Abstract
Background
Hagenia abyssinica leaves have been used traditionally for the management of different diseases including diabetes mellitus (DM) although the antidiabetic effect of different solvent fractions of hydromethanol H. abyssinica leaf extract has not been scientifically studied. Thus, this study was conducted to investigate the in vivo hypoglycemic, antihyperglycemic and antidyslipidemic effects of the solvent fractions of Hagenia abyssinica leaf extract.
Methods
The antidiabetic effect of the solvent fractions was evaluated in normal, oral glucose loaded and streptozotocin-induced diabetic mice. Hypoglycemic, antihyperglycemic, antidyslipidemic activities and effect on body weight change were evaluated after administration of three different doses of the solvent fractions (100, 200, and 400 mg/kg). One-way ANOVA followed by Tukey's post hoc test was used for data analysis, and p<0.05 was considered as statistically significant.
Results
The crude hydromethanol extract of H. abyssinica leaves did not show any sign of toxicity at the dose of 2000 mg/kg in mice. In normoglycemic mice, both aqueous and ethyl acetate fractions of H. abyssinica leaves showed significant (P<0.05) hypoglycemic activity. In oral glucose loaded mice, the two doses of the aqueous fraction, 200 mg/kg (p<0.05) and 400 mg/kg (p<0.001), showed a significant antihyperglycemic effect at 60 and 120 minute post-oral glucose loading while the ethyl acetate fraction showed significant antihyperglycemic effect at 60 (P<0.05 for 200 mg/kg and P<0.001 for 400 mg/kg) and 120 min (P<0.01 for 400 mg/kg) post-oral glucose loading. In single dose-treated diabetic mice, all doses of the solvent fractions caused a significant (P<0.05) reduction in blood glucose level except 100 mg/kg of the aqueous and chloroform fractions. Additionally, repeated daily treatment with the aqueous fraction significantly reduced hyperglycemia, body weight loss, and improved dyslipidemia of diabetic mice.
Conclusion
This study has revealed that the solvent fractions of H. abyssinica leaves possess in vivo blood-glucose-lowering activities on normal, oral glucose loaded, and streptozotocin-induced diabetic mice. Additionally, the aqueous fraction prevented diabetic body weight loss and dyslipidemia in mice after repeated daily dose administration.
Keywords: Antihyperglycemic, Hagenia abyssinica, Leaves, And streptozotocin
1. Background
Diabetes mellitus is a group of metabolic diseases characterized by glycosuria, negative nitrogen balance, hyperglycemia, and sometimes ketonemia resulting from defects in insulin action, insulin secretion, or both. A chronic hyperglycemic condition is linked to long-term damage and dysfunction of different organs such as eyes, heart and blood vessels, kidneys, and nerves [1,2].
Approximately 70–90% of the population in both developing and developed countries use complementary and alternative medicines for their primary healthcare needs as they are comparatively safer and inexpensive [3]. Despite several clinical investigations ongoing, there are still numerous unmet needs of the public. Thus, further investigations are required for more effective and safer antihyperglycemic agents from herbal medicine. Currently, various plant species including Ajuga remota [4], Satvia rebaudiani [5], Calpurnia aurea [6], Datura stramonium [7], Ajuga integrifolia [8], Aloe pulecherrima [9], Bersama abyssinica [10], Pentas schimperiana [11], Moringa stenopetala [12], Otostegia integrifolia [13], and Caylusea Abyssinica [14] have been investigated and their antidiabetic effect was confirmed using animal models.
Hagenia abyssinica is the sole species of the genus Hagenia which belongs to the family Rosaceae [15]. H. abyssinica is found in Ethiopia, Kenya, Tanzania, Uganda, Sudan, Congo, Malawi, Burundi, and Rwanda [16]. The leaf part of H. abyssinica is traditionally used to treat different ailments in Ethiopia [17,18]. Specifically, it has been used for the treatment of DM in Ethiopia. Ethnobotanical surveys which were carried out in Ethiopia reported that the leaf of the plant is taken orally to treat DM [[19], [20], [21]], but the effect of solvent fractions of the leaves on blood glucose has not been scientifically studied.
Pharmacological investigations showed that plants that belong to the Rosaceae family have insulinomimetic and antidiabetic activities [22,23]. H. abyssinica is one of the plant species in the family, Rosaceae [15], suggesting it may have an anti-diabetic property. Additionally, a previous study revealed that the crude extract and solvent fractions of H. abyssinica leaves have in vitro α-amylase and α-glucosidase inhibitory and antioxidant activities [24]. Similarly, the repeated doses of hydromethanole crude extract of H. abyssinica leaves showed significant in vivo antidiabetic activity in streptozotocin-induced diabetic mice [25].
The antidiabetic activity of medicinal plants is due to the presence of phenolic compounds (anthraquinones, C-glycosylated anthrones, 2-hydroxy-3-methyl-anthraquinone, physcion), flavonoids, terpenoids, alkaloids, glycosides, steroid, peptides, lipids, and other constituents [[26], [27], [28]]. Fortunately, phytochemical screening of the hydromethanol leaf extract of H. abyssinica in a previous study revealed the presence of saponins, tannins, terpenoids, phenols, flavonoids, and steroids [29] which are known to have antidiabetic activity. Accordingly, this study was conducted to investigate the in vivo hypoglycemic, antihyperglycemic, and antidyslipidemic effects of the solvent fractions of the 80% hydromethanol extract of Hagenia abyssinica leaves.
2. Methods
2.1. Plant materials
The fresh leaves of H. abyssinica were collected from Kosoye which is 15 km far from Gondar town (located in Amhara region, northwest Ethiopia) in March 2019. The botanical identification and authentication of the plant material were performed by a botanist Mr. Abiyu Enyew, and a voucher specimen (002ZDK/2019) was deposited in the Herbarium of Biology Department, University of Gondar.
2.2. Experimental animals
Healthy Swiss albino mice (weighing 20–28 g and 6–10 weeks of age) were purchased from Ethiopian Public Health Institute (EPHI), Addis Ababa. The animals were kept in polypropylene cages under standard conditions (12 h light & dark cycle, at room temperature) and were allowed free access to a pellet diet and water ad libtum. Animals were acclimatized to the laboratory conditions for 2 weeks before initiating the experiment. All procedures complied with The Guide for the Care and Use of Laboratory Animals, and the study was approved by the research and ethics committee of Wollo University with a Reference number of WU Phar/116/11.
2.3. Preparation of plant crude extract
The preparation of plant crude extract was carried out according to the methods described by Kifle ZD et al. [30]. The leaves of the plant were thoroughly washed with distilled water to remove dirt and then dried under shade at room temperature with optimal ventilation. The dried leaves were ground into coarse powder using an electrical mill. Then, the coarsely powdered leaves were macerated in 80% methanol for 72 h and then the extract was filtered by using Whatman filter paper No. 1. The marc was re-macerated two times with fresh hydromethanol, each for 72 h, and the filtrates obtained from the successive macerations were concentrated under reduced pressure using a rotary evaporator (Hamato, Japan) followed by a hot air oven (Medit-Medizin Technik, Germany) set at 40 °C. The semi-dried residues were then frozen in a refrigerator overnight and then dried using a lyophilizer (Labfreez, China) to completely remove the aqueous residue. The dried leaf extract was kept in a desiccator until used for the experiment.
2.4. Fractionation of the crude hydromethanol extract
Solvent fractionation of the crude leaf extract was carried out according to the methods described by Kifle ZD et al. [30], using water, ethyl acetate, and chloroform. First, 30 g of the crude 80% methanol extract was suspended in 400 ml of warm water, and the suspension was shaken in a separatory funnel by adding an appropriate amount of chloroform. Then, separation was done based on the principle of liquid-liquid extraction. The addition of the extractive solvent proceeded till the solution becomes clear. The same procedure was used to obtain an ethyl acetate fraction. The chloroform and ethyl acetate fractions were concentrated in a rotary evaporator. Finally, the aforementioned fractions were stored in a refrigerator. On the other hand, the aqueous fraction was concentrated in a lyophilizer and kept in a desiccator until used for the experiment.
