Abstract
Background
Consistent guidance for choosing an appropriate probiotic for the treatment of irritable bowel syndrome is lacking.
Methods
Literature databases searched included: PubMed, Google Scholar and NIH registry of clinical trials from inception to June 2021. Inclusion criteria: randomized controlled trials (RCTs) enrolling adult or pediatric IBS patients comparing probiotics against controls and ≥ 2 RCTs with common IBS outcome measures within each type of probiotic. Five common measures of IBS symptoms (changes in global Irritable Bowel Syndrome Severity Scoring System or IBS-SSS scores, frequency of global responders, changes in bloating or abdominal pain scores and frequency of abdominal pain relief) were used. This study was registered at Prospero (#CRD42018109169).
Findings
We screened 521 studies and included 42 randomized controlled trials (45 treatment arms, N = 3856). Four probiotics demonstrated significant reduction in abdominal pain relief: B. coagulans MTCC5260 (RR= 4.9, 95% C.I. 3.3, 7.3), L. plantarum 299v (RR= 4.6, 95% CI 1.9, 11.0), S. boulardii CNCM I-745 (RR= 1.5, 95% C.I. 1.1, 2.1) and S. cerevisiae CNCM I-3856 (RR= 1.3, 95% C.I. 1.04, 1.6). Mild-moderate adverse events were reported in 51% of the trials, none were more associated with the probiotic compared to controls.
Interpretation
Although the analysis of probiotic efficacy was limited by the diversity of IBS outcomes used in trials and lack of confirmatory trials for some strains, six single-strain probiotics and three different types of probiotic mixtures showed significant efficacy for at least one IBS outcome measure. These results might be relevant to clinical practice and policy.
Keywords: Probiotic, Irritable bowel syndrome, Gastrointestinal, Meta-analysis, Diarrhea, Constipation
Research in context.
Evidence before this study
Guidance for choosing an appropriate probiotic for the treatment of irritable bowel syndrome is lacking and evidence from reviews and meta-analyses are conflicting. To assess the efficacy and safety of probiotics for the treatment of irritable bowel syndrome (IBS) within specific strain sub-groups, inclusion criteria required ≥2 trials with common IBS outcome measures within each type of probiotic.
Added value of this study
Fourteen different probiotic types were analyzed (9 single strains and 5 multi-strain mixtures). Nine probiotic types had at least one outcome with significant efficacy, four probiotics showed no efficacy in any of the outcomes. Four probiotics demonstrated significant reduction in abdominal pain relief: B. coagulans MTCC5260, L. plantarum 299v, S. boulardii CNCMI-745 and S. cerevisiae CNCM I-3856.
Implications of all the available evidence
Although the analysis of probiotics was limited by the diversity of IBS outcomes used in trials, the study identified nine specific probiotics that are effective for IBS patients and might inform clinical practice.
Alt-text: Unlabelled box
1. Introduction
Irritable bowel syndrome (IBS) is now included in the spectrum of disorders of gut-brain interactions and is characterized by recurrent abdominal pain, bloating and changes in bowel habits, which may include diarrhea or constipation. The prevalence of IBS varies by geographic region but has been reported in 1–18% of the general population [1,2]. Symptoms are used to classify sub-types of IBS: diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C) or mixed/alternating symptoms (IBS-M). Major risk factors for IBS include female gender, family history of IBS, and environmental triggers such as changes in diet or lifestyle or stress [2]. The pathophysiology of IBS involves chronic mucosal inflammation, alterations in intestinal epithelial and immune functions, visceral hypersensitivity, increased intestinal permeability and dysbiosis of the intestinal microflora. Treatment guidelines recommend medications focused on IBS symptoms, diets low in fermentable types of oligosaccharides, rifaximin and the use of probiotics [3,4]. Choosing an appropriate probiotic for patients can be challenging due to the diversity of different types of available probiotics, efficacy is strain-specific and the paucity of randomized clinical trials for some probiotic strains [5,6]. Recommendations from the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the American Gastroenterology Association (AGA) suggest efficacy be based only on probiotics with at least two randomized, controlled trials (RCTs) and efficacy should be determined for sub-groups of identical probiotic strains [7,8]. Unfortunately, many meta-analyses have not followed these recommendations and have not been able to determine which specific probiotic strains might be effective for IBS patients [9,10]. The aim of this study is to determine which probiotic strains are safe and effective for the treatment of IBS, accounting for both strain-specificity and based on probiotics with at least one confirmatory trial.
2. Methods
2.1. Search strategy and study selection
This study was designed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [11]. The initial protocol for this study is available in the Supplementary appendix (Text 1) and described below with slight revisions. This review is an update from a prior meta-analysis of probiotics for IBS, but includes trials and recommendations published in the subsequent 13 years [12]. PubMed, Google Scholar and NIH registry of clinical trials were searched from database inception to June 2021, unrestricted by language or year of publication. Non-English papers were translated by one author (LM) and reviewed. Search strategy was as follows: (“probiotics” [MeSH Terms] OR “probiotics” [All Fields]) AND “irritable bowel syndrome” [MeSH Terms] OR “irritable bowel syndrome” [All Fields] AND “controlled trials”) OR “probiotics” [MESH Terms] AND “IBS-diarrhea” [All Fields] OR “probiotics” (MeSH Terms] AND “IBS-constipation” [All Fields] OR “probiotics” [MeSH Terms] AND “IBS-mixed” [All Fields]). Additional searches were done using known probiotic types. Secondary searches of gray literature included reference lists, authors, reviews, meeting abstracts websites and clinicaltrials.gov for unpublished trials. A recursive search was also performed, using the bibliographies of all obtained articles.
Inclusion criteria included: randomized, controlled clinical trials (RCTs) assessing a well-defined probiotic, adult or pediatric patients diagnosed with IBS and published in peer-reviewed journals. We included only probiotics fulfilling the standard definition (must be living microbe, of adequate dose and having efficacy for a health effect [13]. This definition excludes dead or heat-killed microbes and prebiotics. Each probiotic type (single strain or multi-species mixture) was required to have at least two RCTs per type sharing at least one common IBS outcome. As bacterial and fungal taxonomies shift over time, the most current strain designations are presented in this review and strain identification was confirmed with the original authors or the manufacturer whenever possible.
Exclusion criteria included: non-human studies, early phase 1 or 2 safety or mechanism of action studies, no control group, probiotic not well-described, reviews and duplicate reports. The protocol was registered with Prospero (Prospero #: CRD42018109169). This study did not require ethical approval as based on published studies.
2.2. Data extraction
Initial screening of studies and data extraction was done independently by one author (LM), then independently reviewed by one of the other two co-authors (TK or AK) following PRISMA guidelines [11]. Any disagreements were discussed until consensus was reached. The data extracted from each study used a standardized data extraction form for PICOS data: (1) patient population (adult/pediatric, age, country, type of IBS), (2) intervention (type of probiotic or controls used, daily doses, formulation, duration and follow-up times), (3) comparisons (type of control group either placebo or open, unblinded), (4) IBS outcomes reported and (5) study design (randomized, controlled trials, either double blinded or open). For data that were required for these analyses but not reported in the published article, we attempted to contact the author or co-authors to obtain the missing data. Data was extracted from summary estimates from published trials from each individual trial.
2.3. Outcomes assessment
As no consensus has been reached for a standardized outcome to evaluate IBS improvements, we included the most common outcomes found in these trials. The primary outcomes that were screened included: (1) “Change in global IBS-Symptom Severity Score [IBS-SSS]”, a continuous outcome comparing the change of overall IBS symptom scores from baseline to end of study; (2) “Frequency of Responders”, a dichotomous outcome defined as the number of patients showing improvement of global IBS symptoms reported by the end of the study (either by physician assessment or by subject interview or diaries); (3) “Change in abdominal pain scores”, a continuous outcome comparing the change of scores for abdominal pain from baseline to end of study; (4) “Frequency reporting abdominal pain relief”, a dichotomous outcome (either by physician assessment or by subject interview or diaries) by the end of the study; (5) “Change in bloating scores”, a continuous outcome comparing the change in scores for bloating from baseline to end of study and (6) “Change in QoL (quality of life) scores”, a continuous outcome comparing the change of scores for quality of life from baseline to end of study. The secondary outcome was the number and types of adverse reactions reported by study participants.
