Abstract
Background & Aims
Autosomal recessive polycystic kidney disease (ARPKD) is caused by mutations in PKHD1, encoding fibrocystin/polyductin (FPC). Severe disease occurs in perinates. Those who survive the neonatal period face a myriad of comorbidities, including systemic and portal hypertension, liver fibrosis, and hepatosplenomegaly. The goal here was to uncover therapeutic strategies for ARPKD.
Methods
We used wild-type and an FPC-mutant cholangiocyte cell line in 3-dimenional cysts and in confluent monolayers to evaluate protein expression using western blotting and protein trafficking using confocal microscopy.
Results
We found that the protein level of the cystic fibrosis transmembrane conductance regulator (CFTR) was downregulated. The levels of heat shock proteins (HSPs) were altered in the FPC-mutant cholangiocytes, with HSP27 being downregulated and HSP90 and HSP70 upregulated. FPC-mutant cholangiocytes formed cysts, but normal cells did not. Cyst growth could be reduced by increasing HSP27 protein levels, by HSP90 and HSP70 inhibitor treatments, by silencing HSP90 through messenger RNA inhibition, or by the novel approach of treating the cysts with the CFTR corrector VX-809. In wild-type cholangiocytes, CFTR is present in both apical and basolateral membranes. FPC malfunction resulted in altered colocalization of CFTR with both apical and basolateral membranes. Whereas, treatment with VX-809, increasing HSP27 or inhibiting HSP70 or HSP90 restored CFTR localization toward normal values.
Conclusions
FPC malfunction induces the formation of cysts, which are fueled by alterations in HSPs and in CFTR protein levels and miss-localization. We suggest that CFTR correctors, already in clinical use to treat cystic fibrosis, could also be used as a treatment for ARPKD.
Keywords: Processing, Trafficking, ARPKD, FPC, ERAD
Abbreviations used in this paper: 3D, 3-dimensional; AHA1, ATPase homolog 1; ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; cAMP, adenosine 3′,5′-cyclic monophosphate; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; ER, endoplasmic reticulum; ERAD, endoplasmic reticulum–associated degradation; FPC, fibrocystin/polyductin; HSP, heat shock protein; PBS, phosphate-buffered saline; WGA, wheat germ agglutinin; WT, wild-type
Graphical abstract
Summary.
Autosomal recessive polycystic kidney disease (ARPKD) causes severe disease in babies in the womb. Those who survive the neonatal period face chronic kidney and liver disease throughout their life. The overall goal of our study was to uncover therapeutic strategies to treat ARPKD.
Autosomal recessive polycystic kidney disease (ARPKD) associated with mutations in PKHD11, 2 is a debilitating autosomal recessive genetic disorder that is responsible for chronic end-stage renal disease in humans.3,4 ARPKD5 occurs in 1 in 20,000 live births in the United States. More than 350,000 people worldwide are affected each year, imposing a large health care burden and resulting in newborn morbidity and mortality. The most severe disease occurs in perinates, who present with massively enlarged, echogenic kidneys and oligohydramnios. Neonates with severe disease have pulmonary hypoplasia and a mortality rate of ~30%.6 Those who survive the neonatal period face a myriad of comorbidities, including systemic and portal hypertension, fibrosis of both the liver and kidney, hepatosplenomegaly,3 and enlarged kidneys, with fusiform dilation of the collecting ducts that progresses to end-stage renal disease.7 ARPKD is caused by mutations in the PKHD1 gene, which encodes a large 4074-amino-acid protein, fibrocystin/polyductin (FPC), with a long extracellular domain and single transmembrane domain.8,9 The existence of several alternate splicing forms suggest that FPC occurs as both an anchored and a secreted protein.10 The gene is expressed predominantly in the fetal and adult liver, kidneys, and pancreas; the protein it encodes is present in the plasma membrane, primary cilium, and cytoplasm.8 Consistent with its multilocational positioning, a functional deficit of FPC has been linked to a number of alterations in cellular integrity, such as changes in their cell adhesion, centrosome positioning, and ability to achieve correct polarization.11
Role of the Cystic Fibrosis Transmembrane Conductance Regulator in ARPKD
The cystic fibrosis transmembrane conductance regulator (CFTR) protein, whose mutation causes CF, has been suggested to also play a role in the growth of the cysts in autosomal dominant polycystic kidney disease (ADPKD). For example, O’Sullivan et al12 reported that a patient cohort with both CF and ADPKD had less severe kidney and liver disease than did patients with ADPKD alone. On the other hand, Persu et al13 were unable to find any difference in kidney volume or renal function between a cohort of ADPKD patients who also bore the F508-del CFTR mutation and a cohort of patients having ADPKD alone. Thus, the impact of CFTR on the progression of ADPKD is equivocal, with some studies pointing to some degree of amelioration and others showing no effect at all. However, in ARPKD, the role of CFTR appears to be quite different from its role in CF. For example, when Nakanishi et al14 crossed a PBK mouse (an ARPKD model) with a CFTR-knockout mouse to create a bpk–/–;cftr–/– double homozygous mouse, they found, surprisingly, that the double mutants exhibited massively enlarged kidneys and died from renal failure at ~24 days after birth.15 Therefore, we have now asked why knocking down CFTR apparently worsens the ARPKD disease phenotype.
Interestingly, liver disease is a frequent complication of CF that is present in about one-third of all CF patients16 and has clear similarities to the liver disease observed in ARPKD. The clinical symptoms include biliary cirrhosis and progressive periportal fibrosis leading to portal hypertension and liver failure.16 The classic thinking is that cholangitis in CF patients is caused by biliary obstruction that results from a deficit in fluid secretion via CFTR and other Cl- channels.17 However, this notion has been questioned. For example, careful electron micrographic examination of liver pathology in patients with varying degrees of liver disease does not support the theory of chronic bile duct obstruction; instead, it shows evidence of increases in collagen deposition and cell proliferation and an abnormal cholangiocyte morphology.18 The authors of the study concluded that their findings do not support obstruction as a major factor in the development of fibrosis in CF.19 Thus, one possible reason that mice with knockout of both CF and FPC exhibit worse disease is the added tendency to liver pathology in both diseases.
Role of Proteostasis
It is well known that an ensemble of proteins referred to as the proteostatic network plays a role in the normal processing of proteins.20,21 According to Balch et al,22 this network participates in disease processes. For example, the heat shock proteins (HSPs) HSP27, HSP70, and HSP90 and the activator of 90-kDa HSP ATPase homolog 1 (AHA1) are all stress-responsive proteins23,24 that are known to be involved in the processing of CFTR.21 HSP90 levels increase during stress in order to guard against misfolded proteins,25 and AHA1 is an activator of HSP90 ATPase.26 HSP70 is upregulated in cancer cells and is associated with a negative outcome.27,28 In addition, HSP70 and HSP90, in combination with AHA1, inhibit the surface expression of CF mutant F508-del CFTR, clearly indicating their role in CFTR trafficking from the endoplasmic reticulum (ER) to the plasma membrane.21 HSP27 (HSPB1), a member of the small HSP family, is thought to guard against protein aggregation during stress.29 We have shown previously that HSP27, HSP70, and HSP90 are all elevated in cystic kidneys derived from mice lacking PC1 when compared with wild-type (WT) control animals. One question to be addressed is whether these same HSPs are involved in ARPKD.
