Abstract
Background and Objectives
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an autoimmune disease primarily affecting the peripheral nervous system. However, several noncontrolled studies have suggested concomitant inflammatory CNS demyelination similar to multiple sclerosis. The aim of this study was to investigate an involvement of the visual pathway in patients with CIDP.
Methods
In this prospective cross-sectional study, we used high-resolution spectral-domain optical coherence tomography to compare the thickness of the peripapillary retinal nerve fiber layer and the deeper macular retinal layers as well as the total macular volume (TMV) in 22 patients with CIDP and 22 age-matched and sex-matched healthy control (HC) individuals. Retinal layers were semiautomatically segmented by the provided software and were correlated with clinical measures and nerve conduction studies.
Results
In patients with CIDP compared with healthy age-matched and sex-matched controls, we found slight but significant volume reductions of the ganglion cell/inner plexiform layer complex (CIDP 1.86 vs HC 1.95 mm3, p = 0.015), the retinal pigment epithelium (CIDP 0.38 vs HC 0.40 mm3, p = 0.02), and the TMV (CIDP 8.48 vs HC 8.75 mm3, p = 0.018). The ganglion cell layer volume and motor nerve conduction velocity were positively associated (B = 0.002, p = 0.02).
Discussion
Our data reveal subtle retinal neurodegeneration in patients with CIDP, providing evidence for visual pathway involvement, detectable by OCT. The results need corroboration in independent, larger cohorts.
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an autoimmune disease of the peripheral nervous system (PNS) characterized by primary myelin sheath destruction and secondary axonal damage, leading to sensory and autonomic deficits, flaccid paresis, and muscle atrophy.1 The disease usually follows a chronic progressive course with or without superimposed relapses or is relapsing, thus resembling multiple sclerosis (MS), the most common primary demyelinating autoimmune disease of the CNS. Forrester and Lascelles2 were the first to describe 2 cases of coincident MS and inflammatory polyneuropathy in 1979. In 1987, 6 additional cases of CIDP with CNS demyelination evidenced by MRI were reported.3 Several subsequent studies suggested CNS involvement in CIDP.4-6 Further investigations associated CIDP specifically with visual pathway disturbance.7-9 Holtkamp et al.10 showed histologically optic nerve inflammation in a patient with CIDP. Japanese and Chinese groups have recently suggested the existence of a condition termed combined central and peripheral demyelination that resembles both MS and CIDP in the same patient.11-13
All these investigations, however, were noncontrolled studies, mainly case reports and case series. In a previous effort, investigating conductivity changes of the visual pathway in patients with CIDP using multifocal visual evoked potentials (mfVEP), we were not able to detect relevant changes compared with age-matched and sex-matched healthy individuals.14 This finding was recently corroborated by another controlled study using full-field VEPs.15
