Abstract
Introduction
Viscosupplementation is widely practiced, to reduce pain in osteoarthritis (OA), using intra articular (IA) injections of hyaluronic acid (HA). In Europe, these products are class III medical devices, for which the Medical Device Regulation (MDR) requires clinical assessment, based on specific studies and/or a bibliographical review of equivalent devices. The purpose of this article is to present a comparative review between a family of devices (ARTHRUM, from LCA Pharmaceuticals, Chartres, France) and an extensive group of presumed equivalent IA HA devices or their controls, whose results have been published in Scientific journals.
Methods
To meet the criteria used in most ARTHRUM studies, the Western Ontario and McMaster Universities’ index sub-scores were selected for pain (WOMAC A), stiffness (WOMAC B) and function (WOMAC C). The main criterion was the variation of the WOMAC A score from T0 (date of inclusion) to T6 (6 months). The other WOMAC criteria were assessed at T1, T3, T6 and complemented by OMERACT-OARSI rates of responders to the treatment. Fifty articles were selected, containing treatment details on more than 12,000 patients. These were divided into three groups: ARTHRUM, EQUIVALENTS and CONTROLS. To get quantitative comparisons, meta-analyses were performed for each criterion individually. The 95% confidence interval of each difference from baseline, was used to assess the clinical relevance, with reference to a minimum validated in OA literature. Comparisons between groups and tolerance assessment completed the investigation.
Results
For the WOMAC A, B and C scores, the full 95% CI was always above the minimal perceptible clinical improvement (MPCI), in the ARTHRUM and EQUIVALENTS groups, but not for all criteria in the CONTROLS group. In the comparisons, both ARTHRUM and EQUIVALENTS groups were significantly better than the CONTROLS group for each criterion. The effect size (ES) on pain, for the ARTHRUM and EQUIVALENTS groups, varied from 0.28 to 0.56 and from 0.23 to 0.27, respectively. Overall, ARTHRUM was estimated always non-inferior to EQUIVALENTS, and sometimes statistically and clinically superior.
Conclusions
The comparison of ARTHRUM clinical studies, with studies selected through bibliographic research, leads to the conclusion that the clinical efficacy of the ARTHRUM medical devices, to reduce pain and improve the function in knee OA, during a six-month period, is at least as great as those of equivalent products. With good tolerance results (lowest rate of adverse events, and none of them serious), the risk benefit ratio favours using viscosupplementation with ARTHRUM.
Abbreviations: AE, adverse event; CD, Cohen's D (effect size); CI, confidence interval (with probability %); CS, chondroitin sulfate; ES, effect size; GAG, glycosaminoglycan; HA, hyaluronic acid (sodium hyaluronate); IA, intra-articular; KL, Kellgren-Lawrence (radiological OA severity scale); MD, mean difference; MDR, Medical Device Regulation; MPCI, minimal perceptible clinical improvement; MSC, mesenchymal cells; Mw, molecular weight (average in weight); NSAID, non-steroidal anti-inflammatory drug; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International; OMERACT, Outcomes Measurements in Rheumatology (international network); PRP, platelet rich plasma; SD, standard deviation; SAE, serious adverse event; SE, standard error; SF, synovial fluid; SSD, smallest detectable difference; WOMAC, Western Ontario & Mac Master Universities (OA index)
Introduction
Osteoarthritis (OA) is a painful and handicapping disease, affecting a large part of the elderly population. OA is characterized by the loss of hyaluronic acid (HA) which is a major component of the cartilage, naturally present in the synovial fluid (SF) of a healthy joint. HA is a long molecular chain, belonging to the glycosaminoglycan (GAG) family. Its role in the joint is complex1, giving viscoelasticity to SF to absorb shocks, lubricate, and protect the cartilage. HA is also involved in biological mechanisms inside the joint. Viscosupplementation of SF is one symptomatic treatment of knee OA, consisting of intra articular (IA) injections of solutions containing HA, to reduce pain in and restore mobility to the OA joint.
The purpose of this study is to assess the clinical results of a family of IA HA devices (ARTHRUM), and compare them to potentially ‘equivalent’ devices in accordance with the definition given in the Medical Device Regulation (MDR) for Europe ECC93/42 and its imminent replacement, 2017/745. This requires that the clinical evidence for class III devices, should be based first on the specific clinical results obtained for the device under evaluation and/or, on a comparison with other devices identified as ‘equivalent’. These belong to the same generic group as they are basically similar, in formulation (same major HA ingredient), presentation, physical characteristics and primary indication. All these devices are used under the same conditions (intra articular injections), by the same medical doctors, on patients suffering from the same disease (knee OA, at the same stage). The available clinical results for these ‘equivalent’ devices are those published in Scientific Journals, preferably peer-reviewed, to ensure quality. To resume the rationale, it appeared relevant to consolidate the assessment of ARTHRUM, with a comparison to ‘equivalent’ devices.
Methods
Data base
For the renewal of the CE mark for ARTHRUM products, a systematic search of the relevant bibliography was undertaken. The clinical results obtained with ARTHRUM were compared with the results available from the literature, for products described as ‘Equivalents’ or for ‘Controls’. The ARTHRUM products were identified as a family group, to give a sample size comparable with the two other groups. The small differences in formulation for two specific ARTHRUM devices were not taken into account, because most studies were carried out with the same product: ARTHRUM H 2% (3 injections). The first variant in the family was ARTHRUM visc 75 – the single-injection version – concentrating 75mg of the same HA, in one injection. The second variant, called ARTHRUM HCS, contains chondroitin sulfate (CS), added to the HA at same proportion (2%). This unique product is discussed at the end of this article.
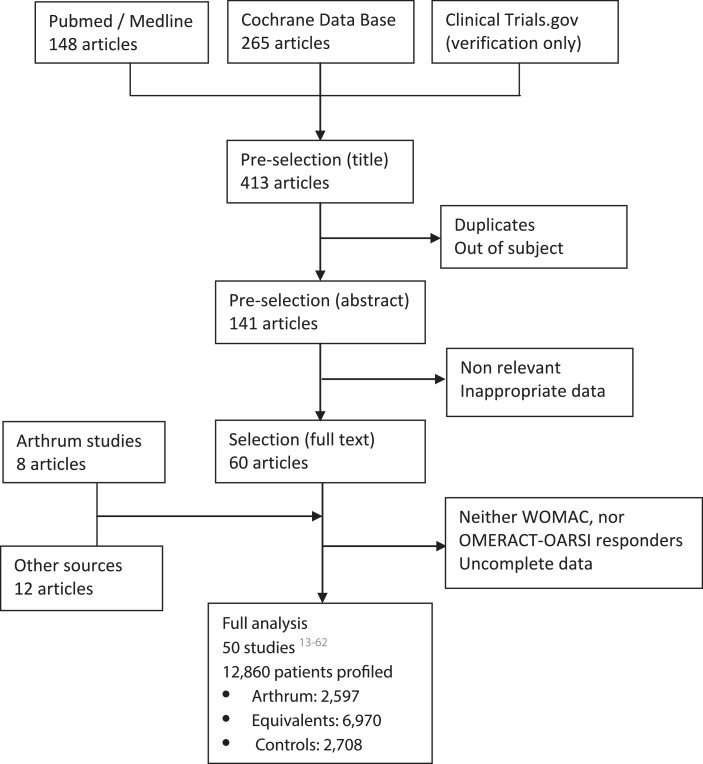
The bibliographical research was carried on PubMed and Cochrane databases, using key words as “hyaluronate”, “viscosupplement”, “knee osteoarthritis”, “intra articular” or “infiltration” as well as “ARTHRUM” and “SYNOVIUM”, to detect any unknown publication on ARTHRUM devices. The word “Human” was used as a filter with PubMed. A control was also done on Clinical Trials, to identify ongoing studies. This research leading to 60 articles, was closed at the end of March 2020 and then it was supplemented with several articles identified in meta-analyses, including those treated with ARTHRUM (Figure 1).
Figure 1.
Data research and collection
The pain sub-score of the Western Ontario and McMaster Universities2 (WOMAC A) index was chosen as the main criterion, as it was used for most of the clinical studies done with ARTHRUM. As secondary measures, the stiffness sub-score (WOMAC B), the function sub-score (WOMAC C) and the OMERACT-OARSI3 rates of responders were also analyzed.
Data collection and treatment has fully been made at LCA. After removal of the articles containing none of above criteria, a comprehensive and complete data was obtained for a large population of 12,860 profiled patients. All patients were assessed, at least from one WOMAC index, or from their OMERACT-OARSI rate of responders (Figure 1). Observation times were those used for the majority of the studies: T0 (at inclusion) then T1, T3 and T6 (months). Separate analyses were performed for each index.
Groups
ARTHRUM devices were grouped-as a family as all were using the same basic ingredients and manufactured under identical strict conditions, including final sterilization. For CONTROLS, the IA placebos were selected, as well as injection shams and arthrocentesis (puncture of SF, without injection). Physical exercises and oral treatments (NSAIDs) were also included. Local active treatments such as IA injections of corticosteroids, platelet rich plasma (PRP) or mesenchymal cells (MSC) were excluded. The group of EQUIVALENTS devices, was the generic group of IA HA products indicated for knee OA symptomatic treatment, called viscosupplements. Any molecular weight (Mw) at any concentration was allowed for the HA, and the presence of an ancillary ingredient was accepted (cross linker agent, mannitol, sorbitol…). This was selected in this way to obtain a realistic representation of the IA HA market.
Statistics
To allow statistical analysis, continuous variables were defined by their mean (score), together with the standard deviation (SD) and the population involved for each observation time. If SD was not available, an estimation of it was made whenever possible (measuring the graph, or starting from the standard error (SE), the 95% CI, or from the p-value). Each score has been converted into a 0-100 base, and all data were treated using Excel (MicroSoft). For discrete variables, data needed to be available under the format of the sub-populations whether satisfying or not, the analyzed criterion.
For each studied data set, the score variation from baseline was assessed, at each available observation time. The result of each measure, has been expressed as a mean difference (MD) from the score at inclusion (baseline), positive in case of improvement for the patient, together with its 95% confidence interval (95% CI). This was done for each arm in individual studies: ARTHRUM devices, CONTROLS, or EQUIVALENTS devices.
Main and secondary criteria
The main criterion is the variation (MD) of the WOMAC pain sub-score (WOMAC A), from T0 (the baseline) to T6 (the final control time). This criterion is satisfied if the lower bound of the 95% CI for the group ARTHRUM, is greater than a clinically recognized minimum, accepted in the current state of the art of medicine. The secondary criteria, include the variations (MD) of the WOMAC A sub-score at other time Intervals, the same for the WOMAC B (stiffness) and WOMAC C (function) sub-scores, and the rates of patients responding to the treatment, according to OMERACT-OARSI criteria, at all available times.
