Abstract
Rheumatoid arthritis (RA) and ankylosing spondylitis (AS) belong to the most common inflammatory rheumatic diseases. MicroRNAs (miRNAs) are small 18–22 RNA molecules that function as posttranscriptional regulators. They are abundantly present within extracellular vesicles (EVs), small intercellular communication vesicles that can be found in bodily fluids and that have key functions in pathological and physiological pathways. Recently, EVs have gained much interest because of their diagnostic and therapeutic potential. Using NanoString profiling technology, the miRNA repertoire of serum EVs was determined and compared in RA and AS patients before and after anti-TNF therapy to assess its potential use as a diagnostic and prognostic biomarker. Furthermore, possible functional effects of those miRNAs that were characterized by the most significant expression changes were evaluated using in silico prediction algorithms. The analysis revealed a unique profile of differentially expressed miRNAs in RA and AS patient serum EVs. We identified 12 miRNAs whose expression profiles enabled differentiation between RA and AS patients before induction of anti-TNF treatment, as well as 4 and 14 miRNAs whose repertoires were significantly changed during the treatment in RA and AS patients, respectively. In conclusion, our findings suggest that extracellular vesicle miRNAs could be used as potential biomarkers associated with RA and AS response to biological treatment.
1. Introduction
MicroRNAs (miRNAs, miRs) are a family of single-stranded, noncoding endogenous regulatory RNAs derived from double-stranded precursors, typically composed of 21-23 nucleotides. They are involved in the regulation of gene expression at the posttranscriptional level [1, 2]. They function by the way of complementary binding to the 3′UTR regions of target mRNA via the RNA-induced silencing complex (RISC), resulting in inhibition of the translation process [3]. One miRNA molecule can regulate the expression of several genes (transcripts), and a transcript may have a 3′-UTR region that is recognized by many miRNAs [4, 5]. It is estimated that encoding miRNA genes constitute 1-5% of all the genes in humans and animals [6]. Regulatory miRNAs and exosomes are becoming increasingly important in identification of molecular markers related to pathogenesis and prognosis of disease [7].
Extracellular vesicles (EVs) are lipid membrane-enclosed vesicles, released by cells into the extracellular space. EVs are a heterogeneous collection of exosomes, microvesicles, and apoptotic bodies, ranging in size from 40 nm to 4000 nm. Classification is based on their size, origin, and biological function. EVs are present in blood, saliva, urine, milk, and amniotic fluid and are secreted by all mammalian cell types [8]. Their main function is the transport of lipids, proteins, miRNAs, and mRNAs. Extracellular vesicles are also mediators in intercellular communication and immune-regulatory processes such as bone remodelling, which implicates them in pathogenesis of rheumatic diseases [9]. Although the unique mechanism of immune complex formation remains unclear, it has been observed that synovial exosomes contain citrullinated peptides, which are well-known autoantigens in RA [10]. It has been reported that EVs widely participate in RA development, including antigen presentation and immune complex formation, inflammation, delivery of miRNA, and destruction of extracellular matrix [11]. Moreover, EVs are considered to be promising biomarkers in joint diseases such as RA [12]. Recent studies have indicated a significantly higher level of plasma EVs in RA patients compared to healthy individuals [13]. Other studies established a role of EVs as biomarkers in arthritis by showing an association of exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 with disease activity in RA [14] and identified higher expression of long noncoding RNA, HOX Transcript Antisense RNA (HOTAIR), in serum exosomes of RA cases [15].
Pathogenesis of rheumatoid arthritis (RA) and ankylosing spondylitis (AS) is considered to be multifactorial, with disease susceptibility being associated with genetic, environmental, and stochastic factors. RA and AS are the most common inflammatory rheumatic diseases [16, 17]. They are characterized by different clinical, laboratory, and imaging hallmarks [18, 19]. RA is an autoimmune disease characterized by symmetric and erosive arthritis typically affecting small- and medium-sized joints. It is marked by inflammation of joint synovial tissue, resulting in progression of cartilage and bone tissue damage, ultimately leading to disability [20]. AS is a systemic inflammatory disorder that affects the sacroiliac joints and the spine and can also affect peripheral joints, causing characteristic inflammatory back pain, which can lead to structural and functional impairments [21].
A number of studies have reported that alteration of miRNA profiles may play an important role in the pathogenesis of rheumatic diseases [22, 23], and thus, these profiles may constitute potential biomarkers [24]. Increased expression of miRNAs has been detected in various cell types of RA patients, and miR-146a was shown to mediate negative feedback in the immune response of RA [25]. This specific miRNA was found to be overexpressed in synovial fibroblasts, synovial tissue, synovial fluid monocytes, peripheral blood mononuclear cells, and serum plasma of RA patients [26]. miR-146a regulates gene expression of TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1) in inflammation and participates in a negative feedback loop [27]. Moreover, single-nucleotide polymorphisms (SNPs) in miR-146a may alter its expression. The rs2910164 SNP was found to reduce the expression level of miR-146a, which led to less efficient inhibition of target genes, including two molecules important for RA development, TRAF6 and IRAK1, suggesting that miR-146a rs2910164 could contribute to RA development [28]. Moreover, our previous study in a group of Polish patients with RA showed an association between rs2910164 C variant and higher expression of miRNA-146a in serum after three months of therapy with TNF inhibitors [29].
Despite the fact that anti-TNF treatment constitutes a breakthrough in management of RA and other rheumatic diseases, approximately 30% of patients do not achieve any improvement. Because patients with RA or AS have a variable response to treatment, identification of biomarkers capable of predicting therapeutic response is imperative.
Both RA and AS would benefit from discovery of biomarkers that could be detected when disease is present, distinguish between the two disorders, that are associated with disease progression and outcome, and help to predict the response to treatment.
This study is aimed at investigating whether analysis and comparison of miRNA profiles in patients with RA and AS could be used (i) for detection of diagnostic miRNA markers to distinguish RA from AS and (ii) before and after TNF-α inhibitor treatment to predict the outcome and effectiveness of this biological therapy on modulation of proinflammatory response.
2. Materials and Methods
2.1. Patients
AS patients were classified according to the 1984 modified New York criteria [30]. Adult Caucasians (age ≥ 18 years) included in the study were characterized with high-disease activity (defined as BASDAI ≥ 4 and back pain ≥ 4) before initiation of anti-TNF therapy and failed to respond to at least two nonsteroidal anti-inflammatory drugs (NSAIDs) for at least four weeks at maximum doses (if there were no contraindications). Effectiveness of current drug therapy was assessed using Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is based on a 0-10 scale measuring discomfort, pain, and fatigue.
RA patients enrolled in this project were classified according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria, as well as the presence of active disease represented by the disease activity score in 28 joints (DAS28 ≥ 5.1). Patients qualified for the study were also before biologic agents therapy initiation and failed to respond to at least two disease-modifying antirheumatic drugs (DMARDs). They were ≥18 years, of Caucasian origin, and with complete medical records.
Exclusion criteria for both RA and AS patients comprised clinically significant impairment of hepatic and renal function, coexistence of connective tissue diseases, infections with hepatotropic viruses, infections resistant to therapy, an ongoing history of cancer or uncontrolled diabetes, alcohol abuse, pregnancy or breastfeeding, and insufficient clinical records.
The ASAS/EULAR criteria were used to assess the clinical outcome of anti-TNF treatment.
All RA patients responded to therapy. Good response was a reduction of the DAS28 score ΔDAS28 > 1.2 to a posttreatment value of DAS28 ≤ 3.2, and moderate response was interpreted as ΔDAS28 > 1.2 and a posttreatment DAS28 > 3.2 or 0.6 < ΔDAS28 ≤ 1.2 and DAS28 ≤ 5.1 [31].
For AS, a good response was determined as a reduction of ΔBASDAI ≥ 2.0 from baseline and BASDAI < 3.0 at the endpoint. A moderate response was defined as a reduction of ΔBASDAI ≥ 2.0 from baseline and BASDAI ≥ 3.0 at the endpoint [32].
The study was approved by the Wrocław Medical University Ethics Committee (identification code KB-625/2016), and written informed consent was obtained from all participants.
Clinical characteristics of the patients are presented in Table 1 as mean and standard deviation (± SD).
Table 1.
Characteristics of RA and AS patients included in the study.
| RA (N = 3) | AS (N = 3) | |
|---|---|---|
| Age [years] | 34.67 ± 11.50 | 5 |
| Sex (female/male) | 1/2 | 1/2 |
| Disease duration [years] | 9.333 ± 9.074 | 15.33 ± 15.63 |
| BMI | 27.02 ± 2.728 | 26.47 ± 8.474 |
| VAS baseline [mm] | 82.67 ± 6.429 | 73.00 ± 18.38 |
| VAS after therapy [mm] | 30.00 ± 20.00 | 19.67 ± 11.68 |
| DAS28 baseline | 5.61 ± 0.71 | — |
| DAS28 after therapy | 1.85 ± 1.20 | — |
| BASDAI baseline | — | 7.90 ± 0.36 |
| BASDAI after therapy | — | 2.00 ± 0.87 |
BMI: body mass index; VAS: visual analogue scale; DAS28: disease activity score 28, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; RA: rheumatoid arthritis; AS: ankylosing spondylitis.
2.2. Sample Preparation
Serum samples were collected from three patients (two men and one woman, aged: 23-46, mean age: 35) with RA and three patients (two men and one woman, aged: 36-70, mean age: 48) with AS at two-time points before and after three months of anti-TNF treatment initialization. All RA patients were treated with Etanercept. In the AS group, two patients were treated with Etanercept and one with Adalimumab. Further characteristics of patients enrolled in this study are detailed in Table 1. Sera were prepared from whole blood samples, which had been collected in 8.5 ml BD Vacutainer® SST™ II advance blood tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with clot activator and acrylic gel separation. Samples were centrifuged at 1500 g for 10 min and stored at −70°C.