2.5. Acute toxicity study
An acute oral toxicity test was carried out for the crude extract of H. abyssinica leaves based on the limit test recommendations of the Organization for Economic Cooperation and Development (OECD) No 425 Guideline. On the first day of the test, one female Swiss albino mouse fasted for 4 h was given 2 g/kg of the extract orally, and the mouse was observed strictly for physical or behavioral changes for one day. Since the sign of toxicity was not observed in the first mouse, other four female mice were recruited on the second day and fasted for 4 h. Then, the mice were given orally a single dose of 2 g/kg of the crude extract, and they were observed strictly in the same manner. The observation was continued for a total of 2 weeks for any sign of toxicity [30,31].
2.6. Grouping and dosing of animals
In all in-vivo experimental studies (normoglycemic, oral glucose loaded, and streptozotocin-induced diabetic mice models) male mice were used [[32], [33], [34]]. In all cases, mice were assigned randomly into different groups of 6 mice each (n=6).
In the normoglycemic and oral glucose loaded mice models, there were a negative control group (groups I) which received distilled water (DW); a positive control group (group II) which received glibenclamide 5 mg/kg (GLC 5 mg/kg); test groups (group III to group V) which received 100 mg/kg, 200 mg/kg and 400 mg/kg of the Aqueous fraction (AQF), respectively. Likewise, test groups (group VI to group VIII) received 100 mg/kg, 200 mg/kg, and 400 mg/kg of the Ethyl acetate fraction (EAF), respectively.
In the single dose-treated diabetic animal model, there were a negative control group (groups I) that received the vehicle, distilled water (DW); a positive control group (group II) which received the standard drug, Glibenclamide 5 mg/kg (GLC 5 mg/kg); and nine test groups (group III-XI) among which group III, IV and V were treated with 100 mg/kg, 200 mg/kg and 400 mg/kg aqueous fraction (AQF), respectively; group VI, VII and VIII were treated with 100 mg/kg, 200 mg/kg and 400 mg/kg of Ethyl acetate fraction (EAF); group IX, X, and XI were treated with 100 mg/kg, 200 mg/kg and 400 mg/kg of Chloroform fraction (CFF), respectively.
In the repeated daily dose-treated diabetic mice model, the animals were randomly divided into six groups (five groups of diabetic mice and 1 group of normal mice). Group I (diabetic mice) was used as diabetic control (DC) which was treated with distilled water (DW), Group II (positive control) received a standard drug, glibenclamide 5 mg/kg (GLC 5 mg/kg), Group III-V (test groups) received different doses of aqueous fraction (100 mg/kg, 200 mg/kg and 400 mg/kg AQF), and Group VI (non-diabetic mice) was used as normal control (NC) that received distilled water (DW) [14,35].
Plant extract doses to be administered were determined based on the result of the acute toxicity study. The volume of administration was 1 ml/100 g of body weight of the mouse [31]. The middle dose was one-tenth of the limit dose, the higher dose was twice the middle dose, and the lower dose was calculated as half of the middle dose. Glibenclamide was selected as a standard drug for the study based on earlier studies [14]. The study was conducted using the oral route of administration because the plant leaves are traditionally used by people via the oral route [[19], [20], [21]].
2.7. Evaluation of the effect of solvent fractions on blood glucose level of normoglycemic mice
To evaluate the effect of the solvent fractions on blood glucose level of normoglycemic mice, we followed the methods of Kifle ZD et al. [30]. Healthy normoglycemic mice were fasted overnight for 14 h but with free access to water. Fasted mice were randomly divided into five different groups (6 mice per group) and treated as described above. Using aseptic conditions, blood samples were collected from the tips of the tail of each mouse to determine blood glucose level (BGL) just before treatment (at 0 h) as a baseline, and then at 1-, 2-, 4-, and 6-h post-treatment.
2.8. Evaluation of the effect of solvent fractions on oral glucose tolerance test (OGTT)
To evaluate the effect of solvent fractions on oral glucose tolerance test, we followed the methods of Kifle ZD et al. [30]. Mice were grouped as described above and the baseline blood glucose level was measured just before administration of treatments. Then, 2 g/kg of glucose solution was administered orally to each mouse 30 min after extract administration. Blood sugar level was measured for each animal at 30, 60, and 120 min following glucose administration.
2.9. Induction of experimental diabetes
In this experimental study, diabetes was induced using streptozotocin (STZ). The drug was dissolved in 0.1 M citrate buffer (pH=4.5). The solution was then administered intraperitoneally at 150 mg/kg dose to mice which were fasted overnight for 14 h before administration. Six hours after the administration of STZ, animals were allowed to drink 5% glucose solution for the next 24 h. Seventy-two hours later, animals were screened for diabetes. Mice that showed fasting blood glucose levels> 200 mg/dl were included in the study as diabetic mice [[36], [37], [38]].
2.10. Antidiabetic activity of a single dose of the solvent fractions in STZ-induced diabetic mice
To assess the antidiabetic activity of a single dose of the solvent fractions in STZ-induced diabetic mice, we followed the methods of Kifle ZD et al. [30]. Following overnight fasting for 14 h, STZ-induced diabetic mice were assigned randomly into 5 groups. Then, distilled water, glibenclamide, and solvent fractions were administered to each group as described above. Blood glucose level was measured just before treatment (at 0 h) as a baseline, and then at 2, 4, 6, and 8-h post-treatment.
2.11. Antidiabetic activity and effect on body weight of repeated dose of the solvent fraction in STZ-induced diabetic mice
To evaluate the antidiabetic activity and effect on body weight of repeated dose of the solvent fraction in STZ-induced diabetic mice, we followed the methods of Kifle ZD et al. [30]. In the STZ-induced diabetic mice model, the mice were randomly grouped into six groups. Then, the distilled water, glibenclamide, and each solvent fraction were administered once daily for 14 days. After overnight fasting (14 h), fasting blood glucose level and body weight of mice were measured just before starting treatment (day 0) and then at the 7th and 14th day of treatment [12,39].
2.12. Effect of the solvent fraction on serum lipid level of diabetic mice
To assess the effect of the solvent fraction on serum lipid level of diabetic mice, we followed the methods of Kifle ZD et al. [30]. On day 15, using a sterile tube blood samples were collected through cardiac puncture under halothane anesthesia from the overnight fasted (14 h) diabetic mice. The blood samples were kept at room temperature for about 2 h and then centrifuged. Then, the supernatant was immediately separated from the pellet to prepare serum samples to determine the serum level of total cholesterol, triglyceride, low-density lipoprotein, and high-density lipoprotein.
2.13. Statistical analysis
Statistical analysis was performed by using a statistical package for social sciences (SPSS) version 24 software. Between and within-group analyses were carried out using one-way ANOVA, followed by Tukey's multiple comparison tests. P values < 0.05 were considered statistically significant.
3. Result
3.1. The percentage yield of plant material extraction
A total of 153 (14.6% w/w) grams of dried hydromethanol crude extract of H. abyssinica leaf was harvested at the end of the extraction process. The yields of the fractions were 47.8% w/w (73.1 g), 29.8% w/w (45.6 g), and 17.5% w/w (26.8 g), for the aqueous, ethyl acetate, and chloroform fractions, respectively.
3.2. Acute toxicity test
The leaf crude extract of H. abyssinica did not show any sign of toxicity at the dose of 2 g/kg in mice. Thus, the median lethal dose (LD50) of the extract is greater than 2 g/kg.