Here, we investigated structural retinal changes as a marker of CNS degeneration in patients with CIDP compared with age-matched and sex-matched healthy individuals using spectral-domain optical coherence tomography (SD-OCT). OCT uses near-infrared light to generate cross-sectional and 3-dimensional images of the retina as part of the CNS. In recent years, OCT has emerged as a reliable and accurate, easy-to-access, and noninvasive technique to assess microscopic retinal pathologies, for example, in MS16-18 and other inflammatory and degenerative CNS diseases.19-24 In MS, spectral-domain OCT has been documented to predict long-term worsening.25
Methods
Patients
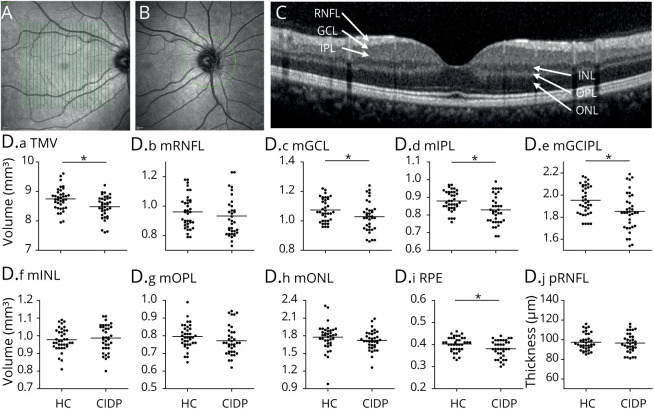
We performed a prospective study at the Department of Neurology, Heinrich-Heine-University Düsseldorf. Subjects were included as previously described.14 Inclusion criteria were of age >18 years, probable or definite CIDP according to the European Federation of Neurological Societies/Peripheral Nerve Society CIDP guidelines and response to immunomodulatory treatment. The flowchart in Figure 1 gives an overview of the subject recruitment. Of 66 subjects (44 patients with probable or definite CIDP and 22 healthy subjects), 22 patients had to be excluded because of diabetes mellitus (n = 13) or concomitant ophthalmologic diseases (n = 9), such as bilateral drusen (4), cataract (2), glaucoma (1), papilledema (1), and choroidal neovascularization (1), which could confound the OCT measurements. Furthermore, 7 single eyes were excluded because of drusen (6) and macular edema (1). Healthy controls (HCs) did not report symptoms or show any clinical signs suspicious of polyneuropathy. Four subjects with CIDP revealed relevant serologic findings (3 had monoclonal gammopathy and 1 was positive for anti-myelin–associated glycoprotein immunoglobulin M antibodies). The Inflammatory Neuropathy Cause and Treatment Overall Disability Sum Score was evaluated in all subjects with CIDP as a measure of disability. Demographic parameters of the study partcipants are listed in the Table. All subjects received neuroophthalmological examinations, including tonometry, slit-lamp examination, and fundoscopy. In addition, 16 of 22 patients with CIDP (73%) were tested for corrected low contrast letter recognition using 2.5% low contrast early treatment of diabetic retinopathy study (ETDRS) charts. Because nerve conduction studies of the lower limbs showed a high rate of signal loss, the right ulnar nerve was used to investigate associations with OCT parameters. Figures 2A–C exemplify the measurement of the macular volumes by consecutive vertical scans (Figure 2A) and the peripapillary retinal nerve fiber layer (pRNFL) thickness (Figure 2B), and illustrate a cross-section through the deeper retinal layers (Figure 2C). The Strengthening the Reporting of Observational Studies in Epidemiology cross-sectional reporting guidelines were used.26
Figure 1. Flowchart of Subject Recruitment.
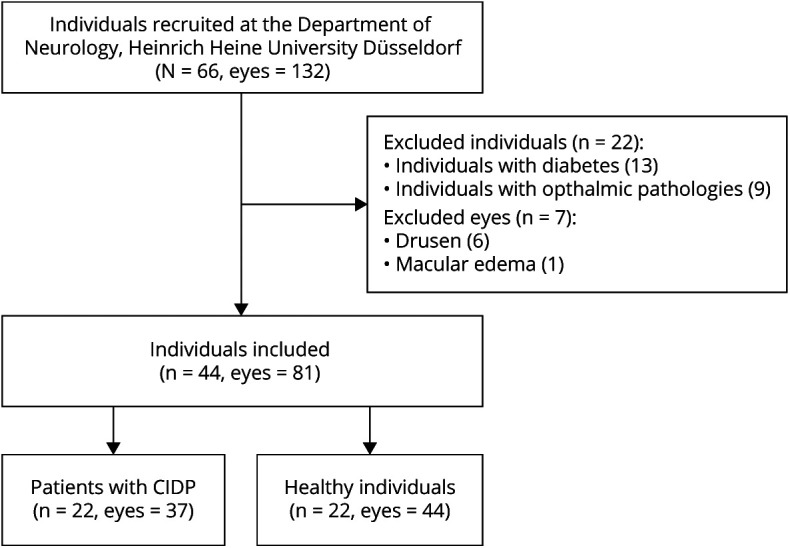
Patients with CIDP and healthy controls were recruited at the Department of Neurology, Heinrich-Heine-University in Düsseldorf, Germany. Of the 66 individuals, 44 were patients with CIDP and 22 healthy control subjects. Of the 44 patients with CIDP who were screened, 22 individuals were excluded because of concomitant diabetes mellitus and ophthalmic pathologies. Additional 7 single eyes were excluded because of drusen and macular edema. Of the remaining individuals, 22 were patients with CIDP and 22 age- and sex-matched controls. CIDP = chronic inflammatory demyelinating polyneuropathy.
