Key Points
Question
When only using randomized clinical trial data, what is the most effective method of bowel preparation among patients undergoing elective colorectal surgery?
Findings
In this network meta-analysis, including data from 8377 patients from 35 randomized clinical trials, the addition of oral antibiotics to intravenous antibiotics (both with and without mechanical bowel preparation) was associated with a reduction in the incidence of incisional surgical site infection by greater than 50%. There were no differences in anastomotic leak or in other clinical outcomes.
Meaning
Bowel preparation should include the addition of oral antibiotics to intravenous antibiotics, as it may reduce incisional surgical site infection among patients undergoing elective colorectal surgery.
This network meta-analysis evaluates the ranking of different bowel preparation treatment strategies for their associations with postoperative outcomes using data from randomized clinical trials.
Abstract
Importance
There are discrepancies in guidelines on preparation for colorectal surgery. While intravenous (IV) antibiotics are usually administered, the use of mechanical bowel preparation (MBP), enemas, and/or oral antibiotics (OA) is controversial.
Objective
To summarize all data from randomized clinical trials (RCTs) that met selection criteria using network meta-analysis (NMA) to determine the ranking of different bowel preparation treatment strategies for their associations with postoperative outcomes.
Data Sources
Data sources included MEDLINE, Embase, Cochrane, and Scopus databases with no language constraints, including abstracts and articles published prior to 2021.
Study Selection
Randomized studies of adults undergoing elective colorectal surgery with appropriate aerobic and anaerobic antibiotic cover that reported on incisional surgical site infection (SSI) or anastomotic leak were selected for inclusion in the analysis. These were selected by multiple reviewers and adjudicated by a separate lead investigator. A total of 167 of 6833 screened studies met initial selection criteria.
Data Extraction and Synthesis
NMA was performed according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. Data were extracted by multiple independent observers and pooled in a random-effects model.
Main Outcomes and Measures
Primary outcomes were incisional SSI and anastomotic leak. Secondary outcomes included other infections, mortality, ileus, and adverse effects of preparation.
Results
A total of 35 RCTs that included 8377 patients were identified. Treatments compared IV antibiotics (2762 patients [33%]), IV antibiotics with enema (222 patients [3%]), IV antibiotics with OA with or without enema (628 patients [7%]), MBP with IV antibiotics (2712 patients [32%]), MBP with IV antibiotics with OA (with good IV antibiotic cover in 925 patients [11%] and with good overall antibiotic cover in 375 patients [4%]), MBP with OA (267 patients [3%]), and OA (486 patients [6%]). The likelihood of incisional SSI was significantly lower for those receiving IV antibiotics with OA with or without enema (rank 1) and MBP with adequate IV antibiotics with OA (rank 2) compared with all other treatment options. The addition of OA to IV antibiotics, both with and without MBP, was associated with a reduction in incisional SSI by greater than 50%. There were minimal differences between treatments in anastomotic leak and in any of the secondary outcomes.
Conclusions and Relevance
This NMA demonstrated that the addition of OA to IV antibiotics were associated with a reduction in incisional SSI by greater than 50%. The results support the addition of OA to IV antibiotics to reduce incisional SSI among patients undergoing elective colorectal surgery.
Introduction
The best way to prepare the bowel among patients undergoing elective colorectal surgery continues to be controversial. Mechanical bowel preparation (MBP) became established in early colorectal surgery because “intuitively it is unfathomable to believe that stool does not have deleterious effects on a healing anastomosis.”1 However, there was no reduction in incisional surgical site infection (SSI) until 1973, when Nichols et al2 introduced an MBP protocol that incorporated oral neomycin and erythromycin. This decreased bacterial colonization with studies confirming a greater than 50% reduction in SSI,3,4 resulting in the widespread introduction of MBP with oral antibiotics (OA). Subsequent studies comparing intravenous (IV) antibiotics and OA demonstrated a greater reduction in infection with IV antibiotics,5,6 although not without some controversy,7 resulting in an increased use of IV antibiotics rather than OA, especially in the United Kingdom. Between 1977 and 1993, 33 randomized clinical trials (RCTs) confirmed that prophylaxis required good cover against both aerobic and anaerobic bacteria.8 Patients’ difficulty taking the MBP9 contributed to questions about its benefits, and a series of well-designed RCTs and meta-analyses have now demonstrated no significant differences between MBP with IV antibiotics (MBP + IV) and IV antibiotics alone.10,11 Because of this, evidence-based early recovery after surgery protocols advise against using MBP among patients undergoing colonic surgery.12
In contrast, in North America, there has been an emphasis on using MBP with both IV antibiotics and OA (MBP + IV + OA).13,14,15,16,17,18 There are 2 main reasons for this. The first is a series of RCTs and meta-analyses showing that MBP + IV + OA has a significantly lower rate of SSI than other options.13,14,15,16,17,19 Unfortunately, many of these RCTs (but certainly not all) compare IV antibiotics regimens providing incomplete aerobic and anaerobic cover with IV antibiotics with OA regimens providing both aerobic and anaerobic cover (eTable 1 in the Supplement), making it unclear if the reduction in infection is due to better antibiotic cover or the additional use of OA. Second, over the last decade, large database reviews using the National Surgical Quality Improvement Program (NSQIP) database have reported that MBP + IV + OA significantly reduces SSI, anastomotic leaks (AL), ileus, and hospital stays.17 While these results reflect real-life practice, they also raise questions. What is the quality of evidence in databases compared with RCTs? Are the groups being compared well matched? Identified differences in the American Society of Anesthesiologists score, disseminated cancer, laparoscopic surgery, and other risk factors in favor of the MBP + IV + OA group16,17 (as well as unknown differences) can be difficult to control for in practice. Also, no data are available on the adequacy of aerobic and anaerobic antibiotic cover between the groups being compared.