3.3. Hypoglycemic activity of the solvent fractions in normoglycemic mice
After administering the solvent fractions and glibenclamide to the mice, the BGL was measured just before treatment (at 0 h) as a baseline, and then at 1-, 2-, 4-, and 6-h post-treatment. Based on the findings designated in Table 1, there was a significant reduction in BGL at the 1st hour post administration of the standard drug (P<0.001) and the higher doses of the solvent fractions (AQF 400 mg/kg, P<0.05 and EAF 400 mg/kg, P<0.05) when compared to the negative control. At 2 h, AQF 400 mg/kg (P<0.05), EAF 200 mg/kg (P<0.05), EAF 400 mg/kg (P<0.01), and GLC 5 mg/kg (P<0.001) showed significant BGL reduction compared to the negative control. A statistical significant BGL reduction was observed at 4 h post-administration of AQF 100 mg/kg (P<0.05), AQF 200 mg/kg (P<0.01), AQF 400 mg/kg (P<0.001), EAF 200 mg/kg (P<0.001), EAF 400 mg/kg (P<0.001) and GLC 5 mg/kg (P<0.001) when compared to the negative control. Likewise, at 6 h, AQF 100 mg/kg (P<0.01), AQF 200 mg/kg (P<0.001), AQF 400 mg/kg (P<0.001), EAF 100 mg/kg (P<0.05), EAF 200 mg/kg (P<0.001), EAF 400 mg/kg (P<0.001), and GLC 5 mg/kg (P<0.001) showed significant BGL reduction compared to the negative control group. The largest dose of both solvent fractions (400 mg/kg) showed the fastest hypoglycemic activity post-treatment, but the lowest dose of the fractions showed delayed action (Table 1).
Table 1.
Hypoglycemic activity of the solvent fractions in normoglycemic mice.
| Group | Blood glucose level (mg/dl) |
||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 4 h | 6 h | |
| DW 10 ml/kg | 117.00 ± 5.01 | 118.83 ± 4.00 | 115.17 ± 4.97 | 117.83 ± 2.32 | 114.67 ± 3.06 |
| GLC 5 mg/kg | 112.83 ± 3.30 | 81.17 ± 2.71a3,β3 | 64.83 ± 3.12a3,β3 | 59.00 ± 3.39a3,β3 | 52.33 ± 2.57a3,β3 |
| AQF 100 mg/kg | 107.83 ± 4.29 | 108.67 ± 4.08n3 | 99.67 ± 1.56n3 | 96.50 ± 4.23a1,n3 | 87.00 ± 6.21a2,β1,n3 |
| AQF 200 mg/kg | 120.00 ± 2.98 | 112.17 ± 3.66n3 | 96.50 ± 4.98n3β2 | 89.17 ± 5.42a2,β3,n3 | 80.00 ± 4.25a3,β3,n2 |
| AQF 400 mg/kg | 116.00 ± 4.70 | 99.33 ± 5.30a1 | 92.83 ± 4.41a1,β2,n3 | 83.17 ± 4.51a3,β3,n1 | 79.33 ± 5.19a3,β3,n1 |
| EAF 100 mg/kg | 114.67 ± 6.41 | 110.83 ± 5.04n3 | 105.50 ± 3.96n3 | 97.50 ± 4.62n3 | 90.83 ± 7.33a1,n3,β1 |
| EAF 200 mg/kg | 114.50 ± 5.76 | 115.17 ± 3.91n3 | 93.00 ± 4.78a1,n3 | 86.17 ± 5.67a3,n2,β2 | 81.17 ± 5.87a3,n2,β3 |
| EAF 400 mg/kg | 110.17 ± 3.05 | 96.83 ± 4.39a1 | 89.17 ± 5.32a2,n2,β1 | 81.50 ± 6.63a3,n1,β2 | 75.67 ± 4.91a3,n1,β3 |
Each value represents mean ± SEM; n = 6 for each treatment. acompared to the negative control, and β compared to baseline blood glucose level, ncompared to GLC5. 1p < 0.05, 2p < 0.01, and 3p < 0.001. AQF =aqueous fraction, EAF= ethyl acetate fraction, DW = distilled water, and GLC = glibenclamide.
3.4. Antihyperglycemic activity of the solvent fractions on oral glucose loaded mice
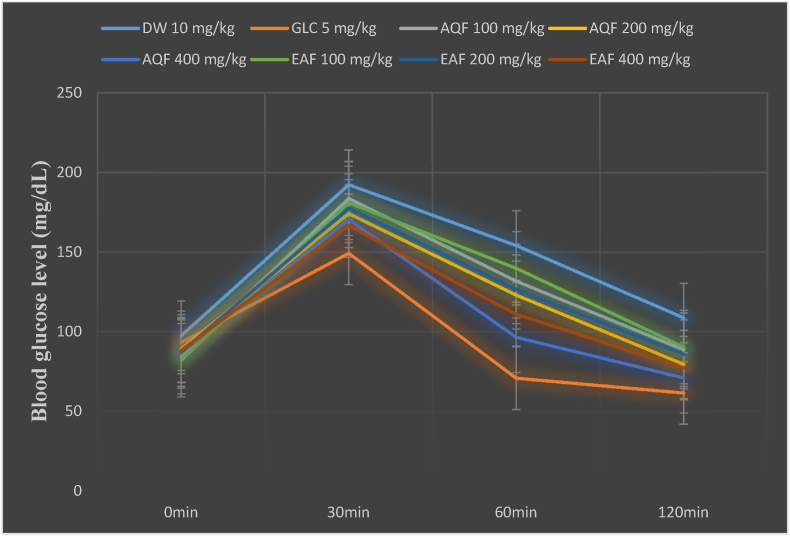
Following oral glucose loading (2 g/kg) to control and test groups, the BGL was raised to the maximum level at 30 min. At 30 min post-oral glucose loading, only the standard drug (GLC, 5 mg/kg) treated group showed a significant (p<0.001) glucose tolerance compared to the negative control group. The two doses of the aqueous fraction, AQF 200 mg/kg (p<0.05) and AQF 400 mg/kg (p<0.001), showed a significant antihyperglycemic effect at 60 and 120 min post-oral glucose loading. Similarly, the ethyl acetate fraction showed a significant antihyperglycemic effect at 60 min (P<0.05 for EAQ 200 mg/kg and P<0.001 for EAF 400 mg/kg) and at 120 min (P<0.01 for EAF 400 mg/kg) post-oral glucose loading. Likewise, GLC 5 mg/kg showed a significant (p<0.001) reduction of BGL at 60- and 120-min post-administration when compared to the negative control group (Fig. 1, Table 2).
Fig. 1.
Effect of Hagenia abyssinica solvent fractions on the blood glucose level of oral glucose loaded mice.
Abbreviations: AQ=aqueous fraction, EAF=ethyl acetate fraction, GLC = glibenclamide, DW= distilled water, and min= minutes.
Table 2.
