Dear Editor,
Although immune checkpoint inhibitors (ICIs) are potential therapeutic agents for hepatocellular carcinoma (HCC), phase III clinical trials failed to show superiority over placebo in terms of prolonging survival [1]. Several reports stated that vascular endothelial signals affected the immunological microenvironment by upregulating regulatory T cells (Treg) in the tumor. We report a case of HCC with a unique clinical course, wherein administration of the tyrosine kinase inhibitor, lenvatinib, was initially ineffective, but elicited a significant response by re-lenvatinib treatment after treatment with pembrolizumab.
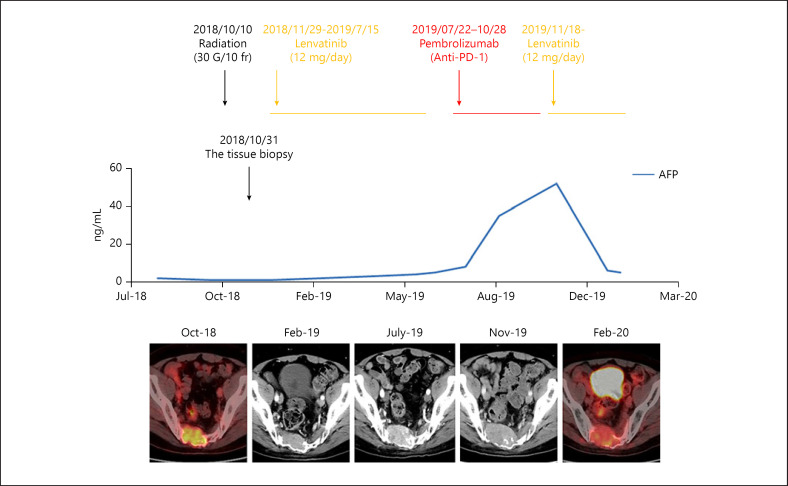
A 56-year-old man was referred to our hospital on April 5, 2011. He was a hepatitis B virus (HBV) carrier with 8.3 log copies/mL of serum HBV-DNA and 52 IU/mL of alanine aminotransferase (ALT). He was treated with entecavir 0.5 mg/day on April 25, 2011, and the serum HBV-DNA level rapidly decreased to an undetectable level. On October 20, 2016, CT revealed HCC measuring 2 cm in the widest diameter in segment 7 (S7) of his liver. Laparoscopic partial hepatectomy was performed on November 22, 2016. In October 2018, he began experiencing dysuria and dyschezia. A metastatic lesion in the sacrum was seen on MRI. This was confirmed by histology through needle biopsy of the sacral lesion. The sacral metastasis was irradiated (30 Gy/10 frequencies) on October 10, 2018. Lenvatinib 12 mg/day was given on November 29, 2018. Despite partial response, tumor progression was observed in July 2019. The microsatellite instability (MSI) detection study (FALCO Biosystems, Kyoto, Japan) using tissues from sacral metastases in 2018 revealed MSI-high phenotype; pembrolizumab was administered on July 22, 2019. Pembrolizumab was discontinued on October 28, 2019, because of rapid tumor growth and increased alpha-fetoprotein (AFP) levels with exacerbation of rectal and bladder disturbance. Because the hepatic reserve was maintained with albumin-bilirubin grade (ALBI) score of −2.93 (Table 1), lenvatinib 12 mg/day was readministered on November 18, 2019, resulting in a rapid decrease in serum AFP levels. On December 23, 2019, serum AFP levels were normal. The metastatic lesion showed significantly decreased fluorodeoxyglucose uptake, revealed by positron emission tomography-CT on January 29, 2020 (clinical courses are shown in Fig. 1).
Table 1.
Laboratory data
| At the time of first administration | At the time of administration after ICI failure | |||
|---|---|---|---|---|
| Blood chemistry | ||||
| TP | 6.9 g/dL | 7.1 g/dL | ||
| Alb | 4.1 g/dL | 4.1 g/dL | ||
| BUN | 16 mg/dL | 15 mg/dL | ||
| Cr | 0.64 mg/dL | 0.69 mg/dL | ||
| T-bil | 0.4 mg/dL | 0.4 mg/dL | ||
| D-bil | 0.1 mg/dL | 0.1 mg/dL | ||
| ALP | 277 U/L | 251 U/L | ||
| AMY | 91 U/L | 70 U/L | ||
| LDH | 189 U/L | 158 U/L | ||
| AST | 16 U/L | 19 U/L | ||
| ALT | 16 U/L | 20 U/L | ||
| γGTP | 20 U/L | 20 U/L | ||
| CRP | 0.158 mg/dL | 0.024 mg/dL | ||
| ALBI score | −2.93 | −2.93 | ||
| ALBI grade | Grade 1 | Grade 1 | ||
|
| ||||
| Coagulation | ||||
| PT | 102.9% | 95.0% | ||
| INR | 0.99 | 1.02 | ||
ICI, immune checkpoint inhibitors; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein; ALBI, albumin-bilirubin; PT, prothrombin time; INR, international normalized ratio.