Unresolved questions have contributed to differences in regional practices. In parts of Europe, there has been an emphasis on IV antibiotics with no MBP (which could be summarized as less is better), while in North America there has been a greater use of MBP + IV + OA (summarized as more is better). In the context of conflicting interpretations and differences in practice, we wanted to synthesize all available RCT data. While meta-analyses are helpful, they can only compare 2 options using pairwise comparisons. To address this controversy, we needed an inclusive, methodologically sound analysis of all the available evidence, which simultaneously integrates data assessing different options for bowel preparation and antibiotic administration. This problem is ideally suited to a network meta-analysis (NMA).20 This can integrate all options into a network where direct evidence from head-to-head comparisons and indirect evidence of comparisons linked within the network are assessed. This allows us to statistically compare and rank different treatment options. This NMA seeks to determine how different strategies for MBP and antibiotic administration rank in terms of their risks and benefits.
Methods
A systematic review of RCTs comparing different methods of bowel preparation among patients undergoing elective colorectal surgery was performed according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. The a priori protocol was published in PROSPERO.21
Data Sources
MEDLINE, Embase, the Cochrane Library (CENTRAL), and Scopus databases and trial registries (ClinicalTrials.gov and World Health Organization International Clinical Trials Registry) were searched. The bibliographies of included studies, clinical practice guidelines, and systematic reviews were hand searched for other relevant articles. There were no language or publication period limitations. The search strategy (eMethods in the Supplement) was based on the keywords: surgery, colorectal, mechanical bowel preparation, and randomized controlled trial. To capture studies observing OA, we searched for OA by their generic name and identified all OA used in RCTs of bowel preparation.
Included Studies
We reviewed the use of IV antibiotics, OA, MBP, enemas, and combinations of these treatments. Exclusion criteria included studies that were not RCTs, studies observing pediatric patients, and studies where results for different bowel preparation interventions were combined.22 As the importance of prophylactic antibiotics is well established, RCTs comparing a treatment with a group with no antibiotics were excluded. To identify the impact of differences in aerobic and anaerobic antibiotic cover on outcomes,8 it was necessary to group the RCTs into 3 models. Model 1 included all RCTs, model 2 included RCTs with good aerobic and anaerobic cover in all groups, taking into account both IV antibiotics and OA, and model 3 included RCTs with good IV aerobic and anaerobic cover. Overall good antibiotic cover required the criterion of effective aerobic and anaerobic cover,8 where effective aerobic cover was defined in line with Clinical and Laboratory Standards Institute guidelines,23 and effective anaerobic cover was defined as a minimum inhibitory concentration to inhibit the growth of 90% of most anaerobic organisms to a factor of less than 16 for most anaerobic pathogens and/or an overall resistance to anaerobic bacteria of less than 20%. This definition of effective anaerobic cover was based on a review of the literature and expert microbiological advice from the Department of Microbiology at the University of Otago in Dunedin, New Zealand. We report the results for model 2 in this article, and the results for all models in eTables 1 to 15 and eFigures 1 to 8 in the Supplement.
Included bowel preparation treatment options were:
MBP + IV
IV antibiotics alone (IV)
IV antibiotics with an enema (IV + E)
IV antibiotics and OA with or without an enema (IV + OA ± E)
-
MBP + IV + OA
with good aerobic and anaerobic IV antibiotic cover and additional OA (MBP + IVA + OA)
with incomplete IV antibiotic cover, but when OA were added, good aerobic and anaerobic cover was achieved (MBP + IVB + OA)
MBP and OA (MBP + OA)
OA alone (OA)
Enemas given with MBP were considered part of the MBP. Studies had to compare at least 2 bowel preparation options.
Outcomes were reported as defined in individual studies, and in line with the following definitions. The primary outcomes were superficial incisional and deep incisional SSI and AL. SSI was described as wound infection in most of the RCTs. AL was defined as clinical disruption of the anastomosis. A radiological diagnosis with no clinical problem was excluded. Secondary outcomes included mortality, defined for the time period reported, which was most commonly 30 days. Deep peritoneal infection or space SSI was defined as an intraperitoneal abscess when AL was not identified. Distant infections included pneumonia, urinary tract infections, and pyrexia of unknown origin. Length of stay (LOS), the number of days from surgery to discharge, was only analyzed in studies performed after 1999. Ileus was as defined within individual studies. The main adverse effects were preoperative severe nausea with or without vomiting, electrolyte problems, and cardiorespiratory problems for MBP; and postoperative diarrhea and Clostridium difficile infection for antibiotics. All measures were recorded as dichotomous variables (number of events), except for LOS, which was recorded as a continuous variable.
Data Extraction
Two researchers (B.S. and G.A.T.) independently screened all citations for eligibility, reviewed abstracts for eligibility, and extracted data, with discrepancies being resolved by the senior author (J.C.W.). Data, including study and patient characteristics, intervention details, and outcome measures, were extracted into forms developed from the Cochrane Collaboration’s data extraction template.24 The corresponding author was contacted to clarify information as required. The methodological quality of studies was assessed by 3 reviewers (K.C., B.S., and G.A.T.) using the Cochrane Collaboration’s risk of bias tool for RCTs. Each domain was assessed as high, low, or unclear risk.
Data Synthesis
Network diagrams illustrated the direct comparisons between the bowel preparation treatments. Random-effects NMA was performed, including direct and indirect comparisons, to determine the pooled relative effect of each treatment compared with every other treatment for the outcomes of interest. Analyses were performed using a frequentist framework in Stata version 15.1 (StataCorp) with routines developed by Chaimani et al,25 including the mvmeta, network, and network graphs packages.26,27 Categorical data were summarized as odds ratios (ORs) with 95% CIs and presented in league tables. The relative ranking of different bowel preparations was estimated for each outcome using the distribution of ranking probabilities and surface under the cumulative ranking curves (SUCRA).25 Between-study heterogeneity was evaluated using τ2. We assessed each model for global and local inconsistency. Global inconsistency was evaluated by assessing the difference between direct and indirect estimates. Local inconsistency (inconsistency factor)28 was evaluated for each independent closed loop in the network. Side-splitting was used to detect disagreements between direct and indirect estimates.29 Funnel plots were used to assess publication bias. Cluster analysis was performed by comparing the SUCRA rankings of the different bowel preparation options for the 2 primary outcomes, SSI and AL.30 Sensitivity analysis for primary outcomes included assessing studies with the highest methodological quality and assessor blinding, assessing the results for models 1, 2, and 3, and reassigning cephalosporin antibiotics with good aerobic and some anaerobic cover (cefoxitin and flomoxef) to good aerobic and anaerobic cover. Subgroup analyses of the primary outcomes were planned for the ascending colon, descending colon, and rectum.