Figure 2. OCT Measurements.
(A) The macular thickness and volume were calculated from consecutive vertical scans centered on the macula. (B) The peripapillary RNFL was evaluated in a circular scan centered on the optic disk. (C) The deeper retinal layers were semiautomatically segmented in a single horizontal foveal scan. The volumes of the retinal layers were assessed by averaging 14 images from vertical scans. (D) Scatter plots display the macular layer volumes or thickness. All values are described as mean ± SD. Each point represents 1 eye. The mean of all eyes is represented by the horizontal line. Statistical differences in patients with CIDP vs healthy controls were assessed by GEE accounting for several measurements in the same individual (2 eyes). GEE = generalized estimation equation; mGCIPL = macular ganglion cell/inner plexiform layer; mGCL = macular ganglion cell layer; mINL = macular inner nuclear layer; mIPL = macular inner plexiform layer; mONL = macular outer nuclear layer; mOPL = macular outer plexiform layer; mRNFL = macular retinal nerve fiber layer; OCT = optical coherence tomography; pRNFL = peripapillary retinal nerve fiber layer; RPE = retinal pigment epithelium; TMV = total macular volume.
Spectral-Domain Optical Coherence Tomography
APOSTEL reporting recommendations were applied for the SD-OCT methodology and results.27 The methods are well established and have also been used and described elsewhere.22,23,28 For the RNFL assessment, using high-resolution scanning mode, we obtained 12° peripapillary, disk-centered ring scans. For macular volume evaluation, we captured 61 fovea-centered vertical scans (30° × 25°, high-speed scanning mode). The total retinal volume and the volumes of the different retinal layers were measured by applying the standard 1-, 3-, and 6-mm ETDRS grid in macular volume scans and using the mean volume of all sectors. For SD-OCT imaging of both eyes, the SPECTRALIS OCT device (Heidelberg Engineering, Germany) with the image alignment eye-tracking software system (TruTrack and Nsite analytics, Heidelberg Engineering) was used. Averaging of macular volume scans was performed from 14 images and of peripapillary ring scans from 100 scans (automatic real time). The threshold for the image quality was beyond 20 dB. Semiautomatic segmentation of all retinal layers using the Heidelberg Eye Explorer software (version HEYEX 1.8.6.0, Viewing Module 5.8.3.0) was manually corrected by a blinded rater. For analysis, we used only scans meeting the OSCAR-IB quality control criteria.29
Statistical Evaluation
SPSS Statistics 24 (IBM) was used for statistical analyses. Generalized estimation equation (GEE) models correcting for sex and age and accounting for within-subject, intersegment, or intereye correlations using an exchangeable working correlation matrix were applied to analyze associations between clinical data and SD-OCT parameters and to test for differences of SD-OCT parameters between controls and patients with CIDP.
Patient Consent and Standard Protocol Approvals
This study was approved by the local ethics committee of Heinrich-Heine-University Düsseldorf (registry number 4389). In accordance with the Declaration of Helsinki, written informed consent was obtained from all study participants.
On reasonable request from any qualified investigator, anonymized data not published within this article will be made available.