CFTR Correctors and Potentiators
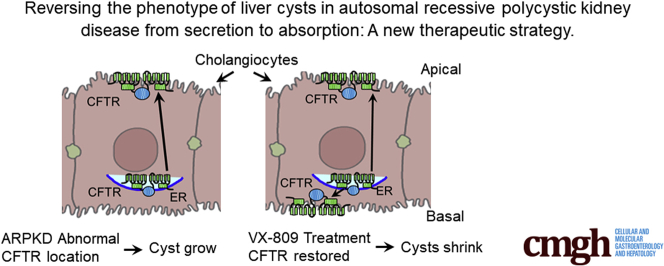
The corrector VX-809 (lumacaftor) was developed to rescue the F508-del CFTR mutant.30 Because CFTR is present in cysts,31 many researchers have suggested that CFTR inhibitors might be therapeutic in reducing fluid secretion.32, 33, 34, 35, 36 However, we have shown previously that the CFTR corrector VX-809 can reduce cysts37 in mouse- and cell-based models of ADPKD. In the cysts, CFTR is present at the apical membrane, which is why CFTR-based inhibitors can reduce cyst growth to some extent. Surprisingly, however, when VX-809 is applied, CFTR moves to the basolateral membrane and promotes an absorptive phenotype, which then shrinks the cysts. The overall goal of our study here is to understand the role of CFTR in ARPKD and uncover therapeutic targets for pharmacological strategies to treat ARPKD.
Results
Pkhd1del4/del4 Cholangiocytes
To begin to identify strategies to treat ARPKD, we conducted experiments in WT and Pkhd1del4/del4 cholangiocytes (hereafter referred to as del4), obtained from the Yale O’Brien Center and isolated as described in the Materials and Methods. The cholangiocytes were isolated from WT and Pkhd1del4/del4 mice, the latter a model of ARPKD.38 These mice, with a disruption in exon 4 of the Pkhd1 gene, display intrahepatic bile duct proliferation, progressive liver cyst formation, and periportal fibrosis, along with pancreatic cysts and splenomegaly.
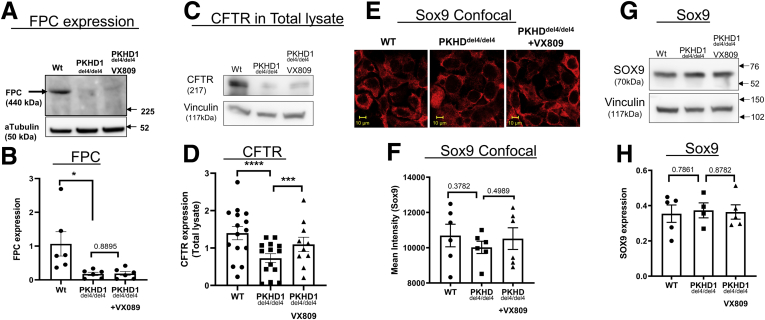
To begin to characterize these mutant cholangiocytes, we measured their steady-state levels of FPC. Figure 1A and B shows that the del4 cholangiocytes have levels of FPC substantially lower than those observed in WT cholangiocytes. Detectable FPC protein in the del4- cholangiocytes is expected because the out-of-frame deletion of exon 4 uncovers a cryptic splice site that creates an in-frame, hypomorphic transcript38 expressing a protein with a 66-amino-acid deletion. Application of 10 uM of VX-809 does not change FPC expression. It is unlikely that this residual protein is functional, given that the mice bearing this mutant have liver disease reminiscent of human pathology.
Figure 1.
FPC in Pkhd1del4/del4(del4) cholangiocytes. (A) Western blotting for FPC. Please note that del4 cells have substantially less FPC. Antibody was obtained from the Baltimore PKD Center. α-Tubulin was used as the loading control. (B) Summary. Reduced CFTR in del4 cholangiocytes. (C) Western blot showing CFTR. (D) Summary. (E, F) Sox9: WT, del4, and VX-809–treated del4 cholangiocytes stained for Sox9 (red), expressed as mean intensity. (G) Western blotting for Sox9. (H) Summary data. Number of Western blot experiments represented in the figure (dots) are from independent 10-cm dishes of cultured cells. Student’s t test. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CFTR Protein Expression
As mentioned previously, the roles of CFTR in ARPKD are unclear. To examine the expression of CFTR in cholangiocytes, we performed Western blotting (Figure 1C and D). We detected a sharp drop in CFTR in del4 cholangiocytes when compared with WT cells. CFTR is well known to exist in an immature, core-glycosylated form that resides primarily in the ER (the B band) and as a mature, fully glycosylated form (the C band), see Cheng et al.39 Thus, it is important to note that in del4 cholangiocytes, the mature fully glycosylated form of CFTR is absent. The processing induced by correctors usually involves the conversion of B bands to C bands as the processing is restored by the corrector30; however, the application of VX-809 does increase CFTR protein levels, but the mature form of CFTR is not fully restored.
Sox9 Expression
Sox9 is present in ductal epithelia such as cholangiocytes.40 Thus, to verify that the cells were cholangiocytes, we assayed the cells for the presence of Sox9. Figure 1E–H shows that Sox9 is expressed in both WT and del4 cholangiocytes. We saw no change in Sox9 either in normal, del4 cholangiocytes or the latter treated with VX-809.
Reducing the Cysts
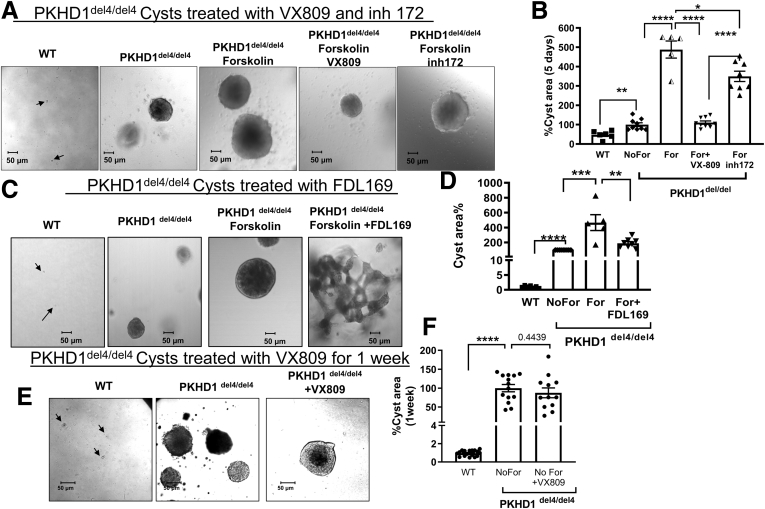
As mentioned previously, the Pkhd1del4/del4 mice develop multiple liver cysts with age.38 To study this phenomenon, we grew WT and del4 cholangiocytes in 3-dimensional (3D) culture. Figure 2 shows that cysts developed in 3D culture from the del4 cholangiocytes but cells from WT animals remain as small clumps. In ADPKD resulting from a malfunction of either PC1 or PC2, cyst growth is accelerated by adenosine 3′,5′-cyclic monophosphate (cAMP).41 Similarly, cyst growth in del4 cholangiocytes was accelerated by forskolin, which stimulates cAMP.42 Please note that 2 different CFTR correctors, VX-809 and FDL169,43 both reduced cyst growth even in the presence of forskolin stimulation. When we used the CFTR inhibitor 172,35 cyst growth was also reduced but to nowhere near the extent seen for VX-809. These data suggest that CFTR modulators such as VX-809 and FDL169 are likely to have greater potential than CFTR inhibitors for use as therapeutics for ARPKD. VX-809 does not have an effect on cyst growth in the absence of forskolin (Figure 1E and F).
Figure 2.