Results
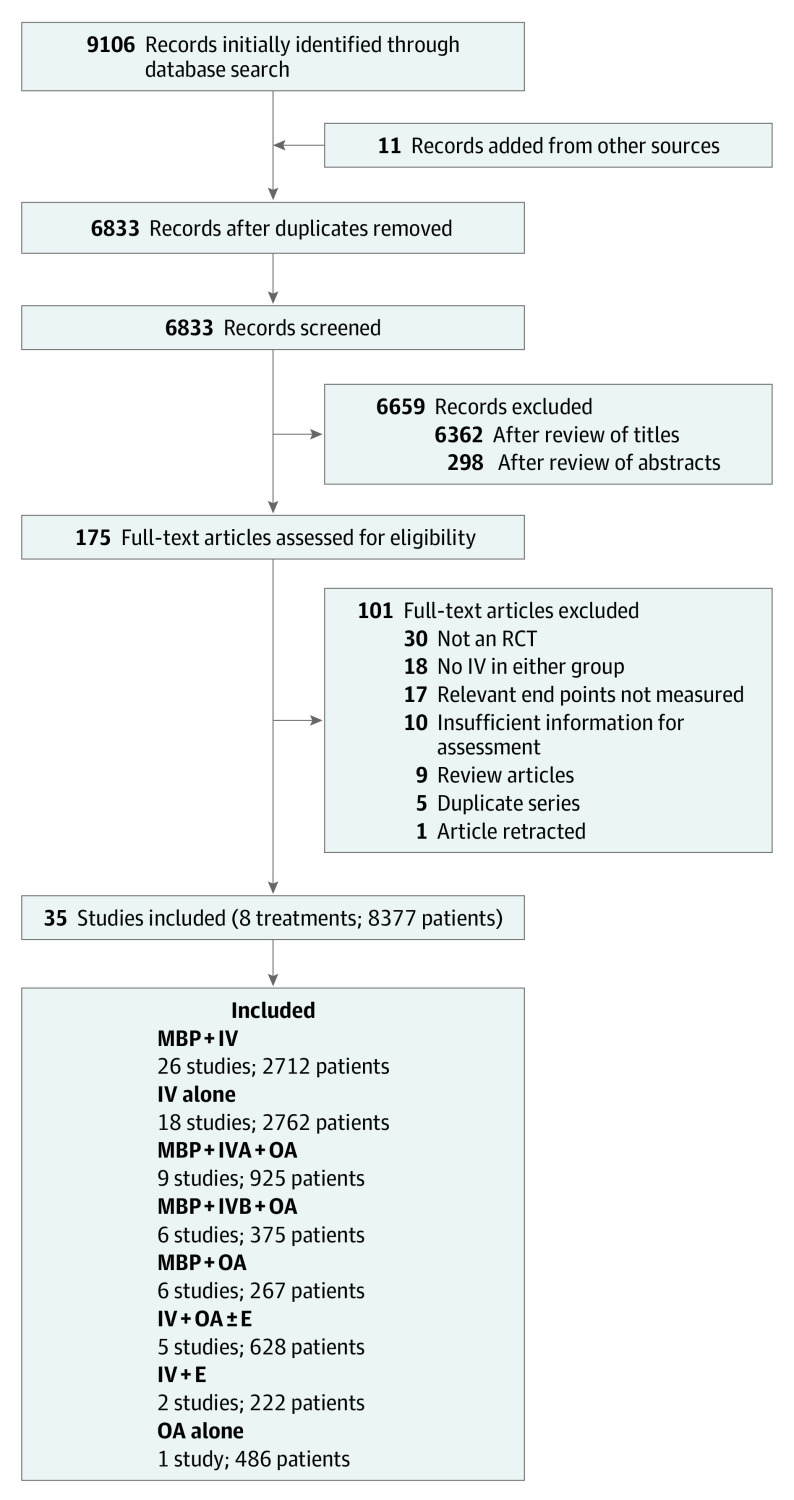
From 6834 titles, 472 abstracts, and 175 articles, 35 eligible RCTs including 8377 patients were identified. The PRISMA flowchart is summarized in Figure 1. Details of identified RCTs are summarized in eTable 1 in the Supplement. The risk of bias assessment (eFigure 1 in the Supplement) shows that in 6 of 7 categories most studies were scored as low or unclear risk of bias. As participant blinding is not possible when one group uses MBP, a number of otherwise high-quality studies were categorized as high risk in this domain. Blinding of assessors was low, unclear, and high risk in 46%, 34%, and 20% of studies, respectively. Eight RCTs (23%) were high risk in zero domains.
Figure 1. PRISMA Flow Diagram for Data Collection.
E indicates enema; IV, intravenous antibiotics; IVA, adequate IV antibiotics; IVB, IV antibiotics with incomplete aerobic and anaerobic cover; MBP, mechanical bowel preparation; OA, oral antibiotics; RCT, randomized clinical trial.
