Abstract
Background and Objectives
In MS, an age-related decline in disease activity and a decreased efficacy of disease-modifying treatment have been linked to immunosenescence, a state of cellular dysfunction associated with chronic inflammation.
Methods
To evaluate age-related immunologic alterations in MS, we compared immune signatures in peripheral blood (PB) and CSF by flow cytometry in patients with relapsing-remitting (RR) (PB n = 38; CSF n = 51) and primary progressive (PP) MS (PB n = 40; CSF n = 36) and respective controls (PB n = 40; CSF n = 85).
Results
Analysis revealed significant age-related changes in blood immune cell composition, especially in the CD8 T-cell compartment of healthy donors (HDs) and patients with MS. However, HDs displayed a strong age-dependent decline in the expression of the immunoregulatory molecules KLRG1, LAG3, and CTLA-4 on memory CD8 T cells, whereas this age-dependent reduction was completely abrogated in patients with MS. An age-dependent increase in the expression of the costimulatory molecule CD226 on memory CD8 T cells was absent in patients with MS. CD226 expression correlated with disability in younger (≤50 years) patients with MS. CSF analysis revealed a significant age-dependent decline in various immune cell populations in PPMS but not RRMS, suggesting a differential effect of aging on the intrathecal compartment in PPMS.
Discussion
Our data illustrate that aging in MS is associated with a dysbalance between costimulatory and immunoregulatory signals provided by CD8 T cells favoring a proinflammatory phenotype and, more importantly, a pattern of premature immune aging in the CD8 T-cell compartment of young patients with MS with potential implications for disease severity.
Because of the increased longevity of the general population worldwide, the concept of immunosenescence is drawing increased attention. Immunosenescence describes the gradual decline of immune system functions with age.1 Functional consequences of immunosenescence comprise a reduced adaptive immune response to vaccinations and an enhanced susceptibility to infections and for the development of distinct autoimmune diseases in elderly individuals.1,2
Aging represents a great challenge in clinical management of multiple sclerosis (MS) as the proportion of patients with a long-term relapsing-remitting MS (RRMS) disease course at an age of around 50 years is increasing.3 To date, there is a wide range of immunotherapy options for patients with RRMS. Nevertheless, a large part of clinical trials did not include patients with RRMS over approximately 50 years of age, which implies that the safety and efficacy of these drugs have not been systematically evaluated in this particular population.3 A meta-analysis of clinical data from patients with RRMS demonstrated that the efficacy of disease-modifying therapies (DMTs) is substantially decreased with age.4 At the same time, the risk of severe adverse events under DMTs increases in patients with RRMS older than 50 years.5,6 The majority of approved treatment approaches for RRMS fail to demonstrate the efficacy in patients with progressive MS usually diagnosed at an age above 50–55 years.3,7,8 These findings indicate that different types of MS exhibit distinct pathophysiologic mechanisms that might appear in an age-dependent manner.
An accelerated aging process has already been implicated in other autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).9-11 In autoimmunity, chronic inflammation drives cells into senescence, which results in a decreased ability for tissue repair and thus contributes to the disease pathogenesis (e.g., as in RA).2,12 The accumulation of CD4+CD28− T cells in the peripheral blood (PB), a hallmark of senescence, might contribute to disease progression in patients with RA.10,11,13-15 The expansion of senescent CD4+CD28− T cells has been found in CNS lesions and PB of patients with MS supporting a crucial role for immunosenescence in MS as well.13,14,16 Cellular senescence might also interfere with recovery in MS, as senescence in neural precursor cells impairs remyelination in CNS lesions of patients with primary progressive MS (PPMS).17 However, comprehensive studies investigating immunosenescence in peripheral immune signatures of RRMS vs PPMS in young vs old individuals are still lacking. The aim of the present study is to investigate the effect of aging on immune signatures of age- and sex-matched patients with RRMS and PPMS in comparison to controls in peripheral and intrathecal compartments. We hypothesize that patients with MS might display signs of accelerated immune aging, which could affect disease progression. To evaluate the underlying pathophysiologic differences of patients and controls during aging, we studied the immune profile with respect to senescence markers and additional relevant immunologic markers by multicolor flow cytometry.
Methods
Study Design
The study comprised 2 independent cohorts (A: peripheral blood mononuclear cell [PBMC] cohort; B: CSF cohort). Demographic data are shown in eTables 1 and 2, links.lww.com/NXI/A620 and links.lww.com/NXI/A621. Cohort A included age- and sex-matched patients with RRMS (n = 38) and PPMS (n = 40) according to the McDonald criteria revised in 201718 and healthy donors (HDs) (n = 40) who underwent comprehensive immunophenotyping in PB. Cohort B consisted of patients with RRMS (n = 51), patients with PPMS (n = 36), and noninflammatory controls (NICs) (n = 85) who underwent comprehensive immunophenotyping in CSF. NICs comprised patients with somatoform disorders or patients who donated CSF during the course of spinal anesthesia as described previously.19 Groups in each cohort were subdivided into young (≤50 years) and old (>50 years) participants. One rationale behind the 50-year cutoff is that the DMT efficacy for RRMS has mostly been tested only in clinical trials with patients up to 50–55 years3,4 Furthermore, a recent meta-analysis shows that there is a lower efficacy of DMTs for the average of patients with MS beyond 53 years.4 Another aspect for choosing this distinct cutoff is that the median age of our main study cohort A is 50 years. As validation, key immunologic markers are also presented as correlation with age.
