Abstract
Background
To determine the impact of COVID-19 infection in patients with chronic limb-threatening ischemia, mainly the limb salvage estimates rate and the overall survival.
Methods
This was a retrospective, consecutive cohort study of chronic limb-threatening ischemia in patients with COVID-19 infection.
Results
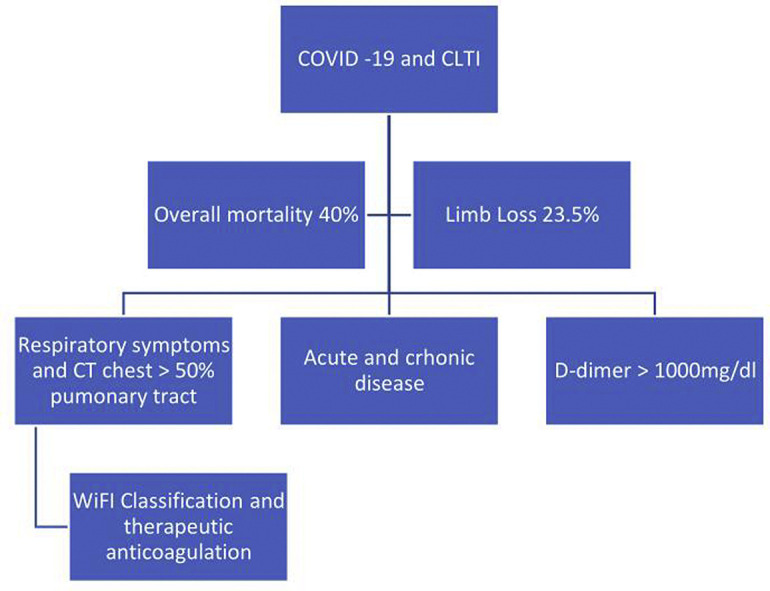
Overall, 35 patients with chronic limb-threatening ischemia and COVID-19 infection were evaluated. The mean age of the patients was 72.51 years, and most of them were male (60%), with arterial hypertension (85.7%), followed by diabetes mellitus (80%) and tobacco user (71.4%). There was a higher prevalence of wound, ischemia and foot infection (WIfI) classification 4 with 58.8% and Rutherford grade 5 (74.3%). The factors related to overall mortality rate were: D-dimer >1,000 mg/dL (hazard ratio = 22.7, P < .001, confidence interval = 10.49–26.52), respiratory symptoms (hazard ratio = 16.6, P < .001, confidence interval = 9.87–20.90), chest computed tomography compromising higher than 50% of the pulmonary tract (hazard ratio = 16,0, P < .001, confidence interval = 10.41–20.55), acute kidney failure (hazard ratio = 21.58, P < .001, confidence interval = 16.5–30.5), chronic kidney disease (hazard ratio = 4.4, P = .036, confidence interval = 1.45–10.1), therapeutic anticoagulation (hazard ratio = 8.37, P = .004, confidence interval = 1.35–8.45), and WIfI classification (hazard ratio = 5.28, P = .022, confidence interval = 1.34–10.01). The following were related to limb loss: D-dimer >1,000 mg/mL (hazard ratio = 5.47, P = .02, confidence interval = 1.94–10.52), respiratory symptoms (hazard ratio = 5.42, P = .02, confidence interval = 1.87–10.90), and WIfI classification (hazard ratio = 4.44, P = .035, confidence interval = 1.34–8.01).
Conclusion
This study concluded that COVID-19 has a catastrophic impact among patients with chronic limb-threatening ischemia. The main factors related to overall mortality were D-dimer >1,000 mg/dL, respiratory symptoms, chest computed tomography compromising higher than 50% of the pulmonary tract, acute kidney failure, chronic kidney disease, therapeutic anticoagulation, and WIfI classification. The factors related to limb loss were WIfI classification, D-dimer >1,000 mg/mL and respiratory symptoms.