Primary Outcomes
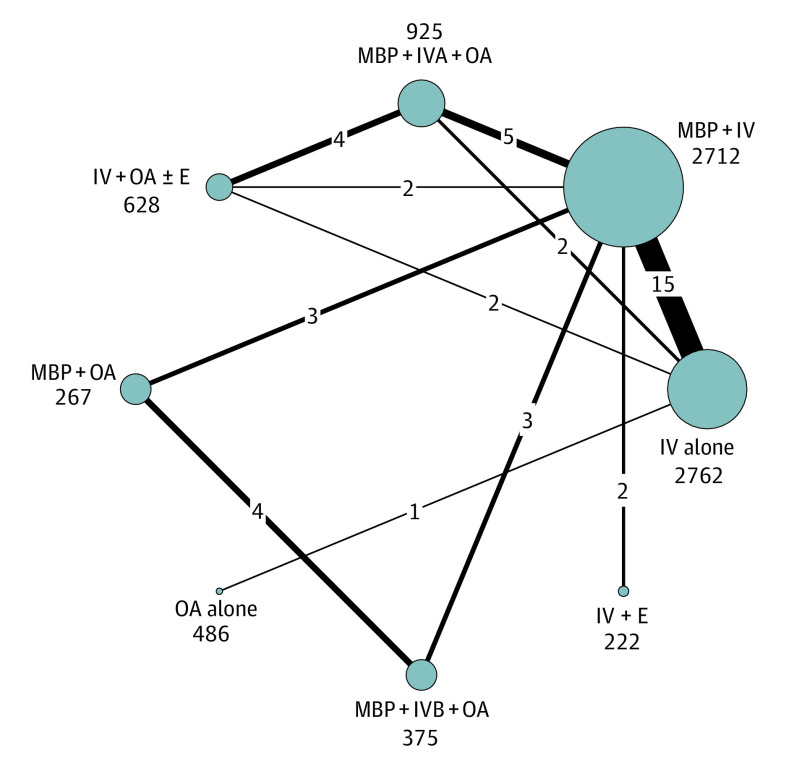
Figure 1 summarizes the number of RCTs and patients for each of the bowel preparation options. All studies assessed SSI and included 8377 patients. Figure 2 illustrates the network for SSI. The results showed good consistency between direct and indirect measurements (χ2 = 6.09; P = .64), good local consistency with no loops with an inconsistency factor greater than 1.1 (eFigure 7 in the Supplement) and no evidence of publication bias (eFigure 8 in the Supplement). The best ranking (Figure 3) of treatments to reduce SSI was achieved with IV + OA ± E (86% chance of being the best treatment option) followed by MBP + IVA + OA (85% chance of being the second best treatment option). In order of ranking, these were followed by IV alone, IV + E, MBP + IV, MBP + IVB + OA, OA alone, and MBP + OA. Patients treated with IV + OA ± E and MBP + IVA + OA had significantly fewer SSIs than all other treatment options (Table). MBP + IV and IV + E were significantly better than MBP + OA. There was no significant difference between IV + OA ± E (628 patients [7%]) and MBP + IV + OA (925 patients [11%]). Sensitivity analyses, as described in the Methods, did not change these results (eFigure 9 in the Supplement). There were insufficient RCT data reporting results separately for ascending colon, descending colon, and rectal surgery to perform the planned subanalysis. A subanalysis using model 1 is presented in the eFigure 4 in Supplement.
Figure 2. Network Plot of Direct Comparisons for Surgical Site Infection .
The size of the individual nodes represents the number of patients studied for each bowel preparation treatment option, and the thickness of the lines is proportional to the number of studies directly comparing the different nodes. E indicates enema; IV, intravenous antibiotics; IVA, adequate IV antibiotics; IVB, IV antibiotics with incomplete aerobic and anaerobic cover; MBP, mechanical bowel preparation; OA, oral antibiotics.
Figure 3. Rankogram for Surgical Site Infection Results.
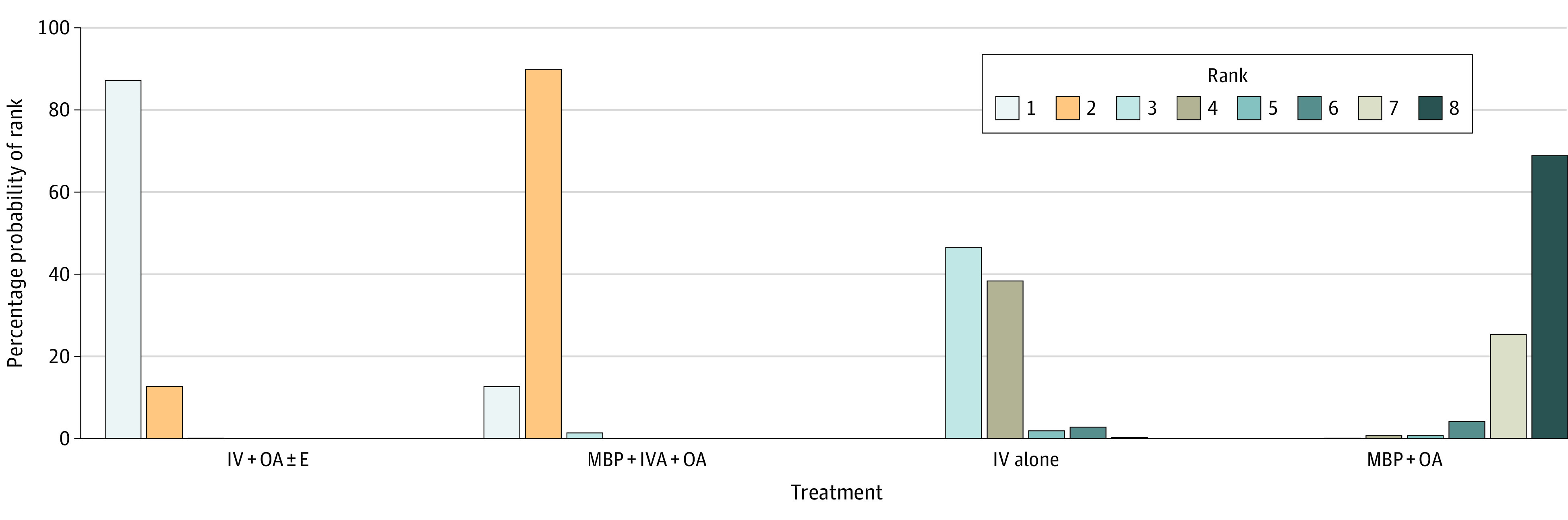
The rankogram shows the probability of each preparation option being ranked best performing to worst performing. For example, intravenous antibiotics with oral antibiotics with or without enema (IV + OA ± E) has an 86% probability of being ranked best, 12.7% probability of being ranked second, and a less than 1% probability for the other options. In comparison, IV antibiotics (IV) alone has a 47% probability of being ranked third, 38% probability of being ranked fourth, 12% probability of being ranked fifth, 2.8% probability of being ranked sixth, and less than 1% probability of the other options. IVA indicates adequate IV antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics.