2.4. Assessment of study quality and risk of bias
Each included RCT was reviewed and scored independently by at least two of the three co-authors using the Cochrane Collaboration's tool for assessing risk of bias [14]. The risk of bias was graded (high, low or not reported) for each of six domains of bias [selection bias (method of randomization and blinded allocation), performance bias (degree of blinding of study personnel and study subjects), detection bias (outcome assessor blinded), attrition bias (attrition different by group), reporting bias (only a priori outcomes reported) and other issues (fraud or miscellaneous)].
2.5. Data synthesis and statistical methods
Statistical analysis and generation of forest plots of pooled summary estimates was performed using Stata software version 16 (Stata Corporation, College Station, Texas) with meta-analysis modules [15]. Summary estimates were based on the pooled data from RCTs using the same type (species) of probiotic and sharing a common IBS outcome measure. Dichotomous outcomes were assessed using relative risks (RR) and 95% confidence intervals (C.I.) and continuous outcomes were assessing using standard mean difference (SMD) and 95% C.I. and heterogeneity across trials was evaluated using the I2 statistic [15]. Random effects models were used for all meta-analyze and results displayed in forest plots. Publication bias was assessed using funnel plots and the Egger test [16,17]. A priori sub-groups included: sub-type of IBS (IBS-C, IBS-D or IBS-M), daily doses of probiotic, type of enrolled subject (adult or pediatric), and study quality.
2.6. Role of the funding source
This study was un-funded. All authors had access to the dataset and decided to submit for publication. This study did not require ethical approval.
3. Results
3.1. Overview of included studies
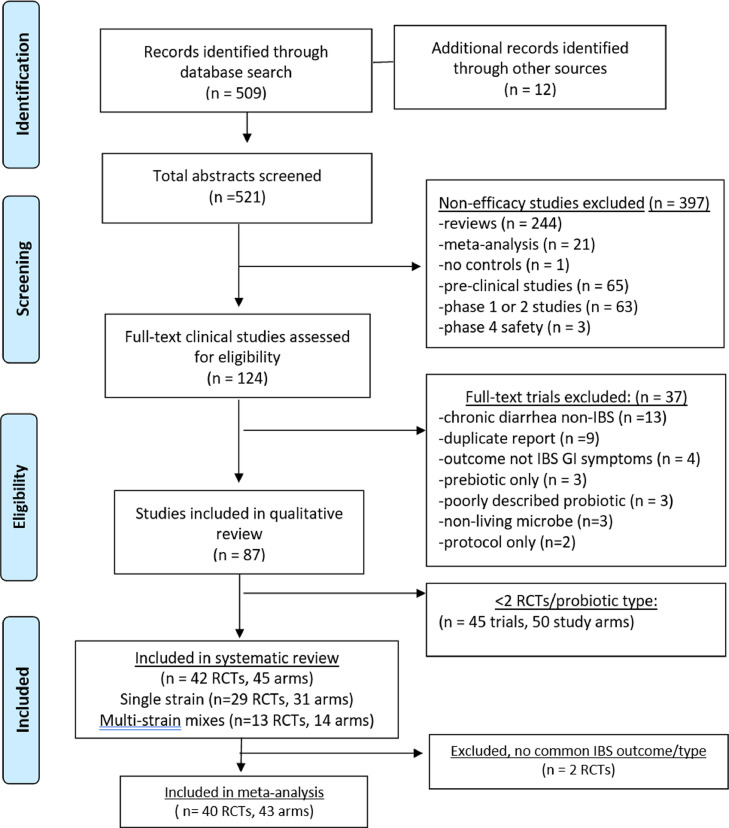
The literature search yielded 521 articles on probiotic use in IBS patients. Reasons for exclusion included non-efficacy studies (n = 397) or failure to meet inclusion criteria (n = 37), as shown in Fig. 1. Of the 87 RCTs on IBS, 45 RCT (50 treatment arms) were then excluded, as there was only one RCT/type (Supplementary appendix, Table 1). As a result, 42 RCTs (45 treatment arms) were included in the systematic review (N = 3856) [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]. For the meta-analysis, two RCTs testing E. coli Nissle 1917 were excluded [27,28] as no common IBS outcome measurement was used. Thus, the meta-analysis included a total of 40 RCTs (43 treatment arms). Two trials were translated from the original French language [39,40].
Fig. 1.
PRISMA study flow-chart of literature search for probiotics for the treatment of irritable bowel syndrome.
Abbreviations: GI, gastrointestinal; IBS, irritable bowel syndrome; RCTs, randomized controlled trials.
Table 1.
Efficacy of probiotics by common IBS outcomes in 42 (45 treatment arms) randomized controlled trials .
| Probiotic | “Responders” Probiotic No. (%) | “Responders” Controls No. (%) | Less abdominal pain Probiotic No. (%) | Less abdominal pain Control No. (%) | Mean ± SD change in global IBS-SSS scores:Probiotic | Mean ± SD change in global IBS-SSS scores:Control | Mean ± SD change in abdominal pain scores:Probiotic | Mean ± SD change in abdominal pain scores:Control | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Bac. coagulans MTCC5260 | nd | nd | 67/72 (93)a | 15/69 (22) | −18.5 ± 3.8a | - 8.7 ± 4.2 | - 2.9 ± 0.8a | - 1.2 + 0.9 | Sudha 2018 [18] |
| Bac. coagulans MTCC5260 | 10/53 (19)a | 0/55 (0) | 45/53 (85)a | 7/55 (13) | −15.8 ± 4.0a | - 5.2 ± 4.1 | - 4.8 ± 2.7a | - 1.6 + 1.7 | Madempudi 2019 [19] |
| Bac. coagulans MTCC5856 | 18/26 (69)a | 1/26 (4) | nd | nd | nd | nd | - 15 ± 28a | - 0.1 ± 22 | Urgesi 2014 [20] |
| Bac. coagulans MTCC5856 + FOS” | nd | nd | nd | nd | nd | nd | - 4.2 ± 0.3a | - 1.9 ± 0.2 | Rogha 2014 [21] |
| Bac. coagulans MTCC5856 | nd | nd | nd | nd | nd | nd | - 3.9 ± 0.3a | - 0.3 ± 0.5 | Majeed 2016 [22] |
| Bifid. infantis 35624 | nd | nd | nd | nd | nd | nd | - 1.0 ± 0.2b | - 0.9 ± 0.2b | O'Mahony 2005 [23] |
| Bifid. infantis 35624 | 33/74 (44) | 32/76 (42) | 32/74 (43) | 39/76 (52) | - 0.36 ± 0.1 | - 0.42± 0.1 | - 0.4 ± 0.1 | - 0.6 ± 0.1 | Whorwell 2006 [24] low dose |
| Bifid. infantis 35624 | 45/72 (62) a | 32/76 (42) | 42/72 (59)a | 39/76 (52) | - 0.76 + 0.1 | - 0.42± 0.1 | - 0.9 ± 0.1 | - 0.6 ± 0.1 | Whorwell 2006 [24] medium dose |
| Bifid. infantis 35624 | 26/71 (37) | 32/76 (42) | 28/71 (39) | 39/76 (52) | - 0.38 ± 0.1 | - 0.42± 0.1 | - 0.7 ± 0.1 | - 0.6 ± 0.1 | Whorwell 2006 [24] high dose |
| Bifid.animalis DN-173010 | 88/135 (65) a | 63/132 (48) | nd | nd | nd | nd | - 0.5 ± 1.0a | - 0.39 ± 0.9 | Guyonnet 2007 [25] |
| Bifid.animalis DN-173010 | nd | nd | nd | nd | - 0.4 ± 0.3b | - 0.1 ± 0.4b | - 0.5 ± 1.6b | - 0.1 ± 1.2b | Agrawal 2009 [26] |
| E. coli Nissle 1917 | 27/51 (53) | 23/48 (48) | 33/60 (55) | 30/60 (50) | nd | nd | nd | nd | Kruis 2012 [27] |
| E. coli Nissle 1917 | nd | nd | nd | nd | - 6.7 ± 6.8 | - 6.7 ± 6.5 | nd | nd | Faghihi 2015 [28] |
| L. plantarum 299v DSN9843 | 11/25 (44) | 7/27 (26) | 9/25 (36) | 5/27 (18) | nd | nd | - 2.0 ± 0.1a | - 1.0 ± 0.3 | Nobaek 2000 [29] |