Effect of the solvent fractions on blood glucose level of oral glucose loaded mice.
| Group | Blood glucose level(mg/dl) |
|||
|---|---|---|---|---|
| 0min | 30min | 60min | 120min | |
| DW 10 mg/kg | 97.50 ± 2.22 | 192.33 ± 11.20 | 154.17 ± 9.68 | 108.67 ± 12.64 |
| GLC 5 mg/kg | 93.33 ± 4.01 | 149.33 ± 8.68a3,β3 | 70.83 ± 12.36a3,β1,μ3 | 61.50 ± 9.87a3,β2,μ3 |
| AQF 100 mg/kg | 84.17 ± 3.03 | 183.67 ± 7.51n2,β3 | 131.83 ± 6.58 β3,μ3 | 88.67 ± 8.34n1,μ3 |
| AQF 200 mg/kg | 89.33 ± 5.01 | 174.17 ± 10.65 β3 | 123.00 ± 11.24a1,β3,μ3 | 79.50 ± 7.54a1,μ3 |
| AQF 400 mg/kg | 86.67 ± 3.46 | 170.50 ± 7.68 β3 | 96.50 ± 5.98a3,μ3 | 71.00 ± 11.36a3,μ3 |
| EAF 100 mg/kg | 82.17 ± 2.78 | 181.00 ± 12.65n2,β3 | 139.83 ± 8.64n3, β3, μ3 | 90.33 ± 5.67n1,μ3 |
| EAF 200 mg/kg | 87.33 ± 3.10 | 177.67 ± 6.59n1,β3 | 126.67 ± 9.54a1,β3,n3,μ3 | 85.83 ± 9.68 μ3 |
| EAF 400 mg/kg | 88.33 ± 1.91 | 166.67 ± 8.64 β3 | 111.00 ± 11.06a3,n3,μ3 | 77.17 ± 12.34a2, μ3 |
Each value represents mean ± SEM; n = 6 for each treatment. acompared to the negative control, μompared to the blood glucose level at 30 minute and βcompared to baseline blood glucose level, ncompared to GLC5. 1p < 0.05, 2p < 0.01, and 3p < 0.001. AQF =aqueous fraction, EAF= ethyl acetate fraction, DW = distilled water, and GLC = glibenclamide.
3.5. Antihyperglycemic activity of the single dose of the solvent fractions of Hagenia abysinica leaves on STZ-induced diabetic mice
The effects of H. abyssinica solvent fractions on BGL of STZ-induced diabetic mice are shown in Table 3. Between and within-group analyses were performed to see BGL differences across the various groups and time points, respectively. The between-group analysis indicated no significant disparity in baseline fasting BGL across all groups. Likewise, there was no significant disparity in BGL at all time points when groups treated with plant extract were compared to each other.
Table 3.
Antihyperglycemic effect of the single dose of the solvent fractions on STZ-induced diabetic mice.
| Group | Blood glucose level (mg/dl) |
||||
|---|---|---|---|---|---|
| 0hr | 2hr | 4hr | 6hr | 8hr | |
| DW 10 mg/kg | 320.33 ± 6.94 | 318.00 ± 9.54 | 326.67 ± 5.67 | 327.33 ± 8.65 | 322.67 ± 5.94 |
| GLC 5 mg/kg | 343.83 ± 11.25 | 268.50 ± 16.54β3 | 205.00 ± 6.89β3,n3 | 180.33 ± 16.54β3,n3 | 166.00 ± 11.25β3,n3 |
| AQF 100 mg/kg | 309.67 ± 9.65 | 292.33 ± 10.36 | 282.17 ± 11.02 | 268.83 ± 11.25 | 262.67 ± 13.52 |
| AQF 200 mg/kg | 325.00 ± 15.32 | 280.33 ± 9.67 | 267.50 ± 5.67β1 | 254.83 ± 19.54β2,n1 | 238.17 ± 9.65β3,n3 |
| AQF 400 mg/kg | 298.17 ± 9.67 | 250.17 ± 12.54β3\1 | 245.50 ± 13.54β2,n1 | 231.17 ± 15.68β3,n3 | 183.00 ± 14.52β3,n3 |
| EAF 100 mg/kg | 327.67 ± 14.25 | 299.17 ± 8.67 | 281.83 ± 9.82 | 255.67 ± 13.24n1 | 247.17 ± 8.69β1,n2 |
| EAF 200 mg/kg | 325.50 ± 11.07 | 267.67 ± 15.64β2 | 255.33 ± 7.68β2,n1 | 247.83 ± 17.36β3,n2 | 242.50 ± 10.36β3,n2 |
| EAF 400 mg/kg | 314.83 ± 8.87 | 257.67 ± 13.54β1 | 245.33 ± 14.25β2,n1 | 236.50 ± 9.58β2,n3 | 224.00 ± 9.58β3,n3 |
| CHF 100 mg/kg | 307.83 ± 10.24 | 287.17 ± 16.54 | 273.50 ± 14.56 | 268.00 ± 15.21 | 262.00 ± 14.27n1 |
| CHF 200 mg/kg | 318.00 ± 9.54 | 286.83 ± 12.34 | 274.67 ± 9.87 | 269.67 ± 9.58 | 255.17 ± 11.38β1,n1 |
| CHF 400 mg/kg | 328.50 ± 7.58 | 286.33 ± 11.58 | 269.67 ± 13.24β1 | 260.67 ± 10.57β2,n1 | 254.17 ± 9.68β3,n1 |
Each value represents mean ± SEM; n=6 for each treatment. nCompared to the negative control, βcompared to baseline blood glucose level. 1p < 0.05, 2p <0.01, and 3p < 0.001, AQF100=aqueous fraction 100 mg/kg, AQF200=aqueous fraction 200 mg/kg, AQF400=aqueous fraction 400 mg/kg, EAF100=ethyl acetate fraction 100 mg/kg, EAF200=ethyl acetate fraction 200 mg/kg, EAF400=ethyl acetate fraction 400mg/kg, CFF100=chloroform fraction 100 mg/kg, CFF200=chloroform fraction 200 mg/kg, CFF400=chloroform fraction 400 mg/kg, DW10=Distilled water 10 ml/kg and GLC5=glibenclamide 5 mg/kg.
The standard drug (GLC 5 mg/kg) produced a significant BGL reduction at the 4th (p<0.05), 6th (p < 0.01), and 8th (p<0.001) hours compared to the negative control. Similarly, significant reduction in BGL was observed with AQF200 at the 6th (p < 0.05), and 8th (p<0.001) hours; AQF400 at the 4th (p<0.05), 6th (p < 0.001), and 8th (p<0.001) hours; EAF100 at the 6th (p < 0.05) and 8th (p<0.01) hours; EAF200 at the 4th (p<0.05), 6th (p < 0.01), and 8th (p<0.01) hours; EAF400 at the 4th (p<0.05), 6th (p < 0.001), and 8th (p<0.001) hours; CHF100 and CHF200 at the 8th (p<0.05) hours; CHF400 at the 6th (p < 0.05), and 8th (p<0.05) hours compared to the negative control. The greatest percent reductions in BGL were recorded as 22.63% in CHF400, 28.85% in EAF400, 38.62% in AQF400, and 51.72% in GLC5 treated groups at the 8th hour compared to the respective baseline fasting BGL level.
The standard drug, GLC5 also produced a significant BGL reduction at the 2nd (p<0.001), 4th (p<0.001), 6th (p < 0.001), and 8th (p<0.001) hours compared to baseline blood glucose level. Similarly, except AQF100 and CHF100, all doses of the solvent fractions produced a significant (p>0.05) BGL reduction at different time points compared to baseline blood glucose level (Table 3).
3.6. Antihyperglycemic activity of repeated daily doses of the aqueous fraction in diabetic mice
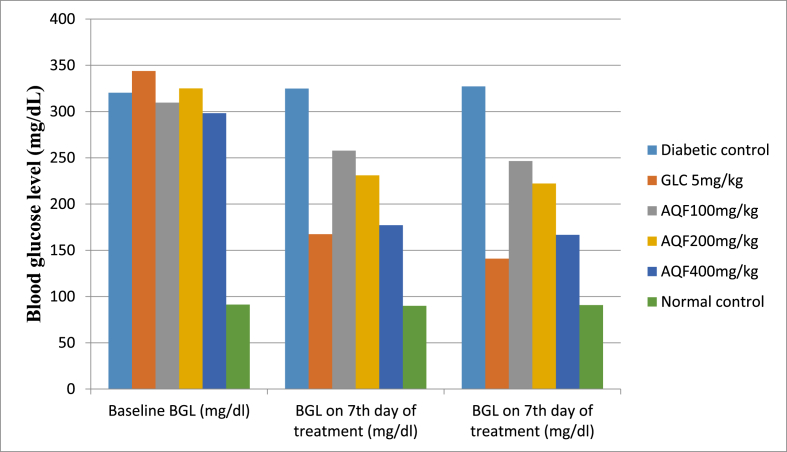
The BGL was measured once weekly in the control and tested group given DW 10 ml/kg, AQF 100, mg/kg, AQF 200, mg/kg, AQF 400, mg/kg, and GLC 5 mg/kg after the induction of DM. The findings are shown in Table 4. Following the induction of experimental DM, diabetic mice displayed significant disparity in BGL when compared to normal mice (p<0.001). However, there was no significant disparity in baseline fasting blood glucose levels across all diabetic mice. Repeated daily dose administration of the aqueous fraction at 100 mg/kg, 200 mg/kg, and 400 mg/kg doses showed a significant (P<0.001) antihyperglycemic effect at the 7th and 14th day of treatment. The largest % reduction in blood glucose level (40.58% on the 7th day and 44.10% on the 14th day) was recorded in 400 mg/kg AQF treated group. Likewise, the standard drug (GLC 5 mg/kg) showed a significant (P<0.001) antihyperglycemic effect on the 7th and 14th day of treatment (Fig. 2, Table 4).
