Abstract
Background and Aims
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with altered gut microbiota composition. Phylogenetic groups of gut bacteria involved in the metabolism of short chain fatty acids (SCFAs) were depleted in SARS-CoV-2–infected patients. We aimed to characterize a functional profile of the gut microbiome in patients with COVID-19 before and after disease resolution.
Methods
We performed shotgun metagenomic sequencing on fecal samples from 66 antibiotics-naïve patients with COVID-19 and 70 non–COVID-19 controls. Serial fecal samples were collected (at up to 6 times points) during hospitalization and beyond 1 month after discharge. We assessed gut microbial pathways in association with disease severity and blood inflammatory markers. We also determined changes of microbial functions in fecal samples before and after disease resolution and validated these functions using targeted analysis of fecal metabolites.
Results
Compared with non–COVID-19 controls, patients with COVID-19 with severe/critical illness showed significant alterations in gut microbiome functionality (P < .001), characterized by impaired capacity of gut microbiome for SCFA and L-isoleucine biosynthesis and enhanced capacity for urea production. Impaired SCFA and L-isoleucine biosynthesis in gut microbiome persisted beyond 30 days after recovery in patients with COVID-19. Targeted analysis of fecal metabolites showed significantly lower fecal concentrations of SCFAs and L-isoleucine in patients with COVID-19 before and after disease resolution. Lack of SCFA and L-isoleucine biosynthesis significantly correlated with disease severity and increased plasma concentrations of CXCL-10, NT- proB-type natriuretic peptide, and C-reactive protein (all P < .05).
Conclusions
Gut microbiome of patients with COVID-19 displayed impaired capacity for SCFA and L-isoleucine biosynthesis that persisted even after disease resolution. These 2 microbial functions correlated with host immune response underscoring the importance of gut microbial functions in SARS-CoV-2 infection pathogenesis and outcome.
Keywords: Coronavirus, Gut Microbiome, Microbial Functions, SCFAs
Abbreviations used in this paper: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; FDR, false discovery rate; IQR, interquartile range; LDH, lactate dehydrogenase; MaAsLin, multivariate analysis by linear models; NT-proBNP, N-terminal B-type natriuretic peptide; PLT, levels of platelet count; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCFA, short chain fatty acid
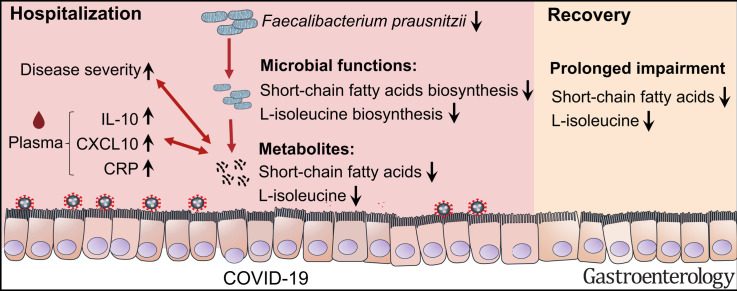
Graphical abstract
Gut microbiome of patients with coronavirus disease 2019 displayed impaired capacity for short chain fatty acids and L-isoleucine biosynthesis. Further studies are needed to understand how impaired gut microbial functions are involved in disease outcome.
See editorial on page 394.
What You Need to Know.
Background and Context
Coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with altered gut microbiota composition. The authors characterized a functional profile of the gut microbiome and examined fecal metabolites in patients with COVID-19 before and after disease resolution.
New Findings
Patients with COVID-19 displayed impaired capacity for short-chain fatty acid (SCFA) and L-isoleucine biosynthesis in their gut microbiome that persisted after recovery and correlated with disease severity and host immune responses.
Limitations
It is an observational study without clear cause or consequence effect established. Further studies are required to determine whether these changes to the gut microbiome functions directly affect COVID-19 severity.
Impact
These findings indicate perturbations of gut microbial functions including decreased SCFA and L-isoleucine biosynthesis in COVID-19 before and after disease resolution. Strategies to supplement SCFA or L-isoleucine might be developed to improve disease outcome.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily infects the respiratory system but also affects other organs including the gastrointestinal tract.1, 2, 3, 4 Recent studies have reported altered gut microbiome in SARS-CoV-2 infection,5, 6, 7 characterized by depletion of beneficial (butyrate-producing) bacteria, such as several genera from the Ruminococcaceae and Lachnospiraceae families and enrichment of opportunistic pathogens including Streptococcus, Rothia, Veillonella, and Actinomyces. 8 These alterations persisted even after recovery from COVID-19. Under-representation of several gut commensals with known immunomodulatory potential in fecal samples of patients with COVID-19, including Faecalibacterium prausnitzii, Eubacterium rectale, and Bifidobacterium, reflects disease severity and dysfunctional host immune responses.9
The gut microbiota is known to regulate host immune responses to respiratory viral infections.10, 11, 12 Metabolites secreted by gut microbiome (such as tyrosine) could protect from influenza through the production of type I interferons and inflammasome-dependent cytokines by pulmonary cells.13 , 14 Whether microbial-derived metabolites also regulate host immune response to SARS-CoV-2 infections remains unknown. Interestingly, an in vitro experiment demonstrated that butyrate down-regulated genes essential for SARS-CoV-2 infection, such as angiotensin-converting enzyme 2, and up-regulated toll-like receptor antiviral pathways in gut epithelial organoids.15
Important microbial activities are a reflection of cumulative functions of the whole community of gut microbiota and the balance of the community and its output determines the net contribution to health or disease.16 Deciphering the role of microbial-derived butyrate or other metabolites in SARS-CoV-2 pathogenesis and severity can shed light on our understanding of possible mechanistic links between gut microbiota and host defense against SARS-CoV-2.
In this study, we prospectively recruited 66 antibiotic treatment-naïve patients with COVID-19 and followed them up from hospital admission until 30 days after discharge. We characterized alterations and longitudinal dynamics of the functions of the gut microbiome in association with disease severity and immune response using metagenomic analysis. We further validated our findings using targeted metabolomics analysis to examine alterations in fecal microbial metabolites.
Methods
Subject Recruitment and Sample Collection
This study was approved by the Clinical Research Ethics Committee (reference number 2020.076), and all patients provided written informed consent. As described in our previous study,6 , 9 COVID-19 subjects were recruited at the Prince of Wales and United Christian Hospitals in Hong Kong. Inclusion criteria included: SARS-CoV-2 reverse transcription polymerase chain reaction positivity based on respiratory specimens, hospitalization, and no probiotics, prebiotics, and antibiotics use within 3 months before enrollment. Patients were classified into 4 severity groups based on symptoms as reported by Wu et al.17 Briefly, patients were classified as mild if there were no radiographic indications of pneumonia, moderate if pneumonia with fever and respiratory tract symptoms were detected, severe if there was respiratory rate ≥30 breaths per minute, oxygen saturation ≤93% when breathing ambient air, or PaO2/FiO2 ratio ≤300 mm Hg, and critical if there was respiratory failure requiring mechanical ventilation or organ failure requiring intensive care. Blood and stools from hospitalized patients were collected by hospital staff, whereas discharged patients provided stools during follow-up visits. Samples were stored at -80°C until processing. The pneumonia controls who were hospitalized with community-acquired pneumonia were recruited. Inclusion criteria included: SARS-CoV-2 reverse transcription polymerase chain reaction–negative status based on respiratory specimens, hospitalization, and no probiotics, prebiotics, and antibiotics use within 3 months before enrollment. The non–COVID-19 subjects were recruited at the Prince of Wales Hospital in Hong Kong from the general population in 2019 before the pandemic as part of a microbiome survey. Inclusion criteria included the following: 18 years of age or older, and had not used probiotics, prebiotics, or antibiotics within 3 months before enrollment.
Stool DNA Extraction
Detailed methods are described in Zuo et al.6 Briefly, approximately 0.1 g fecal sample was prewashed with 1 mL ddH2O and pelleted by centrifugation at 13,000g for 1 min. The fecal DNA was subsequently extracted from the pellet using Maxwell RSC PureFood GMO and Authentication Kit (Promega, Madison, WI) following the manufacturer’s instructions.
Shotgun Metagenomics Sequencing and Profiling
Sequencing libraries were prepared from extracted DNA using the Nextera DNA Flex Library Prep Kit (Illumina, San Diego, CA), and sequenced on an Illumina NovaSeq 6000 System at the Centre for Gut Microbiota Research, Chinese University of Hong Kong. An average of 32 ± 4.6 million reads (6G data) per sample were obtained.