| L. plantarum 299v DSN9843 | 19/20 (95)a | 3/20 (15) | 20/20 (100)a | 4/20 (20) | nd | nd | nd | nd | Niedezielin 2001 [30] |
| L. plantarum 299v DSN9843 | 10/29 (35) | 11/29 (38) | nd | nd | - 16 ± 82 | - 60 ± 45 | nd | nd | Simren 2006 [31] |
| L. plantarum 299v DSN9843 | 82/105 (78)a | 8/99 (8) | 79/98 (81) | 8/92 (9) | nd | nd | - 0.6 ± nd | - 0.3 ± nd | Ducrotte 2012 [32] |
| L. plantarum 299v DSN9843 | nd | nd | nd | nd | nd | nd | - 60.5 ± 2.8 | - 54.1 ± 6.9 | Stevenson 2014 [33] |
| L. rhamnosus GG | nd | nd | 11/25 (44) | 10/25 (40) | nd | nd | - 13 ± 0.3 | - 1.7 ± 0.6 | Bausserman 2005 [34] |
| L. rhamnosus GG | nd | nd | 6/18 (33)a | 1/19 (5) | nd | nd | - 1.5 ± 1.5 | - 1.0 ± 1.2 | Gawronska 2007 [35] |
| L. rhamnosus GG | nd | nd | 34/42 (82)a | 17/38 (45) | nd | nd | - 1.9 ± 1.6 a | - 1.0 ± 2.5 | Francavilla 201,036 |
| L. rhamnosus GG | nd | nd | nd | nd | - 68 ± 107a | - 34 ± 95 | nd | nd | Pedersen 2014 [37] |
| L. rhamnosus GG | nd | nd | nd | nd | nd | nd | - 1.7 ± 0.9 a | - 1.2 ± 0.8 | Kianifar 2015 [38] |
| S. boulardii CNCM I-745 | 13/16 (81) a | 13/18 (72) | 6/15 (40) | 3/18 (17) | nd | nd | nd | nd | Maupas 1983 [39] |
| S. boulardii CNCM I-745 | nd | nd | 22/25 (81)a | 18/30 (56) | nd | nd | nd | nd | Bennani 1990 [40] |
| S. boulardii CNCM I-745 | 11/45 (25) | 9/45 (20) | nd | nd | −0.5 ± 0.8 | - 0.5 ± 0.8 | - 0.3 ± 1.0 | - 0.. ± 1.0 | Choi 2011 [41] |
| S. boulardii CNCM I-745 | nd | nd | nd | nd | nd | nd | −0.4 ± 0.7 | - 0.8 ± 0.7 | Kabir 2011 [42] |
| S. boulardii CNCM I-745 | nd | nd | nd | nd | nd | nd | - 0.04 ± 0.9 | - 0.3 ± 0.5a | Abbas 2014 [43] |
| S. cerevisiae I-3856 | nd | nd | 54/86 (63)a | 44/93 (47) | nd | nd | −1.2 ± 1.3 | - 0.85 ± 1.4 | Pineton de Chambrun 2015 [44] |
| S. cerevisiae I-3856 | nd | nd | 57/177 (32) | 47/175 (27) | - 3.6 ± 4.3 | −3.3 ± 4.0 | nd | nd | Spiller 2016 [45] |
| S. cerevisiae I-3856 | nd | nd | nd | nd | nd | nd | - 1.7 ± 0.9a | - 0.4 ± 0.7 | Gayathri 2020 [46] |
| 3 strain mix | 14/37 (38) | 10/37 (27) | nd | nd | - 30 ± 80 | - 60 ± 90 | - 8 ± 30 | - 3 ± 30 | Simren 2010 [47] |
| 3 strain mix | 9/27 (33) | 7/25 (28) | nd | nd | - 89 ± 140 | - 47 ± 120 | - 17 ± 15 | - 9 ± 27 | Sondergaard 2011 [48] |
| 3 strain mix | 35/67 (52) | 26/64 (41) | nd | nd | −0.1 ± 1.0 | - 0.2 ± 1.0 | - 0.1 ± 1.2 | - 0.6 ± 1.2 | Begtrup 2013 [49] |
| 4 strain mix | 31/41 (76)a | 17/40 (43) | 27/41 (66)a | 17/40 (43) | - 7.7 ± 2.0a | - 1.2 ± 2.0 | - 4.2 ± nd | - 5.8± nd | Kajander 2005 [50] |
| 4 strain mix | nd | nd | nd | nd | - 14 ± 10 a,b | - 3 ± 9b | - 3 ± 2.2a,b | 0 ± 3.0b | Kajander 2008 [51] |
| 6 strain mix | 17/25 (68) a | 9/24 (37) | nd | nd | nd | nd | - 1.2 ± 0.2 | - 0.5 ± 0.3 | Yoon 2014 [52] |
| 6 strain mix | 29/39 (74) | 26/42 (62) | nd | nd | nd | nd | - 2 ± 1 | - 1.2 ± 1.0 | Yoon 2015 [53] |
| 7 strain mix | 12/25 (48)a | 3/25 (12) | nd | nd | - 1.0 ± 0.2 | - 0.8 ± 0.06 | - 0.6 ± 0.05 | - 0.3 ± 0.1 | Ki Cha 2012 [54] |
| 2/4 txt arms: 7 strain mix vs placebo | nd | nd | 10/14 (71)a | 3/12 (25) | - 19. . ± 5.0 | - 9.8 ± 0.5 | - 12.1 ± 1.0 | - 7.8 ± 7.1 | Ko 2013 [55] |
| 2/4 txt arms: Herbal txt + 7 strain mix vs herbal control | nd | nd | 10/13 (77) | 11/14 (78) | - 14.5 ± 1.3 | - 5.8 ± 0.8 | - 10.2 ± 0.2 | - 3.0 ± 0.6 | Ko 2013 [55] |
| 8-strain mix | 4/12 (33) a | 5/13 (38) | nd | nd | - 35 ± 32 | - 7.0 ± 31 | - 8 ± 12 | - 0 ± 8 | Kim 2003 [56] |
| 8-strain mix | 11/24 (46) | 8/24 (33) | nd | nd | nd | nd | nd | nd | Kim 2005 [57] |
| 8-strain mix | 44/59 (75)a | 2/59 (3) | 40/59 (68)a | 0/59 (0) | nd | nd | - 1.0 ± 0.2a | - 0.5 ± 0.2 | Guandalini 2010 [58] |
| 8-strain mix | 30/53 (57)a | 19/51 (37) | nd | nd | - 82 ± 78 | - 78 ± 96 | nd | nd | Staudaeher 2017 [59] |
Abbreviations: Bac., Bacillus; Bifid., Bifidobacterium; E., Enterococcus; L., Lactobacillus; No., number; nd, not done or not reported; P., Propionibacterium; S., Saccharomyces; SD, standard deviation; Strept., Streptococcus;3 strain mix, “Cultura®”: L. paracasei 19, L. acido La5, Bifido lactis Bb12; 4 strain mix, L. rhamnosus GG, L. rhamnosus LC705, Bifido. breve Bb99, P. freudenreichii ssp. shermanii JS; 6 strain mix, “LacClean®” L. acidophilus 11906BP, L. rhamnosus 12202BP, Bifido. bifidum 12199BP, Bifido. lactis 11,904 BP, Bifido. longum 12,200 BP, Strept. thermophilus 11870BP; 7 strain mix, “DuoLac®:: Bifido. brevis 11858BP, Bifido. lactis 11903BP, Bifido. longum 11860BP, L. acidophilus 11906BP, L. rhamnosus 11868BP, L. plantarum 11867BP, Strept. thermophilus 11870BP; 8 strain mix,Bifido. breve DSM24732, Bifido. longum DSM24736, Bifido. infantis DSM24737, L. acidophilus DSM24735, L. plantarum DSM24730, L. paracasei DSM24733, L. delbruckii subsp. bulgaricus DSM24734, Strept. thermophilus DSM24731, originally named VSL#3®, now either Visbiome™ or Vivomixx™ using the De Simone formulation.
P < 0.05 compared to control.
Estimated standard deviation.
3.2. Study population and study design characteristics
The study population were mostly adults (86%), female (66%), with mixed IBS sub-types (60%), diagnosed with Rome II/III criteria (93%), and enrolled in one of 17 countries (Supplementary appendix Table 2). The mean number of participants per RCT was 92/trial. Attrition was generally low (0–5% in 31% of 42 RCTs) or moderate (6–24% in 60% of RCTs).