For the composition of the study cohort, the following exclusion criteria were fulfilled:
Patients with a chronic disease of the immune system in addition to MS who requires long-term immunotherapy or is associated with a known immunodeficiency
Patients with RRMS who presented with an acute clinical relapse within the preceding 6 weeks
Patients with RRMS and PPMS who presented an enhanced T2 lesion load or a gadolinium-enhanced cerebral or spinal cord T1-weighted lesions in an MRI scan, which was performed no longer than 1 month ago
Patients who received ongoing immunotherapy
-
Patients with RRMS with the following treatment history:
- IV cortisone pulse therapy within 3 months before inclusion
- Interferon-beta, glatiramer acetate, teriflunomide, or dimethyl fumarate within 2 years before inclusion
- Fingolimod or natalizumab within 6 months before inclusion
- Previous treatment with ocrelizumab, rituximab, alemtuzumab, or cladribine
-
Patients with PPMS with the following treatment history:
- IV cortisone pulse therapy within 3 months of inclusion
- Mitoxantrone within 12 months before inclusion
- Previous treatment with ocrelizumab or rituximab
- Other immunomodulatory therapies within 3 months before inclusion
Because this was a proof-of-concept study, pilot group size was used. As possible confounders such as sex, disease duration, Expanded Disability Status Scale (EDSS), and MRI lesion load might impair the read out, we adjusted data from patients with MS of our main PBMC cohort for these covariates (eFigure 1, links.lww.com/NXI/A607; eTable 3, links.lww.com/NXI/A622).
Sampling and Flow Cytometric Analysis of PBMCs
Whole-blood samples were collected from study subjects. PBMCs were isolated by Ficoll (Sigma-Aldrich) density gradient centrifugation and stored in liquid nitrogen according to our standard operating procedure.20 Freshly thawed or stimulated PBMCs were stained with fluorochrome-conjugated antibodies. For further information, please see eMethods, links.lww.com/NXI/A626.
Data Analysis
For data analysis, we used Kaluza Flow Cytometry Analysis software version 2.1. We used scripts generated in R version 4.0.2 (“Taking Off Again”)21 and RStudio version 1.3.959 for streamlined batch generation of box plots and age correlation plots.
Statistical Analysis
For continuous variables, the independent-sample t test or Mann-Whitney U test and analysis of variance with post hoc Bonferroni testing were conducted as appropriate. To assess the relationship between age and immune parameters, we computed Pearson product-moment correlation coefficients (Pearson R). To adjust for possible confounders, we used median regression (R package quantreg) including age, sex, disease duration, EDSS, and lesion load as covariates. Differences were considered statistically significant with the following p values: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Nevertheless, all analyses should be considered as exploratory. If possible, investigators were blinded throughout data acquisition and analysis. Clinical scoring was independent of flow cytometric analysis.
Standard Protocol Approvals, Registrations, and Patient Consents
The local ethics committee (2016-053-f-S) approved blood and CSF sampling, and all subjects provided written informed consent before entering the study. This trial was conducted in accordance with the Declaration of Helsinki.
Data Availability
All data associated with this study are present in the article or the supplementary material. Data and codes of all scripts are available from the corresponding author on reasonable request. All models were created using publicly available packages and functions in the R programming language. For further information, please see eMethods (links.lww.com/NXI/A626).
Results
Age-Dependent Alterations in Peripheral Immune Cell Composition in MS
We investigated potential age-related changes in peripheral immune signatures by multiparameter flow cytometry using a cohort of age- and sex-matched patients with RRMS (n = 38) and PPMS (n = 40) and HDs (n = 40) (young ≤50 years; old >50 years). None of the patients received any DMT for at least 3 months before blood sampling. Demographic data and key information of clinical characteristics are depicted in eTable 1, links.lww.com/NXI/A620.
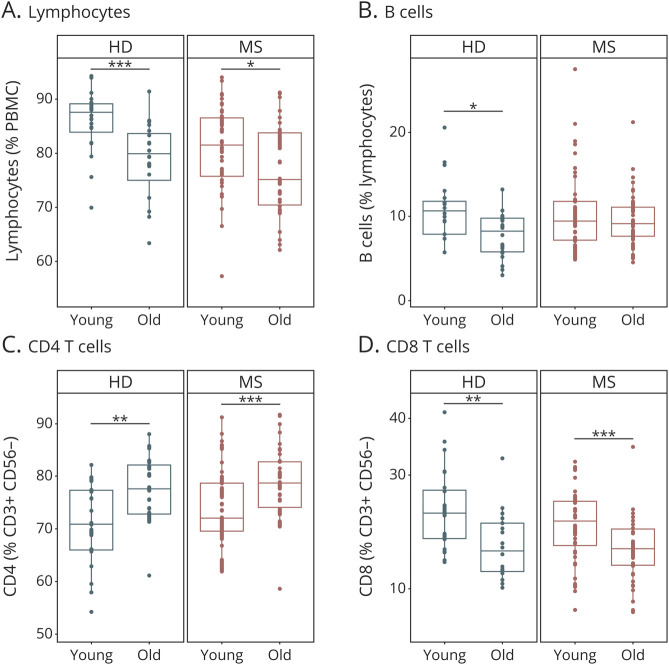
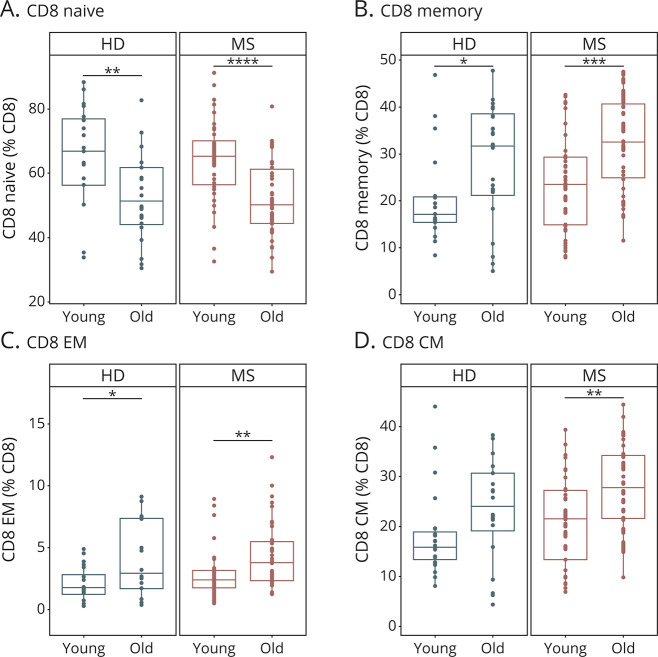
In accordance to the literature,22,23 characterization of major immune cell populations revealed an age-dependent decrease in the proportions of lymphocytes, B cells and CD8 T cells with a concomitant increase in CD4 T cells in HDs (Figure 1, eFigure 2, links.lww.com/NXI/A608). In patients with MS, these age-dependent alterations were less pronounced albeit still significant, at least for lymphocytes, CD4 and CD8 T cells (Figure 1, eFigure 2, links.lww.com/NXI/A608). The CD8 T-cell compartment exhibited a particularly strong age dependency in controls and in patients with MS, with a decrease in naive CD8 T cells and a reciprocal increase in CD8 memory T cells reflecting an increase in effector and central memory (EM and CM) subsets (Figure 2, eFigure 3, links.lww.com/NXI/A609). Direct comparison of patients with RRMS and PPMS revealed that age-dependent changes within the CD8 T-cell compartment were more pronounced in patients with PPMS compared with patients with RRMS (eFigure 3B, links.lww.com/NXI/A609). CD4 T-cell subsets did not exhibit pronounced age-dependent alterations, neither in controls nor in patients with MS (eFigure 4, links.lww.com/NXI/A610). Overall, these data reveal strong age-related alterations within the lymphocyte compartment of HDs and patients with MS, which are particularly pronounced in the CD8 T-cell compartment.
