Abstract
Introduction
Equitable COVID-19 vaccine access is imperative to mitigating negative COVID-19 impacts among racial/ethnic minorities. U.S. racial/ethnic minorities have lower COVID-19 vaccination rates than Whites despite higher COVID-19 death/case rates. The Veterans Health Administration provides the unique context of a managed care system with few access barriers. This study evaluates race/ethnicity as a predictor of Veterans Health Administration COVID-19 vaccination.
Methods
The cohort was composed of Veterans Health Administration outpatient users aged ≥65 years (N=3,474,874). COVID-19 vaccination was assessed between December 14, 2020 and February 23, 2021. Multivariable logistic regressions were conducted, controlling for demographics, medical comorbidity, and influenza vaccination history. Proximity to Indian Health Service Contract Health Service Delivery Areas was tested as a moderator. Data analyses were conducted during 2021.
Results
Blacks (OR=1.28, 95% CI=1.17, 1.40), Hispanics (OR=1.15, 95% CI=1.05, 1.25), and Asians (OR=1.21, 95% CI=1.02, 1.43) were more likely than Whites to receive Veterans Health Administration COVID-19 vaccinations. American Indian/Alaska Natives were less likely than Whites to receive Veterans Health Administration COVID-19 vaccinations, but only those residing in Contract Health Service Delivery Area counties (OR= 0.58, 95% CI= 0.47, 0.72). Influenza vaccine history positively predicted COVID-19 vaccine uptake (OR= 2.28, 95% CI=2.22, 2.34).
Conclusions
In the Veterans Health Administration, compared with the general U.S. population, COVID-19 vaccine receipt is higher among most racial/ethnic minority groups than Whites, suggesting reduced vaccination barriers . The Indian Health Service may provide a safety net for American Indian/Alaska Native populations. Addressing vaccination access barriers in non–Veterans Health Administration settings can potentially reduce racial/ethnic disparities.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic revealed longstanding racial/ethnic healthcare disparities. Black, Hispanic, American Indian/Alaska Native (AI/AN), and Asian subgroups are at higher risk for COVID-19 infection, hospitalization, and death than Whites.1 Explanatory factors include over-representation of racial/ethnic minorities among essential workers, higher population density in racial/ethnic minority neighborhoods, and reduced medical care access.2 These disadvantages reflect the adverse effects of systemic racism on social determinants of health (e.g., education, income, employment, housing) and health outcomes in racial/ethnic minority communities.3
Equitable vaccination access is a key concern for the U.S. COVID-19 vaccine rollout, with Blacks and Hispanics in the U.S. having lower COVID-19 vaccination rates than Whites.4 In prior instances of vaccine distribution,5 structural barriers, including limited healthcare access, limited time for medical appointments, and vaccine supply chain issues, disproportionately affected racial/ethnic minorities.6 Attitudinal barriers, such as institutional mistrust owing to both current and past discrimination in the medical system, also contribute to lower vaccine uptake among racial/ethnic minorities.7 For example, despite greater perceived COVID-19 risk, Blacks report more COVID-19 vaccine hesitancy than Whites.8
The Veterans Health Administration (VA) began administering COVID-19 vaccinations shortly after the U.S vaccine rollout began, allowing examination of COVID-19 vaccination rates among racial/ethnic minorities in a managed care system with few access barriers. This study aims to examine the association between racial/ethnic minority status and VA COVID-19 vaccine uptake. Based on media reports of higher vaccination rates among AI/ANs owing to Indian Health Services (IHSs) use,9 residential proximity to a federally recognized tribal reservation was tested as a moderator of the association between race/ethnicity and vaccine uptake.
METHODS
The cohort was composed of Veterans aged ≥65 years with VA outpatient use between March 1, 2018 and February 29, 2020. Data analyses were conducted during 2021.