Results
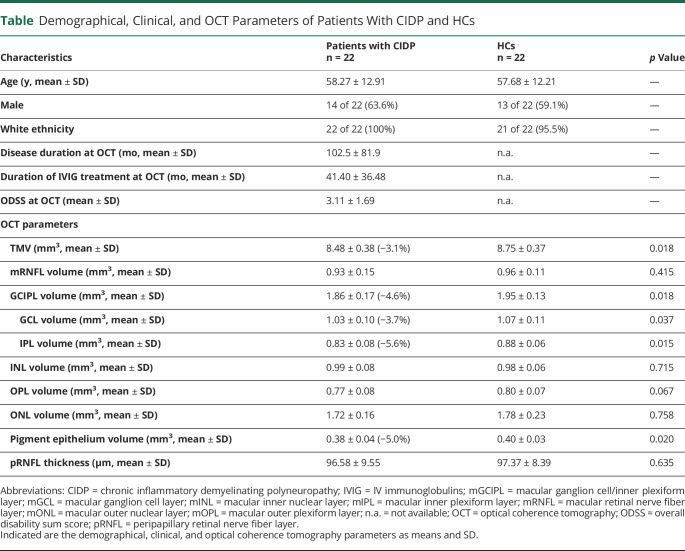
The mean total macular volume (TMV) was significantly reduced in patients with CIDP (8.48 mm3, ± 0.38) compared with HCs (8.75, ± 0.37 mm3; p = 0.018, GEE, Figure 2D). Further analyses of the different macular layers revealed a significant thinning of the ganglion cell layer (GCL) in patients with CIDP (1.03 ± 0.10 mm3) compared with HCs (1.07 ± 0.11 mm3, p = 0.037) and of the inner plexiform layer (IPL) in patients with CIDP (0.83 ± 0.08 mm3) vs healthy individuals (0.88 ± 0.06 mm3, p = 0.015). Consequently, the often-used combined ganglion cell IPL (GCIPL) volume also showed a significant difference (CIDP 1.86 ± 0.17 vs HCs 1.95 ± 0.13 mm3, p = 0.018). Furthermore, the pigment epithelium was significantly reduced in patients with CIDP compared with HCs (0.38 ± 0.04 vs 0.40 ± 0.03 mm3, p = 0.02). No changes were observed for the macular retinal nerve fiber layer (0.93 ± 0.15 vs 0.96 ± 0.11 mm3; p = 0.415), the inner nuclear layer (0.99 ± 0.08 vs 0.98 ± 0.06 mm3; p = 0.715), the outer plexiform layer (0.77 ± 0.08 vs 0.80 ± 0.07 mm3; p = 0.067), and the outer nuclear layer (1.72 ± 0.16 vs 1.78 ± 0.23 mm3; p = 0.758). Moreover, the peripapillary retinal nerve fiber layer (pRNFL) thickness did not differ between patients with CIDP (96.58 ± 9.55 µm) and HCs (97.37 ± 8.39 µm; p = 0.635) (Table).
Table.
Demographical, Clinical, and OCT Parameters of Patients With CIDP and HCs
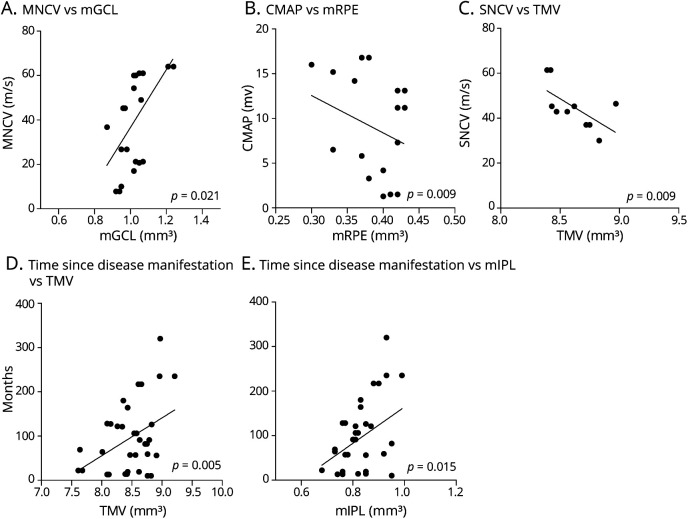
In an exploratory approach, we correlated the significantly altered retinal layers (TMV, GCIPL, and pigment epithelium) with neurographical and clinical parameters in the CIDP group. We found a positive association of the motor nerve conduction velocity of the right ulnar nerve with the GCL (p = 0.02, B = 0.002, Figure 3A) and negative associations of the compound muscle action potential with the retinal pigment epithelium (RPE, p = 0.009, B = −0.003, Figure 3B) and of the sensory nerve conduction velocity with the TMV (p = 0.009, B = −0.01, Figure 3C). Regarding clinical parameters, positive associations were detected regarding the time since clinical disease manifestation and the TMV (p = 0.005, B = 0.003, Figure 3D) and the IPL (p = 0.015, B = 0.001, Figure 3E). No associations were observed between retinal layers that were found to be changed in CIDP and low-contrast visual acuity, clinical severity of the disease as assessed by ODSS, time since diagnosis, therapy duration, and CSF protein levels.