2.3. EV and RNA Isolation
Extracellular vesicles (EVs) were isolated from 3 ml of serum using Total Exosome Isolation Reagent (from Serum) (ThermoFisher Scientific), following supplier's guidelines. EV pellets were stored at -80°C.
The EV pellets were resuspended in particle-free PBS, and RNA was isolated using Total Exosome RNA and Protein Isolation Kit (ThermoFisher Scientific), following supplier's protocol. RNA was concentrated incorporating Amicon Ultra-0.5 centrifugal filter unit with Ultracel-3 membrane (Merck Millipore), according to NanoString recommendations.
RNA concentration was determined using the 2100 Bioanalyzer and the RNA 6000 Pico Kit (Agilent Technologies). While accurate RIN numbers could not be generated, due to the lack of ribosomal RNA in EVs, RNA concentration and peak area were assessed. All RNA was stored at −80°C.
2.4. EV Morphology and Size Measurement
To find out whether EVs were properly isolated, EV morphology and size distribution were assessed. For EV morphology, transmission electron microscopy (TEM) was used. 300-mesh grids were filmed with Pioloform® resin (SPI Supplies), carbon coated, and plasma etched before use. EVs were directly applied on the grid, stained with 10 μl of 2% aqueous uranyl, and air-dried. Examination was conducted on a Hitachi HT7800 transmission electron microscope equipped with an Emsis Xarosa camera with Radius software, in cooperation with the Electron Microscopy Research Services, Newcastle University.
For EV size distribution analysis, nanoparticle tracking analysis (NTA) was performed employing a NanoSight LM10 microscope supplied with NTA software version 3.0 (NanoSight Ltd., UK). Background extraction with blur settings and maximum jump distance was applied automatically, and 5 × 60 second recordings were taken for each sample.
2.5. miRNA Profiling
Serum EV microRNA expression profiling was performed using the nCounter® Human v3 miRNA Expression Assay kit (NanoString Technologies) as previously described [33]. This code set comprises 98% of microRNA sequences found in miRbase v22 and includes 798 mature microRNAs, six positive and eight negative controls, six ligation controls, and five reference controls. The procedure was performed according to manufacturer's guidelines. Data normalization was performed using nSolver Analysis Software v4.0 (NanoString Technologies), with positive control normalization using the geometric mean and normalization flagging outside the normalization factor range 0.3-3.0. Codeset content normalization was performed using the top 100 microRNAs for normalization, based on geometric mean and flagging outside the normalization factor range 0.1-10.0.
2.6. Statistical Analysis
Data normalization and fold change (FC) expression differences between groups were conducted using nSolver v4.0 software (NanoString Technologies). Further analyses were performed using the R software (version 3.6.1) with RStudio 1.2.5001 (RStudio, Inc., USA) applying an analysis pipeline designed by Newcastle University, Haematological Sciences Department. “ggplot2” (v2.1.0) package function was used to construct Volcano plots. Heatmaps with unsupervised clustering were generated using “gplots” (v2.17.0) and “RColorBrewer” (v1.1-2). p values between the two groups were calculated using a two-tailed t-test. Significance was set at p < 0.05.
2.7. Target Prediction and Pathway Analysis
To perform gene prediction and pathway analyses for miRNAs obtained from NanoString, we incorporated an approach described by Lou et al. [34]. Potential microRNA gene targets were identified by the miRNet database (http://www.mirnet.ca/) [35]. The STRING database (http://string-db.org) was used to construct networks of protein-protein interactions based on target genes obtained from miRNet [36]. Hub genes, or genes from the protein-protein interaction network that have the highest degree of connectivity, were determined using the Cytoscape software (version 3.7.2) [37]. They were subsequently used as input in the KEGG pathway enrichment analysis performed in the Database for Annotation, Visualization and Integrated Discovery (DAVID, http://david.abcc.ncifc http://rf.gov/) [38].
3. Results
3.1. NanoString Experimental Setup and MicroRNA Expression Counts
In this study, serum samples from three RA patients and three AS patients before and after administration of biological treatment were analysed. Samples were labelled according to diagnosis (RA or AS), time points of sample collection: before anti-TNF treatment induction (indicated by odd numbers, for example: RA1 and RA3) and after three month of drug administration (indicated by respective even numbers, RA2 and RA4). This labelling method resulted in the following groups: six RA samples, including three before treatment (RA1, RA3, and RA5) and three after therapy (RA2, RA4, and RA6), and similarly six AS samples, three before treatment (AS1, AS3, and AS5) and additional three samples after three months after treatment initialization (AS2, AS4, and AS6).
All NanoString samples passed quality control parameters according to their microRNA expression profiles. A total of 159 microRNAs were positively expressed in >2 RA and AS samples and were included in the final analysis.
3.2. MicroRNA Expression Analysis in RA EV Samples before vs. after Therapy
Unsupervised hierarchical clustering analysis separated RA serum EV samples before and after three months of anti-TNF treatment as shown in Figure 1(a). EVs from serum collected after three months of anti-TNF therapy were characterized by a unique miRNA signature consisting of four miRNA molecules that were overexpressed (p < 0.05), compared to samples collected before treatment: miR-520 h (4.77-fold, p = 0.038), miR-498 (3.28-fold, p = 0.042), miR-548n (1.66-fold, p = 0.023), and miR-19b-3p (1.35-fold, p = 0.047) (Figures 1(b) and 1(c), Table 2).
Figure 1.
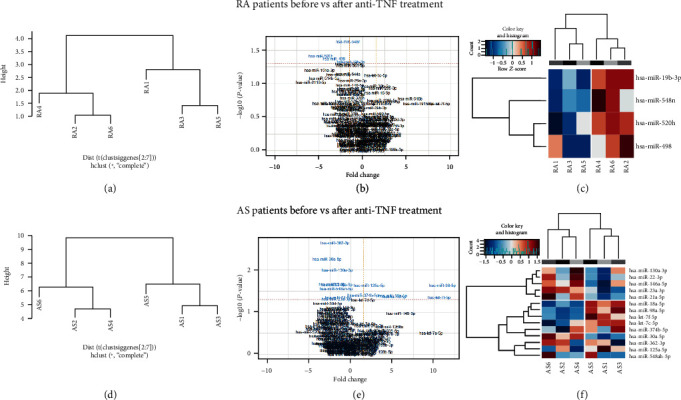
Serum EV microRNA expression in RA and AS patients before vs. after anti-TNF treatment. (a, d) Unsupervised hierarchical clustering analysis. Samples before (1, 3, and 5) vs. after (2, 4, and 6) anti-TNF therapy. (b, e) Volcano plots showing the relationship between fold change and significance for RA and AS patients before and after anti-TNF therapy. The horizontal dashed line indicates cutoff for significance p < 0.05 (−log10 p value > 1.3) and the vertical lines for fold change ≥ 1.5/≤−1.5. Significantly different miRNAs are highlighted in blue. (c) Heatmaps showing unsupervised hierarchical clustering of differentially expressed miRNAs in serum EVs of patients before (n = 3) vs. after Etanercept treatment. The colour scale indicates relative fold change (red: high; blue: low).
Table 2.
Comparison of differentially expressed miRNAs in RA and AS patients before and after three months of the anti-TNF treatment. Statistical characteristics of depicted miRNAs. FC: fold change.
| RA samples before vs. after therapy | AS samples before vs. after therapy | RA vs. AS patients before anti-TNF treatment | RA vs. AS patients after anti-TNF treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| miRNA | p | FC | miRNA | p | FC | miRNA | p | FC | miRNA | p | FC |
| hsa-miR-19b-3p | 0.047 | -1.35 | hsa-let-7c-5p | 0.042 | 5.21 | hsa-miR-125a-5p | 0.028 | 4.31 | hsa-let-7e-5p | 0.005 | 6.13 |
| hsa-miR-548n | 0.023 | -1.66 | hsa-let-7f-5p | 0.044 | 10.61 | hsa-miR-130b-3p | 0.031 | 4.91 | hsa-let-7i-5p | 0.004 | -1.77 |
| hsa-miR-520 h | 0.038 | -4.77 | hsa-miR-125a-5p | 0.023 | 2.23 | hsa-miR-151a-5p | 0.012 | 11.58 | hsa-miR-125a-5p | 0.020 | 6.00 |
| hsa-miR-498 | 0.042 | -3.28 | hsa-miR-130a-3p | 0.010 | -1.65 | hsa-miR-301a-3p | 0.029 | 4.61 | hsa-miR-145-5p | 0.012 | 12.76 |
| hsa-miR-146a-5p | 0.022 | -1.67 | hsa-miR-324-5p | 0.031 | 5.45 | hsa-miR-151a-3p | 0.027 | 3.42 | |||
| hsa-miR-18a-5p | 0.040 | 5.05 | hsa-miR-376c-3p | 0.018 | -1.08 | hsa-miR-151a-5p | 0.027 | 2.96 | |||
| hsa-miR-21-5p | 0.045 | -1.49 | hsa-miR-378 h | 0.048 | -2.47 | hsa-miR-1915-3p | 0.033 | -2.62 | |||
| hsa-miR-22-3p | 0.022 | -2.22 | hsa-miR-411-5p | 0.037 | -1.77 | hsa-miR-221-3p | 0.021 | 1.62 | |||
| hsa-miR-23a-3p | 0.048 | -1.74 | hsa-miR-548a-5p | 0.008 | -3.50 | hsa-miR-28-3p | 0.044 | 3.04 | |||
| hsa-miR-30a-5p | 0.005 | -2.82 | hsa-miR-548n | 0.001 | -2.11 | hsa-miR-30a-5p | 0.050 | -2.27 | |||
| hsa-miR-362-3p | 0.002 | -1.86 | hsa-miR-548q | 0.013 | -1.95 | hsa-miR-3158-3p | 0.033 | -2.62 | |||
| hsa-miR-374b-5p | 0.040 | 1.48 | hsa-miR-579-3p | 0.029 | -1.60 | hsa-miR-324-5p | 0.019 | 4.56 | |||
| hsa-miR-548a-5p | 0.028 | -1.72 | hsa-miR-379-5p | 0.039 | -2.84 | ||||||
| hsa-miR-98-5p | 0.023 | 10.99 | hsa-miR-496 | 0.030 | -2.83 | ||||||
| hsa-miR-503-5p | 0.012 | 2.90 | |||||||||
| hsa-miR-612 | 0.024 | -3.08 | |||||||||
| hsa-miR-649 | 0.033 | -2.62 | |||||||||
| hsa-miR-98-5p | 0.006 | 7.32 | |||||||||
3.3. MicroRNA Expression Analysis in AS EV Samples before vs. after Therapy
AS samples were similarly evaluated and unsupervised clustering analysis separated serum EV samples taken before and after three months of anti-TNF treatment (Figure 1(d)). Expression profiling analysis identified 14 miRNAs significantly (p < 0.05) differentially expressed in AS after therapy, of which eight were overexpressed: miR-130a-3p, miR-146a-5p, miR-21-5p, miR-22-3p, miR-23a-3p, miR-30a-5p, miR-362-3p, miR-548ah-5p (with FC range 1.49-2.82, p value range: 0.002-0.048), while 6 were downregulated: let-7c-5p, let-7f-5p, miR-125a-5p, miR-18a-5p, miR-374b-5p, and miR-98-5p (FC range: 1.48-10.99, p value range: 0.023-0.044). The greatest fold change (FC = 10.99) was detected for hsa-miR-98-5p (Figures 1(e) and 1(f), Table 2).
