Abstract
Extralobar pulmonary sequestration is a rare congenital pulmonary malformation that may present early in life or remain asymptomatic. Here we present a case of torsion of an extralobar pulmonary sequestration on its vascular pedicle. Although the patient's initial symptomatology suggested intraabdominal pathology, the correct preoperative diagnosis was determined in large part by the lesion's MRI characteristics, which strongly suggested tissue infarction.
Keywords: Sequestration, MRI, CT
Introduction
Pulmonary sequestration is a rare malformation of nonfunctioning lung tissue that lacks a communication with the tracheobronchial tree. Although these lesions may exist on a “sequestration spectrum,” they are typically divided into intralobar and extralobar subtypes. While the more common intralobar pulmonary sequestration (ILS) is enclosed by the visceral pleura of the adjacent normal lung tissue, the less common extralobar pulmonary sequestration (ELS) is separate from the normal lung tissue—and sometimes the thoracic cavity—contained within its own visceral pleura. While the origins of ILS are hypothesized to includ congenital and acquired mechanisms, ELS is certainly congenitally derived. ELS is believed to arise during the fourth to eighth week of gestation as an accessory lung bud inferior to the normal lung bud, likely accounting for its most common location between the lower pulmonary lobes and diaphragm. Although typically arising from the lung bud, ELS can arise within the abdomen, diaphragm, or pericardium. ELS is more often left sided and is usually fed by a single systemic artery, typically arising from the thoracic or abdominal aorta and draining into the systemic venous system [1].
ELS is commonly detected antenatally, especially given its relatively high association (50%-60%) with other congenital anomalies, including congenital diaphragmatic hernia, congenital heart disease, and congenital pulmonary airway malformation [1,2]. After birth, infants with ELS may experience respiratory distress, hemorrhage, malabsorption, or high-output congestive heart failure secondary to shunting [1]. However, asymptomatic cases may remain undetected until discovered incidentally [3]. ELS symptomatic in adulthood have been reported to cause massive hemothorax or recurrent massive pleural effusion [1]. One uncommon complication of ELS is torsion on its vascular pedicle, resulting in infarction of the sequestration, as we describe in this case report. Given the overall infrequency of ELS, torsion of an ELS is a truly extraordinary occurrence, with their description in the English literature limited only to case reports. Additionally, ELS torsion may present with counterintuitive symptomatology, complicating evaluation, and delaying diagnosis. Imaging can play a key role in establishing the correct diagnosis, thereby facilitating prompt intervention, which generally results in excellent outcomes [2], [3], [4], [5], [6], [7].
Case report
A 13-year-old boy suddenly developed severe abdominal pain that progressively worsened over the next 3 days. No clear inciting event could be identified. The patient was previously healthy and active, having won a track competition a few days prior to onset of his pain as well as bicycling and mowing the lawn the preceding day. At the time he presented to an outside hospital, his pain had increased to the point he was having difficulty walking and taking deep breaths. He had also had several episodes of non–bloody, non–bilious emesis. After an abdominal radiograph raised concern for a bowel obstruction, he was transferred to our tertiary children's hospital for further care.
On exam, he was tender to palpation in the left upper quadrant and flank with abdominal guarding and splinted respirations. He was also noted to have decreased breath sounds at the left lung base. Laboratory evaluation was notable for an elevated C-reactive protein (5.7 mg/dL), D-dimer (996 ng/mL), fibrinogen (565 mg/dL), white blood cell count (10.78 thousand), and neutrophil count (8.98 thousand).