Table 4.
Antihyperglycemic activity of repeated daily doses of the aqueous fraction in diabetic mice.
| Fasting blood glucose level (mg/dl) |
% reduction |
||||
|---|---|---|---|---|---|
| Group | Baseline | 7th day | 14th day | 7th day | 14th day |
| DC | 320.33 ± 10.25 | 324.83 ± 8.26 | 327.17 ± 5.67 | −1.40 | −2.13 |
| GLC 5 mg/kg | 343.83 ± 16.52n3 | 167.50 ± 10.23a3,β3,n3 | 141.00 ± 20.21a3,β3,n3 | 51.28 | 58.99 |
| AQF 100 mg/kg | 309.67 ± 21.25n3 | 257.67 ± 8.52a3,β3,n3 | 246.50 ± 9.54a3,β3,n3 | 16.79 | 20.39 |
| AQF 200 mg/kg | 325.00 ± 14.59n3 | 231.00 ± 14.25a3,β3,n3 | 222.17 ± 11.23a3,β3,n3 | 28.92 | 31.64 |
| AQF 400 mg/kg | 298.17 ± 10.26n3 | 177.17 ± 16.32a3,β3,n3 | 166.67 ± 14.25a3,β3,n3 | 40.58 | 44.10 |
| NC | 91.34 ± 9.54a3 | 89.98 ± 10.23a3 | 90.78 ± 11.36a3 | 1.49 | 0.61 |
Each value represents mean ± SEM; n=6 for each group. acompared to the diabetic control, ncompared to the normal control, and β compared to baseline blood glucose level. 1p<0.05, 2p<0.01, and 3p<0.001, AQF =aqueous fraction, GLC= glibenclamide, DC = Diabetic control, NC = Normal control.
Fig. 2.
Effect of repeated daily doses of Hagenia abyssinica on blood glucose level of diabetic mice.
Abbreviations: AQF= aqueous fraction, GLC = glibenclamide, and BGL = blood glucose level, DC = diabetic control, NC = normal control.
3.7. Effect of repeated daily doses of the aqueous fraction on body weight of diabetic mice
The aqueous fraction at the dose of 400 mg/kg significantly (P<0.001) improved the body weight of diabetic mice on the 7th and 14th day of treatment, whereas lower doses (AQF 100 mg/kg and AQF 200 mg/kg) showed delayed improvement in body weight (P<0.001) at 14th day of treatment as compared to the diabetic control (Table 5).
Table 5.
Effect of repeated daily doses of the aqueous fraction on body weight of diabetic mice.
| Body weight (g) | ||||
|---|---|---|---|---|
| Group | Before induction of DM | Baseline | 7th day of treatment | 14th day of treatment |
| DC | 28.55 ± 0.68 | 27.07 ± 0.67 | 25.58 ± 1.04 | 24.08 ± 0.94 |
| GLC 5 mg/kg | 27.77 ± 1.02 | 26.70 ± 0.57 | 28.97 ± 0.86β1 | 29.46 ± 1.02a3,β2 |
| AQF 100 mg/kg | 29.63 ± 0.67 | 28.15 ± 0.57 | 28.44 ± 0.54 | 29.18 ± 0.34a3 |
| AQF 200 mg/kg | 28.89 ± 0.57 | 27.39 ± 0.39 | 28.39 ± 0.67 | 29.35 ± 0.67a3 |
| AQF 400 mg/kg | 31.88 ± 0.77 | 29.12 ± 0.84 | 31.51 ± 0.33a3, β1 | 31.94 ± 1.21a3,β1 |
| NC | 27.01 ± 1.21 | 26.94 ± 0.37 | 28.67 ± 0.47 | 28.93 ± 0.67a2 |
The result presented as mean ± SEM; n=6 for each group. acompared to the diabetic control, ncompared to the normal control, and β compared to baseline bodyweight. 1p<0.05, 2p<0.01, and 3p<0.001. AQF =aqueous fraction, GLC= glibenclamide, DC= Diabetic control, NC = normal control.
3.8. Effect of repeated daily doses of the aqueous fraction on serum lipid level of diabetic mice
The two doses of the aqueous fraction (AQF100 mg/kg, P<0.05 and AQF 200 mg/kg, P<0.01) significantly reduced LDL-cholesterol after daily treatment for 14 days. Additionally, AQF 400 mg/kg of the aqueous fraction significantly reduced serum level of total cholesterol (P<0.001), triglyceride (P<0.01), very low-density lipoprotein-cholesterol (P<0.05), and low-density lipoprotein-cholesterol (P<0.001) while significantly increasing the high-density lipoprotein-cholesterol (P<0.01) (Table 6).
Table 6.
Effect of repeated daily doses of the aqueous fraction on serum lipid level of diabetic mice.
| Serum lipid level (mg/dl) | |||||
|---|---|---|---|---|---|
| Group | STC(mg/dl) | STG(mg/dl | HDL-c(mg/dl) | VLDL-c(mg/dl) | LDL-c(mg/dl) |
| DC | 179.67 ± 1.35 | 164.83 ± 2.31 | 24.50 ± 0.67 | 34.66 ± 0.68 | 123.01 ± 1.41 |
| GLC5 | 95.33 ± 0.98a3,n1 | 91.83 ± 0.98a3,n1 | 37.17 ± 1.24a3 | 19.67 ± 1.67a3 | 41.65 ± 0.83a3 |
| AQF100 | 173.67 ± 0.67n3 | 158.83 ± 0.97n3 | 26.50 ± 1.37n3 | 33.00 ± 0.66n3 | 115.76 ± 1.07a1,n3 |
| AQF200 | 168.83 ± 0.58n3 | 154.33 ± 1.04n3 | 27.67 ± 0.85n3 | 32.12 ± 0.79n3 | 108.67 ± 0.87a2,n3 |
| AQF400 | 161.83 ± 0.77a3,n3 | 148.83 ± 0.67a2,n3 | 31.17 ± 0.67a2,n3 | 28.87 ± 1.07a1,n3 | 99.76 ± 0.73a3,n3 |
| NC | 88.17 ± 1.21a3 | 86.17 ± 0.85a3 | 40.17 ± 0.79a3 | 18.01 ± 0.83a3 | 30.76 ± 2.34a3 |
Results are expressed in mean ± S.E.M, n = 6; acompared to the diabetic control, ncompared to the normal control; 1p < 0.05, 2p < 0.01, 3p < 0.001; AQF =aqueous fraction, GLC= glibenclamide, DC = diabetic control; NC = normal control; GLC = glibenclamide; STC = serum total cholesterol; STG = serum triglyceride; HDL-c = high-density lipoprotein cholesterol; VLDL-c = very low-density lipoprotein cholesterol; LDL-c = low-density lipoprotein cholesterol.