3.4. Comparison of RA vs. AS EV miRNA Expression before Anti-TNF Therapy Implementation
To assess whether RA and AS patients can be distinguished based on their serum EV expression profiles, we compared miRNA repertoires at the baseline, before induction of biological treatment (Figure 2(a)). The comparison of RA and AS serum EVs before anti-TNF treatment implementation identified 12 miRNAs with significantly different expression (FC range: 1.08-11.58, p value range: 0.001-0.048) (Figures 2(b) and 2(c)). Among those miRNA molecules, higher expression of miR-125a-5p, miR-130b-3p, miR-151a-5p, miR-301a-3p, and miR-324-5p characterized patients with RA (FC range: 4.31-11.58, p value range: 0.012-0.031), while miR-376c-3p, miR-378h, miR-411-5p, miR-548a-5p, miR-548n, miR-548q, and miR-579-3p were overexpressed in AS patients, but with a smaller FC range (1.08-3.50, p value range: 0.001-0.048) (Figures 2(b) and 2(c), Table 2).
Figure 2.
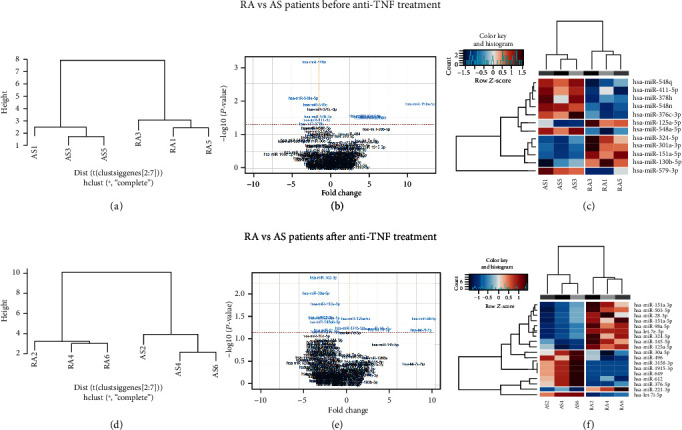
Serum EV microRNA expression in RA vs. AS patients before and three months after anti-TNF treatment. (a, d) Unsupervised hierarchical clustering analysis. RA vs. AS patients before biological treatment initialization and after agent administration. (b, e) Volcano plots showing the relationship between fold change and significance for RA vs. AS patients before and after anti-TNF therapy. The horizontal dashed line indicates cutoff for significance p < 0.05 (−log10 p value > 1.3) and the vertical lines for fold change ≥ 1.5/≤−1.5. Significantly different miRNAs are highlighted in blue. (c) Heatmaps showing unsupervised hierarchical clustering of differentially expressed miRNAs in serum EVs of RA vs. AS patients before (n = 3) and after treatment. The colour scale indicates relative fold change (red: high; blue: low).
3.5. Comparison of RA vs. AS EV miRNA Expression after Therapy
A similar comparison of miRNA repertoires between patients with RA vs. AS was performed on the RA and AS samples collected three months after initialization of anti-TNF treatment. A total of 18 microRNAs were significantly differentially expressed after therapy in RA compared to AS patients (FC range: 1.77-12.76, p value range: 0.004-0.050), of which eight were upregulated in AS (miR-7i-5p, miR-1915-3p, miR-30a-5p, miR-3158-3p, miR-379-5p, miR-496, miR-612, and miR-649; FC range: 1.77-3.08, p value range: 0.004-0.050) (Figures 2(e) and 2(f), Table 2).
Furthermore, miR-30a was upregulated in AS after therapy in comparison to its expression before treatment and its expression in patients with RA after anti-TNF drug administration. On the other hand, miR-98 was downregulated after initialization of anti-TNF therapy in both cases. Interestingly, miR-151a and miR-125a were characterized with lower expression levels in AS patients compared to those with RA after, as well as before biological therapy.
3.6. Identification of Target Genes and Potential Pathways
The potential target genes were analysed separately for upregulated and downregulated microRNAs. 10 hub genes were identified for each of the analysed groups. In RA patients, all upregulated microRNAs after therapy were linked to TP53, EP300, PTEN, MAPK1, STAT3, ESR1, EZH2, CCNB1, BRCA1, and CASP3. In AS patients, both upregulated and downregulated microRNAs after treatment were associated with TP53, AKT1, MYC, UBC, EGFR, and IL6. Additionally, downregulated microRNAs after therapy were linked to UBA52, CCND1, PTEN, and STAT3, whereas the upregulated ones were associated with RPS27A, MAPK1, UBB, and VEGFA. In comparison between RA and AS patients before treatment, downregulated microRNAs were connected to TP53, AKT1, MYC, UBC, EGFR, RPS27A, MAPK1, UBB, HSPA8, and PTEN, while upregulated microRNAs were linked to HSP90AA1, CCNB1, STAT3, CDC5L, MDM2, CASP3, ATM, SKP1, ACTB, and IGF1R. The hub genes detected in comparison between RA and AS patients after therapy linked to downregulated, as well as upregulated microRNAs were TP53, AKT1, MYC, EGFR, and CTNNB1, although downregulated microRNAs were also related to UBC, PTEN, CCND1, HSPA8, and VEGFA and upregulated to MAPK1, JUN, HDAC1, NOTCH1, and MAPK8.
The KEGG pathway enrichment analysis showed that targets of miRNAs identified as upregulated/downregulated in the treatment-related analyses (RA before vs. after, AS before vs. after) were enriched for pathways associated with response to drugs and cellular response to drugs. Additionally, targets of miRNAs upregulated in AS vs. RA (after therapy) and in AS patients after treatment were enriched in pathways associated with the TNF signalling pathway.
4. Discussion
Anti-TNF treatment constitutes a breakthrough in management of rheumatic diseases, although many patients do not achieve significant or any improvement. This makes it essential to find new biomarkers capable of predicting therapeutic response. In the present study, we examined whether serum EV miRNA profiles could be used to distinguish rheumatoid arthritis from ankylosing spondylitis, as well as to predict outcome of TNF inhibitor treatment in both of these rheumatic diseases. We found that in RA patients, four miRNAs (miR-520h, miR-498, miR-548n, and miR-19b-3p) were differentially expressed after anti-TNF treatment, while fourteen were differentially expressed in AS patients (miR-130a-3p, miR-146a-5p, miR-21-5p, miR-22-3p, miR-23a-3p, miR-30a-5p, miR-362-3p, miR-548ah-5p, let-7c-5p, let-7f-5p, miR-125a-5p, miR-18a-5p, miR-374b-5p, and miR-98-5p). Additionally, we identified twelve miRNAs that distinguished the two diseases (miR-125a-5p, miR-130b-3p, miR-151a-5p, miR-301a-3p, miR-324-5p, miR-376c-3p, miR-378h, miR-411-5p, miR-548a-5p, miR-548n, miR-548q, and miR-579-3p).
Recently, there have been many studies investigating EV miRNAs as markers for evaluating RA progression. To date, the association between miRNAs and parameters of disease activity were reported in East Asian populations, specifically in Korean [39] and Chinese [40] RA patients. Wang et al. showed downregulation of exosome-delivered miR-548a-3p in serum of RA patients in contrast with healthy controls, as well as a lower level of miR-548a-3p correlating with higher levels of CRP, RF, and ESR [40]. Similarly, serum exosomal miR-6089 was significantly decreased in RA Chinese patients compared to controls [41]. Regarding plasma exosomes, secretion of miR-17, miR-19b, and miR-121 was significantly higher in RA patients compared to healthy individuals. Moreover, it was found that transport of miR-17 into T cells represses Treg induction and differentiation [42].
MiRNAs delivered by exosomes were also described in the context of their therapeutic potential in RA. miR-150-5p reduced joint destruction by inhibiting angiogenesis mediated by downregulation of matrix metalloproteinase 14 (MMP14) and vascular endothelial growth factor (VEGF) [43]. However, upregulation of exosomal miR-92 boosted bone destruction by blocking apoptosis of fibroblast-like synoviocytes (FLSs) and inflammatory cytokine release [44]. Another microRNA, miR-let-7b, and its ligation to TLR-7 were able to induce joint inflammation through M1 macrophage differentiation [45]. Furthermore, overexpression of miR-221-3p in exosomes isolated from inflamed FLSs might suppress bone formation at erosion sites [46]. These findings provide evidence that exosomal miRNAs can modulate inflammatory responses during RA pathogenesis.