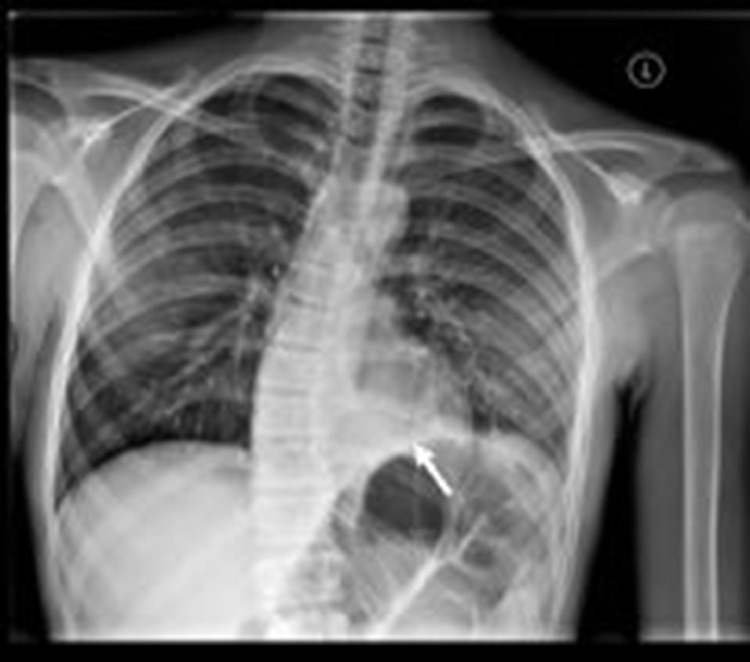
Chest radiographs showed a left lower lobe airspace opacity and probable small left pleural effusion with dextrocurvature of the thoracic spine, which was attributed to splinting from pain. (Fig. 1) The outside abdominal radiograph was re-examined and felt to be most consistent with colonic ileus. A right lower quadrant ultrasound was performed to evaluate for appendicitis, but visualization of the appendix was obscured by bowel gas and stool in the cecum.
Fig. 1.
PA chest radiograph demonstrates a left lower lobe (retrocardiac) opacity (white arrow), dextrocurvature of the thoracolumbar spine, and a small left pleural effusion.
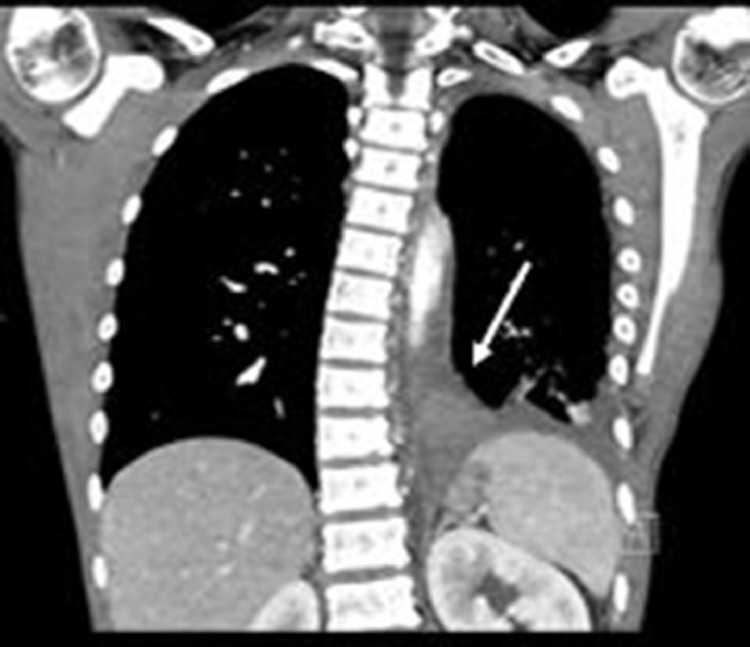
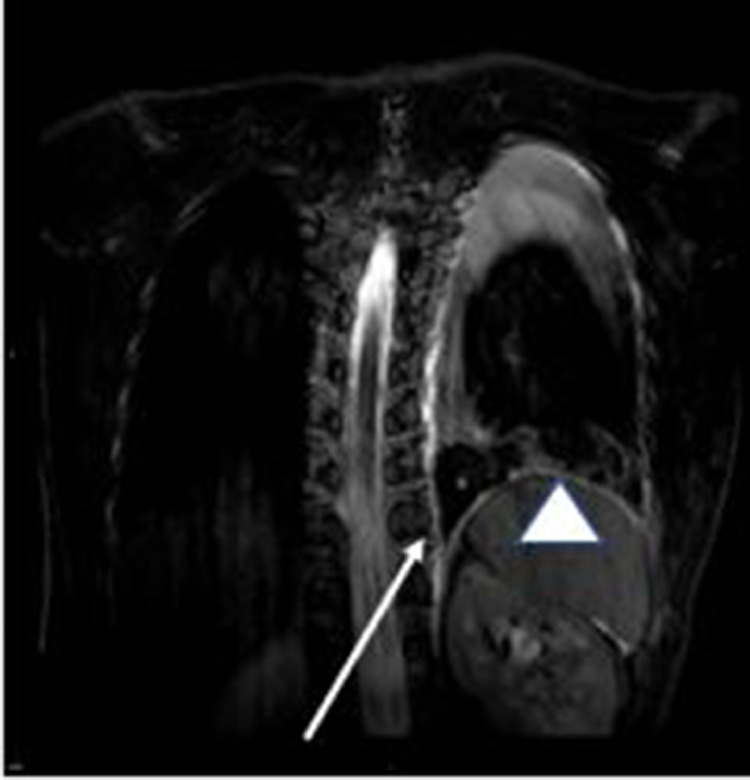
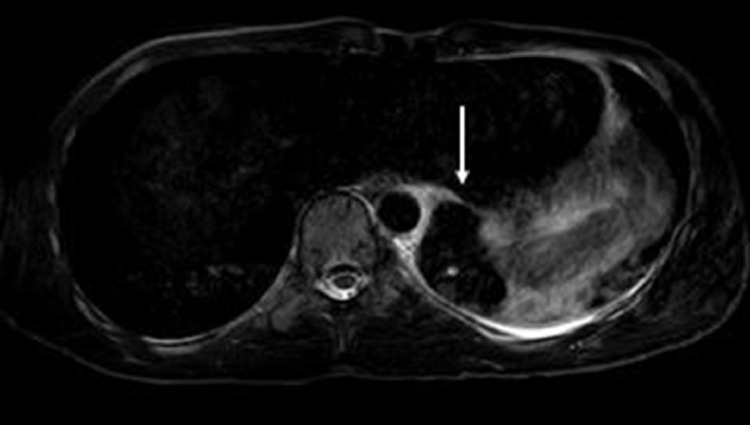
Following the administration of analgesics, his abdominal guarding had resolved and the pain was localized to the left upper quadrant and back. Given the absence of fever and cough to suggest pneumonia, contrast-enhanced computed tomography (CECT) of the chest was performed to better determine the etiology of the left lower lobe opacity and effusion seen on chest radiography. (Fig. 2) This demonstrated a small left pleural effusion with a hyperdense polygonal mass in the inferior medial left pleural space without clear vascularity, postulated to relate to blood products or tumor. Contrast-enhanced magnetic resonance imaging (MRI) of the chest showed a well-encapsulated medial left lower pleural space nonenhancing mass, characterized by homogeneous hypointense signal on T2-weighted imaging and slightly hyperintense signal on T1-weighted imaging, measuring 3.8 cm x 3.1 cm x 3.5 cm. (Figs. 3, 4, and 5) The associated small left pleural effusion demonstrated slightly hyperintense signal on both T2- and T1-weighted imaging, suggesting proteinaceous or hemorrhagic contents. Smooth pleural enhancement was consistent with pleuritis. (Fig. 6) Although no feeding vessel was identified by CECT or MRI, the location, morphology, and MRI signal characteristics of the mass raised the possibility of a pulmonary sequestration with infarction and hemorrhage, with blood clot, and solitary fibrous tumor considered to be less likely alternative diagnoses.
Fig. 2.
Coronal CT image of the Chest with intravenous contrast shows a hyperdense polygonal mass (white arrow) in the inferior medial left pleural space without clear feeding vessel and a small left pleural effusion. There is dextrocurvature of the spine which was attributed to splinting from pain.