Figure 1. Basic Immune Cell Subset Composition in Young and Old Patients With MS and HDs.
Immune cell subset composition in the peripheral blood of young (≤50 years) and old (>50 years) patients with multiple sclerosis (MS) (young: n = 40, old: n = 38) and HDs (young: n = 20, old: n = 20). Demographic data of study subjects are depicted in eTable 1 (A–D, links.lww.com/NXI/A620). Frequencies of lymphocytes (A), B cells (B), CD4 (C), and CD8 (D) T cells. Data are displayed as boxplots of the median and the 25th and 75th percentile ± interquartile range. Statistical analysis was conducted by the 2-tailed Mann-Whitney test. Differences were considered statistically significant with the following p values: *p < 0.05, **p < 0.01, and ***p < 0.001. HD = healthy donor.
Figure 2. Age-Related Changes in the CD8 T-Cell Compartment in Patients With MS and HDs.
Composition of the CD8 T-cell compartment in the peripheral blood of young (≤50 years) and old (>50 years) patients with MS (young: n = 40, old: n = 38) and HDs (young: n = 20, old: n = 20). Demographic data of study subjects are depicted in eTable 1 (A–D, links.lww.com/NXI/A620). Proportions of naive (A), memory (B), effector memory (C), and central memory (D) CD8 T cells. Data are displayed as boxplots of the median and the 25th and 75th percentile ± interquartile range. Statistical analysis was conducted by the 2-tailed Mann-Whitney test. Differences were considered statistically significant with the following P values: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. CM = central memory; EM = effector memory; HD = healthy donor.
Features of Premature Immune Aging in Young Patients With MS
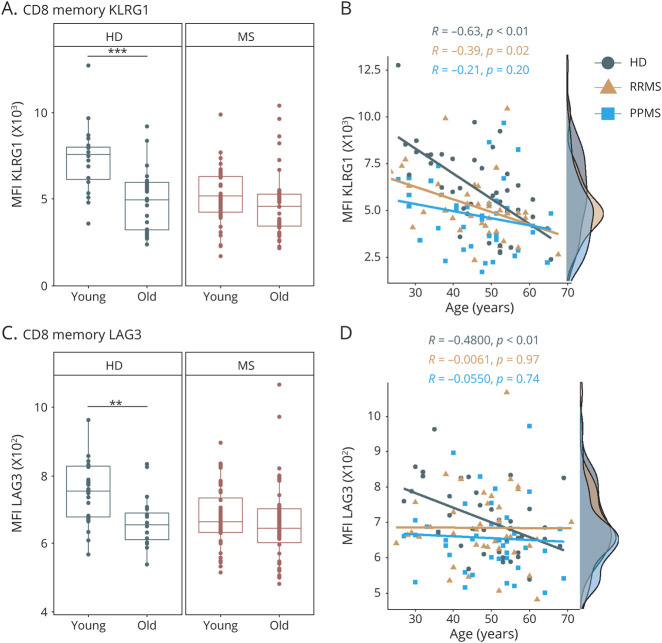
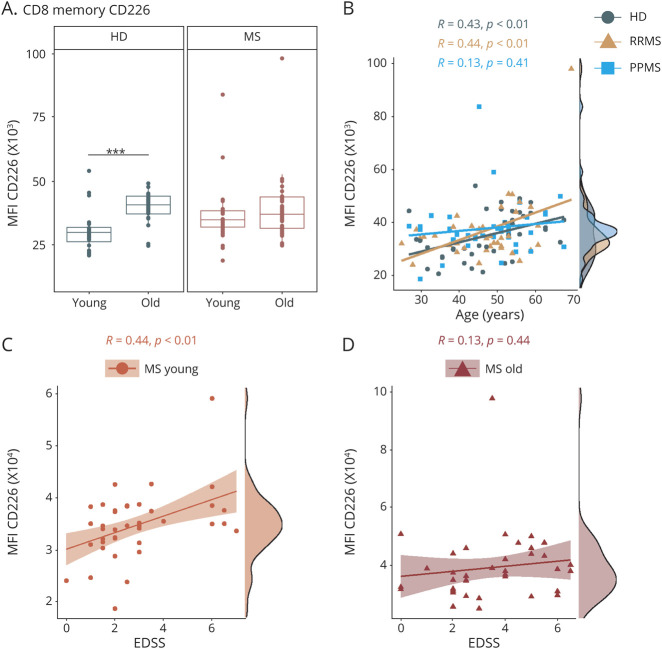
Next, we evaluated age-dependent changes in immunoregulatory and immunosenescence-related molecules within the T-cell compartment (eTable 4, links.lww.com/NXI/A623). Notably, we observed an age-related increase in CD4+CD28− T cells in HDs and patients with MS (eFigure 5A, links.lww.com/NXI/A611). Nevertheless, we did not detect age-dependent changes in frequencies of CD8+CD28− T cells and CD57+ T cells neither in controls nor in patients with MS (eFigure 5, links.lww.com/NXI/A623). We did notice a strong age-dependent decrease in the expression of several immunoinhibitory molecules such as killer cell lectin-like receptor subfamily G member 1 (KLRG1), lymphocyte-activation gene 3 (LAG3), and cytotoxic T lymphocyte–associated protein 4 (CTLA-4) on memory CD8 T cells and respective subsets from HDs (Figure 3, eFigure 6, links.lww.com/NXI/A612). Importantly, this age-dependent regulation was abrogated in patients with MS, as young patients already exhibited expression levels of these molecules comparable to those from old patients with MS or old HDs (Figure 3, eFigure 6, links.lww.com/NXI/A612). Direct comparison of patients with RRMS and PPMS revealed that this loss of age-dependent regulation was slightly more pronounced in patients with PPMS compared with patients with RRMS (Figure 3, B and D, eFigure 6, links.lww.com/NXI/A612). With regard to functional properties of T cells, we showed that CD8 T cells of elderly had an increased expression of CD107a, a marker for degranulation of cytolytic vesicles on T cell receptor stimulation (eFigure 7A, links.lww.com/NXI/A613). T cells of old patients with MS displayed significantly increased intracellular expression of interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) on stimulation, which was less pronounced in the HD cohort (eFigure 7, B–D, links.lww.com/NXI/A613). Of note, HDs exhibited a strong age-dependent increase in the costimulatory molecule CD226 (DNAX accessory molecule-1, DNAM-1) on memory and EM CD8 T cells (Figure 4, eFigure 8, links.lww.com/NXI/A614). Again, this age-dependent modulation was much less pronounced in patients with MS, in particular in patients with PPMS, due to elevated levels already in the young MS population (Figure 4B, eFigure 8A, links.lww.com/NXI/A614). Notably, for CD226 expression on memory and EM CD8 T cells, we observed a significant correlation with disability as reflected by the EDSS in young but not in old patients (Figure 4, C and D, eFigure 8B, links.lww.com/NXI/A614). In a similar line of evidence, we observed a distinct correlation between serum neurofilament light chain (sNfL) levels and the presence of senescence-associated T-cell traits, i.e., CD28 negativity and CD57 and KLRG1 expression specifically in young but not old patients with MS (eFigure 9, links.lww.com/NXI/A615), possibly suggesting a link between the loss of age-dependent regulation and clinical disease manifestation. In a subgroup analysis of only treatment-naive patients with MS, we could confirm those premature immune aging signatures of KLRG1, LAG3, CTLA-4, and CD226 in young patients with MS (eFigure 10, links.lww.com/NXI/A616). When we divided our study cohort into tertiles by age, the premature immune aging signatures in the CD8 memory compartment were also evident (eFigure 11, links.lww.com/NXI/A617).
Figure 3. Immunoregulatory Markers on T Cells in Young and Old Patients With MS and HDs.
Flow cytometric analysis of frozen peripheral blood mononuclear cells from young (≤50 years) and old (>50 years) patients with MS (MS: young: n = 40, old: n = 38; RRMS: young: n = 20, old: n = 18; PPMS: young: n = 20, old: n = 20) and HDs (young: n = 20, old: n = 20). Demographic data of study subjects are depicted in eTable 1, links.lww.com/NXI/A620. (A) MFI of KLRG1 on memory CD8 T cells of patients with MS and HDs. (B) Correlation analysis of MFI of KLRG1 on memory CD8 T cells with age of HDs (n = 40), patients with RRMS (n = 38), and patients with PPMS (n = 40). (C) MFI of LAG3 on memory CD8 T cells of patients with MS and HDs. (D) Correlation analysis of MFI of LAG3 on memory CD8 T cells with age of HDs (n = 40), patients with RRMS (n = 38), and patients with PPMS (n = 40). Data are displayed as boxplots of the median and the 25th and 75th percentile ± interquartile range. Statistical analysis was conducted by the 2-tailed Mann-Whitney test. For correlation analysis, the Pearson product-moment correlation coefficients (Pearson R) were computed. Differences were considered statistically significant with the following p values: **p < 0.01 and ***p < 0.001. HD = healthy donor; KLRG1 = killer cell lectin-like receptor subfamily G member 1; LAG3 = lymphocyte-activation gene 3; MFI = mean fluorescence intensity; MS = multiple sclerosis; PPMS = primary progressive MS; RRMS = relapsing-remitting MS.
Figure 4. Age-Related Expression of the Costimulatory Molecule CD226 on T Cells in Patients With MS and HDs.
Flow cytometric analysis of frozen peripheral blood mononuclear cells from young (≤50 years) and old (>50 years) patients with MS (MS: young: n = 40, old: n = 38; RRMS: young: n = 20, old: n = 18; PPMS: young: n = 20, old: n = 20) and HDs (young: n = 20, old: n = 20). Demographic data of study subjects are depicted in eTable 1, links.lww.com/NXI/A620. (A) MFI of CD226 (DNAM-1) on memory CD8 T cells. (B) Correlation analysis of MFI of CD226 on memory CD8 T cells with age of HDs (n = 40), patients with RRMS (n = 38), and patients with PPMS (n = 40). (C, D) Correlation analysis of CD226 expression on memory CD8 T cells with the EDSS score of young (C) and old (D) patients with MS. Data are displayed as boxplots of the median and the 25th and 75th percentile ± interquartile range. Statistical analysis was conducted by the 2-tailed Mann-Whitney test. For correlation analysis, the Pearson product-moment correlation coefficients (Pearson R) were computed. Differences were considered statistically significant with the following p values: **p < 0.01 and ***p < 0.001. EDSS = Expanded Disability Status Scale; HD = healthy donor; MS = multiple sclerosis; MFI = mean fluorescence intensity; PPMS = primary progressive MS; RRMS = relapsing-remitting MS.