4. Discussion
In the acute oral toxicity study of the 80% methanolic crude leaf extract of H. abyssinica, there was no mortality or any signs of behavioral changes or toxicity observed after oral administration of the extract at the dose level of 2000 mg/kg in mice. This indicates that the plant has a good safety profile.
Evaluation of the antidiabetic effect of H. abyssinica was done on streptozotocin-induced diabetic mice, which is similar to human DM [40,41]. N-methyl nitro carbamoyl-d-glucosamine popularly known as streptozotocin is a well-known diabetogenic agent for evaluating the antidiabetic activities of medicinal plants in different animal models [42,43]. This compound is a potent methylating agent for DNA and acts as a nitric oxide donor in pancreatic cells. Pancreatic cells are particularly sensitive to damage by nitric oxide (via inhibition of aconitase activity) and free radicals because of their low levels of free radical scavenging enzymes [44]. Basically, STZ induces diabetes by destroying the insulin-secreting pancreatic β-cells, resulting in a decreased insulin secretion. In accordance, findings from the current investigation showed that STZ administration (i.p) at 150 mg/kg effectively induced diabetes mellitus in physiologically normal mice as reflected by hyperglycemia.
Repeated daily dose administration of all doses of the aqueous fraction showed a significant (P<0.001) antihyperglycemic effect at the 7th and 14th day of treatment. The largest % reduction in blood glucose level (40.58% on the 7th day and 44.10% on the 14th day) was induced by 400 mg/kg of the aqueous fraction. In the single-dose STZ-induced diabetic mice model, the solvent fractions of H. abyssinica revealed significant glucose-lowering activity when compared to the negative control group. This finding is in agreement with previous similar studies [2,9,37]. Previous findings revealed that GLC possesses hypoglycemic activity in normal mice through stimulation of pancreatic β-cells to release insulin [45]. It has also been stated that the leaf solvent fraction of H. abyssinica contains terpenes, steroidal compounds, flavonoids, phenolic compounds alkaloids, tannins, and saponins [46], and these phytoconstituents were reported to stimulate insulin release from pancreatic β-cells [47]. Thus, the antihyperglycemic effect of the solvent fraction of H. abyssinica may be due to the availability of any of these secondary metabolites that act synergistically or individually to either mimic insulin action or stimulate insulin secretion.
The oral glucose tolerance test model was employed to evaluate the effect of solvent fractions on glucose metabolism [[48], [49], [50]]. It is reported that a high level of glucose in blood leads to insulin release, and insulin reduces the blood glucose level to normal level in two to 3 h [45,49]. Following glucose loading to all groups of mice, the BGL was increased to the maximum level at 30 minute. The two doses of the aqueous fraction, AQF 200 mg/kg (p<0.05) and AQF 400 mg/kg (p<0.001), showed a significant antihyperglycemic effect at 60 and 120 min post-oral glucose loading. Similarly, the ethyl acetate fraction showed a significant antihyperglycemic effect at 60 min (P<0.05 for EAQ 200 mg/kg and P<0.001 for EAF 400 mg/kg) and at 120 min (P<0.01 for EAF 400 mg/kg) post-oral glucose loading. These findings showed that the improvement in glucose tolerance by the solvent fractions may be because of insulin-sensitizing effect of H. abyssinica possibly through peroxisome proliferator-activated receptor-gamma activation or simulation of β-cells of the pancreas to release insulin leading to increased glucose utilization by peripheral tissues [50,51]. The mechanism involved in decreased postprandial hyperglycemia is inhibition of enzymes like α-amylase and α-glycosidase in the gastrointestinal system which avoids postprandial hyperglycemia [52,53]. In a previous study, the crude extract of H. abyssinica leaves showed significant in vitro α-glucosidase and α-amylase inhibitory activities [24]. The α-glucosidase and α-amylase inhibitors control BGL particularly postprandial BGL by delaying the gastric emptying time and the absorption of fructose and glucose in the gastrointestinal tract [54]. Furthermore, decreasing the rate of starch metabolism may endorse weight loss through reduced accessibility of carbohydrate-derived calories [55]. The other possible mechanism of antidiabetic activity of H. abyssinica on the oral glucose tolerance test may be secondary to the availability of phytoconstituents like flavonoids [56], because flavonoids are known to regenerate pancreatic β-cells, inhibit α glycosidase and glucose transporter in the intestine, and enhance the peripheral glucose utilization [57].
In the normoglycemic mice model, the hypoglycemic effect of the standard drug was apparent due to the inhibition of glucagon secretion and stimulation of insulin release from pancreatic β-cells [58]. The solvent fractions of H. abyssinica might stimulate insulin secretion from β -cells or have an insulin-like effect. Phytoconstituents like tannins and flavonoids are reported to stimulate insulin release from pancreatic β-cells [59].
Dyslipidemia is one of the major complications of diabetes mellitus. Dyslipidemia has been implicated as the major cause of cardiovascular complications like cardiomyopathies and atherosclerosis [[60], [61], [62]]. In this study, the two doses of the aqueous fraction (100 mg/kg, P<0.05 and 200 mg/kg, P<0.01) significantly reduced LDL-cholesterol after daily treatment for 14 days. Additionally, 400 mg/kg of the aqueous fraction significantly reduced serum level of total cholesterol (P<0.001), triglyceride (P<0.01), very low-density lipoprotein-cholesterol (P<0.05), and low-density lipoprotein-cholesterol (P<0.001) while significantly increasing the high-density lipoprotein-cholesterol (P<0.01). Thus, the aqueous fraction induced significant improvement in the lipid profile of the diabetic mice. Moreover, the high dose of the aqueous fraction (400 mg/kg) showed more prominent anti-dyslipidemic activity. This antidyslipidemic effect is directly related to the enhancement of insulin action, in addition to other possible lipid-lowering mechanisms [62].
Previous preliminary phytochemical analysis indicated the leaf crude extract contains saponins, tannins, terpenoids, phenols, flavonoids, glycosides, and anthraquinones [29]. Many secondary metabolites isolated from different plant species have been reported to have potent antidiabetic effect. These secondary metabolites include flavonoids [63,64], sterols/triterpenoids [65], alkaloids, and phenols [66]. The antidiabetic effect might be achieved by facilitating insulin release from pancreatic ß-cells, inhibiting glucose absorption in the gut, stimulating glycogenesis in the liver, and/or increasing glucose utilization by the body [67]. Additionally, phytochemicals are known to induce regeneration of the damaged beta cells and inhibit oxidative stress in beta cells of experimental diabetic rats [68].
This study didn't identify the exact molecular mechanism of antidiabetic activity of the solvent fractions as limitation. Additionally, this study didn't isolate and identify the exact secondary metabolites responsible for the antidiabetic activity.
5. Conclusions
The findings of the present study confirmed that the solvent fractions of Hagenia abyssinica leaves possess antidiabetic activity in normoglycemic, oral glucose loaded and STZ-induced diabetic mice. Additionally, the solvent fractions induced improvement in diabetes associated body weight change and serum lipid profile. Thus, this study validates the traditional use of Hagenia abyssinica in the management of DM.
CRediT authorship contribution statement
Zemene Demelash Kifle: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Roles/Writing – original draft, Writing – review & editing. Alem Endeshaw Woldeyohanin: Formal analysis, Funding acquisition, Investigation. Faisel Dula Sema: Methodology, Project administration, Resources. Simachew Gidey Debeb: Software, Supervision, Validation, Visualization, Writing – original draft, Roles/Writing – original draft, Writing – review & editing. Asmamaw Emagn Kasahun: Software, Supervision, Validation, Visualization, Writing – original draft, Roles/Writing – original draft, Writing – review & editing. Chilot Abiyu Demeke: Methodology, Project administration, Resources. Yaschilal Muche Belayneh: Conceptualization, Data curation.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgment
The authors would like to thank Wollo University for allowing us to use the laboratory facility for the experimental works of the study.