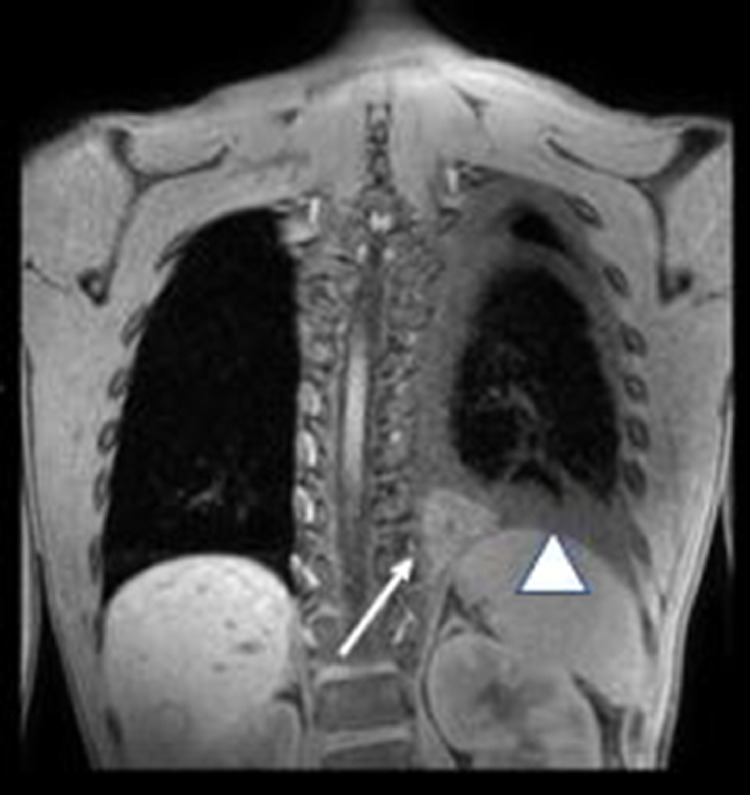
Fig. 3.
T2FS Coronal MRI image demonstrate the hypointense polygonal mass (white arrow) in the inferior medial left pleural space, with a small left T2 hyperintense pleural effusion (white arrowhead).
Fig. 4.
T2FS Axial MRI image demonstrate the hypointense polygonal mass (white arrow) in the inferior medial left pleural space.
Fig. 5.
T1 weighted fat saturated Coronal image showing the hyperintense polygonal mass (white arrow) in the inferior medial left pleural space, with a slightly hyperintense small left pleural fluid consistent with hemorrhage (white arrowhead).
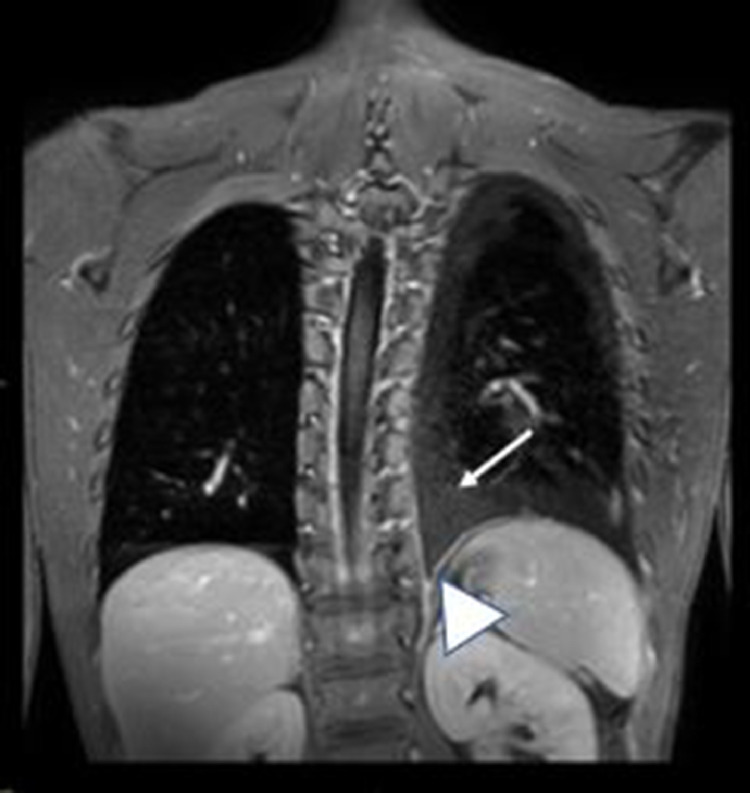
Fig. 6.