Meta-analyses of the WOMAC sub-scores results
To make a global and comparative assessment of ARTHRUM devices vs CONTROLS, and EQUIVALENTS groups, a meta-analysis has been done using Mix2.0 (BioStatXL), in the mode “random effect”, to determine MD (95% CI) for each group, at times T1, T3 and T6. This has been repeated for each WOMAC sub-score. To complete the analysis, the results for each group were compared together. The result for the ARTHRUM group of devices was considered satisfactory when the average gain from baseline was:
-
•
Greater – for its whole 95% CI – than the Minimum Perceptible Clinical Improvement (MPCI), defined by Ehrich4, for the WOMAC A, B or C sub-scores.
-
•
Clinically non-inferior to the EQUIVALENTS group, verifying that the lower bound of 95% CI for the ARTHRUM group, was above a non-inferiority limit. This limit was defined as MD for the EQUIVALENTS group, reduced by a non-inferiority margin, arbitrarily chosen to be equal to the SDD (Smallest Detectable Difference) defined by Angst et al5, for each WOMAC sub-score.
-
•
Statistically better than its homologues in the CONTROLS group (p < 0,05) and not worse than same in the EQUIVALENTS group.
Comparison between the groups was also made and, to give further confidence in the results, the effect size (ES) vs CONTROLS was calculated as defined by Cohen6 (CD).
OMERACT-OARSI responders
The percentage of patients responding to OMERACT-OARSI criteria, has been re-assessed from the populations described in the studies, following two interpretation hypotheses:
-
•
Definition “strict”: base = population with sufficient criteria answered, to determine that patients were either certified responders, or certified non-responders.
-
•
Definition “minimal”: base = population with partial answers to the criteria, and for which a fraction of patients remains uncertain. In such cases, the percentage of responders is lower, as it is relative to a larger base (which can be up to the whole population included in the study). This is sometimes proposed, but is a matter for discussion, as some patients, potentially responders, may have been discarded, just because data were missing.
After re-evaluation or control of the rate of responders found in each study, a global assessment has been done for each group - ARTHRUM, EQUIVALENTS and CONTROLS. These results were then compared, using the chi2 test and p-value.
Results
Patient profiles
The patient profiles described in the various studies (12,860 patients), were summarized to verify their representativeness regarding the target population for viscosupplementation, as it is recognized. Also, they were detailed and compared between groups, to ensure a minimal homogeneity. The profile assessment included following parameters: gender (%); age: mean (SD), [minimum-maximum]; OA anteriority: mean (SD); body mass index (BMI): mean (SD), [minimum-maximum]; and Kellgren & Lawrence7 radiological grades, for the severity of the disease: KL I (%), KL II (%), KL III (%) and KL IV (%).
ARTHRUM group includes 2,597 patients: 61.8% women, aged 63.6 (10.4) years [20-97], anteriority 4.1 (4.2) years, BMI 27.3 (4.5) kg/m2 [16.2-60.0], KL I (11.0%), KL II (39.7%), KL III (40.6%) and KL IV (8.7%)
EQUIVALENTS group includes 6,970 patients: 66.7% women, aged 63.3 (8.9) years [29-90], anteriority 4.9 (4.7) years, BMI 28.1 (4.2) kg/m2 [18.0-61.1], KL I (4.8%), KL II (41.5%), KL III (47.6%) and KL IV (6.1%)
CONTROLS group includes 2,708 patients: 61.1% women, aged 63.3 (8.2) years [30-86], anteriority 5.1 (5.0) years, BMI 29.1 (4.5) kg/m2 [18.4-54.6], KL I (5.6%), KL II (37.2%), KL III (50.3%) and KL IV (6.9%)
All these profiles meet those found in literature, and are close together, eliminating this potential cause of heterogeneity in the global comparisons. However, distribution of above parameters is wide in each group. Detailed tables for patients profiles are available (annexed to this article).
Data survey
A detailed and critical survey of the data13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, was undertaken to ensure full representation of the current practice of viscosupplementation for knee OA, according to the standard protocols. Following this, two studies and one arm have been rejected from their protocol. These were:
-
•
Karlsson52 (2002): only patients with an ultimately progressed OA (Ahlbäck grades), almost corresponding to the grade KL IV (indication for surgery with total knee replacement), leading to poor clinical results, outside the usual indication for viscosupplementation.
-
•
Kearey46 (2016): very high scores, due to a probable over-rating of the answers given by patients, assessed by phone at long distance (experimental protocol, in Australia). Also, this open study (no control group) was for a product already well represented in this analysis (Synvisc-One).
-
•
Neustadt37 (2005): one arm studying the effect of 4 successive injections of Orthovisc, has been removed from the analysis, as the product information suggests 3 injections.
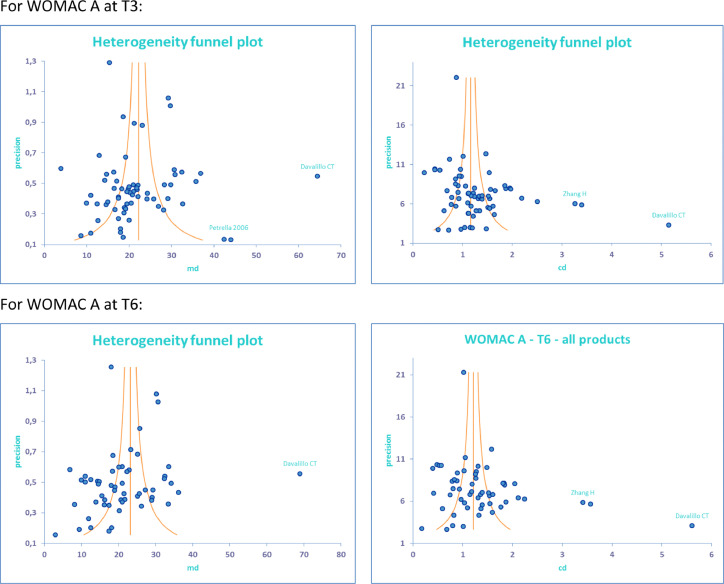
A search for outlier studies, providing inconsistent results and heterogeneity, was done on the whole selected population. Preliminary meta-analysis tests were carried out, using funnel plots to assess MD and ES (CD), that must remain coherent (Figure 2). This yielded:
-
•
Petrella34 (2006): uncertain results (very low precision) and risk of bias
-
•
Davalillo19 (2015): absurd results, and very important bias
-
•
Zhang20 (2015): under-rated SD (and SE) giving abnormally high results for ES (CD), and risk of bias
Figure 2.
Funnel plots to identify doubtful studies.
In these graphs, each study result is represented by a point function of gain for MD or CD (x axis) and of study precision (y axis). The average mean is represented by a vertical line. The surrounding curves represent the 95% CI, which reduces as precision is increased (higher population, smaller SE). Abnormally high effect sizes (CD) in contradiction with MD (as for Zhang), demonstrate the under-rating of SD and SE.
Note: 2 points below an author's name, describe 2 products (device and/or control)
Following the removal of these doubtful studies, the remaining selected studies were split into three groups, ARTHRUM, CONTROLS and EQUIVALENTS for each criterion WOMAC A, B or C. All details are given for WOMAC A (Table 1). Completed data (mostly SD) are highlighted using a gray background, in these tables.
Table 1.
Selected studies for meta-analyses and synthesis of WOMAC results
The following tables, given per group, provide detailed results for the WOMAC A (pain sub-score)
| WOMAC A |
Baseline |
T1 |
T3 |
T6 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTHOR | PUB | ARTHRUM | N | MEAN | SD | N | MEAN | SD | N | MEAN | SD | N | MEAN | SD |
| Maravic (ART-QUALIVIE)53 | 2019 | Arthrum H 2% | 134 | 50,6 | 15,1 | 111 | 26,3 | 19,9 | 111 | 23,4 | 19,1 | |||
| Baron (ART-ONE 75)54 | 2018 | Arthrum visc 75 | 218 | 50,3 | 15,6 | 207 | 28 | 20 | 180 | 19,6 | 17,8 | 183 | 16,7 | 17,4 |
| Thomas55 | 2017 | Arthrum H 2% | 202 | 49,9 | 17,2 | 202 | 33,5 | 17,9 | 202 | 27,6 | 18,2 | |||
| Hilliquin (< 60 ANS)56 | 2017 | Arthrum H 2% | 182 | 32,4 | 18,4 | 182 | 17,7 | 15,6 | 182 | 12,3 | 13,0 | |||
| Germonville57 | 2015 | Arthrum H 2% | 126 | 49,1 | 17,4 | 122 | 28,3 | 19,6 | 120 | 23,5 | 19,4 | |||
| Hilliquin (DOULEUR & HANDICAP)60 | 2021 | Arthrum H 2% | 451 | 51,0 | 19,2 | 430 | 31,3 | 17,9 | 427 | 26,9 | 19,4 | |||
| Vincent (LONG TERME)58 | 2020 | Arthrum H 2% | 1177 | 46,6 | 16,4 | 970 | 31,2 | 19,0 | 904 | 28,6 | 19,2 | |||