Table. Odds Ratios (ORs) for Number of Surgical Site Infections.
| Treatment | OR (95% CIa) | |
|---|---|---|
| IV + OA | MBP + IV + OA | |
| OA alone | 0.14 (0.06-0.33)b | 0.19 (0.08-0.43)b |
| MBP + OA | 0.10 (0.04-0.25)b | 0.14 (0.07-0.31)b |
| MBP + IVB + OA | 0.18 (0.08-0.41)b | 0.25 (0.12-0.51)b |
| MBP + IV | 0.22 (0.12-0.40)b | 0.31 (0.20-0.48)b |
| IV alone | 0.27 (0.15-0.50)b | 0.38 (0.24-0.62)b |
| IV + E | 0.26 (0.11-0.63)c | 0.37 (0.17-0.81)d |
| MBP + IV + OA | 0.71 (0.41-1.21) | NA |
| IV + OA ± E | NA | 1.41 (0.83-2.42) |
Abbreviations: E, enema; IV, intravenous antibiotics; IVB, inadequate IV antibiotics; MBP, mechanical bowel preparation; OA, oral antibiotics.
OR less than 1 means that surgical site infection is less likely after the treatment in the column compared with the treatment in the corresponding row. For example, an OR of 0.5 means that the occurrence of a surgical site infection is half as likely for the treatment in the column than for the treatment in the corresponding row.
P < .001.
P < .01.
P = .01.
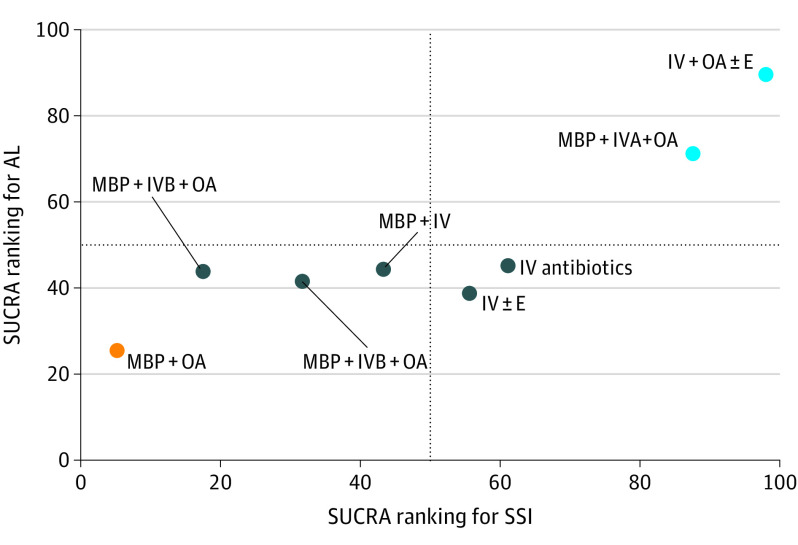
Analysis of AL included 7788 patients (93%) in 31 RCTs (89%), with good consistency and no evidence of publication bias. There were no significant differences between different options, although IV + OA ± E (628 patients [7%]) and MBP + IVA + OA (908 patients [11%]) had lower AL rates than other options (eTable 5 in the Supplement). There was insufficient information to assess the severity of AL.31 Sensitivity analysis, including models 1 and 3 (eTable 5 in the Supplement), did not change these results. To further assess the association of OA with AL, we summarized the data into the 4 options reported by Toh et al32 and by Garfinkle et al17: MBP + OA, MBP alone, OA alone, or none (neither MBP or OA). While Toh et al32 ignored the importance of IV antibiotics and differences in aerobic and anaerobic cover, our analysis only included studies with good overall antibiotic cover. Analysis of 7325 patients in 27 RCTs showed no significant differences although there was a trend in favor of options with OA (eTable 15 in the Supplement). The cluster plot for the primary outcomes (Figure 4) showed 3 clusters where IV + OA ± E and MBP + IVA + OA formed a cluster characterized by similar associations with SSI and AL, which was superior to other bowel preparation options.
Figure 4. Clustered Ranking Plot of Treatment Associations of Bowel Preparation With Surgical Site Infection (SSI) and Anastomotic Leaks (AL).
The colors represent different clusters. The clusters representing similar effects for treating SSI and AL are IV antibiotics and OA with or without an enema (IV + OA ± E), MBP with OA and adequate IV antibiotics (MBP + IVA + OA) (as the best-performing cluster) then IV antibiotics alone (IV), IV antibiotics with an enema (IV + E), MBP with IV antibiotics (MBP + IV), MBP with OA and IV antibiotics with incomplete aerobic and anaerobic cover (MBP + IVB + OA), OA alone (OA), and finally MBP + OA (as the worst-performing cluster). The surface area under the cumulative ranking (SUCRA) curve represents the overall rank for each treatment with regards to the likelihood of the outcome of interest. For IV + OA ± E, this is 98% for SSI and 90% for AL. In contrast, for IV, this is 61% for SSI and 45% for AL.
Secondary Outcomes
NMA showed good consistency and no significant differences between treatment options for the following secondary end points: mortality (5764 patients [69%], 23 RCTs [66%]), deep peritoneal infection (7332 patients [88%], 29 RCTs [83%]), pneumonia (5340 patients [64%], 14 RCTs [43%]), urinary tract infection (5493 patients [66%], 15 RCTs [43%]), pyrexia of unknown origin (2780 patients [33%], 10 RCTs [29%]), and LOS (5801 patients [69%], 16 RCTs [46%]). Details are presented in eTable 7 through eTable 12 in the Supplement. Results for ileus, adverse effects of antibiotics, and adverse effects of bowel preparation were reported with insufficient numbers for analysis; however, results using model 1 are presented in eTable 13 through eTable 15 in the Supplement. No significant differences were reported for ileus.