Raw sequence reads were filtered and quality-trimmed using Trimmomatic v0.36 18 as follows: (1) trimming low-quality base (quality score <20), (2) removing reads shorter than 50 bp, and (3) tracing and cutting off sequencing adapters. Contaminating human reads were filtered using Kneaddata v0.7.3 (https://bitbucket.org/biobakery/kneaddata/wiki/Home; reference database: GRCh38 p12) with default parameters.
Profiling of bacterial taxonomy and functional composition was extracted using humann2 v0.11.1 19 from metagenomes, which included taxonomic identification via MetaPhlAn2 by mapping reads to clade-specific markers,20 annotation of species pangenomes through Bowtie2 v2.3 21 with reference to the ChocoPhlAn database, translated search of unmapped reads with DIAMOND v2.0.4 22 against the UniRef90 universal protein reference database,23 and pathway collection from the generated gene list with reference to the Metacyc database.24
Plasma Measurements
Laboratory results at admission including blood count test (platelet count, white blood cell count, neutrophil count) and the plasma concentrations of lactate dehydrogenase (LDH), C-reactive protein (CRP), albumin, hemoglobin, alkaline phosphatase and aspartate aminotransferase, alanine aminotransferase, total bilirubin, and creatinine, were extracted from the electronic medical records in the Hong Kong Hospital Authority clinical management system. Concentrations of cytokines (interleukin [IL]10, IL12, IL1b, IL6, and tumor necrosis factor–α) and chemokines (CXCL8, CXCL10, and CCL2) in patients at admission were measured using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay (Merck Millipore, MA) on a Bio-Plex 200 System (Bio-Rad Laboratories, CA). Concentration of N-terminal B-type natriuretic peptide (NT-proBNP) was measured using Human NT-proBNP enzyme-linked immunosorbent assay kits (Abcam, Cambridge, UK).
Quantification of Fecal Metabolites
The quantification of fecal metabolites was performed by Metware Biotechnology Co., Ltd. (Wuhan, China). Short chain fatty acids (SCFAs), including acetic, propionic, isobutyric, butyric, isovaleric, valeric, and hexanoic acid, were detected using gas chromatography-tandem mass spectrometry analysis. Agilent 7890B gas chromatograph coupled to a 7000D mass spectrometer with a DB-5MS column (30 m length × 0.25 mm inner diameter × 0.25 μm film thickness; J&W Scientific, Folsom, CA) was used. Helium was used as carrier gas, at a flow rate of 1.2 mL/min. Injections were made in the splitless mode and the injection volume was 2 μL. The oven temperature was held at 90°C for 1 minute, raised to 100°C at a rate of 25°C/min, raised to 150°C at a rate of 20°C/min and held at 150°C for 0.6 minutes, further raised to 200°C at a rate of 25°C/min, and held at 200°C for 0.5 minutes. After running for 3 minutes, all samples were analyzed in multiple reaction monitoring mode. The temperature of injector inlet and transfer line were held at 200°C and 230°C, respectively.
L-isoleucine was detected using liquid chromatography-mass spectrometry analysis. Liquid chromatography-electrospray ionization tandem mass spectrometry system (ultra performance liquid chromatography, ExionLC AD, https://sciex.com.cn/; MS, QTRAP 6500+ System, https://sciex.com/) was used for analysis. The analytical liquid chromatography-mass spectrometry conditions for L-isoleucine were as follows: high performance liquid chromatography, column, Waters ACQUITY ultra performance liquid chromatography HSS T3 C18 (100 mm × 2.1 mm inner diameter; 1.8 μm); solvent system, water with 0.05% formic acid (A), acetonitrile with 0.05% formic acid (B). The gradient was started at 5% B (0–10 minutes), increased to 95% B (10–11 minutes), and ramped back to 5% B (11–14 minutes); flow rate was 0.35 mL/min, temperature was 40°C, and injection volume was 2 μL. The electrospray ionization source operation parameters were as follows: ion source, turbo spray; source temperature 550°C; ion spray voltage 5500 V (Positive), -4500 V (Negative); declustering potential and collision energy for individual multiple reaction monitoring transitions was done with further declustering potential and collision energy optimization.
Statistical Analysis
Data on the abundance of bacterial taxa and functionality were imported into R v3.5.1. Data on the functionality was normalized based on relative log expression by Deseq2 (v1.26.0). Nonmetric multidimensional scaling analysis were performed based on Bray-Curtis dissimilarities using vegan package (v2.5-3). Differential microbial functional pathway between patients with COVID-19 and non–COVID-19 controls were identified using Deseq2 (v1.26.0). Associations of microbial pathways with disease severity were identified using the multivariate analysis by linear models (MaAsLin) statistical frameworks implemented in the Huttenhower Lab Galaxy Server (http://huttenhower.sph.harvard.edu/galaxy/). Spearman correlations between microbial pathways, metabolites and the patients’ plasma parameters were assessed using cor and cor.test functions. Heat maps were generated using the pheatmap package (v1.0.10).
Results
Subjects’ Clinical Characteristics
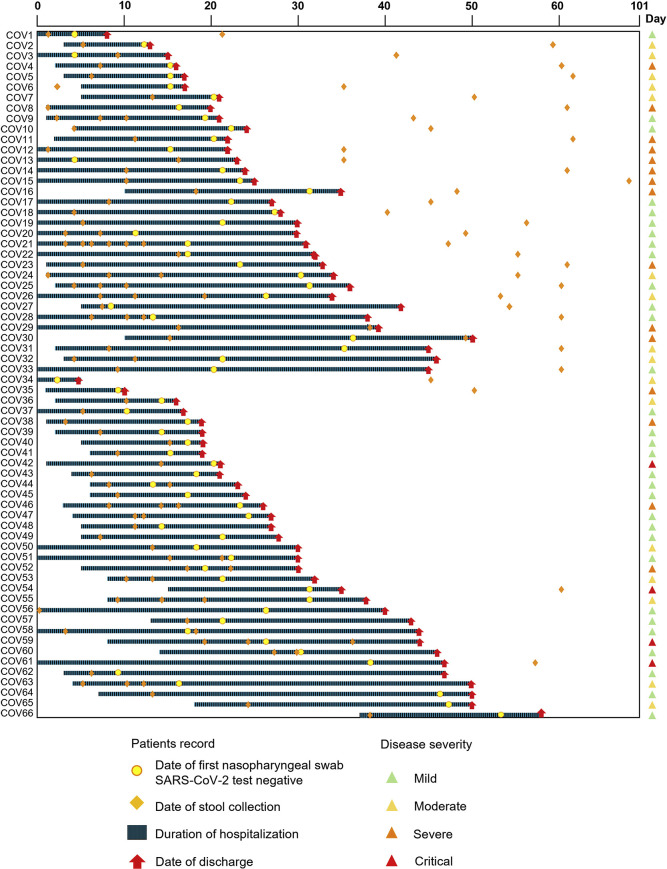
Sixty-six hospitalized patients with laboratory-confirmed SARS-CoV-2 infection (29 males; mean ± standard deviation [SD] age of 42.6 ± 19.0 years) and 70 age- and sex-matched controls (non–COVID-19 controls) were studied. Demographic, clinical characteristics and stool collection schedule are shown in Table 1 and Figure 1 . Median duration of hospitalization for patients with COVID-19 was 24 days (interquartile range [IQR], 4–46 days). Thirty-five patients were followed up after discharge (median [IQR], 28 [18–41] days). All patients were antibiotic-naive and 35 patients (53.0%) received at least 1 anti-viral drug. At admission, 35 patients (53.0%) presented with fever, 8 patients (12.1%) had diarrhea, and 47 patients (71.2%) showed at least 1 respiratory symptom. Patients were stratified as critical (6.1%; n = 4), severe (22.7%; n = 15), moderate (24.2%; n = 16), and mild (47.0%; n = 31), according to the COVID-19 severity classification criteria.17
Table 1.