Fig. 1.
Tumor control by the reintroduction of lenvatinib. The sacral metastasis was irradiated (30 Gy/10 frequencies) on October 10, 2018. After this, lenvatinib 12 mg/day was given on November 29, 2018. A partial response was noticed probably by radiation therapy, but tumor progression was observed in July 2019. The patient received pembrolizumab on July 22, 2019, because of the microsatellite instability-high phenotype confirmed by the MSI detection test (FALCO Biosystems, Kyoto, Japan). Pembrolizumab was discontinued on October 28, 2019, because of the rapid tumor growth and increased AFP levels, accompanied by exacerbation of rectal and bladder disturbance. Lenvatinib 12 mg/day was reintroduced on November 18, 2019, leading to a rapid decrease in serum AFP level. On December 23, 2019, serum AFP was reduced to the normal level with a significant decrease in FDG uptake in the metastatic lesion, shown by positron emission tomography-CT on January 29, 2020. AFP, alpha-fetoprotein; FDG, fluorodeoxyglucose.
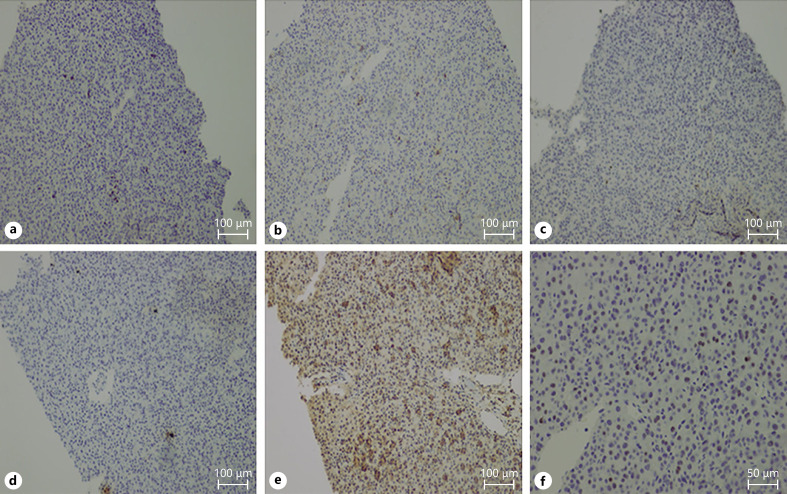
Immunostaining of the needle biopsy specimen of the sacral metastases showed CD4+ and CD8+ cells. CD4+ was predominant in the tumor-infiltrated lymphocytes (TILs) (Fig. 2b, c). We also found the expression of programmed cell death 1 (PD-1) in 10% of the TILs (Fig. 2d). Dense expression of programmed cell death ligand 1 (PD-L1) in the tumor was noted (Fig. 2e). Furthermore, a considerable number of Foxp3+ TILs was seen in the HCC tissues (Fig. 2f).
Fig. 2.
Immunostaining using a needle biopsy specimen of sacral metastases. CD3+ (a), CD4+, and CD8+ cells were observed. CD4+ cells were predominant in the TILs (b, c). We also found the expression of PD-1 in 10% of TILs (d), along with a dense expression of PD-L1 in the tumor (e). (f) A considerable number of Foxp3+ TILs were also observed in HCC tissues. The antibodies used were CD3 and CD4 (Roche Diagnostics, Tokyo, Japan), CD8 (Nichirei Bioscience, Tokyo, Japan), PD-1, PD-L1, and FoxP3 (Abcam, Cambridge, UK). CD3 staining (a), CD4 staining (b), CD8 staining (c), PD-1 staining (d), PD-L1 staining (e), and FOXP3 staining (f). Bar, ×200. TILs, tumor infiltrated lymphocytes; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; HCC, hepatocellular carcinoma.