Discussion
The main finding of this NMA was that adding IV + OA resulted in a significant reduction in SSI by greater than 50% compared with other options. This was the case both with and without MBP. The finding that IV + OA ± E is an excellent (perhaps the best) option in colorectal surgery is interesting from 2 perspectives. First, it avoids the potential adverse effects of full MBP while having the benefit of combining both IV antibiotics and OA. Second, this option has not been widely used, and therefore requires further assessment. This network identified 5 RCTs 33,34,35,36,37 including the recent ORALEV38 and SELECT39 studies that assessed this option. Large database studies, which summarize data into MBP + OA, MBP alone, OA alone, and none (neither MBP or OA), although not assessing IV antibiotic use and differences in aerobic and anaerobic antibiotic cover, also provide some evidence supporting the use of IV + OA ± E. The Veterans Affairs Surgical Quality Improvement Program (VASQIP) study40 showed an SSI rate of 8.3% for OA, 9.2% for MBP + OA, and approximately 20% for groups without any OA. In a study using NSQIP data, Morris et al18 showed an SSI of 9.4% for OA and 6.3% for MBP + OA. Similarly Garfinkle et al17 showed that OA reduced SSI and that there were no significant differences in the results for OA (similar to IV + OA ± E) compared with MBP + OA (similar to MBP + IV + OA). The results of this NMA, the 2020 ORALEV study,38 and large database evidence highlight the importance of this underused option.
The advantage of MBP + IVA + OA in reducing SSI is extensively supported by the literature. This includes RCTs as reported in our NMA,19,34,36,41,42,43,44,45,46,47 meta-analyses,13,14,15 and NSQIP database studies.16,17,18 This NMA demonstrates that the advantages of MBP + IV + OA are also present when only studies with good antibiotic cover in all groups being compared are assessed, confirming that OA confer a benefit beyond improving the spectrum of antibiotic cover.
With respect to AL, there was no significant advantage in favor of any option. However, OA was associated with a nonsignificant reduction in AL both in our NMA and when using the approach used by Toh et al.32 This result, based on RCT data, is different to conclusions reached in large database studies,16,17,18 where AL was significantly reduced when OA were given. The reason for this difference is unclear. Important advantages of RCTs include consistency of protocol and the ability to control for unknown confounders through randomization. In the context of only 908 patients (11%) receiving MBP + IVA + OA in this analysis, there will continue to be discussion over how to best interpret these results.
Limitations
This study has limitations. A potential criticism of our article is that it is too complex, comparing too many bowel preparation options. As our protocol included all eligible RCTs, the choice of bowel preparation options was determined by the RCT data. This has the advantage of documenting the effectiveness and rankings of these different regimens. One challenge was how best to incorporate all RCTs into an NMA while accounting for differences in antibiotic cover. This was achieved by using 3 models. In this article, we included RCTs with good aerobic and anaerobic antibiotic cover in all groups being compared (model 2), in line with contemporary colorectal practice. With respect to the potential impact of changes in practice over time on the NMA, the results for SSI and AL were consistently the same, indicating that changes, such as the introduction of laparoscopic surgery, equally impacted the different bowel preparation options being compared. A review of all RCTs may also have problems introduced by differences in the definitions and methods used to diagnose end points. When end points were different to our study definitions, such as AL including radiologically diagnosed asymptomatic leaks, these data were not included in the analysis. Variations, in line with study definitions and applied equally to all groups being compared, were included in the analysis.
The main weakness of the NMA is the limited number of studies and patients included, with less than 500 patients for 4 of the bowel preparation options. With NMA, there also needs to be some caution interpreting the results when an option with few studies is ranked at either extreme of the network. For example, IV + OA ± E (5 RCTs) performed as the best option for SSI, and OA alone (1 RCT) as the worst option. The relatively limited data (628 patients [7%]) in the IV + OA ± E group was expected, as this has only been assessed in a few studies. However, with the long history of using MBP + IV + OA and the amount of literature discussing this, 925 patients (11%) in the MBP + IVA + OA group was less than expected, and is perhaps the main reason for ongoing discussion around the advantages of MBP + IV + OA.
These weaknesses demonstrate the need for further research, including larger studies with standardized methods for diagnosing end points. For SSI, more RCTs examining the role of IV + OA ± E and MBP + IVA + OA among patients undergoing both colon and rectal surgery are indicated. Microbiologically, while we know that adding OA to MBP reduces bacterial concentration in the colon,2 the impact of OA without MBP on the microflora of the colon requires further study. Studies assessing the role of enemas would also be of interest. Also, since some RCTs have insufficient details in the Methods sections, it is unclear if enemas were used in left-sided colon surgery without MBP. This makes results from the IV + E group more difficult to interpret. For AL, larger RCTs with good antibiotic cover in all groups being compared and assessing the impact of combining IV + OA will clarify if OA significantly impacts rates of AL. In contrast, good data exist comparing MBP + IV and IV alone, and no further studies comparing these options are required. Another consequence of low patient numbers was that we were unable to perform planned subanalyses of the ascending and descending colons and rectum. Also, as most of the data on IV + OA ± E was in colon surgery, this has not yet been adequately assessed in rectal surgery.