Figure 3. Analysis of Associations With Neurographic and Clinical Features.
Scatter plots of associations of retinal layers with nerve conduction and compound action potential amplitudes of the right ulnar nerve, as well as time since disease manifestation in patients with CIDP vs. healthy controls. Each dot represents 1 eye, p values (GEE method), and regression lines are provided. (A) Positive association of MNCV with mGCL (p = 0.021). (B) Negative association of CMAP with mRPE (p = 0.009). (C) Negative association of SNCV with TMV (p = 0.009). (D and E) Positive association of the time of OCT measurement since disease manifestation with TMV (D, p = 0.005) and mIPL (E, p = 0.015). CIDP = chronic inflammatory demyelinating polyneuropathy; CMAP = compound motor action potential; GEE = generalized estimation equation; mGCL, macular ganglion cell layer; mIPL, macular inner plexiform layer; MNCV, motor nerve conduction velocity; mRPE, macular retinal pigment epithelium; SNCV, sensory nerve conduction velocity; TMV, total macular volume.
Discussion
We herein present data demonstrating subtle retinal degeneration in patients with CIDP detected by optical coherence tomography. Our observations provide further evidence for a CNS involvement in CIDP. A number of case reports or small series have implicated some connection between autoimmune peripheral demyelination and specific CNS pathologies. Two case-control studies have recently been published. We looked at mfVEPs in CIDP and found no clear-cut evidence of CIDP-dependent alterations.14 Dziadkowski et al.15 compared multimodal evoked potentials in patients with CIDP and HCs and detected prolonged latencies in VEP, brainstem acoustic evoked potentials, and somatosensory-evoked potentials.
Because CIDP is an autoimmune and primary demyelinating PNS disease, its most obvious CNS counterpart is MS. Classic OCT alteration in MS occurs in the pRNFL, which is decreased in thickness especially after optic neuritis,30 but also independent of optic neuritis.31 Here, we did not observe any alterations in pRNFL thickness in CIDP. GCIPL, which contains the neurons forming nonmyelinated axons of the RNFL and their dendritic arbors, has been shown to be also affected in patients with MS and displayed robust differences between patient and control eyes.32,33 It has been suggested to be equivalent to the pRNFL, concerning its utility in diagnosis, monitoring, and research in MS.16 In the GCIPL, as well as the GCL and the IPL separately, we observed a significant volume reduction in CIDP compared with HC eyes. The GCL is believed to be a sensitive biomarker of neurodegeneration34 and may even have advantages compared with the RNFL because it is not inflicted by edema in optic neuritis in MS, and therefore, atrophy becomes detectable earlier.35,36 In addition, GCL seems to reflect MRI changes in MS better than pRNFL, and it seems to have a lower variability in cross-sectional data.37-39 Whether these findings are applicable in the context of CIDP, however, is unclear because we have previously found that mfVEP was not markedly altered in patients with CIDP.14 Because mfVEP is quite sensitive in detecting optic nerve damage, the significant changes in the GCL may therefore point toward a retinal, rather than optic nerve pathology. Another explanation may be a degenerative process in the posterior visual pathway, which could lead to retrograde transsynaptic degeneration in the retina. However, the lack of MRI brain imaging limits this study in assessing for that possibility.
We also noted a decrease in RPE layer volumes in patients with CIDP. Concerning embryology, the RPE stems from neuroectodermal cells, and thus, these epithelial cells can be considered as CNS cells.40 The biological context of this finding seems unclear. The TMV was significantly decreased in patients with CIDP, which rather reflects a thinning of single retinal layers.