To date, researchers have focused mostly on microRNAs extracted from whole blood, serum/plasma, peripheral blood mononuclear cells (PBMC), and rheumatoid arthritis synovial fibroblasts (RASF) to investigate their role in RA progression [23, 47, 48]. Herein, we present isolated serum exosomes as a novel source of microRNAs. Various techniques can be used to study the miRNA profile. The most common practice is to employ microarray profiling to analyse a wide range of microRNAs on a qRT-PCR instrument [49]. However, a novel technique, NanoString, described previously in a study on serum miRNAs in patients with graft-versus-host disease (GvHD) [33, 50], allows analysis of around 800 microRNAs using digital barcoding technology. This removes the need for reverse transcription or preamplification of RNA, as the technology is able to directly count isolated microRNA molecules. During our study, NanoString was used to assess the repertoire of serum EV microRNAs in RA and AS cases subjected to biological treatment with anti-TNF agents. In the present study, we established for the first time that miR-498, miR-520h, and miR-548n are present in serum EVs of RA patients. Furthermore, expression of miR-7c-5p, miR-7f-5p, miR-362-3p, miR-3746-5p, and miR-548ah-5p has been characterized by us for the first time in AS EVs; in fact, these miRNAs have never been described in any rheumatoid disease at all.
As described in the literature (summary presented in Table 3), miR-155 [51] and miR-146a [52] are among the most widely studied microRNAs in RA and AS patients. Although our analysis did not identify miR-155 expression as significantly different between RA and AS patients, our results regarding miR-146a are consistent with those previously described. Higher expression of miR-146a in RA is well-characterized in multiple components such as synovial fibroblasts [53], CD4+ T cells [54], serum [55], and peripheral blood cells [26]. Moreover, upregulation of miR-146a in various samples from RA patients can distinguish them from osteoarthritis (OA) patients [56]. Interestingly, miR-146a is an NF-κB-dependent gene and controls inflammatory responses through inhibition of IL-1 receptor-associated kinase 1 and TNF receptor-associated factor 6 proteins [25] and downregulation of TLR4/NF-κB pathway [57]. By studying exosomes from rheumatoid arthritis synovial fibroblast cell line, Takamura et al. observed upregulation of miR-146a caused by TNF-α stimulation [58]. A positive correlation between the miR-146a level and IL-1β, IL-6, and TNF-α expression was also observed in AS [59].
Table 3.
MicroRNAs differently expressed in RA and AS patients compared to healthy controls.
| miRNA | Disease | Country | Ethnicity | Source | No. of cases/controls | Investigation method | Changes of miR expression | Target gene | References |
|---|---|---|---|---|---|---|---|---|---|
| miR-146a | RA | Egypt | Arab | Whole blood | 25/25 | qPCR | ↑ | — | [104] |
| RA | Japan | Asian | PBMC | 6/5 | qPCR∗ | ↑ | — | [62] | |
| RA | China | Asian | PBMC | 69/69 | qPCR | ↑ | — | [105] | |
| RA | Egypt | Arab | PBMC | 52/56 | qPCR | ↑ | — | [55] | |
| RA | Egypt | Arab | PBMC | 70/60 | qPCR | ↑ | — | [106] | |
| RA | Canada | — | PBMC | 11/10 | qPCR | ↑ | — | [107] | |
| RA | USA | — | PBMC | 16/9 | qPCR | ↑ | TRAF6, IRAK1 | [26] | |
| RA | Switzerland | European | Serum | 34/16 | qPCR | ↓ | — | [60] | |
| RA | Poland | European | Serum | 13/16 | qPCR | ↓ | — | [29] | |
| RA | USA | European | Plasma | 168/91 | qPCR | ↑ | — | [108] | |
| RA | China | Asian | Plasma | 25/20 | qPCR∗ | ↓ | — | [109] | |
| RA | Japan | Asian | Plasma, synovial fluid | 30/30 | qPCR | ↑ | — | [61] | |
| RA | China | Asian | Synovial tissue | 17/3 | qPCR∗ | ↑ | — | [102] | |
| RA | China | Asian | FLS | 12/10 | qPCR | ↓ | TLR4 | [57] | |
| RA | Japan | Asian | CD4+ T | 33/12 | qPCR | ↑ | FAF1 | [54] | |
| RA | Germany | European | Treg | 61/49 | qPCR | ↓ | STAT1 | [110] | |
| AS | China | Asian | Serum | 70/68 | qPCR† | ↑ | — | [66] | |
| AS | Spain | European | Plasma | 53/57 | qPCR‡ | ↑ | — | [74] | |
| AS | Switzerland | European | Plasma | 24/29 | qPCR∗ | ↓ | — | [69] | |
| AS | China | Asian | Hip capsule | 30/30 | qPCR | ↑ | DKK-1 | [67] | |
| miR-155 | RA | Canada | Native American | Whole blood | 18/12 | qPCR | ↑ | — | [111] |
| RA | Japan | Asian | PBMC | 6/5 | qPCR∗ | ↑ | — | [62] | |
| RA | China | Asian | PBMC | 45/25 | qPCR | ↑ | SOCS1, TNFa, IL-1B | [112] | |
| RA | Canada | — | PBMC | 11/10 | qPCR | ↑ | — | [107] | |
| RA | USA | — | PBMC | 16/9 | qPCR | ↑ | — | [26] | |
| RA | China | Asian | PBMC, FLS | 26/23 | qPCR∗ | ↑ | IKBKE | [113] | |
| RA | Egypt | Arab | Serum | 100/100 | qPCR | ↑ | — | [114] | |
| RA | Japan | Asian | Plasma, synovial fluid | 30/30 | qPCR | ↑ | — | [61] | |
| RA | China | Asian | Plasma | 25/20 | qPCR∗ | ↓ | — | [109] | |
| RA | USA | European | Plasma | 168/91 | qPCR | ↑ | — | [108] | |
| RA | China | Asian | FLS | 89/49 | qPCR | ↑ | FOXO3a | [115] | |
| RA | UK | European | CD14+ T | 9/8 | qPCR∗ | ↑ | — | [116] | |
| RA | Germany | European | Treg | 61/49 | qPCR | ↓ | — | [110] | |
| RA | UK | European | Monocyte | 24/22 | qPCR | ↑ | CCR2, CCR7 | [117] | |
| AS | China | Asian | Serum | 70/68 | qPCR | ↑ | — | [66] | |
| miR-21 | RA | China | Asian | Plasma | 25/20 | qPCR∗ | ↑ | — | [109] |
| RA | Italy | European | Plasma | 28/20 | qPCR | ↓ | — | [94] | |
| RA | China | Asian | CD4+ T, PBMC | 25/20 | qPCR | ↓ | STAT3, STAT5 | [93] | |
| AS | China | Asian | Whole blood | 122/122 | qPCR | ↑ | PDCD4 | [91] | |
| miR-22 | RA | China | Asian | RASF | 40/40 | qPCR | ↓ | SIRT1 | [118] |
| RA | UK | European | Serum | 12/12 | Microarray | ↑ | — | [119] | |
| RA | China | Asian | FLS | 48/30 (OA) | qPCR | ↓ | Cyr61 | [120] | |
| AS | Spain | European | Plasma | 53/57 | qPCR‡ | ↑ | — | [74] | |
| miR-30a | RA | China | Asian | Synovial tissue | 7/12 (OA) | qPCR | ↓ | Beclin-1 | [121] |
| AS | USA | — | Plasma | 15/5 | Microarray | ↓ | — | [98] | |
| miR-125a | RA | China | Asian | Plasma | 25/20 | qPCR∗ | ↓ | — | [109] |
| RA | Japan | Asian | Plasma | 102/104 | qPCR∗ | ↑ | — | [122] | |
| RA | USA | European | Plasma | 168/91 | qPCR | ↑ | — | [108] | |
| AS | Spain | European | Plasma | 53/57 | qPCR‡ | ↑ | — | [74] | |
| miR-221 | RA | Egypt | Arab | PBMC | 30/20 | qPCR | ↑ | — | [123] |
| RA | China | Asian | Serum, FLS | 22/18 | qPCR | ↑ | — | [124] | |
| AS | Switzerland | European | Plasma | 24/29 | qPCR∗ | ↓ | — | [69] | |
| miR-29a | RA | China | Asian | Serum, synovial tissue, FLS | 20/10 | qPCR | ↓ | STAT3 | [87] |
| AS | Switzerland | European | Plasma | 24/29 | qPCR∗ | ↓ | — | [69] |
↑/↓: up/downregulation; RA: rheumatoid arthritis; AS: ankylosing spondylitis; PBMC: peripheral blood mononuclear cell; FLS: fibroblast-like synoviocytes; RASF: RA synovial fibroblasts; ∗microarray screening; †sequencing screening; ‡NanoString screening.
There is some evidence to suggest a role for miR-146a in the course of the disease. Filková et al. characterized a decreased level of circulating miR-146a at an early stage of RA, compared to established disease [60]. Furthermore, the expression of miR-146a correlated positively with disease activity in RA [61, 62]. A baseline level of miR-146a was lower in methotrexate responders than in nonresponders [63]. Regarding anti-TNF therapy, previous studies reported increased levels of serum miR-146a in patients under treatment [29, 64]. Besides, Liu et al. demonstrated the potential predictive value of miR-146a measurement for biological agent therapy outcome in RA patients [65].