List of abbreviations
- BGL
Blood Glucose Level
- DNSA
3, 5-Dinitrosalicylic Acid
- DM
Diabetes Mellitus
- DPPH
2, 2-Diphenyl-1-Picrylhydrazyl
- OECD
Organization for Economic Cooperation and Development
- OGTT
Oral Glucose Tolerance Test
- STZ
Streptozotocin
Declarations
Ethics Approval:
Ethical clearance was obtained from the research and ethics committee of the department of pharmacy, Wollo University with a Reference number of WU Phar/116/11 to conduct the experiment.
Availability of data and materials
The data used to support the findings of this study are included in the article.
Funding
No funding was received.
References
- 1.Holman N., Young B., Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med: a journal of the British Diabetic Association. 2015;32(9):1119–1120. doi: 10.1111/dme.12791. [DOI] [PubMed] [Google Scholar]
- 2.Kifle Z.D., Enyew E.F. Evaluation of in vivo antidiabetic, in vitro α-amylase inhibitory, and in vitro antioxidant activity of leaves crude extract and solvent fractions of bersama abyssinica fresen (melianthaceae) Journal of Evidence-Based Integrative Medicine. 2020;25 doi: 10.1177/2515690X20935827. 2515690X20935827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson M.M., Zhang X. vol. 3. The world medicines situation; 2011. (Traditional medicines: global situation, issues and challenges). [Google Scholar]
- 4.Assefa F., Seifu D., Makonnen E. Antihyperglycemic and antihyperlipidemic activities of ethanol extract of Ajuga remota Benth (Harmegusa) leaves in streptozotocin induced diabetic rats. African Journal of Pharmacy and Pharmacology. 2017;11(2):17–24. [Google Scholar]
- 5.Bekele T., Hymete A., Tadesse M., Mekonnen Y. Department of Pharmaceutical Chemistry, School of Pharmacy, Addis Ababa University April; 2008. Antidiabetic activity and phytochemical screening of crude extracts of Stevia rebaudiana Bertoni and Ajuga remota Benth grown in Ethiopia on alloxan-induced diabetic mice. [Google Scholar]
- 6.Belayneh Y.M., Birru E.M. Evidence-Based Complementary and Alternative Medicine2018; 2018. Antidiabetic activities of hydromethanolic Leaf Extract of Calpurnia aurea (Ait.) Benth. Subspecies aurea (Fabaceae) in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melaku B.C., Amare G.G. Evaluation of antidiabetic and antioxidant potential of hydromethanolic seed extract of Datura stramonium linn (solanaceae) J Exp Pharmacol. 2020;12:181. doi: 10.2147/JEP.S258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alene M., Abdelwuhab M., Belay A., Yazie T.S. Evidence-Based Complementary and Alternative Medicine2020; 2020. Evaluation of antidiabetic activity of Ajuga integrifolia (Lamiaceae) Root Extract and Solvent Fractions in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amare G.G., Meharie B.G., Belayneh Y.M. Evidence-Based Complementary and Alternative Medicine2020; 2020. Evaluation of antidiabetic activity of the Leaf Latex of Aloe pulcherrima Gilbert and Sebsebe (Aloaceae) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kifle Z.D., Anteneh D.A., Atnafie S.A. Hypoglycemic, anti-hyperglycemic and anti-hyperlipidemic effects of bersama abyssinica fresen (melianthaceae) leaves' solvent fractions in normoglycemic and streptozotocin-induced diabetic mice. J Exp Pharmacol. 2020;12:385. doi: 10.2147/JEP.S273959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinku T., Tadesse S., Asres K. Antidiabetic activity of the leafextracts of Pentas schimperiana subsp. schimperiana (A. Rich) Vatke on alloxaninduced diabetic mice. Ethiop Pharm J. 2010;28:22–26. [Google Scholar]
- 12.Toma A., Makonnen E., Mekonnen Y., Debella A., Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Compl Alternative Med. 2015;15(1):242. doi: 10.1186/s12906-015-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shewamene Z., Abdelwuhab M., Birhanu Z. Methanolic leaf exctract of Otostegia integrifolia Benth reduces blood glucose levels in diabetic, glucose loaded and normal rodents. BMC Compl Alternative Med. 2015;15(1):19. doi: 10.1186/s12906-015-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamiru W., Engidawork E., Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Compl Alternative Med. 2012;12(1):151. doi: 10.1186/1472-6882-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feyissa T. Bruce) JF Gmel; 2006. Micropropagation, transformation and genetic diversity of Hagenia abyssinica. [Google Scholar]
- 16.Beentje H., Adamson J., Bhanderi D. National Museums of Kenya; 1994. Kenya trees, shrubs, and lianas. [Google Scholar]
- 17.Assefa B., Glatzel G., Buchmann C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) JF Gmel. among rural communities of Ethiopia. J Ethnobiol Ethnomed. 2010;6(1):20. doi: 10.1186/1746-4269-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enyew A., Asfaw Z., Kelbessa E., Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. [Google Scholar]
- 19.Lunyera J., Wang D., Maro V., Karia F., Boyd D., Omolo J. Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: an ethnomedical survey. BMC Compl Alternative Med. 2016;16(1):282. doi: 10.1186/s12906-016-1262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekuria A.B., Belachew S.A., Tegegn H.G., Ali D.S., Netere A.K., Lemlemu E. Prevalence and correlates of herbal medicine use among type 2 diabetic patients in Teaching Hospital in Ethiopia: a cross-sectional study. BMC Compl Alternative Med. 2018;18(1):85. doi: 10.1186/s12906-018-2147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habte B.M., Kebede T., Fenta T.G., Boon H. Explanatory models of adult patients with type 2 diabetes mellitus from urban centers of central Ethiopia. BMC research notes. 2016;9(1):441. doi: 10.1186/s13104-016-2248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bnouham M., Ziyyat A., Mekhfi H., Tahri A., Legssyer A. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990-2000) Int J Diabetes Metabol. 2006;14(1):1. [Google Scholar]
- 23.Patel D., Prasad S., Kumar R., Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific journal of tropical biomedicine. 2012;2(4):320. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kifle Z.D., Debeb S.G., Belayneh Y.M. BioMed Research International2021; 2021. Vitro α-amylase and α-Glucosidase Inhibitory and antioxidant activities of the Crude Extract and Solvent Fractions of Hagenia abyssinica Leaves. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kifle Z.D., Belayneh Y.M. Antidiabetic and anti-hyperlipidemic effects of the crude hydromethanol extract of hagenia abyssinica (rosaceae) leaves in streptozotocin-induced diabetic mice. Diabetes, Metab Syndrome Obes Targets Ther. 2020;13:4085. doi: 10.2147/DMSO.S279475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demissew S., Friis I., Awas T., Wilkin P., Weber O., Bachman S. Four new species of Aloe (Aloaceae) from Ethiopia, with notes on the ethics of describing new taxa from foreign countries. Kew Bull. 2011;66(1):111–121. [Google Scholar]
- 27.Grover J., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 28.Vuksan V., Sievenpiper J.L. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metabol Cardiovasc Dis. 2005;15(3):149–160. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Wolde T., Bizuayehu B., Hailemariam T., Tiruha K. Phytochemical analysis and Antimicrobial activity of HAGENIA ABYSSINICA. Indian J Pharm Pharmacol. 2016;3(3):127–134. [Google Scholar]
- 30.Kifle Z.D., Yesuf J.S., Atnafie S.A. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of hagenia abyssinica (rosaceae) J Exp Pharmacol. 2020;12:151. doi: 10.2147/JEP.S249964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.OCDE O. Acute oral toxicity: up and down procedure. OECD Guidel Test Chem. 2008;425:1–2. [Google Scholar]