At this point, it can only be speculated how retinal degeneration and immune mediated peripheral nerve damage are linked biologically. Retinal atrophy can result from retrograde degeneration after an insult to the optic nerve, activation of apoptotic pathways, and glial activation.41-44 Because CIDP is an autoimmune disease, one possibility would be the presence of a common antigen in peripheral nerves and the optic nerve or the retina, but to date, none has been identified.
A direct immune attack on the retina remains a theoretical possibility, but likewise, nothing is known about antigens shared with peripheral nerves. To learn more about possible clinical implications, we investigated relations between the retinal layer volumes and nerve conduction and clinical parameters. In an exploratory approach, we found a number of associations. The most remarkable was a positive association between the macular GCL volume and the motor nerve conduction velocity of the right ulnar nerve.
The study has obvious limitations. The cohort size of 22 individuals per group does allow for statistical evaluation, but it does not withstand corrections for multiple testing. Because the study is explicitly of exploratory nature, we deem this to be acceptable. Furthermore, our findings suggest that an in-depth investigation of the visual pathway is warranted in CIDP by, for example, applying OCT side-by-side with MRI covering the optic radiation. Although this was not part of the current investigation, it would be important and of interest to correlate subclinical MRI involvement with OCT alterations in CIDP.
Taken together, the data suggest degenerative processes in the retina and thus the CNS in CIDP. Particularly, the changes in the GCIPL could point to underlying processes that may be functionally and biologically relevant. To consolidate these intriguing findings, an independent longitudinal study of a larger patient cohort should be conducted.
Glossary
- CIDP
chronic inflammatory demyelinating polyneuropathy
- ETDRS
early treatment of diabetic retinopathy study
- GCIPL
ganglion cell IPL
- GCL
ganglion cell layer
- GEE
generalized estimation equation
- HC
healthy control
- INL
inner nuclear layer
- IPL
inner plexiform layer
- mfVEP
multifocal visual evoked potential
- MNCV
motor nerve conduction velocity
- MS
multiple sclerosis
- OPL
outer plexiform layer
- PNS
peripheral nervous system
- pRNFL
peripapillary retinal nerve fiber layer
- SD-OCT
spectral-domain optical coherence tomography
Appendix. Authors
Contributor Information
Jens Ingwersen, Email: jens.ingwersen@hhu.de.
Jonas Graf, Email: jonas.graf@hhu.de.
Julia Kluge, Email: j.kluge@ymail.com.
Margit Weise, Email: margit.weise@med.uni-duesseldorf.de.
Michael Dietrich, Email: michael.dietrich@med.uni-duesseldorf.de.
John-Ih Lee, Email: john-ih.lee@uni-duesseldorf.de.
Jens Harmel, Email: jens.harmel@artemed.de.
Hans-Peter Hartung, Email: hans-peter.hartung@uni-duesseldorf.de.
Tobias Ruck, Email: tobias.ruck@med.uni-duesseldorf.de.
Sven G. Meuth, Email: sven.meuth@uni-duesseldorf.de.
Philipp Albrecht, Email: phil.albrecht@gmail.com.
Orhan Aktas, Email: orhan.aktas@hhu.de.
Study Funding
No targeted funding reported.