| Rivera59 | 2016 | Arthrum HCS | 112 | 52,1 | 15,2 | 111 | 25,7 | 17,4 | 111 | 20,4 | 16,3 | 109 | 20,5 | 19,7 |
| 2602 | 318 | 2308 | 2238 | |||||||||||
| AUTHOR | PUB | CONTROL | N | MEAN | SD | N | MEAN | SD | N | MEAN | SD | N | MEAN | SD |
| Van Der Weegen18 | 2015 | Placebo IA | 97 | 44,4 | 15,0 | 97 | 33,0 | 22,5 | 97 | 25,5 | 24,6 | 96 | 30,5 | 21,8 |
| Arden22 | 2013 | Placebo IA | 110 | 49,2 | 10,2 | 110 | 36,9 | 17,2 | ||||||
| Strand24 | 2012 | Placebo IA | 128 | 68,0 | 13,1 | 128 | 55,9 | 21,4 | 119 | 53,4 | 27,6 | |||
| DeCaria25 | 2012 | Placebo IA | 15 | 36,4 | 18,8 | 15 | 32,4 | 13,4 | 15 | 27,8 | 16,0 | 15 | 33,4 | 16,2 |
| Huang28 | 2011 | Placebo IA | 100 | 45,4 | 13,1 | 98 | 23,9 | 19,4 | ||||||
| Diraçoglu29 | 2009 | Placebo IA | 21 | 56,0 | 11,3 | 20 | 51,9 | 11,5 | ||||||
| Neustadt37 | 2005 | Arthrocentesis | 114 | 58,8 | 11,7 | 114 | 32,9 | 24,3 | 114 | 33,6 | 23,5 | |||
| Altman38 | 2004 | Placebo IA | 174 | 52,1 | 11,4 | 139 | 35,2 | 19,1 | 139 | 35,0 | 20,5 | 139 | 37,7 | 20,9 |
| Petrella39 | 2002 | NSAID | 26 | 42,2 | 32,5 | 26 | 28,6 | 27,5 | ||||||
| Placebo IA | 28 | 36,2 | 27,1 | 25 | 31,9 | 28,1 | ||||||||
| Brandt40 | 2001 | Placebo IA | 112 | 81,5 | 13,5 | 69 | 62,3 | 9,0 | 69 | 62,3 | 22,5 | 69 | 65,3 | 21,2 |
| Takamura41 | 2018 | Placebo IA | 159 | 63,5 | 9,2 | 159 | 48,0 | 22,9 | 159 | 47,0 | 25,5 | 159 | 48,7 | 23,3 |
| Chevalier49 | 2010 | Placebo IA | 129 | 56,3 | 10,3 | 117 | 38,3 | 15,5 | 117 | 38,0 | 21,2 | 117 | 41,7 | 19,9 |
| Cubukçu50 | 2004 | Placebo IA | 10 | 88,0 | 7,1 | 10 | 70,5 | 7,6 | ||||||
| Day51 | 2004 | Placebo IA | 115 | 43,4 | 18,6 | 115 | 22,9 | 16,0 | 115 | 23,2 | 15,8 | |||
| Baron (ART-ONE 75)54 | 2018 | Placebo IA | 326 | 61,4 | 11,5 | 326 | 43,5 | 19,6 | 326 | 42,2 | 24,3 | 198 | 40,4 | 21,6 |
| Thomas (CELTIPHARM)55 | 2017 | NSAID | 199 | 50,4 | 16,1 | 199 | 46,5 | 17,3 | 199 | 43,5 | 18,1 | |||
| 1863 | 1356 | 1469 | 1204 | |||||||||||
| WOMAC A |
Baseline |
T1 |
T3 |
T6 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTHOR | PUB | EQUIVALENT | N | MEAN | SD | N | MEAN | SD | N | MEAN | SD | N | MEAN | SD |
| Maheu E14 | 2019 | Ostenil Plus | 144 | 58,4 | 11,5 | 139 | 25,0 | 17,5 | 139 | 21,5 | 17,5 | 113 | 24,1 | 19,1 |
| Synvisc-One | 148 | 58,3 | 12,0 | 141 | 27,0 | 20,0 | 141 | 22,5 | 20,0 | 112 | 22,1 | 22,2 | ||
| Diraçoglu D15 | 2016 | Monovisc | 21 | 55,0 | 15,9 | 20 | 40,5 | 15,9 | 20 | 37,0 | 15,9 | 20 | 42,5 | 15,9 |
| Adant | 20 | 52,5 | 17,7 | 20 | 37,5 | 17,7 | 20 | 34,5 | 17,7 | 20 | 35,0 | 17,7 | ||
| Saccomanno MF16 | 2016 | Orthovisc | 55 | 48,2 | 20,4 | 53 | 35,4 | 19,6 | 53 | 35,5 | 20,1 | 53 | 36,3 | 19,6 |
| Van Der Weegen18 | 2015 | Fermathron Plus | 99 | 40,2 | 17,6 | 99 | 30,0 | 22,5 | 99 | 23,5 | 24,6 | 97 | 32,0 | 21,8 |
| Leighton R21 | 2014 | Durolane | 218 | 50,5 | 11,0 | 185 | 29,0 | 29,9 | 185 | 28,4 | 31,3 | 171 | 29,8 | 32,7 |
| Arden NK22 | 2013 | Durolane | 108 | 49,9 | 10,5 | 103 | 37,1 | 19,0 | ||||||
| Berenbaum F23 | 2012 | Go-On | 217 | 47,5 | 14,3 | 217 | 27,0 | 18,8 | 217 | 28,0 | 18,9 | 217 | 24,6 | 21,0 |
| Hyalgan | 209 | 48,8 | 14,9 | 209 | 31,0 | 16,5 | 209 | 29,5 | 18,6 | 209 | 30,4 | 20,4 | ||
| Strand V24 | 2012 | Gel One | 247 | 70,7 | 14,4 | 247 | 50,5 | 21,4 | 231 | 49,7 | 27,7 | |||
| DeCaria JE25 | 2012 | Hyalgan | 15 | 26,0 | 17,2 | 15 | 16,0 | 13,3 | 15 | 15,0 | 14,2 | 15 | 16,6 | 10,7 |
| Pavelka27 | 2011 | Sinovial | 192 | 55,2 | 10,8 | 183 | 25,4 | 18,9 | 183 | 22,7 | 21,1 | 183 | 22,7 | 22,8 |
| Synvisc | 188 | 55,5 | 10,9 | 171 | 27,1 | 18,9 | 171 | 24,6 | 21,1 | 171 | 23,1 | 22,8 | ||
| Huang T28 | 2011 | Hyalgan | 100 | 45,7 | 11,2 | 100 | 16,4 | 19,2 | ||||||
| Diraçoglu D29 | 2009 | Synvisc | 42 | 58,4 | 13,2 | 40 | 41,8 | 14,2 | ||||||
| Onel E30 | 2008 | Euflexxa | 160 | 49,2 | 13,9 | 156 | 22,5 | 16,0 | 156 | 19,3 | 21,5 | |||
| Synvisc | 161 | 51,1 | 14,0 | 158 | 25,0 | 17,0 | 158 | 22,7 | 21,6 | |||||
| Jüni P32 | 2007 | Synvisc | 222 | 45,0 | 18,0 | 221 | 34,0 | 30,4 | 219 | 35,0 | 22,6 | |||
| Orthovisc | 219 | 46,0 | 19,0 | 218 | 31,0 | 34,0 | 215 | 35,0 | 22,5 | |||||
| Ostenil | 219 | 46,0 | 18,0 | 219 | 35,0 | 30,2 | 217 | 35,0 | 20,7 | |||||
| Mazières B33 | 2007 | Suplasyn | 294 | 48,5 | 15,5 | 285 | 35,5 | 19,5 | 275 | 30,0 | 19,5 | |||
| Lee PB35 | 2006 | Hyruan-Plus | 75 | 47,5 | 20,0 | 75 | 30,0 | 21,3 | 75 | 30,0 | 25,0 | |||
| Hyal | 71 | 50,0 | 22,5 | 71 | 31,5 | 21,3 | 71 | 30,0 | 23,8 | |||||
| Arensi F36 | 2006 | Go-On | 20 | 30,5 | 5,0 | 20 | 20,5 | 7,5 | 20 | 18,0 | 11,2 | |||
| Hyalgan | 20 | 23,5 | 4,5 | 20 | 14,5 | 7,5 | 20 | 13,5 | 11,2 | |||||
| Neustadt D37 | 2005 | Orthovisc 3 inj | 107 | 57,8 | 10,1 | 107 | 33,6 | 24,1 | 107 | 36,1 | 24,9 | |||
| Altman RD38 | 2004 | Durolane | 172 | 49,5 | 11,4 | 134 | 33,8 | 19,5 | 134 | 35,2 | 19,9 | 134 | 37,0 | 20,0 |
| Petrella RJ39 | 2002 | Suplasyn | 25 | 33,2 | 24,2 | 25 | 24,2 | 23,4 | ||||||
| Brandt KD40 | 2001 | Orthovisc | 114 | 82,0 | 14,0 | 66 | 59,5 | 9,0 | 66 | 53,8 | 22,5 | 66 | 55,8 | 21,2 |
| Takamura J41 | 2018 | Gel-One | 152 | 63,4 | 9,1 | 152 | 48,6 | 22,9 | 152 | 41,3 | 23,6 | 152 | 42,4 | 23,3 |
| Tuan S42 | 2018 | Hya-joint Plus | 46 | 38,1 | 17,0 | 46 | 23,5 | 23,7 | 46 | 19,3 | 14,2 | 46 | 17,9 | 13,6 |
| Ha CW43 | 2017 | Hyruan Plus | 146 | 52,8 | 16,6 | 111 | 36,8 | 15,8 | 111 | 31,7 | 18,5 | |||
| Hyruan One | 137 | 51,3 | 15,3 | 97 | 37,5 | 16,4 | 97 | 31,5 | 16,9 | |||||
| Sun SF44 | 2017 | Hya-joint Plus | 66 | 49,5 | 17,0 | 62 | 32,0 | 20,0 | 62 | 29,0 | 13,5 | 62 | 28,5 | 13,5 |
| Synvisc-One | 66 | 49,0 | 16,5 | 59 | 32,5 | 18,5 | 59 | 29,5 | 14,0 | 59 | 31,5 | 15,5 | ||
| Conrozier T45 | 2016 | Happy Cross | 40 | 43,0 | 19,6 | 10 | 24,4 | 19,4 | 40 | 24,8 | 24,6 | |||
| Pal S47 | 2014 | Synvisc-One | 394 | 55,5 | 13,6 | 380 | 37,9 | 16,2 | 380 | 32,4 | 17,7 | 380 | 29,8 | 18,6 |
| Borras-Verdera A48 | 2012 | Ostenil Plus | 80 | 57,7 | 16,5 | 79 | 33,9 | 18,9 | 78 | 30,7 | 19,4 | 77 | 28,7 | 16,5 |
| Chevalier X49 | 2010 | Synvisc | 124 | 57,5 | 11,0 | 115 | 36,3 | 15,2 | 115 | 35,6 | 20,8 | 115 | 38,4 | 20,4 |
| Cubukçu D50 | 2004 | Synvisc | 30 | 78,6 | 12,9 | 30 | 57,0 | 11,2 | ||||||
| Day R51 | 2004 | Artz | 108 | 39,8 | 15,5 | 108 | 19,8 | 16,0 | 108 | 18,0 | 15,8 | |||
| Germonville T57 | 2015 | Hyalgan | 122 | 47,9 | 17,2 | 121 | 30,4 | 21,2 | 119 | 31,6 | 22,8 | |||
| 5413 | 3806 | 4772 | 3764 | |||||||||||
Representativeness of selected studies
With more than 12,000 patients included in the studies, this review and meta-analyses compare favorably in size with other large meta-analyses carried out for viscosupplementation. Another necessary aspect was to verify that the EQUIVALENTS group was truly representative of the market. This was done for the main criterion population (base 5,413 patients):
Synvisc 915, Durolane 498, Orthovisc 495, Hyalgan 466, Synvisc One 460, Gel-One 399, Suplasyn 319, GoOn 237, Ostenil Plus 224, Hyruan Plus 221, Ostenil 219, Sinovial 192, Euflexxa 160, Hyruan One 137, Hya Joint Plus 112, Artz 108, Fermathron Plus 99, Hyal 71, Happy-Cross 40, Monovisc 21, Adant 20
More than 20 devices are represented. With the advantage of more published studies, Synvisc devices comprise 25% of patients in the EQUIVALENTS group. Some products present on other markets (USA or Asia), have also been included here (16% of patients), but their presence on the European market has not been established or confirmed. In this group, single injection devices have been used for 33% of the patients, showing the expansion of this regime.
