Abstract
Purpose
Locoregional therapy at primary or secondary sites in breast cancer may be associated with improved survival as compared to systemic therapy alone. We explored the sociodemographic and clinicopathologic factors associated with the use of radiation versus surgical resection of metastatic sites (metastasectomy) in patients with de novo stage IV breast cancer, followed by the associated overall survival.
Methods
We sampled the National Cancer Database for patients with de novo stage IV breast cancer, (2010–2017) and described cohort's characteristics using univariate analyses. We identified 5 subgroups based on malignant site involvement: 1. Bone only, 2. Brain only, 3. Liver only, 4. Lung only, and 5. Metastasis involving >1 site. Kaplan-Meier modeling with log-rank testing and multivariate Cox Regression analysis were used to explore differences in overall survival between those that received radiation at secondary sites and those that underwent metastasectomy.
Results
N = 22,749patients were included in this analysis. Radiation (81.2%) was used more commonly than metastasectomy (28.8%). Metastasectomy was associated with better median overall survival across all 5 cohorts (p < .001), with the survival benefit being the most pronounced with lung only (OS: 56.9 months; HR 0.8, 95% CI 0.7–0.9, p = .032), or liver only (OS: 41.6 months; HR: 0.9; 95% CI: 0.7–1.1, p < .001) metastasis.
Conclusion
Metastasectomy in patients with de novo stage IV breast cancer may be associated with improved overall survival as compared to radiation of secondary lesions, particularly in those with only liver or lung involvement. Prospective randomized controlled trials investigating surgical resection of metastatic sites in patients with breast cancer are warranted.
Keywords: Breast neoplasms, Metastasectomy, Targeted radiotherapy, Metastasis
Highlights
-
•
Metastasectomy in Stage IV breast cancer leads to improved survival regardless of organ involvement and number of lesions.
-
•
The survival benefit of metastasectomy is most apparent when the breast cancer metastasis involves the liver or the lung.
-
•
Radiation and metastasectomy should be considered for treatment of secondary breast cancer lesions in clinical practice.
1. Introduction
Breast cancer (BC) remains the most commonly diagnosed cancer in women. At diagnosis, 6% of patients with BC will already have distant organ metastasis [1]; referred to as de novo stage IV BC. Patients with de novo stage IV have an estimated 5-year overall survival (OS) rate of 27% [2]. The mainstay of treatment for metastatic BC remains systemic therapy, with the goal of palliating symptoms. In metastatic breast cancer, surgical resection may be used for palliative purposes [3]. While mounting evidence suggests that locoregional treatment (LRT), including radiation or surgical resection of the primary tumor, may be associated with improved survival, both in large registry analysis [4] and prospective trials [5], this has yet to be corroborated consistently [6,7]. Thus far, however, these studies have focused primarily on the impact of lumpectomy or mastectomy alone of primary lesions only, and did not explore the surgical resection of secondary BC lesions.
Targeting a secondary metastatic site is an important therapeutic consideration in metastatic BC. We previously showed that a combined approach involving resection of primary and secondary sites (i.e. ‘metastasectomy’) in patients with limited metastasis was associated with improved OS in stage IV BC [8]. We also showed that metastasectomy alone, without primary site surgical resection, was associated with improved OS compared to no surgery [9]. Retrospective and single-arm prospective studies have investigated the survival benefit of locoregional therapy in lung [[10], [11], [12]], prostate [13], and colorectal cancer [14]. No study to this date has compared the benefit associated with different LRT approaches for secondary BC lesions.
In this study, we sought to first explore the sociodemographic and clinicopathologic factors associated with the use of metastasectomy versus radiation of secondary sites in patients with de novo stage IV BC. Thereafter, we investigated whether radiation therapy or metastasectomy was associated with improved overall survival (OS).
2. Patients and methods
2.1. Patient data
We sampled the National Cancer Database (NCDB) for patients with de novo stage IV BC, diagnosed between 2010 and 2017. Access to the NCDB was achieved based on a Participant User File (PUF) award granted to the principal investigator (Z.N). The NCDB is a large de-identified national database in the United States, jointly supported by the American College of Surgeons (ACS) and the Commission on Cancer. It encompasses an estimated 70% of all cancer diagnoses from more than 1500 institutions nationwide [15].