Clinical Characteristics of COVID-19 Cases and Non–COVID-19 Controls
| Characteristic | COVID-19 cases | Non–COVID-19 controls |
|---|---|---|
| Number of subjects | 66 | 70 |
| Male (%) | 29 (59.1) | 29 (41.0) |
| Age, y (mean ± SD) | 42.6 (± 19.0) | 45.8 (±13.7) |
| Co-morbidities, n (%) | 18 (27.3) | 20 (29.0) |
| Hypertension | 6 (9.1) | 10 (14.3) |
| Hyperlipidemia | 7 (10.6) | 0 (0.0) |
| Heart disease | 1 (1.5) | 0 (0.0) |
| Eczema | 1 (1.5) | 2 (2.9) |
| Morbid obesity | 0 () | 0 () |
| HIV | 2 (3.0) | 0 (0.0) |
| Allergic rhinitis | 2 (3.0) | 7 (10) |
| Asthma | 3 (4.5) | 2 (2.9) |
| Gastric ulcer | 0 (0.0) | 0 (0.0) |
| Acid reflux disease | 0 (0.0) | 0 (0.0) |
| Bowel disease | 0 (0.0) | 5 (7.1) |
| Hemorrhoids | 0 (0.0) | 4 (5.7) |
| Diabetes | 3 (4.5) | 0 (0.0) |
| HBsAg | 3 (4.5) | 0 (0.0) |
| Pulmonary disease | 0 (0.0) | 0 (0.0) |
| Duration of hospitalization | 24 (4–46) | |
| Symptoms at admission, n (%) | ||
| Fever | 35 (53.0) | |
| Gastrointestinal symptoms | ||
| Diarrhea | 8 (12.1) | |
| Respiratory symptoms | 47 (71.2) | |
| Cough | 24 (36.4) | |
| Sputum | 12 (18.2) | |
| Rhinorrhea (runny nose) | 14 (21.2) | |
| Shortness of breath (dyspnea) | 9 (13.6) | |
| Blood result | ||
| Lymphocyte counts (x109/L, normal range, 1.1–2.9, median [IQR]) | 1.2 = (1.0, 1.7) | |
| Antiviral therapy, n (%) | ||
| Kaletra | 30 (45.5) | |
| Oseltamivir | 2 (3.0) | |
| Ribavirin | 14 (21.2) | |
| Interferon Beta-1B | 23 (34.8) | |
| Remdesivir | 9 (13.6) | |
| Death, n (%) | 1 (1.5) |
Figure 1.
Schematic diagram of stool sample collection and hospitalization duration in patients with COVID-19 (n = 66). “CoV” denotes patient with COVID-19. “D0” denotes when the patients reported illness onset.
Altered Gut Microbiome Functional Profile in Patients With COVID-19
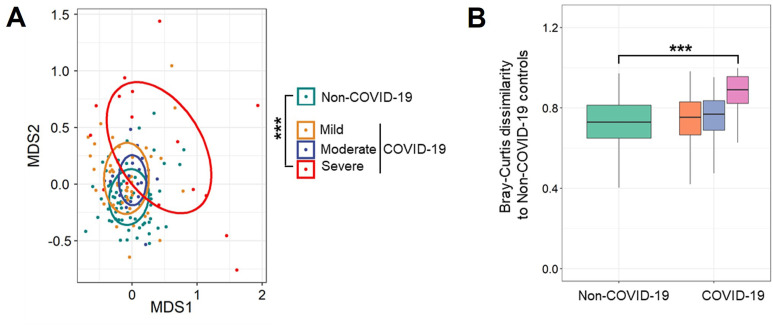
To understand how SARS-CoV-2 infection influences the gut microbiome in antibiotics-naïve patients with COVID-19, ultra-deep metagenomic sequencing was performed on fecal samples from patients with COVID-19 and controls, followed by taxonomic profiling of the fecal microbiome. We first investigated taxonomic composition of gut microbiota in patients with COVID-19 with mild, moderate, and severe/critical illness at baseline (the first stool sample collected after admission). The taxonomic composition in patients with COVID-19 with severe/critical illness was significantly different from that of non–COVID-19 controls (Supplementary Figure 1 A; permutational multivariate analysis of variance [PERMANOVA] test; P < .001), and their Bray-Curtis dissimilarity to controls was significantly higher than that within non–COVID-19 individuals (Supplementary Figure 1 B; Mann-Whitney U test; P < .05). The patients with COVID-19 were primarily characterized by a depletion of Bifidobacterium adolescentis, Ruminococcus bromii, and F prausnitzii and an enrichment of Bacteroides ovatus, Bacteroides dorei, and Bacteroides thetaiotaomicron compared with non–COVID-19 controls (MaAalin2 adjusting for age, gender, and comorbidities; all adjusted P <.05; Supplementary Table 1). Interestingly, decreased Bifidobacterium adolescentis and F prausnitzii in patients with COVID-19 significantly associated with more severe symptom (MaAalin2 adjusting for age, gender, and comorbidities; all adjusted P <.02; Supplementary Table 2). These results suggest that SARS-COV-2 infection is associated with altered composition of gut microbiome.
Supplementary Figure 1.
Altered taxonomic composition of gut microbiome in patients with COVID-19 during hospitalization. (A) The taxonomic composition of gut microbiome in patients with COVID-19 with mild, moderate, and severe/critical illness and non–COVID-19 individuals, viewed using NMDS plot based on Bray-Curtis dissimilarities. The P value of the significance was determined using PERMANOVA analysis and was indicated as ∗∗∗P <.001. (B) The Bray-Curtis dissimilarity of patients with COVID-19 with mild, moderate, and severe/critical illness to non–COVID-19 controls based on relative abundance of species. The P value of the significance was determined using Mann-Whitney U test, and was indicated as ∗P < .05. The first stool sample collected after admission from COVID-19 was used for the analyses.
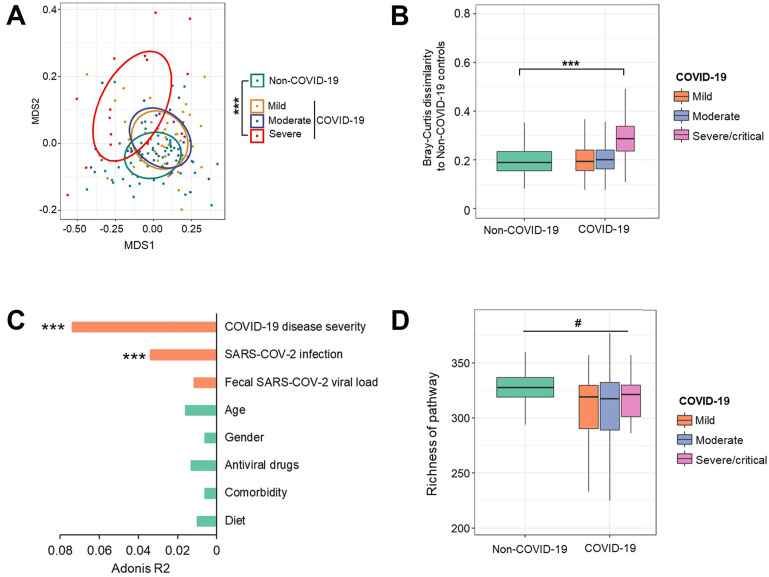
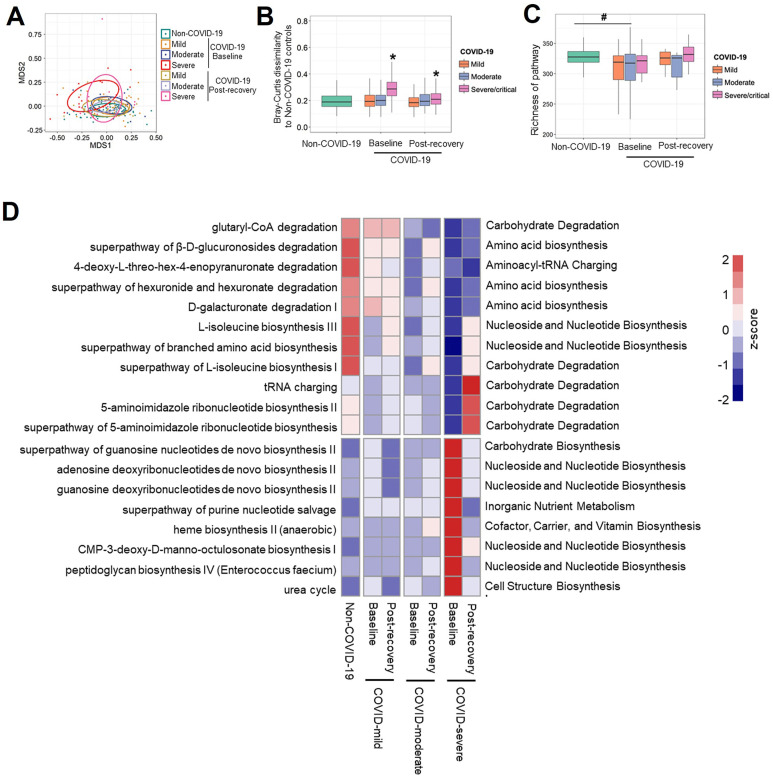
Because there was a difference in gut microbial composition between COVID-19 and non-COVID subjects, we examined whether these compositional differences translated into differences at the functional level by profiling the functional capacity of the gut microbiome, including the functional genes abundance and their corresponding pathways. We first investigated composition of microbial pathways of patients with COVID-19 with mild, moderate, and severe/critical illness at baseline (the first stool sample collected after admission). The patients whose first stool sample were collected after clearance of SARS-CoV-2 were excluded. The composition of microbial pathways in patients with COVID-19 with severe/critical illness was significantly different from that of non–COVID-19 controls (Figure 2 A; PERMANOVA test; P < .001), and their Bray-Curtis dissimilarity to controls was significantly higher than that within non–COVID-19 individuals (Mann-Whitney U test; P < .05; Figure 2 B). Among all host factors examined (age, gender, co-morbidities, COVID-19 disease severity, fecal SARS-CoV-2 viral load, antiviral drugs, and diet), both SARS-CoV-2 infection and COVID-19 disease severity significantly impacted composition of microbial functional pathways, with COVID-19 disease severity showing the largest effect size (R 2 = 0.073; P <.001; PERMANOVA test; Figure 2 C). Diet over the course of hospitalization (Supplementary Table 3) did not show significant effect in the variation of microbial functional pathway (PERMANOVA test; R 2 = 0.01; P = 0.23; Figure 2 C). In addition, patients with COVID-19 showed significantly lower richness of microbial pathways in their feces compared with non–COVID-19 controls (Figure 2 D; P < .05). These results suggest that SARS-COV-2 infection is associated with altered functional profile of gut microbiome as well.