Age-Dependent Alterations of Immune Cell Subsets in the CSF
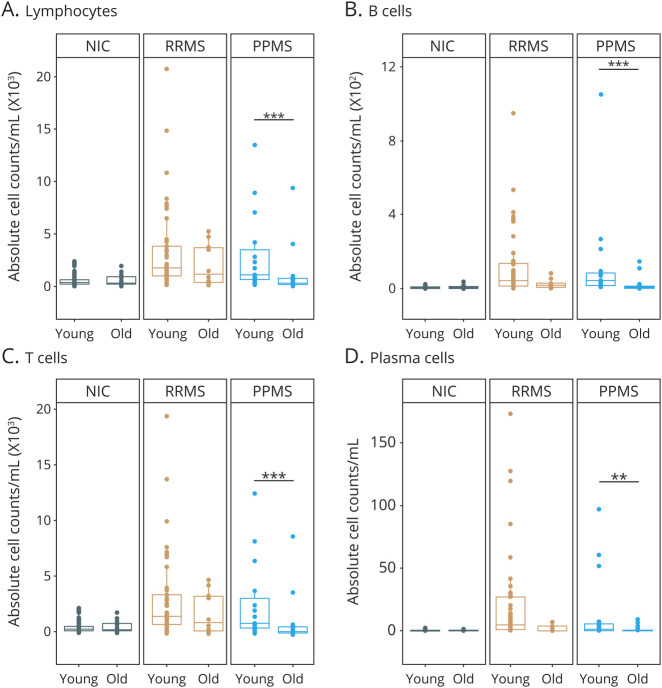
In an additional independent cohort, we evaluated potential age-dependent alterations in immune cell composition and activation status in the CSF of 51 patients with RRMS and 36 patients with PPMS and 85 NICs by multiparameter flow cytometry. Demographic data and key information of clinical characteristics are depicted in eTable 2, links.lww.com/NXI/A621. In patients with RRMS, lumbar puncture was performed at least 6 weeks after the last acute clinical disease activity to avoid potential relapse-associated alterations in CSF subset composition. Notably, in controls, we did not observe any age-dependent alteration in total cell numbers of major immune cell populations as well as human leukocyte antigen-DR expressing T cells (Figure 5, eFigure 12, links.lww.com/NXI/A618). In contrast, we observed an age-dependent decrease in counts of lymphocytes, B and T cells, as well as plasma cells and natural killer (NK) cells in patients with PPMS but not in patients with RRMS (Figure 5, eFigure 12, links.lww.com/NXI/A618), suggesting an age-dependent decrease in immune cell infiltration into the CSF in the context of PPMS.
Figure 5. Age-Dependent Immune Cell Subset Composition in the CSF of Patients With MS and Noninflammatory Controls.
Immune cell subset composition in the in the CSF of young (≤50 years) and old (>50 years) NICs (young n = 60; old: n = 25) and patients with RRMS (young: n = 41; old: n = 10) and PPMS (young: n = 15; old: n = 21). Demographic data of study subjects are depicted in eTable 2, links.lww.com/NXI/A621. (A–D) Absolute numbers of lymphocytes (A), B cells (B), T cells (C), and plasma cells (D). Data are displayed as boxplots of the median and the 25th and 75th percentile ± interquartile range. Statistical analysis was conducted by the 2-tailed Mann-Whitney test. Differences were considered statistically significant with the following p-values: **p < 0.01 and ***p < 0.001. NIC = noninflammatory control; PPMS = primary progressive MS; RRMS = relapsing-remitting MS.
Discussion
Our study provides a comprehensive overview of age-related changes in peripheral immune signatures of patients with RRMS and PPMS compared with controls. We reveal that especially the CD8 T-cell compartment of young patients with MS exhibits signs of premature immune aging.
In accordance to the literature,22,23 we could verify that elderly HDs and patients with MS exhibit decreased proportions of lymphocytes and CD8 T cells with a concomitant increase in CD4 T-cell proportions. Particularly, the composition of the CD8 T-cell compartment shifts toward a higher proportion of memory T cells in the elderly, whereas the naive T-cell frequency decreases in the PB of HDs, as described previously.24,25 Decreasing lymphocyte and naive T-cell proportions across the lifespan are driven by thymic involution and decreased hematopoietic output by the bone marrow of aged individuals.26 Although we showed that CD4 T-cell frequencies correlate with age, the composition of the CD4 T-cell compartment is less impaired in aging compared with the CD8 T-cell compartment.24,27
Of interest, we observed elevated proportions of senescence-associated CD4+CD28− T cells in the periphery of older HDs and patients with MS, whereas we found no increase in CD8+CD28− T cells.15,23,28 The finding of elevated CD4+CD28− T-cell proportions is in line with other human studies that showed a relative increase of CD4+CD28− T cells with age in the periphery of patients with MS and patients with other autoimmune diseases such as RA and SLE.14,28,29 CD4+CD28− T cells in brain lesions of patients with MS have been identified to exhibit pathogenic properties contributing to tissue damage.16,28 Of interest, we did not detect an increase in the expression of another prominent senescence marker CD57 on T cells of elderly subjects.30
In our study, we aimed to focus on a comprehensive characterization of age-dependent changes of immunoregulatory molecules in age- and sex-matched as well as untreated patients with RRMS and PPMS. We observed that the CD8 T-cell compartment of young patients with MS displayed signs of premature immune aging, as young patients with MS displayed alterations in immunoregulatory and costimulatory molecules that were comparable to those observed in elderly HDs. Especially, the comprised decrease in the levels of the coinhibitory markers KLRG1 and LAG3 and the concomitant increase of the costimulatory molecule CD226 were even more pronounced in young patients with PPMS compared with patients with RRMS, pointing toward an accelerated aging process in patients with progressive MS. Similar to the HDs in our study, aged patients with MS showed a pronounced relative increase of the nonclassical (CD14+CD16high) monocyte subset (eFigure 13A and B, links.lww.com/NXI/A619). However, different to the T-cell compartment, we only detected age-related changes but no indicators for a premature aging process in MS in the monocyte compartment (eFigure 13, links.lww.com/NXI/A619).