Recent studies also highlight a role of miR-146a in AS. Significantly higher expression levels have been found in patient serum samples compared to healthy controls [66]. miR-146a overexpression can also cause inhibition of fibroblast proliferation and osteogenic potential while its knockdown blocked disease progression by regulating Dickkopf Wnt Signalling Pathway Inhibitor 1 (DKK1) expression [67]. Other results suggested a positive correlation between miR-146a expression in peripheral blood mononuclear cells and duration of morning stiffness, ESR, CRP, and BASDAI [68]. Prajzlerová et al. also established the role of miR-146a in the pathogenesis of Axial Spondyloarthritis (AxSpA) [69]. Our current study does not show any significant differences in miR-146a expression between RA patients in two time points (before and after three months of the treatment) or RA group compared to AS. However, we detected upregulation of miR-146a-5p in AS cases after anti-TNF therapy. This observation is consistent with previous results obtained by our group for RA patients [29] where the expression level of miR-146a was lower in serum of patients before therapy compared to that after three months of treatment. Taken together, those conclusions implicate miR-146a as a potential noninvasive biomarker in RA and AS prognosis.
Another microRNA, miR-125b, can also serve as a predictor of anti-TNF therapy response [70]. We identified downregulation of miR-125a-5p in AS patient serum EVs after therapy. In contrast, Castro-Villegas et al. observed that patients responding to anti-TNF/DMARD combination therapy exhibited overexpression of miR125b-5p after treatment and lower RF, CRP, TNFα, IL-17, and IL-6 levels [64]. Previous studies confirmed that the upregulation of miR-125a and miR-125b directly affects NF-κB [71, 72]. Likewise, decreased miR-125a-5p expression enhanced the protein level of TNFRSF1B and reduced osteoclast activity [73].
On the other hand, Perez-Sanchez et al. found that expression levels of miR-146a-5p, miR125a-5p, miR-151-3p, miR-22-3p, and miR-451a were higher in AS patients compared to psoriatic arthritis patients. Furthermore, miR-146a-5p, miR-125a-5p, and miR-22-3p can distinguish the active and nonactive stage of the disease. Expression of miR-125a-5p, miR-151a-3p, miR-150-5p, and miR-451a was also related to the presence of syndesmophytes in AS patients [74]. Although we demonstrated that miR-146a-5p and miR-22-3p were upregulated in AS patient EVs after therapy, miR-125a-5p was downregulated. Moreover, miR-125a-5p was characterized by lower expression in AS patient EVs compared to RA EVs before and after treatment. Similarly, miR-151-5p was decreased in AS EV samples before and after anti-TNF treatment compared to RA samples. These results implicate miR-125a-5p as a potential diagnostic biomarker in AS.
Little is known about miR-130a and miR-301a-3p. In AS patients after biological therapy, we observed an upregulation of miR-130a. A molecular mechanism involving histone deacetylase 3 (HDAC3) was published by Jiang and Wang [75]. A study on miR-130a in human chondrocytes identified its crucial role in regulating TNF-α expression [76]. miR-301a-3p was found to be overexpressed in the PBMCs and associated with Th17 cell frequency in RA patients [77]. We hypothesize that miR-130b-3p and miR301a-3p EV expression may distinguish RA patients from AS patients based on our observation that those microRNAs are higher in RA cases.
To date, publications describing miR-18a referred to osteoarthritis [78, 79], primary Sjögren's syndrome [80], and rheumatoid arthritis [81]. Herein, we demonstrate for the first time downregulation of miR-18a-5p in AS patient EVs after anti-TNF therapy.
We also observed that EVs isolated from sera of RA patients showed overexpression of miR-19b-3p, miR-498, miR-520h, and miR-548n. miR-19b-3p was previously investigated in knee osteoarthritis patients and associated with disease severity [82]. Duan et al. revealed miR-19b-3p involvement in OA through the GRK6-NF-κB pathway [83]. Gantier et al. suggested that miR-19 regulates NF-κB signalling [84]; however, data collected from an experiment with RA FLSs underline the role of miR-19a/b in the stimulation of TLR2 expression [85].
To the best of our knowledge, we showed for the first time that miR-548n and miR-548ah-5p are overexpressed after therapy in RA and AS patients, respectively. Moreover, we identified miR-548n, miR-548ah-5p, and miR-548q as possible biomarkers of AS. Even though no results describing miR-548n, miR-548ah-5p, or miR-548q in rheumatic diseases have been published before, our results are consistent with those reporting about miR-548a-3p, which belongs to the hsa-miR-548 family. miR-548a-3p has previously been shown to be downregulated in serum EVs of RA compared to healthy individuals. Furthermore, expression was negatively correlated with ESR, CRP, and RF levels in patients [40]. In our study, expression of miR-548a-5p was also decreased in RA patients compared to AS patients, before initialization of anti-TNF therapy.
Several studies reported the differential expression of miR-29a and miR-21, making them potentially involved in AS pathogenesis [86–89]. Additionally, overexpression of miR-29a was described in PBMCs from AS patients after etanercept treatment [90]. However, we did not observe differential expression of miR-29a in either RA or AS samples. The analysis showed a significant increase in expression of miR-21 in AS patients after anti-TNF treatment. In contrast, Huang et al. reported greater miR-21 expression in whole blood of patients with AS compared to controls [91]. Recently, Zou et al. showed that upregulation of miR-21 was associated with radiographic severity of AS [92]. The studies regarding RA reported decreased miR-21 level in PBMCs, CD4+ T cells [93], and plasma [94]. Balzano et al. hypothesized that low expression of miR-21-5p was a result of corticosteroids that inhibit NF-κB [94]. Further analyses revealed that miR-21 may be implicated in several signalling pathways such as the IL-34/STAT3/miR-21 pathway, essential for synovial fibroblast survival in RA [95], as well as a mediator between inflammation and bone formation through the JAK2/STAT3 pathway in AS [96]. Additionally, miR-21 plays a role in mediation of RANKL-induced osteoclastogenesis and downregulation of programmed cell death 4 (PDCD4) protein levels [97].
Other microRNA considered to be involved in AS are miR-30a and let-7i. MiR-30a was downregulated in serum/plasma of radiographic axial spondyloarthritis patients compared to healthy individuals [98]. In turn, let-7i was upregulated in T cells [99] and plasma [100] of AS patients compared to controls. In our analysis, both miR-30a-5p and let-7i-5p levels were higher in AS patient EVs after therapy compared to that before treatment and RA cases, respectively. In the study conducted by Lai et al., expression of let-7i in T cells was also positively correlated with the Bath Ankylosing Spondylitis Radiology Index (BASRI) of the lumbar spine, a scoring system which we did not include in our analysis. The authors concluded that overexpression of these microRNAs suppressed TLR-4 expression, which led to downregulation of TLR-4 [99].
The present work is in line with the previous finding identifying miR-23a as a potential predictor of etanercept response [101]. miR-23b can repress IL-17-associated autoimmune inflammation in human fibroblast-like synoviocytes [102]. This observation was confirmed for miR-23a in articular cartilage tissues from RA patients [103].
In summary, to the best of our knowledge, the present study identified for the first time 12 microRNAs differently expressed in serum EVs between RA and AS patients before biological agent administration. miR-125a-5p, miR-130b-3p, miR-151a-5p, miR-301a-3p, and miR-324-5p were upregulated in RA EVs, and miR-376c-3p, miR-378h, miR-411-5p, miR-548a-5p, miR-548n, miR-548q, and miR-579-3p in AS EVs. We believe that these microRNAs have the potential to distinguish RA pathogenesis from AS. The pathway prediction analysis performed using mirPATH v.3 DIANA tools (data not shown) found that all the microRNAs are involved in the Wnt signalling pathway.
5. Conclusions
Our analysis has revealed a unique profile of differentially expressed miRNAs in RA and AS patient serum EVs, both before and three months after anti-TNF treatment administration. These results suggest that EV miRNA profiling of RA and AS patients can be used for detection of diagnostic and predictive biomarkers. They confirm a potential role of these miRNAs in distinguishing the two diseases and in the prediction of response to treatment in RA and AS patients. Nevertheless, the results reported herein should be considered as a pilot study that was conducted on a limited number of patients; therefore, validation in larger verification cohorts is required.
Acknowledgments
This research was funded by the National Science Centre (Poland) (grant number 2016/21/B/NZ5/01901).