Administrative data were used to identify VA COVID-19 vaccination medical procedure codes. A binary variable was created to identify COVID-19 vaccination receipt (0=No, 1=Yes) between December 14, 2020 and February 23, 2021.
Administrative VA data were used to identify demographics. A combined race/ethnicity variable was created (Hispanic, non-Hispanic AI/AN, Asian, Black, Native Hawaiian or other Pacific Islander, Multi-race, White, Unknown). Because VA's initial vaccination efforts targeted older adults,10 analyses were limited to individuals aged ≥65 years. A 2-category age variable (65–74, ≥75 years) was created based on higher COVID-19 illness severity and vaccination rates among older adults.10 , 11 Sex was included based on evidence of higher COVID-19 vaccination rates among women than men.11 , 12
Medical comorbidities associated with COVID-19 severe illness (asthma, end-stage renal disease, chronic pulmonary disease, diabetes, heart disease, immunocompromised, liver disease, severe obesity) were selected as covariates because they may affect vaccination priority status and behavior. Presence or absence of each condition was determined using ICD-10 codes for March 1, 2018‒February 29, 2020 care. To control for health status, Care Assessment Needs scores, which predict likelihood of hospitalization and mortality in VA populations, were included as of February 28, 2020.13
To represent potential access to IHS care,9 Contract Health Service Delivery Area (CHSDA) county designation, which indicates residence in/adjacent to federally reserved tribal lands, was assessed.
To address potential correlation between influenza vaccination and COVID-19 vaccination uptake, influenza vaccination between March 1, 2018 and February 29, 2020 (0=No, 1=Yes) was included as a covariate.
Two logistic regression models were conducted with VA COVID-19 vaccination (0=No, 1=Yes) as the dependent variable. Model 1 included all covariates, and Model 2 added a race/ethnicity X CHSDA interaction term. SEs were clustered by VA facility. Statistical analyses using were conducted using Stata, version 15.1.
This project received a determination of nonresearch from the IRB at the Greater Los Angeles VA Healthcare System.
RESULTS
Veterans (N=3,474,874) age ≥65 years who used VA services between March 1, 2018 and February 29, 2020 were identified (Table 1 ). Most (97%) were male. Overall, 75% were White, 11% Black, 4% Hispanic, 0.7% Asian, 0.6% AI/AN, 0.6% Native Hawaiian or other Pacific Islander, and 0.6% Multi-race. Black and Hispanic patients were younger than Whites and had a higher percentage of women. Overall, 25% of the cohort resided in CHSDA counties compared with 53% of AI/ANs.
Table 1.
Patient Characteristics by COVID-19 Vaccination Status