T1 weighted fat saturated post contrast Coronal image showing the non–enhancing polygonal mass (white arrow) in the inferior medial left pleural space, with inferomedial left pleural enhancement consistent with pleuritis (white arrowhead).
Given these imaging findings and the patient's symptoms, video-assisted thoracoscopic surgery was performed, which revealed a necrotic extralobar pulmonary sequestration, torsed on its vascular pedicle. (Fig. 7) The vascular pedicle was ligated at its exit from the diaphragm with clips, and the sequestration resected. (Figs. 8,9) Final diagnosis on pathology was pulmonary sequestration with prominent hemorrhage, consistent with torsion. The patient experienced immediate relief of his pain following operation and was discharged on the first postoperative day. He was continuing to do well at the time of his follow-up appointment two weeks later with no plans for any additional follow up.
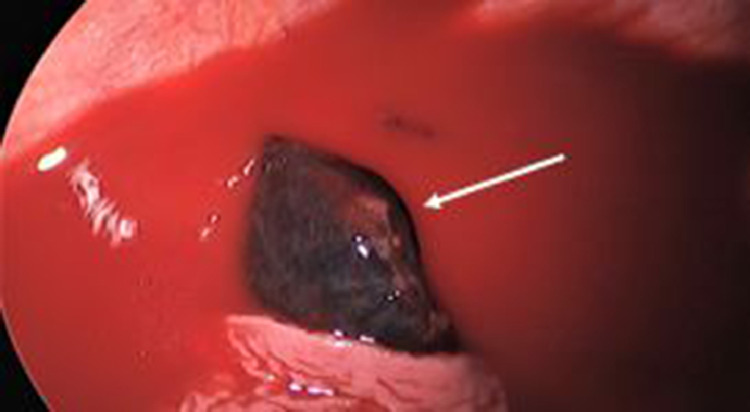
Fig. 7.
Image obtained during Video assisted thoracotomy showing the necrotic sequestration (white arrow) in the pleural space with hemorrhagic pleural fluid.
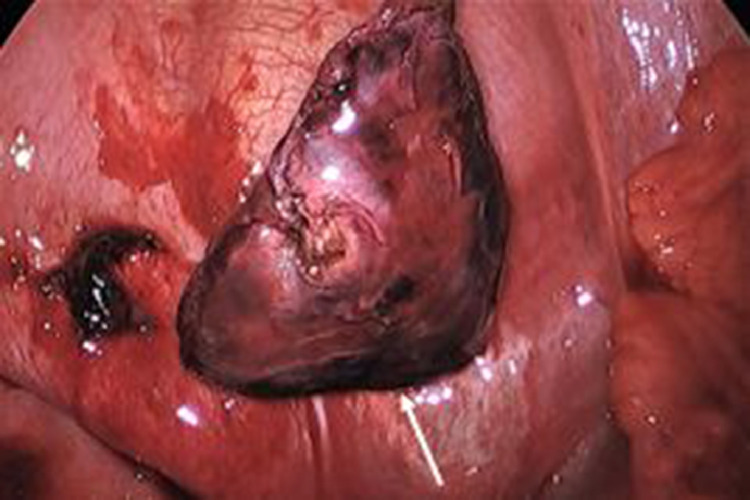
Fig. 8.
Image obtained during video assisted thoracotomy showing the necrotic extralobar sequestration (white arrow) after ligating the feeding vessel and suctioning the hemorrhagic pleural fluid.
Fig. 9.
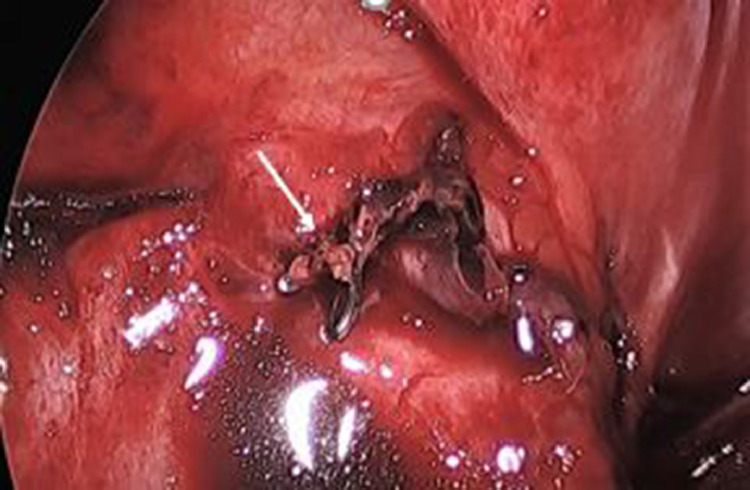
Image obtained during video assisted thoracotomy showing the ligated feeding vessel to the sequestration (white arrow).