Graphical abstract
Introduction
Since the outbreak of COVID-19 infections, it is well known that the virus affects disproportionately patients with cardiovascular disease.1 , 2 Furthermore, SARS-CoV-2, the causative agent of COVID-19, has been shown to establish itself in the host by exploiting angiotensin-converting enzyme 2 as its cellular receptor.3 Moreover, diabetes was present in 42.3% of 26 fatalities due to COVID-19 in Wuhan, China.4 , 5
Notwithstanding the COVID-19 pandemic, chronic limb-threatening ischemia (CLTI) continues to be a life-threatening condition, requiring appropriate intervention to avoid mortality and major amputations.5 The impact of COVID-19 infection on patients with CLTI has not yet been thoroughly studied in the literature. There are case reports in literature showing that patients with CLTI are linked to poorer prognosis when infected with SARS-Cov-2, due to medical comorbidities such as advanced age, hypertension, cardiovascular disease, and diabetes.5
Patients with COVID-19 infection are at risk of developing disseminated intravascular coagulation and thrombotic complications.6 There are studies reporting higher failure rate of revascularization surgery in patients with acute limb ischemia, CLTI, and COVID-19 pneumonia.7 Frequent early recurrent thrombosis and absence of forefoot microcirculation are also described as factors related to major amputations.7
Therefore, the main objective of this study was to determine the impact of COVID-19 infection in patients with CLTI, mainly the limb salvage estimates rate and the overall survival.
Methods
Study design
The study was approved by the Ethical Committee for Research. All patients treated in our institution consented to the use of anonymized and aggregate data linked to the data basis for the purposes of research. No further patient contact was required. This was a retrospective, consecutive cohort study of CLTI patients with COVID-19 infection admitted at the Vascular and Endovascular Surgery Service of the Hospital do Servidor Público Estadual de São Paulo, between March 2020 and March 2021.
Patient data were obtained from the service database using the Microsoft Access software. The patients’ medical records were also consulted as necessary. Information regarding the surgical procedures was obtained from the service database and the patients’ medical records.
Patients
Patients with COVID-19 infection, either symptomatic or asymptomatic, who were admitted with CLTI at our vascular surgery department. The patients were submitted either to revascularization (endovascular or open surgery), debridement, minor or major amputations and clinical treatment, depending on the clinical condition of the patient. Initial technical success of angioplasty was defined as no residual stenosis >30% or dissection at the end of the procedure, together with the prompt restoration of the circulation in the previously stenotic or occluded artery. Procedures such as debridement and minor amputations were performed, as necessary, during hospitalization. The COVID-19 infection was treated and controlled both by the vascular surgeon team and by the infectious disease team, and, if necessary, by the intensivist. All patients admitted at the hospital were submitted to reverse transcription polymerase chain reaction test for SARS-Cov-2. If necessary, serologic tests were performed for SARS-Cov-2. Patients with positive diagnostic for COVID-19 were systematically submitted to chest CT to stratify the pulmonary infection in less or more than 50% of the lungs, or normal. Aspirin at a dose of 100 mg/day and statins were prescribed for all patients before the procedures and continued after the endovascular surgery. Moreover, all patients submitted to endovascular therapy received clopidogrel with a loading dose of 300 mg immediately after the procedure and a maintenance dose of 75 mg/day for 6 months. Therapeutic anticoagulation was administered according to COVID-19 infection and the patient’s clinical condition.
Wound, ischemia and foot infection classification
Wounds were classified according to the Society for Vascular Surgery wound, ischemia and foot infection (WIfI) classification system8 based on 2 blinded and independent providers consensus at initial presentation. The WIfI classification scores wounds on the basis of their wound, infection, and ischemia characteristics to generate a clinical stage that has been shown to correlate with wound healing.
The indications for angioplasty instead of bypass surgeries were essentially based on those defined by the TASC-II and the BASIL study (Bypass versus Angioplasty in Severe Ischaemia of the Leg),8 , 9 and based on the clinical condition of the patients. Patients with severe infection of COVID-19 were submitted to clinical treatment and, in cases of severe infection of the legs, to primary major amputation.
Major amputation was defined by amputation above the ankle. Debridements and minor amputations were performed when necessary. Dressings and antibiotics were administered for patients to treat their infection related to foot infection.
Outcomes
The primary outcome variable was the limb salvage rate and overall mortality. The secondary outcome variables were the factors related to overall mortality, prognosis of the COVID-19 infection and CLTI and factors related to limb loss.