| Characteristics | No COVID-19 vaccine | COVID-19 vaccine | Total |
|---|---|---|---|
| COVID-19 vaccination status, n (%) | 2,630,980 (75.7) | 843,894 (24.3) | 3,474,874 (100.0) |
| Race/ethnicity, n (%) | |||
| American Indian/Alaska Native | 16,761 (0.6) | 3,416 (0.4) | 20,177 (0.6) |
| Asian | 16,483 (0.6) | 6,195 (0.7) | 22,678 (0.7) |
| Black | 278,965 (10.6) | 112,771 (13.4) | 391,736 (11.3) |
| Hispanic | 111,814 (4.2) | 42,903 (5.1) | 154,717 (4.5) |
| Native Hawaiian/Other Pacific Islander | 15,265 (0.6) | 5,111 (0.6) | 20,376 (0.6) |
| Multi-race | 14,617 (0.6) | 4,633 (0.5) | 19,250 (0.6) |
| Unknown | 187,117 (7.1) | 41,116 (4.9) | 228,233 (6.6) |
| White | 1,989,958 (75.6) | 627,749 (74.4) | 2,617,707 (75.3) |
| Age group, n (%) | |||
| 65–74 years | 1,481,001 (56.3) | 451,531 (53.5) | 1,932,538 (55.6) |
| ≥75 years | 1,149,979 (43.7) | 392,363 (46.5) | 1,542,344 (44.4) |
| Sex, n (%) | |||
| Male | 2,559,753 (97.3) | 821,868 (97.4) | 3,381,621 (97.3) |
| Female | 71,227 (2.7) | 22,026 (2.6) | 93,253 (2.7) |
| Rurality, n (%) | |||
| Urban | 1,562,523 (59.4) | 586,464 (69.5) | 2,148,987 (61.8) |
| Rural | 1,068,457 (40.6) | 257,430 (30.5) | 1,325,887 (38.2) |
| Residence in CHSDA county, n (%) | |||
| Non-CHSDA resident | 1,974,273 (75.0) | 643,499 (76.3) | 2,617,772 (75.3) |
| CHSDA resident | 656,535 (25.0) | 200,364 (23.7) | 856,899 (24.7) |
| Missing | 172 (0.0) | 31 (0.0) | 203 (0.0) |
| Influenza vaccine, n (%) | |||
| No influenza vaccine | 1,037,698 (39.4) | 153,683 (18.2) | 1,191,381 (34.3) |
| Influenza vaccine | 1,593,282 (60.6) | 690,211 (81.8) | 2,283,493 (65.7) |
| Medical comorbidities, n (%) | |||
| Asthma | 80,350 (3.1) | 36,603 (4.3) | 116,953 (3.4) |
| End-stage renal disease | 32,388 (1.2) | 9,180 (1.1) | 41,568 (1.2) |
| Chronic pulmonary disease | 518,024 (19.7) | 182,611 (21.6) | 700,635 (20.2) |
| Diabetes | 872,390 (33.2) | 324,287 (38.4) | 1,196,677 (34.4) |
| Heart disease | 990,093 (37.6) | 362,623 (43.0) | 1,352,716 (38.9) |
| Immunocompromised | 136,204 (5.2) | 52,637 (6.2) | 188,841 (5.4) |
| Liver disease | 126,344 (4.8) | 48,543 (5.8) | 174,887 (5.0) |
| Severe obesity | 95,662 (3.6) | 34,587 (4.1) | 130,249 (3.7) |
| Care assessment need score, mean (SD) | 61.0 (26.1) | 55.1 (26.7) | 56.8 (26.7) |
CHSDA, Indian Health Service Contract Health Service Delivery Area.
Overall, 24% of the cohort received at least 1 COVID-19 vaccine dose as of February 23, 2021. Black (29%), Hispanic (27%), and Asian (27%) patients were significantly more likely than White (24%) patients to receive a COVID-19 vaccination through VA, whereas AI/AN patients were less likely (Table 2 ; Model 1).
Table 2.
Logistic Regression of Associations Between Race/Ethnicity and Veterans Health Administration COVID-19 Vaccination