3.3. Probiotic intervention characteristics
The daily dose of the probiotic was typically given at 109–1010/day in 81% of RCTs for 4–8 weeks (in 83% of RCTs), as either capsules (57%) or in beverages (16%) and more details are given in Supplementary appendix Table 2. Nearly half (48%) of the 42 RCTs did not include a post-probiotic/control follow-up period, but 21% did follow patients for 2 weeks and 31% of the RCTs had longer follow-up times (3 weeks-1 year).
From the 42 RCTs (total of 45 intervention arms), a total of 14 different types of probiotic products were assessed (Table 1). Sufficient evidence (≥ 2 RCTs) were found for nine different types of single strains of probiotics: Bacillus (Bac.) coagulans MTCC5260 (2 RCTs), Bac. coagulans MTCC5856 (3 RCTs), Bifidobacterium (Bif.) infantis 35624 (2 RCTs, 4 treatment arms), Bif. animalis DN-173010 (2 RCTs), E. coli Nissle 1917 (2 RCTs), Lactobacillus (L.) plantarum 299v (5 RCTs), L. rhamnosus GG (5 RCTs), Saccharomyces (S.) boulardii CNCM I-745 (5 RCTs) and S. cerevisiae (I-3856 (3 RCTs). Sufficient evidence (≥ 2 RCTs) was also found for five different types of multi-strain mixtures: a three-strain mixture of L. paracasei 19, L. acidophilus La5 and Bif. lactis Bb12 (3 RCTs), a four-strain mixture of L. rhamnosus GG, L. rhamnosus LC705, Bif. breve Bb99 and P. shermanii (2 RCTs), a six-strain mixture of L. acidophilus 11906BP, L. rhamnosus 12202BP, Bif. bifidum 12199BP, Bif. lactis 11904, Bif. longum 12200, Streptococcus (Strept.) thermophilus 11870BP (2 RCTs), a seven-strain mixture of Bif. brevis 11858BP, Bif. lactis 11903BP, Bif. longum 11860BP, L. acidophilus 11868BP, L. rhamnosus 11868BP, L. plantarum 11867BP and Strept. thermophilus 11870BP (2 RCTs, 3 treatment arms) and an eight-strain mixture of Bif. breve DSM24732, Bif. longum DSM24736, Bif. infantis DSM24737, L. acidophilus DSM24735, L. plantarum DSM24730, L. paracasei DSM24733, L. delbruckii subsp. bulgaricus DSM24734 and Strept. thermophilus DSM24731 (4 RCTs).
3.4. Assessment of study quality
Of the 42 RCTs (and 45 treatment arms) included in the systematic review, six types of bias were evaluated (Supplementary appendix Fig. 1). Most (98%) had an overall low risk of bias. We were unable to perform a sub-group analysis comparing efficacy of specific probiotic strains done in low-risk versus high-risk bias trials, as only one trial had a high risk of overall bias.46 Within bias domains, most high risk bias ratings were due to two reasons: 48% did not report if the study group allocator was blinded and 31% did not report the method of randomization used. Of the other domains of study bias (performance, detection, attrition, reporting and other), most were rated of low bias in each of these domains.
3.5. Safety
Of the 42 RCTs reviewed, seven (17%) did not report any adverse reaction (AE) data (Supplementary appendix Table 3), while 17 (40%) only included a statement ‘No adverse reactions were noted in the study’, but did not provide further data on frequency by study group or by type of adverse reaction. Three (7%) RCTs only reported total adverse reactions, but not by study group. Only 15 (36%) of the RCTs reported adverse event data by study group (probiotic versus placebo). With the exception of one RCT [21], there were no significant difference in the frequency or types of mild-moderate AEs in probiotic versus control groups. The most common types of reported adverse reactions were mild gastrointestinal complaints (69%) or rash (15%). Infrequent serious AEs associated with probiotic use (vertigo) was reported in one trial [32] and two trials reported serious AEs (hospitalization or not described) in the placebo group [25,27].
3.6. Meta-analysis of efficacy for IBS
Among the 40 RCTs included in the meta-analysis, many different IBS outcomes were used, ranging from changes in global IBS symptoms to changes in specific IBS symptoms (abdominal pain, bloating, gas, etc.) to the quality of life indicators or changes in immune system markers. Four of the most commonly reported IBS outcomes are provided in Table 1, which shows the inconsistency of commonly shared IBS outcomes used in these trials, even within the same type of probiotic strain(s). The two most common IBS outcomes assessed improvement in global (overall) IBS symptoms (change in IBS-SSS scores) and the frequency of ‘Responders). Symptom-specific outcomes were commonly reported (change in abdominal pain scores, frequency reporting abdominal pain relief and changes in bloating scores). Other outcomes including IBS quality of life and changes in other IBS symptoms were not consistently reported or used different scoring scales and thus were not used in the meta-analyses. Five IBS outcomes had at least two RCTs with the same outcome and had at least two probiotics of the same strain or mixture sharing that common IBS outcome. Publication bias was difficult to assess by probiotic type, as the number of trials per type was limited. To overcome this limitation, we pooled all probiotic types together for the 20 trials reporting a common outcome (frequency of no abdominal pain by study end) and the funnel plot (Supplementary appendix Fig. 2) showed there was significant publication bias (Egger's test P = 0.005). Similar results were found for trials using the other IBS outcomes once we pooled all probiotic types together (data not shown).
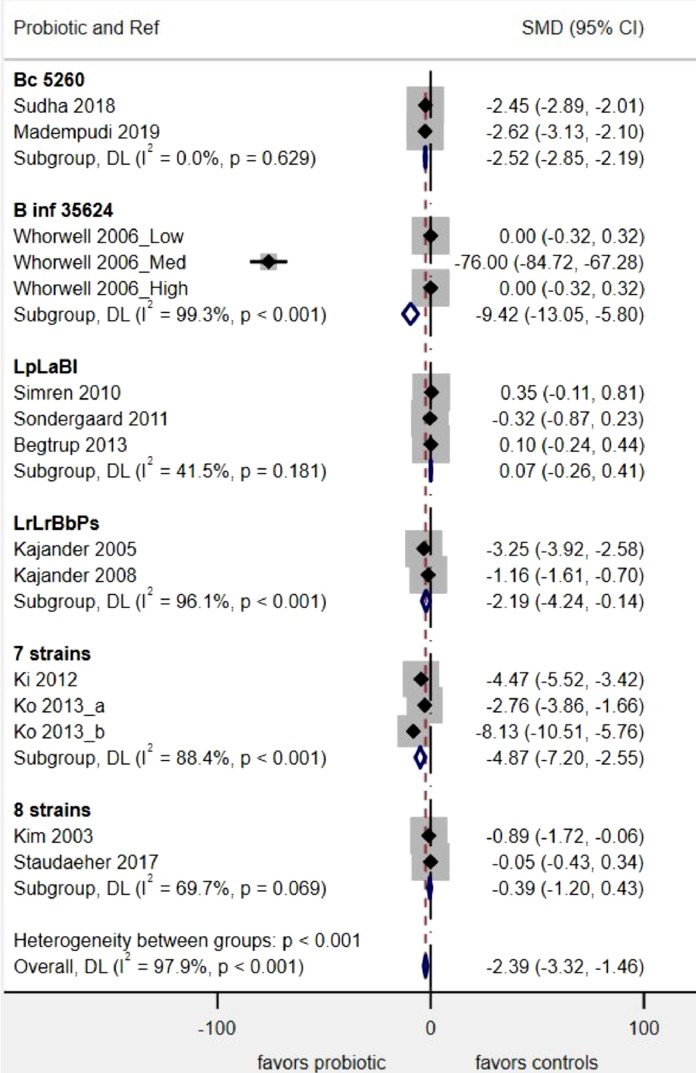
Fig. 2.
Forest plot of probiotics for the change in IBS symptom scores. Boxes indicate each study's relative risk and horizontal lines indicate each study's 95% confidence intervals, Diamond indicates subgroup's pooled RR and 95% C.I.