Figure 2.
Altered gut microbiome functional profile in patients with COVID-19 at baseline. (A) The composition of microbial functional pathways in patients with COVID-19 with mild, moderate, and severe/critical illness and non–COVID-19 individuals, viewed using nonmetric multidimensional scaling (NMDS) plot based on Bray-Curtis dissimilarities. The P value of the significance was determined using PERMANOVA analysis and was indicated as ∗∗∗P < .001. (B) The Bray-Curtis dissimilarity of patients with COVID-19 with mild, moderate, and severe/critical illness to non–COVID-19 controls based on abundance of pathways. The P value of the significance was determined using Mann-Whitney U test, and was indicated as ∗∗∗P < .001. (C) The effect size of host factors on the composition of microbial pathways. The effect size and statistical significance was determined via PERMANOVA analysis. ∗, ∗∗, ∗∗∗ indicate P < .05, P < .01, and P < .001, respectively. (D) Richness of microbial pathways in patients with COVID-19 with mild, moderate, and severe/critical illness and non–COVID-19 individuals, evaluated based on Chao1 index. The statistical significance between patients with COVID-19 with non–COVID-19 controls was determined using Mann-Whitney U test, and was indicated as #P < .05.
Depletion of Beneficial Microbial Functions and its Correlation With COVID-19 Severity
We next examined which microbial functions primarily drive the difference in patients with COVID-19 and non-COVID-19 controls. Nineteen microbial pathways were depleted, and 19 pathways were enriched in fecal samples from patients with COVID-19 at baseline compared with non–COVID-19 controls, which were determined using DESeq2 (all adjusted P < .05) and adjusted for age and gender using MaAalin2 (all adjusted P <.05). Seven of nineteen depleted pathways in patients with COVID-19 were related to carbohydrate degradation indicating SARS-CoV-2 infection impaired the gut microbiome’s capacity to degrade carbohydrate. Bifidobacterium shunt pathway involved in biosynthesis of acetic acid showed the greatest reduction (2.4-fold) whereas urea cycle pathway displayed the greatest increase (2.3-fold) in patients with COVID-19 compared with non–COVID-19 controls (Supplementary Table 4). These data suggest that patients with COVID-19 demonstrated impaired capacity for SCFA production and showed enhanced capacity for urea cycle. We then correlated the 38 differential pathways with COVID-19 disease severity via MaAsLin2 analysis adjusting for age, gender, and comorbidities, 11 microbial pathways mainly related to sugar derivative degradation, L-isoleucine biosynthesis, and purine nucleotide biosynthesis, which showed significantly negative correlation with COVID-19 severity (Table 2 ; false discovery rate [FDR] corrected P < .2), and 8 microbial pathways associated with carbohydrate biosynthesis, purine nucleotide biosynthesis, heme biosynthesis, and peptidoglycan biosynthesis, which showed significantly positive correlation with COVID-19 severity (Table 2; FDR corrected P < .2). Sugar derivative degradation was associated with production of pyruvate, which is the key metabolite for SCFA fermentation.25 , 26 L-isoleucine is a crucial mediator in the microbiota-host crosstalk and it plays an important role in regulating host innate and adaptive immunity.27, 28, 29 Alterations in the microbial functions in the gut of patients with COVID-19 may have a significant impact in host physiology and functions.
Table 2.
Microbial Pathways Significantly Correlated With Disease Severity
| Pathway | Subclass | Coefficient | FDR- corrected P value | |
|---|---|---|---|---|
| PWY-5177: glutaryl-CoA degradation | Glutaryl-CoA degradation | -267.0167 | .0143 | Negative correlation with disease severity |
| PWY-3001: superpathway of L-isoleucine biosynthesis I | L-isoleucine biosynthesis | -557.1294 | .0387 | |
| TRNA-CHARGING-PWY: tRNA charging | Aminoacyl-tRNA Charging | -602.3432 | .0387 | |
| PWY-5103: L-isoleucine biosynthesis III | L-isoleucine biosynthesis | -654.6095 | .0387 | |
| BRANCHED-CHAIN-AA-SYN-PWY: superpathway of branched amino acid biosynthesis | Branched amino acid biosynthesis | -647.6653 | .0387 | |
| PWY-6122: 5-aminoimidazole ribonucleotide biosynthesis II | Purine nucleotide biosynthesis | -849.5535 | .0429 | |
| PWY-6277: superpathway of 5-aminoimidazole ribonucleotide biosynthesis | Purine nucleotide biosynthesis | -849.5535 | .0429 | |
| GALACTUROCAT-PWY: D-galacturonate degradation I | Sugar derivative degradation | -236.7136 | .0611 | |
| GALACT-GLUCUROCAT-PWY: superpathway of hexuronide and hexuronate degradation | Sugar derivative degradation | -204.1731 | .0885 | |
| GLUCUROCAT-PWY: superpathway of β-D-glucuronosides degradation | Sugar derivative degradation | -199.9632 | .0885 | |
| PWY-6507: 4-deoxy-L-threo-hex-4-enopyranuronate degradation | Sugar derivative degradation | -174.8413 | .1631 | |
| PWY-1269: CMP-3-deoxy-D-manno-octulosonate biosynthesis I | Sugar nucleotide biosynthesis | 727.2037 | .0043 | Positive correlation with disease severity |
| PWY-7220: adenosine deoxyribonucleotides de novo biosynthesis II | Purine nucleotide biosynthesis | 757.6133 | .0043 | |
| PWY-7222: guanosine deoxyribonucleotides de novo biosynthesis II | Purine nucleotide biosynthesis | 757.6133 | .0043 | |
| PWY-4984: urea cycle | Nitrogen compound metabolism | 337.4701 | .0043 | |
| HEMESYN2-PWY: heme biosynthesis II (anaerobic) | Heme biosynthesis | 519.5936 | .0043 | |
| PWY-6125: superpathway of guanosine nucleotides de novo biosynthesis II | Purine nucleotide biosynthesis | 818.4742 | .0048 | |
| PWY66-409: superpathway of purine nucleotide salvage | Purine nucleotide biosynthesis | 476.2132 | .0170 | |
| PWY-6471: peptidoglycan biosynthesis IV (Enterococcus faecium) | Peptidoglycan biosynthesis | 205.5374 | .0515 |
NOTE. The correlations between pathways and disease severity were determined using MaAsLin2, adjusting for age, gender, and comorbidities. Only the pathways with FDR-corrected P value <0.2 were shown.
