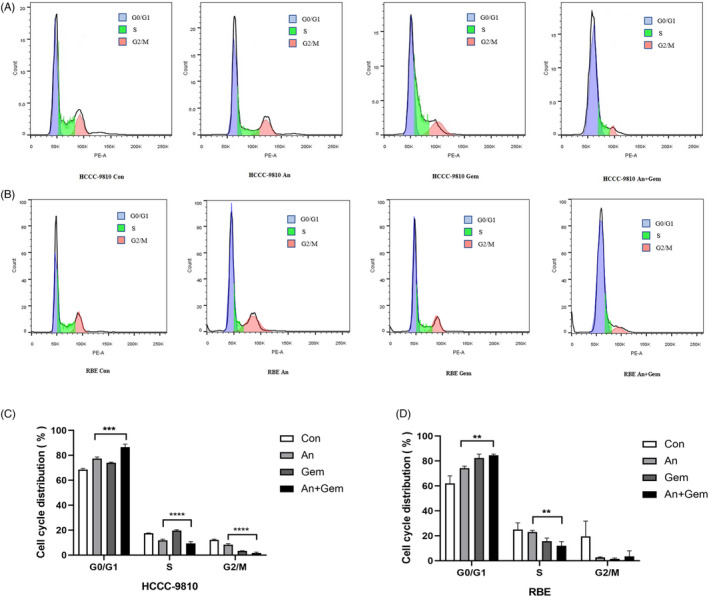
Anlotinib and gemcitabine combination can promote cell cycle arrest in the G0/G1 phase compared with the single drug group. (A, B, C, and D) The ICC cell lines were treated with anlotinib (2 μM) and gemcitabine (0.025 μM) for 48 h, as described above. G0/G1 phase cell cycle arrest was significantly observed in the combination of anlotinib and gemcitabine group compared with the single drug group by FCM. (n = 3, ** p < 0.01, *** p < 0.001, **** p < 0.0001)
4. DISCUSSION
ICC is a highly malignant tumor prone to early metastasis, with short survival and poor prognosis. 20 However, effective therapeutic methods for ICC are not yet well established. Surgery can improve survival in patients with early‐stage ICC; however, most ICC patients are diagnosed at advanced stages. 6 A combined chemotherapeutic regimen of gemcitabine and cisplatin for advanced ICC patients confers a median overall survival of less than 12 months. 4 Unfortunately, survival time was not significantly improved in the past decade. Consequently, the therapeutic effect of chemotherapy for ICC treatment is unsatisfactory, and there is a lack of standard targeted therapeutic drugs. Anlotinib is a multi‐target TKI independently developed in China. The ALTER 0303 study was a multicenter, double‐blinded, phase III randomized clinical trial. 11 Based on the clinical trial, anlotinib has been approved as a third‐line treatment for NSCLC in China. 21 Currently, preclinical studies of other solid tumors are gradually being performed. 17 , 22 However, research on targeted therapy combined with chemotherapy is limited for the treatment of ICC.
Clinical studies have shown that antiangiogenetic‐targeted drugs combined with chemotherapy drugs can improve the efficacy of anti‐tumor therapy. 15 In this study, we found that anlotinib has significant anti‐tumor activity against ICC cell lines. The IC50 value at 24 and 48 h of anlotinib was 45.51 μM (95% CI 38.18–56.16) and 21.33 μM (95% CI 17.7–26.11) in HCCC‐9810 cell line, and 49.37 μM (95% CI 39.25–66.96) and 7.18 μM (95% CI 5.30–9.73) in RBE cell line, respectively. The IC50 value at 24 and 48 h of gemcitabine was 3277862 μM (95% CI 168851–537181042) and 2.08 μM (95% CI 1.08–4.16) in HCCC‐9810 cell line, and 153735 μM (95% CI 12688–13674180) and 4.92 μM (95% CI 1.62–17.34) in RBE cell line, respectively. The significant IC50 difference between HCCC‐9810 and RBE cell lines at 24 h and 48 h of anlotinib and gemcitabine was due to their time‐ and dose‐dependence. The results of this study are consistent with the results of Song et al. study, 23 which provides the scientific basis for this study. However, Song et al. mainly studied the inhibition of proliferation, invasion, and promotion of apoptosis of tumor cells by anlotinib monotherapy through the VEGFR2/PI3K/Akt cell signaling pathway. Our study is the first to investigate the synergistic anti‐tumor effect of the anlotinib multi‐targeted drug combined with gemcitabine chemotherapy. Using Compusyn software, the CI of anlotinib and gemcitabine was less than 1.0 at low concentrations, suggesting the synergism of the two drugs. It is well established that single traditional chemotherapy is not effective. Currently, more researchers focus on targeted drugs combined with chemotherapy to achieve synergistic effects. 15 Interestingly, we found that HCCC‐9810 cell lines were sensitive to gemcitabine, and RBE cell lines were relatively sensitive to anlotinib at 48 h; however, they are less sensitive to the other drug. The combination of the two drugs improved the poor sensitivity of HCCC‐9810 and RBE cell lines to targeted drugs and chemotherapy. Hence, it has a synergistic anti‐tumor effect.
Gemcitabine is a cell cycle‐specific chemotherapeutic and an antimetabolic chemotherapeutic drug. It can arrest the cell cycle in the G1/S phase and inhibit DNA synthesis. 24 We found that anlotinib combined with gemcitabine could promote apoptosis. Previous in vitro experiments showed that anlotinib combined with chemotherapeutic drugs could promote the apoptosis of tumor cells, 8 , 17 which was also confirmed by our results. Western blotting further confirmed that the protein expression rate of cleaved PARP/PARP and cleaved caspase‐3/caspase‐3 was notably increased in the combination group of anlotinib and gemcitabine compared with the monotherapy group, suggesting that the combination of the two drugs promotes apoptosis and has a synergistic effect. Through our research, we found that anlotinib combined with gemcitabine significantly increased the effects of gemcitabine on G0/G1 cell cycle arrest and promoted apoptosis of gemcitabine in the G0/G1 phase for ICC cell lines. Therefore, anlotinib has a synergistic effect in the two‐drug combination regimen.