Discussion
ELS is a rare congenital anomaly, representing 25% of all pulmonary sequestrations, which have an overall estimated incidence of only 0.15%-1.7% [1,8]. Unlike ILS, which are contained within the visceral pleura of the normal lung, ELS are invested in their own pleural lining, and lack the ligamentous attachments of the normal lung. This allows the ELS to rotate on its systemic vascular pedicle and may result in strangulation of the sequestration [7]. In the English literature, we could find only 18 cases of ELS with torsion, excluding a couple of other case reports of ELS infarction that were not clearly attributed to torsion [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. These case reports of ELS torsion mostly involve children and adolescents, although adult cases have been described, along with a suspected fetal case [3,14,16,18]. Like our patient, the chief presenting complaint of many of these children is abdominal pain, sometimes with vomiting, and accompanying chest symptoms [2,4,5,[7], [8], [9], [10],12,13,[15], [16], [17], [18], [19]]. Given the presenting complaints, initial evaluation is often focused on possible abdominal pathology, although most are found to have pleural effusion with associated mass or opacity [[2], [3], [4], [5], [6], [7], [8], [9], [10],[12], [13], [14], [15], [16], [17], [18], [19]]. The list of possible differential diagnoses is long, and reported workups have included routine CT abdomen, CT enterography, CT chest, ultrasound (US), MRI, positron emission tomography, metaiodobenzylguanidine scan, thoracentesis, and endoscopy [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. In many cases, the diagnosis remained undetermined until the time of exploratory thoracotomy or thoracoscopy [3,[8], [9], [10], [11], [12],[14], [15], [16], [17],19]. Among the previously published case reports, the correct diagnosis of ELS torsion was suggested preoperatively in only a minority of cases, as it was in our case [2,4,5,6,7,18].
The correct preoperative diagnosis of ELS torsion in these cases relied on CT, US, and MRI findings [2,[4], [5], [6], [7],18]. In some cases, imaging was able to visualize the characteristic aberrant systemic vessel of ELS [2,6,18]. However, due to the inherent compromise of the vessel in torsion, a feeding vessel is not always seen, as in our case [4,5,7,9]. Interestingly, in the previously reported case of fetal ELS, a feeding vessel was intermittently apparent on imaging, which was attributed to intermittent torsion [18].
In our case, the preoperative diagnosis of ELS torsion was heavily dependent on the lesion's MRI features. The lesion's polygonal, well-circumscribed shape, and location in the in the left inferior medial pleural space were classic for imaging appearance ELS [7]. Hypointense signal on T2-weighted imaging with slight hyperintense signal on T1-weighted imaging suggested the hemorrhagic nature of the infarcted ELS. Lack of contrast enhancement was also supportive of infarction and decreased the likelihood that the mass represented a neoplasm, an important alternative diagnosis. The associated pleural effusion in our patient is also nearly universally described by other authors and has been attributed to blockage of the draining lymphatics by torsion of the vascular pedicle [8,18].
In the scientific literature, the reported MRI characteristics of ELS with torsion are variable. Like ours, some cases have demonstrated low T2 signal intensity of the torsed ELS, [3,7,10] while others have demonstrated increased or intermediate T2 signal intensity [18,9]. We interpreted the low T2 signal intensity in our case to indicate hemorrhage, which was also supported by the increased T1 signal intensity of the torsed ELS. Increased T1 signal intensity of the torsed ELS has been previously described and attributed to hemorrhage [2,3]. Yokota et al. noted low T1 signal intensity in their case of ELS torsion and emphasized the usefulness of chemical shift subtraction in suggesting hemorrhage. Alternately, ELS with torsion has also been reported to have heterogeneous signal intensity, characterized by branching foci of T2 hyperintensity, which was not seen in our patient [2,3].