In contrast to what has been suggested in the literature regarding senescence and autoimmunity,3,31 we did not find evidence of enhanced age-dependent alterations of immune signatures in elderly patients with MS compared with controls, at least not by our multiparameter flow cytometry approach. Instead, we observed that overall, the immune signatures of HDs and patients with MS rather similarly correlated with age. Therefore, our data do not support the idea, as proposed by a meta-analysis4 that elderly patients with MS should exhibit enhanced or accelerated immune aging, which had been conceptionally linked to the observed reduced response to immunomodulatory treatment in older patients with MS. In addition, our data underpin recent clinical observations that MS-related autoimmunity might not lead to a significantly increased risk of severe infectious diseases like COVID-19. It rather seems that besides age, the extent of disability and a higher degree of immunosuppression in patients with MS might drive COVID-19 susceptibility.32 Of interest, we observed that T cells from older controls and patients with MS exhibit an elevated degranulation of cytolytic vesicles and display an age-related increase in the production of IFN-γ and TNF-α, pointing toward a proinflammatory phenotype in aging. Based on our setup, we cannot exclude that antigen-specific immune responses, e.g., against viruses, might be impaired in the elderly population, as these more complex immune functions are not properly mirrored by our flow cytometry analysis approach. Overall, our data support the notion of an enhanced proinflammatory state of T cells in elderly individuals rather than an impaired immune function in this population.
Based on our data, we can conclude that the CD8 T-cell compartment of younger patients with MS is pathophysiologically distinct to the one of age-matched HDs. However, whether these signs of premature aging are a cause or consequence of the inflammatory activity in MS cannot be revealed from our data. Notably, these changes were more pronounced in patients with PPMS than in patients with RRMS. This finding was somewhat surprising in light of the fact that in clinical practice, the majority of immunotherapies used in patients with RRMS are not effective in patients with PPMS.7,8 It has been suggested that this might be due to a relatively low inflammatory vs neurodegenerative component within the pathophysiologic trajectories of PPMS.3,33 However, our data rather suggest that also PPMS is characterized by a distinct proinflammatory component, which might be different from the one known from RRMS and is characterized by premature signs of immune aging. This interpretation is further supported by our analysis of the CSF compartment of patients with PPMS vs patients with RRMS: Here, young patients with PPMS exhibited a pronounced immune cell infiltration of different immune cell subsets, with a significant decrease with age. This illustrates that especially in young patients with PPMS, there is a notable immune cell infiltration into the CSF as a clear indicator of inflammatory disease activity. Together, our data suggest that PPMS—particularly in younger patients—is indeed characterized by a distinct proinflammatory phenotype, which might explain the positive results of anti-inflammatory DMTs, especially in young patients with PPMS.8,34 In a similar line, another study described that patients with PPMS with a higher T2 lesion load display in vitro increased rates of lymphocyte migration and enhanced IFN-γ production compared with patients with PPMS with a lower lesion load.35 As different pathomechanisms are suggested to underlie this distinct proinflammatory phenotype, therapeutic approaches might indeed need distinct immunologic strategies compared with RRMS. Along this line, it is tempting to speculate that drugs, which target senescent cells (senolytics), could provide a suitable therapeutic strategy in PPMS. In animal models of neurodegenerative and other autoimmune diseases, targeted depletion of senescent cells by senolytics has already achieved success.36-38
With respect to a potential clinical implication of our findings, we did not only observe an age-related upregulation of the costimulatory molecule CD226 (DNAM-1) on memory and EM CD8 T cells, but also identified a significant correlation between CD226 expression levels and the EDSS score only in young patients with MS, suggesting a link between the degree of premature immune aging and disease severity. CD226 is expressed constitutively on the majority of NK cells and T cells, mediating adhesion, costimulation, and cytotoxicity of immune cells.39,40 Initially, CD226 was described as a key molecule on proinflammatory Th1-differentiated cells and treatment with an α-CD226 monoclonal antibody reduced the onset and severity of disease in experimental autoimmune encephalomyelitis (EAE), an animal model for MS.40 In the context of autoimmune diabetes, the knockout of CD226 led to a decreased disease severity in the nonobese diabetes mouse model.41 In humans, genetic variations of CD226 were linked to a higher susceptibility to develop MS and other autoimmune diseases.42 These findings, together with our data, imply that CD226 is regulated in an age-dependent manner and plays an important role in modulating autoimmunity. This concept is further supported by our observation of a correlation between sNfL levels and several other senescence-associated T-cell traits, and intriguingly, this was confined to young patients with MS, again supporting the idea that premature aging in MS might be associated with features of clinical disease manifestation. We are fully aware that caution is warranted due to limited sample size and lack of standardized long-term follow-up data from our patients and therefore suggest that these intriguing findings need to be corroborated in another independent cohort.
In conclusion, we identified that young patients with MS display signs of premature immune aging in the CD8 T-cell compartment, especially with respect to immunoregulatory molecules, whereas age-related changes in immune signatures in elderly patients with MS were comparable to those of HDs. This phenomenon of premature immune aging could be caused by chronic inflammation driving sustained alterations in immune-regulatory networks and ultimately cellular senescence, as it has been suggested in RA and SLE.9,11,43 Further mechanistic studies are needed to determine the relationship between the premature aging phenotype (altered regulation of immunoregulatory molecules) and disease pathogenesis in MS.