Data Availability
All relevant data are within the manuscript. More detailed information cannot be shared publicly because it allows identification of the patients. It can be accessed from the Hirszfeld Institute of Immunology and Experimental Therapy (contact via katarzyna.bogunia-kubik@hirszfeld.pl) for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Filipowicz W., Bhattacharyya S. N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature Reviews. Genetics . 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 2.Satoh J. I., Tabunoki H. Comprehensive analysis of human microRNA target networks. BioData Mining . 2011;4 doi: 10.1186/1756-0381-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell . 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Gulyaeva L. F., Kushlinskiy N. E. Regulatory mechanisms of microRNA expression. Journal of Translational Medicine . 2016;14:p. 143. doi: 10.1186/s12967-016-0893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritz J. V., Heintz-Buschart A., Ghosal A., et al. Sources and functions of extracellular small RNAs in human circulation. Annual Review of Nutrition . 2016;36:301–336. doi: 10.1146/annurev-nutr-071715-050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang H., Huang L., Cao J., Zen K., Chen X., Zhang C.-Y. Regulation of mammalian gene expression by exogenous microRNAs. WIREs RNA . 2012;2012:733–742. doi: 10.1002/wrna.1127. [DOI] [PubMed] [Google Scholar]
- 7.Zakeri Z., Salmaninejad A., Hosseini N., et al. MicroRNA and exosome: key players in rheumatoid arthritis. Journal of Cellular Biochemistry . 2019;120(7):10930–10944. doi: 10.1002/jcb.28499. [DOI] [PubMed] [Google Scholar]
- 8.Pourakbari R., Khodadadi M., Aghebati-Maleki A., Aghebati-Maleki L., Yousefi M. The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sciences . 2019;236:p. 116861. doi: 10.1016/j.lfs.2019.116861. [DOI] [PubMed] [Google Scholar]
- 9.Behera J., Tyagi N. Exosomes: mediators of bone diseases, protection, and therapeutics potential. Oncoscience . 2018;5:181–195. doi: 10.18632/oncoscience.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skriner K., Adolph K., Jungblut P. R., Burmester G. R. Association of citrullinated proteins with synovial exosomes. Arthritis and Rheumatism . 2006;54:3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 11.Withrow J., Murphy C., Liu Y., Hunter M., Fulzele S., Hamrick M. W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Research & Therapy . 2016;18:p. 286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malda J., Boere J., Van De Lest C. H. A., Van Weeren P. R., Wauben M. H. M. Extracellular vesicles - new tool for joint repair and regeneration. Nature Reviews Rheumatology . 2016;12:243–249. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellam J., Proulle V., Jüngel A., et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Research & Therapy . 2009;11:p. R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo J., Lee S. K., Lim M., Sheen D., Choi E. H., Kim S. A. Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Research & Therapy . 2017;19 doi: 10.1186/s13075-017-1334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J., Kim D., Han J., Kim Y., Lee M., Jin E. J. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clinical and Experimental Medicine . 2014;15:121–126. doi: 10.1007/s10238-013-0271-4. [DOI] [PubMed] [Google Scholar]
- 16.Braun J., Sieper J. Ankylosing spondylitis. Lancet . 2007;369:1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 17.Scott D. L., Wolfe F., Huizinga T. W. J. Rheumatoid arthritis. Lancet . 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 18.Michelsen B., Fiane R., Diamantopoulos A. P., et al. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS One . 2015;10, article e0123582 doi: 10.1371/journal.pone.0123582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schett G., Coates L. C., Ash Z. R., Finzel S., Conaghan P. G. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Research & Therapy . 2011;13:p. S4. doi: 10.1186/1478-6354-13-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung S., Kim K., Yang C., Park S., Ju J. Cytokine-mediated bone destruction in rheumatoid arthritis. Journal of Immunology Research . 2014;2014 doi: 10.1155/2014/263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turina M. C., de Winter J. J., Paramarta J. E., et al. Clinical and imaging signs of spondyloarthritis in first-degree relatives of HLA–B27–positive ankylosing spondylitis patients: the pre-spondyloarthritis (pre-SpA) cohort study. Arthritis & Rhematology . 2016;68:2444–2455. doi: 10.1002/art.39766. [DOI] [PubMed] [Google Scholar]
- 22.Alevizos I., Illei G. G. MicroRNAs as biomarkers in rheumatic diseases. Nature Reviews Rheumatology . 2010;6:391–398. doi: 10.1038/nrrheum.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evangelatos G., Fragoulis G. E., Koulouri V., Lambrou G. I. MicroRNAs in rheumatoid arthritis: from pathogenesis to clinical impact. Autoimmunity Reviews . 2019;18:p. 102391. doi: 10.1016/j.autrev.2019.102391. [DOI] [PubMed] [Google Scholar]
- 24.Wielinska J., Bogunia-Kubik K. miRNAs as potential biomarkers of treatment outcome in rheumatoid arthritis and ankylosing spondylitis. Pharmacogenomics . 2021;22:291–301. doi: 10.2217/pgs-2020-0148. [DOI] [PubMed] [Google Scholar]
- 25.Taganov K. D., Boldin M. P., Chang K.-J., Baltimore D. NF-B-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. PNAS . 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauley K. M., Satoh M., Chan A. L., Bubb M. R., Reeves W. H., Chan E. K. L. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Research & Therapy . 2008;10:1–10. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatzikyriakidou A., Voulgari P. V., Georgiou I., Drosos A. A. The role of microRNA-146a (miR-146a) and its target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scandinavian Journal of Immunology . 2010;71:382–385. doi: 10.1111/j.1365-3083.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 28.Jazdzewski K., Murray E. L., Franssila K., Jarzab B., Schoenberg D. R., De La Chapelle A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proceedings of the National Academy of Sciences of the United States of America . 2008;105:7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogunia-Kubik K., Wysoczańska B., Piątek D., Iwaszko M., Ciechomska M., Świerkot J. Significance of polymorphism and expression of miR-146a and NFkB1 genetic variants in patients with rheumatoid arthritis. Archivum Immunologiae et Therapiae Experimentalis (Warsz) . 2016;64:131–136. doi: 10.1007/s00005-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Linden S., Valkenburg H. A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis and Rheumatism . 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 31.Fransen J., van Riel P. L. C. M. The disease activity score and the EULAR response criteria. Clinical and Experimental Rheumatology . 2005;23:S93–S99. doi: 10.1016/j.rdc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Zochling J., Braun J. Assessment of ankylosing spondylitis. Clinical and Experimental Rheumatology . 2005;23(5 Suppl 39):S133–S141. [PubMed] [Google Scholar]
- 33.Crossland R. E., Norden J., Juric M. K., et al. Expression of serum microRNAs is altered during acute graft-versus-host disease. Frontiers in Immunology . 2017;8:1–14. doi: 10.3389/fimmu.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lou W., Liu J., Ding B., et al. Identification of potential miRNA–mRNA regulatory network contributing to pathogenesis of HBV-related HCC. Journal of Translational Medicine . 2019;17 doi: 10.1186/s12967-018-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan Y., Siklenka K., Arora S. K., Ribeiro P., Kimmins S., Xia J. miRNet-dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Research . 2016;44:135–141. doi: 10.1093/nar/gkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szklarczyk D., Franceschini A., Wyder S., et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Research . 2015;43:D447–D452. doi: 10.1093/NAR/GKU1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shannon P. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research . 2003;13:2498–2504. doi: 10.1101/GR.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Da W., Sherman B., Lempicki R. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols . 2009;4(1):44–57. doi: 10.1038/NPROT.2008.211. [DOI] [PubMed] [Google Scholar]
- 39.Yoo J., Lim M.-K., Sheen D.-H. MicroRNA-1915-3P in serum exosome is associated with disease activity of rheumatoid arthritis in Korea. Osteoarthritis and Cartilage. Elsevier BV . 2019;34:p. S100. doi: 10.1016/j.joca.2019.02.145. [DOI] [Google Scholar]
- 40.Wang Y., Zheng F., Gao G., et al. MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis. Journal of Cellular Biochemistry . 2019;120:1133–1140. doi: 10.1002/jcb.26659. [DOI] [PubMed] [Google Scholar]
- 41.Xu D., Song M., Chai C., et al. Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. Journal of Cellular Physiology . 2019;234:1502–1511. doi: 10.1002/jcp.27014. [DOI] [PubMed] [Google Scholar]
- 42.Wang L., Wang C., Jia X., Yu J. Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cellular Physiology and Biochemistry . 2018;50:1754–1763. doi: 10.1159/000494793. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z., Wang H., Xia Y., Yan F., Lu Y. Therapeutic potential of mesenchymal cell–derived miRNA-150-5p–expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. Journal of Immunology . 2018;201:2472–2482. doi: 10.4049/jimmunol.1800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xin Y., Yang Z. S., Fei X. Y., et al. Plasma exosomal MIR-92A are involved in the occurrence and development of bone destruction in ra patients by inhibiting apoptosis of fibroblast-like synoviocytes. Ann Rheum Dis . 2018;77 doi: 10.1136/annrheumdis-2018-eular.5065. [DOI] [Google Scholar]
- 45.Kim S.-j., Chen Z., Essani A. B., et al. Identification of a novel toll-like receptor 7 endogenous ligand in rheumatoid arthritis synovial fluid that can provoke arthritic joint inflammation. Arthritis & Rhematology . 2016;68:1099–1110. doi: 10.1002/art.39544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeda Y., Farina N. H., Matzelle M. M., Fanning P. J., Lian J. B., Gravallese E. M. Synovium-derived microRNAs regulate bone pathways in rheumatoid arthritis. Journal of Bone and Mineral Research . 2017;32:461–472. doi: 10.1002/jbmr.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Churov A. V., Oleinik E. K., Knip M. MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmunity Reviews . 2015;14:1029–1037. doi: 10.1016/j.autrev.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Lam I. K. Y., Chow J. X., Lau C. S., Chan V. S. F. MicroRNA-mediated immune regulation in rheumatic diseases. Cancer Letters . 2018;431:201–212. doi: 10.1016/j.canlet.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 49.Romo-García M. F., Bastian Y., Zapata-Zuñiga M., et al. Identification of putative miRNA biomarkers in early rheumatoid arthritis by genome-wide microarray profiling: a pilot study. Gene . 2019;720:p. 144081. doi: 10.1016/j.gene.2019.144081. [DOI] [PubMed] [Google Scholar]