About heterogeneity
It is generally recognized that heterogeneity is important in most meta-analyses8, 9, 10, 11, 12 made on clinical studies about viscosupplementation, showing all difficulties. Beyond the internal heterogeneity of the studies (wide distribution of patient's profiles and variations between patient's responses…), there are big differences between studies (greater differences between patient profiles at inclusion, different investigators, and possible variations depending on country, approach or education). Moreover, the risks of bias exist, and could be important in their influence on final results. Research for and exclusion of outlier studies, was therefore essential.
For the WOMAC A at T6 (main criterion), the true heterogeneity index (inside studies) varied from I2 = 95% for all studies before exclusions, to I2 = 93% for the EQUIVALENTS group, I2 = 86% for the CONTROLS group, and I2 = 93% for the ARTHRUM group. Despite this slight improvement, heterogeneity remained important. In the same conditions for the WOMAC A at T6, the heterogeneity index τ2 (between studies, and dimensionless) was clearly improved, moving from τ2 = 0.34 for all studies, to τ2 = 0.24 for the EQUIVALENTS group, τ2 = 0.11 for the CONTROLS group, and τ2 = 0.14 for the ARTHRUM group. This greater improvement is clearly the result of the exclusion of these divergent protocol and outlier studies.
Forest plots
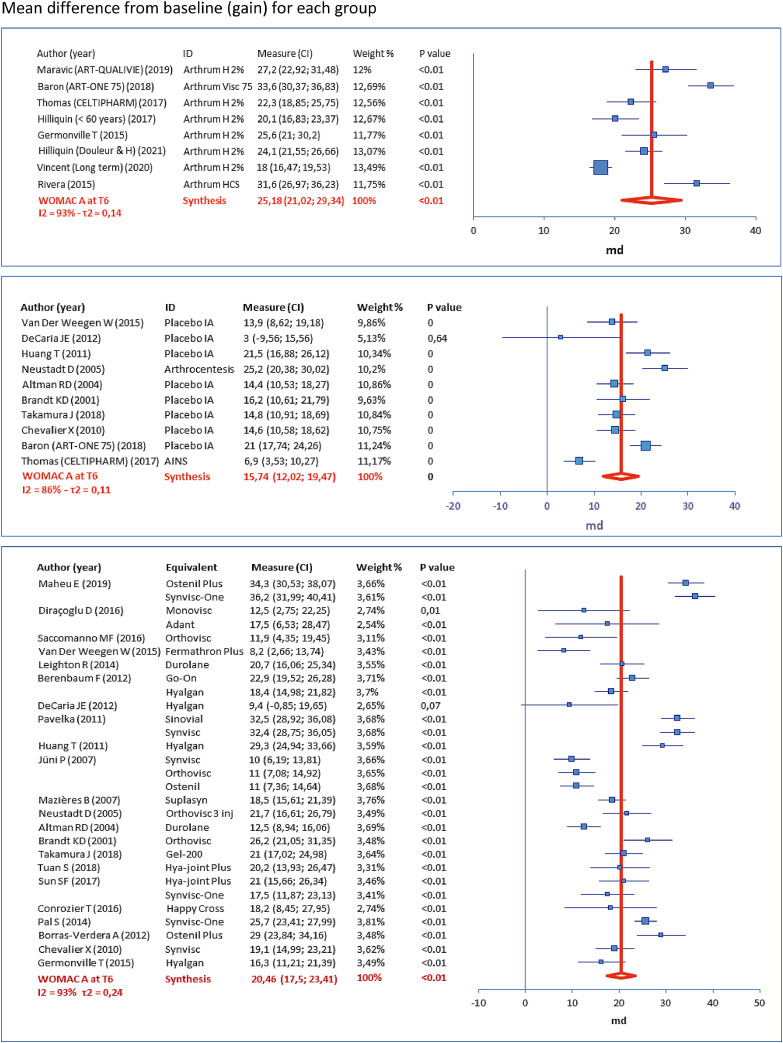
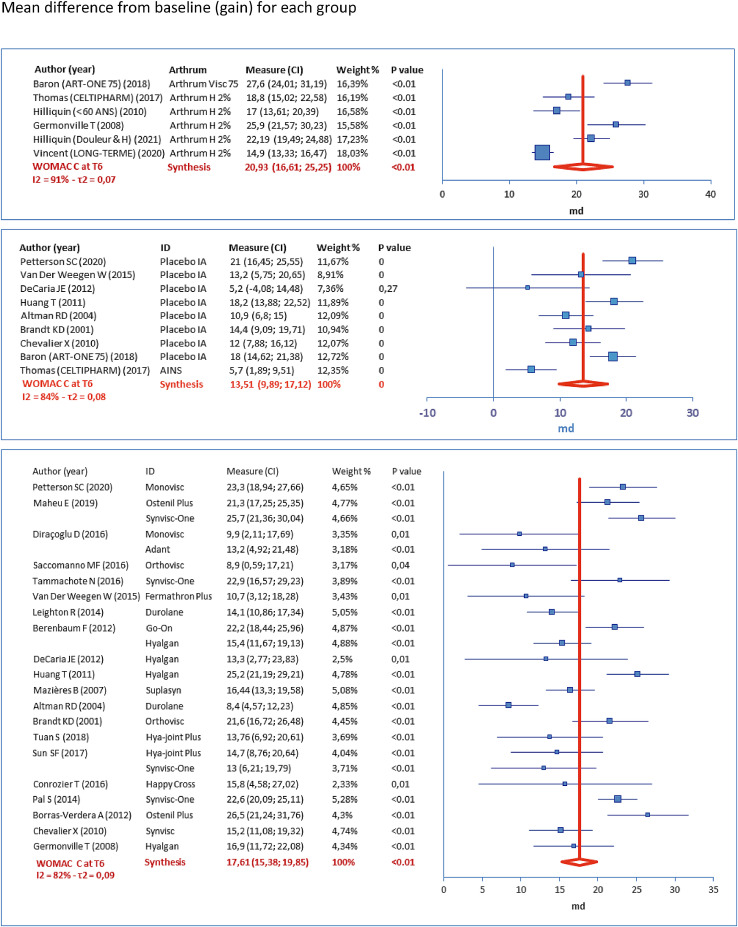
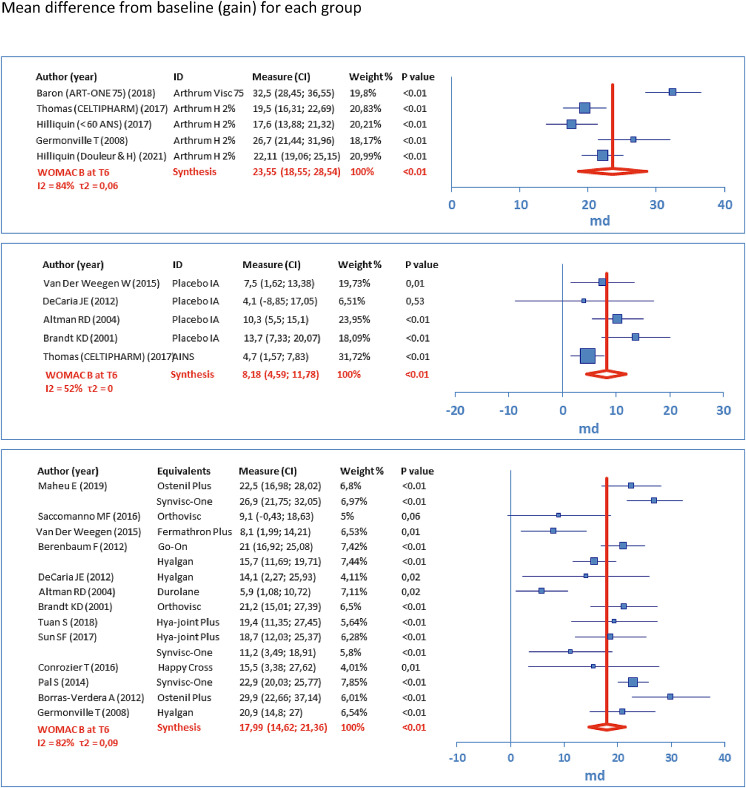
The following forest plots (Figures 3–5) represent the score variations from baseline (scale 0-100). For each study arm, the mean difference (MD) is represented by a square, and its 95% CI is represented by the associated horizontal bar. Synthesis is given by the vertical dark red bar (mean) and the horizontal width of the lozenge below represents 95% CI, for the group. The table to the left of each plot gives the numerical results, with product identity (ID), weighting (%) used to calculate the overall mean (inverse variance, random effects). These results are given at T6, respectively for WOMAC A (Figure 3), WOMAC B (Figure 4) and WOMAC C (Figure 5).
Figure 3.
Results for the main criterion (WOMAC A at T6)
Figure 5.
Results for a secondary criterion (WOMAC C at T6)
Figure 4.
Results for a secondary criterion (WOMAC B at T6)
Results interpretation
All the combined results (MD 95%CI) of the meta-analyses are detailed by group and WOMAC sub-score at times T1, T3 and T6 (Table 2). Populations are given at inclusion (N0) and at the observation time (N). To assess the importance of the gain obtained from inclusion (baseline), the minimal perceptible clinical improvement (MPCI) from Ehrich4 has been used: 9.7 for the WOMAC A, 10.0 for the WOMAC B and 9.3 for the WOMAC C (scale 0-100). When these values are exceeded, the answer “Yes” is given on the table.
Table 2.