Abbreviations: Bc, Bacillus coagulans; B inf, Bifidobacterium infantis; L., Lactobacillus;LpLaBl, “Cultura®”: L. paracasei 19, L. acido La5, Bifido lactis Bb12; LrLrBbPs, L. rhamnosus GG, L. rhamnosus LC705, Bifido. breve Bb99, P. freudenreichii ssp. shermanii JS; 7 strains, “DuoLac®:: Bifido. brevis 11858BP, Bifido. lactis 11903BP, Bifido. longum 11860BP, L. acidophilus 11906BP, L. rhamnosus 11868BP, L. plantarum 11867BP, Strept. thermophilus 11870BP; 8 strains, Bifido. breve DSM24732, Bifido. longum DSM24736, Bifido. infantis DSM24737, L. acidophilus DSM24735, L. plantarum DSM24730, L. paracasei DSM24733, L. delbruckii subsp. bulgaricus DSM24734, Strept. thermophiles DSM24731, originally named VSL#3®, now either Visbiome™ or Vivomixx™ using the De Simone formulation.
3.6.1. Change in global IBS-SSS scores
Of the 14 different types of probiotics, six had ≥ 2 RCTs/probiotic type and also reported this shared IBS outcome. Four probiotic types significantly reduced global IBS-SSS scores by the end of the study, as shown in Fig. 2: Bif. infantis 35624 (SMD= −9.4, 95% C.I. −13.0, −5.8, P < 0.001), a 7-strain mixture (SMD= −4.9, 95% C.I. −7.2, −2.5, P < 0.001), Bac. coagulans MTCC5260 (SMD= −2.5, 95% C.I. −2.8, −2.2, P < 0.001) and a 4-strain mixture (SMD= −2.2, 95% C.I. −4.2, −0.1, P = 0.04), while two other types of probiotics (a 3 strain mixture and an 8-strain mixture) showed no significant reduction in IBS-SSS scores. Overall, the heterogeneity in the 21 RCTs with this outcome was high (I2= 97.9%), but by stratifying by probiotic type, low heterogeneity (from I2=0–41%) was achieved for 2/6 (33%) of the probiotic sub-groups.
3.6.2. Frequency of global responders
Six different types of probiotics had sufficient RCTs/type to be included for this outcome, but none showed a significant increase in the number of responders in global IBS symptoms by the end of the study, as shown in Supplementary appendix Fig. 3. Two probiotics showed a non-statistically significant trend in better responder rates: L. plantarum 299v (P = 0.06) and a 3-strain mixture (P = 0.09). Overall, the heterogeneity in the 16 RCTs with this outcome was high (I2= 75%), but by stratifying by probiotic type, low heterogeneity (from I2=0–34%) was achieved for 4/6 (67%) of the probiotic sub-groups.
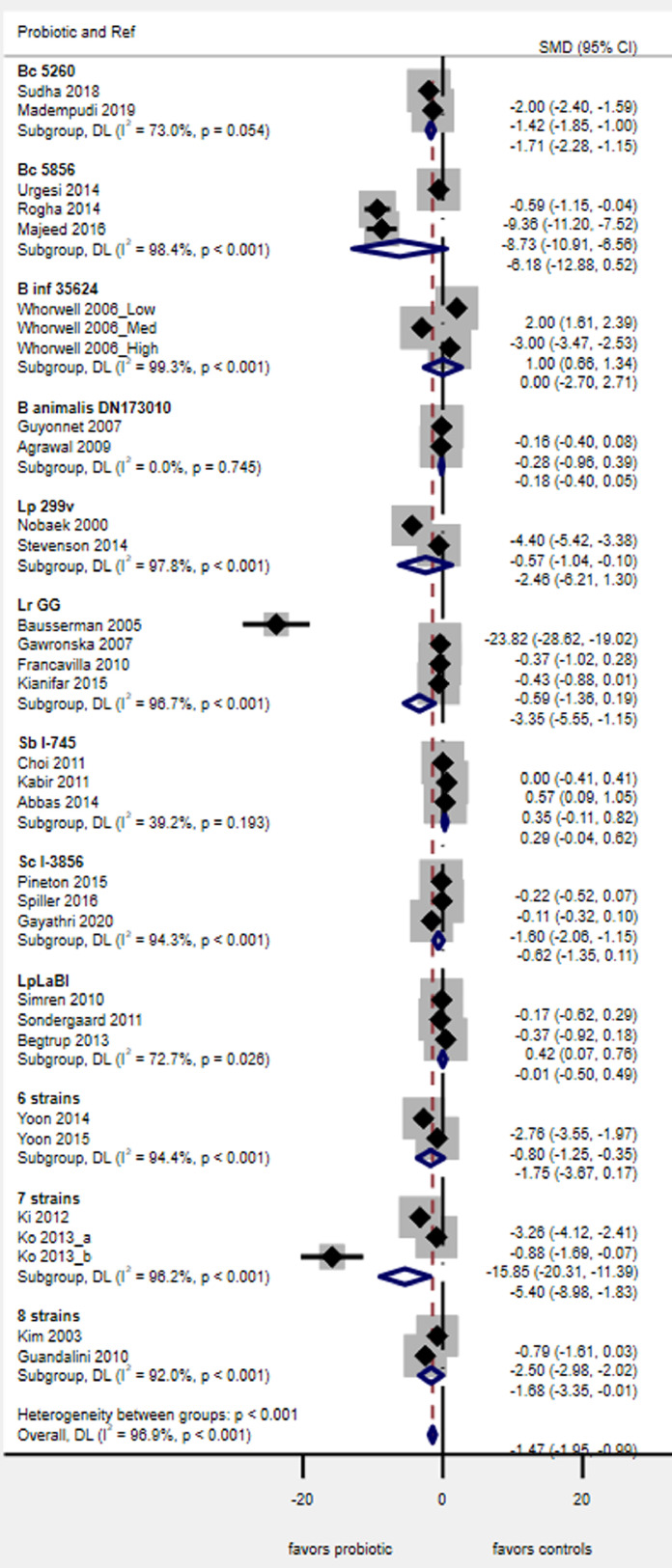
Fig. 3.
Forest plot of probiotics for the change in IBS abdominal pain scores. Boxes indicate each study's relative risk and horizontal lines indicate each study's 95% confidence intervals, Diamond indicates subgroup's pooled RR and 95% C.I.
Abbreviations: B, Bifidobacterium; Bc,Bacillus coagulans;Binf, Bifidobacterium infantis;Lp,Lactobacillus plantarumLr, L. rhamnosus;Sb, Saccharomyces boulardii; Sc, Saccharomyces cerevisiae; LpLaBl, “Cultura®”: L. paracasei 19, L. acido La5, Bifido lactis Bb12; 6 strains, “LacClean®” L. acidophilus 11906BP, L. rhamnosus 12202BP, Bifido. bifidum 12199BP, Bifido. lactis 11,904 BP, Bifido. longum 12,200 BP, Strept. thermophilus 11870BP; 7 strains, “DuoLac®: Bifido. brevis 11858BP, Bifido. lactis 11903BP, Bifido. longum 11860BP, L. acidophilus 11906BP, L. rhamnosus 11868BP, L. plantarum 11867BP, Strept. thermophilus 11870BP; 8 strains, Bifido. breve DSM24732, Bifido. longum DSM24736, Bifido. infantis DSM24737, L. acidophilus DSM24735, L. plantarum DSM24730, L. paracasei DSM24733, L. delbruckii subsp. bulgaricus DSM24734, Strept. thermophiles DSM24731, originally named VSL#3®, now either Visbiome™ or Vivomixx™ using the De Simone formulation.
3.6.3. Change in abdominal pain scores
Of the 14 different probiotic types, 12 had at least two RCTs/type for this outcome. This was the most commonly reported IBS outcome in all the 40 RCTs. Four probiotics showed a significant reduction in abdominal pain scores (Fig. 3): Bac. coagulans MTCC5260 (SMD= −1.7, 95% C.I. −2.3, −1.1, P < 0.001), L. rhamnosus GG (SMD= −3.3, 95% C.I. −5.65, −1.1, P = 0.003), a 7-strain mixture (SMD= −5.4, 95% C.I. −8.9, −1.8, P = 0.003), and an 8-strain mixture (SMD= −1.7, 95% C.I. −3.3, −0.01, P = 0.049), while a non-statistically significant trend was seen for S. boulardii CNCM I-745 (P = 0.09), a 6-strain mixture (P = 0.07) and Bac. coagulans MTCC5856 (P = 0.07). No significant reduction in pain scores were seen for the other five probiotics. Overall, the heterogeneity in the 29 RCTs with this outcome was high (I2= 95.9%), but by stratifying by probiotic type, low heterogeneity (from I2= 0–39%) was achieved for 2/12 (17%) of the probiotic sub-groups.