2.2. Statistical analysis
Data were analyzed by using version 27.0 of the Statistical Package for the Social Sciences (SPSS) software (IBM, Version 23.0. Armonk, NY: IBM Corp). Bivariate analysis with chi-squared statistics was performed to compare patient sociodemographic (including age, sex, race, etc.) and clinicopathologic characteristics (such as receptor status, Charlson/Deyo comorbidity scoring, etc.) between two patient groups, based on the LRT approach adopted. We then performed a multivariate regression model to identify the most important factors associated with the choice of LRT at the secondary site.
We identified 5 cohorts of patients by the site of metastatic involvement: 1. Bone only, 2. Brain only, 3. Liver only, 4. Lung only, and 5. Metastasis involving >1 site. For each cohort, we used Kaplan-Meier (KM) models and log-rank testing to explore differences in OS by the approach of LRT used for secondary sites (radiation vs. metastasectomy). We adjusted KM models according to identified potential confounders. Multivariate Cox regression for OS was also performed for each cohort to ensure that the LRT approach was significantly independent of other potential confounders documented by the NCDB, including patient age, race, breast cancer receptor subtype, and Charlson/Deyo comorbidity scoring.
3. Results
3.1. Patient characteristics
A total of n = 22,749 patients with de novo stage IV BC were included in this analysis. These cases were included due to the availability of data on LRT approaches at secondary sites (radiotherapy vs. metastasectomy), as well as and survival. Most patients had metastasis involving more than 1 site (63.1%). In patients with secondary involvement to only one site, bone involvement was most common (31.6%), followed by brain involvement (2.9%), lung involvement (1.3%), and liver involvement (1.1%).
Interestingly, amongst patient characteristics, race did not play a statistically significant role in the choice of LRT modality (p = .059). Focusing on disease characteristics, breast cancer subtype per receptor status was not a significant predictor of LRT approach, as all breast cancer subtypes had similar rates of likelihood of metastasectomy: 17.1% for the HR+/HER2+ BC subtype, 19.4% for the HR+/HER2- BC subtype, for 17.8% for the HR-/HER2+ BC subtype and 18.4% for the HR-/HER2- BC subtype (p = .084). Similarly, tumor grade did not significantly predict the LRT modality adopted with metastasectomy rates of 17.8% for Grade I, 15.6% for Grade II and 16.7% for Grade III (p = .064).
Overall, radiation therapy (n = 18,469, 81.2%) was used more commonly to target secondary sites LRT when compared to metastasectomy (n = 4,280, 18.8%) (Table 1).
Table 1.
Sociodemographic and clinical characteristics associated with the use of surgical resection at secondary sites over radiation in patients with de novo stage IV breast cancer.
| Variable N (%) | LRT |
Chi-square p-value | Multiple logistic regression |
||
|---|---|---|---|---|---|
| Radiation therapy N = 18,469 (81.2%) | Metastasectomy N = 4280 (18.2%) | OR (95% CI) | p-value | ||
| Metastatic site involvement | <.001 | ||||
| Bone | 6057 (84.3) | 1124 (15.7) | <.001 | 1 | |
| Brain | 490 (73.6) | 176 (26.4) | 1.9 (1.6–2.3) | <.001 | |
| Liver | 100 (39.2) | 155 (60.8) | 7.8 (6.0–10.2) | <.001 | |
| Lung | 87 (29.7) | 206 (70.3) | 12.9 (9.9–16.7) | <.001 | |
| More than 1 site | 11,735 (81.8) | 2619 (18.2) | 1.2 (1.1–1.3) | <.001 | |
| Age | <.001 | ||||
| < 50 | 3635 (78.4) | 1000 (21.6) | <.001 | 1 | |
| 50–70 | 10,507 (81.1) | 2443 (18.9) | 0.8 (0.7–0.9) | <.001 | |
| > 70 | 4327 (83.8) | 837 (16.2) | 0.7 (0.6–0.8) | <.001 | |
| Race | |||||
| White | 14,797 (81.0) | 3462 (19.0) | .059 | – | – |
| Black | 2890 (82.5) | 613 (17.5) | – | – | |
| Asian | 400 (78.1) | 112 (29.2) | – | – | |
| Other | 182 (80.5) | 44 (19.5) | – | – | |
| Insurance Status | <.001 | ||||
| Not insured | 1089 (85.3) | 188 (14.7) | <.001 | 1 | |
| Private Insurance | 7610 (78.8) | 2052 (21.2) | 1.6 (1.3–1.9) | <.001 | |