Conclusions
In conclusion, a review of all RCT evidence demonstrates that adding OA to IV antibiotics among patients undergoing elective colorectal surgery may reduce SSI. While there were no significant differences in favor of any option for AL, the options associated with reductions in SSI were also favorably ranked in terms of reducing AL. There were no convincing differences in secondary outcomes.
eMethods. Search strategy for network meta-analysis on bowel preparation for elective colorectal surgery.
eTable 1. Summary of 3 models used for mechanical bowel preparation network meta-analysis model parameters.
eTable 2. Data available for analysis for primary and secondary end points using model 1.
eTable 3. Data available for analysis for primary and secondary end points using model 2.
eFigure 1. Risk of bias assessment summary.
eTable 4. SSI league tables.
eTable 5. AL league tables.
eTable 6. Odds ratios (95% CI) for anastomotic leak analysis with nodes according to Toh et al.
eTable 7. Mortality league tables.
eTable 8. Deep peritoneal infection league tables.
eTable 9. Pneumonia league tables.
eTable 10. Urinary tract infection league tables.
eTable 11. Pyrexia of unknown origin league tables.
eTable 12. Length of stay league tables.
eTable 13. Ileus league table.
eTable 14. Antibiotics adverse effects (significant postoperative diarrhea) league table.
eTable 15. Mechanical bowel preparation adverse effects (significant preoperative nausea and/or vomiting).
eFigure 2. SSI network plots for surgical site infection results for models 1, 2, and 3.
eFigure 3. SSI rankograms.
eFigure 4. SSI forest plots model 1.
eFigure 5. SSI forest plots model 2.
eFigure 6. SSI forest plots model 3.
eFigure 7. SSI IF plots.
eFigure 8. SSI funnel plots.
eFigure 9. Surface area under the curve (SUCRA) plots for sensitivity analyses.
eReferences.
References
- 1.Gordon P, Nivatvongs S. Principles and Practice of Surgery for the Colon, Rectum, and Anus. 2nd ed. Quality Medical Publishing. 1999. [Google Scholar]
- 2.Nichols RL, Broido P, Condon RE, Gorbach SL, Nyhus LM. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg. 1973;178(4):453-462. doi: 10.1097/00000658-197310000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matheson DM, Arabi Y, Baxter-Smith D, Alexander-Williams J, Keighley MR. Randomized multicentre trial of oral bowel preparation and antimicrobials for elective colorectal operations. Br J Surg. 1978;65(9):597-600. doi: 10.1002/bjs.1800650902 [DOI] [PubMed] [Google Scholar]
- 4.Clarke JS, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg. 1977;186(3):251-259. doi: 10.1097/00000658-197709000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keighley MR., Arabi Y, Alexander-Williams J. Which is the best route of antibiotic prophylaxis in elective colorectal surgery: oral or parenteral? Gut. 1979;20:A453. [Google Scholar]
- 6.Aeberhard P, Fluckiger M, Berger J, et al. Antibiotic bowel preparation or perioperative parenteral shielding in colon surgery? Langenbeck’s Arch Surg. 1981;353:233–240. doi: 10.1007/BF01266008 [DOI] [PubMed] [Google Scholar]
- 7.Weaver M, Burdon DW, Youngs DJ, Keighley MR. Oral neomycin and erythromycin compared with single-dose systemic metronidazole and ceftriaxone prophylaxis in elective colorectal surgery. Am J Surg. 1986;151(4):437-442. doi: 10.1016/0002-9610(86)90097-8 [DOI] [PubMed] [Google Scholar]
- 8.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2014;(5):CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung B, Påhlman L, Nyström PO, Nilsson E; Mechanical Bowel Preparation Study Group . Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg. 2007;94(6):689-695. doi: 10.1002/bjs.5816 [DOI] [PubMed] [Google Scholar]
- 10.Dahabreh IJ, Steele DW, Shah N, Trikalinos TA. Oral mechanical bowel preparation for colorectal surgery: systematic review and meta-analysis. Dis Colon Rectum. 2015;58(7):698-707. doi: 10.1097/DCR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 11.Wille-Jørgensen P, Guenaga KF, Matos D, Castro AA. Pre-operative mechanical bowel cleansing or not? an updated meta-analysis. Colorectal Dis. 2005;7(4):304-310. doi: 10.1111/j.1463-1318.2005.00804.x [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations: 2018. World J Surg. 2019;43(3):659-695. doi: 10.1007/s00268-018-4844-y [DOI] [PubMed] [Google Scholar]
- 13.Bellows CF, Mills KT, Kelly TN, Gagliardi G. Combination of oral non-absorbable and intravenous antibiotics versus intravenous antibiotics alone in the prevention of surgical site infections after colorectal surgery: a meta-analysis of randomized controlled trials. Tech Coloproctol. 2011;15(4):385-395. doi: 10.1007/s10151-011-0714-4 [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum. 2016;59(1):70-78. doi: 10.1097/DCR.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 15.Koullouros M, Khan N, Aly EH. The role of oral antibiotics prophylaxis in prevention of surgical site infection in colorectal surgery. Int J Colorectal Dis. 2017;32(1):1-18. doi: 10.1007/s00384-016-2662-y [DOI] [PubMed] [Google Scholar]
- 16.Kiran RP, Murray ACA, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262(3):416-425. doi: 10.1097/SLA.0000000000001416 [DOI] [PubMed] [Google Scholar]
- 17.Garfinkle R, Abou-Khalil J, Morin N, et al. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum. 2017;60(7):729-737. doi: 10.1097/DCR.0000000000000851 [DOI] [PubMed] [Google Scholar]
- 18.Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg. 2015;261(6):1034-1040. doi: 10.1097/SLA.0000000000001125 [DOI] [PubMed] [Google Scholar]
- 19.Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg. 2002;45(3):173-180. [PMC free article] [PubMed] [Google Scholar]
- 20.Mills EJ, Thorlund K, Ioannidis JPA. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914 [DOI] [PubMed] [Google Scholar]
- 21.Network meta-analysis of bowel preparation in elective colorectal surgery. PROSPERO identifier:CRD42017059746. Updated November 4, 2020. Accessed April 16, 2021. https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017059746&ID=CRD42017059746