VX-809–mediated reduction in cyst formation. WT and del4 cholangiocytes were grown on Matrigel and monitored for 1 week. Cholangiocytes treated with (A, B) VX-809 or (C, D) FDL169. A smaller total cystic area was observed after treatment with either corrector. Cysts were induced with forskolin (For) (10 μM) for 24 hours on day 6. Arrows indicate WT cells. (E, F) Cholangiocytes were treated with VX-809 in the absence of forskolin. Cyst growth was monitored every other day. Cysts were treated with VX-809 (10 μM) for 10 days, with the medium was replaced every 48 hours. Number of cysts measured are represented in the figure as dots. Student’s t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Location of CFTR in Cholangiocytes
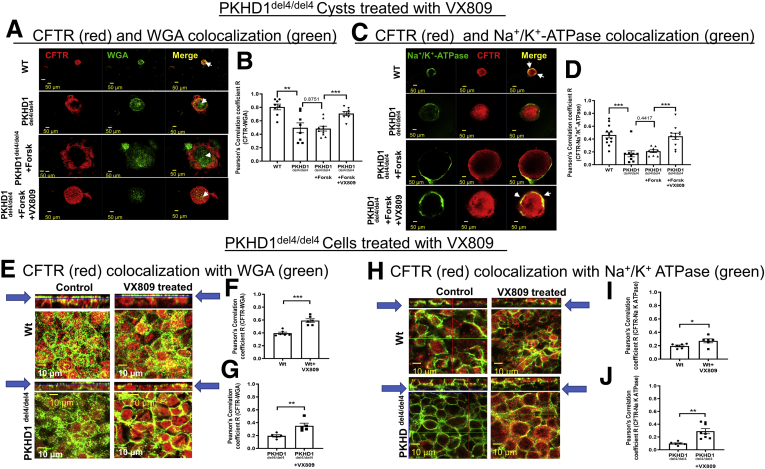
Next, we determined the location of CFTR in cysts (Figure 3A–D). Consistent with its role in fluid secretion in the bile duct,44 CFTR colocalizes with the apical marker wheat germ agglutinin (WGA) in WT cholangiocytes. In del4 cholangiocytes there is a reduction in colocalization of CFTR with the apical and most dramatically with the basolateral membrane. VX-809 increased the colocalization of CFTR with both the apical and basolateral membrane markers. To take this one step forward, we determined the location of CFTR in cholangiocytes grown on permeable supports using confocal microscopy (Figure 3E–J). Note that CFTR colocalized with the apical membrane marker WGA in WT and del4 cholangiocytes. Importantly, the colocalization was increased in the presence of VX-809. Interestingly CFTR also colocalizes with the basolateral membrane marker, Na+/K+ ATPase, but less so. VX-809 caused an increase in colocalization with the basolateral membrane marker Na+/K+ ATPase in both WT and del4 cholangiocytes.
Figure 3.
Colocalization of CFTR with (A, B) WGA and (C, D) Na+/K+ATPase in VX-809–treated WT or mutant (Pkhd1del4/del4) cholangiocyte cysts. WT or mutant del4 cholangiocytes were grown on Matrigel. For immunostaining, 3D cultures were treated with collagenase 1 to improve the permeability through the Matrigel and then fixed with 4% PFA. Double-labeled immunofluorescence images stained for CFTR (red), (A) WGA or (C) Na+/K+ ATPase (green), and the merged image. Yellow denotes colocalization. (B, D) Pearson's correlation coefficient (R). Scale bar = 50 μm. White arrows indicate the colocalization of CFTR and WGA or CFTR and Na+/K+ ATPase at the apical or basolateral regions of the cells, respectively. Each set of data is from multiple regions of 7-8 individual cysts. CFTR expression and localization: Localization of CFTR to (E) apical vs (H) basolateral membranes. (E–G) CFTR (red) and WGA (green) in WT or del4 cholangiocytes treated with VX-809 (10 μM). Pearson's correlation coefficient R. (H–J) CFTR (red) and Na+/K+ ATPase (green) in WT or del4 cholangiocytes treated with VX-809 (10 μM). Pearson's correlation coefficient R. Cells were grown on Transwell membranes. Strips = Z-stacks of confocal images acquired at 0.4 μm using a 63× oil objective. Arrows point to the Z-stack image corresponding to the accompanying figure immediately below. In this figure and all subsequent confocal images, 1 Z-stack plane is shown. As noted by the arrows, Z-stack planes for apical localization were selected toward the apical pole of the cells. In contrast, Z-stack planes for basolateral localization were selected toward the basolateral pole of the cells. Scale bar = 10 μm. All data are expressed as mean ± SEM. P values were determined by Student’s t test. For quantification of colocalization, 2–3 random areas were measured along a single z-plane as depicted by the arrows, from 3 individual slides for each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
HSPs Play an Important Role in ARPKD
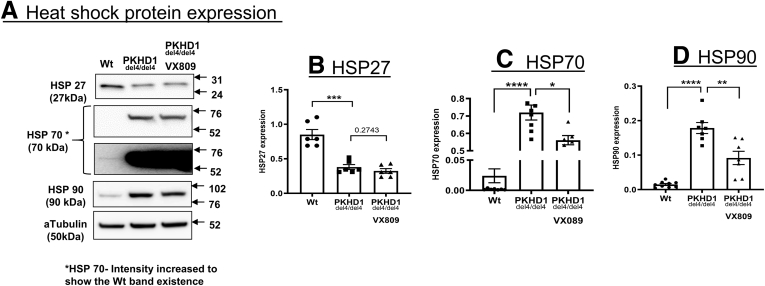
A myriad of proteins forming a proteostatic network are involved in the processing and trafficking of CFTR. To delineate the role of HSPs in ARPKD, we measured their protein expression by Western blotting. The absence of FPC had a profound effect on the HSPs, causing a large reduction in the protein levels of HSP27 and dramatic increases in HSP90 and HSP70 (Figure 4). The next question we asked was whether these changes affected cyst growth and the localization of CFTR.
Figure 4.
ERAD protein expression in ARPKD cells. (A) Western blot of HSP27, HSP70, and HSP90 in WT or del4 cholangiocytes and del4 cells treated with VX-809 (10 μM). α-tubulin was used as the loading control. (B–D) Summary data. All data are expressed as mean ± SEM. P values were determined by Student’s t test. Please note that the same gel for HSP70 is presented at 2 different exposure times. Number of Western blot experiments represented in the figure (dots) are from independent 10-cm dishes of cultured cells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
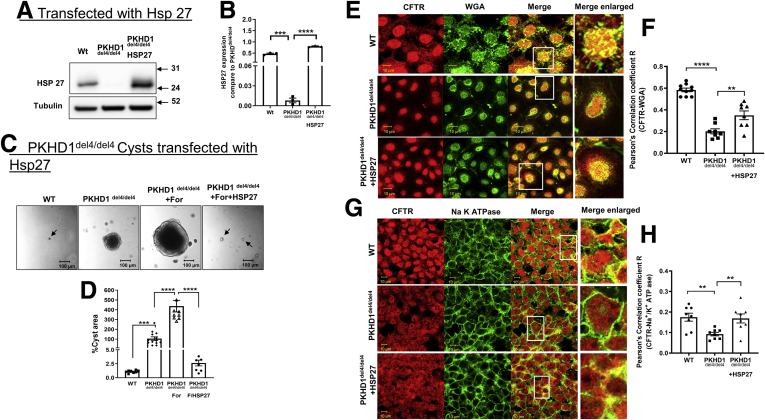
To evaluate this question, we first focused on HSP27. To determine whether low levels of HSP27 affected cyst growth, we transfected additional HSP27 into del4 cholangiocytes. Transfecting additional HSP27 into del4 cholangiocytes (see Figure 5A and B) to restore the protein levels to near WT values induced a profound reduction in cyst growth (Figure 5C and D). Note that similar to VX-809, transfecting additional HSP27 caused an increase in colocalization with the apical marker WGA and the basolateral membrane marker Na+/K+ ATPase in del4 cholangiocytes toward WT values (Figure 5E–H).
Figure 5.