| Medicare | 2592 (83.1) | 527 (16.9) | 1.2 (1.0–1.4) | .087 | |
| Medicaid | 6849 (82.4) | 1459 (17.6) | 1.4 (1.2–1.7) | <.001 | |
| Facility Type | <.001 | ||||
| Community CP | 1931 (88.0) | 264 (12.0) | <.001 | 1 | |
| Comprehensive Community CP | 7389 (84.4) | 1363 (15.6) | 1.3 (1.2–1.6) | <.001 | |
| Academic/Research CP | 5490 (75.4) | 1795 (24.6) | 2.4 (2.1–2.7) | <.001 | |
| Integrated Network CP | 2530 (81.8) | 562 (18.2) | 1.6 (1.4–1.9) | <.001 | |
| Charlson/Deyo Comorbidity Score | <.001 | ||||
| 0 | 15,210 (81.7) | 3385 (18.3) | <.001 | 1 | |
| 1 | 2381 (79.5) | 615 (20.5) | 1.2 (1.1–1.3) | <.001 | |
| 2 | 675 (76.3) | 210 (23.7) | 1.5 (1.3–1.8) | <.001 | |
| 3 | 293 (80.7) | 70 (19.3) | 1.2 (0.9–1.5) | .218 | |
| Receptor Status | |||||
| HR+/HER2+ | 1799 (82.9) | 372 (17.1) | .084 | – | – |
| HR+/HER2- | 7207 (80.6) | 1730 (19.4) | – | – | |
| HR-/HER2+ | 796 (82.2) | 172 (17.8) | – | – | |
| HR-/HER2- | 1333 (81.6) | 300 (18.4) | – | – | |
| Grade | |||||
| I | 1114 (82.2) | 242 (17.8) | .064 | – | – |
| II | 6097 (84.4) | 1129 (15.6) | – | – | |
| III | 6121 (83.3) | 1224 (16.7%) | – | – | |
CP = Cancer Program.
3.2. Use of either radiation or metastasectomy at secondary sites
Patient profile and clinical characteristics were significantly associated with different LRT approaches targeting secondary BC lesions. 1. Metastatic site involvement: Compared to patients with bone metastasis only, patients with liver only (OR 7.8; 95% CI 6.0–10.2, p < .001) or lung only (OR 12.9, 95% CI 9.9–16.7, p < .001) were more likely to receive metastasectomy.2. Age: Patients aged 50–70 (OR 0.8, 95% CI 0.7–0.9, p < .001) were less likely to receive metastasectomy as compared to patients <50 years old, while patients >70 years old (OR 0.7, 95% CI 0.6–0.8, p < .001) were the least likely to receive metastasectomy 3. Insurance status: Patients with private insurance were the most likely to receive metastasectomy (OR 1.6, 95% CI 1.3–1.9, p < .001), followed by patients on Medicaid (OR 1.4, 95% CI 1.2–1.7, p < .001), compared to uninsured patients. 4. Facility-type Community Cancer Programs (CP) were the least likely to use metastasectomy for the treatment of secondary cancer lesions in their patients overall P < .001). Academic/Research CP were the most likely (OR 2.4, 95% CI2.1–2.7, p < .001) to use metastasectomy in their patients, followed by Integrated Network CP (OR 1.6, 95% CI 1.4–1.9, p < .001) and Comprehensive community CP (OR 1.3, 95% CI 1.2–1.6, p < .001) 5. Charlson/Deyo comorbidity score was also significantly associated with the LRT treatment approach at secondary sites (p < .001). Increase in comorbidity score was associated with increased likelihood of metastasectomy as opposed to radiation when the score was 1 (OR: 1.2; 95% CI: 1.1–1.3, p < .001) or 2 (OR: 1.5, 95% CI: 1.3–1.8, p < .001), but not when it was 3 or above (OR: 1.2, 95% CI: 0.9–1.5, p = .218) (Table 1).
Race, BC receptor status, and tumor grade were not significantly associated with differences in the LRT approach for secondary BC lesions (Table 1).
3.3. Survival analysis
Multivariate Cox regression modeling (Table 2) was conducted to evaluate for differences in overall survival by treatment approach used for secondary sites, controlling for known predictors of survival documented by the NCDB. Survival curves were also constructed using the Kaplan-Meier method with log-rank testing on the 5 subgroups of analysis (i.e. patients with only bone involvement, brain involvement, liver involvement, lung involvement, or involvement of multiple sites) (Fig. 1 and Fig. 2). A summary of KM statistics can be found in Table 3. Of potential confounders, age was significantly different in patients with bone metastasis only receiving radiation versus metastasectomy, so KM modeling controlled for patients aged 50–70. Additionally, age, Charlson/Deyo comorbidity scoring, and receptor were all controlled for in patients with more than 1 metastatic site as bivariate analysis showed they were potential confounders.