Statistics
Statistical analyses were performed using SPSS 22.0 for MacApple (SPSS Inc, Chicago, IL). The frequencies of patients and descriptive statistics were calculated. The χ2 test or Student’s t test were used for univariate analyses. Analyses of the factors related to overall mortality rate and limb loss were made by univariate and multivariable analysis using logistic regression. Statistical significance was defined as a P value of < .05.
Results
All patients were evaluated during hospitalization until discharge or death. Overall, 35 patients with CLTI and COVID-19 infection were evaluated. The mean age of the patients was 72,51 years, and most of them were male (60%). Regarding the comorbidities, arterial hypertension was the most prevalent (85.7%), followed by diabetes mellitus (80%) and tobacco user (71.4%). There was a higher prevalence of WIfI classification 4 with 58.8% and Rutherford grade 5 (74.3%). All of those data are described in Table I .
Table I.
Patient characteristics
| Variable | Total (N = 35) |
|---|---|
| Age, years | 72.51 ± 10.06 |
| Males | 21 (60%) |
| Hypertension | 30 (85.7%) |
| Diabetes | 28 (80%) |
| Heart disease | 21 (60%) |
| Chronic renal failure | 12 (34.2%) |
| Claudication | 13 (37.1%) |
| WIfI 1 | 18 (51.4%) |
| WIfI 2 | 1 (2.9%) |
| WIfI 3 | 3 (8.8%) |
| WIfI 4 | 20 (58.8%) |
| Rutherford grade | 10 (29.4%) |
| 4 | 3 (8.57%) |
| 5 | 26 (74.3%) |
| 6 | 6 (14.3%) |
Regarding the type of treatment performed, 2 (5.7%) patients were submitted to open bypass surgery (1 iliacfemoral bypass with Dacron and 1 iliacfemoral bypass with basilic arm vein), 15 patients (44.1%) were submitted to endovascular treatment, 2 patients to a hybrid procedure (5.7%), 4 patients to debridements (11.4%), 5 patients to primary major amputations (14.3%) due to WIfI clinical stage 5 (unsalvageable limb at presentation), and 7 patients submitted to a clinical treatment, without surgery (20.6%). The lesion location most prevalent were the toes, with 64.5% of cases. The overall mortality rate was 40%, and the major amputation rate was 23.5% (8 patients), transfemoral amputation 4 patients, and transtibial amputation 4 patients. A subanalysis was performed regarding the type of treatment and mortality rate. Among the 5 patients submitted to primary major amputations, the mortality rate was 80% (4 patients died); the patients submitted to clinical treatment had 42.9% mortality rate (3 patients died). Among the patients submitted to endovascular treatment, the mortality rate was 26.7% (4 patients), and in the open surgical group of patients, there were no deaths. All those data are summarized on Table II . Regarding the overall mortality rate, 100% of the patients died due to COVID-19 pneumonia infection. All patients who died with COVID-19 were ventilator dependent.
Table II.
Patients’ treatment data
| Variable | Total (N = 35) |
|---|---|
| Type of treatment | |
| Open bypass | 2 (5.7%) |
| Endovascular | 15 (44.1%) |
| Hybrid (Femoral endarterectomy + iliac angioplasty) | 2 (5.7%) |
| Debridements | 4 (11.4%) |
| Primary major amputations | 5 (14.3%) |
| Clinical treatment | 7 (20.6%) |
| Endovascular arterial segment | 15 (44.1%) |
| Aortoiliac | 5 (29.4%) |
| Femoropopliteal | 7 (41.2%) |
| Infrapopliteal | 5 (29.4%) |
| Lesion location | |
| Toes | 20 (64.5%) |
| Forefoot | 7 (22.6%) |
| Calcaneus | 4 (12.9%) |
Overall mortality rate 14 (40%)
Major amputation 8 (23.5%)
Regarding the COVID-19 infection, 18 patients (52.9%) had reverse transcription polymerase chain reaction test for SARS-Cov-2 positive. Twenty-three patients (65.7%) had a serology immunoglobulin M (IgM) positive, and 12 patients (34.3%) had a serology immunoglobulin G (IgG) positive. Eighteen patients (51.4%) had respiratory symptoms, and 10 patients (28.6%) had a chest CT showing pulmonary infection compromising >50% of the lungs. Five patients (14.3%) presented acute kidney failure, needing hemodialysis. There were 33 patients (94.3%) who received any kind of anticoagulant drug, most of them endovenous unfractionated heparin (17 patients, 51.5%), and therapeutic anticoagulation was administered for 5 patients (14.3%). Moreover, 11 patients (31.4%) received endovenous dexamethasone. D-dimer was higher than 1,000 mg/dL in 18 patients (51.4%), mean 1,521 mg/dL. Those data are summarized in Table III .