Consolidated WOMAC results
| ARTHRUM | Time | N0 | N | MD | SD | MPCI | < 95% CI |
|---|---|---|---|---|---|---|---|
| WOMAC A | T1 | 330 | 318 | 24.14 (20.14; 28.13) | 17.0 | 9.7 | Yes |
| T3 | 2602 | 2308 | 21.61 (17.15; 26.07) | 17.3 | 9.7 | Yes | |
| T6 | 2602 | 2238 | 25.18 (21.02; 29.34) | 17.5 | 9.7 | Yes | |
| WOMAC B | T1 | 218 | 208 | 21.20 (17.02; 25.38) | 22.1 | 10.0 | Yes |
| T3 | 1179 | 1117 | 20.02 (13.62; 26.41) | 20.2 | 10.0 | Yes | |
| T6 | 1179 | 1113 | 23.55 (18.55; 28.54) | 19.8 | 10.0 | Yes | |
| WOMAC C | T1 | 218 | 185 | 18.30 (14.70; 21.90) | 18.3 | 9.3 | Yes |
| T3 | 2356 | 2066 | 16.72 (12.45; 21.00) | 18.4 | 9.3 | Yes | |
| T6 | 2356 | 2000 | 20.93 (16.61; 25.25) | 18.2 | 9.3 | Yes | |
| EQUIVALENTS | Time | N0 | N | MD | SD | MPCI | < 95% CI |
| WOMAC A | T1 | 4090 | 3806 | 18.97 (16.75; 21.19) | 15.5 | 9.7 | Yes |
| T3 | 4682 | 4346 | 20.91 (18.38; 23.44) | 17.3 | 9.7 | Yes | |
| T6 | 4063 | 3764 | 20.46 (17.50; 23.41) | 17.3 | 9.7 | Yes | |
| WOMAC B | T1 | 2069 | 1874 | 15.29 (13.11; 17.48) | 19.9 | 10.0 | Yes |
| T3 | 2461 | 2249 | 18.73 (16.35; 21.12) | 20.6 | 10.0 | Yes | |
| T6 | 1987 | 1799 | 17.99 (14.62; 21.36) | 21.4 | 10.0 | Yes | |
| WOMAC C | T1 | 2671 | 2462 | 14.09 (12.16; 16.03) | 16.2 | 9.3 | Yes |
| T3 | 3242 | 3011 | 17.62 (15.4; 19.85) | 17.3 | 9.3 | Yes | |
| T6 | 2994 | 2731 | 17.61 (15.38; 19.85) | 17.8 | 9.3 | Yes | |
| CONTROLS | Time | N0 | N | MD | SD | MPCI | < 95% CI |
| WOMAC A | T1 | 1450 | 1356 | 14.97 (12.76; 17.17) | 16.3 | 9.7 | Yes |
| T3 | 1568 | 1469 | 16.84 (13.03; 20.65) | 18.1 | 9.7 | Yes | |
| T6 | 1425 | 1204 | 15.74 (12.02; 19.47) | 16.9 | 9.7 | Yes | |
| WOMAC B | T1 | 596 | 557 | 10.20 (6.98; 13.41) | 20.0 | 10.0 | No |
| T3 | 712 | 634 | 11.27 (5.38; 17.15) | 20.1 | 10.0 | No | |
| T6 | 597 | 518 | 8.18 (4.59; 11.78) | 20.0 | 10.0 | No | |
| WOMAC C | T1 | 1090 | 1032 | 12.26 (9.18; 15.34) | 15.5 | 9.3 | No |
| T3 | 1094 | 1028 | 14.98 (9.83; 20.13) | 17.6 | 9.3 | Yes | |
| T6 | 1208 | 1108 | 13.51 (9.89; 17.12) | 18.0 | 9.3 | Yes | |
For the main criterion WOMAC A at T6:
-
•
Each lower bound of the 95% CI, for individual studies in the ARTHRUM group, demonstrates an important gain, higher than the MPCI (= 9.7). This, a fortiori, also applies to the lower bound of the 95% CI estimated at 21.02 for the group as a whole.
-
•
In the EQUIVALENTS group, the lower bounds of 95% CIs are below the MPCI for 35% of individual studies. However, the lower bound of the 95% CI, estimated at 17.50 for the group as a whole, is above the MPCI.
-
•
For the CONTROLS group, the results are similar to the EQUIVALENTS group with clinical efficacy of the IA placebo being observed. The lower bound of the 95% CI for the group as a whole, estimated at 12.02 is also above the MPCI.
For the secondary criterion WOMAC C at T6, the same observations can be made, as the MPCI (= 9.3) is below each lower bound for each individual study for the ARTHRUM group, and below the lower bound of the overall 95% CI, for each group. For the other criteria at any time, the MPCI is always below the 95% CI for the ARTRUM and EQUIVALENTS groups. However, for the CONTROLS groups, there are several cases with the MPCI greater than the 95% CI lower bound (Table 2), demonstrating that an IA placebo, does not always attain the minimum efficacy to be clinically relevant.
Inter groups comparisons
The Inter group comparisons have been done on two groups at a time, giving three comparisons (Table 3). Results are presented for each WOMAC sub-score, at each observation time. The differences between the score variations (MD) from baseline, are given with SDpooled and SEpooled, allowing the t-test, to determine whether the differences are significant (p<0.05). The non-inferiority of ARTHRUM vs EQUIVALENTS, was determined using SDD5 as non-inferiority margin, converted into a 0-100 base, giving -8.1 for the WOMAC A, -9.6 for the WOMAC B and -7.8 for the WOMAC C. The lowest bound of 95% CI (kept at the same size as in Table 2) was always above this limit, confirming the non-inferiority of ARTHRUM. In the comparisons vs CONTROLS, ES was calculated. The analyses demonstrated that ARTHRUM and EQUIVALENTS were significantly better than CONTROLS for each measurement time, for all criteria. For the WOMAC A at T3 and T6, ES was respectively 0.28 and 0.56 for ARTHRUM vs 0.23 and 0.27 for EQUIVALENTS. This difference in favour of ARTHRUM was significant at T6. Similar results were observed for the other WOMAC sub-scores.
Table 3.
WOMAC comparisons between groups
| Criterium | Time | Difference | NI limit | NI | SD pooled | SE pooled | P-value | ES |
|---|---|---|---|---|---|---|---|---|
| ARTHRUM vs EQUIVALENTS | ||||||||
| WOMAC A | T1 | 5.17 (1.18; 9.17) | -8.1 | Yes | 15.67 | 0.91 | < 0.001 | NA |
| T3 | 0.70 (-3.76; 5.16) | -8.1 | Yes | 17.28 | 0.45 | 0.12 | NA | |
| T6 | 4.72 (0.56; 8.88) | -8.1 | Yes | 17.39 | 0.46 | < 0.001 | NA | |
| WOMAC B | T1 | 5.91 (1.73; 10.09) | -9.6 | Yes | 20.09 | 1.47 | < 0.001 | NA |
| T3 | 1.29 (-5.11; 7.69) | -9.6 | Yes | 20.46 | 0.75 | 0.085 | NA | |
| T6 | 5.56 (0.57; 10.56) | -9.6 | Yes | 20.81 | 0.79 | < 0.001 | NA | |
| WOMAC C | T1 | 4.21 (0.61; 7.81) | -7.8 | Yes | 16.35 | 1.25 | < 0.001 | NA |
| T3 | -0.90 (-5.18; 3.38) | -7.8 | Yes | 17.73 | 0.51 | 0.076 | NA | |
| T6 | 3.32 (-1.00; 7.64) | -7.8 | Yes | 17.96 | 0.53 | < 0.001 | NA | |
| ARTHRUM vs CONTROLS | ||||||||
| WOMAC A | T1 | 9.17 | NA | NA | 16.41 | 1.02 | < 0.001 | 0.56 |
| T3 | 4.77 | NA | NA | 17.61 | 0.59 | < 0.001 | 0.27 | |
| T6 | 9.44 | NA | NA | 17.29 | 0.62 | < 0.001 | 0.55 | |
| WOMAC B | T1 | 11.00 | NA | NA | 20.59 | 1.67 | < 0.001 | 0.53 |
| T3 | 8.75 | NA | NA | 20.19 | 1.00 | < 0.001 | 0.43 | |
| T6 | 15.37 | NA | NA | 19.84 | 1.06 | < 0.001 | 0.77 | |
| WOMAC C | T1 | 6.04 | NA | NA | 15.97 | 1.28 | < 0.001 | 0.38 |
| T3 | 1.74 | NA | NA | 18.13 | 0.69 | 0.012 | 0.10 | |
| T6 | 7.42 | NA | NA | 18.13 | 0.68 | < 0.001 | 0.41 | |
| EQUIVALENTS vs CONTROLS | ||||||||
| WOMAC A | T1 | 4.00 | NA | NA | 15.74 | 0.50 | < 0.001 | 0.25 |
| T3 | 4.07 | NA | NA | 17.49 | 0.53 | < 0.001 | 0.23 | |
| T6 | 4.72 | NA | NA | 17.24 | 0.57 | < 0.001 | 0.27 | |
| WOMAC B | T1 | 5.09 | NA | NA | 19.89 | 0.96 | < 0.001 | 0.26 |
| T3 | 7.46 | NA | NA | 20.48 | 0.92 | < 0.001 | 0.36 | |
| T6 | 9.81 | NA | NA | 21.10 | 1.05 | < 0.001 | 0.46 | |
| WOMAC C | T1 | 1.83 | NA | NA | 16.00 | 0.59 | 0.002 | 0.11 |
| T3 | 2.64 | NA | NA | 17.36 | 0.63 | < 0.001 | 0.15 | |
| T6 | 4.10 | NA | NA | 17.85 | 0.64 | < 0.001 | 0.23 | |
NI limit = - SDD (Angst)
OMERACT-OARSI responders
Results for OMERACT-OARSI responders (Table 4), have been collected from 13 studies (3 for ARTHRUM). Data were insufficient at T1 or T2. Results at T3 and T4 have been pooled when it appeared relevant. The rates of patients who were strictly responders, according to OMERACT-OARSI, varied from:
-
•
63.4% to 88.6% at T3-4 and 64.7% to 91.2% at T6, for the ARTHRUM group
-
•
49.5% to 71.3% at T3-4 and 52.4% to 85.7% at T6, for the EQUIVALENTS group
-
•
54.6% to 60.9% at T3-4 and 41.8% to 58.7% at T6, for the CONTROLS group
Table 4.