Table 2.
Multivariable Cox Regression model for overall survival.
| HR (95% CI) | p-value | |
|---|---|---|
| LRT | ||
| Radiation | 1 | – |
| Metastasectomy | 0.7 (0.6–0.7) | <.001 |
| Metastatic site involvement | <.001 | |
| Bone | 1 | |
| Brain | 1.6 (1.5–1.8) | <.001 |
| Liver | 0.9 (0.7–1.1) | <.001 |
| Lung | 0.8 (0.7–0.9) | .032 |
| More than 1 site | 1.7 (1.6–1.8) | <.001 |
| Age | <.001 | |
| < 50 | 1 | |
| 50–70 | 1.3 (1.2–1.4) | <.001 |
| >70 | 1.9 (1.7–2.0) | <.001 |
| Race | <.001 | |
| White | 1 | |
| Black | 1.2 (1.1–1.2) | <.001 |
| Asian | 0.8 (0.7–0.9) | .020 |
| Other | 0.7 (0.5–0.9) | .002 |
| Charlson/Deyo Comorbidity Score | <.001 | |
| 0 | 1 | |
| 1 | 1.2 (1.2–1.3) | <.001 |
| 2 | 1.5 (1.3–1.7) | <.001 |
| 3 | 1.7 (1.4–2.0) | <.001 |
| Receptor Status | <.001 | |
| HR+/HER2+ | 1 | |
| HR+/HER2- | 1.1 (1.1–1.2) | <.001 |
| HR-/HER2+ | 1.3 (1.2–1.5) | <.001 |
| HR-/HER2- | 3.2 (2.9–3.4) | <.001 |
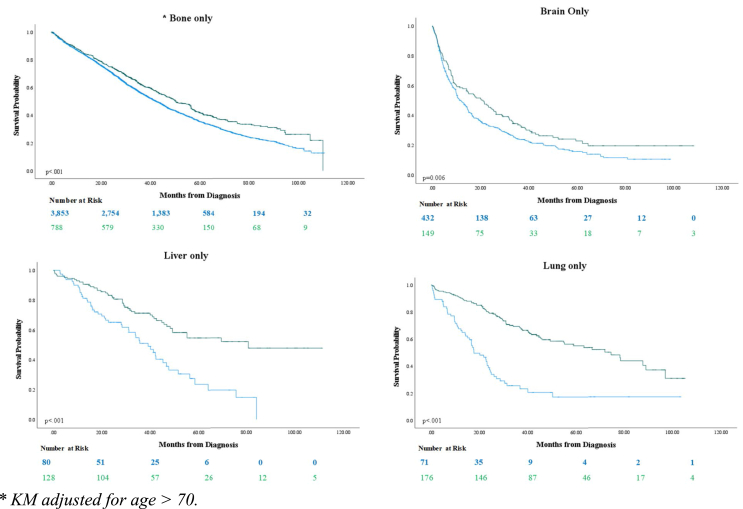
Fig. 1.
Kaplan-Meier models depicting overall survival of patients with breast cancer metastatic to only one site, by treatment approach at the secondary site (radiation versus metastasectomy).
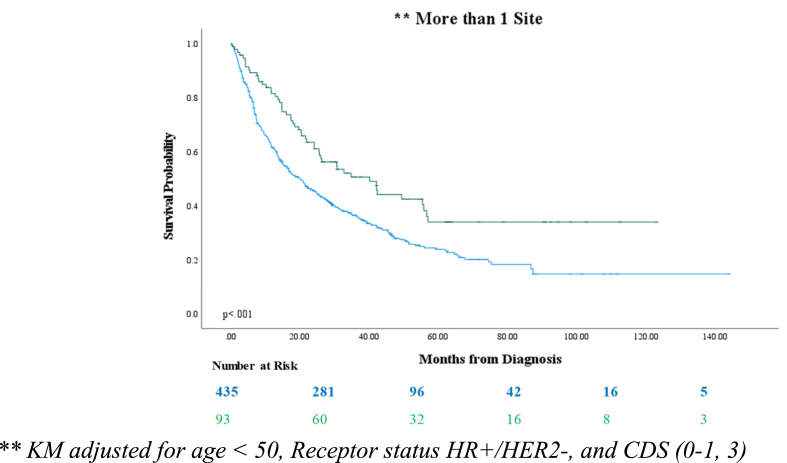
Fig. 2.