In this case, lenvatinib was administered after radiation, and the tumor showed a partial response for 30 weeks probably because of the effect of radiotherapy. However, the tumor was noticed to have progressed approximately 8 months after the introduction of lenvatinib. Since the metastatic lesion was MSI-high, pembrolizumab was given as the second-line treatment, resulting in disease progression. The response of pembrolizumab to MSI-high tumor was reportedly 30–35% of cases [2]. ICIs were reportedly refractory to bone lesions in nonsmall-cell lung cancer cases [3]. In addition, a significant number of Treg in the tumor was observed. PD-1 and PD-L1 expression was observed in TILs and tumor cells, respectively. Although the frequency of MSI-H is rare in HCC, which is as low as 1.11% [4], and thus there are no reports for Treg infiltration in HCC with HSI-H, it has been reported that Treg infiltration is high in colorectal cancer with MSI-H [5].
We chose to readminister lenvatinib because the patient refused surgical treatment and expectation of immune modulation effect during long-lasting binding of pembrolizumab to T cells. This lenvatinib re-treatment led to successful shrinkage of the tumor. Vascular endothelial growth factor (VEGF) induces Treg, tumor-associated macrophage, and myeloid-derived suppressor cell. It contributes to the establishment of an immunosuppressive tumor microenvironment [6]. Therefore, VEGF inhibition improves the immunosuppressive microenvironment and restores the antitumor immunity of ICIs. Previous reports have shown that mixed culture of VEGF-producing HCC cells with PBMC increased % Tregs, which was suppressed by the addition of bevacizumab [7]. Previous reports showed that the ability of ICIs to bind with T lymphocytes persisted for at least 20 weeks after the discontinuation of ICIs [8]. In the present case, monotherapy with either lenvatinib or pembrolizumab failed to elicit an antitumor response. However, sequential therapy with ICI followed by readministration of lenvatinib induced a positive response. Immunohistochemical staining showed infiltration of CD4+ and CD8+ cells with expression of PD-1 and PD-L1 on TILs and tumor cells, respectively. These findings suggested that the tumor was sensitive to ICIs. However, dense infiltration of Tregs hampered the effect of ICI monotherapy. The anti-VEGF effect of lenvatinib prevented the antitumor immunity of Treg, promoted the sustained effect of pembrolizumab, and resulted in successful treatment of the tumor. In addition, in this case, lenvatinib could be reintroduced, where preserved hepatic function was considered to be critical for ensuring the efficacy and safety of lenvatinib [9]. In conclusion, not only combination but also sequential therapy using ICI followed by VEGF inhibitors effectively treated a case of HCC that was refractory to anti-VEGF or ICI monotherapy.
Conflict of Interest Statement
Satoru Hagiwara reports honoraria and consulting or advisory fees from Bayer AG and Eisai Co. Ltd.; honoraria from Bayer AG, Bristol Myers Squibb, EA Pharma Co., Ltd., Eisai Co. Ltd., and Merck Sharp & Dohme; and research funding from Bayer AG, Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Merck Sharp & Dohme, Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., and Taiho Pharmaceutical. Masatoshi Kudo is an Editor-in-Chief of Liver Cancer. Naoshi Nishida is an Editorial Board Member of Liver Cancer.
Funding Sources
The authors did not receive any funding.
Author Contributions
All authors contributed to the formal analysis. Conceptualization and methodology: Satoru Hagiwara, Naoshi Nishida, and Masatoshi Kudo; resources: Satoru Hagiwara; writing (original draft): Satoru Hagiwara, Naoshi Nishida, and Masatoshi Kudo.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020 Jan;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 2.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II keynote-158 study. J Clin Oncol. 2020 Jan;38((1)):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landi L, D'Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019 Nov;7((1)):316. doi: 10.1186/s40425-019-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akagi K, Oki E, Taniguchi H, Nakatani K, Aoki D, Kuwata T, et al. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021 Mar;112((3)):1105–13. doi: 10.1111/cas.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, et al. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008 Dec 2;99((11)):1867–73. doi: 10.1038/sj.bjc.6604756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015 Feb;212:139–48. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K, et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009 Mar;29((3)):881–8. [PubMed] [Google Scholar]
- 8.Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018 Oct;3((19)):e59125. doi: 10.1172/jci.insight.59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruta S, Ogasawara S, Ooka Y, Obu M, Inoue M, Itokawa N, et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020 Aug;9((4)):382–96. doi: 10.1159/000507022. [DOI] [PMC free article] [PubMed] [Google Scholar]