| Variables | Model 1 |
Model 2 |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Race/ethnicity | ||||
| American Indian/Alaska Native | 0.66 (0.58, 0.76) | <0.01 | 0.86 (0.80, 0.92) | <0.01 |
| Asian | 1.21 (1.02, 1.43) | 0.03 | 1.22 (0.99, 1.50) | 0.06 |
| Black | 1.28 (1.17, 1.40) | <0.01 | 1.28 (1.16, 1.42) | <0.01 |
| Hispanic | 1.14 (1.05, 1.25) | <0.01 | 1.12 (1.01, 1.24) | 0.03 |
| NHOPI | 1.03 (0.96, 1.10) | 0.38 | 1.04 (0.96, 1.13) | 0.29 |
| Multi-race | 0.98 (0.89, 1.08) | 0.69 | 1.01 (0.90, 1.13) | 0.85 |
| Unknown | 0.81 (0.76, 0.87) | <0.01 | 0.82 (0.76, 0.90) | <0.01 |
| Age group, years (ref: 65‒74) | ||||
| ≥75 | 1.23 (1.14, 1.33) | <0.01 | 1.23 (1.14, 1.33) | <0.01 |
| Sex (ref: male) | ||||
| Female | 0.92 (0.89, 0.95) | <0.01 | 0.92 (0.89, 0.95) | <0.01 |
| Care assessment needs score | 1.01 (1.01, 1.01) | <0.01 | 1.01 (1.01, 1.01) | <0.01 |
| Asthma | 1.28 (1.25, 1.32) | <0.01 | 1.28 (1.25, 1.32) | <0.01 |
| End-stage renal disease | 0.84 (0.80, 0.88) | <0.01 | 0.84 (0.80, 0.88) | <0.01 |
| Chronic pulmonary disease | 0.89 (0.88, 0.91) | <0.01 | 0.89 (0.88, 0.91) | <0.01 |
| Diabetes | 1.01 (0.99, 1.03) | 0.44 | 1.01 (0.99, 1.03) | 0.42 |
| Heart disease | 1.02 (1.01, 1.04) | <0.01 | 1.02 (1.01, 1.04) | <0.01 |
| Immunocompromised | 1.12 (1.10, 1.15) | <0.01 | 1.12 (1.10, 1.15) | <0.01 |
| Liver disease | 1.08 (1.05, 1.11) | <0.01 | 1.08 (1.05, 1.11) | <0.01 |
| Severe obesity (ref: no obesity) | 0.95 (0.93, 0.98) | <0.01 | 0.95 (0.93, 0.98) | <0.01 |
| Obesity assessment missing | 0.88 (0.80, 0.97) | 0.01 | 0.88 (0.80, 0.97) | 0.01 |
| Influenza vaccination | 2.28 (2.22, 2.34) | <0.01 | 2.28 (2.22, 2.34) | <0.01 |
| CHSDA | 0.98 (0.83, 1.15) | 0.80 | 0.98 (0.84, 1.15) | 0.82 |
| Race/ethnicity X CHSDA | ||||
| American Indian/Alaska Native X CHSDA | — | — | 0.58 (0.47, 0.72) | <0.01 |
| Asian X CHSDA | — | — | 0.97 (0.74, 1.27) | 0.82 |
| Black X CHSDA | — | — | 1.00 (0.79, 1.27) | 1.00 |
| Hispanic X CHSDA | — | — | 1.10 (0.91, 1.33) | 0.35 |
| NHOPI X CHSDA | — | — | 0.96 (0.84, 1.09) | 0.51 |
| Multi-race X CHSDA | — | — | 0.89 (0.74, 1.07) | 0.22 |
| Unknown X CHSDA | — | — | 0.95 (0.85, 1.07) | 0.42 |
Note: Boldface indicates statistical significance (p<0.05).
Model 1: adjusted for age, gender medical morbidities, CHSDA, and influenza vaccination. Model 2: Model 1 covariates and Race X Time interaction.
CHSDA, Indian Health Service Contract Health Service Delivery Area; NHOPI, Native Hawaiian/Other Pacific Islander.
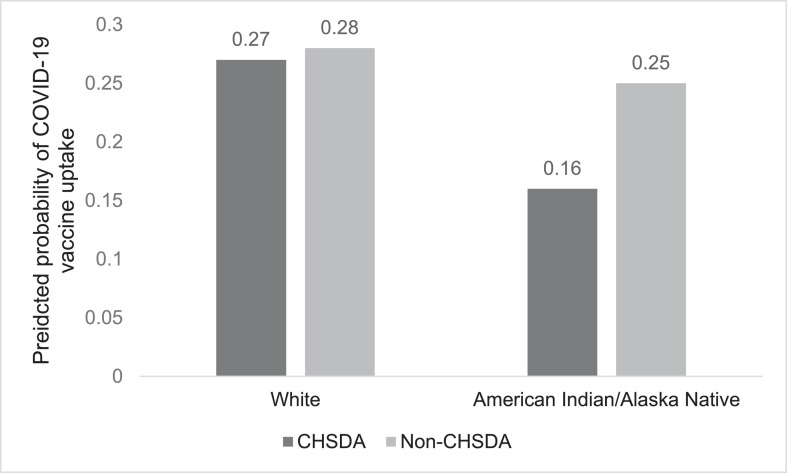
However, a significant race/ethnicity X CHSDA interaction occurred (Model 2): only AI/ANs in CHSDA counties were less likely than Whites to be vaccinated (Figure 1 ). Influenza vaccination history was positively associated with COVID-19 uptake.