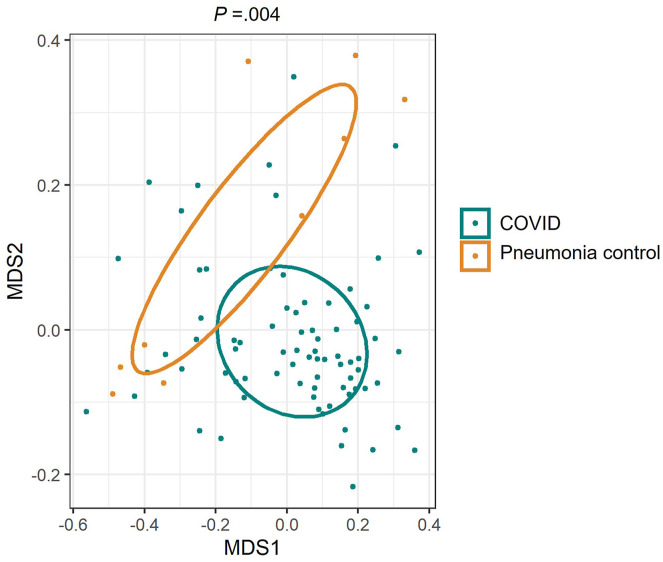
To determine whether hospitalization may contribute to changes of gut microbiome, we have also included patients who were hospitalized with community-acquired pneumonia but who were negative for COVID-19 (Supplementary Table 5). We found that patients with COVID-19 had significantly different gut microbiome function compared with pneumonia cases (Supplementary Figure 3; P = .004; PERMANOVA test). Thirty-eight enriched or depleted microbial pathways were identified by comparing COVID-19 and non–COVID-19 controls (Supplementary Table 4). Twenty-one (51%) were also different between patients with COVID-19 and pneumonia cases including pathways associated with fecal SCFA synthesis and isoleucine production (Supplementary Table 6; FDR corrected P < .2). These data suggest that alterations of gut microbiome functions in patients with COVID-19 are unlikely the result of hospitalization.
Supplementary Figure 3.
The composition of microbial functional pathways in patients with COVID-19 and hospitalized pneumonia controls. NMDS plot was based on Bray-Curtis dissimilarities. The P value of the significance was determined using PERMANOVA analysis.
Correlations Between Microbial Functions of SCFA and L-Isoleucine Biosynthesis and Plasma Measurements
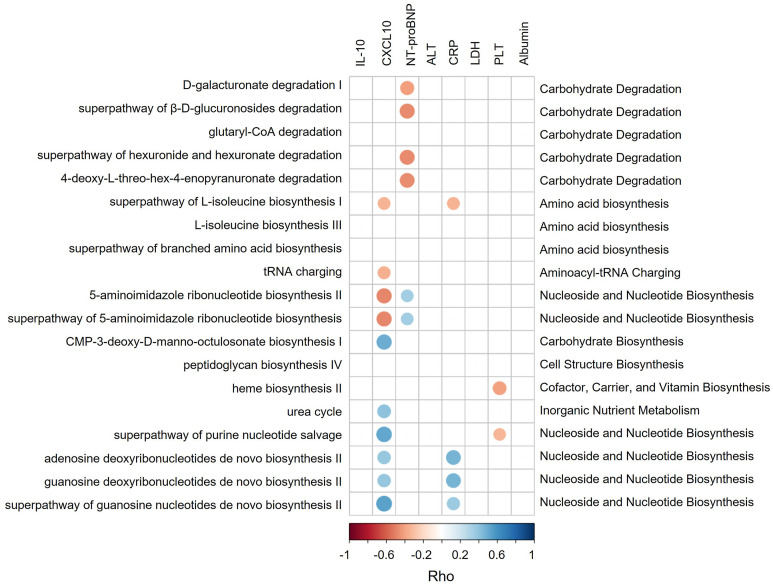
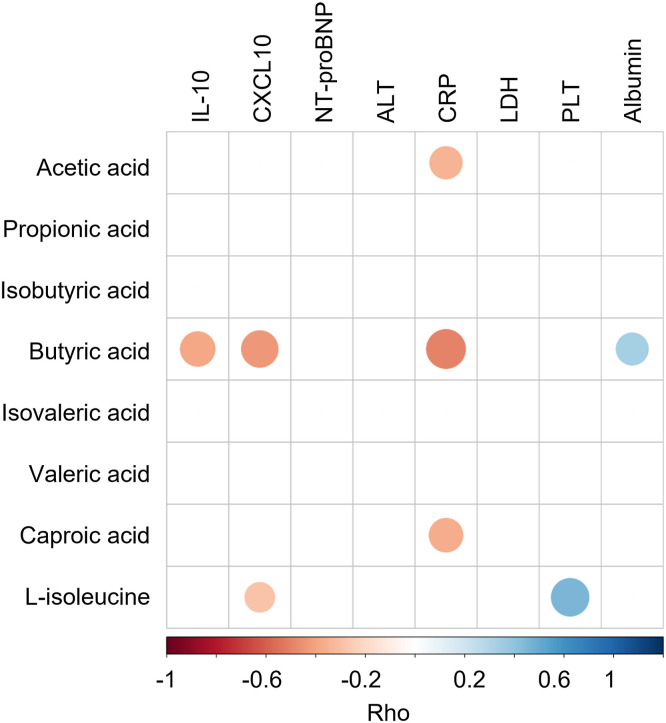
SARS-CoV-2 infection could induce dysfunctional immune responses and consequently cytokine storm syndrome in a subset of patients with COVID-19 leading to more severe disease outcomes.30 Here, we found increased plasma levels of NT-proBNP, IL10, CXCL10, LDH, CRP, and alanine aminotransferase and decreased levels of platelet count (PLT), albumin, and hemoglobin (MaAsLin2; FDR corrected P < .05) significantly associated with more severe symptoms in patients with COVID-19 (Supplementary Table 7), suggesting SARS-CoV-2 infection could induce those changes consequently leading to more severe disease outcomes. We then link these changes in blood measurements (CXCL-10, IL10, NT-proBNP, LDH, CRP, alanine aminotransferase, PLT, and albumin) to the findings of microbial pathways (Table 2) to assess whether microbial functions play a role in dysregulation of the immune response. Four pathways (D-galacturonate degradation I, superpathway of hexuronide and hexuronate degradation, superpathway of β-D-glucuronosides degradation, and 4-deoxy-L-threo-hex-4-enopyranuronate degradation) involved in SCFA production significantly negatively correlated with NT-proBNP (Rho = -0.41, -0.48, -0.48, and -0.47 respectively; Figure 3 ). Superpathway of L-isoleucine biosynthesis I negatively correlated with plasma levels of CXCL-10 (Rho = -0.35) and CRP (Rho = -0.35) (Figure 3). NT-proBNP is heart failure marker that was associated with more adverse clinical outcomes in patients with COVID-19 and increased significantly during the course of hospitalization in those who ultimately died.31 CXCL10 is a pro-inflammatory chemokine known to be associated with poor outcome in COVID-19.32 CRP levels in plasma increased in response to inflammation.33 , 34 These pathways may be involved in preventing overaggressive inflammation in COVID-19. In contrast, urea cycle pathway exhibited positive correlation with CXCL-10 (Rho = 0.40), and heme biosynthesis II pathway showed negative correlation with PLT (Rho = -0.40) (Figure 3). Low platelet count was associated with increased risk of severe disease and mortality in COVID-19.35 Hence, overexpression of these 2 pathways may be associated with more dysfunctional immune responses. Altogether, our data highlight that the gut microbiome might functionally calibrate host immunity against SARS-CoV-2 infection thereby affecting COVID-19 severity.
Figure 3.
Spearman correlations between microbial pathways and plasma measurements. Those pathways or plasma measurements that were significantly correlated with disease severity were plotted. Blue circles and positive values indicate positive correlations, and red circles and negative values indicate inverse correlations. The size and shading indicate the magnitude of the correlation, where darker shades showed higher correlations than lighter ones.