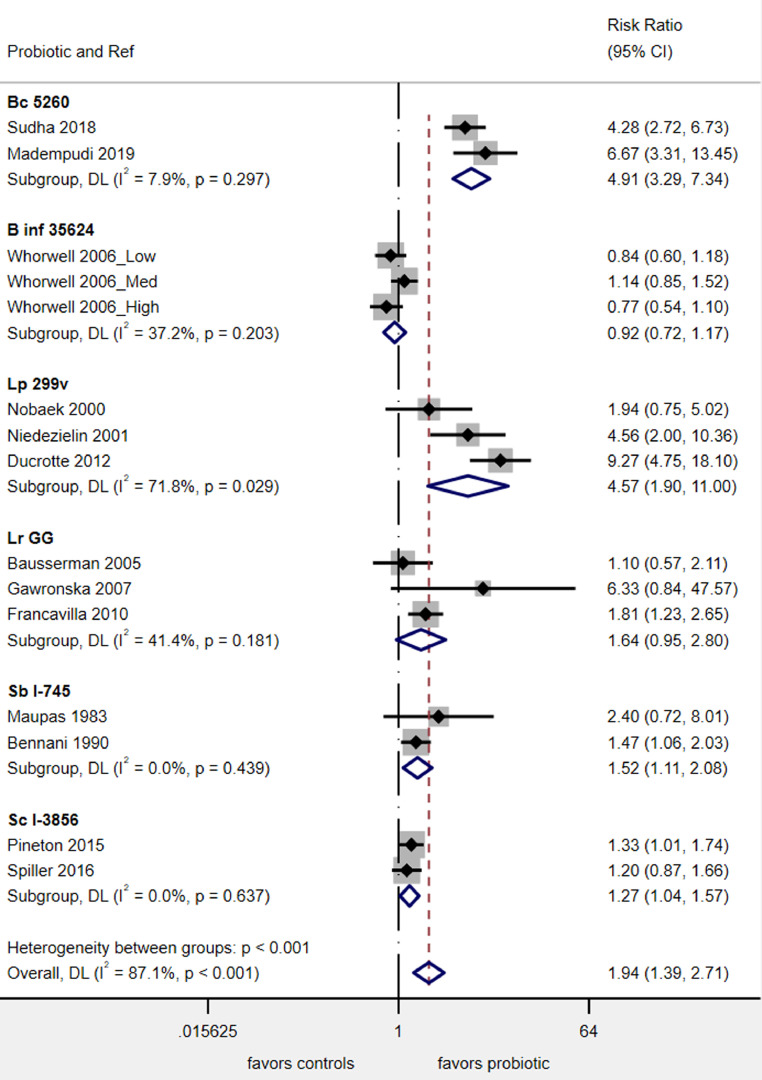
3.6.4. Frequency of abdominal pain relief
Six of the 14 probiotic types had at least two RCTs for this outcome (Fig. 4). Four probiotics significantly improved the report of abdominal pain by the end of the study: Bac. coagulans MTCC5260 (RR= 4.9, 95% C.I. 3.3, 7.3, P < 0.001), L. plantarum 299v (RR= 4.6, 95% C.I. 1.9, 11.0, P = 0.001), S. boulardii CNCM I-745 (RR= 1.5, 95% C.I. 1.1, 2.1, P = 0.009) and S. cerevisiae CNCM I-3856 (RR= 1.3, 95% C.I. 1.04, 1.6, P = 0.02). L. rhamnosus GG had a non-statistically significant trend (P = 0.07) but Bif. infantis 35624 had no significant impact on abdominal pain relief. Overall, the heterogeneity in the 13 RCTs with this outcome was high (I2=87%), but by stratifying by probiotic type, low heterogeneity (from I2= 0–37%) was achieved for 5/6 (83%) of the probiotic sub-groups.
Fig. 4.
Forest plot of probiotics for frequency reporting less abdominal pain by study end. Boxes indicate each study's relative risk and horizontal lines indicate each study's 95% confidence intervals, Diamond indicates subgroup's pooled RR and 95% C.I.
Abbreviations: Bc, Bacillus coagulans; B inf,Bifidobacterium infantis; L., Lactobacillus; Lp, L. plantarum; Lr, L. rhamnosus; Sb, Saccharomyces boulardii; Sc, Saccharomyces cerevisiae.
3.6.5. Change in bloating severity scores
Only nine RCTs (10 treatment arms) measured the change in bloating severity scores. Two single strain probiotics and two multi-strain mixtures were eligible for this analysis, but none demonstrated significant efficacy for this outcome (Supplementary appendix Fig. 4). Overall, the heterogeneity in the 9 RCTs with this outcome was high (I2= 87%), but by stratifying by probiotic type, low heterogeneity (from I2= 0–6%) was achieved for all 4 (100%) of the probiotic sub-groups.
3.7. Sub-group analyses
IBS sub-types. Most RCTs enrolled a mixed population of different of IBS subtypes, but failed to analyze outcomes by specific IBS subtypes. Of the 42 RCTs, only 14 (33%) reported outcome data separately by IBS subtypes, either as sub-groups in mixed types of enrolled IBS patients [24,37,45,46,50,53] or only enrolled patients with IBS-D [22,42,43,[54], [55], [56]] or only IBS-C [25,26]. Only 8 RCTs with common outcomes and at least two RCTs/type could be analyzed. Of the 4 RCTs IBS-D patients [42,43,54,55] two probiotics were analyzed and only the 7-strain mixture significantly reduced abdominal pain scores (SMD= −5.8, 95% C.I. −9.7, −1.9, P = 0.004), as shown in Supplementary appendix Fig. 5. Stratifying by probiotic strain types reduced overall heterogeneity for the four IBS-D trials from I2= 96.7% to 0%, but only for S. boulardii I-745. Of the 4 RCTs in IBS-C patients,[25,26,45,46] neither S. cerevisiae CNCM I-3856 nor Bif. animalis DN173010 had a significant reduction in abdominal pain scores, as shown in Supplementary appendix Fig. 6. Stratifying by probiotic strain types reduced overall heterogeneity for the four IBS-C trials from I2=48.5% to 0%, but only for Bif. animalis DN173010.
Daily dose of probiotic. Overall, in the 45 treatment study arms, 16 (36%) used the probiotic at a dose of 106–109 cfu/day, 23 (51%) used a higher dose (1010/day), five (11%) used the highest dose at 1011/day, while one study did not report the daily dose used [28]. Once we stratified on the type of probiotic and a common outcome, our analysis was limited due to either low numbers of trials or similar daily doses used within the same type of probiotic. Only two types of probiotics were tested with different daily doses: Bif. infantis 35624 (106, 108 and 1010) [24] and the 4-strain mixture (109) [50] and (1010) [51]. A dose-response was only seen for the 108 dose of Bif. infantis 35624 and the 1010 dose of the 4-strain mixture (Table 1).
Adult versus pediatric patients The majority of the study participants were adults (86% of the trials) and only six RCTs were done in children assessing either Bac. coagulans 5260 [18] or L. rhamnosus GG [[34], [35], [36],38] or the 8-strain mixture [58]. Using the most common IBS outcome (change in abdominal pain scores), the reduction in pain scores appeared to be similar in adult and pediatric trials (data not shown), but we were unable to conduct a strain-specific meta-analysis by type as there was only one trial in adults and one in children for each of the two the probiotic types (B. coagulans 5260 and the 8-strain mixture) and there were no trials done in adults with a common IBS outcome to compare to the four pediatric trials using L. rhamnosus GG.
4. Discussion
To the best of our knowledge, this is the first meta-analysis that accounted for probiotic strain-specificity when assessing efficacy for the treatment of IBS. Overall, this study showed six single-strain probiotics (Bac. coagulans MTCC5260, Bif. infantis 35624, L. plantarum 299v, L. rhamnosus GG, S. boulardii CNCM I-745 and S. cerevisiae CNCM I-3856) and three mixtures (a 4-strain mixture, a 7-strain mixture and an 8-strain mixture) showed significant improvement in at least one IBS outcome measure. As the choice of an appropriate type of probiotic can be challenging for clinicians and policy-makers [5], this analysis offers nine probiotics supported by rigorous analyses following recommended guidelines for strain-specificity and having at least two RCTs per probiotic type [7,8].