Figure 1.
Race/ethnicity and CHSDA residence as predictors of COVID-19 vaccine uptake.
CHSDA, Indian Health Service Contract Health Service Delivery Area.
DISCUSSION
Black, Hispanic, and Asian VA users were more likely than Whites to receive a VA COVID-19 vaccination. By contrast, data from the U.S. general population as of March 1, 2021 showed higher vaccination rates among Whites relative to Blacks, Hispanics, and Asians.4 These findings may reflect factors such as reduced logistical barriers for VA patients than private health care, greater use of non-VA community care among Whites, or higher perceived and actual risk for COVID-19 infection and death among Veterans from racial/ethnic minority communities.1 , 14 VA is a geographically distributed managed healthcare system offering low-cost care to qualifying patients. To maximize COVID-19 vaccine access, VA applied strategies including offering both technology-based and nontechnologic appointment scheduling, walk-ins, and calling eligible Veterans to schedule vaccinations. These measures potentially mitigated structural barriers including cost, convenience, and supply chain disruptions.6
Nonstructural factors may also contribute to higher rates of vaccination among Blacks, Hispanics, and Asians in the VA population. U.S. racial/ethnic minorities report lower trust in the healthcare system than Whites,7 , 15 which may contribute to vaccine hesitancy.15 , 16 Based on evidence of vaccine hesitancy among racial/ethnic minorities, VA used strategies such as listening sessions with diverse Veterans and staff, targeted electronic communications, and outreach activities (e.g., vaccination events) to foster trust before and during the COVID-19 vaccine rollout.
In this study, AI/ANs were less likely than Whites to receive VA vaccinations, but only those residing in CHSDA counties, who presumably had access to IHS care. This finding points to the role of IHS-delivered COVID-19 vaccinations among CHSDA-dwelling AI/ANs.9 Media reports note that AI/ANs prefer receiving COVID-19 vaccination guidance from community members with a shared cultural knowledge, many of whom have partnered with IHS to promote vaccination efforts.9 Thus, lower structural barriers to IHS care because of physical proximity and higher institutional trust in IHS relative to VA may contribute to lower VA COVID-19 vaccination among AI/ANs in CHSDA counties.
Limitations
Because COVID-19 vaccine uptake was measured from VA encounter data, the effects of potential racial/ethnic differences in use of non-VA/alternative vaccination sites cannot be determined. Analogous national data that would contextualize this study's findings by comparing the results against vaccine uptake in the U.S. population among individuals aged ≥65 years were not accessible. Although it is posited that lower vaccine use among AI/ANs could relate to IHS use, that was not assessed directly. The findings may not be generalizable to non-VA Veteran populations.
CONCLUSIONS
Compared with community care, lower logistical barriers to VA use may mitigate some of the racial/ethnic disparities in COVID-19 vaccine uptake. Because AI/ANs were less likely to obtain COVID-19 vaccinations through VA when close to a tribal area, IHS may provide a safety net that is effective at reaching this population despite disparities in other contexts. Addressing vaccination access barriers in non-VA settings can potentially reduce racial/ethnic disparities in COVID-19 vaccination.