The enhancement pattern of ELS with torsion is also variable. Like the case reported by Gawlitza et al., our patient's torsed ELS did not enhance following contrast administration. Choe et al. described irregular central enhancement with decreased peripheral enhancement of a torsed ELS, while Takeuchi et al. reported only peripheral enhancement in a case of ELS torsion, distinct from the strong enhancement of their comparison case of a ELS without torsion. The amount of enhancement may relate to the degree of torsion of the feeding vessel, with lack of enhancement likely in those with complete occlusion of vascular flow.
While the MRI imaging characteristics of our case of ELS with torsion are shared by some of the previously reported cases in the literature, its particular combination of low signal intensity on both T2-, and T1-weighted imaging with complete lack of enhancement has not been previously described. Perhaps these features reflect complete occlusion of the feeding vessel with an advanced degree of infarction of the torsed ELS, as the patient's symptoms began three days prior to presentation.
One question not explained by imaging or surgical findings is why the torsion of the ELS occurred. Our patient had participated in an athletic competition prior to onset of this pain, although the two events were separated by three days. However, he had also been active just the day before his presentation, bicycling, and mowing the lawn. This raises the possibility that activity or respiratory exertion may predispose an ELS to torsion. Yokota et al. has endorsed vigorous exercise as a potential precipitating factor, noting that their patient with ELS torsion had been playing tennis at the time his symptoms began. In contrast, in an adult case of ELS torsion, Lima et al. proposed that the mass effect of a recent pregnancy had promoted torsion of the ELS.
In conclusion, ELS with torsion is a rare complication of a rare congenital anomaly with an often-nonspecific clinical presentation. The correct diagnosis can be made by imaging, and MRI may be particularly helpful, as it was in our case. Though the MRI signal characteristics of ELS with torsion may be variable, certain features are fairly consistent, including inferior medial pleural location, well-defined contours, and associated pleural effusion. Importantly, though an aberrant systemic feeding vessel is the hallmark of ELS, this is often not demonstrated in the setting of torsion. Accurate preoperative diagnosis allows for treatment with sequestrectomy, which is well tolerated, and often abruptly terminates the patient's symptoms [3,10,12,14].
Patient consent
Written informed consent was obtained for this case report.
Footnotes
Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gabelloni M, Faggioni L, Accogli S, Aringhieri G, Neri E. Pulmonary sequestration: what the radiologist should know. Clin Imaging. 2021;73:61–72. doi: 10.1016/j.clinimag.2020.11.040. [DOI] [PubMed] [Google Scholar]
- 2.Choe J, Woo Goo H. Extralobar pulmonary sequestration with hemorrhagic infarction in a child: preoperative imaging diagnosis and pathological correlation. Korean J Radiol. 2015;16:662–667. doi: 10.3348/kjr.2015.16.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi K, Ono A, Yamada A, Toyooka M, Takahashi T, Shigematsu Y. Two adult cases of extralobar pulmonary sequestration: a non-complicated case and a necrotic case with torsion. Pol J Radiol. 2014;79:145–149. doi: 10.12659/PJR.890662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Son SA, Do YW, Kim YE, Lee SM, Lee DH. Infarction of torsed extralobar pulmonary sequestration in adolescence. Gen Thorac Cardiovasc Surg. 2020;68:77–80. doi: 10.1007/s11748-019-01105-7. [DOI] [PubMed] [Google Scholar]