- 50.Crossland R. E., Perutelli F., Bogunia-Kubik K., et al. Potential novel biomarkers in chronic graft-versus-host disease. Frontiers in Immunology . 2020;11:p. 3374. doi: 10.3389/fimmu.2020.602547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurowska-Stolarska M., Alivernini S., Ballantine L. E., et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proceedings of the National Academy of Sciences of the United States of America . 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smigielska-Czepiel K., van den Berg A., Jellema P., et al. Comprehensive analysis of miRNA expression in T-cell subsets of rheumatoid arthritis patients reveals defined signatures of naive and memory Tregs. Genes and Immunity . 2014;15:115–125. doi: 10.1038/gene.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanczyk J., Leslie Pedrioli D. M., Brentano F., et al. Altered expression of microRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis and Rheumatism . 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Wan Y., Guo Q., et al. Altered microRNA expression profile with miR-146a upregulation in CD4+T cells from patients with rheumatoid arthritis. Arthritis Research & Therapy . 2010;12:p. R81. doi: 10.1186/ar3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayeldeen G., Nassar Y., Ahmed H., Shaker O., Gheita T. Possible use of miRNAs-146a and -499 expression and their polymorphisms as diagnostic markers for rheumatoid arthritis. Molecular and Cellular Biochemistry . 2018;449(1-2):145–156. doi: 10.1007/s11010-018-3351-7. [DOI] [PubMed] [Google Scholar]
- 56.Kriegsmann M., Randau T. M., Gravius S., et al. Expression of miR-146a, miR-155, and miR-223 in formalin-fixed paraffin-embedded synovial tissues of patients with rheumatoid arthritis and osteoarthritis. Virchows Archiv . 2016;469:93–100. doi: 10.1007/s00428-016-1939-4. [DOI] [PubMed] [Google Scholar]
- 57.Liu W., Wu Y.-H., Zhang L., et al. MicroRNA-146a suppresses rheumatoid arthritis fibroblast-like synoviocytes proliferation and inflammatory responses by inhibiting the TLR4/NF-kB signaling. Oncotarget . 2018;9(35):23944–23959. doi: 10.18632/oncotarget.24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takamura Y., Aoki W., Satomura A., Shibasaki S., Ueda M. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation. PLoS One . 2018;13:1–18. doi: 10.1371/journal.pone.0201851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Zhang S., Zhang C., Wang M. LncRNA MEG3 inhibits the inflammatory response of ankylosing spondylitis by targeting miR-146a. Molecular and Cellular Biochemistry . 2020;466:17–24. doi: 10.1007/s11010-019-03681-x. [DOI] [PubMed] [Google Scholar]
- 60.Filková M., Aradi B., Šenolt L., et al. Association of circulating miR-223 and miR-16 with disease activity in patients with early rheumatoid arthritis. Annals of the Rheumatic Diseases . 2014;73:1898–1904. doi: 10.1136/annrheumdis-2012-202815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murata K., Yoshitomi H., Tanida S., et al. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Research & Therapy . 2010;12:p. R86. doi: 10.1186/ar3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niimoto T., Nakasa T., Ishikawa M., et al. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskeletal Disorders . 2010;11:p. 209. doi: 10.1186/1471-2474-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh A., Patro P. S., Aggarwal A. MicroRNA-132, miR-146a, and miR-155 as potential biomarkers of methotrexate response in patients with rheumatoid arthritis. Clinical Rheumatology . 2019;38:877–884. doi: 10.1007/s10067-018-4380-z. [DOI] [PubMed] [Google Scholar]
- 64.Castro-Villegas C., Pérez-Sánchez C., Escudero A., et al. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα. Arthritis Research & Therapy . 2015;17:p. 49. doi: 10.1186/s13075-015-0555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Han Y., Qu H., Fang J., Ye M., Yin W. Correlation of microRNA expression profile with clinical response to tumor necrosis factor inhibitor in treating rheumatoid arthritis patients: a prospective cohort study. Journal of Clinical Laboratory Analysis . 2019;33 doi: 10.1002/jcla.22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qian B.-p., Ji M.-l., Qiu Y., et al. Identification of serum miR-146a and miR-155 as novel noninvasive complementary biomarkers for ankylosing spondylitis. Spine (Phila Pa 1976) . 2016;41:735–742. doi: 10.1097/BRS.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 67.Di G., Kong L., Zhao Q., Ding T. MicroRNA-146a knockdown suppresses the progression of ankylosing spondylitis by targeting dickkopf 1. Biomedicine & Pharmacotherapy . 2018;97:1243–1249. doi: 10.1016/j.biopha.2017.11.067. [DOI] [PubMed] [Google Scholar]
- 68.Wei C., Zhang H., Wei C., Mao Y. Correlation of the expression of miR-146a in peripheral blood mononuclear cells of patients with ankylosing spondylitis and inflammatory factors. Experimental and Therapeutic Medicine . 2017;14:5027–5031. doi: 10.3892/etm.2017.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prajzlerová K., Grobelná K., Hušáková M., et al. Association between circulating miRNAs and spinal involvement in patients with axial spondyloarthritis. PLoS One . 2017;12, article e0185323 doi: 10.1371/journal.pone.0185323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duroux-Richard I., Pers Y.-M., Fabre S., et al. Circulating miRNA-125b is a potential biomarker predicting response to rituximab in rheumatoid arthritis. Mediators of Inflammation . 2014;2014:9. doi: 10.1155/2014/342524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de la Rica L., García-Gómez A., Comet N. R., et al. NF-κB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biology . 2015;16:p. 2. doi: 10.1186/s13059-014-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang B., Wang L. S., Zhou Y. H. Elevated microRNA-125b promotes inflammation in rheumatoid arthritis by activation of NF-κB pathway. Biomedicine & Pharmacotherapy . 2017;93:1151–1157. doi: 10.1016/j.biopha.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 73.Sun L., Lian J. X., Meng S. MiR-125a-5p promotes osteoclastogenesis by targeting TNFRSF1B. Cellular & Molecular Biology Letters . 2019;24 doi: 10.1186/s11658-019-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez-Sanchez C., Font-Ugalde P., Ruiz-Limon P., Lopez-Pedrera C., Castro-Villegas M. C., Abalos-Aguilera M. C. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Human Molecular Genetics . 2018;27:875–890. doi: 10.1093/hmg/ddy008/4797103. [DOI] [PubMed] [Google Scholar]
- 75.Jiang Y., Wang L. Role of histone deacetylase 3 in ankylosing spondylitis via negative feedback loop with microRNA-130a and enhancement of tumor necrosis factor-1α expression in peripheral blood mononuclear cells. Molecular Medicine Reports . 2016;13:35–40. doi: 10.3892/mmr.2015.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z.-C., Han N., Li X., Li G., Liu Y.-Z., Sun G.-X. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. International Journal of Clinical and Experimental Pathology . 2015;8(3):2555–2564. [PMC free article] [PubMed] [Google Scholar]
- 77.Tang X., Yin K., Zhu H., et al. Correlation between the expression of microRNA-301a-3p and the proportion of Th17 cells in patients with rheumatoid arthritis. Inflammation . 2016;39:759–767. doi: 10.1007/s10753-016-0304-8. [DOI] [PubMed] [Google Scholar]
- 78.Ding B., Xu S., Sun X., Gao J., Nie W., Xu H. miR-18a-3p encourages apoptosis of chondrocyte in osteoarthritis via HOXA1 pathway. Curr Molecular Pharmacology . 2020;13 doi: 10.2174/1874467213666200204143740. [DOI] [PubMed] [Google Scholar]
- 79.Li B., Bai L., Shen P., Sun Y., Chen Z., Wen Y. Identification of differentially expressed microRNAs in knee anterior cruciate ligament tissues surgically removed from patients with osteoarthritis. International Journal of Molecular Medicine . 2017;40:1105–1113. doi: 10.3892/ijmm.2017.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang W., Yan X., Xia Q., et al. Predisposition of six well-characterized microRNAs to syndesmophytes among Chinese patients with ankylosing spondylitis. Modern Rheumatology . 2019;29:173–180. doi: 10.1080/14397595.2018.1453277. [DOI] [PubMed] [Google Scholar]
- 81.Trenkmann M., Brock M., Gay R. E., Michel B. A., Gay S., Huber L. C. Tumor necrosis factor α-induced microRNA-18a activates rheumatoid arthritis synovial fibroblasts through a feedback loop in NF-κB signaling. Arthritis and Rheumatism . 2013;65:916–927. doi: 10.1002/art.37834. [DOI] [PubMed] [Google Scholar]
- 82.Kong R., Gao J., Si Y., Zhao D. Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p expressions correlates with risk and disease severity of knee osteoarthritis. American Journal of Translational Research . 2017;9(6):2852–2864. [PMC free article] [PubMed] [Google Scholar]
- 83.Duan L., Duan D., Wei W., et al. MiR-19b-3p attenuates IL-1β induced extracellular matrix degradation and inflammatory injury in chondrocytes by targeting GRK6. Molecular and Cellular Biochemistry . 2019;459:205–214. doi: 10.1007/s11010-019-03563-2. [DOI] [PubMed] [Google Scholar]
- 84.Gantier M. P., Stunden H. J., McCoy C. E., et al. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Research . 2012;40:8048–8058. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Philippe L., Alsaleh G., Suffert G., et al. TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. Journal of Immunology . 2012;188:454–461. doi: 10.4049/jimmunol.1102348. [DOI] [PubMed] [Google Scholar]
- 86.Huang J., Song G., Yin Z., Luo X., Ye Z. Elevated miR-29a expression is not correlated with disease activity index in PBMCs of patients with ankylosing spondylitis. Modern Rheumatology . 2014;24:331–334. doi: 10.3109/14397595.2013.854077. [DOI] [PubMed] [Google Scholar]
- 87.Liu J., Fei D., Xing J., Du J. MicroRNA-29a inhibits proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by repressing STAT3. Biomedicine & Pharmacotherapy . 2017;96:173–181. doi: 10.1016/j.biopha.2017.09.120. [DOI] [PubMed] [Google Scholar]