This review has several strengths, in that it was an extensive and broad-based literature review. The trial selection, data extraction and quality scoring was done independently by multiple researchers to minimize bias and transcription errors. The meta-analysis accounted for both strain-specific assessment of probiotics and type of outcome measures. Another strength is that most included trials were of moderate-high quality that had at least one confirmatory trial using the identical probiotic strain or strains.
The review does have several limitations. Of 64 different types of probiotics screened, 50 had insufficient numbers of randomized trials (n < 2) to be included in this analysis. We cannot conclude these 50 probiotic types may not be effective for IBS patients, rather we just lacked confirmatory trials to form a robust conclusion of efficacy. These 50 types of probiotics may be potential candidates for IBS, but more RCTs are required for these strains. IBS trials also had diverse outcomes used to measure efficacy: responders (usually improved symptoms), changes in symptom severity scores, quality of life, stool consistency, etc. and different scales and score ranges used by different studies hampered the comparisons of a common outcome measure. Studies often had a heterogeneity of IBS sub-groups included or failed to report outcome efficacy by IBS sub-types, thus limiting analysis by IBS sub-types. Variable study designs (different doses, varying duration of treatments, lack of adequate follow-up to determine if the effect was sustainable, lack of adverse event data) made it difficult to determine the role of daily probiotic dose and duration for the various probiotics. The formulation of the probiotic was also diverse and fermented milk products may have prebiotic effects, which were not addressed in the trials. The generalizability of these results is also limited, as most of the trials were done with adults and there was no documentation of other risk factors (such as diet), which may impact IBS symptoms.
Probiotics have been investigated as IBS treatments but as the efficacy of probiotics has been determined to be strain-specific [6], previous meta-analyses of IBS may have reached erroneous conclusions if the strain-specificity was not accounted for. An extensive systematic review of 66 RCTs of probiotics, prebiotics, synbiotics and antibiotics for the treatment of IBS could not conclude which treatments might be more effective due to significant heterogeneity between studies and different types of treatments [9]. Even recent meta-analyses have inappropriately pooled different types of probiotic strains at a genus level (that is, pooling all trials of any type of Lactobacilli or Bifidobacterium strains into genus-level groups) and then concluding all Lactobacilli or all Bifidobacteria may be effective or not [3,9,60,61]. One meta-analysis included both trials of IBS patients and non-IBS patients with chronic constipation (but no other Rome criteria) and based their conclusions on this mixed group of diseases and also incorrectly pooled different types of probiotics together [10]. More recent meta-analyses focused on including a single type of probiotic in an effort to account for strain-specificity. Yuan et al. pooled six RCTs that used Bifidobacterium infantis 35624, but they erroneously included two trials of different probiotic mixtures that used a different strain of Bifidobacteria [62,63]. Another meta-analysis limited included trials to those testing S. cerevisiae CNCM I-3856, but found only two RCTs with inconsistent results [64]. Other meta-analyses of probiotics for IBS have also included probiotics with a single trial, pooling this data into a larger dataset of all probiotic strains. The British Society of Gastroenterology published their 2021 guidelines for the treatment of IBS and recommended probiotics, in general, as a first line treatment, but as their analysis pooled dissimilar strains of probiotics, they could not identify which specific probiotics might be useful [3]. This meta-analysis might help to overcome this limitation.
Controversies exist for the use of probiotics, mainly including which probiotics have proven efficacies. This has been addressed by limiting our analysis to probiotic types with sufficient number of RCTs and common IBS outcomes. Future trials are needed for other probiotic candidates currently supported by only a single trial. Another issue is what role dead or heat-modified bacterial or fungal microbes or prebiotics may have for the efficacy of disease. As the efficacy of modified microbes is debated, [65], [66], [67] we limited our analysis to living probiotics, as this follows the international consensus that probiotics must be alive [13].
For future studies, there is a need to have standardized IBS outcomes, using at least one global improvement measure, so that different trial results may be compared. In addition, many studies used different scales with varied ranges of values, making comparisons between studies difficult. Future reviews and meta-analysis need to analyze probiotics separately by the type of probiotic and have at least one confirmatory trial for each outcome. As IBS criteria for diagnosis were revised in 2016 as Rome IV and future studies should use this basis for IBS diagnosis and to report efficacy data by the IBS subtypes. Acidified or fermented milk usage as probiotic form causes prebiotic effect, so capsule or granule forms should be advocated. Exclusion criteria should be expanded because of comorbidities may affect the study, such as small intestinal bacterial overgrowth. Synchronous prebiotic or over the counter usage should be restricted to demonstrate actual probiotic effect. As safety of investigational treatments is an important consideration, future studies need to report adverse event data in more detail. As we found 17% of the trials in our review did not report any safety data, it is important to fully document safety data. The paucity of adverse event data published in probiotic trials should be rectified in future studies, as this is an important clinical consideration. As 50 types of probiotics did not have a confirmatory trial, this opens an opportunity for future research to identify more potential probiotics for the treatment of IBS. As research defining microbiome profiles continues, it may be possible to determine if different microbiome profiles respond better to specific probiotic types or if individual microbiome profiles may be helpful in determining treatment responses for IBS patients.
Although the analysis of probiotics was limited by the diversity of IBS outcomes used in trials, six single-strain probiotics and three different types of probiotic mixtures showed significant efficacy for at least one IBS outcome measure.
Funding
This study was unfunded.
Contributors
LVM designed the study; LVM oversaw the conduct of the study; LVM designed and conducted the analysis; LVM designed and conducted the statistical analysis; LVM, TK and AK independently reviewed and scored all reviewed papers and all had access to all data; LVM, TK and AK wrote the manuscript and all authors reviewed and contributed to the final manuscript. LVM, TK and AK all verified the underlying data.
Data sharing
Data collected for this meta-analysis have already been published in other studies. Data extracted from these published articles are available in the supplementary materials. Protocol and statistical analysis available at: https://www.crd.york.ac.uk/PROSPERO (CRD42018109169). There are no individual participant data due to the nature of this meta-analysis. The data will be made available upon publication and ending 5 years following article publication.
Declaration of Competing Interest
LVM is on the Scientific Advisory Board of Bio-K+ (Bio-K+, a Kerry company) and on the Biocodex Microbiome Board (Biocodex, France) and has received honoraria from Bio-K+ and Biocodex. TK and AK declared no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101154.
Contributor Information
Lynne V. McFarland, Email: mcfarland.lynne.v@gmail.com.
Tarkan Karakan, Email: tkarakan@gazi.edu.tr.
Ali Karatas, Email: akaratas85@hotmail.com.