- 22.Jung B, Lannerstad O, Påhlman L, Arodell M, Unosson M, Nilsson E. Preoperative mechanical preparation of the colon: the patient’s experience. BMC Surg. 2007;7:5. doi: 10.1186/1471-2482-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein MP. M100 Performance Standards for Antimicrobial Susceptibility Testing, 31st ed. Clinical and Laboratory Standards Institute; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochrane. Data collection form. Accessed December 16, 2019. https://dplp.cochrane.org/data-extraction-forms
- 25.Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White I. NETWORK: Stata module to perform network meta-analysis. Stata J. Published online December 1, 2015. doi: 10.1177/1536867X1501500403 [DOI] [Google Scholar]
- 27.Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J. Published online December 1, 2015. doi: 10.1177/1536867X1501500402 [DOI] [Google Scholar]
- 28.White I. MVMETA: Stata module to perform multivariate random-effects meta-analysis. Accessed April 16, 2021. https://econpapers.repec.org/software/bocbocode/s456970.htm
- 29.Shim S, Yoon B-H, Shin I-S, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. doi: 10.4178/epih.e2017047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romesburg CH. Cluster Analysis for Researchers. Lulu Press; 1984. [Google Scholar]
- 31.Pittet O, Nocito A, Balke H, et al. Rectal enema is an alternative to full mechanical bowel preparation for primary rectal cancer surgery. Colorectal Dis. 2015;17(11):1007-1010. doi: 10.1111/codi.12974 [DOI] [PubMed] [Google Scholar]
- 32.Toh JWT, Phan K, Hitos K, et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection: a network meta-analysis. JAMA Netw Open. 2018;1(6):e183226. doi: 10.1001/jamanetworkopen.2018.3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abis GSA, Stockmann HBAC, Bonjer HJ, et al. ; SELECT trial study group . Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg. 2019;106(4):355-363. doi: 10.1002/bjs.11117 [DOI] [PubMed] [Google Scholar]
- 34.Alcantara Moral M, Serra Aracil X, Bombardó Juncá J, et al. A prospective, randomised, controlled study on the need to mechanically prepare the colon in scheduled colorectal surgery. Cir Esp. 2009;85(1):20-25. doi: 10.1016/S0009-739X(09)70082-X [DOI] [PubMed] [Google Scholar]
- 35.Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(8):729-738. doi: 10.1016/S2468-1253(20)30075-3 [DOI] [PubMed] [Google Scholar]
- 36.Verma GR, Pareek S, Singh R. Mechanical bowel preparation in elective colo-rectal surgery: a practice to purge or promote? Indian J Gastroenterol. 2007;26(3):142-143. [PubMed] [Google Scholar]
- 37.Zmora O, Mahajna A, Bar-Zakai B, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg. 2003;237(3):363-367. doi: 10.1097/01.SLA.0000055222.90581.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;1253:1-10. doi: 10.1016/S2468-1253(20)30075-3 [DOI] [PubMed] [Google Scholar]
- 39.Abis GSA, Stockmann HBAC, Bonjer HJ, et al. ; SELECT trial study group . Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg. 2019;106(4):355-363. doi: 10.1002/bjs.11117 [DOI] [PubMed] [Google Scholar]
- 40.Cannon JA, Altom LK, Deierhoi RJ, et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum. 2012;55(11):1160-1166. doi: 10.1097/DCR.0b013e3182684fac [DOI] [PubMed] [Google Scholar]
- 41.Anjum N, Ren J, Wang G, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum. 2017;60(12):1291-1298. doi: 10.1097/DCR.0000000000000927 [DOI] [PubMed] [Google Scholar]
- 42.Lau WY, Chu KW, Poon GP, Ho KK. Prophylactic antibiotics in elective colorectal surgery. Br J Surg. 1988;75(8):782-785. doi: 10.1002/bjs.1800750819 [DOI] [PubMed] [Google Scholar]
- 43.Coppa GF, Eng K, Gouge TH, Ranson JH, Localio SA. Parenteral and oral antibiotics in elective colon and rectal surgery. a prospective, randomized trial. Am J Surg. 1983;145(1):62-65. doi: 10.1016/0002-9610(83)90167-8 [DOI] [PubMed] [Google Scholar]
- 44.Eisenberg HW. Cefamandole preparation for colonic surgery. Dis Colon Rectum. 1981;24(8):610-612. doi: 10.1007/BF02605757 [DOI] [PubMed] [Google Scholar]
- 45.Kaiser AB, Herrington JL Jr, Jacobs JK, Mulherin JL Jr, Roach AC, Sawyers JL. Cefoxitin versus erythromycin, neomycin, and cefazolin in colorectal operations. importance of the duration of the surgical procedure. Ann Surg. 1983;198(4):525-530. doi: 10.1097/00000658-198310000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peruzzo L, Savio S, De Lalla F. Systemic versus systemic plus oral chemoprophylaxis in elective colorectal surgery. Chemioterapia. 1987;6(2)(suppl):601-603. [PubMed] [Google Scholar]
- 47.Sertoli MR, Cafiero F, Campora E, et al. A randomized trial of systemic versus oral prophylactic antibiotic treatment in colo-rectal surgery. Chemioterapia. 1982;1:375-378. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search strategy for network meta-analysis on bowel preparation for elective colorectal surgery.
eTable 1. Summary of 3 models used for mechanical bowel preparation network meta-analysis model parameters.
eTable 2. Data available for analysis for primary and secondary end points using model 1.
eTable 3. Data available for analysis for primary and secondary end points using model 2.
eFigure 1. Risk of bias assessment summary.
eTable 4. SSI league tables.
eTable 5. AL league tables.
eTable 6. Odds ratios (95% CI) for anastomotic leak analysis with nodes according to Toh et al.
eTable 7. Mortality league tables.
eTable 8. Deep peritoneal infection league tables.
eTable 9. Pneumonia league tables.
eTable 10. Urinary tract infection league tables.
eTable 11. Pyrexia of unknown origin league tables.
eTable 12. Length of stay league tables.
eTable 13. Ileus league table.
eTable 14. Antibiotics adverse effects (significant postoperative diarrhea) league table.
eTable 15. Mechanical bowel preparation adverse effects (significant preoperative nausea and/or vomiting).
eFigure 2. SSI network plots for surgical site infection results for models 1, 2, and 3.
eFigure 3. SSI rankograms.
eFigure 4. SSI forest plots model 1.
eFigure 5. SSI forest plots model 2.
eFigure 6. SSI forest plots model 3.
eFigure 7. SSI IF plots.
eFigure 8. SSI funnel plots.
eFigure 9. Surface area under the curve (SUCRA) plots for sensitivity analyses.
eReferences.