- 88.Huang J., Song G., Yin Z., Fu Z., Zhang L. Altered expression of microRNAs targeting Dkk-1 in peripheral blood mononuclear cells of patients with ankylosing spondylitis. Cent Eur J Immunol. . 2019;44:59–64. doi: 10.5114/ceji.2019.84018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X., Lv Q., Tu L., et al. Aberrant expression of microRNAs in peripheral blood mononuclear cells as candidate biomarkers in patients with axial spondyloarthritis. International Journal of Rheumatic Diseases . 2019;22:1188–1195. doi: 10.1111/1756-185X.13563. [DOI] [PubMed] [Google Scholar]
- 90.Lv Q., Li Q., Zhang P., et al. Disorders of microRNAs in peripheral blood mononuclear cells: as novel biomarkers of ankylosing spondylitis and provocative therapeutic targets. BioMed Research International . 2015;2015:7. doi: 10.1155/2015/504208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang C.-H., Wei J. C.-C., Chang W.-C., et al. Higher expression of whole blood microRNA-21 in patients with ankylosing spondylitis associated with programmed cell death 4 mRNA expression and collagen cross-linked C-telopeptide concentration. The Journal of Rheumatology . 2014;41:1104–1111. doi: 10.3899/jrheum.130515. [DOI] [PubMed] [Google Scholar]
- 92.Zou Y. C., Gao Y. P., Yin H. D., Liu G. Serum miR-21 expression correlates with radiographic progression but also low bone mineral density in patients with ankylosing spondylitis: a cross-sectional study. Innate Immunity . 2019;25:314–321. doi: 10.1177/1753425919842932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong L., Wang X., Tan J., et al. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. Journal of Cellular and Molecular Medicine . 2014;18:2213–2224. doi: 10.1111/jcmm.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balzano F., Deiana M., Giudici S. D., et al. MicroRNA expression analysis of centenarians and rheumatoid arthritis patients reveals a common expression pattern. International Journal of Medical Sciences . 2017;14:622–628. doi: 10.7150/ijms.18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang S., Jiang S., Wang Y., Tu S., Wang Z., Chen Z. Interleukin 34 upregulation contributes to the increment of microRNA 21 expression through STAT3 activation associated with disease activity in rheumatoid arthritis. The Journal of Rheumatology . 2016;43(7):1312–1319. doi: 10.3899/jrheum.151253. [DOI] [PubMed] [Google Scholar]
- 96.Zou Y. C., Yan L. M., Gao Y. P., Wang Z. Y., Liu G. miR-21 may act as a potential mediator between inflammation and abnormal bone formation in ankylosing spondylitis based on TNF-α concentration-dependent manner through the JAK2/STAT3 pathway. Dose-Response . 2020;18 doi: 10.1177/1559325819901239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sugatani T., Vacher J., Hruska K. A. A microRNA expression signature of osteoclastogenesis. Blood . 2011;117:3648–3657. doi: 10.1182/blood-2010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Magrey M. N., Haqqi T., Haseeb A. Identification of plasma microRNA expression profile in radiographic axial spondyloarthritis—a pilot study. Clinical Rheumatology . 2016;35:1323–1327. doi: 10.1007/s10067-015-3123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai N. S., Yu H. C., Chen H. C., Yu C. L., Huang H. B., Lu M. C. Aberrant expression of microRNAs in T cells from patients with ankylosing spondylitis contributes to the immunopathogenesis. Clinical and Experimental Immunology . 2013;173:47–57. doi: 10.1111/cei.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reyes-Loyola P., Rodríguez-Henríquez P., Ballinas-Verdugo M. A., et al. Plasma let-7i, miR-16, and miR-221 levels as candidate biomarkers for the assessment of ankylosing spondylitis in Mexican patients naïve to anti-TNF therapy. Clinical Rheumatology . 2019;38:1367–1373. doi: 10.1007/s10067-019-04509-1. [DOI] [PubMed] [Google Scholar]
- 101.Cuppen B. V. J., on behalf of all SRU investigators, Rossato M., et al. Can baseline serum microRNAs predict response to TNF-alpha inhibitors in rheumatoid arthritis? Arthritis Research & Therapy . 2016;18:p. 189. doi: 10.1186/S13075-016-1085-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu S., Pan W., Song X., et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nature Medicine . 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 103.Hu J., Zhai C., Hu J., et al. MiR-23a inhibited IL-17-mediated proinflammatory mediators expression via targeting IKKα in articular chondrocytes. International Immunopharmacology . 2017;43:1–6. doi: 10.1016/j.intimp.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 104.Elsayed H. M. A., Khater W. S., Ibrahim A. A., Hamdy M. S. E., Morshedy N. A. MicroRNA-146a expression as a potential biomarker for rheumatoid arthritis in Egypt. Egypt J Med Hum Genet. . 2017;18:173–179. doi: 10.1016/j.ejmhg.2016.07.001. [DOI] [Google Scholar]
- 105.Chen Z. Z., Zhang X. D., Chen Y., Wu Y. B. The role of circulating miR-146a in patients with rheumatoid arthritis treated by Tripterygium wilfordii Hook F. Med (United States) . 2017;96 doi: 10.1097/MD.0000000000006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abou-Zeid A., Saad M., Soliman E. MicroRNA 146a expression in rheumatoid arthritis: association with tumor necrosis factor-alpha and disease activity. Genetic Testing and Molecular Biomarkers . 2011;15:807–812. doi: 10.1089/gtmb.2011.0026. [DOI] [PubMed] [Google Scholar]
- 107.Mookherjee N., El-Gabalawy H. S. High degree of correlation between whole blood and PBMC expression levels of miR-155 and miR-146a in healthy controls and rheumatoid arthritis patients. Journal of Immunological Methods . 2013;400–401:106–110. doi: 10.1016/j.jim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 108.Ormseth M. J., Solus J. F., Vickers K. C., Oeser A. M., Raggi P., Stein C. M. Utility of select plasma microRNA for disease and cardiovascular risk assessment in patients with rheumatoid arthritis. The Journal of Rheumatology . 2015;42:1746–1751. doi: 10.3899/jrheum.150232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H., Peng W., Ouyang X., Li W., Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Translational Research . 2012;160:198–206. doi: 10.1016/j.trsl.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Q., Haupt S., Kreuzer J. T., et al. Decreased expression of miR-146a and miR-155 contributes to an abnormal Treg phenotype in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases . 2015;74:1265–1274. doi: 10.1136/annrheumdis-2013-204377. [DOI] [PubMed] [Google Scholar]
- 111.Anaparti V., Smolik I., Meng X., Spicer V., Mookherjee N., El-Gabalawy H. Whole blood microRNA expression pattern differentiates patients with rheumatoid arthritis, their seropositive first-degree relatives, and healthy unrelated control subjects. Arthritis Research & Therapy . 2017;19:p. 249. doi: 10.1186/s13075-017-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li X., Tian F., Wang F. Rheumatoid arthritis-associated microrna-155 targets socs1 and upregulates TNF-α and IL-1β in PBMCs. International Journal of Molecular Sciences . 2013;14:23910–23921. doi: 10.3390/ijms141223910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Long L., Yu P., Liu Y., et al. Upregulated microRNA-155 expression in peripheral blood mononuclear cells and fibroblast-like synoviocytes in rheumatoid arthritis. Clinical & Developmental Immunology . 2013;2013:1–10. doi: 10.1155/2013/296139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abdul-Maksoud R. S., Sediq A. M., Kattaia A. A. A., et al. Serum miR-210 and miR-155 expression levels as novel biomarkers for rheumatoid arthritis diagnosis. British Journal of Biomedical Science . 2017;74:209–213. doi: 10.1080/09674845.2017.1343545. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y., Feng T., Duan S., et al. miR-155 promotes fibroblast-like synoviocyte proliferation and inflammatory cytokine secretion in rheumatoid arthritis by targeting FOXO3a. Experimental and Therapeutic Medicine . 2020;19:1288–1296. doi: 10.3892/etm.2019.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajasekhar M., Olsson A. M., Steel K. J. A., et al. MicroRNA-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis. Journal of Autoimmunity . 2017;79:53–62. doi: 10.1016/j.jaut.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elmesmari A., Fraser A. R., Wood C., et al. MicroRNA-155 regulates monocyte chemokine and chemokine receptor expression in rheumatoid arthritis. Rheumatol (United Kingdom) . 2016;55:2056–2065. doi: 10.1093/rheumatology/kew272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C., Fang L., Liu X., et al. miR-22 inhibits synovial fibroblasts proliferation and proinflammatory cytokine production in RASF via targeting SIRT1. Gene . 2020;724 doi: 10.1016/j.gene.2019.144144. [DOI] [PubMed] [Google Scholar]
- 119.Ouboussad L., Hunt L., Hensor E. M. A., et al. Profiling microRNAs in individuals at risk of progression to rheumatoid arthritis. Arthritis Research & Therapy . 2017;19:p. 288. doi: 10.1186/s13075-017-1492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin J., Huo R., Xiao L., et al. A novel p53/microRNA-22/Cyr61 axis in synovial cells regulates inflammation in rheumatoid arthritis. Arthritis & Rhematology . 2014;66:49–59. doi: 10.1002/art.38142. [DOI] [PubMed] [Google Scholar]
- 121.Xu K., Xu P., Yao J. F., Zhang Y. G., Hou W. K., Lu S. M. Reduced apoptosis correlates with enhanced autophagy in synovial tissues of rheumatoid arthritis. Inflammation Research . 2013;62:229–237. doi: 10.1007/s00011-012-0572-1. [DOI] [PubMed] [Google Scholar]
- 122.Murata K., Furu M., Yoshitomi H., et al. Comprehensive microRNA analysis identifies miR-24 and miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PLoS One . 2013;8, article e69118 doi: 10.1371/journal.pone.0069118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abo ElAtta A. S., Ali Y. B. M., Bassyouni I. H., Talaat R. M. Upregulation of miR-221/222 expression in rheumatoid arthritis (RA) patients: correlation with disease activity. Clinical and Experimental Medicine . 2019;19:47–53. doi: 10.1007/s10238-018-0524-3. [DOI] [PubMed] [Google Scholar]
- 124.Yang S., Yang Y. Downregulation of microRNA-221 decreases migration and invasion in fibroblast-like synoviocytes in rheumatoid arthritis. Molecular Medicine Reports . 2015;12:2395–2401. doi: 10.3892/mmr.2015.3642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript. More detailed information cannot be shared publicly because it allows identification of the patients. It can be accessed from the Hirszfeld Institute of Immunology and Experimental Therapy (contact via katarzyna.bogunia-kubik@hirszfeld.pl) for researchers who meet the criteria for access to confidential data.