Appendix. Supplementary materials
References
- 1.Sperber A.D., Bangdiwala S., Drossman D.A. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterol. 2021;160:99–114. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Van den Houte K., Carbone F., Pannemans J. Prevalence and impact of self-reported irritable bowel symptoms in the general population. United Euro Gastroenterol J. 2019;7(2):307–315. doi: 10.1177/2050640618821804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasant D.H., Paine P.A., Black C.J. British society of gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70(7):1214–1240. doi: 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- 4.Altobelli E., Del Negro V., Angeletti P.M. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9(940):1–19. doi: 10.3390/nu9090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sniffen J.C., McFarland L.V., Evans C.T., Goldstein E.J.C. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS ONE. 2018;13(12) doi: 10.1371/journal.pone.0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland L.V., Evans C.T., Goldstein E.J.C. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med. 2018;5(124):1–14. doi: 10.3389/fmed.2018.00124. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szajewska H., Canani R.B., Guarino A. ESPGHAN working group for probiotics prebiotics. probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62(3):495–506. doi: 10.1097/MPG.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 8.Su G.L., Ko C.W., Bercik P., Falck-Ytter Y., Sultan S., Weizman A.V., Morgan R.L. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159(2):697–705. doi: 10.1053/j.gastro.2020.05.059. [DOI] [PubMed] [Google Scholar]
- 9.Ford A.C., Harris L.A., Lacy B.E. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 10.Wen Y., Li J., Long Q. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: a systematic review and meta-analysis based on seventeen randomized controlled trials. Intl J Surg. 2020;79:111–119. doi: 10.1016/j.ijsu.2020.04.063. [DOI] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland L.V., Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel disease. World J Gastroenterol. 2008;14(17):2650–2661. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill C., Guarner F., Reid G. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Green S. The Cochrane Collaboration; 2011. Cochrane handbook for systematic reviews of interventions. version 5.1.0 [updated march 2011] editors. Available from www.cochrane-handbook.org. Accessed June 3, 2020. [Google Scholar]
- 15.Palmer T.M., Sterne J.A.C. Published by Stata Press; College Station, Texas: 2016. Meta-analysis in Stata: an updated collection from the Stata journal. editor. 2nd Ed. [Google Scholar]
- 16.McGuinness L.A. 2019. Robvis: an r package and web application for visualising Risk-Of-Bias Assessments.https://github.com/mcguinlu/robvis Available at. Accessed June 3, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Egger M., Davey-Smith G., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudha M.R., Jayanthi N., Aasin M. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: a double blind, randomised placebo controlled study. Benef Microbes. 2018;9(4):563–572. doi: 10.3920/BM2017.0129. [DOI] [PubMed] [Google Scholar]
- 19.Madempudi R.S., Ahire J.J., Neelamraju J. Randomized clinical trial: the effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci Rep. 2019;9:12210. doi: 10.1038/s41598-019-48554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urgesi R., Casale C., Pistelli R. A randomized double-blind placebo-controlled clinical trial on efficacy and safety of association of simethicone and Bacillus coagulans (Colinox®) in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2014;18(9):1344–1353. [PubMed] [Google Scholar]
- 21.Rogha M., Esfahani M.Z., Zargarzadeh A.H. The efficacy of a synbiotic containing Bacillus coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench. 2014;7(3):156–163. [PMC free article] [PubMed] [Google Scholar]
- 22.Majeed M., Nagabhushanam K., Natarajan S. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J. 2016;15:21. doi: 10.1186/s12937-016-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Mahony L., McCarthy J., Kelly P. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterol. 2005;128(3):541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Whorwell P.J., Altringer L., Morel J. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 25.Guyonnet D., Chassany O., Ducrotte P. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26(3):475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal A., Houghton L.A., Morris J. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Alim Pharmacolo Therap. 2009;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 27.Kruis W., Chrubasik S., Boehm S. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27(4):467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faghihi A.H., Agah S., Masoudi M. Efficacy of probiotic escherichia coli Nissle 1917 in patients with irritable bowel syndrome: a double blind placebo-controlled randomized trial. Acta Med Indones. 2015;47(3):201–208. [PubMed] [Google Scholar]
- 29.Nobaek S., Johansson M.L., Molin G. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroentero. 2000;95(5):1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 30.Niedzielin K., Kordecki H., Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13(10):1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Simren M., Syrous A., Lindh A. Effects of Lactobacillus plantarum 299v on symptoms and rectal sensitivity in patients with irritable bowel syndrome (IBS) - a randomized, double-blind controlled trial. Presented at Digestive Disease Week, May 20-25, 2006, Los Angeles California. Gastroenterol. 2006;130(4/S2):A600. A600 (abstr) [Google Scholar]
- 32.Ducrotté P., Sawant P., Jayanthi V. Clinical trial: lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18(30):4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson C., Blaauw R., Fredericks E. Randomized clinical trial: effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition. 2014;30(10):1151–1157. doi: 10.1016/j.nut.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Bausserman M., Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediat. 2005;147(2):197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Gawrońska A., Dziechciarz P., Horvath A. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25(2):177–184. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 36.Francavilla R., Miniello V., Magistà A.M. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126(6):e1445–e1452. doi: 10.1542/peds.2010-0467. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen N., Andersen N.N., Végh Z. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20(43):16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kianifar H., Jafari S.A., Kiani M. Probiotic for irritable bowel syndrome in pediatric patients: a randomized controlled clinical trial. Electron Physician. 2015;7(5):1255–1260. doi: 10.14661/1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maupas J.L., Champemont P., Delforge M. Traitement des colopathies fonctionnelles. Essai en double aveugle de I’ULTRA-LEVURE [Treatment of irritable bowel syndrome double blind trial of Saccharomyces boulardii] Med Chir Dig. 1983;12(1):77–79. [Google Scholar]
- 40.Bennani A. Essai randomise de Saccharomyces boulardii dans le traitement des colopathies fonctionnelles.[Randomized study of S. boulardii for the treatment of functional gastroenteropathies.] L'Objectif. Médical. 1990;73:56–61. [Google Scholar]
- 41.Choi C.H., SY Jo, Park H.J. A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45(8):679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 42.Kabir M.A., Ishaque S.M., Ali M.S. Role of Saccharomyces boulardii in diarrhea predominant irritable bowel syndrome. Mymensingh Med J. 2011;20(3):397–401. [PubMed] [Google Scholar]
- 43.Abbas Z., Yakoob J., Jafri W. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial. Eur J Gastroenterol Hepatol. 2014;26(6):630–639. doi: 10.1097/MEG.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 44.Pineton de Chambrun P., Neut C., Chau A. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liv Dis. 2015;47(2):119–124. doi: 10.1016/j.dld.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Spiller R., Pélerin F., Cayzeele Decherf A. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United Eur Gastroenterol J. 2016;4(3):353–362. doi: 10.1177/2050640615602571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gayathri R., Aruna T., Malar S. Efficacy of Saccharomyces cerevisiae CNCM I-3856 as an add-on therapy for irritable bowel syndrome. Interl J Colorect Dis. 2020;35(1):139–145. doi: 10.1007/s00384-019-03462-4. [DOI] [PubMed] [Google Scholar]
- 47.Simrén M., Ohman L., Olsson J. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome-a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 48.Sondergaard B., Olsson J., Ohlson K. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–672. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 49.Begtrup L.M., de Muckadell O.B., Kjeldsen J. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome–a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48(10):1127–1135. doi: 10.3109/00365521.2013.825314. [DOI] [PubMed] [Google Scholar]
- 50.Kajander K., Hatakka K., Poussa T. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6 month intervention. Aliment Pharmacol Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 51.Kajander K., Myllyluoma E., Rajilić-Stojanović M. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27(1):48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoon J.S., Sohn W., Lee O. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52–59. doi: 10.1111/jgh.12322. [DOI] [PubMed] [Google Scholar]
- 53.Yoon H., Park Y.S., Lee D.H. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Biochem Nutr. 2015;57(2):129–134. doi: 10.3164/jcbn.15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ki Cha B., Mun Jung S., Hwan Choi C. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 55.Ko S.J., Han G., Kim S.K. Effect of Korean herbal medicine combined with a probiotic mixture on diarrhea-dominant irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/824605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H.J., Camilleri M., McKinzie S. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim H.J., Vazquez Roque M.I., Camilleri M. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 58.Guandalini S., Magazzù G., Chiaro A. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51(1):24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 59.Staudacher H.M., Lomer M.C.E., Farquharson F.M. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153(4):936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Liang D., Longgui N., Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine. 2019;98(27):e16068. doi: 10.1097/MD.0000000000016068. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niu H., Xiao J. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Intl J Surg. 2020;75:116–127. doi: 10.1016/j.ijsu.2020.01.142. [DOI] [PubMed] [Google Scholar]
- 62.Yuan F., Ni H., Asche C.V. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33(7):1191–1197. doi: 10.1080/03007995.2017.1292230. [DOI] [PubMed] [Google Scholar]
- 63.Allen A.P., Clarke G., Cryan J.F. Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms and mechanisms. Curr Med Res Opin. 2017;33(7):1349–1351. doi: 10.1080/03007995.2017.1322571. [DOI] [PubMed] [Google Scholar]
- 64.Cayzeele-Decherf A., Pélerin F., Leuillet S. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: an individual subject meta-analysis. World J Gastroenterol. 2017;23(2):336–344. doi: 10.3748/wjg.v23.i2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zorzela L., Ardestani S.K., McFarland L.V., Vohra S. Is there a role for modified probiotics as beneficial microbes: a systematic review of the literature. Benef Microbes. 2017;8(5):739–754. doi: 10.3920/BM2017.0032. [DOI] [PubMed] [Google Scholar]
- 66.Sugahara H., Yao R., Odamaki T., Xiao J.Z. Differences between live and heat-killed bifidobacteria in the regulation of immune function and the intestinal environment. Benef Microbes. 2017;8(3):463–472. doi: 10.3920/BM2016.0158. [DOI] [PubMed] [Google Scholar]
- 67.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., Vinderola G. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.