Maneuvers that reduce cysts increasing HSP27. WT, del4 cholangiocytes, and del4 cholangiocytes transfected with HSP27. (A, B) Western blot confirming HSP27 transfection. α-Tubulin was used as the loading control. WT and del4 cholangiocytes were grown on Matrigel and monitored for 1 week. (C, D) Significant reduction in cyst area as compared with WT cholangiocytes was observed in HSP27-transfected cholangiocytes. Localization of CFTR to (E, F) apical vs (G, H) basolateral membranes. (F) CFTR (red) and WGA (green) in WT or del4 cholangiocytes treated with VX-809 (10 μM). Pearson's correlation coefficient R. (G, H) CFTR (red) and Na+/K+ ATPase (green) in WT or Del4 cholangiocytes. Cells were grown on Transwell membranes. Z-stacks of confocal images acquired at 0.4 μm using a 63× oil objective but representative images of the apical location for WGA vs the more basolateral image for Na+/K+ ATPase. A portion of the image demarcated by a white box was enlarged on the right. Scale bar = 10 μm. All data are expressed as mean ± SEM. P values were determined by Student’s t test. For quantification of colocalization, 2–3 random areas were measured along a single z-plane as depicted by the arrows, from 3 individual slides for each group. Number of cysts represented in the figure (dots) are from individual cysts. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
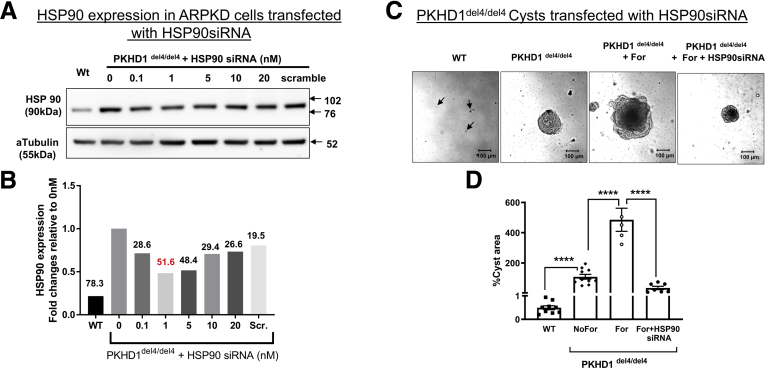
Next, we focused on HSP90. Because HSP90 expression is elevated in del4 cholangiocytes, we silenced HSP90 using small interfering RNA to reduce its protein levels (Figure 6A and B). Reducing HSP90 protein levels using small interfering RNA had a profound effect on cyst size even in the presence of forskolin (Figure 6C and D).
Figure 6.
Silencing HSP90. (A, B) Western blots showing optimization of HSP90 silencing. WT and del4 cholangiocytes were grown on Matrigel and monitored for 1 week. (C, D) Cyst growth is inhibited by silencing HSP90. All data are expressed as mean ± SEM. P values were determined by Student’s t test. Number of cysts represented in the figure (dots) are from individual cysts. Number of Western blot experiments represented in the figure (dots) are from independent 10-cm dishes of cultured cells. ∗∗∗∗P < .0001. siRNA, small interfering RNA.
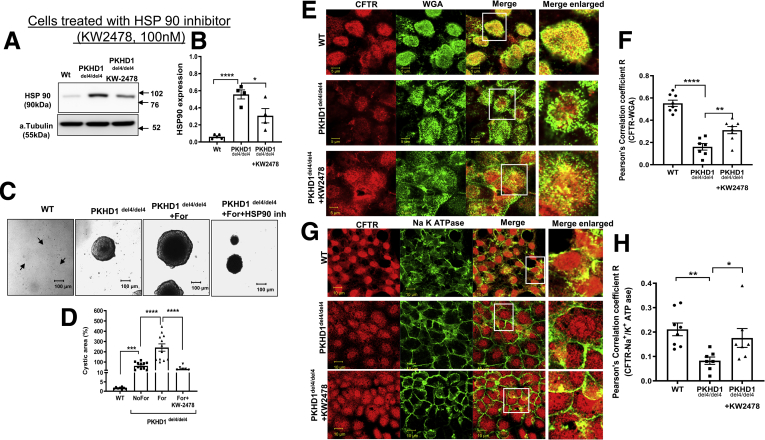
We also applied the HSP90 inhibitor KW-247845 to reduce HSP90 protein and activity. Note that application of KW-2478 reduced HSP90 protein levels (Figure 7A and B) and reduced cyst growth (Figure 7C and D). Note that similar to VX-809, the HSP90 inhibitor KW-2478 caused an increase in colocalization of CFTR with the apical marker WGA and the basolateral membrane marker Na+/K+ ATPase in del4 cholangiocytes toward WT values (Figure 7E–H).
Figure 7.
Maneuvers that reduce cysts inhibiting HSP90. (A, B) Western blot of WT and del4 cholangiocytes treated with KW-2478. WT and del4 cholangiocytes were grown on Matrigel and monitored for 1 week. (C, D) A significant difference from WT in cyst area was observed in HSP90 inhibitor treated cholangiocytes. Localization of CFTR to (E, F) apical vs (G, H) basolateral membranes. CFTR (red) and WGA (green) in WT or del4 cholangiocytes treated with VX-809 (10 μM). (F, H) Pearson's correlation coefficient R. (G, H) CFTR (red) and Na+/K+ ATPase (green) in WT or Del4 cholangiocytes. Cells were grown on Transwell membranes. Z-stacks of confocal images acquired at 0.4 μm using a 63× oil objective but representative images of the apical location for WGA vs. the more basolateral image for Na+/K+ ATPase. A portion of the image demarcated by a white box was enlarged on the right. Scale bar = 10 μm. All data are expressed as mean ± SEM. P values were determined by Student’s t test. For quantification of colocalization, 2–3 random areas were measured along a single z-plane as depicted by the arrows, from 3 individual slides for each group. Number of cysts represented in the figure (dots) are from 3 independent experiments. Number of Western blot experiments represented in the figure (dots) are from independent 10 cm dishes of cultured cells. Number of Western blot experiments represented in the figure (dots) are from independent 10 cm dishes of cultured cells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
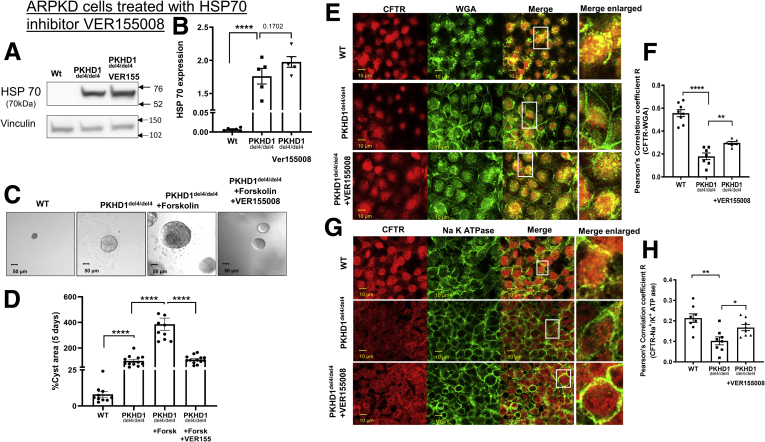
Finally, we focused on HSP70. Because HSP70 expression is elevated in del4 cholangiocytes, we applied the HSP70 inhibitor, VER155008,46 which did not affect the steady state levels of HSP70 (Figure 8A and B). Similar to HSP90, the HSP70 inhibitor reduced cyst growth (Figure 8C and D). Note that similar to VX-809, the HSP70 inhibitor caused an increase in colocalization of CFTR with the apical marker WGA and the basolateral membrane marker Na+/K+ ATPase in del4 cholangiocytes toward WT values (Figure 8E–H).
Figure 8.