In summary, the current study demonstrated that anlotinib combined with gemcitabine increased the anti‐tumor effect mainly through cell cycle arrest, promoting the apoptosis of ICC cell lines. Anti‐vascular targeting drugs combined with conventional chemotherapy can improve the sensitivity of the ICC cell lines to chemotherapy. It may be used as an alternative treatment for ICC and has potential clinical application. However, based on the above conclusions, further prospective clinical trials are needed to verify these effects.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
We thank Chia Tai Tianqing Pharmaceutical Group Co., Ltd (Nanjing, China) for kindly providing the multi‐TKI anlotinib.
Fan S, Ge Y, Liu J, et al. Combination of anlotinib and gemcitabine promotes the G0/G1 cell cycle arrest and apoptosis of intrahepatic cholangiocarcinoma in vitro . J Clin Lab Anal. 2021;35:e23986. 10.1002/jcla.23986
DATA AVAILABILITY STATEMENT
All data are included in this article.
REFERENCES
- 1. Olthof SC, Othman A, Clasen S, Schraml C, Nikolaou K, Bongers M. Imaging of cholangiocarcinoma. Visceral Medicine. 2016;32(6):402‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel R, Miller K, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3. Vitale A, Spolverato G, Bagante F, et al. A multi‐institutional analysis of elderly patients undergoing a liver resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2016;113(4):420‐426. [DOI] [PubMed] [Google Scholar]
- 4. Cai Y, Zhang B, Li J, et al. A novel nomogram based on hepatic and coagulation function for evaluating outcomes of intrahepatic cholangiocarcinoma after curative hepatectomy: a multi‐center study of 653 patients. Front Oncol. 2021;11:711061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol. 2017;67(3):632‐644. 10.1016/j.jhep.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao GS, Li WY, Chen DH, et al. A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther. 2011;10(11):2200‐2210. [DOI] [PubMed] [Google Scholar]
- 8. He C, Wu T, Hao Y. Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem Biophys Res Comm. 2018;503(4):3093‐3099. [DOI] [PubMed] [Google Scholar]
- 9. Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third‐line treatment on patients with advanced non‐small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Translational Lung Cancer Research. 2019;8(5):575‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third‐line or further treatment on overall survival of patients with advanced non‐small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. Jama Oncol. 2018;4(11):1569‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C, An L, Cheng Y, Luo X, Li Z, Liu X. Clinical outcomes and prognosis factors of nivolumab plus chemotherapy or multitarget tyrosine kinase inhibitor in multi‐line therapy for recurrent hepatitis B virus‐related hepatocellular carcinoma: a retrospective analysis. Front Oncol. 2020;10:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang Q, Xu J, Qiang H, et al. EGFR Tyrosine Kinase Inhibitor (TKI) combined with concurrent or sequential chemotherapy for patients with advanced lung cancer and gradual progression after first‐line EGFR‐TKI therapy: a randomized controlled study. Clin Lung Cancer. 2021;22(3):e395‐e404. [DOI] [PubMed] [Google Scholar]
- 14. Chang Q, Xu J, Qiang H, et al. EGFR Tyrosine Kinase Inhibitor (TKI) Combined With Concurrent or Sequential Chemotherapy for Patients With Advanced Lung Cancer and Gradual Progression After First‐Line EGFR‐TKI Therapy: A Randomized Controlled Study. Clin Lung Cancer. 2021;22(3):e395‐e404. [DOI] [PubMed] [Google Scholar]
- 15. Xie XH, Wang F, Lin XQ, et al. Anlotinib plus S‐1 for patients with EGFR mutation‐negative advanced squamous cell lung cancer with PS scores of 2–3 after progression of second‐line or later‐line treatment. Cancer Manag Res. 2020;12:12709‐12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waissi W, Amé JC, Mura C, Noël G, Burckel H. Gemcitabine‐based chemoradiotherapy enhanced by a PARP inhibitor in pancreatic cancer cell lines. Int J Mol Sci. 2021;22(13):6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang G, Sun M, Jiang Y, et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. 2019;145(4):979‐993. [DOI] [PubMed] [Google Scholar]
- 18. Miller AL, Garcia PL, Fehling SC, et al. The BET inhibitor JQ1 augments the antitumor efficacy of gemcitabine in preclinical models of pancreatic cancer. Cancers. 2021;13(14):3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torres C, Linares A, Alejandre M, Palomino‐Morales R, Delgado J, Perales S. Interplay between gemcitabine and erlotinib over pancreatic adenocarcinoma cells. Pancreas. 2016;45(2):269‐280. [DOI] [PubMed] [Google Scholar]
- 20. Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long‐term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121(22):3998‐4006. [DOI] [PubMed] [Google Scholar]
- 21. Zhou M, Chen X, Zhang H, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non‐small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond). 2019;39(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruan X, Shi X, Dong Q, et al. Antitumor effects of anlotinib in thyroid cancer. Endocr Relat Cancer. 2019;26(1):153‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song F, Hu B, Cheng JW, et al. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis. 2020;11(7):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian H, Li H, Xie J, et al. Immunity‐related gene signature identifies subtypes benefitting from adjuvant chemotherapy or potentially responding to PD1/PD‐L1 blockage in pancreatic cancer. Front Cell Dev Biol. 2021;9:682261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this article.