Kaplan-Meier model depicting overall survival of patients with breast cancer metastatic to >1 site, by treatment approach at the secondary site (radiation versus metastasectomy).
Table 3.
Summary of Kaplan-Meier modeling across all 5 cohorts of investigation by site of malignant involvement: 1. Bone only, 2. Brain only, 3. Liver only, 4. Lung only, and 5. Metastasis involving >1 site.
| KM Median OS (Months) |
ΔmOS (months) | Log-rank p-value | |||
|---|---|---|---|---|---|
| Radiation | Metastasectomy | ||||
| 1 site of metastasis | Bone Only ∗ | 42.4 | 50.3 | 8.9 | <0.001 |
| Brain Only | 11.9 | 21.0 | 9.1 | <0.001 | |
| Liver Only | 39.2 | 80.8 | 41.6 | <0.001 | |
| Lung Only | 17.7 | 74.6 | 56.9 | <0.001 | |
| > 1 site of metastasis ∗∗ | 20.1 | 40.1 | 20.0 | <0.001 | |
∗ KM adjusted for age > 70.
∗∗ KM adjusted for age < 50, Receptor status HR+/HER2-, and CDS (0–1, 3).
The multivariate Cox Regression model that controlled for other confounders of survival showed that patients who underwent metastasectomy of a secondary site had better OS compared to those that underwent radiation therapy (HR 0.7, 95% CI 0.6–0.7, p < .001). Metastatic site involvement was an additional independent predictor of OS: as compared to patients with bone metastasis, patients with metastasis to the lung only (HR 0.8, 95% CI 0.7–0.9, p = .032) had better OS while patients with brain only (HR 1.6, 95% CI 1.5–1.8, p < .001) or more than 1 metastatic site (HR 1.7, 95% CI 1.6–1.8, p < .001) had worse OS.
Age was a significant factor predicting OS (p < .001), with decreasing OS associated with an increase in age: age 50–70 (HR 1.3, 95% CI 1.2–1.4, p < .001), age >70 (HR 1.9, 95% CI 1.7–2.0, p < .001) as compared to those aged <50. Black patients had better OS (HR 1.2, 95% CI 1.1–1.2, p < .001) than white patients, while Asian patients (HR 0.8, 95% CI 0.7–0.9, p = .020) or patients from other races (HR 0.7, 95% CI 0.5–0.9, p = .002) had worse OS. Higher Charlson/Deyo comorbidity scoring was also associated with worse OS outcomes (p < .001).
Patients with receptor status HR+/HER2+ had the best OS as compared to other receptor subtypes (p < .001), with the worst OS seen in patients with receptor type HR-/HER2- (HR 3.2, 95% CI 2.9–3.4, p < .001).
Kaplan-Meier modeling indicated a significant benefit in OS observed with metastasectomy versus as radiation therapy in patients with stage IV BC as opposed to radiation across all 5 cohorts (p < .001) (Table 3). The difference in median OS (ΔmOS) by the LRT approach was the most pronounced when metastasis involved only the lung (ΔmOS: 56.9 months), followed by the liver (ΔmOS: 41.6 months. Metastasectomy improved median OS by ΔmOS = 20.0 months in patients with >1 metastatic site, as compared to radiation therapy, versus only the brain (ΔmOS: 9.1 months) or bone (ΔmOS: 8.9 months) (Table 3).
4. Discussion
This study used multivariate survival models on a large sample to investigate the impact of LRT on survival in metastatic BC. Our findings suggest that local treatment targeting secondary lesions, through either radiation therapy or surgical resection, results in improved OS, regardless of the treatment modality of primary lesions.
A number of clinical characteristics were predictive of the use of radiation therapy versus metastasectomy at the secondary sites. As would be expected, patients with metastasis to the liver or the lungs were more likely to undergo metastasectomy while patients with metastasis to the bone, brain, or with multiple metastatic sites were more likely to undergo radiation therapy. The current widespread use of surgical resection as treatment for primary and secondary liver malignancies may explain the higher rates of metastasectomy in this population [16]. Inconsistent evidence is found when looking at the survival benefit of surgical resection of liver metastasis [17], a finding that contrasts with ours.