Table III.
Patients; COVID-19 infection data
| Variable | Total = 35 patients |
|---|---|
| RT-PCR test positive | 18 (52.9%) |
| IgM positive serology | 23 (65.7%) |
| IgG positive serology | 12 (34.3%) |
| Respiratory symptoms | 18 (52.9%) |
| Chest CT pulmonary | |
| Normal | 13 (37.1%) |
| Less than 50% | 12 (34.3%) |
| Higher than 50% | 10 (28.6%) |
| Acute kidney failure | 12 (34.3%) |
| Anticoagulation usage | 33 (94.3%) |
| Type of anticoagulation | |
| Enoxaparin | 16 (45.7%) |
| Unfractionated heparin | 17 (48.6%) |
| Full anticoagulation | 5 (14.3%) |
| Dexamethasone usage | 11 (31.4%) |
| D-dimer >1,000 mg/dL | 18 (51.4%) |
CT, computed tomography; RT-PCR, reverse transcription polymerase chain reaction.
A univariate and multivariate logistic regression analysis was performed to analyze the factors related to overall mortality. Among the factors evaluated, the following were related to overall mortality rate: D-dimer >1,000 mg/dL (HR = 22.7, P < .001, CI = 10.49–26.52), respiratory symptoms (HR = 16.6, P < .001, CI = 9.87–20.90), chest CT compromising higher than 50% of the pulmonary tract (HR = 16,0, P < .001, CI = 10.41–20.55), acute kidney failure (HR = 21.58, P < .001, CI = 16.5–30.5), chronic kidney disease (HR = 4.4, P = .036, CI = 1.45–10.1), therapeutic anticoagulation (HR = 8.37, P = .004, CI = 1.35–8.45), and WIfI classification (HR = 5.28, P = .022, CI = 1.34–10.01). These data are summarized in Table IV .
Table IV.
Logistic regression analysis of factors associated with overall mortality rate
| Variable | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| B | HR | 95% CI | P | B | HR | 95% CI | P | |
| Rutherford classification | 0.988 | 0.065 | 0.330–2.760 | .931 | 0.597 | 0.065 | 0.226–10.060 | .880 |
| D-dimer >1,000 mg/dL | 1.805 | 22.7 | 10.49–26.52 | <.001 | 1.296 | 22.7 | 10.49–26.52 | <.001 |
| Chronic kidney disease | 1.592 | 4.40 | 1.45–10.1 | .036 | 2.592 | 4.40 | 1.45–10.1 | .036 |
| Diabetes WIfI classification Respiratory symptoms Chest CT >50% Acute kidney failure Therapeutic anticoagulation |
0.546 3.047 2.285 1.888 2.907 20.220 |
0.220 5.280 5.422 16.0 21.58 8.370 |
0.341–1.272 1.34–10.01 9.87–20.90 10.41–20.55 16.5–30.5 1.35–8.45 |
.339 .022 <.001 <.001 <.001 .004 |
0.564 4.089 2.185 1.978 2.796 20.220 |
0.220 5.280 5.422 16.0 21.58 8.370 |
0.311–14.294 1.34–10.01 9.87–20.90 10.41-20.55 16.5-30.5 1.35–8.45 |
.339 .022 <.001 <.001 <.001 .004 |
CI, confidence interval; CT, computed tomography; HR, hazard ratio.
Patients with WIfI 3-4 had higher mortality rate than WIfI 1-2 (38.2% vs 2.9%, P = .009), in a crosstabs analysis. Furthermore, patients who received therapeutic anticoagulation had higher mortality rate than patients who received prophylactic doses (100% vs 30%, P = .006), in a crosstabs analysis. Among the patients with D-dimer higher than 1,000 mg/dL, 88.9% had pulmonary symptoms (P < .001), and 55.6% had chest CT showing pulmonary infection compromising >50% of the lungs (P = .001).