Acknowledgment
The authors thank J. Meyer and M.L. Frankenberg (University Hospital Münster, Münster, Germany) for excellent technical assistance.
Glossary
- CM
central memory
- CTLA-4
cytotoxic T lymphocyte–associated protein 4
- DMT
disease-modifying therapy
- DNAM-1
DNAX accessory molecule-1
- EAE
experimental autoimmune encephalomyelitis
- EDSS
Expanded Disability Status Scale
- EM
effector memory
- HD
healthy donor
- IFN-γ
interferon-gamma
- KLRG1
killer cell lectin-like receptor subfamily G member 1
- LAG3
lymphocyte-activation gene 3
- MFI
mean fluorescence intensity
- MS
multiple sclerosis
- NIC
noninflammatory control
- NK
natural killer
- PB
peripheral blood
- PBMC
peripheral blood mononuclear cell
- PPMS
primary progressive multiple sclerosis
- RA
rheumatoid arthritis
- RRMS
relapsing-remitting multiple sclerosis
- sNfL
serum neurofilament light chain
- SLE
systemic lupus erythematosus
- TNF-α
tumor necrosis factor alpha
Appendix. Authors
Contributor Information
Melanie Eschborn, Email: melanie.eschborn@ukmuenster.de.
Marc Pawlitzki, Email: marc.pawlitzki@ukmuenster.de.
Timo Wirth, Email: timo.wirth@ukmuenster.de.
Christopher Nelke, Email: christopher.nelke@ukmuenster.de.
Steffen Pfeuffer, Email: steffen.pfeuffer@lwl.org.
Andreas Schulte-Mecklenbeck, Email: andreas.schulte-mecklenbeck@ukmuenster.de.
Lisa Lohmann, Email: lisa.lohmann@ukmuenster.de.
Leoni Rolfes, Email: leoni.rolfes@gmail.com.
Katrin Pape, Email: katrin.pape@unimedizin-mainz.de.
Maria Eveslage, Email: maria.eveslage@ukmuenster.de.
Stefan Bittner, Email: stefan.bittner@unimedizin-mainz.de.
Catharina C. Gross, Email: catharina.gross@ukmuenster.de.
Tobias Ruck, Email: tobias.ruck@med.uni-duesseldorf.de.
Heinz Wiendl, Email: heinz.wiendl@ukmuenster.de.
Sven G. Meuth, Email: sven.meuth@uni-duesseldorf.de.
Study Funding
This study was funded by the Deutsche Multiple Sklerose Gesellschaft (DMSG) Landesverband Nordrhein-Westfalen (NRW) (to M.E. and M.P.), Innovative Medical Research (IMF) program of the Westfälische Wilhelms-University Münster (ES112007 to M.E. and M.P.), Interdisciplinary Center for Clinical Research (IZKF) Münster (Kl03/010/19 to L.K.), Deutsche Forschungsgemeinschaft (DFG) SFB TR128 (project A08 to L.K.), and Novartis (to L.K.).
Disclosure
M. Eschborn received speaker honoraria and travel support from Sanofi-Genzyme. She received research support from the Deutsche Multiple Sklerose Gesellschaft (DMSG) Landesverband Nordrhein-Westfalen (NRW) and the Innovative Medical Research (IMF) program of the University Münster. M. Pawlitzki received speaker honoraria and travel support from Novartis. He received research support from the DMSG Landesverband NRW and the IMF program of the University Münster. T. Wirth and C. Nelke report no disclosures. S. Pfeuffer received travel grants from Sanofi-Genzyme and Merck Serono, lecturing honoraria from Sanofi-Genzyme, Mylan Healthcare, and Biogen, and research support from Diamed, Merck Serono, and the DMSG Landesverband NRW. A. Schulte-Mecklenbeck received research and travel support from Novartis. L. Lohmann, L. Rolfes, K. Pape, and M. Eveslage report no disclosures. S. Bittner has received honoraria and compensation for travel from Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, and Roche. C.C. Gross received speaker honoraria from Mylan, Bayer HealthCare, and Sanofi-Genzyme and travel/accommodation/meeting expenses from Bayer HealthCare, Biogen, Euroimmun, Novartis, and Sanofi-Genzyme. She also received research support from Biogen and Novartis. T. Ruck has received honoraria and consultation fee support from Celgene/BMS, Biogen, Roche, Sanofi-Aventis, Alexion, Novartis, and Teva and has received personal support from Merck Serono. H. Wiendl receives honoraria for acting as a member of scientific advisory boards for Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, and Sanofi-Aventis as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, Teva, and WebMD Global. Prof. Wiendl is acting as a paid consultant for Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis, and the Swiss rheuma Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, the European Union, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and Biogen, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi-Genzyme. S.G. Meuth received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer HealthCare, Biogen Idec, Celgene, Diamed, Sanofi-Aventis, MedDay, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, and by Alexion, Almirall, Amicus Therapeutics Germany, Biogen Idec, Diamed, Fresenius Medical Care, Sanofi-Aventis, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. L. Klotz received compensation for serving on scientific advisory boards for Alexion, Genzyme, Janssen, Merck Serono, Novartis, and Roche. She received speaker honoraria and travel support from Bayer, Biogen, Genzyme, Grifols, Merck Serono, Novartis, Roche, Santhera, and Teva. She receives research support from the German Research Foundation, the IZKF Münster, IMF Münster, Biogen, Immunic AG, Novartis, and Merck Serono. Go to Neurology.org/NN for full disclosures.