Results for OMERACT-OARSI responders
| OMERACT-OARSI |
T3-4 |
T6 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTHOR | PUB | PRODUCT | N | NR | NNR | % Min | % Strict | N | NR | NNR | Min | % Strict |
| Maheu14 | 2019 | Ostenil Plus | 134 | 93 | 19 | 69.4 | 83.0 | |||||
| Synvisc-One | 132 | 96 | 16 | 72.7 | 85.7 | |||||||
| Berenbaum23 | 2012 | Go-On | 217 | 151 | 66 | 69.6 | 69.6 | 217 | 159 | 58 | 73.3 | 73.3 |
| Hyalgan | 209 | 126 | 83 | 60.3 | 60.3 | 209 | 122 | 87 | 58.4 | 58.4 | ||
| Strand24 | 2012 | Gel One | 247 | 141 | 90 | 57.1 | 61.0 | |||||
| Placebo | 128 | 65 | 54 | 50.8 | 54.6 | |||||||
| Kawasaki26 | 2009 | Artz | 42 | 22 | 20 | 52.4 | 52.4 | |||||
| Exercices | 45 | 25 | 20 | 55.6 | 55.6 | |||||||
| Onel30 | 2008 | Euflexxa | 157 | 112 | 45 | 71.3 | 71.3 | |||||
| Synvisc | 158 | 99 | 59 | 62.7 | 62.7 | |||||||
| Lundsgaard31 | 2008 | Hyalgan | 84 | 50 | 32 | 59.5 | 61.0 | |||||
| Placebo | 84 | 33 | 46 | 39.3 | 41.8 | |||||||
| Ha43 | 2017 | Hyruan | 111 | 55 | 56 | 49.5 | 49.5 | |||||
| Hyruan Plus | 97 | 57 | 40 | 58.8 | 58.8 | |||||||
| Maheu61 | 2011 | Structovial | 119 | 76 | 43 | 63.9 | 63.9 | 119 | 77 | 42 | 64.7 | 64.7 |
| Synvisc | 117 | 70 | 47 | 59.8 | 59.8 | 117 | 79 | 38 | 67.5 | 67.5 | ||
| Chevalier49 | 2010 | Synvisc | 124 | 73 | 43 | 58.9 | 62.9 | |||||
| Placebo | 129 | 66 | 52 | 51.2 | 55.9 | |||||||
| Altman62 | 2009 | Euflexxa | 291 | 173 | 90 | 59.5 | 65.8 | 291 | 169 | 85 | 58.1 | 66.5 |
| Placebo | 295 | 167 | 107 | 56.6 | 60.9 | 295 | 155 | 109 | 52.5 | 58.7 | ||
| Baron54 | 2018 | Arthrum visc 75 | 214 | 156 | 20 | 72.9 | 88.6 | 214 | 165 | 16 | 77.1 | 91.2 |
| Germonville57 | 2008 | Arthrum H 2% | 126 | 96 | 26 | 76.2 | 78.7 | 126 | 102 | 18 | 81.0 | 85.0 |
| Hyalgan | 122 | 77 | 44 | 63.1 | 63.6 | 122 | 77 | 42 | 63.1 | 64.7 | ||
| Vincent58 | 2020 | Arthrum H 2% | 970 | 615 | 355 | 63.4 | 63.4 | 904 | 658 | 246 | 72.8 | 72.8 |
| TOTAL | ARTHRUM | 1310 | 867 | 401 | 66.2 | 68.4 | 1244 | 925 | 280 | 74.4 | 76.8 | |
| EQUIVALENTS | 1845 | 1137 | 663 | 61.6 | 63.2 | 1591 | 1017 | 482 | 63.9 | 67.8 | ||
| CONTROLS | 423 | 232 | 161 | 54.8 | 59.0 | 553 | 279 | 227 | 50.5 | 55.1 | ||
N Population studied for OMERACT-OARSI responders
NR Number of patient responders
NNR Number of patient non-responders
At T3-4 and T6 respectively, the average “strict” rates were better for ARTHRUM, 68.4% - 76.8%, vs 63.2% - 67.8% for EQUIVALENTS, and 59.0% - 55.1% for CONTROLS. With the “minimum” concept, these rates at T3-4 and T6, were respectively 66.2% - 74.4% for ARTHRUM, vs 61.6% - 63.9% for EQUIVALENTS, and 54.8% - 50.5% for CONTROLS. Statistical comparisons were made with the chi2 test (Table 5). Significantly better rates for OMERACT-OARSI responders were obtained with ARTHRUM devices (p<0.01).
Table 5.
Comparative statistics for OMERACT-OARSI responders
| OMERACT-OARSI | Time | Calculation | chi2 | P-value | Conclusion |
|---|---|---|---|---|---|
| ARTHRUM vs CONTROLS | T3-4 | Strict | 11.7 | 0.0006 | ARTHRUM better |
| Mini | 17.7 | < 0.0001 | |||
| T6 | Strict | 79.9 | < 0.0001 | ||
| Mini | 98.9 | < 0.0001 | |||
| ARTHRUM vs EQUIVALENTS | T3-4 | Strict | 8.92 | 0.0028 | ARTHRUM better |
| Mini | 6.86 | 0.0088 | |||
| T6 | Strict | 26.3 | < 0.0001 | ||
| Mini | 35.2 | < 0.0001 | |||
| EQUIVALENTS vs CONTROLS | T3-4 | Strict | 2.36 | 0.12 | unclear |
| Mini | 6.62 | 0.010 | EQUIVALENTS better | ||
| T6 | Strict | 761 | < 0.0001 | ||
| Mini | 31.1 | < 0.0001 |
Tolerance
Tolerance was not a target for this meta-analysis. However, the tolerance results recorded from the bibliographical research have been evaluated for 59 articles, representing 7,031 EQUIVALENTS patients, 2,959 CONTROLS patients and 5,831 ARTHRUM patients. For the CONTROLS group, any comparator was accepted, except for IA HA patients, that were included in the EQUIVALENTS group, even when other treatments were associated. For this total population of 15,821 patients, no serious adverse event (SAE) has been reported.
The Assessment of lower grade adverse events – possibly related to the treatment – was more delicate, because transient minor events (almost all at the site of injection), were unequally reported in the various studies. By limiting the investigation to the comparative studies (including those comparing several treatments from the same group), the results became more consistent. The rates of these minor adverse events (AE) were 13.2% for EQUIVALENTS (6,481 patients), 12.5% for CONTROLS (2,959 patients) and 6.5% for ARTHRUM (718 patients). Overall, one can conclude that the tolerance of IA HA is good, as the rate of AE was similar between the EQUIVALENTS and the CONTROLS group (most with IA placebo). Looking in greater detail, the presence of 29% patients receiving a modified (cross-linked) HA – Synvisc, Synvisc-One, Durolane, Monovisc or Gel-One – show two differentiated subgroups within the EQUIVALENTS group: for the cross-linked fraction (1,896 patients) the rate of AE became 18.8%, whereas for the non-cross-linked devices the rate was 10.8%.
Discussion
Equivalence between devices
According to MDR, a strictly equivalent device should be identical to the one which is assessed in all aspects: formulation, ingredients, presentation and all physical, chemical and biological properties. For the main active ingredient (HA) contained in viscosupplements, this should include the molecular weight (Mw), the nature of the molecule (native or modified by cross-linking), and their impact upon rheology or residence time. This should also include the whole manufacturing process, and therefore it could be difficult to achieve apart from within the same device family.
Inside the ARTHRUM group, two devices present slight differences from the original ARTHRUM H 2%: a single-injection product (ARTHRUM visc 75) containing 75mg of the same HA (in a 3 mL syringe), and a device containing 40 mg chondroitin sulfate (ARTHRUM HCS) in addition to the 40 mg HA (in each 2 mL syringe). These differences were considered as minor, in relation to the general properties of the devices.
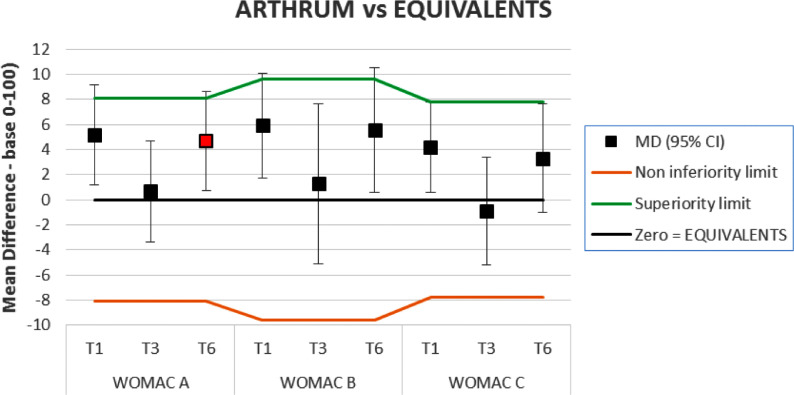
On the clinical side, ‘equivalence’ is defined as non-inferiority combined with non-superiority. This was assessed when comparative results were available. In this study, the smallest detectable difference (SDD) from Angst5, was used as non-inferiority and superiority margins as in the Results chapter. Thus, for clinical equivalence, the whole 95% CI must remain between the 2 limits (Figure 6), which was observed at T3 for all scores and for the WOMAC C at T6. In other cases, ARTHRUM was non-inferior and superior.
Figure 6.
Non inferiority or clinical equivalence.
The graph illustrates the difference between the ARTHRUM and the EQUIVALENTS groups, for each WOMAC sub-score, at all observation times. Positive results are in favour of ARTHRUM. The squares represent the mean difference (MD) – filled in red for the main criterion – and the vertical bars represent the 95% CI. The orange and green lines respectively represent the non-inferiority limit for ARTHRUM (which is always observed) and its superiority limit. When the 95% CI intervals are totally between these two limits, it is reasonable to infer clinical equivalence between ARTHRUM and EQUIVALENTS devices. But when the upper bound of the CI interval is above the green limit, one can assume a combined non-inferiority and superiority of ARTHRUM. Also, when the lower bound of CI is above zero. a statistical superiority of ARTHRUM over EQUIVALENTS is observed (p<0.05).
About the method
Our approach may certainly be questioned for several elements, notably for the design of the CONTROLS group, which is heterogenous. From this, it was impossible to make one-to-one comparisons with individual IA HA arms of the studies. However, the method works “globally” as our quantitative results meet those described in the literature, and in particular from Bannuru10 (ES = 0.20 à 0.46), and from Rutjes11 (ES = 0.37), confirming our results for the groups ARTHRUM and EQUIVALENTS.
About results
Compared with the baseline, the improvements observed over time for the ARTHRUM group are statistically significant and clinically relevant for each WOMAC sub-score, at all time points. Compared with the CONTROLS group, these improvements are also significantly in favor of ARTHRUM group, for the same criteria, at all time points. Based on ES (dimensionless value), these improvements are low to moderate at T3 (ES = 0.10 to 0.43), then clearly better at T6 (ES = 0.41 to 0.77). From Cohen6, ES is low at 0.2, medium at 0.5 and high at 0.8. Overall, these ES results for pain, match those published by authors of other major meta-analyses8, 9, 10, 11. At T6, there is some clinical superiority in favor of ARTHRUM for the WOMAC A and B sub-scores (Figure 6). The clinical superiority of ARTHRUM compared with EQUIVALENTS, is also observed (p<0.01) from the rates of OMERACT-OARSI responders (Table 5). The results for ARTHRUM at T1, should be treated with some caution as they are based on only two studies, although both gave excellent results (Baron54 and Rivera59). One can conclude that long term clinical efficacy is demonstrated for the ARTHRUM family of devices.
About intra-articular placebo
The importance of the therapeutic efficacy of the IA placebo, can be clearly seen from this study, as the gain on pain index is greater than the MPCI from Ehrich4, for its pooled 95% CI (Table 2). Such a gain is greater than a simple placebo effect: in an OA joint, the injection of a physiological liquid (pH balanced) has a certain beneficial effect that may persist three months or more. Expert authors such as Altman12 or Bannuru9 have observed this phenomenon. It is therefore a positive benefit of IA HA, that an improvement (even modest ES = 0.23 to 0.56) over an IA placebo is obtained. The position of the IA placebo in OA viscosupplementation studies should therefore be considered. For the patient an improvement is perceived from the baseline, and this should be considered as relevant. This supports the use of such indicators as the rates of OMERACT-OARSI responders, in real live studies.