Regarding major amputation rates, patients with WIfI 3-4 had higher incidence rates than WIfI 1-2 (27.5% vs 0%, P = .01). Furthermore, D-dimer >1,000 mg/dL was also related to higher amputation levels than D-dimer <1,000 mg/dL (20.6% vs 2.9%, P = .018), in a crosstabs analysis. Among the patients submitted to endovascular treatment, there was a 13.3% major amputation rate (2 patients); in the surgical group of patients, there were no major amputations, and in the clinical group of patients, there was a 14.2% major amputation rate (1 patient).
Moreover, a univariate and multivariate logistic regression also was performed to test the factors related to major amputation rate. Among the factors evaluated, the following were related to limb loss: D-dimer >1,000 mg/mL (HR = 5.47, P = .02, CI = 1.94–10.52), respiratory symptoms (HR = 5.42, P = .02, CI = 1.87–10.90), and WIfI classification (HR = 4.44, P = .035, CI = 1.34–8.01). These data are summarized in Table V .
Table V.
Logistic regression analysis of factors associated with limb loss
| Variable | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| B | HR | 95% CI | P | B | HR | 95% CI | P | |
| Rutherford classification | 0.988 | 0.065 | 0.330–2.760 | .931 | 0.597 | 0.065 | 0.226–10.060 | .880 |
| D-dimer >1,000 mg/dL | 3.805 | 5.47 | 1.94–10.52 | .02 | 3.296 | 5.47 | 1.94–10.52 | .02 |
| Chronic kidney disease | 3.582 | 0.220 | 0.45–10.1 | .89 | 0.592 | 0.40 | 0.45–10.1 | .98 |
| Diabetes WIfI classification Respiratory symptoms Chest CT >50% Acute kidney failure |
1.646 3.047 4.286 2.888 3.907 |
1.220 4.40 5.420 0.898 1.58 |
0.341–1.272 1.34–8.01 1.87–10.90 0.41–20.55 065–30.5 |
.339 .035 .02 .78 .98 |
1.564 5.089 4.185 12.978 1.796 |
1.220 4.40 5.420 16.0 0.220 |
0.311–14.294 1.34–8.01 1.87–10.90 0.41–20.55 0.509–3.5 |
.339 .035 .02 .78 .98 |
CI, confidence interval; HR, hazard ratio.
We have performed a control group with CLTI patients treated during the same timeframe 1 year before the onset of the COVID pandemic in our vascular department; 95 infrapopliteal endovascular procedures were performed in 95 patients. The mean age was 72.97 ± 8.3 years, with higher prevalence of male gender (55.6%). Regarding the WIfI classification subanalysis, there were 22 patients WIfI 1-2 (23.2%) and 73 patients WIfI 3-4 (76.8%). The overall mortality rate was 2.1%. The limb salvage rate at 360 days was 92.6%. A simple comparison among this present study, showing a 40% overall mortality rate, demonstrates an almost 20-fold increase in mortality rate, and a major amputation rate of 23.5%, demonstrating a 3-fold increase in limb loss rate with the same period 1 year before the COVID-19 pandemic crisis.