Disclosure
J. Ingwersen reports no conflicts of interest; J. Graf received travel/meeting/accommodation reimbursements from Biogen, Merck Serono, Sanofi Genzyme, Grifols, and a Research Fellowship from the Deutsche Forschungsgemeinschaft (project number 438899010); J. Kluge and M. Weise report no conflicts of interest; M. Dietrich received speaker honoraria from Merck; J.-I. Lee has received honoraria for speaking/consultation from Bayer Healthcare, Boehringer Ingelheim, Allergan, Novartis, Ipsen, Teva, and Daiichi-Sankyo as well as travel grants from Bayer Healthcare, Merz, Allergan, and Ipsen, all outside the submitted work; J. Harmel reports no conflicts of interest; T. Ruck reports grants from German Ministry of Education, Science, Research and Technology, grants and personal fees from Sanofi Genzyme and Alexion, personal fees from Biogen, Roche and Teva, personal fees and nonfinancial support from Merck Serono, outside the submitted work; H.P. Hartung received, with approval of the Rector of Heinrich-Heine-University and the CEO of University of Düsseldorf Hospital honoraria for consulting, serving on steering committees and speaking from Bayer, Biogen, Celgene BMS, GeNeuro, MedImmune, Merck, Novartis, Octapharma, Roche, Sanofi Genzyme, and TG Therapeutics; S.G. Meuth receives honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, DiaMed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and Alexion, Almirall, Amicus Therapeutics Germany, Biogen, DiaMed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva; P. Albrecht received compensation for serving on Scientific Advisory Boards for Ipsen, Novartis, Biogen; he received speaker honoraria and travel support from Novartis, Teva, Biogen, Genzyme, Merz Pharmaceuticals, Ipsen, Janssen Healthcare, Allergan, Bayer Healthcare, UCB and Glaxo Smith Kline; he received research support from Allergan, Novartis, Biogen, Celgene, Teva, Merz Pharmaceuticals, Ipsen, and Roche. All not related to the content of this article; O. Aktas has received personal fees from Alexion, Bayer Healthcare, Biogen, Celgene, Merck Serono, MedImmune, Novartis, Roche, Teva, and Zambon, outside of the submitted work. His research is funded by the German Ministry for Education and Research (BMBF) and the German Research Foundation (DFG); M. Ringelstein received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, Roche, and Merck, none related to this study. Go to Neurology.org/NN for full disclosures.
References
- 1.Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2019;90(9):981-987. [DOI] [PubMed] [Google Scholar]
- 2.Forrester C, Lascelles RG. Association between polyneuritis and multiple sclerosis. J Neurol Neurosurg Psychiatry. 1979;42(9):864-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas PK, Walker RW, Rudge P, et al. Chronic demyelinating peripheral neuropathy associated with multifocal central nervous system demyelination. Brain. 1987;110(pt 1):53-76. [DOI] [PubMed] [Google Scholar]
- 4.Mendell JR, Kolkin S, Kissel JT, Weiss KL, Chakeres DW, Rammohan KW. Evidence for central nervous system demyelination in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1987;37(8):1291-1294. [DOI] [PubMed] [Google Scholar]
- 5.Ormerod IE, Waddy HM, Kermode AG, Murray NM, Thomas PK. Involvement of the central nervous system in chronic inflammatory demyelinating polyneuropathy: a clinical, electrophysiological and magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 1990;53(9):789-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtake T, Komori T, Hirose K, Tanabe H. CNS involvement in Japanese patients with chronic inflammatory demyelinating polyradiculoneuropathy. Acta Neurol Scand. 1990;81(2):108-112. [DOI] [PubMed] [Google Scholar]
- 7.Hickman SJ, Allen JA, Baisre A, et al. Neuro-ophthalmological complications of chronic inflammatory demyelinating polyradiculoneuropathy. Neuroophthalmology. 2013;37(4):146-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopp M, Leese RJ, Martin-Lamb D, Rajabally YA. Optic and auditory pathway dysfunction in demyelinating neuropathies. Acta Neurol Scand. 2014;130(1):53-57. [DOI] [PubMed] [Google Scholar]
- 9.Takeda M, Tachibana H, Tuda K, Wada S, Kasama S. CNS involvement in chronic inflammatory demyelinating polyneuropathy: a visual evoked potential study. J Neurol Neurophysiol. 2010;1:105-108. [Google Scholar]
- 10.Holtkamp M, Zschenderlein R, Brück W, Weber JR. Chronic inflammatory demyelinating polyradiculoneuropathy with histologically proven optic neuritis. Acta Neuropathol. 2001;101(5):529-531. [DOI] [PubMed] [Google Scholar]
- 11.Ogata H, Matsuse D, Yamasaki R, et al. A nationwide survey of combined central and peripheral demyelination in Japan. J Neurol Neurosurg Psychiatry. 2016;87(1):29-36. [DOI] [PubMed] [Google Scholar]
- 12.Wang YQ, Chen H, Zhuang WP, Li HL. The clinical features of combined central and peripheral demyelination in Chinese patients. J Neuroimmunol. 2018;317:32-36. [DOI] [PubMed] [Google Scholar]