Some evidence does however suggest improved survival from radiation to liver metastasis from colon cancer [18], notably in un-resectable tumors [19]. This finding that was corroborated when looking at 491 patients with BC, who demonstrated a median survival of 25.7 months after receiving radiation targeting their liver metastasis at a mean follow-up period of 26 months [20]. Focusing on lung metastasis, the clinical characteristics of lung metastases from BC – notably the peripheral location of most secondary BC lesions to the lung [21] – may also justify the high rates of secondary site surgical resection observed in our study population. A multicenter prospective study looking at 467 patients, complete resection was possible in 84% of lung metastasis [22]. Additionally, small population studies suggests survival benefit in women with isolated lung metastasis who underwent surgical resection of their secondary tumors [23]. One prospective study showed a median survival benefit of 79.2 months (6.6 years), similar to our findings which showed a survival benefit of 74.6 months [24]. Conversely, patients with brain metastasis are traditionally treated with whole-brain radiation [25]. Scant evidence is available on the role of surgery for the palliation of bone metastasis in BC. Similarly, radiation therapy is the mainstay of treatment for bone metastases [26], and provides excellent palliation for pain management [27,28]. Evidence on survival outcomes remains scarce. Data on sternal resection has shown good long-term local tumor of the term and adequate pain relief, but did not provide information on survival benefit [29,30]. These current management practices go in hand with our analysis findings that the survival benefit in a patient with liver (ΔmOS = 41.6 months) and lung (ΔmOS = 56.9 months) metastasis is much greater than that observed in patients with brain (ΔmOS = 8.9 months) and bone (ΔmOS = 9.1 months) metastasis.
Furthermore, this study also suggests that socioeconomic factors affecting healthcare disparity do not play a major role in the decision to surgically manage secondary lesions versus use radiation therapy. However, as would be expected, advanced age, higher comorbidity as measured by the Charlson/Deyo score, and receptor status were independent negative predictors of worsened outcome. These findings were consistent across all 5 patient cohorts. The main takeaway remains the survival benefit observed with either radiation therapy or metastasectomy after controlling for these factors negatively impacting survival. Metastasectomy also remained an independent predictor of better survival outcomes as compared to radiation therapy, after these factors were controlled for. Current National Comprehensive Cancer Network (NCCN) treatment guidelines recommend systemic therapy as the mainstay of treatment for stage IV BC, in the form of chemotherapy or endocrine therapy, depending on the molecular receptor status of the specific cancer [3]. With the observed survival benefit with LRT – either as radiation therapy or surgical resection – clinical practice needs to consider both options in patients with stage IV BS, with the appropriate use of either radiation therapy or metastasectomy depending on the clinical indication as part of a multidisciplinary discussion. As per the NCCN, clinical trials remain the best management of any patient with cancer. Which patients ultimately benefit the most when pursuing a metastasectomy vs localized treatment with radiation therapy needs to be answered in future prospective trials. The latter would also need to investigate these outcomes in relation to the various modalities of radiation therapy. Future trials are already looking to expand the use of radiation treatment for patients with limited sites of metastatic disease specifically oligometastatic disease [31].
An additional consideration to be investigated in clinical trials is the quality of life of patients during the increased survival period. Factors such as physical health, mental health, pain levels, etc. need to be explored prospectively. Physical symptoms, notably pain, mobility, physical activity, appetite, and sleep are all quality of life issues that women with stage IV BC face [32]. A Turkish trial in 2020 evaluated the quality of life in patients with Stage IV BC who received LRT and survived at least 3 years and found similar physical and mental health outcomes in this population as compared to patients with Stage IV BC who never received LRT [33]. There is however not enough evidence of the association between LRT and quality of life in Stage IV BC patients [34]. In fact, some prospective studies showed no improvement in quality of life when patients receive LRT for Stage IV BC [35], thus raising the need for this matter to be explored thoroughly in any prospective trial investigating the survival outcomes of LRT in this patient population.