Maneuvers that reduce cysts inhibiting HSP70. (A, B) Western blot of WT and del4 cholangiocytes treated with VER155008. WT and del4 cholangiocytes were grown on Matrigel and monitored for 1 week. (C, D) Cystic cells were treated with HSP70 inhibitor (VER155008). A significant difference from WT in cyst area was observed in HSP70 inhibitor treated cholangiocytes. Localization of CFTR to (E, F) apical vs (G, H) basolateral membranes. CFTR (red) and WGA (green) in WT or del4 cholangiocytes treated with VER155008 (10 μM). Pearson's correlation coefficient R. (F) CFTR (red) and (H) Na+/K+ ATPase (green) in WT or del4 cholangiocytes. Cells were grown on Transwell membranes. Z-stacks of confocal images acquired at 0.4 μm using a 63× oil objective but representative images of the apical location for WGA vs. the more basolateral image for Na+/K+ ATPase. A portion of the image demarcated by a white box was enlarged on the right. Scale bar = 10 μm. All data are expressed as mean ± SEM. P values were determined by Student’s t test. For quantification of colocalization, 2–3 random areas were measured along a single z-plane as depicted by the arrows, from 3 individual slides for each group. Number of Western blot experiments represented in the figure (dots) are from independent 10-cm dishes of cultured cells. Number of cysts represented in the figure (dots) are from individual cysts. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Thus, in their entirety, our data show that the HSPs play an important role in fueling cyst growth in ARPKD and altering the localization of CFTR.
Discussion
Defective Processing of CFTR
We show here that FPC malfunction in cholangiocytes affects several cellular processes that have a profound impact on the trafficking and processing of proteins such as CFTR. We observed that CFTR is expressed in the cholangiocytes at a much lower level in del4 cholangiocytes than in WT cells, demonstrating that in the absence of FPC, the steady-state levels of CFTR fall. This result is interesting, given that Nakanishi et al14 showed that creating a double knockout of CFTR and FPC makes the phenotype of the mouse worse than that of a mouse bearing only a mutant FPC gene. These data regarding the double-knockout mice, together with our observed reduction in CFTR levels in del4 cholangiocytes, suggests that a deficit in CFTR expression contributes to the pathologies noted in ARPKD. Interestingly, the reduction in CFTR protein in del4 cholangiocytes was associated with an increase in the presence of the immature, lower-molecular-weight B-band of CFTR. CFTR is well characterized with regard to its processing and exit from the ER, and the presence of the also B-band is well known to indicate arrested processing and trafficking from the ER.47 Thus, the lower levels of CFTR are suggestive of defective processing in del4 cholangiocytes.
ER-Associated Protein Degradation
Because we noted changes in CFTR that point to its misprocessing, we decided to determine whether key ER–associated degradation (ERAD) proteins, including HSPs,48 are altered in the del4 cholangiocytes. We were surprised to detect a drop in HSP27 because we have previously shown that when PC1 malfunctions, HSP27 is upregulated.49 A reduction in HSP27 levels clearly plays a role in this process because transfecting additional HSP27 reduces cyst growth. HSP27 plays many roles in cells, ranging from protecting the cells from misfolded proteins to regulating actin stability and inflammation.50 Interestingly, both upregulation and downregulation of HSP27 levels are associated with many diseases.50 For example, in certain cancers such as bladder cancer, HSP27 is upregulated to prevent aggregation of proteins. In this instance, reducing HSP27 reduces cancer progression.51 At the opposite extreme, in retinopathy, high glucose in retinal capillary cells causes a downregulation of HSP27 induced by proinflammatory cytokines that stimulates apoptosis, thereby potentially contributing to the retinopathy.52 In our experience, the most common mutation in CFTR, F508-del, increases HSP27 in airway cells, most likely protecting them from the misfolded F508-del protein.53 On the other hand, in some mutant forms of ABCA4 associated with Stargardt disease, HSP27 levels are downregulated.54 Recently, we have shown that a reduction in HSP27 is one of the outcomes of Clostridium difficile infection.55 These polar differences in disease-associated changes in HSP27 reflect the different pathological outcomes associated with the disease process. For example, in cancer as well as ADPKD, high HSP27 levels may protect the dividing cells from unfolded proteins and apoptosis, allowing them to grow pathologically, whereas in C. difficile, a reduction in HSP27 is associated with a reduction in the epithelial barrier in infected GI tract cells that leads to inflammatory diarrhea.
HSP27 is known to bind to actin and increase the stability of F-actin (filamentous actin),56 1 of the 2 forms of actin in cells. The dynamic interchange between the 2 actin forms, F-actin and G-actin (globular actin), is critical for the proper functioning of the cellular cytoskeleton.56 A reduction in HSP27 has been shown to have a deleterious effect on cells, particularly with regard to disrupting actin dynamics.56 The phenotype in ARPKD differs from ADPKD in the development of ductal plate abnormalities and fusiform dilation in the collecting ducts.9 It is interesting that the cortical location of the actin cytoskeleton observed in normal inner medullary collecting duct cells is disrupted by silencing Pkhd1, which in turn leads to an epithelial-to-mesenchymal transition,57 perhaps as a result of enhanced RhoA levels in kidney cells.
Here, we have also demonstrated that HSP90 and HSP70 are upregulated in cholangiocytes. Furthermore, we have shown that reducing HSP90, either by silencing its transcription or by the use of an inhibitor, reduces cyst growth. This result is consistent with our data showing that CFTR protein levels are reduced in del4 cholangiocytes. Given that HSP70 and 90 are known to play a role in the processing of CFTR21, these data demonstrate clearly the role of these proteins in CFTR trafficking from the ER to the plasma membrane.
Along these lines, HSP90 is overactive in ADPKD cells, and a reduction in its function decreases cyst growth.58,59 Seeger-Nukpezah et al59 showed that application of the small molecule inhibitor STA-2842 over a 10-week period not only inhibits the initial formation of cysts, but also slows the progression of preexisting cysts, not unlike our observations here. Thus, elevations in HSP90 and HSP70 appear to be common to both ARPKD and ADPKD.
Role in CFTR trafficking
CFTR is normally found in the apical membrane of cholangiocytes, where it participates in fluid secretion.60 Consistent with this localization, we found that in WT cholangiocytes, CFTR colocalizes principally with apical membrane markers. CFTR also colocalizes with the basolateral membrane marker Na+/K+ ATPase, albeit less strongly. It is known that in secretory epithelia, newly processed CFTR first traffics to the basolateral membrane and then is transcytosed to the apical membrane.61 Thus, it is not unusual to detect CFTR colocalization within the basolateral membrane. One can readily see that in del4 cholangiocytes, the colocalization of CFTR with the basolateral marker is reduced, likely contributing to cyst formation in a manner similar to that in ADPKD.31 VX-809 treatment increased CFTR expression in del4 cholangiocytes at the apical and basolateral membranes. We have previously demonstrated a similar pattern in PC1-null cells derived from the kidney.37 In that study, we showed that the trafficking and function of transporters involved in Na+ absorption, such as NHE3 and ENaC, are also restored by VX-809, reversing the cyst phenotype from secretion to absorption.
CFTR Modulators
We have shown here that the CFTR corrector VX-809, which was designed to rescue F508-del CFTR, reduces cyst growth in del4 cholangiocytes essentially as it does in PC1-deficient proximal tubule cells and in a PC1-null model of ADPKD.37,49 The overarching similarity in these scenarios is that VX-809 increases the localization of CFTR in the basolateral membrane. We suggested in our previous work that this movement of CFTR to the basolateral membrane reduces the phenotype of the cysts from secretory to absorptive.37 VX-809 does not have any effect on the cysts in the absence of forskolin. This is not surprising because CFTR is not the only channel driving fluid secretion in the cysts.31 For example, the calcium activated chloride channel, Anoctamin 1 also supports cyst growth, particularly in response to signal transduction mechanisms which increase Ca2+.62 Moreover, VX-809 increases the processing and trafficking of CFTR but does not active it.30 CFTR is activated though protein kinase A via cAMP.63 Thus, in our scenario, VX-809 moves CFTR to the basolateral membrane and then forskolin activates and increase in cAMP and subsequent increase in active protein kinase A, thereby reducing the cysts.
It is also informative to determine what VX-809 fails to rescue. Although our transfection experiments show that HSP27 plays a role in cyst growth, VX-809 does not restore HSP27 toward WT values, indicating that VX-809 and HSP27 reduce cyst growth by different mechanisms.