Prolonged Impairment of Microbial Functions of SCFA and L-Isoleucine Biosynthesis After Recovery of COVID-19
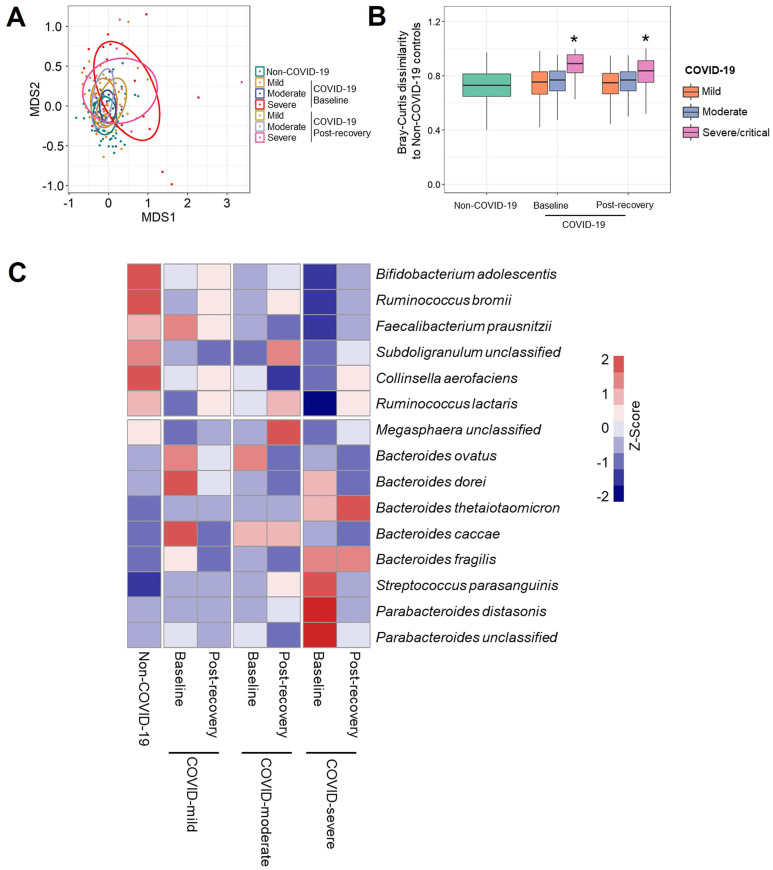
To explore whether gut microbiome and their functions was restored in patients after recovery, fecal samples after discharge (postdischarge days, median [IQR], 28 [18–41] days) were collected from 35 patients (15 with mild disease, 17 with moderate disease, and 13 with severe disease; Figure 1). The patients with severe/critical illness still displayed a significantly different microbiome composition and functional capacity from that of non–COVID-19 controls after disease resolution (Supplementary Figure 2 A and B and Figure 4 A and B), although the richness of the microbial pathway increased to a comparable level with non–COVID-19 controls (Figure 4 C). We then investigated whether differential taxa (Supplementary Table 1) or microbial functional pathways (Table 2) were restored in patients with COVID-19 after disease resolution. Seven bacteria taxa, including Bifidobacterium adolescentis, Ruminococcus bromii, and F prausnitzii, which were depleted in patients’ baseline samples, showed sustainable lower abundance after discharge when compared with non–COVID-19 controls. However, Bacteroides thetaiotaomicron and Bacteroides caccae enriched at baseline still displayed a higher abundance in patients with COVID-19 than non–COVID-19 controls, even after disease resolution (Supplementary Figure 2 C). In terms of microbial functions, all over-represented pathways in severe patients’ baseline samples returned to comparable level with non–COVID-19 controls after discharge. In contrast, 9 of 11 pathways, which were involved in SCFA and L-isoleucine biosynthesis, showed persistent depletion in severe patients after disease resolution (Figure 4 D). These data suggest that gut microbiome functionality in patients was persistently impaired after COVID-19 resolution.
Supplementary Figure 2.
Altered taxonomic composition of gut microbiome in patients with COVID-19 after recovery. (A) The taxonomic composition of gut microbiome in non–COVID-19 controls as well as patients with COVID-19 at baseline and after discharge, viewed using NMDS plot based on Bray-Curtis dissimilarities. (B) The Bray-Curtis dissimilarity of patients with COVID-19 at baseline and after discharge to non–COVID-19 controls based on relative abundance of species. The P value of the significance was determined using Mann-Whitney U test, and was indicated as ∗P < .05. (C) Heat map summarizing changes in gut microbiome at species level in patients with COVID-19 after discharge. Species with higher relative abundances are red, whereas those with lower relative abundances are blue.
Figure 4.
Prolonged impairment of microbial functions of SCFAs and L-isoleucine biosynthesis after recovery of COVID-19. (A) The composition of microbial pathways in non–COVID-19 controls as well as patients with COVID-19 at baseline and after discharge, viewed using NMDS plot based on Bray-Curtis dissimilarities. (B) The Bray-Curtis dissimilarity of patients with COVID-19 at baseline and after discharge to non–COVID-19 controls. The P value of the significance was determined using Mann-Whitney U test and was indicated as ∗P < .05. (C) Richness of microbial pathways in non–COVID-19 controls as well as patients with COVID-19 at baseline and after discharge, evaluated based on Chao1 index. #P < .05 indicates statistical significance for patients with COVID-19 versus non–COVID-19 controls, determined using Mann-Whitney U test. (D) Heat map summarizing changes in gut microbiome functionality in patients with COVID-19 after discharge. The labels on the right side indicate microbial pathways. Pathways with higher abundances are red, whereas those with low relative abundances are blue.
Reduced Fecal Concentrations of SCFAs and L-Isoleucine in COVID-19
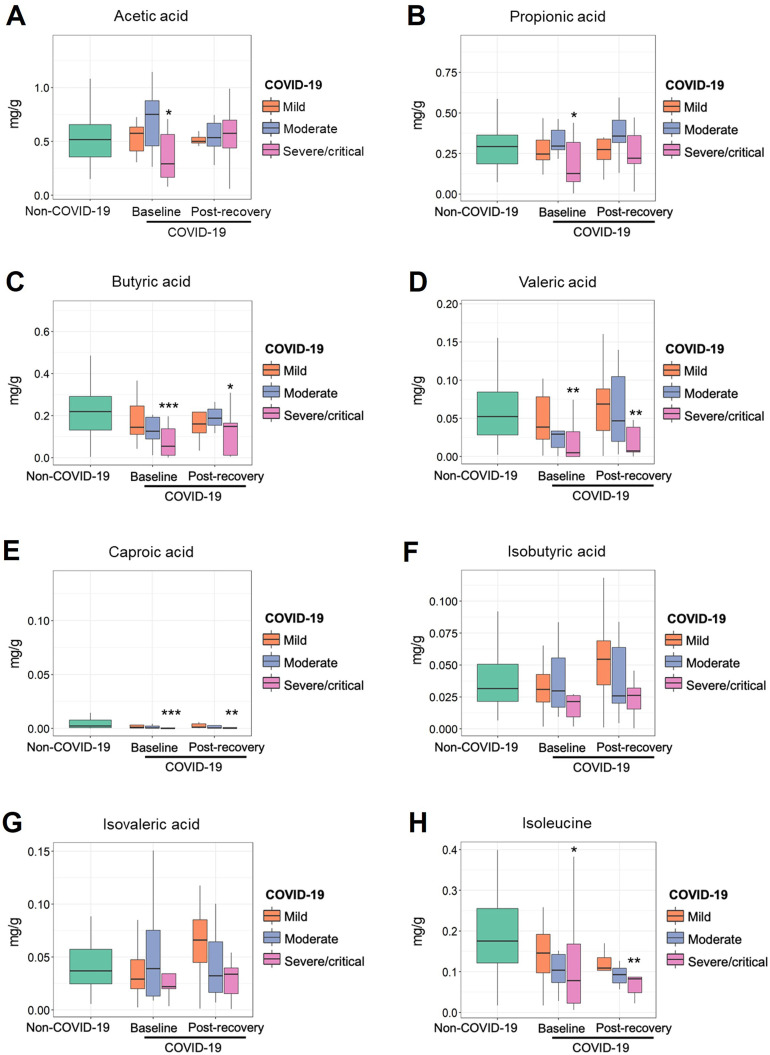
Fecal metabolome provides a functional readout of microbial activity and can be used as a medium for mediating host-microbiome interaction.36 Based on targeted analysis of fecal metabolites in patients with COVID-19 and controls, we found that the changes of gut microbial functions were consistent with alterations of gut microbial metabolites. Several pathways associated with production of SCFAs and L-isoleucine were depleted in patients with COVID-19, especially in those patients with severe/critical disease (Supplementary Table 4 and Table 2). We found fecal concentrations of SCFAs, including acetic acid, propionic acid, butyric acid, valeric acid, and caproic acid, were significantly lower in patients with COVID-19 with severe/critical illness at baseline (the first stool sample collected after admission) than that in non–COVID-19 controls (Figure 5 A–G). The concentrations of L-isoleucine in patients with COVID-19 were lower than that in controls as well (P < .05; Figure 5 H). These results further demonstrated SARS-CoV-2 infection may be associated with impaired capacity of the gut microbiome for SCFA production and L-isoleucine biosynthesis. Intriguingly, beyond 30 days after disease resolution, patients who had severe/critical illness still showed a significantly lower concentration of butyric acid, valeric acid, caproic acid, and L-isoleucine, supporting the idea that SARS-CoV-2 infection may cause a long-lasting effect on gut microbiome function. In addition, we found fecal butyrate level in patients with COVID-19 showed significantly negative correlation with plasma IL10, CXCL-10, and CRP, and positive correlation with albumin (all P < .05; Supplementary Figure 4). L-isoleucine negatively correlated with CXCL-10 and positively correlated with PLT (all P < .05; Supplementary Figure 4). These results suggest that microbiota-derived butyrate and L-isoleucine may be involved in preventing overaggressive inflammation in COVID-19.