Acknowledgments
ACKNOWLEDGMENTS
The views expressed within represent those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
This work was funded by the Department of Veterans Affairs Office of Health Equity and the Quality Enhancement Research Initiative through Grant #PEC-15-239 to the Department of Veterans Affairs Office of Health Equity/Quality Enhancement Research Initiative National Partnered Evaluation Center and by the Department of Veterans Affairs Health Services Research and Development Service through Grant #S DR-20-402.
No financial disclosures were reported by the authors of this paper.
CRediT AUTHOR STATEMENT
Taona P. Haderlein: Formal analysis; Investigation; Software; Writing-original draft. Michelle S. Wong: Formal analysis; Software; Writing-review and editing. Kenneth T. Jones: Conceptualization; Formal analysis; Software; Writing-review and editing. Ernest M. Moy: Conceptualization; Writing-review and editing. Anita Yuan: Formal analysis; Software; Writing-review and editing. Donna L. Washington: Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Writing-original draft.
REFERENCES
- 1.Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Updated September 9, 2021. Accessed April 2, 2021.
- 2.Introduction to COVID-19 racial and ethnic health disparities. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/index.html. Updated December 10, 2020. Accessed April 2, 2021.
- 3.Gray DM, 2nd, Anyane-Yeboa A, Balzora S, Issaka RB, May FP. COVID-19 and the other pandemic: populations made vulnerable by systemic inequity. Nat Rev Gastroenterol Hepatol. 2020;17(9):520–522. doi: 10.1038/s41575-020-0330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndugga N, Hill L, Artiga S, Haldar S. Kaiser Family Foundation; San Francisco, CA: Published October 6, 2021. Latest data on COVID-19 vaccinations race/ethnicity.https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/ [Google Scholar]
- 5.Burger AE, Reither EN, Mamelund SE, Lim S. Black–White disparities in 2009 H1N1 vaccination among adults in the United States: a cautionary tale for the COVID-19 pandemic. Vaccine. 2021;39(6):943–951. doi: 10.1016/j.vaccine.2020.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Fisk RJ. Barriers to vaccination for coronavirus disease 2019 (COVID-19) control: experience from the United States. Glob Health J. 2021;5(1):51–55. doi: 10.1016/j.glohj.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamison AM, Quinn SC, Freimuth VS. “You don't trust a government vaccine”: narratives of institutional trust and influenza vaccination among African American and white adults. Soc Sci Med. 2019;221:87–94. doi: 10.1016/j.socscimed.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunch L. A tale of two crises: addressing Covid-19 vaccine hesitancy as promoting racial justice [published correction appears in HEC Forum. 2021;33(1–2):155] HEC Forum. 2021;33(1–2):143–154. doi: 10.1007/s10730-021-09440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown A. Hard-hit Indian country, tribes rapidly roll out vaccines. Pew Stateline. February 9, 2021 https://pew.org/3a18pyx [Google Scholar]
- 10.How CDC Is making COVID-19 vaccine recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations.html. Updated September 30, 2021. Accessed November 4, 2021.
- 11.People with certain medical conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Updated October 14, 2021. Accessed November 4, 2021.
- 12.COVID data tracker. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker. Updated November 3, 2021. Accessed November 4, 2021.
- 13.Nelson KM, Chang ET, Zulman DM, Rubenstein LV, Kirkland FD, Fihn SD. Using predictive analytics to guide patient care and research in a national health system. J Gen Intern Med. 2019;34(8):1379–1380. doi: 10.1007/s11606-019-04961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niño M, Harris C, Drawve G, Fitzpatrick KM. Race and ethnicity, gender, and age on perceived threats and fear of COVID-19: evidence from two national data sources. SSM Popul Health. 2021;13 doi: 10.1016/j.ssmph.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesch GS, Schwirian KP. Social and political determinants of vaccine hesitancy: lessons learned from the H1N1 pandemic of 2009-2010. Am J Infect Control. 2015;43(11):1161–1165. doi: 10.1016/j.ajic.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the U.S. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]