Conclusion
We found that a deficit of FPC in cholangiocytes leads to a reduction in CFTR and profound changes in an ensemble of ERAD proteins that are known to play a role in CFTR processing such as HSP27, HSP90, and HSP70.64 VX-809 also reduces ERAD proteins, which are implicated in retaining CFTR in the ER and thus promoting the trafficking of CFTR to the basolateral membrane, where it can participate in reducing cysts. These data suggest that CFTR correctors could be useful and therapeutics for ARPKD. Given that they are already approved for use in humans,65 they can be fast-tracked for clinical use for ADPKD.
Materials and Methods
Reagents
Forskolin (#11018) was purchased from Sigma-Aldrich (St Louis, MO); VX-809 (#S1565) and FDL169 (#S8795) were purchased from Selleck Chemicals (Houston, TX); HSP27 (SC13132), HSP70 (SC66048) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX); Sox9 (ab185230) and Na+/K+ ATPase (#ab76020) antibodies were purchased from Abcam (Cambridge, United Kingdom); WGA (#W11261) was purchased from Invitrogen (Waltham, MA); and HSP90 (ADI-SPA-830F) antibody was purchased from Enzo Life Sciences (Farmingdale, NY).
Isolation of Cholangiocytes From WT-C57Bl/6 and Pkhd1del4-/del4 Mice
To create the cholangiocyte cultures, WT-C57Bl/6 and Pkhd1del/4/del4 mice66 were crossed with mice transgenic for the Sv40 large T antigen (strain name SV40 T-antigen, JAX strain #002233). Segments of intrahepatic bile ducts were microdissected from livers that had been perfused via the portal vein with molten agarose containing toluidine blue to mark the veins. The liver parenchyma was stripped until the biliary tree was identified, and the bile duct units were seeded onto rat type I collagen-coated plates. The cells were cultured under non-permissive conditions in the following mixed medium: Dulbecco’s modified Eagle medium/F12 with 5% fetal bovine serum, 10-μL/mL insulin-transferrin-selenium, 3.4-μg/mL tri-iodothryrodine, and 50,000 units of penicillin/streptomycin under humidified conditions at 37°C in 5% CO2. After 1 week, the cells were cultured under permissive conditions in the presence of 10 units of interferon-γ at 33°C in 5% CO2. When confluent, the cells were passaged by digesting the collagen slab below the cells (60 minutes; 37°C; Dulbecco’s modified Eagle medium/F12 with 2-mg/mL dispase and 1-mg/mL collagenase type 11), then washed twice in phosphate-buffered saline (PBS) and plated onto collagen-coated support systems. Clonal cell lines were generated by single-cell isolation. For experimental use, the cells were differentiated for 10 days at 37°C without interferon-γ.
In Vitro Cystogenesis
To induce differentiation, cells were kept at 37ºC for at least 6 days without interferon-γ. After 1 week, the cells were used for 3D culture or other experiments. Growth factor–reduced Matrigel #354230 (Corning, Corning, NY) was used here, and cell dilutions were prepared so that there were approximately 6000 cells in 25 μL of medium; 25 μL of cell preparation was mixed with 50 μL of Matrigel, see Yanda et al.67 Pictures were taken with a Zeiss Axio microscope (Zeiss, Oberkochen, Germany). Cystic areas were analyzed with ImageJ 1.50i (provided by the National Institutes of Health, Bethesda, MD).
Immunofluorescent Staining
WT or del4 cells were grown on Transwell membranes of a 6-well plate for 4 days. Following the confluence of cells each Transwell membrane apparatus was transferred to a new plate and membranes were washed with PBS for 3 times. Cells on membranes were fixed with 4% formaldehyde. Cells were then washed 3 times with PBS and blocked, permeabilized with PBS containing 1% bovine serum albumin, 0.2% Triton X-100. Following blocking, membranes were incubated with primary antibodies for overnight at 4°C and then processed for secondary antibody. Following antibody staining and washings, membranes were mounted on glass slides using Fluoro-gel (EMS#17985-10) and covered with coverslips. Images were captured using Zeiss LSM 880 confocal microscope.
Western Blot
WT or del4 cells were harvested and solubilized in NP40 lysis buffer (1% NP40, 50-mM Tris HC, 150-mM NaCl), containing protease inhibitor cocktail (catalog# 78438; Thermo Fisher Scientific, Waltham, MA). Lysates were rotated for 20 minutes at 4°C. Following the lysis samples were centrifuged at 14,000 g for 15 minutes at 4°C to pellet the insoluble material. Supernatant was carefully transferred to a new clean tube for protein concentration measurements. Supernatants were mixed with 2X Laemmli sample buffer (#1610737; Bio-Rad, Hercules, CA). Each 50 μg of protein sample was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis using 4%–15% protein TGX precast gel (#4561084; Bio-Rad). Proteins were transferred onto polyvinylidene difluoride membrane (#1704157; Bio-Rad) using Bio-Rad turbo transfer system, followed by blocking the membrane in 5% non-fat milk in TBST for 1hr at room temperature. Membranes were washed twice with TBST (tris-buffered saline with 0.05% tween20 and incubated with primary antibody (1:1000) diluted in blocking buffer for overnight at 4°C. Following the primary antibody incubation, membranes were washed 3 times and incubated with horseradish peroxidase conjugated secondary antibody for 1h at room temperature followed by washing with TBST for 3 times. The protein of interest was detected using Enhanced chemiluminescence (SuperSignal West Dura Extended Duration Substrate, catalog no. 34075; Thermo Fisher Scientific) reagent. The chemiluminescent signal on the polyvinylidene difluoride membrane was directly captured using Amersham Imager 600 (Amersham, Amersham, United Kingdom) equipped with a cooled CCD camera. ImageQuant TL 8.1 software (Cytiva, Marlborough, MA) was used for quantification of the bands.
Statistics
Averages ± SEM are given for all data. Data were analyzed by Student’s t test. Brackets indicate the compared values. The number of independent experiments is represented in each figure as a dots. The del4 and control cells originated each from 1 animal. Experiments on cultured cells were conducted on independent cultures of cells. For quantification of colocalization, 2–3 random areas were measured along a single z-plane from 3 individual slides for each group. For cysts, n indicates the number of individual cysts. All experiments were replicated until statistically significant at P < .05. Statistical significance was determined by Student’s t test. All experiments were unblinded. All data points are depicted in the figures.
Acknowledgments
The authors thank William B. Guggino, PhD, for reading the manuscript and Deborah McClellan, PhD, for editorial assistance. The authors thank Anna-Rachael Gallagher, PhD, and Stephen Somlo, MD, at the Yale O’Brian Center for the cholangiocytes.
CRediT Authorship Contributions
Murali K Yanda, Ph.D (Data curation: Lead; Formal analysis: Lead; Investigation:Supporting; Methodology: Supporting; Validation: Supporting; Visualization:Supporting; Writing – review & editing: Supporting)
Vartika Tomar, Ph.D (Data curation: Supporting; Formal analysis: Supporting;Methodology: Supporting; Writing – review & editing: Supporting)
Liudmila Cebotaru, MD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Resources: Lead; Supervision: Lead; Validation: Lead; Visualization: Lead; Writing –original draft: Lead)
Footnotes
Conflict of interest This author discloses the following: Liudmila Cebotaru has license and consulting agreements with RA Capital. These did not influence the experiments conducted in this manuscript. The remaining authors disclose no conflicts.
Funding The work was funded by National Institutes of Health Grant No. DK125272 (to Liudmila Cebotaru).