- 13.Aktas O. Shifting borders, crossing boundaries: the case of combined central and peripheral demyelination. Mult Scler. 2018;24(4):550-551. [DOI] [PubMed] [Google Scholar]
- 14.Graf J, Jansen L, Ingwersen J, et al. Multifocal visual evoked potentials in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. 2018;5(8):952-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dziadkowiak E, Ejma M, Wieczorek M, et al. Abnormality of multimodal evoked potentials in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Neurol Sci. 2020;41(9):2495-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797-812. [DOI] [PubMed] [Google Scholar]
- 17.Sotirchos ES, Gonzalez Caldito N, Filippatou A, et al. Progressive multiple sclerosis is associated with faster and specific retinal layer atrophy. Ann Neurol. 2020;87(6):885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisa M, Guerrieri S, Di Maggio G, et al. No evidence of disease activity is associated with reduced rate of axonal retinal atrophy in MS. Neurology. 2017;89(24):2469-2475. [DOI] [PubMed] [Google Scholar]
- 19.Schneider E, Zimmermann H, Oberwahrenbrock T, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One. 2013;8(6):e66151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung CY, Ong YT, Hilal S, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2015;45(1):45-56. [DOI] [PubMed] [Google Scholar]
- 21.Roth NM, Saidha S, Zimmermann H, et al. Photoreceptor layer thinning in idiopathic Parkinson's disease. Mov Disord. 2014;29(9):1163-1170. [DOI] [PubMed] [Google Scholar]
- 22.Ringelstein M, Albrecht P, Südmeyer M, et al. Subtle retinal pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2014;1(4):290-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albrecht P, Müller AK, Ringelstein M, et al. Retinal neurodegeneration in Wilson's disease revealed by spectral domain optical coherence tomography. PLoS One. 2012;7(11):e49825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oertel FC, Havla J, Roca-Fernández A, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry. 2018;89(12):1259-1265. [DOI] [PubMed] [Google Scholar]
- 25.Lambe J, Fitzgerald KC, Murphy OC, et al. . Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology. 2021;96(16):e2058-e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. [DOI] [PubMed] [Google Scholar]
- 27.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JI, Gemerzki L, Boerker L, et al. No alteration of optical coherence tomography and multifocal visual evoked potentials in eyes with symptomatic carotid artery disease. Front Neurol. 2019;10:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balk LJ, Twisk JWR, Steenwijk MD, et al. . A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2014;85(7):782-789. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht P, Ringelstein M, Müller AK, et al. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler. 2012;18(10):1422-1429. [DOI] [PubMed] [Google Scholar]
- 32.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(pt 2):521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberwahrenbrock T, Ringelstein M, Jentschke S, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19(14):1887-1895. [DOI] [PubMed] [Google Scholar]
- 34.Motamedi S, Gawlik K, Ayadi N, et al. Normative data and minimally detectable change for inner retinal layer thicknesses using a semi-automated OCT image segmentation pipeline. Front Neurol. 2019;10:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2016;22(5):641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77(3):517-528. [DOI] [PubMed] [Google Scholar]
- 37.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolan-Kenney RC, Liu M, Akhand O, et al. . Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann Neurol. 2019;85(5):618-629. [DOI] [PubMed] [Google Scholar]
- 39.Aktas O, Hartung H-PCSI. Multiple sclerosis. Tracing optic nerve involvement by standardized optical coherence tomography. Ann Neurol. 2019;85(5):615-617. [DOI] [PubMed] [Google Scholar]
- 40.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845-881. [DOI] [PubMed] [Google Scholar]
- 41.Horstmann L, Schmid H, Heinen AP, Kurschus FC, Dick HB, Joachim SC. Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J Neuroinflammation. 2013;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz-Herranz A, Dietrich M, Hilla AM, et al. . Monitoring retinal changes with optical coherence tomography predicts neuronal loss in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2019;16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manogaran P, Samardzija M, Schad AN, et al. Retinal pathology in experimental optic neuritis is characterized by retrograde degeneration and gliosis. Acta Neuropathol Commun. 2019;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(pt 6):1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]