- 5.Uchida DA, Moore KR, Wood KE, Pysher TJ, Downey EC. Infarction of an extralobar pulmonary sequestration in a young child: diagnosis and excision by video-assisted thoracoscopy. J Laparoendosc Adv Surg Tech A. 2010;20:399–401. doi: 10.1089/lap.2009.0217. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Yang G. Extralobar pulmonary sequestration with a complication of torsion. Medicine. 2020;99:29. doi: 10.1097/MD.0000000000021104. (e21104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokota R, Sakamoto K, Urakawa H, Takeshita M, Yoshimitsu K. Torsion of right lung sequestration mimicking a posterior mediastinal mass presenting as acute abdomen: usefulness of MR imaging. Radiol Case Rep. 2019;14:551–554. doi: 10.1016/j.radcr.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Wagner L, Boyd T, Nagarajan R, Dasgupta R. Extralobar pulmonary sequestration presenting with torsion: a case report and review of literature. J Pediatr Surg. 2011;46:2025–2028. doi: 10.1016/j.jpedsurg.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Gawlitza M, Hirsch W, Weiber M, Ritter L, Metzger RP. Torsion of extralobar lung sequestration – lack of contrast medium enhancement could facilitate MRI-based diagnosis. Klin Padiatr. 2014;226:38–39. doi: 10.1055/s-0033-1351319. [DOI] [PubMed] [Google Scholar]
- 10.Huang EY, Monforte HL, Shaul DB. Extralobar pulmonary sequestration presenting with torsion. Pediatr Surg Int. 2004;20:218–220. doi: 10.1007/s00383-004-1156-0. [DOI] [PubMed] [Google Scholar]
- 11.Kanauchi N, Kato H, Endo M, Okazaki T. Torsion of extralobar pulmonary sequestration. Eur J Cardiothorac Surg. 2011;39:e31. doi: 10.1016/j.ejcts.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Kirkendall ES, Guiot AB. Torsed pulmonary sequestration presenting with gastrointestinal manifestations. Clin Pediatr. 2013;52:981–984. doi: 10.1177/0009922812453197. [DOI] [PubMed] [Google Scholar]
- 13.Lima M, Randi B, Gargano T, Tani G, Pession A, Gregori G. Extralobar pulmonary sequestration presenting with torsion and associated hydrothorax. Eur J Pediatr Surg. 2010;20:208–210. doi: 10.1055/s-0029-1241837. [DOI] [PubMed] [Google Scholar]
- 14.Maull KI, McElvein RB. Infarcted extralobar pulmonary sequestration. Chest. 1975;68:98–99. doi: 10.1378/chest.68.1.98. [DOI] [PubMed] [Google Scholar]
- 15.Nakano T, Tetsuka K, Yamamoto S, Endo S. Strangulation of aberrant artery in extralobar pulmonary sequestration on video imaging. Interact Cardiovasc Thorac Surg. 2014;19:324–325. doi: 10.1093/icvts/ivu103. [DOI] [PubMed] [Google Scholar]
- 16.Pinto Filho DR, Avino AJ, Brandao SL. Extralobar pulmonary sequestration with hemothorax secondary to pulmonary infarction. J Bras Pneumol. 2009;35:99–102. doi: 10.1590/s1806-37132009000100015. [DOI] [PubMed] [Google Scholar]
- 17.Shah R, Carver TW, Rivard DC. Torsed pulmonary sequestration presenting as a painful chest mass. Pediatr Radiol. 2010;40:1434–1435. doi: 10.1007/s00247-010-1558-1. [DOI] [PubMed] [Google Scholar]
- 18.Coleman AM, Merrow AC, Crombleholme TM, Jaekle R, Lim FY. Fetal MRI of torsed bronchopulmonary sequestration with tension hydrothorax and hydrops in a twin gestation. Fetal Diagn Ther. 2016;40:156–160. doi: 10.1159/000371513. [DOI] [PubMed] [Google Scholar]
- 19.Zucker EJ, Tracy DA, Chwals WJ, Solky AC, Lee EY. Paediatric torsed extralobar sequestration containing calcification: imaging findings with pathological correlation. Clin Radiol. 2013;68:94–97. doi: 10.1016/j.crad.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Chong NV, Polley TZ, Geiger JD. Infarction of extralobar pulmonary sequestration after blunt trauma. J Pediatr Surg. 2007;42:1127–1129. doi: 10.1016/j.jpedsurg.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 21.Tetsuka K, Endo S, Kanai Y, Yamamoto S. Extralobar pulmonary sequestration presenting as hemothorax. Interact Cardiovasc Thorac Surg. 2009;9:547–548. doi: 10.1510/icvts.2009.209254. [DOI] [PubMed] [Google Scholar]