Figure 5.
The fecal concentration of acetic acid (A), propionic acid (B), butyric acid (C), valeric acid (D), hexanoic acid (E), isobutyric acid (F), isovaleric acid (G), and. L-isoleucine (H) in patients with COVID-19 and non–COVID-19 controls. The first stool sample collected after admission (baseline group) and the stool collected after discharge (postrecovery group) were used for the targeted metabolome analysis. ∗P <.05, ∗∗P < .01, and ∗∗P<.001 indicate statistical significance for mild, moderate, and severe/critical patients vs non–COVID-19 controls, determined using Mann-Whitney U test.
Supplementary Figure 4.
Spearman correlations between fecal metabolites and plasma measurements. Those plasma measurements significantly correlated with disease severity were plotted. Blue circles and positive values indicate positive correlations, and red circles and negative values indicate inverse correlations. The size and shading indicate the magnitude of the correlation, where darker shades showed higher correlations than lighter ones.
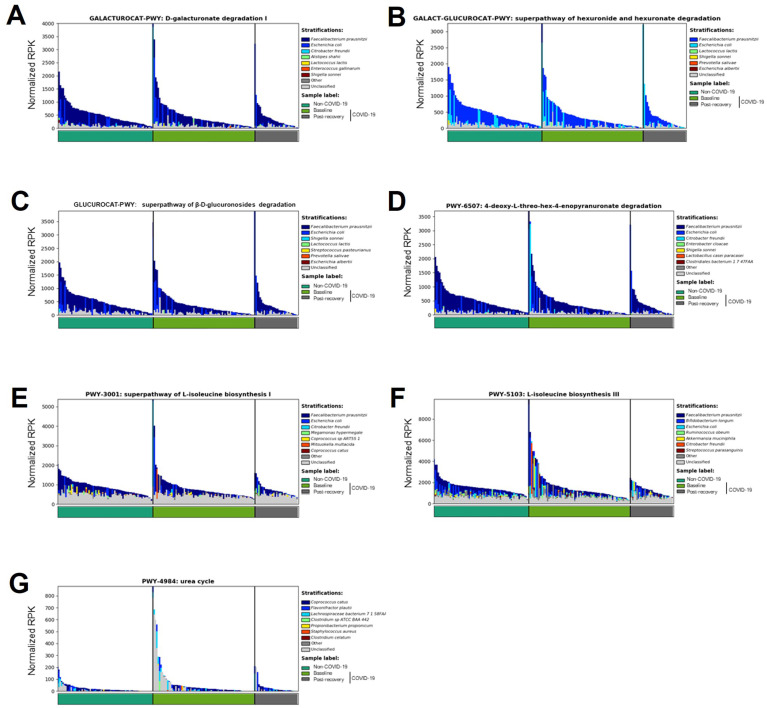
We further examined which bacterial species contributed most to the changes of these microbial pathways. None of the pathways were dominated by any single species, suggesting that alterations of these pathways were likely accounted for by a community-level shift in their functions (Supplementary Figure 5). Notably, the bacterium F prausnitzii was a primary contributor to SCFA-producing pathways (D-galacturonate degradation I, superpathway of hexuronide and hexuronate degradation, and superpathway of β-D-glucuronosides degradation) (Supplementary Figure 5 A–D) and L-isoleucine-producing pathways (L-isoleucine biosynthesis I and L-isoleucine biosynthesis III) (Supplementary Figure 5 E and F), implicating F prausnitzii may have a beneficial role in combating SARS-CoV-2 infection.
Supplementary Figure 5.
Contribution of organisms to microbial pathways in COVID-19 baseline and non–COVID-19 fecal samples. The abundance of microbial pathway was normalized based on relative log expression by Deseq2.
Discussion
Previous studies identified depleted bacterial taxa including Eubacterium, Faecalibacterium, Roseburia, Bifidobacterium, and Lachnospiraceae in patients with COVID-19 compared with controls and inferred that these bacteria may have a functional role in the treatment of COVID-19.6 , 8 , 9 However, mechanistic insights of such findings were limited until recently. To the best of our knowledge, this is the first study to delineate the functional potential and metabolic output of the entire gut microbiome community in COVID-19, which helps support development of microbiota-based therapy for COVID-19.
SCFAs, including butyrate, along with propionate and acetate, can exert anti-inflammatory effects through activating anti-inflammatory immune cells and inhibiting inflammatory signaling pathways.37 In addition, butyrate can maintain the integrity of the gut barrier to prevent translocations and circulation of gut endotoxins and bacteria, thus reducing systemic inflammatory responses.38 Recently, butyrate was found to protect the host from viral infection via down-regulating genes essential for SARS-CoV-2 infection, such as angiotensin-converting enzyme 2, and up-regulating toll-like receptor antiviral pathways in gut epithelial organoids models.15 In this study, we provided direct evidence that SCFA depletion was associated with severe COVID-19 by fecal metabolite measurements and demonstrated that decreased fecal butyrate level was associated with increased plasma levels of pro-inflammatory cytokine IL10 and chemokine CXCL-10, which highlights the importance of SCFAs in COVID-19 pathogenesis and disease severity. Supplementation with SCFAs or SCFA-producing probiotics may have the potential for improvement of disease outcome, although this hypothesis requires further confirmation. Notably, impaired capacity for SCFA biosynthesis and deficient fecal metabolite SCFAs in patients with COVD-19 persisted beyond 30 days after disease resolution which may cause the long-term health complications due to the importance of SCFA in host immunity and metabolism.
The gut microbiota also play a crucial role in manipulating the amino acid pool and profile over the course of amino acid digestion and absorption, thereby mediating the physiological aspects of the host.39 We for the first time found that gut microbiota of patients with COVID-19 had an attenuated capacity for L-isoleucine biosynthesis and they had lower fecal concentrations of L-isoleucine compared with controls. Recent studies have shown that L-isoleucine may induce expression of host defense peptides (ie, β-defensins) that can regulate host innate and adaptive immunity and alleviate detrimental effects of pathogens on humans and animals.28 , 29 In line with that, we found that both L-isoleucine biosynthesis I pathway and fecal L-isoleucine displayed negative correlation with disease severity and a pro-inflammatory chemokine (CXCL-10). These results collectively support that L-isoleucine produced by gut microbiota may alleviate severity of COVID-19 by modulating the immune response of the host to SARS-COV-2 infection. In addition, L-isoleucine is one of the branched chain amino acids considered to be a nutritional supplement to improve central and muscular fatigue by increasing serum concentration of fatigue substances (lactate, ammonia, and 5-HT), energy metabolites (glucose and free fatty acids), and muscle soreness substances (LDH and creatine kinase).40 , 41 Interestingly, we observed that patients after recovery still have persistently impaired L-isoleucine biosynthesis and deficient fecal metabolite L-isoleucine. This may in part explain why COVID-19 survivors reported persistent symptoms of fatigue and muscle weakness.42 Notably, we found the depletion of F prausnitzii primarily contributed to the impaired capacity for SCFA and L-isoleucine biosynthesis in patients with COVID-19. Beyond a depletion of F prausnitzii reported by previous study, we provide possible modes of action that F prausnitzii contributes to disease progression, underscoring the important role of this bacterium in COVID-19.