References
- 1.Ward C.J., Hogan M.C., Rossetti S., Walker D., Sneddon T., Wang X., Kubly V., Cunningham J.M., Bacallao R., Ishibashi M., Milliner D.S., Torres V.E., Harris P.C. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa Y., Matthiesen S., Onuchic L.F., Hou X., Bergmann C., Esquivel E., Senderek J., Ren Z., Zeltner R., Furu L., Avner E., Moser M., Somlo S., Guay-Woodford L., Buttner R., Zerres K., Germino G.G. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol. 2002;13:2246–2258. doi: 10.1097/01.asn.0000030392.19694.9d. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney W.E., Jr., Avner E.D. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD) Cell Tissue Res. 2006;326:671–685. doi: 10.1007/s00441-006-0226-0. [DOI] [PubMed] [Google Scholar]
- 4.Zerres K., Mucher G., Becker J., Steinkamm C., Rudnik-Schoneborn S., Heikkila P., Rapola J., Salonen R., Germino G.G., Onuchic L., Somlo S., Avner E.D., Harman L.A., Stockwin J.M., Guay-Woodford L.M. Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): molecular genetics, clinical experience, and fetal morphology. Am J Med Genet. 1998;76:137–144. [PubMed] [Google Scholar]
- 5.Guay-Woodford L.M., Muecher G., Hopkins S.D., Avner E.D., Germino G.G., Guillot A.P., Herrin J., Holleman R., Irons D.A., Primack W., Thompson P.D., Waldo F.B., Lunt P.W., Zerres K. The severe perinatal form of autosomal recessive polycystic kidney disease maps to chromosome 6p21.1-p12: implications for genetic counseling. Am J Hum Genet. 1995;56:1101–1107. [PMC free article] [PubMed] [Google Scholar]
- 6.Roy S., Dillon M.J., Trompeter R.S., Barratt T.M. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997;11:302–306. doi: 10.1007/s004670050281. [DOI] [PubMed] [Google Scholar]
- 7.Hartung E.A., Guay-Woodford L.M. Autosomal recessive polycystic kidney disease: a hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics. 2014;134:e833–e845. doi: 10.1542/peds.2013-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onuchic L.F., Furu L., Nagasawa Y., Hou X., Eggermann T., Ren Z., Bergmann C., Senderek J., Esquivel E., Zeltner R., Rudnik-Schoneborn S., Mrug M., Sweeney W., Avner E.D., Zerres K., Guay-Woodford L.M., Somlo S., Germino G.G. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris P.C., Rossetti S. Molecular genetics of autosomal recessive polycystic kidney disease. Mol Genet Metab. 2004;81:75–85. doi: 10.1016/j.ymgme.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Menezes L.F., Cai Y., Nagasawa Y., Silva A.M., Watkins M.L., Da Silva A.M., Somlo S., Guay-Woodford L.M., Germino G.G., Onuchic L.F. Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int. 2004;66:1345–1355. doi: 10.1111/j.1523-1755.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler W.H., Soetje B., Marten L.P., Wiese J., Burute M., Haffner D. Fibrocystin Is Essential to Cellular Control of Adhesion and Epithelial Morphogenesis. Int J Mol Sci. 2020;21:5140. doi: 10.3390/ijms21145140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Sullivan D.A., Torres V.E., Gabow P.A., Thibodeau S.N., King B.F., Bergstralh E.J. Cystic fibrosis and the phenotypic expression of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32:976–983. doi: 10.1016/s0272-6386(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 13.Persu A., Devuyst O., Lannoy N., Materne R., Brosnahan G., Gabow P.A., Pirson Y., Verellen-Dumoulin C. CF gene and cystic fibrosis transmembrane conductance regulator expression in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:2285–2296. doi: 10.1681/ASN.V11122285. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi K., Sweeney W.E., Jr., Macrae Dell K., Cotton C.U., Avner E.D. Role of CFTR in autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2001;12:719–725. doi: 10.1681/ASN.V124719. [DOI] [PubMed] [Google Scholar]
- 15.Nauta J., Ozawa Y., Sweeney W.E., Jr., Rutledge J.C., Avner E.D. Renal and biliary abnormalities in a new murine model of autosomal recessive polycystic kidney disease. Pediatr Nephrol. 1993;7:163–172. doi: 10.1007/BF00864387. [DOI] [PubMed] [Google Scholar]
- 16.Colombo C., Battezzati P.M., Strazzabosco M., Podda M. Liver and biliary problems in cystic fibrosis. Semin Liver Dis. 1998;18:227–235. doi: 10.1055/s-2007-1007159. [DOI] [PubMed] [Google Scholar]
- 17.Tabibian J.H., Masyuk A.I., Masyuk T.V., O'Hara S.P., LaRusso N.F. Physiology of cholangiocytes. Compr Physiol. 2013;3:541–565. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo C., Russo M.C., Zazzeron L., Romano G. Liver disease in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S49–S55. doi: 10.1097/01.mpg.0000226390.02355.52. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad A., Hultcrantz R., Strandvik B. Bile-duct destruction and collagen deposition: a prominent ultrastructural feature of the liver in cystic fibrosis. Hepatology. 1992;16:372–381. doi: 10.1002/hep.1840160215. [DOI] [PubMed] [Google Scholar]
- 20.Balch W.E., Roth D.M., Hutt D.M. Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb Perspect Biol. 2011;3:a004499. doi: 10.1101/cshperspect.a004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skach W.R. CFTR: new members join the fold. Cell. 2006;127:673–675. doi: 10.1016/j.cell.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Roth D.M., Balch W.E. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol. 2011;23:126–134. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borkan S.C., Gullans S.R. Molecular chaperones in the kidney. Annu Rev Physiol. 2002;64:503–527. doi: 10.1146/annurev.physiol.64.081501.155819. [DOI] [PubMed] [Google Scholar]
- 24.Seit-Nebi A.S., Gusev N.B. Versatility of the small heat shock protein HSPB6 (Hsp20) Cell Stress Chaperones. 2010;15:233–236. doi: 10.1007/s12192-009-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taipale M., Jarosz D.F., Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 26.Stiegler S.C., Rubbelke M., Korotkov V.S., Weiwad M., John C., Fischer G., Sieber S.A., Sattler M., Buchner J. A chemical compound inhibiting the Aha1-Hsp90 chaperone complex. J Biol Chem. 2017;292:17073–17083. doi: 10.1074/jbc.M117.797829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M.E. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyrd-Hansen M., Nylandsted J., Jaattela M. Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle. 2004;3:1484–1485. doi: 10.4161/cc.3.12.1287. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.E., Hipp M.S., Bracher A., Hayer-Hartl M., Hartl F.U. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 30.Van Goor F., Hadida S., Grootenhuis P.D., Burton B., Stack J.H., Straley K.S., Decker C.J., Miller M., McCartney J., Olson E.R., Wine J.J., Frizzell R.A., Ashlock M., Negulescu P.A. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanaoka K., Devuyst O., Schwiebert E.M., Wilson P.D., Guggino W.B. A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol. 1996;270:C389–C399. doi: 10.1152/ajpcell.1996.270.1.C389. [DOI] [PubMed] [Google Scholar]
- 32.Hanaoka K., Guggino W.B. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 33.Tradtrantip L., Sonawane N.D., Namkung W., Verkman A.S. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52:6447–6455. doi: 10.1021/jm9009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verkman A.S., Lukacs G.L., Galietta L.J. CFTR chloride channel drug discovery--inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des. 2006;12:2235–2247. doi: 10.2174/138161206777585148. [DOI] [PubMed] [Google Scholar]