- 32.Deeds M., Anderson J., Armstrong A., Gastineau D., Hiddinga H., Jahangir A. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furman B.L. Streptozotocin‐induced diabetic models in mice and rats. Current Protocols in Pharmacology. 2015;5.47 doi: 10.1002/0471141755.ph0547s70. 1-5. 20. [DOI] [PubMed] [Google Scholar]
- 34.Vital P.L.E., Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol. 2006;190(2):425–432. doi: 10.1677/joe.1.06596. [DOI] [PubMed] [Google Scholar]
- 35.Tesfaye A., Makonnen E., Gedamu S. Hypoglycemic and anti-hyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharmaceut Sci Res. 2016;7(2):107–113. [Google Scholar]
- 36.Baquer N.Z., Kumar P., Taha A., Kale R., Cowsik S., McLean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36(2):383–396. doi: 10.1007/s12038-011-9042-0. [DOI] [PubMed] [Google Scholar]
- 37.Belayneh Y.M., Birru E.M., Ambikar D. Evaluation of hypoglycemic, antihyperglycemic and antihyperlipidemic activities of 80% methanolic seed extract of Calpurnia aurea (Ait.) Benth.(Fabaceae) in mice. J Exp Pharmacol. 2019;11:73. doi: 10.2147/JEP.S212206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belayneh Y.M., Birru E.M. Vol. 9. Evidence-Based Complementary and Alternative Medicine2018; 2018. (Antidiabetic Activities of Hydromethanolic Leaf Extract of Calpurnia aurea (Ait.) Benth. Subspecies aurea (Fabaceae) in Mice). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S., Choudhary M., Bhardwaj S., Choudhary N., Rana A.C. Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in streptozotocin induced diabetes in albino mice. Bull Fac Pharm Cairo Univ. 2014;52(2):211–217. [Google Scholar]
- 40.Adisa R.A., Choudhary M.I., Olorunsogo O.O. Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in streptozotocin-induced diabetic rats and mice. Exp Toxicol Pathol. 2011;63(7-8):619–625. doi: 10.1016/j.etp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Hammeso W.W., Emiru Y.K., Ayalew Getahun K., Kahaliw W. Evidence-Based Complementary and Alternative Medicine2019; 2019. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimo T., Rakotonirina S.V., Tan P.V., Azay J., Dongo E., Kamtchouing P. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol. 2007;110(3):434–438. doi: 10.1016/j.jep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira H.C., dos Santos M.P., Grigulo R., Lima L.L., Martins D.T., Lima J.C. Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol. 2008;115(3):515–519. doi: 10.1016/j.jep.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Spinas G.A. The dual role of nitric oxide in islet β-cells. Physiology. 1999;14(2):49–54. doi: 10.1152/physiologyonline.1999.14.2.49. [DOI] [PubMed] [Google Scholar]
- 45.Gebreyohannis T., Shibeshi W., Asres K. Effects of solvent fractions of caylusea abyssinica (fresen.) fisch. and mey. on blood glucose levels of normoglycemic, glucose loaded and streptozotocin-induced diabetic rodents. J Nat Remedies. 2013;14(1):67–75. [Google Scholar]
- 46.Kifle Z.D., Kidanu B.B., Tadesse T.Y., Belachew T.F., Atnafie S.A. Evidence-Based Complementary and Alternative Medicine2021; 2021. Evaluation of in vivo antidiarrheal activity of solvent fractions of Hagenia abyssinica (Rosaceae) in Swiss albino mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanko Y., Goji A., Mohammed A., Musa K. Hypoglycaemic effects of the methanolic extract of Aerial part of Chrysanthellum indicum in rats. J Nat Prod Plant Resour. 2011;1(2):1–7. [Google Scholar]
- 48.Tesfaye A., Makonnen E., Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7(2):110–113. [Google Scholar]
- 49.Al-Sadi A.M., Al-Oweisi F.A., Edwards S.G., Al-Nadabi H., Al-Fahdi A.M. Genetic analysis reveals diversity and genetic relationship among Trichoderma isolates from potting media, cultivated soil and uncultivated soil. BMC Microbiol. 2015;15(1):1–11. doi: 10.1186/s12866-015-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagmoti D.M., Kothavade P.S., Bulani V.D., Gawali N.B., Juvekar A.R. Antidiabetic and antihyperlipidemic activity of Pithecellobium dulce (Roxb.) Benth seeds extract in streptozotocin-induced diabetic rats. European Journal of Integrative Medicine. 2015;7(3):263–273. [Google Scholar]
- 51.Chávez-Silva F., Cerón-Romero L., Arias-Durán L., Navarrete-Vázquez G., Almanza-Pérez J., Román-Ramos R. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J Ethnopharmacol. 2018;212:1–7. doi: 10.1016/j.jep.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Tekulu G.H., Araya E.M., Mengesha H.G. In vitro α-amylase inhibitory effect of TLC isolates of Aloe megalacantha baker and Aloe monticola Reynolds. BMC Compl Alternative Med. 2019;19(1):1–7. doi: 10.1186/s12906-019-2622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aloulou A., Hamden K., Elloumi D., Ali M.B., Hargafi K., Jaouadi B. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Compl Alternative Med. 2012;12(1):1–9. doi: 10.1186/1472-6882-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He K., Shi J.-C., Mao X.-M. Safety and efficacy of acarbose in the treatment of diabetes in Chinese patients. Therapeut Clin Risk Manag. 2014;10:505. doi: 10.2147/TCRM.S50362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Celleno L., Tolaini M.V., D'Amore A., Perricone N.V., Preuss H.G. A dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int J Med Sci. 2007;4(1):45. doi: 10.7150/ijms.4.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beshir K., Shibeshi W., Ejigu A., Engidawork E. In-vivo wound healing activity of 70% ethanol leaf extract of Beciumgrandiflorum Lam.(Lamiaceae) in mice. Ethiopian Pharmarmaceutical Journal. 2016;32:117–130. [Google Scholar]
- 57.Jadhav R., Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Group. 2012;1:100g. [Google Scholar]
- 58.Ramkumar K.M., Vanitha P., Uma C., Suganya N., Bhakkiyalakshmi E., Sujatha J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49(12):3390–3394. doi: 10.1016/j.fct.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 59.Tanko AGJ Y., Mohammed A.D.T.A., Musa K.Y. Hypoglycaemic effects of the methanolic extract of aerial part of Chrysanthellum indicuminrats. J Nat Prod Plant Resour. 2011;1:1–7. [Google Scholar]
- 60.Abou-Seif M.A., Youssef A.-A. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346(2):161–170. doi: 10.1016/j.cccn.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 61.Elnasri H., Ahmed A. Patterns of lipid changes among type 2 diabetes patients in Sudan. EMHJ-Eastern Mediterranean Health Journal. 2008;14(2):314–324. 2008. [PubMed] [Google Scholar]
- 62.Ikonen E. Mechanisms for cellular cholesterol transport: defects and human disease. Physiol Rev. 2006;86(4):1237–1261. doi: 10.1152/physrev.00022.2005. [DOI] [PubMed] [Google Scholar]
- 63.Sharma B., Salunke R., Balomajumder C., Daniel S., Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127(2):457–462. doi: 10.1016/j.jep.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Oliver-Bever B. Cambridge university press; 1986. Medicinal plants in tropical West Africa. [Google Scholar]
- 65.Ivorra M., Paya M., Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27(3):243–275. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 66.Kameswara Rao B.G.R., Kesavulu M.M., Appa Rao C. Herbal medicine: in the management of diabetes mellitus. Manphar Vaidhya Patrika. 1997;1(4):33–35. [Google Scholar]
- 67.Sezik E., Aslan M., Yesilada E., Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76(11):1223–1238. doi: 10.1016/j.lfs.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 68.Alexandru V., Balan M., Gaspar A., Coroiu V. Antioxidant activity, phenolics and flavonoid content of some selected Romanian medicinal plants. Planta Med. 2007;73(9):P_261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.