Furthermore, gut microbiota–dependent urea metabolism is known to correlate with host urea balance and is implicated in disease progression.43 We found that gut microbiome–related urea cycle was enriched in COVID-19 samples and positively correlated with disease severity. Shen at al44 also found that patients with COVID-19 had higher serum concentrations of urea than non–COVID-19 cases. Altered gut microbiome may be associated with disruption of urea cycle functions during COVID-19 infection. However, how disruption of the urea cycle impacts disease outcomes needs further investigation.
This study has several strengths. Subjects were all naïve to antibiotics (which is known to affect the microbiome) and followed up until after discharge with serial stool sample collection. The particularly striking finding was that those distinct characteristics in a person’s gut microbial functions (ie, impaired SCFA biosynthesis) persisted after viral clearance and it is possible that these changes could contribute to the symptoms of so-called “Long-COVID,” including fatigue and muscle weakness. Although this remains speculative, it demands further investigation. Because a hospital diet may contribute to the alteration of microbiome functions, we collected dietary record of patients over the course of hospitalization and found the hospital diet had no significant effect on the variations of the microbiome functions in patients during hospitalization. Collectively, the change of diet is unlikely to account for our findings. This study has some limitations. We unfortunately did not capture the diet of non–COVD-19 controls or that of patients with COVID-19 before disease onset, which precludes us to investigate the virtual effect of diet in gut microbiome alteration between COVID-19 and non–COVID-19, although the hospital diet was aligned with the habitual diets commonly consumed by Hong Kong Chinese people. Moreover, it is an observational study and cannot indicate whether variations in gut microbial functions are determining COVID-19 severity or whether the virus itself has caused this variation. Further studies are required to determine whether these changes to the gut microbiome functions directly affect the severity of COVID-19 in patients, or whether they are simply a consequence of the effects of the infection on the gut and the immune system. Future mechanistic studies are warranted to confirm the impact of SCFA and L-isoleucine on disease severity and their role in preventing viral infection. It is also not certain whether similar changes are observed in patients in other geographic regions.
In conclusion, this study showed perturbations of gut microbial functions including decreased SCAF and L-isoleucine biosynthesis and increased urea production in COVID-19. This highlights a potential link between impaired gut microbiome functions and COVID-19 severity and paves the way to understand the role of the gut microbiome in disease onset and progression in COVID-19.
Acknowledgments
We thank all health care workers working in the isolation wards of Prince of Wales Hospital, Hong Kong, China. We thank Miu Ling Chin, Apple C.M. Yeung, Wendy C.S. Ho, Rity Wong, Vickie Li, and other staff/students for their technical contribution to this study including sample collection, inventory, and processing, and Yao Zeng and Nan Chen for assistance with DNA extraction.
CRediT Authorship Contributions
Siew C. Ng, PhD (Funding acquisition: Lead; Supervision: Lead; Writing – review & editing: Lead). Fen Zhang, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Methodology: Lead; Visualization: Lead; Writing – original draft: Lead). Ya Ting Wan, PhD (Data curation: Lead; Formal analysis: Supporting; Methodology: Supporting; Visualization: Supporting; Writing – original draft: Supporting). Tao Zuo, PhD (Conceptualization: Supporting; Supervision: Supporting; Writing – review & editing: Supporting). Yun Kit Yeoh, PhD (Writing – review & editing: Supporting). Qin Liu, PhD (Methodology: Supporting). Lin Zhang, PhD (Writing – original draft: Supporting). Hui Zhan, PhD (Methodology: Supporting). Wen Qi Lu, PhD (Methodology: Supporting). Wen Ye Xu, PhD (Methodology: Supporting). Grace C.Y. Lui, PhD (Resources: Supporting). Amy Y.L. Li, PhD (Methodology: Supporting). Chun Pan Cheung, PhD (Methodology: Supporting). Chun Kwok Wong, PhD (Resources: Supporting). Paul K.S. Chan, PhD (Resources: Supporting). Francis K.L. Chan, PhD (Resources: Supporting).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by a donation from Koon Wah Mirror Group and was additionally supported in part by InnoHK, The Government of Hong Kong, Special Administrative Region of the People’s Republic of China.
Data availability Raw sequence data generated for this study are available in the Sequence Read (archive under BioProject accession PRJNA689961;. https://www.ncbi.nlm.nih.gov/bioproject/ PRJNA689961).
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2021.10.013.
Supplementary Material
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo T., Liu Q., Zhang F., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y., Zhao Z., Wang Y., et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao F., Sun J., Xu Y., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L., Tong Y., Shen G., et al. Immunodepletion with hypoxemia: a potential high risk subtype of coronavirus disease 2019. MedRxiv. 2020 03.03.20030650. [Google Scholar]
- 6.Zuo T., Zhang F., Lui G.C., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo T., Zhan H., Zhang F., et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159:1302–1310.e5. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu S., Chen Y., Wu Z., et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeoh Y.K., Zuo T., Lui G.C.-Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichinohe T., Pang I.K., Kumamoto Y., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar D., Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abt M.C., Osborne L.C., Monticelli L.A., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steed A.L., Christophi G.P., Kaiko G.E., et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson B.A., Hage A., Kalveram B., et al. Peptidoglycan-associated cyclic lipopeptide disrupts viral infectivity. J Virol. 2019;93:e01282–19. doi: 10.1128/JVI.01282-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J., Richards E.M., Handberg E.M., et al. Butyrate regulates COVID-19-relevant genes in gut epithelial organoids from normotensive rats. Hypertension. 2021;77:e13–e16. doi: 10.1161/HYPERTENSIONAHA.120.16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 17.Wu J., Liu J., Zhao X., et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzosa E.A., McIver L.J., Rahnavard G., et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15:962–968. doi: 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segata N., Izard J., Waldron L., et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 23.Suzek B.E., Wang Y., Huang H., et al. UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics. 2014;31:926–932. doi: 10.1093/bioinformatics/btu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caspi R., Altman T., Billington R., et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2013;42:D459–D471. doi: 10.1093/nar/gkt1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González Hernández M.A., Canfora E.E., Jocken J.W., Blaak E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11:1943. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinson L.F., Gay M.C., Koleva P.T., et al. Human milk from atopic mothers has lower levels of short chain fatty acids. Front Immunol. 2020;11:1427. doi: 10.3389/fimmu.2020.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshii K., Hosomi K., Sawane K., et al. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu C., Mao X., Chen D., et al. Isoleucine plays an important role for maintaining immune function. Curr Protein Peptide Sci. 2019;20:644–651. doi: 10.2174/1389203720666190305163135. [DOI] [PubMed] [Google Scholar]
- 29.Mao X., Gu C., Ren M., et al. L-isoleucine administration alleviates rotavirus infection and immune response in the weaned piglet model. Front Immunol. 2018;9:1654. doi: 10.3389/fimmu.2018.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao L., Jiang D., Wen X.-S., et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:1–7. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliviero A., de Castro F., Coperchini F., et al. COVID-19 pulmonary and olfactory dysfunctions: is the chemokine CXCL10 the common denominator? Neuroscientist. 2021;27:214–221. doi: 10.1177/1073858420939033. [DOI] [PubMed] [Google Scholar]
- 33.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W., Li C., Wang Z., et al. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Science China Life Sci. 2020;63:1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clinica Chimica Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zierer J., Jackson M.A., Kastenmüller G., et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018;50:790–795. doi: 10.1038/s41588-018-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Y., Cai X., Fei W., et al. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2020:1–12. doi: 10.1080/10408398.2020.1854675. [DOI] [PubMed] [Google Scholar]
- 38.Geirnaert A., Calatayud M., Grootaert C., et al. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin R., Liu W., Piao M., et al. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. 2017;49:2083–2090. doi: 10.1007/s00726-017-2493-3. [DOI] [PubMed] [Google Scholar]
- 40.Hormoznejad R., Javid A.Z., Mansoori A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: a systematic review with meta-analysis. Sport Sci Health. 2019;15:265–279. [Google Scholar]
- 41.AbuMoh’d M.F., Matalqah L., Al-Abdulla Z. Effects of oral branched-chain amino acids (BCAAs) intake on muscular and central fatigue during an incremental exercise. J Hum Kinet. 2020;72:69–78. doi: 10.2478/hukin-2019-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J., Tang L., Shen C.-L., et al. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague–Dawley rats. J Nutr Biochem. 2020;81:108395. doi: 10.1016/j.jnutbio.2020.108395. [DOI] [PubMed] [Google Scholar]
- 44.Shen B., Yi X., Sun Y., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.