References
- 1.Pawelec G. Age and immunity: What is “immunosenescence”? Exp Gerontol. 2018;105:4-9. [DOI] [PubMed] [Google Scholar]
- 2.Ray D, Yung R. Immune senescence, epigenetics and autoimmunity. Clin Immunol. 2018;196:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughn CB, Jakimovski D, Kavak KS, et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. 2019;15(6):329-342. [DOI] [PubMed] [Google Scholar]
- 4.Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanai SA, Saini V, Benedict RH, et al. Aging and multiple sclerosis. Mult Scler. 2016;22(6):717-725. [DOI] [PubMed] [Google Scholar]
- 6.Mills EA, Mao-Draayer Y. Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult Scler. 2018;24(8):1014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075-1084. [DOI] [PubMed] [Google Scholar]
- 8.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471. [DOI] [PubMed] [Google Scholar]
- 9.Kalim H, Pratama MZ, Mahardini E, Winoto ES, Krisna PA, Handono K. Accelerated immune aging was correlated with lupus-associated brain fog in reproductive-age systemic lupus erythematosus patients. Int J Rheum Dis. 2020;23(5):620-626. [DOI] [PubMed] [Google Scholar]
- 10.Petersen LE, Schuch JB, de Azeredo LA, et al. Characterization of senescence biomarkers in rheumatoid arthritis: relevance to disease progression. Clin Rheumatol. 2019;38(10):2909-2915. [DOI] [PubMed] [Google Scholar]
- 11.Bauer ME. Accelerated immunosenescence in rheumatoid arthritis: impact on clinical progression. Immun Ageing. 2020;17:6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(suppl 5):S422-S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broux B, Markovic-Plese S, Stinissen P, Hellings N. Pathogenic features of CD4+CD28- T cells in immune disorders. Trends Mol Med. 2012;18(8):446-453. [DOI] [PubMed] [Google Scholar]
- 14.Thewissen M, Somers V, Venken K, et al. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007;123(2):209-218. [DOI] [PubMed] [Google Scholar]
- 15.Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broux B, Mizee MR, Vanheusden M, et al. IL-15 amplifies the pathogenic properties of CD4+CD28- T cells in multiple sclerosis. J Immunol. 2015;194(5):2099-2109. [DOI] [PubMed] [Google Scholar]
- 17.Nicaise AM, Wagstaff LJ, Willis CM, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci USA. 2019;116(18):9030-9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. [DOI] [PubMed] [Google Scholar]
- 19.Gross CC, Schulte-Mecklenbeck A, Madireddy L, et al. Classification of neurological diseases using multi-dimensional cerebrospinal fluid analysis. Brain 2021:awab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posevitz-Fejfár A, Posevitz V, Gross CC, et al. Effects of blood transportation on human peripheral mononuclear cell yield, phenotype and function: implications for immune cell biobanking. Plos One. 2014;9(12):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. 2020. [Google Scholar]
- 22.Jentsch-Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets' reference ranges in an age- and gender-balanced population of 100 healthy adults - a monocentric German study. Clin Immunol. 2005;116(2):192-197. [DOI] [PubMed] [Google Scholar]
- 23.Qin L, Jing X, Qiu Z, et al. Aging of immune system: immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging (Albany NY). 2016;8(5):848-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wertheimer AM, Bennett MS, Park B, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192(5):2143-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194(9):4073-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. 2020;134:110887. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broux B, Pannemans K, Zhang X, et al. CX3CR1 drives cytotoxic CD4+CD28-T cells into the brain of multiple sclerosis patients. J Autoimmun. 2012;38(1):10-19. [DOI] [PubMed] [Google Scholar]
- 29.Thewissen M, Linsen L, Somers V, Geusens P, Raus J, Stinissen P. Premature immunosenescence in rheumatoid arthritis and multiple sclerosis patients. Ann N Y Acad Sci. 2005;1051:255-262. [DOI] [PubMed] [Google Scholar]
- 30.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711-2720. [DOI] [PubMed] [Google Scholar]
- 31.Oost W, Talma N, Meilof JF, Laman JD. Targeting senescence to delay progression of multiple sclerosis. J Mol Med (Berl). 2018;96(11):1153-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015;14(4):406-419. [DOI] [PubMed] [Google Scholar]
- 34.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2016;376(3):209-220. [DOI] [PubMed] [Google Scholar]
- 35.Prat A, Pelletier D, Duquette P, Arnold DL, Antel JP. Heterogeneity of T-lymphocyte function in primary progressive multiple sclerosis: relation to magnetic resonance imaging lesion volume. Ann Neurol. 2000;47(2):234-237. [PubMed] [Google Scholar]
- 36.Chinta SJ, Woods G, Demaria M, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson's disease. Cell Rep. 2018;22(4):930-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang P, Kishimoto Y, Grammatikakis I, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci. 2019;22(5):719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 2019;29(5):1045–1060.e10.ko [DOI] [PubMed] [Google Scholar]
- 39.Hafler JP, Maier LM, Cooper JD, et al. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10(1):5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dardalhon V, Schubart AS, Reddy J, et al. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J Immunol. 2005;175(3):1558-1565. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro MR, Yeh WI, Longfield JR, et al. CD226 deletion reduces type 1 diabetes in the NOD mouse by impairing thymocyte development and peripheral T cell activation. Front Immunol. 2020;11:2180-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piédavent-Salomon M, Willing A, Engler JB, et al. Multiple sclerosis associated genetic variants of CD226 impair regulatory T cell function. Brain. 2015;138(Pt 11):3263-3274. [DOI] [PubMed] [Google Scholar]
- 43.Tsai CY, Shen CY, Liao HT, et al. Molecular and cellular bases of immunosenescence, inflammation, and cardiovascular complications mimicking “inflammaging” in patients with systemic lupus erythematosus. Int J Mol Sci. 2019;20(16):3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data associated with this study are present in the article or the supplementary material. Data and codes of all scripts are available from the corresponding author on reasonable request. All models were created using publicly available packages and functions in the R programming language. For further information, please see eMethods (links.lww.com/NXI/A626).