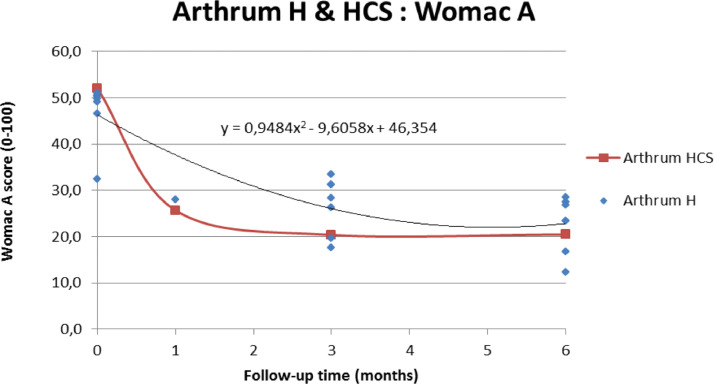
About ARTHRUM HCS
The original formulation of ARTHRUM HCS (2% HA + 2% CS), was only represented in this meta-analysis by the study made by Rivera59, giving results for the WOMAC A at T1, T3 and T6. A graph (Figure 7) allows comparison of the evolution of the WOMAC A (pain) score for ARTHRUM HCS, with those of the other ARTHRUM devices (based on HA alone), represented as a scatter plot (with associated tendency curve). This representation of the advantage of ARTHRUM HCS, is somewhat biased because of the relatively high heterogeneity of ARTHRUM H trials, which include a large diversity of patients, some with a limited potential for improvement: some with ultimate radiological grades (KL IV) and conversely, young patients with early OA symptoms. Therefore, other comparison tests were made between Rivera59 and Baron54 studies, both of which gave consistent results. The difference (base 0-100, positive in favor of ARTHRUM HCS in the score variation of MD (SD) from baseline, for the WOMAC A (pain sub-score) were: 4.1(17.4) at T1, 1.0(16.3) at T3 and -2.0(16.9) at T6. As in the main results, the improvement was significantly better at T1 (p=0.045) with ARTHRUM HCS. At times T3 and T6, the differences were not significant. This confirms the feed-back information given by doctors prescribing ARTHRUM HCS, that a very fast response on pain is obtained. This presence of CS is beneficial from the beginning of the treatment (as seen at T1), and should be recommended if the OA is painful at the time of treatment.
Figure 7.
ARTHRUM HCS compared to other ARTHRUM devices.
The WOMAC A (pain) scores are represented: ARTHRUM HCS (red curve) vs other ARTHRUM (scatter plots completed with the tendency curve and its equation): the lack of data at T1 explains the difference in the overall curve shape.
Conclusions
The comparison of ARTHRUM clinical studies (all devices), with studies selected after systematic bibliographical research (LCA, 2020), leads to the conclusion that the clinical efficacy of the ARTHRUM medical devices, in reducing pain and improving function, in knee osteoarthritis, for a period of up to 6 months has been demonstrated. Non inferiority and also superiority to equivalent IA HA devices, present on the market, were observed. With good tolerance results (lowest rate of AE, and none of them serious), the risk benefit ratio clearly favors viscosupplementation with ARTHRUM.
Conflicts of Interest
As share-holder and employee of LCA Pharmaceutical (Chartres, France), I have interest in the family of the medical devices “ARTHRUM”, intended for the viscosupplementation of osteoarthritic joints.
I am the sole author of this review article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2021.100637.
Appendix. Supplementary materials
References
- 1.Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review - Seminars in Arthritis and Rheumatism, 2019; 48(4):563-572, doi: 10.1016/j.semarthrit.2018.06.002. [DOI] [PubMed]
- 2.Bellamy N., Buchanan WW., Goldsmith CH., Campbell J., Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with of the hip or knee. J Rheumatol. 1988;15(12):1833–1844. [PubMed] [Google Scholar]
- 3.Pham T., van der Heijde D., Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T., Dougados M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis and Cartilage. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Ehrich EW., Davies GM., Watson DJ., Bolognese JA., Seidenberg BC., Bellamy N. Minimal Perceptible Clinical Improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessment in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–2641. Nov. [PubMed] [Google Scholar]
- 5.Angst F., Aeschlimann A., Stucki G. Smallest Detectable and Minimal Clinically Important Differences of rehabilitation intervention with their implication for required sample sizes using WOMAC and SF-36 Quality of Life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Care & Research. 2001;45:384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. 2nd edition. Lawrence Erlbaum Associates; Hillsdale. NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 7.Kellgren JH., Lawrence JS. Radiological assessment of osteoarthritis – Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller LE., Block JE. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthitis: Systematic Review and Meta-Analysis of Randomized. Saline-Controlled Trials – Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. http://dx.doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannuru RR., Schmid CH., Kent DM., Vaysbrot EE., Wong JB, McAlindon TE. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis - A Systematic Review and Network Meta-analysis. Annals of Internal Medicine. 2015;162(1):46–55. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 10.Bannuru RR., Natov NS., Dasi UR., Schmid CH., McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in the treatment of knee osteoarthritis – meta-analysis. Osteoarthritis Cartilage. 2011;19:611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutjes AWS., Jüni P., da Costa BR., Trelle S., Nüesch E., Reichenbach S. Viscosupplementation for Osteoarthritis of the Knee. A systematic review and meta-analysis – Annals of Internal Medicine. 2012;157(3):180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 12.Altman RD., Devji T., Bhandari M., Fierlinger A., Niazi F., Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Seminars in Arthritis and Rheumatism. 2016;46:151–159. doi: 10.1016/j.semarthrit.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Petterson SC., Plancher KD. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter. double-blind. randomized. placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1992–2002. doi: 10.1007/s00167-018-5114-0. Jun. [DOI] [PubMed] [Google Scholar]
- 14.Maheu E., Avouac B., Dreiser RL., Bardin T. A single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate vs 0.8% hylan G-F 20 in the treatment of symptomatic knee osteoarthritis: a 6-month. multicenter. randomized. controlled non-inferiority trial - PloS one. 2019;14(12) doi: 10.1371/journal.pone.0226007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dıraçoğlu D., Tunçay TB., Şahbaz T., Aksoy C. Single versus multiple dose hyaluronic acid: Comparison of the results. J Back Musculoskelet Rehabil. 2016 Nov 21;29(4):881–886. doi: 10.3233/BMR-160714. [DOI] [PubMed] [Google Scholar]
- 16.Saccomanno MF., Donati F., Careri S., Bartoli M., Severini G., Milano G. Efficacy of intra-articular hyaluronic acid injections and exercise-based rehabilitation programme. administered as isolated or integrated therapeutic regimens for the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1686–1694. doi: 10.1007/s00167-015-3917-9. May. [DOI] [PubMed] [Google Scholar]
- 17.Tammachote N., Kanitnate S., Yakumpor T., Panichkul P. Intra-articular. single-shot Hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind. randomized controlled trial. J Bone Joint Surg Am. 2016;98:885–892. doi: 10.2106/JBJS.15.00544. [DOI] [PubMed] [Google Scholar]
- 18.Van der Weegen W., Wullems JA., Bos E., Noten H., van Drumpt RA. No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: a randomized. controlled. double-blind trial. J Arthroplasty. 2015;30(5):754–757. doi: 10.1016/j.arth.2014.12.012. May. [DOI] [PubMed] [Google Scholar]
- 19.Davalillo CAT. Vasavilbaso CT. Alvarez JMN. Granado PC. Jimenez OAG. del Sol MG. Orbezo FG. Clinical efficacy of intra-articular injections in knee osteoarthritis: a prospective randomized study comparing hyaluronic acid and betamethasone - Open access rheumatology: research and reviews. 2015. 7. 9-18.https://doi.org/ 10.2147/OARRR.S74553 [DOI] [PMC free article] [PubMed]
- 20.Zhang H., Zhang K., Zhang X., Zhu Z., Yan S., Sun T., Guo A., Jones J., Steen RG, Zhang J., Lin J. Comparison of two hyaluronic acid formulations for safety and efficacy (CHASE) study in knee osteoarthritis: a multicenter. randomized. double-blind. 26-week non-inferiority trial comparing Durolane to Artz. Arthritis Research & Therapy. 2015;17:51. doi: 10.1186/s13075-015-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leighton R., Akermark C., Therrien R., Richardson JB, Andersson M., Todman MG, Arden NK, DUROLANE Study Group NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: a prospective. multi-centre. randomized. non-inferiority trial. Osteoarthritis Cartilage. 2014;22(1):17–25. doi: 10.1016/j.joca.2013.10.009. JanEpub 2013 Nov 1PMID: 24185114. [DOI] [PubMed] [Google Scholar]
- 22.Arden NK, Åkermark C., Andersson M., Todman MG, Altman RD. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr Med Res Opin. 2014;30(2):279–286. doi: 10.1185/03007995.2013.855631. Feb. [DOI] [PubMed] [Google Scholar]
- 23.Berenbaum F., Grifka J., Cazzaniga S., D'Amato M., Giacovelli G., Chevalier X., Rannou F., Rovati LC, Maheu E. A randomised. double-blind. controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71(9):1454–1460. doi: 10.1136/annrheumdis-2011-200972. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strand V., Baraf HSB, Lavin PT, Lim S., Hosokawa H. A multicenter. randomized controlled trial comparing a single intra-articular injection of Gel-200. a new cross-linked formulation of hyaluronic acid. to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350–356. doi: 10.1016/j.joca.2012.01.013. May. [DOI] [PubMed] [Google Scholar]
- 25.DeCaria JE., Montero-Odasso M., Wolfe D., Chesworth BM., Petrella RJ. The effect of intra-articular hyaluronic acid treatment on gait velocity in older knee osteoarthritis patients: a randomized. controlled study. Arch Gerontol Geriatr. 2012;55(2):310–315. doi: 10.1016/j.archger.2011.11.007. Sep-Oct. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki T, Kurosawa H, Ikeda H, Takazawa Y, Ishijima M, Kubota M, Kajihara H, Maruyama Y, Kim SG, Kanazawa H, Doi T. Therapeutic home exercise versus intraarticular hyaluronate injection for osteoarthritis of the knee: 6-month prospective randomized open-labeled trial. J Orthop Sci. 2009;14(2):182–191. doi: 10.1007/s00776-008-1312-9. Mar. [DOI] [PubMed] [Google Scholar]
- 27.Pavelka K., Uebelhart D. Efficacy evaluation of highly purified intra-articular hyaluronic acid (Sinovial) vs hylan G-F20 (Synvisc) in the treatment of symptomatic knee osteoarthritis. A double-blind. controlled. randomized. parallel-group non-inferiority study. Osteoarthritis and cartilage. 2011;19(11):1294–1300. doi: 10.1016/j.joca.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Huang T-L, Chang C-C, Lee C-H, Chen S-C, Lai C-H, Tsai C-L. Intra-articular injections of sodium hyaluronate (Hyalgan) in osteoarthritis of the knee. a randomized. controlled. double-blind. multicenter trial in the asian population. BMC musculoskeletal disorders. 2011;12 doi: 10.1186/1471-2474-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diracoglu D., Vural M., Baskent A., Dikici F., Aksoy C. The effect of viscosupplementation on neuromuscular control of the knee in patients with osteoarthritis. J Back Musculoskelet Rehabil. 2009;22(1):1–9. doi: 10.3233/BMR-2009-0207. [DOI] [PubMed] [Google Scholar]