The strength in the study lies in the consistency and reliability of the NCDB data, which undergoes continuous internal auditing, and reflects real practice on a national level. However, the limitations of this study also lie in the limitations of the NCDB and those of a retrospective study. Characteristics of the secondary BC lesions, such as size or relative location (peripheral vs. central) are not available and may limit the generalizability of our findings. Information on metastasis is limited to the following secondary sites: bone, brain, liver, lungs, distant lymph nodes, and “other”. Only bone, brain, liver, and lung were included for specificity. There is no documentation on the number of secondary sites that underwent surgical resection (e.g. resection of bone and brain metastases), versus metastasectomy at only one site. It was thus not possible to assess whether limited versus radical resection at metastatic sites is associated with improved survival. Furthermore, the NCDB does not document whether disease involvement of visceral organs is unifocal or multifocal. Survival outcomes, and the potential utility of the LRT approach to secondary lesions, may differ based on this pattern of involvement. This raises the need of focusing on the clinicopathologic characteristics of secondary BC lesions in clinical practice, with the aim of evaluating the possibility of surgical resection. The subsequent inclusion of this information in the NCDB and other major databases must ensue. Additionally, the NCDB does not report information on disease progression, or any patient outcome apart from OS such as disease control rate or progression free survival (PFS).
Despite these limitations, notably pertaining to the underestimation of the true extent of metastasis in Stage IV BC patients, a significant survival benefit was observed in our analysis. Both radiation therapy and metastasectomy should be considered in addition to systemic therapy in clinical practice.
5. Conclusion
Surgical resection of metastatic sites in patients with de novo Stage IV is associated with significantly improved OS as compared to radiation of these secondary lesions, regardless of organ involvement and number of secondary lesions. Improved OS is however most apparent when the BC metastasis involves the liver or the lung. Both radiation therapy and metastasectomy should be considered in addition to systemic therapy in clinical practice, depending on the clinical indication as part of a multidisciplinary discussion.
Prospective randomized controlled trials investigating radiation and/or surgical resection of metastatic sites in patients with stage IV BC are warranted. These trials need to additionally focus on the quality of life patients will have post-metastasectomy, and evaluate whether or not the treatment approach influences quality of life outcomes.
Funding
None of the authors have any funding to declare.
Availability of data and material
The data that support the findings of this study are available from the American College of Surgeons/American Cancer Society but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the American College of Surgeons/American Cancer Society.
Code availability
Not applicable.
Authors' contributions
N·B., M.Y., O.M., and M.N. are major contributors in study design, conduction of statistics and data interpretation, and manuscript writing. Z.N. participated in study design, interpretation of the data, and writing the manuscript. H.L. participated in the conduction of statistics. I.J., C.R., L.E., and D.S. participated in writing and editing. All authors read and approved the final manuscript.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Ethics approval
Ethical approval was obtained from the Cleveland Clinic Institutional Review Board (IRB) before conducting this study. All patient data were strictly de-identified and provided, with approval, from the American College of Surgeons as part of the National Cancer Database.
Consent to participate
Ethical approval was obtained from the Cleveland Clinic Institutional Review Board (IRB) prior to conducting this study. All patient data were strictly de-identified and provided, with approval, from the American College of Surgeons as part of the National Cancer Database.
Consent for publication
No individual person's data were included; all data is reported in an aggregated manner.
Declaration of Competing interest
The authors declare that they have no conflict of interest. The authors declare that they have no competing interests.
Contributor Information
Nadeem Bilani, Email: nadeem.bilani@mountsinai.org.
Marita Yaghi, Email: yaghim@ccf.org.
Mihir Naik, Email: NAIKM@ccf.org.
Iktej Jabbal, Email: jabbali@ccf.org.
Carlos Rivera, Email: RIVERAC13@ccf.org.
Leah Elson, Email: lcelson@gmail.com.
Hong Liang, Email: LIANGH2@ccf.org.
Diana Saravia, Email: SARAVID@ccf.org.
Zeina Nahleh, Email: nahlehz@ccf.org.