Discussion
The recent COVID-19 pandemic has significantly increased pressure on the healthcare system around the world, leading to important changes in the treatment of patients without COVID-19, resulting in the most difficult access to care with delays in diagnosis and treatment.8, 10 Especially patients with CLTI require rapid revascularization to avoid tissue loss and amputation. Some publications reported that the number of amputations performed in 2020 was significantly greater than the number performed in the same period in 2019, an increase of almost 50%.10, 11 Similarly, in this present article we have noticed an increase in amputation rate, higher than 50%, when compared to past articles published in our department in 2016, 2018, and 2019 (23.5%; 7.7%, 10%, 11%).12, 13, 14 The possible explanation to this increased amputation rate may be related to an introduction of the lockdown in Brazil, which led to the closure of outpatient activities and prevented early observation of patients with CLTI. Patients arrived with more severe forms of CLTI, such as septic ulcers and gangrene, at our vascular surgery department. Furthermore, COVID-19 infection is related to thrombotic complications. Patients infected by this disease are at risk of developing disseminated intravascular coagulation.15 Increased levels of D-dimer and fibrin degradation products and prolonged prothrombin time have been associated with poor prognosis in patients affected by the novel coronavirus.7 Similarly, in this present study, D-dimer >1,000 mg/dL was related to higher major amputation rates than D-dimer <1,000 mg/dL (20.6% vs 2.9%, P = .018). These patients had more severe clinical conditions; some of them were submitted to primary major amputation, without attempt for revascularization, due to high risk and irreversible conditions of the limbs. Another factor related to major amputation rate in this present study was WIfI 3-4, both in a crosstabs analysis and in a logistic regression. Those data are comparable with overall literature, especially in patients with COVID-19 infection, whose severe clinical conditions make the revascularization procedures and limb salvage even more difficult. In the overall literature,11 , 15 , 16 , 17 the WIfI classification was considered more important to limb salvage rates and ulcer/wound healing rates than angiosome concept. Moreover, the WIfI classification 1-2 was associated with faster and higher wound/ulcer healing rates than WIfI classification 3-4. The time to heal the ulcer was faster in the WIfI 1-2 groups than WIfI 3-4 groups (164.82 days vs 251.48; P = .017). Furthermore, WIfI stage is strongly associated with wound healing, improvement of Rutherford stage, amputation rate, and long-term mortality.12 , 15 , 16 In patients with COVID-19 and concomitant CLTI, the WIfI stage continues to be a predictor or mortality and major amputations.
Another result that needs further discussion is the high overall mortality rate among patients with CLTI and COVID-19 infection. D-dimer >1,000 mg/dL and therapeutic anticoagulation were associated with overall mortality rate (HR = 22.7, P < .001, CI = 10.49–26.52; HR = 8.37, P = .004, CI = 1.35–8.35, respectively). Reported evidence of coagulopathy in COVID-19 infection showed increased levels of D-dimer, lactate dehydrogenase, mild to no changes in PT and PTT, and increased levels of antiphospholipid antibodies.17 , 18 A recent systematic review19, 20 showed that elevated levels of D-dimer in patients with COVID-19 are associated with poor prognosis. According to this study, the latest literature review suggests that the D-dimer test can be a reliable predictor of thrombosis growth in COVID-19 and its prognosis. Similarly, therapeutic anticoagulation in this present study was associated with a higher mortality. These findings are similar to those found by Lopes et al,21 in a study comparing the efficacy and safety of therapeutic versus prophylactic anticoagulation in patients hospitalized with COVID-19. The authors concluded that in-hospital therapeutic anticoagulation with rivaroxaban or enoxaparin followed by rivaroxaban to day 30 did not improve clinical outcomes and increased bleeding compared with prophylactic anticoagulation. Therefore, use of therapeutic-dose rivaroxaban, and other direct oral anticoagulants, should be avoided in these patients in the absence of an evidence-based indication for oral anticoagulation. Furthermore, the higher mortality rate associated with patients under therapeutic anticoagulation should be explained by the severe clinical condition of these patients and the pro-coagulation clinical status.
Another factor related to overall mortality rate in the present article was acute and chronic kidney failure (HR = 21.58, P < .001, CI = 16.5–30.5; HR = 4.4, P = .036, CI = 1.45–10.1). These data regarding kidney dysfunction and COVID-19 infection mortality rate are comparable with the global literature. A recent meta-analysis by Ali et al22 showed that mortality is significantly higher in patients with COVID-19 with severe acute kidney injury. According to this meta-analysis, acute kidney injury stage III occurred in 14/701 (2%) of the patients and was associated with an increased risk of in-hospital mortality (hazard ratio = 9.81, 95% CI 5.46–17.65). Another meta-analysis23 showed significant association of chronic kidney disease with severe COVID-19, with no relevant heterogeneity (OR 3.03 [95% CI 1.09–8.47], I2 = 0.0%, Cochran’s Q, P = 0.84). The authors concluded that chronic kidney disease should be regarded as an important factor in future risk stratification models for COVID-19.