- 35.Yang B., Sonawane N.D., Zhao D., Somlo S., Verkman A.S. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H., Sheppard D.N. Therapeutic potential of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors in polycystic kidney disease. Biodrugs. 2009;23:203–216. doi: 10.2165/11313570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Yanda M.K., Cha B., Cebotaru C.V., Cebotaru L. Pharmacological reversal of renal cysts from secretion to absorption suggests a potential therapeutic strategy for managing autosomal dominant polycystic kidney disease. J Biol Chem. 2019;294:17090–17104. doi: 10.1074/jbc.RA119.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallagher A.R., Esquivel E.L., Briere T.S., Tian X., Mitobe M., Menezes L.F., Markowitz G.S., Jain D., Onuchic L.F., Somlo S. Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol. 2008;172:417–429. doi: 10.2353/ajpath.2008.070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S.H., Gregory R.J., Marshall J., Paul S., Souza D.W., White G.A., O'Riordan C.R., Smith A.E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A., Jagannathan N. Cytokeratin: A review on current concepts. Int J Orofac Biol. 2018;2:6–11. [Google Scholar]
- 41.Grantham J.J. Mechanisms of progression in autosomal dominant polycystic kidney disease. Kidney Int Suppl. 1997;63:S93–S97. [PubMed] [Google Scholar]
- 42.Seamon K.B., Daly J.W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7:201–224. [PubMed] [Google Scholar]
- 43.Ghelani D.P., Schneider-Futschik E.K. Emerging cystic fibrosis transmembrane conductance regulator modulators as new drugs for cystic fibrosis: a portrait of in vitro pharmacology and clinical translation. ACS Pharmacol Transl Sci. 2019;3:4–10. doi: 10.1021/acsptsci.9b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uc A., Giriyappa R., Meyerholz D.K., Griffin M., Ostedgaard L.S., Tang X.X., Abu-El-Haija M., Stoltz D.A., Ludwig P., Pezzulo A., Abu-El-Haija M., Taft P., Welsh M.J. Pancreatic and biliary secretion are both altered in cystic fibrosis pigs. Am J Physiol Gastrointest Liver Physiol. 2012;303:G961–G968. doi: 10.1152/ajpgi.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakashima T., Ishii T., Tagaya H., Seike T., Nakagawa H., Kanda Y., Akinaga S., Soga S., Shiotsu Y. New molecular and biological mechanism of antitumor activities of KW–2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res. 2010;16:2792–2802. doi: 10.1158/1078-0432.CCR-09-3112. [DOI] [PubMed] [Google Scholar]
- 46.Wen W., Liu W., Shao Y., Chen L. VER–155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp Biol Med (Maywood) 2014;239:638–645. doi: 10.1177/1535370214527899. [DOI] [PubMed] [Google Scholar]
- 47.Ostedgaard L.S., Meyerholz D.K., Chen J.H., Pezzulo A.A., Karp P.H., Rokhlina T., Ernst S.E., Hanfland R.A., Reznikov L.R., Ludwig P.S., Rogan M.P., Davis G.J., Dohrn C.L., Wohlford-Lenane C., Taft P.J., Rector M.V., Hornick E., Nassar B.S., Samuel M., Zhang Y., Richter S.S., Uc A., Shilyansky J., Prather R.S., McCray P.B., Jr., Zabner J., Welsh M.J., Stoltz D.A. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001868. 74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodsky J.L. The protective and destructive roles played by molecular chaperones during ERAD (endoplasmic-reticulum-associated degradation) Biochem J. 2007;404:353–363. doi: 10.1042/BJ20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanda M.K., Liu Q., Cebotaru L. A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J Biol Chem. 2018;293:11513–11526. doi: 10.1074/jbc.RA118.001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mymrikov E.V., Seit-Nebi A.S., Gusev N.B. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 51.Kamada M., So A., Muramaki M., Rocchi P., Beraldi E., Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther. 2007;6:299–308. doi: 10.1158/1535-7163.MCT-06-0417. [DOI] [PubMed] [Google Scholar]
- 52.Nahomi R.B., Palmer A., Green K.M., Fort P.E., Nagaraj R.H. Pro-inflammatory cytokines downregulate Hsp27 and cause apoptosis of human retinal capillary endothelial cells. Biochim Biophys Acta. 2014;1842:164–174. doi: 10.1016/j.bbadis.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopes-Pacheco M., Boinot C., Sabirzhanova I., Morales M.M., Guggino W.B., Cebotaru L. Combination of Correctors Rescue DeltaF508-CFTR by Reducing Its Association with Hsp40 and Hsp27. J Biol Chem. 2015;290:25636–25645. doi: 10.1074/jbc.M115.671925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabirzhanova I., Lopes Pacheco M., Rapino D., Grover R., Handa J.T., Guggino W.B., Cebotaru L. Rescuing Trafficking Mutants of the ATP-binding Cassette Protein, ABCA4, with Small Molecule Correctors as a Treatment for Stargardt Eye Disease. J Biol Chem. 2015;290:19743–19755. doi: 10.1074/jbc.M115.647685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanda M.K., Guggino W.B., Cebotaru L. A new role for heat shock factor 27 in the pathophysiology of Clostridium difficile toxin B. Am J Physiol Gastrointest Liver Physiol. 2020;318:G120–G129. doi: 10.1152/ajpgi.00166.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doshi B.M., Hightower L.E., Lee J. HSPB1, actin filament dynamics, and aging cells. Ann N Y Acad Sci. 2010;1197:76–84. doi: 10.1111/j.1749-6632.2010.05191.x. [DOI] [PubMed] [Google Scholar]
- 57.Mai W., Chen D., Ding T., Kim I., Park S., Cho S.Y., Chu J.S., Liang D., Wang N., Wu D., Li S., Zhao P., Zent R., Wu G. Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells. Mol Biol Cell. 2005;16:4398–4409. doi: 10.1091/mbc.E04-11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proia D., Golemis E., Seeger-Nukpezah T. Treating polycystic kidney disease with hsp90 inhibitory compounds. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014063080 Available at: Accessed October 8, 2015.
- 59.Seeger-Nukpezah T., Proia D.A., Egleston B.L., Nikonova A.S., Kent T., Cai K.Q., Hensley H.H., Ying W., Chimmanamada D., Serebriiskii I.G., Golemis E.A. Inhibiting the HSP90 chaperone slows cyst growth in a mouse model of autosomal dominant polycystic kidney disease. Proc Natl Acad Sci U S A. 2013;110:12786–12791. doi: 10.1073/pnas.1301904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bertolini A., Bodewes F.A.J.A., Slae M., Wilschanski M. In: Cystic Fibrosis. Davis S.D., Rosenfeld M., Chmiel J., editors. Springer International; Cham, Switzerland: 2020. Hepatobiliary Involvement in Cystic Fibrosis; pp. 299–320. Accessed October 8, 2015. [Google Scholar]
- 61.Bidaud-Meynard A., Bossard F., Schnur A., Fukuda R., Veit G., Xu H., Lukacs G.L. Transcytosis maintains CFTR apical polarity in the face of constitutive and mutation-induced basolateral missorting. J Cell Sci. 2019;132:jcs226886. doi: 10.1242/jcs.226886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchholz B., Faria D., Schley G., Schreiber R., Eckardt K.-U., Kunzelmann K. Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst–forming epithelial cells. Kidney Int. 2014;85:1058–1067. doi: 10.1038/ki.2013.418. [DOI] [PubMed] [Google Scholar]
- 63.Fuller C.M., Benos D.J. CFTR! Am J Physiol. 1992;263:C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- 64.Skach W.R. Defects in processing and trafficking of the cystic fibrosis transmembrane conductance regulator. Kidney Int. 2000;57:825–831. doi: 10.1046/j.1523-1755.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 65.Elborn J.S., Ramsey B.W., Boyle M.P., Konstan M.W., Huang X., Marigowda G., Waltz D., Wainwright C.E. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med. 2016;4:617–626. doi: 10.1016/S2213-2600(16)30121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Gonzalez M.A., Menezes L.F., Piontek K.B., Kaimori J., Huso D.L., Watnick T., Onuchic L.F., Guay-Woodford L.M., Germino G.G. Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet. 2007;16:1940–1950. doi: 10.1093/hmg/ddm141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanda M.K., Liu Q., Cebotaru V., Guggino W.B., Cebotaru L. Histone deacetylase 6 inhibition reduces cysts by decreasing cAMP and Ca(2+) in knock-out mouse models of polycystic kidney disease. J Biol Chem. 2017;292:17897–17908. doi: 10.1074/jbc.M117.803775. [DOI] [PMC free article] [PubMed] [Google Scholar]