References
- 1.Atlanta: American Cancer Society . 2020. Breast cancer facts & figures 2019-2020.https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf 2020. Available from: [Google Scholar]
- 2.Howlader N., Noone A.M., Krapcho M. 2020. SEER cancer statistics review, 1975-2016.https://seer.cancer.gov/archive/csr/1975_2016/ Available from: [Google Scholar]
- 3.National Comprehensive Cancer Network . 7/26/2021. Breast cancer NCCN guidelines version 5.2021.https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf 2021. Available from: [Google Scholar]
- 4.Gera R. 2020. Locoregional therapy of the primary tumour in de novo stage IV breast cancer in 216 066 patients: a meta-analysis; pp. 1–11. 10(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soran A. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25(11):3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 6.Khan S.A. American Society of Clinical Oncology; 2020. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: a trial of the ECOG-ACRIN Research Group (E2108) [Google Scholar]
- 7.Fitzal F. Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE trial. Ann Surg. 2019;269(6):1163–1169. doi: 10.1097/SLA.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 8.Bilani N. Survival benefit of a combined surgical approach in patients with metastatic breast cancer. J Surg Oncol. 2021 doi: 10.1002/jso.26656. [DOI] [PubMed] [Google Scholar]
- 9.Bilani N. 2021. Effect of surgery at primary and metastatic sites in patients with stage IV breast cancer; pp. 170–180. 21(3) [DOI] [PubMed] [Google Scholar]
- 10.Iyengar P. 2018. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: a phase 2 randomized clinical trial. 4(1)e173501-e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez D.R. 2016. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study; pp. 1672–1682. 17(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez D.R. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II. randomized study. 2019;37(18):1558. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ost P R.D., Decaestecker K. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 14.Ruers T. 2017. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. 109(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilimoria K.Y. 2008. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States; pp. 683–690. 15(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinrich S., Lang H.J.I.s.s. 2017. Hepatic resection for primary and secondary liver malignancies; pp. 1–8. 2(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadot E. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg. 2016;264(1):147. doi: 10.1097/SLA.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Baere T. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. Am J Roentgenol. 2000;175(6):1619–1625. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 19.Wood T.F. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7(8):593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 20.Bortolotto C. Radiofrequency ablation of metastatic lesions from breast cancer. Journal of ultrasound. 2012;15(3):199–205. doi: 10.1016/j.jus.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung J.I. 2004. Thoracic manifestations of breast cancer and its therapy; pp. 1269–1285. 24(5) [DOI] [PubMed] [Google Scholar]
- 22.Friedel G. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur J Cardio Thorac Surg. 2002;22(3):335–344. doi: 10.1016/s1010-7940(02)00331-7. [DOI] [PubMed] [Google Scholar]
- 23.Planchard D. Uncertain benefit from surgery in patients with lung metastases from breast carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2004;100(1):28–35. doi: 10.1002/cncr.11881. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto M. Favourable long-term results after surgical removal of lung metastases of breast cancer. Breast Cancer Res Treat. 2008;110(3):485–491. doi: 10.1007/s10549-007-9747-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin X., DeAngelis L.M. Treatment of brain metastases. 2015;33(30):3475. doi: 10.1200/JCO.2015.60.9503. J.J.o.c.o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz S. 2011. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline; pp. 965–976. 79(4) [DOI] [PubMed] [Google Scholar]
- 27.Lutz S. Palliative radiation therapy for bone metastases: update of an ASTRO Evidence-Based Guideline. Practical radiation oncology. 2017;7(1):4–12. doi: 10.1016/j.prro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Chow R. Single vs multiple fraction palliative radiation therapy for bone metastases: cumulative meta-analysis. Radiother Oncol. 2019;141:56–61. doi: 10.1016/j.radonc.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Noble J. Sternal/para-sternal resection for parasternal local recurrence in breast cancer. Breast. 2010;19(5):350–354. doi: 10.1016/j.breast.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Dürr H.R. 2002. Surgical treatment of bone metastases in patients with breast cancer; pp. 191–196. 396. [PubMed] [Google Scholar]
- 31.Kent C.L., McDuff S.G., Salama J.K. 2020. Oligometastatic breast cancer: where are we now and where are we headed?—a narrative review; pp. 62–72. 9. [DOI] [PubMed] [Google Scholar]
- 32.Aranda S. 2005. Mapping the quality of life and unmet needs of urban women with metastatic breast cancer; pp. 211–222. 14(3) [DOI] [PubMed] [Google Scholar]
- 33.Soran A. 2021. The role of loco-regional treatment in long-term quality of life in de novo stage IV breast cancer patients: protocol MF07-01Q; pp. 3823–3830. 29(7) [DOI] [PubMed] [Google Scholar]
- 34.Reinhorn D. vol. 58. 2021. Locoregional therapy in de novo metastatic breast cancer: systemic review and meta-analysis; pp. 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjelic-Radisic V. Primary surgery versus no surgery in synchronous metastatic breast cancer: patient-reported quality-of-life outcomes of the prospective randomized multicenter ABCSG-28 Posytive Trial. BMC Cancer. 2020;20(1):1–15. doi: 10.1186/s12885-020-06894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the American College of Surgeons/American Cancer Society but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the American College of Surgeons/American Cancer Society.