In the present study, overall mortality was 40%, and patients with chest CT scan showing severe pulmonary disease and pulmonary symptomatic patients were related to overall mortality in a logistic regression analysis. These data are similar to those found in several studies in literature,23 , 24 especially a Brazilian study among 250,000 patients hospital admissions for COVID-19. The authors showed that in-hospital mortality was 38% (87,515 of 232,036 patients) overall, 59% (47,002 of 79 687) among patients admitted to the ICU, and 80% (36,046 of 45,205) among those who were mechanically ventilated. Moreover, in-hospital mortality among patients younger than 60 years was 31% (4,204 of 13,468) in the Northeast versus 15% (1694 of 11 196) in the South.
This study has some limitations, mainly the sample size and noncomparable analysis with patients with CLTI and nonconcomitant COVID-19 infection. Notably, very few studies have analyzed the impact of COVID-19 infection among patients with CLTI.
In conclusion, this study concluded that COVID-19 has a catastrophic impact among patients with CLTI. The main factors related to overall mortality were D-dimer >1,000 mg/dL, respiratory symptoms, chest CT compromising higher than 50% of the pulmonary tract, acute kidney failure, chronic kidney disease, therapeutic anticoagulation, and WIfI classification. The factors related to limb loss were WIfI classification, D-dimer >1,000 mg/mL, and respiratory symptoms.
Funding/Support
None disclosed.
Conflict of interest/Disclosure
There is no conflict of interest in the present study.
References
- 1.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iida O., Soga Y., Hirano K., et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg. 2012;55:363–370. doi: 10.1016/j.jvs.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;18:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9:575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soares R.A., Vedovello R.S., Medeiros S.C.G., Nunes C.Z., Sian C.A., Jorge P.D.M. COVID-19 diagnosis in a patient with critical limb ischemia: complications and clinical outcomes. J Vasc Bras. 2020;19 doi: 10.1590/1677-5449.200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., et al. China Medical Treatment Expert Group for COVID-19: clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellosta R., Luzzani L., Natalini G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver M.L., Hicks C.W., Canner J.K., et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing better than direct angiosome perfusion in diabetic foot wounds. J Vasc Surg. 2018;68:1473–1481. doi: 10.1016/j.jvs.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58(1S) doi: 10.1016/j.ejvs.2019.05.006. S1–S109.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sena G., Gallelli G. An increased severity of peripheral arterial disease in the COVID-19 era foot wounds. J Vasc Surg. 2020;72:758. doi: 10.1016/j.jvs.2020.04.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Athayde Soares R., Matielo M.F., Brochado-Neto F.C., et al. WIfI classification versus angiosome concept: a change in the infrapopliteal angioplasties paradigm. Ann Vasc Surg. 2021;71:338–345. doi: 10.1016/j.avsg.2020.07.049. [DOI] [PubMed] [Google Scholar]
- 13.de Athayde Soares R., Matielo M.F., Brochado Neto F.C., et al. Impact of calcification and infrapopliteal outflow on the outcome of endovascular treatment of femoropopliteal occlusive disease. JRSM Cardiovasc Dis. 2019;8 doi: 10.1177/2048004019828941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Athayde Soares R., Matielo M.F., Brochado Neto F.C., Martins Cury M.V., Marques R.C., Sacilotto R. Number of infrapopliteal arteries undergoing endovascular treatment is not associated with the limb salvage rate in patients with critical limb ischemia. J Vasc Surg. 2016;64:1344–1350. doi: 10.1016/j.jvs.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills J.L., Sr., Conte M.S., Armstrong D.G., et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–234.e1–2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S) doi: 10.1016/j.jvs.2019.02.016. 3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi C, Wang C, Wang H, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. medRxiv. 2020 2020.2003.2028.20046144. [DOI] [PMC free article] [PubMed]
- 19.Zhang Y., Xiao M., Zhang S., et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rostami M., Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. 2020;13:1265–1275. doi: 10.1080/17474086.2020.1831383. [DOI] [PubMed] [Google Scholar]
- 21.Lopes RD, Furtado RHM, Macedo AVS, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 397:2253–2263. [DOI] [PMC free article] [PubMed]
- 22.Ali H., Daoud A., Mohamed M.M., et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren Fail. 2020;42:393–397. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020:1–2. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhaskaran K., Bacon S., Evans S.J.W., et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet. 2021;6:100109. doi: 10.1016/j.lanepe.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]