- 30.Onel E., Kolsun K., Kauffman JI. Post-Hoc analysis of a head-to-head hyaluronic acid comparison in knee osteoarthritis using the 2004 OMERACT-OARSI responder criteria. Clin Drug Investig. 2008;28(1):37–45. doi: 10.2165/00044011-200828010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Lundsgaard C., Dufour N., Fallentin E., Winkel P., Gluud C. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol. 2008;37(2):142–150. doi: 10.1080/03009740701813103. Mar-AprPMID: 18415773. [DOI] [PubMed] [Google Scholar]
- 32.Jüni P., Reichenbach S., Trelle S., Tschannen B., Wandel S., Jordi B., Züllig M., Guetg R., Häuselmann HJ, Schwarz H., Theiler R., Ziswiler HR, Dieppe PA, Villiger PM. Egger M; Swiss Viscosupplementation Trial Group. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;56(11):3610–3619. doi: 10.1002/art.23026. Nov. [DOI] [PubMed] [Google Scholar]
- 33.Mazières B., Bard H., Ligier M., Bru I. d'Orsay GG., Le Pen C. Medicoeconomic evaluation of hyaluronic acid for knee osteoarthritis in everyday practice: the MESSAGE study. Joint Bone Spine. 2007;74(5):453–460. doi: 10.1016/j.jbspin.2007.01.037. OctEpub 2007 Aug 9. [DOI] [PubMed] [Google Scholar]
- 34.Petrella RJ, Petrella M. A prospective. randomized. double-blind. placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33(5):951–956. May. [PubMed] [Google Scholar]
- 35.Lee PB., Kim YC., Lim YJ., Lee CJ., Sim WS., Ha CW., Bin SI., Lim KB., Choi SS., Lee SC. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label. randomized. multicentre clinical trial. J Int Med Res. 2006;34(1):77–87. doi: 10.1177/147323000603400110. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- 36.Arensi F Comparison of efficacy and therapeutic safety of two treatments based on hyaluronic acid (Go-On and Hyalgan) in knee osteoarthritis - Minerva ortopedica e traumatologica. 2006. 57(3). 105-111
- 37.Neustadt D., Caldwell J., Bell M., Wade J., Gimbel J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: a randomized. controlled. multicenter trial. J Rheumatol. 2005;32(10):1928–1936. Oct. [PubMed] [Google Scholar]
- 38.Altman RD., Akermark C., Beaulieu AD., Schnitzer T, Durolane International Study Group Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2004;12(8):642–649. doi: 10.1016/j.joca.2004.04.010. Aug. [DOI] [PubMed] [Google Scholar]
- 39.Petrella RJ., DiSilvestro MD., Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee: a randomized. double-blind. placebo-controlled clinical trial. Arch Intern Med. 2002;162(3):292–298. doi: 10.1001/archinte.162.3.292. Feb 11. [DOI] [PubMed] [Google Scholar]
- 40.Brandt KD., Block JA., Michalski JP., Moreland LW., Caldwell JR., Lavin PT. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin Orthop Relat Res. 2001;(385):130–143. doi: 10.1097/00003086-200104000-00021. Apr. [DOI] [PubMed] [Google Scholar]
- 41.Takamura J., Seo T., Strand V. A Single Intra-Articular Injection of Gel-200 for Treatment of Symptomatic Osteoarthritis of the Knee Is More Effective than Phosphate Buffered Saline at 6 Months: A Subgroup Analysis of a Multicenter. Randomized Controlled Trial. Cartilage. 2019;10(4):417–422. doi: 10.1177/1947603518768015. OctEpub 2018 Apr 12. PMID: 29644875; PMCID: PMC6755876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuan S., Liou I., Su H., Tsai Y., Chen G., Sun S. Improvement of self-reported functional scores and thickening of quadriceps and femoral intercondylar cartilage under ultrasonography after single intra-articular injection of a novel cross-linked hyaluronic acid in the treatment of knee osteoarthritis. Journal of Back and Musculoskeletal Rehabilitation. 2018;1:1–10. doi: 10.3233/BMR-170950. [DOI] [PubMed] [Google Scholar]
- 43.HA CW, Park YB, Choi CH, Kyung HS, Lee JH, Yoo JD, Yoo JH, Choi CH, Kim CW, Kim HC, Oh KJ, Bin SI, Lee MC. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: a double-blind. randomized. multi-center. non-inferiority study - BMC Musculoskeletal Disorders. 2017;18:223. doi: 10.1186/s12891-017-1591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun SF., Hsu CW., Lin HS., Liou IH., Chen YH., Hung CL. Comparison of Single Intra-Articular Injection of Novel Hyaluronan (HYA-JOINT Plus) with Synvisc-One for Knee Osteoarthritis: A Randomized. Controlled. Double-Blind Trial of Efficacy and Safety. J Bone Joint Surg Am. 2017;99:462–471. doi: 10.2106/JBJS.16.00469. [DOI] [PubMed] [Google Scholar]
- 45.Conrozier T., Bozgan AM., Bossert M., Sondag M., Lohse-Walliser A., Balblanc JC. Standardized Follow-up of Patients with Symptomatic Knee Osteoarthritis Treated with a Single Intra-articular Injection of a Combination of Cross-Linked Hyaluronic Acid and Mannitol. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2016;9:1–5. doi: 10.4137/CMAMD.S39432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearey P., Popple AE, Warren J., Davis T., Bellamy N, LOBRAS Study Group Improvement in condition-specific and generic quality of life outcomes in patients with knee osteoarthritis following single-injection Synvisc: results from the LOBRAS study. Curr Med Res Opin. 2017;33(3):409–419. doi: 10.1080/03007995.2016.1260533. Mar. [DOI] [PubMed] [Google Scholar]
- 47.Pal S., Thuppal S., Reddy KJ., Avasthi S., Aggarwal A., Bansal H., Mohanasundaram S., Bailleul F. Long-Term (1-Year) Safety and Efficacy of a Single 6-mL Injection of Hylan G-F 20 in Indian Patients with Symptomatic Knee Osteoarthritis. The Open Rheumatology Journal. 2014;8:54–68. doi: 10.2174/1874312901408010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borras-Verdera A., Calcedo-Bernal V., Ojeda-Levenfeld J. Clavel-Sainz C. Efficacy and safety of a single injection of 2% hyaluronic acid and mannitol in knee osteoarthritis over a 6-month period. Rev Esp Cir Ortop Traumatol. 2012;56(4):274–280. doi: 10.1016/j.recot.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Chevalier X., Jerosch J., Goupille P., van Dijk N., Luyten FP, Scott DL, Bailleul F., Pavelka K. Single. intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised. multicentre. double-blind. placebo controlled trial. Ann Rheum Dis. 2010;69(1):113–119. doi: 10.1136/ard.2008.094623. JanPMID: 19304567; PMCID: PMC2789938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cubukçu D., Ardiç F., Karabulut N., Topuz O. Hylan G-F 20 efficacy on articular cartilage quality in patients with knee osteoarthritis: clinical and MRI assessment. Clin Rheumatol. 2005;24(4):336–341. doi: 10.1007/s10067-004-1043-z. AugEpub 2004 Dec 14. PMID: 15599642. [DOI] [PubMed] [Google Scholar]
- 51.Day R., Brooks P., Conaghan PG. Petersen M; Multicenter Trial Group. A double blind. randomized. multicenter. parallel group study of the effectiveness and tolerance of intra-articular hyaluronan in osteoarthritis of the knee. J Rheumatol. 2004;31:775–782. [PubMed] [Google Scholar]
- 52.Karlsson J., Sjögren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled. randomized. double-blind. parallel-design multicenter study. Rheumatology (Oxford) 2002;41:1240–1248. doi: 10.1093/rheumatology/41.11.1240. [DOI] [PubMed] [Google Scholar]
- 53.Maravic M. Pascaretti C. Insalaco P. Lesort A. Vincent P. Miotti H. Bardoulat I. Maillard C. ART-QUALIVIE: Assessment of the Quality of Life of patients with knee osteoarthritis six months after treatment with three intra-articular injections of ARTHRUM H 2% - Ann Rheum Dis: first published as 10.113/annrheumdis-2019-eular.2937.
- 54.Baron D, Flin C, Porterie J, Despaux J., Vincent P. Hyaluronic Acid Single Intra-Articular Injection in Knee Osteoarthritis: A Multicenter Open Prospective Study (ART-ONE 75) with Placebo Post Hoc Comparison. Curr Ther Res Clin Exp. 2018 Apr 18;88:35–46. doi: 10.1016/j.curtheres.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas T., Amouroux F., Vincent P. Intra articular hyaluronic acid in the management of knee osteoarthritis: Pharmaco-economic study from the perspective of the national health insurance system. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173683. Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hilliquin P. Mesure de l'efficacité en vie réelle de trois injections intra-articulaires du produit de santé ARTHRUM H 2% chez les sujets de moins de 60 ans atteints de gonarthrose. RéfleXions Rhumatologiques. 2017;195(21):30–32. Nov. [Google Scholar]
- 57.Germonville T., Prudat M., Vincent P. Pain care in knee osteoarthritis by intra-articular injections of hyaluronic acid (ARTHRUM H 2%): a randomized double-blind controlled trial versus another hyaluronic acid (HYALGAN) Minerva Ortopedica e Traumatologica. 2015;66:235–253. [Google Scholar]
- 58.Vincent P., Lucas de Couville T., Thomas T. Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: A Postmarket. Open-Label. Long-Term Historical Control Study with Analysis Detailed per Kellgren-Lawrence Radiologic Osteoarthritis Scale Grade. Curr Ther Res Clin Exp. 2020 Feb 26;92 doi: 10.1016/j.curtheres.2020.100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivera F., Bertignone L., Grandi G., Camisassa R., Comaschi G., Trentini D., Zanone M., Teppex G., Vasario G., Fortina G. Effectiveness of intra-articular injections of sodium hyaluronate-chondroitin sulfate in knee osteoarthritis: a multicenter prospective study. J Orthop Traumatol. 2016;17(1):27–33. doi: 10.1007/s10195-015-0388-1. MarEpub 2015 Nov 14. Erratum in: J Orthop Traumatol. 2016 Mar;17(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hilliquin P. Prise en charge de la douleur et du handicap des patients atteints de gonarthrose par injection intra-articulaire d'Arthrum H 2% Réflexions Rhumatologiques. 2021;229(25):56–58. [Google Scholar]
- 61.Maheu E, Zaim M, Appelboom T, Jeka S, Trc T, Maasalu K, Berenbaum F. Comparative efficacy and safety of two different molecular weight (MW) hyaluronans F60027 and Hylan G-F20 in symptomatic osteoarthritis of the knee (KOA). Results of a non-inferiority, prospective, randomized, controlled trial. Clin Exp Rheumatol. 2011;29(3):527–535. [PubMed] [Google Scholar]
- 62.Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial) Semin Arthritis Rheum. 2009;39(1):1–9. doi: 10.1016/j.semarthrit.2009.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.